- 1Division of Nephrology, Taichung Veterans General Hospital, Taichung, Taiwan
- 2Graduate Institute of Public Health, China Medical University, Taichung, Taiwan
- 3Department of Internal Medicine, College of Medicine, China Medical University, Taichung, Taiwan
- 4Department of Post-Baccalaureate Medicine, College of Medicine, National Chung Hsing University, Taichung, Taiwan
- 5Department of Pediatrics, Taipei City Hospital Zhongxiao Branch, Taipei, Taiwan
- 6Department of Health and Welfare, University of Taipei, Taipei, Taiwan
- 7School of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan
- 8Graduate Institute of Business Administration, Fu Jen Catholic University, New Taipei, Taiwan
- 9Department of Nephrology, Kaohsiung Medical University Baccalaureate Medicine, Kaohsiung, Taiwan
- 10Graduate Institute of Biomedical Sciences and School of Medicine, College of Medicine, China Medical University, Taichung, Taiwan
- 11Management Office for Health Data, China Medical University Hospital, Taichung, Taiwan
- 12Department of Bioinformatics and Medical Engineering, Asia University, Taichung, Taiwan
- 13Department of Nuclear Medicine and PET Center, China Medical University Hospital, Taichung, Taiwan
- 14Artificial Intelligence Center, China Medical University Hospital, Taichung, Taiwan
Introduction: Denosumab demonstrates efficacy in reducing the incidence of hip, vertebral, and nonvertebral fractures in postmenopausal women with osteoporosis. We present a population-based national cohort study to evaluate the infection risks in patients with osteoporosis after long-term denosumab therapy.
Methods: We used the Taiwan National Health Insurance Research Database (NHIRD) to identify patients with osteoporosis. The case cohort comprised patients treated with denosumab. Propensity score (PS) matching was used to select denosumab nonusers for the control cohort. The study period was between August 2011 and December 2017. Our study comprised 30,106 pairs of case and control patients.
Results: Patients receiving denosumab therapy had high risks of the following infections: pneumonia and influenza (adjusted hazard ratio [aHR]: 1.33; 95% confidence interval [CI]: 1.27 -1.39), urinary tract infection (aHR: 1.36; 95% CI:1.32 -1.40), tuberculosis (aHR: 1.60; 95% CI: 1.36 -1.87), fungal infection (aHR: 1.67; 95% CI:1.46 -1.90), candidiasis (aHR: 1.68; 95% CI: 1.47 -1.93), herpes zoster infection (aHR: 1.27; 95% CI: 1.19 -1.35), sepsis (aHR: 1.54; 95% CI:1.43 -1.66), and death (aHR: 1.26; 95% CI: 1.20 -1.32). However, the longer the duration of denosumab treatment, the lower the risk patients had of developing infections.
Discussion: Denosumab therapy is associated with a higher infection risk at the early periods of treatment. Nevertheless, the risk attenuates significantly after the 2nd year of therapy. Clinicians should closely monitor infection status in patients with osteoporosis during the initial stages of denosumab therapy.
Introduction
Osteoporosis is a progressive condition of bone fragility leading to risks of fracture. Denosumab, a fully human monoclonal antibody, binds with high specificity to the RANK ligand and reduces the number and activity of osteoclasts (OCs). In a 3-year randomized FREEDOM clinical trial, with a 7-year extension to evaluate efficacy and safety, denosumab demonstrated significant efficacy in reducing the incidence of hip, vertebral, and nonvertebral fractures of postmenopausal women with osteoporosis (1). Once age-related osteoporosis develops, patients will likely require lifelong treatment. This exposes patients to the adverse events of cumulative drug exposure, such as osteonecrosis of the jaw. Despite the benefits of anti-osteoporosis treatment, the risks of long-term exposure to osteoporosis drugs need to be evaluated, particularly in the older adult population (2).
The RANKL–RANK–OPG (osteoprotegerin) pathway involves the tumor necrosis factor (TNF) and TNF receptor superfamilies, which have many common signaling characteristics (3). The RANKL–RANK–OPG pathway was first discovered in the late 1990s and was considered important to immunity, primarily through its actions on dendritic cells (DCs) (4, 5). Concomitantly, it was determined to be critical to bone homeostasis through its regulation of OCs (6, 7). Denosumab (formerly AMG 162) is an effective compound that binds to and inhibits RANKL (8). Similar to OPG, denosumab inhibits bone loss by inhibiting RANKL’s action on the receptor RANK.
Reports concerning the relationship between denosumab and the risk of infection have been inconsistent. The relevant studies are limited by small sample sizes and short study periods. More importantly, infection risk was not the primary outcome in these studies. Accordingly, we conducted a population-based national cohort study to evaluate the infection risk in patients with osteoporosis after long-term denosumab treatment.
Methods
Data source
We used the Taiwan National Health Insurance Research Database (NHIRD), which is a valuable resource for public health research. The NHIRD contains comprehensive claims records related to outpatient and inpatient medical care, comprising the demographic information of beneficiaries; hospital care data; laboratory tests; diagnostic codes; interventional procedures; and drug prescription data such as drug quantity, expenditure, and dosage. All data are encrypted and deidentified to protect personal privacy. The diagnoses in the database are based on the International Classification of Diseases, 9th Revision and 10th Revision, Clinical Modification (ICD-9-CM & ICD-10-CM, respectively). Our study was approved by the Institutional Review Board of China Medical University Hospital Research Ethics Committee (CMUH109-REC2-031[CR-2]), and was conducted in accordance with the guidelines of the World Medical Association’s Declaration of Helsinki.
