
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol., 15 June 2023
Sec. Diabetes: Molecular Mechanisms
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1179161
This article is part of the Research TopicCell Cross-talk in Diabetic Kidney Diseases, Volume IIView all 8 articles
Diabetic nephropathy (DN) is one of the most common and intractable microvascular complications of diabetes worldwide, serving as the main cause of terminal renal disease. Due to the lack of early specific symptoms and diagnostic markers, DN severely threatens the sufferer’s life. MicroRNA-192 (miR-192) was early identified in human renal cortical tissue and stored and excreted in urine as microvesicles. MiR-192 was found to be involved in the development of DN. For the first time, the present review summarized all the current evidence on the topic of the roles of miR-192 in DN. Finally, 28 studies (ten clinical trials and eighteen experimental studies) were eligible for thorough reviewing. Most of the clinical trials (7/10, 70%) indicated miR-192 might be a protective factor for DN development and progression, while the majority of experimental studies (14/18, 78%) suggested miR-192 might be a pathogenic factor for DN. Mechanistically, miR-192 interacts with various direct targeted proteins (i.e., ZEB1, ZEB2, SIP1, GLP1R, and Egr1) and signaling cascades (i.e., SMAD/TGF-β and PTEN/PI3K/AKT), together contribute to the pathogenesis of DN through epithelial-to-mesenchymal transition (EMT), extracellular matrix deposition, and fibrosis formation. The current review highlights the dual role of miR-192 in the development of DN. Low serum miR-192 expression could be applied for the early prediction of DN (the early stage of DN), while the high miR-192 level in renal tissues and urine may imply the progression of DN (the late stage of DN). Further investigations are still warranted to illustrate this inconsistent phenomenon, which may facilitate promoting the therapeutic applications of miR-192 in predicting and treating DN.
Diabetic nephropathy (DN), a serious renal disease, is found to be correlated with the development of proteinuria, glomerular enlargement, reduction in glomerular filtration, and renal fibrosis (1). It affects roughly 9% of the global adult population in those suffering from either type 1 (T1DM) or type 2 diabetes mellitus (T2DM). In China, the prevalence of diabetes in adults was recorded at about 11% (2), while the prevalence of DN was up to 21.8% (3). There is a positive association between DN and the risk of the development of terminal renal disease (4). In diabetic patients with terminal kidney disease, the five-year survival rate is only 20%. There is a significant increase in deaths and morbidity associated with DN-associated heart failure with unchanged ejection fraction (5, 6). Renal replacement therapy is necessary in a substantial proportion of cases due to there are few effective treatments. At present, the therapeutic strategies for DN aim to reduce its progression but most of them turned out to be ineffective (7). As a result, an effective, rapid, and non-invasive method for detecting DN early and predicting its prognosis is critical. In the past two decades, multiple microRNAs (miRNAs) have been identified to involve in the pathophysiological action in DN.
MiRNAs are short, endogenous, and noncoding RNA molecules consisting of 19-24 nucleotides. A miRNA achieves its biological function by binding to the 3’ untranslated region of a target gene mRNA. By promoting the degradation of mRNA or causing translational repression, miRNAs effectively regulate gene expression post-transcriptionally (8, 9). MiRNAs are involved in the regulation of multiple cellular biological processes, either physiologic or pathologic conditions (e.g., proliferation, differentiation, programmed death, apoptosis, or passive cell death) (8). The dysregulation and aberrant expression of several miRNAs are also found to be associated with the development and progression of DN, such as miR-21, miR-126, miR-29, miR-192, miR-214, miR