Study population
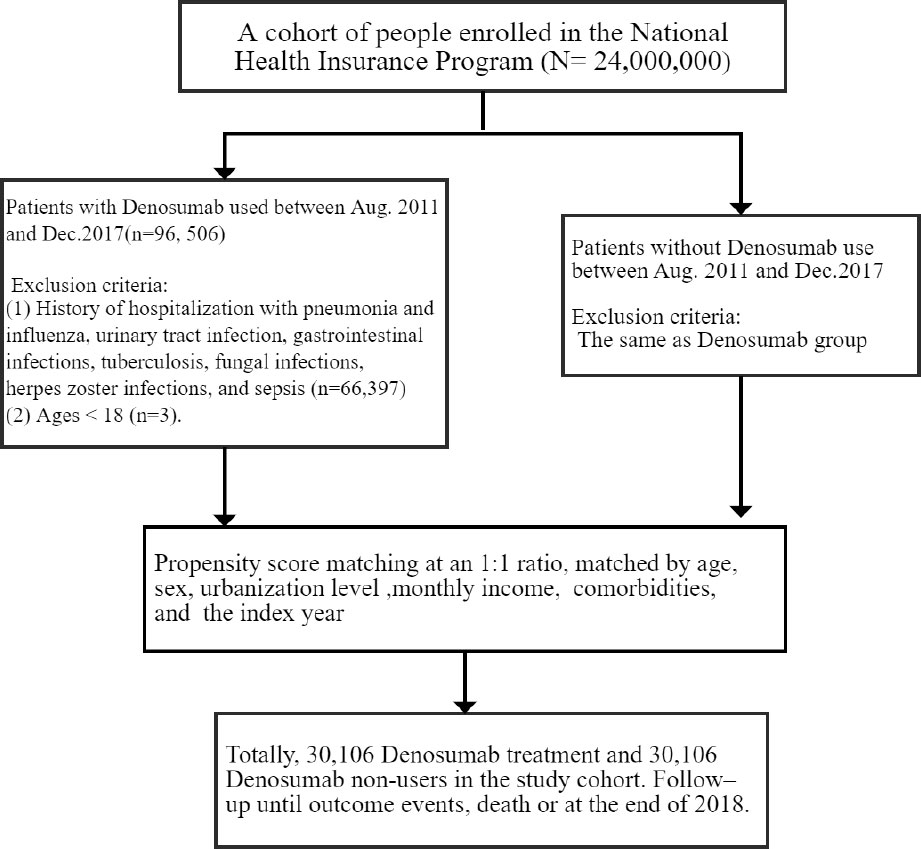
This retrospective cohort study focused on patients with osteoporosis who had or had not received denosumab therapy. The case cohort comprised patients who were treated with denosumab. The index date was the first day of denosumab therapy. Denosumab users received a subcutaneous dose of 60 mg every 6 months depending on clinical responses. The control cohort comprised patients who had not received denosumab therapy; these patients were selected on the basis of propensity score (PS) matching for balancing heterogenous factors such as age, gender, index year, comorbidities, and other medicines, and they were assigned to the control cohort at a 1:1 ratio to the case patients. Each member of the control cohort was assigned a random index date that postdated their diagnosis of osteoporosis. The exclusion criteria for both cohorts were age <18 years and outcomes (namely, hospitalization for infection) before the index date. The enrollment period was between August 2011 and December 2017 (Figure 1).
Outcome measurement and covariate
The study outcomes were the following infections: pneumonia and influenza (ICD-9-CM codes 480–488; ICD-10-CM code J09–J18); urinary tract infection (ICD-9-CM code 599; ICD-10-CM code N390); gastrointestinal infection (ICD-9-CM code 0088; ICD-10-CM codes A084, A088); tuberculosis (ICD-9-CM codes 010–018; ICD-10-CM codes A15–A19); fungal infection (ICD-9-CM codes 112, 1173, 1363, 3210, 4846, 51636, 5186; ICD-10-CM codes B37, B44, B45, B59, J84114), herpes zoster infection (ICD-9-CM codes 053, 054; ICD-10-CM codes B00, B02) and sepsis (ICD-9-CM code 99591; ICD-10-CM code A419). Patients were censored at the date of death, withdrawal from the health insurance system, or on Dec 31, 2018, whichever came first. Patients who died or left the program before the end of study were censored.
The following confounders were compared between cohorts: age, sex, urbanization level, monthly income, hypertension, type 2 diabetes mellitus (T2DM), hyperlipidemia, chronic obstructive pulmonary disease (COPD), obesity, cirrhosis, chronic kidney disease, heart failure, and fracture.
Statistical analysis
Data are presented as percentages and mean values. We used the chi-square test to compare proportional differences in baseline variables between case and control patients. A Wilcoxon signed-rank test was used to compare the median ages between the two groups. Incidence rates are presented as the number of events of 1000 person–years. The Cox proportional hazard regression model was used to compare the risks of developing outcome events between case and control patients. Adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated, with adjustment for important risk factors for developing study outcomes, including age, sex, urbanization level, monthly income, and comorbidities. We also considered death as a competing factor to estimate subhazard ratios (SHRs) and 95% confidence intervals (CIs) using the competing-risks regression models. The risk of study outcomes over time for patients who received denosumab therapy as compared with controls was determined using survival analysis with the Kaplan–Meier method, and the differences were evaluated using a log-rank test. All statistical analyses were performed using SAS (v.9.4; SAS Institute, Cary, NC), and R software (v.4.1.0, R Foundation, Vienna, Austria). Statistical significance was defined as a p value of <0.05.
Results
A total of 30,106 pairs of PS-matched case and control patients were analyzed. In the denosumab cohort, the median age was 76 years, and the majority of participants were women (86.1%); 48% of the participants lived in high-urbanization areas, and 56% had a monthly income between NT$20,001 and NT$39,999. The prevalent comorbidities in the denosumab cohort were hypertension (72.1%), hyperlipidemia (54.1%), and COPD (28%). During the follow-up period, 69.5% of the participants in the denosumab cohort received <4 injections of denosumab, 69.5% received 5–8 injections, and 8.09% received ≥9 injections (Table 1).
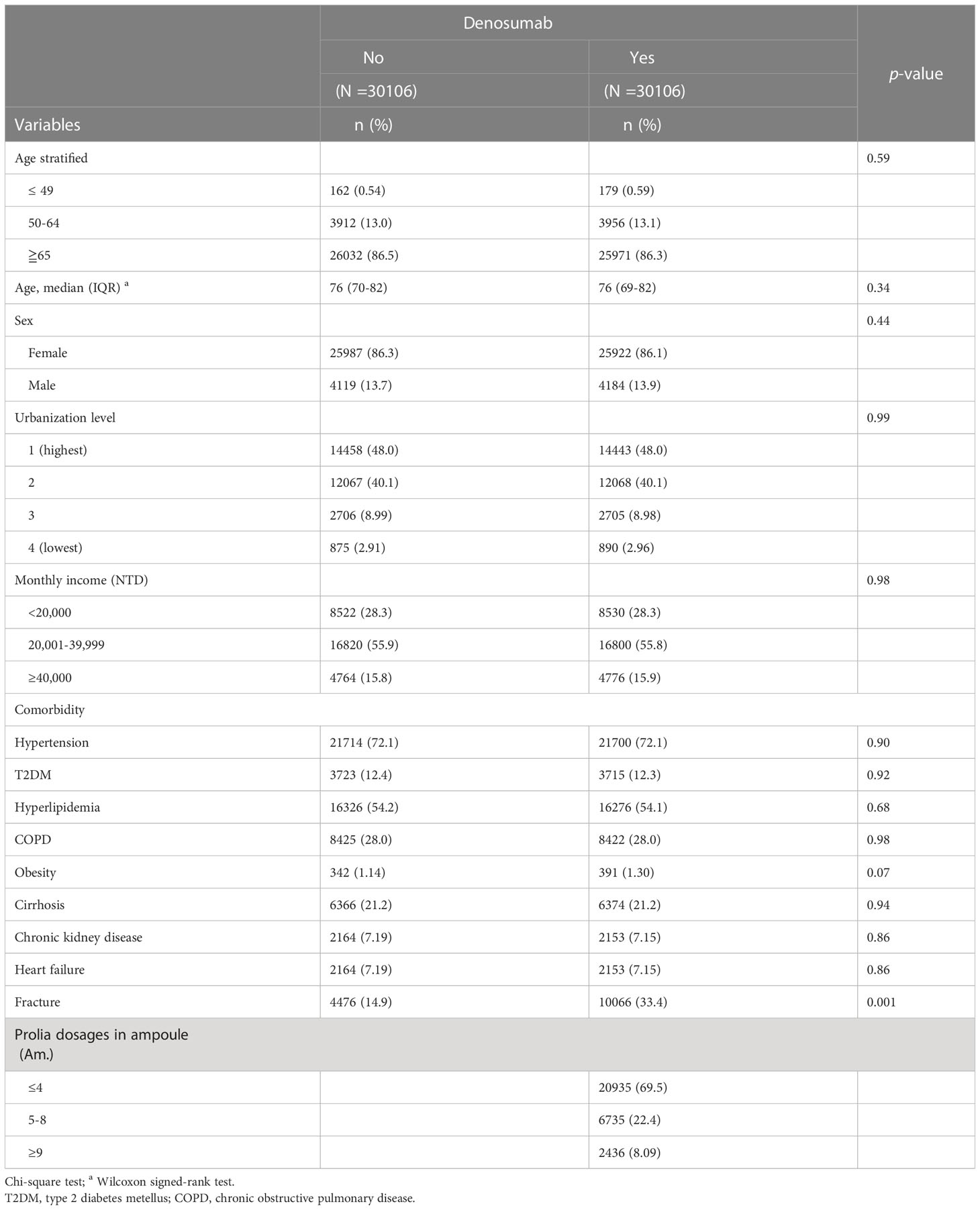
Table 1 Demographic characteristics, comorbidities and medications in patients with osteoporosis with and without denosumab use.
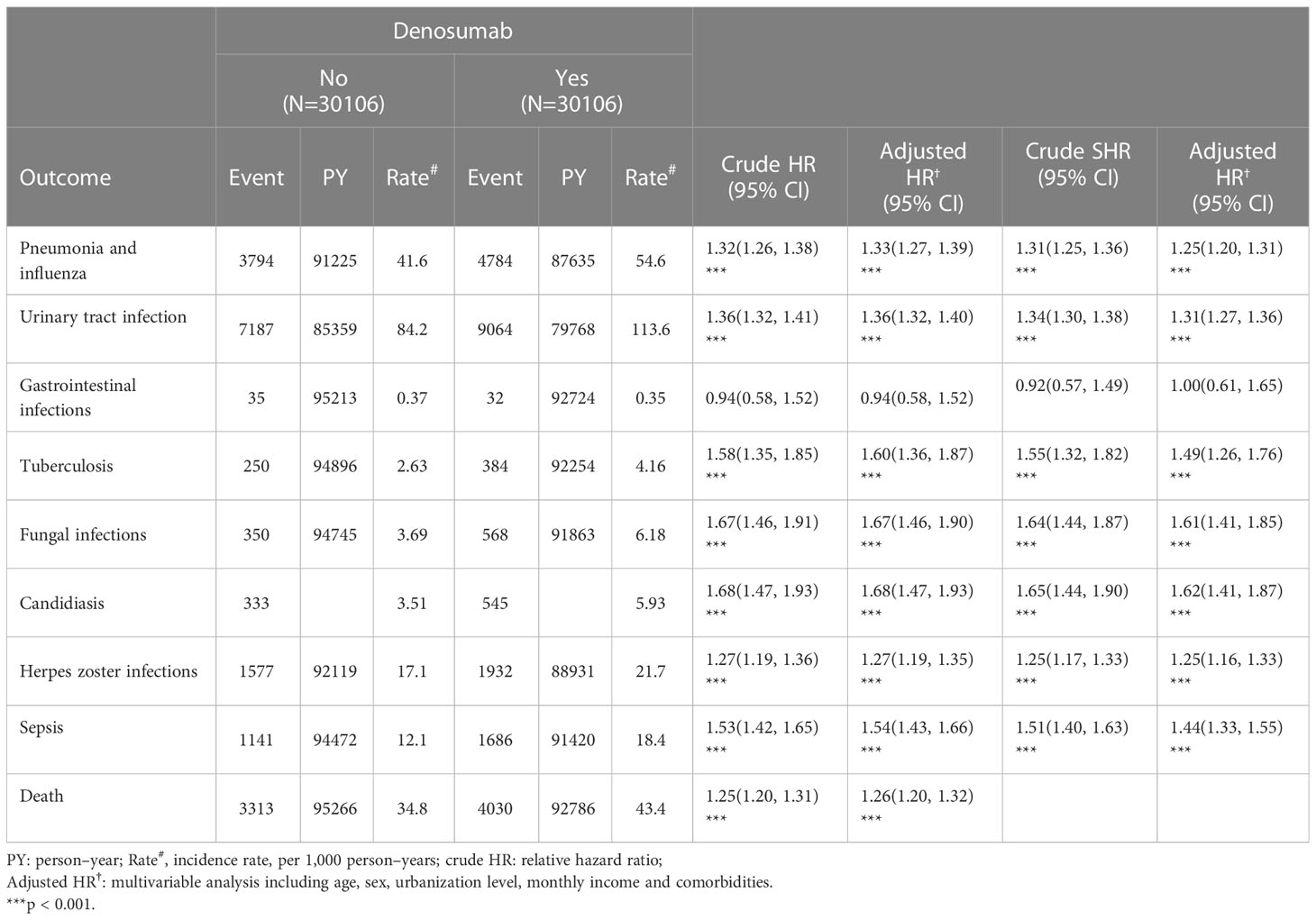
The outcomes and incidence rates of infections and death are presented in Table 2. In the denosumab cohort, urinary tract infection had the highest incidence rate (113.6 per 1000 person–years), followed by pneumonia and influenza (54.6 per 1000 person–years), herpes zoster infection (21.7 per 1000 person–years), and serious adverse events of sepsis (18.4 per 1000 person–years). Compared with the control group, patients receiving denosumab therapy had significantly higher risks of pneumonia and influenza (adjusted HR [aHR]: 1.33; 95% confidence interval [CI]: 1.27–1.39), urinary tract infection (aHR: 1.36; 95% CI: 1.32–1.40), tuberculosis (aHR: 1.60; 95% CI: 1.36–1.87), fungal infection (aHR: 1.67; 95% CI: 1.46–1.90), candidiasis (aHR: 1.68; 95% CI: 1.47–1.93), herpes zoster infection (aHR: 1.27; 95% CI: 1.19–1.35), and sepsis (aHR: 1.54; 95% CI: 1.43–1.66). Additionally, patients receiving denosumab therapy had a 26% higher risk of death (aHR: 1.26; 95% CI: 1.20–1.32; p < 0.001) than controls. Table 2 shows the subhazard ratios of outcome for patients receiving denosumab therapy, with consideration of the competing risk of death. We found that the denosumab cohort still remained a significantly higher risk of outcome than did in the control group, except for gastrointestinal infections.
Denosumab use was not significantly associated with gastrointestinal infection. As demonstrated in Figures 2, 3, the cumulative risks of infection and death in the denosumab cohort were significantly higher than those in the control cohort in terms of pneumonia and influenza, urinary tract infection, tuberculosis, fungal infection, herpes zoster infection, and sepsis, with a follow-up duration longer than 6 years.
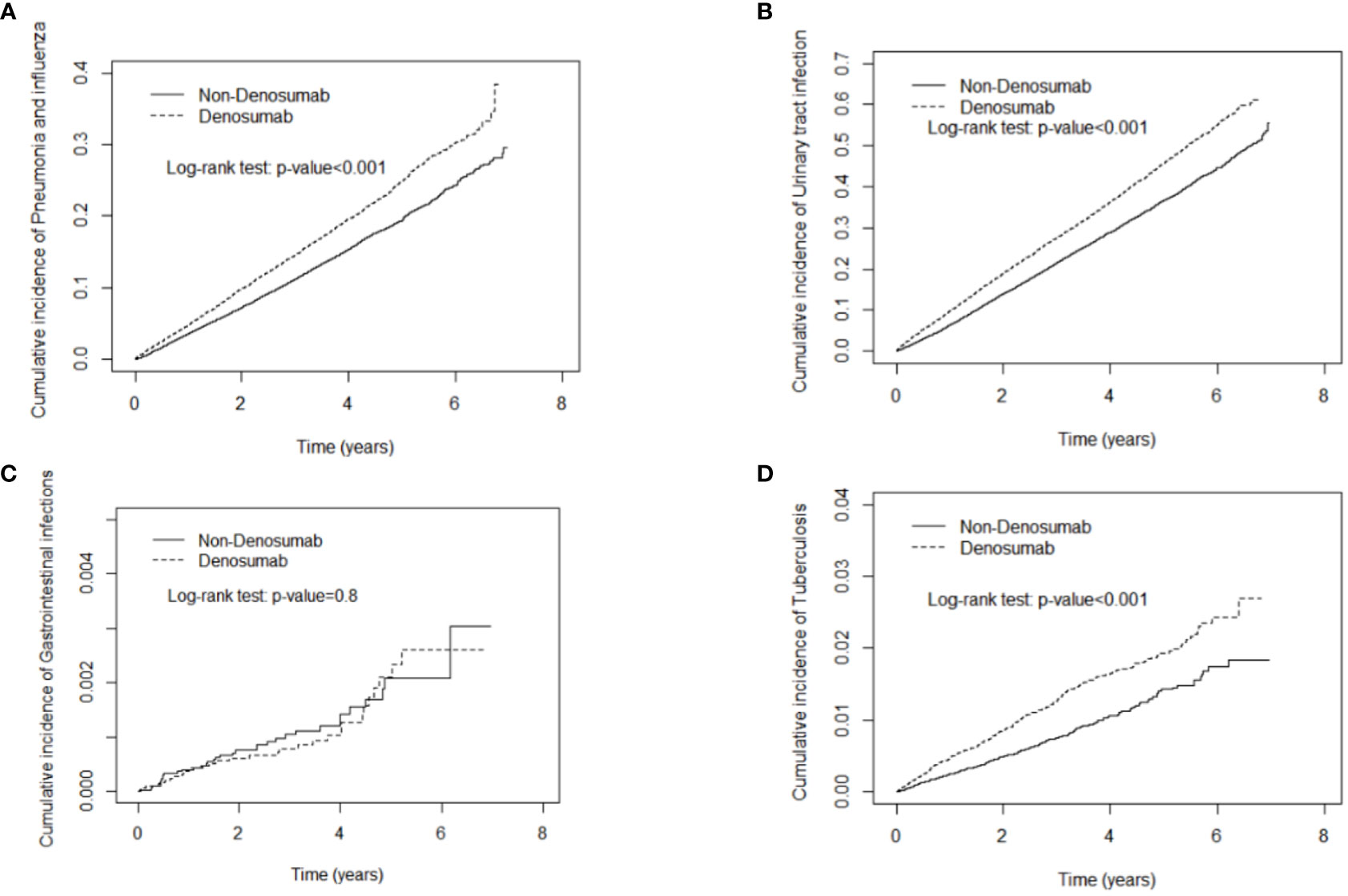
Figure 2 Cumulative incidence curves of pneumonia and influenza (A), urinary tract infection (B), gastrointestinal infections (C), tuberculosis (D) in patients with and without denosumab use.
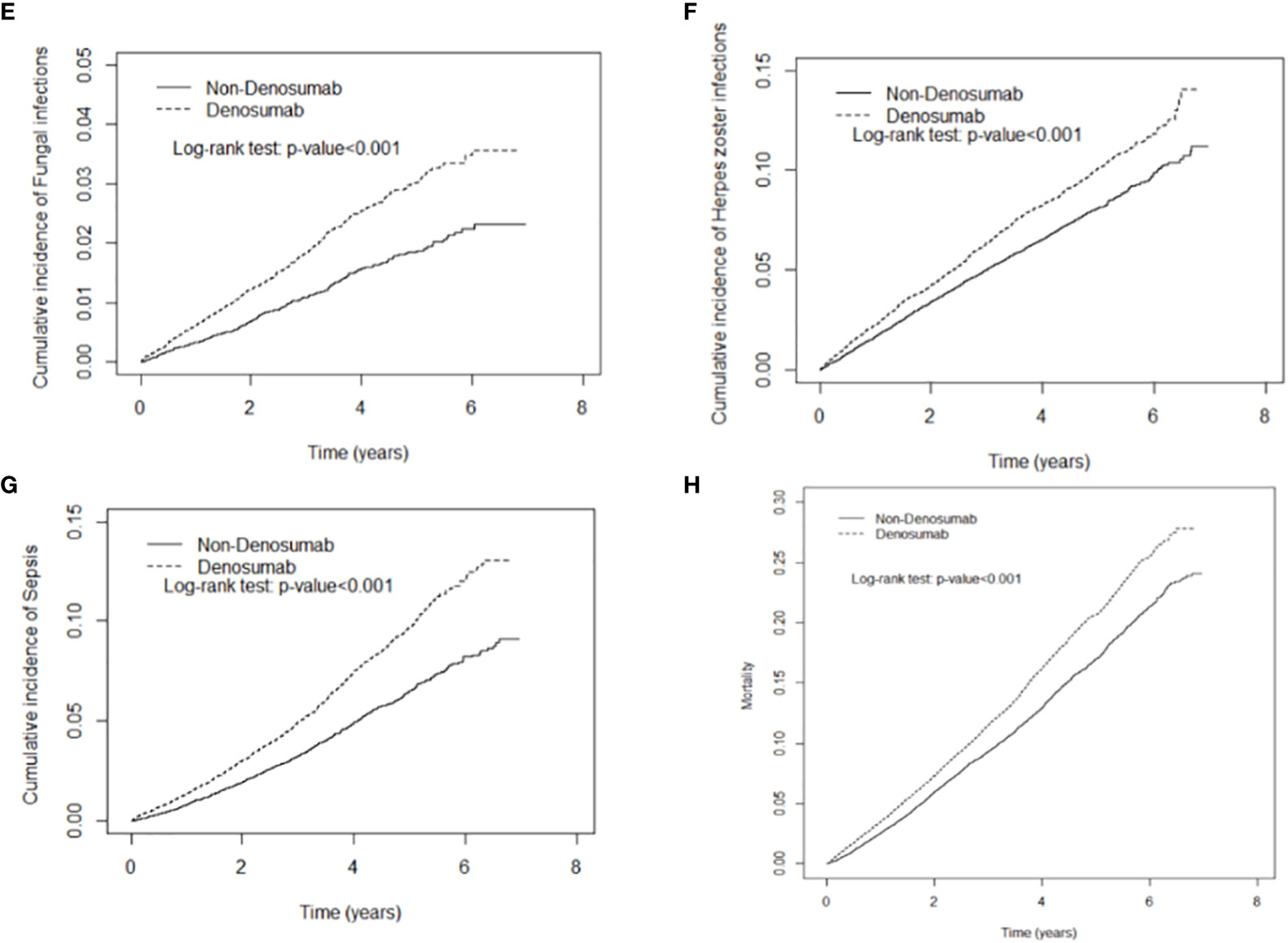
Figure 3 Cumulative incidence curves of fungal infection (E), herpes zoster infection (F), sepsis (G), mortality (H) in patients with and without denosumab use.
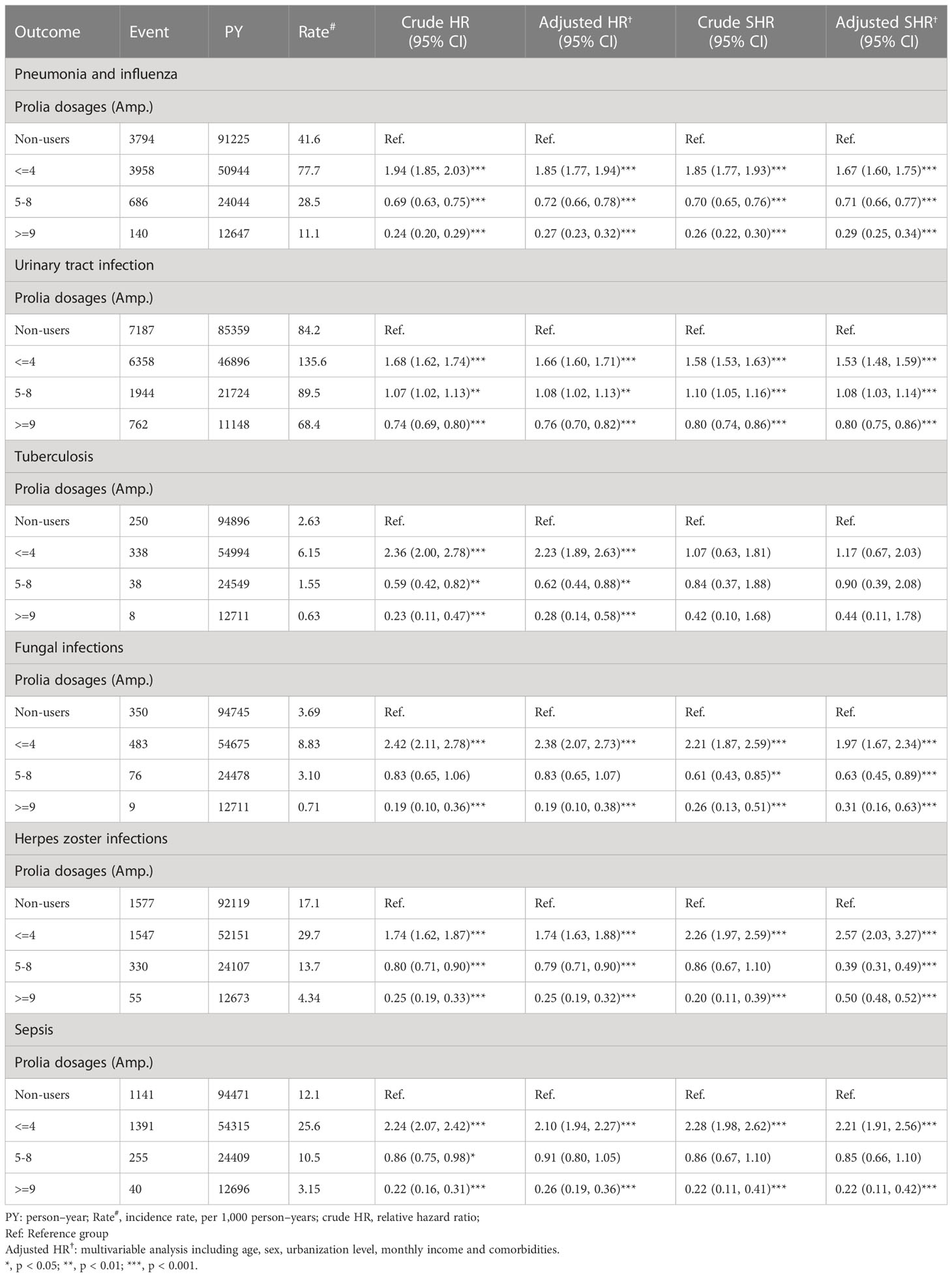
Table 3 displays the dose effect of denosumab on infectious outcomes. Compared with the control cohort, denosumab users receiving ≤4 cumulative doses had significantly higher risks of pneumonia and influenza (aHR: 1.85; 95% CI: 1.77–1.94), urinary tract infection (aHR: 1.66; 95% CI: 1.60–1.71), tuberculosis (aHR: 2.23; 95% CI: 1.89–2.63), fungal infection (aHR: 2.38; 95% CI: 2.07–2.73), herpes zoster infection (aHR: 1.74; 95% CI: 1.63–1.88), and sepsis (aHR: 2.10; 95% CI: 1.94–2.27). However, after cumulative denosumab use exceeded 5 doses, higher cumulative doses were associated with a lower risk of certain infections. Compared with controls, patients who received 5–8 injections of denosumab had a 28% lower risk of pneumonia and influenza (aHR: 0.72; 95% CI: 0.66–0.78), and those receiving ≥9 injections had a 73% lower risk (aHR: 0.27; 95% CI: 0.230–0.32). The association between higher cumulative doses of denosumab and lower risk of infection was also observed for tuberculosis, fungal infection, and herpes zoster infection. The consistent results were shown in the competing-risks regression models.
Discussion
In this large-scale 10-year observational study of 30,160 patients receiving denosumab therapy, infection risks were investigated as the primary outcome. After adjustment for confounders including diabetes, chronic kidney disease, obesity, liver cirrhosis, chronic pulmonary disease, heart failure and fracture, the multivariate analysis revealed a significantly higher infection rate in patients with osteoporosis who received denosumab treatment than in those who did not. Additionally, we compared patient survival rates and observed a 1.26-fold higher risk of death in patients treated with denosumab compared with controls. A meta-analysis of 22,253 patients who received denosumab therapy revealed a 1.21-fold increase in severe adverse events infection (SAEI) compared with controls (9). Our findings are consistent with the aforementioned result.
The present study investigated the rates of infections, including pneumonia and influenza, urinary tract infection, tuberculosis, candidiasis, fungal infection (Aspergillosis or Cryptococcosis), herpes zoster infection, and sepsis, in the denosumab cohort and the control cohort. Intergroup comparisons revealed a consistent trend across the various infections: denosumab treatment was significantly associated with a higher infection risk in patients with osteoporosis. Specifically, we found a 1.60-fold higher risk of tuberculosis infection, 1.67-fold higher risk of fungal infection, 1.68-fold higher risk of candidiasis infection, and 1.27-fold higher risk of herpes zoster infection. To investigate whether mortality was associated with the effect of denosumab on infection risks, competing risk analysis with mortality was done as well. Our findings revealed that consistent results regarding the effect of denosumab on infection risks were observed in patients with osteoporosis. To the best of our knowledge, the current study is the first to report on the higher risks of infections in patients receiving denosumab treatment.
Substantial evidence suggests that RANK or RANKL mRNA expression is detected in immune tissues apart from osteoblasts, osteocytes, and bone stroma. For example, RANK expression has been identified in immune cells including T lymphocytes and B cells in the lung (6, 7, 10, 11). RANK expression is predominant in mature DCs. DCs are believed to share a common lineage with OCs and macrophages (4, 5). T cell activation via major histocompatibility complex and antigen–TCR interactions is likely mediated by DCs (3). RANKL expression plays a critical role in T cell function and presumably promotes T cell activation, which is dependent on its action on DCs. This in turn strengthens the survival of DCs in addition to triggering a mutual immune reaction. The subsequent engagement of T cells with DCs induces the differentiation of subsets of T cells for their transformation into Th1, Th2, and Th17 cells (3). Although RANKL inhibits bone metabolism, it is unclear whether RANKL inhibition might also attenuate the systemic immune response.
No consistent results on this issue related to the immune response (mentioned above) have been presented thus far. A previous study reported that in some specific tissues such as the skin, the RANKL–RANK interaction may alter the intensity of the inflammatory reaction beyond an immunosuppression response (12, 13). By contrast, a relevant study explored the differences between RANKL pathway inhibition and the complete absence of RANK or RANKL expression. The findings in an animal model revealed that the inhibition of RANKL could reduce bone loss by increasing bone density, but with no effect on inflammatory reactions (14). Our key finding is that denosumab use is significantly associated with a higher infection risk, including tuberculosis, candidiasis, herpes zoster virus, and fungal infection. This finding suggests that a modest attenuated cellular immunity response occurs in patients with osteoporosis under denosumab treatment.
Additionally, we investigated the immunosuppressive effects of cumulative denosumab dosages on infection risk in separate time periods. We observed that the infection risk was significantly higher after the first 4 consecutive doses of denosumab within 2 years; however, the risk attenuated thereafter. To our knowledge, it is the first study to disclose the reciprocal relationship between infection risk in osteoporosis patients and the immunosuppression nature by accumulative dosage of denosumab. The finding was novel which has never been identified previously.
The reasons to explain the infection risk and transient immunosuppressive effect of denosumab need to be elucidated further. In certain conditions, a high infection risk post-transplantation developed in new organ transplant recipients who received immunosuppression initially but the risk declined gradually in the later period post-transplantation, which was similar to our findings in this study to some degree. For example, a previous study reported that the incidence rate of herpes virus infection was highest in the first year after transplantation but the risk attenuated thereafter, which suggests that acute impairment of T cellular immunity occurs in the first several months following transplantation (15).
Altogether, we hypothesize that the acute immunosuppressive effect of denosumab developed initially but lasted transiently. More evidence is needed to investigate the sophisticated mechanism in the future.
Limitations
Our study results should be interpreted with caution due to some inherent limitations. First, information on lifestyle, body mass index (or obesity) and personal behaviors (such as smoking and alcoholic drinking) is not available from the NHIRD; therefore, we applied proxy variables for comorbidities. For example, the COPD variable was replaced by smoking to attenuate the confounding effect. Second, our data were retrieved from an inpatient database, meaning that only instances of severe infection were analyzed in this study. Patients with infectious diseases of mild severity may have been overlooked; thus, the actual risks of infections were likely underestimated for the denosumab cohort. Third, lack of individual laboratory data such as circulating 25(OH) D levels and imaging finding in the NHIRD may be the other study limitation.
Finally, despite our best attempts to control for all possible disease-associated confounders, there are still many unknown or uncontrolled confounding factors and some inherent bias may have affected the study findings. The retrospective study is usually lower evidence than the randomized controlled trials. Such shortcoming can be overcome by conducting a randomized control trial in the future.
Conclusion
Our findings provide evidence that denosumab use is associated with a high risk of opportunistic infection during the first 2 years of administration. Based on the findings, the effects of immunosuppression associated with denosumab use may be attributed to RANKL inhibition.
Data availability statement
The original contributions presented in the study are included in the article/supplementary materials. Further inquiries can be directed to the corresponding authors.
Ethics statement
Our study was approved by the Institutional Review Board of China Medical University Hospital Research Ethics Committee (CMUH109-REC2-031[CR-2]), and was conducted in accordance with the guidelines of the World Medical Association’s Declaration of Helsinki. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
All authors have contributed significantly, and that all authors are in agreement with the content of the manuscript: Conception/Design: S-TH, T-MY. Provision of study materials: C-LL, C-HK. Collection and/or assembly of data: C-LL. Data analysis and interpretation: all authors. Manuscript writing: all authors. Final approval of manuscript: all authors.
Funding
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial Center (MOHW110-TDU-B-212-124004), Ministry of Science and Technology (MOST 110-2321-B-039-003), and China Medical University Hospital (DMR-111-105, DMR-112-072, DMR-112-073). We are grateful to Health Data Science Center, China Medical University Hospital for providing administrative, technical and funding support. The funders had no role in the study design, data collection and analysis, the decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bone HG, Wagman RB, Brandi ML, Brown JP, Chapurlat R, Cummings SR, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol (2017) 5:513–23. doi: 10.1016/S2213-8587(17)30138-9
2. Compston J. Safety of long-term denosumab therapy for osteoporosis. Lancet Diabetes Endocrinol (2017) 5:485–7. doi: 10.1016/S2213-8587(17)30178-X
3. Walsh MC, Choi Y. Biology of the RANKL–RANK–OPG system in immunity, bone, and beyond. Front Immunol (2014) 5:511. doi: 10.3389/fimmu.2014.00511
4. Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, et al. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature (1997) 390:175–9. doi: 10.1038/36593
5. Wong BR, Josien R, Lee SY, Sauter B, Li HL, Steinman RM, et al. TRANCE (tumor necrosis factor [TNF]-related activation-induced cytokine), a new TNF family member predominantly expressed in T cells, is a dendritic cell-specific survival factor. J Exp Med (1997) 186:2075–80. doi: 10.1084/jem.186.12.2075
6. Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclasts differentiation and activation. Cell (1998) 93:165–76. doi: 10.1016/S0092-8674(00)81569-X
7. Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/ osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA (1998) 95:3597–602. doi: 10.1073/pnas.95.7.3597
8. Bekker PJ, Holloway DL, Rasmussen AS, Murphy R, Martin SW, Leese PT, et al. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J Bone Miner Res (2004) 19:1059–66. doi: 10.1359/JBMR.040305
9. Diker-Cohen T, Rosenberg D, Avni T, Shepshelovich D, Tsvetov G, Gafter-Gvili A, et al. Risk for infections during treatment with denosumab for osteoporosis: a systematic review and meta-analysis. J Clin Endocrinol Metab (2020) 105:dgz322. doi: 10.1210/clinem/dgz322
10. Kartsogiannis V, Zhou H, Horwood NJ, Thomas RJ, Hards DK, Quinn JM, et al. Localization of RANKL (receptor activator of NF kappa b ligand) mRNA and protein in skeletal and extraskeletal tissues. Bone (1999) 25:525–34. doi: 10.1016/S8756-3282(99)00214-8
11. Zhao S, Kato Y, Zhang Y, Harris S, Ahuja SS, Bonewald LF, et al. MLO-Y4 osteocyte-like cells support osteoclast formation and activation. J Bone Miner Res (2002) 17:2068–79. doi: 10.1359/jbmr.2002.17.11.2068
12. Barbaroux J-BO, Beleut M, Brisken C, Mueller CG, Groves RW, et al. Epidermal receptor activator of NF-kappaB ligand controls langerhans cells numbers and proliferation. J Immunol (2008) 181:1103–8. doi: 10.4049/jimmunol.181.2.1103
13. Loser K, Mehling A, Loeser S, Apelt J, Kuhn A, Grabbe S, et al. Epidermal RANKL controls regulatory T-cell numbers via activation of dendritic cells. Nat Med (2006) 12:1372–9. doi: 10.1038/nm1518
14. Stolina M, Dwyer D, Ominsky MS, Corbin T, Van G, Bolon B, et al. Continuous RANKL inhibition in osteoprotegerin transgenic mice and rats suppresses bone resorption without impairing lymphorganogenesis or functional immune responses. J Immunol (2007) 179:7497–505. doi: 10.4049/jimmunol.179.11.7497
Keywords: denosumab, immunity, RANKL inhibition, T-cell, osteoporosis
Citation: Huang S-T, Chiu T-F, Chiu C-W, Kao Y-N, Wang I-K, Chang C-T, Li C-Y, Sun C-S, Lin C-L, Yu T-M and Kao C-H (2023) Denosumab treatment and infection risks in patients with osteoporosis: propensity score matching analysis of a national-wide population-based cohort study. Front. Endocrinol. 14:1182753. doi: 10.3389/fendo.2023.1182753
Received: 09 March 2023; Accepted: 04 May 2023;
Published: 19 May 2023.
Edited by:
Melissa Orlandin Premaor, Federal University of Minas Gerais, BrazilCopyright © 2023 Huang, Chiu, Chiu, Kao, Wang, Chang, Li, Sun, Lin, Yu and Kao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chia-Hung Kao, RDEwMDQwQG1haWwuY211aC5vcmcudHc=; ZHIua2FvY2hpYWh1bmdAZ21haWwuY29t; Tung-Min Yu, eXU1NTIzQGdtYWlsLmNvbQ==
†These authors have contributed equally to this work
 Shih-Ting Huang
Shih-Ting Huang Ting-Fang Chiu5,6,7,8†
Ting-Fang Chiu5,6,7,8† Chih-Wei Chiu
Chih-Wei Chiu I-Kang Wang
I-Kang Wang Chi-Tzung Chang
Chi-Tzung Chang Chi-Yuan Li
Chi-Yuan Li Cheng-Li Lin
Cheng-Li Lin Tung-Min Yu
Tung-Min Yu Chia-Hung Kao
Chia-Hung Kao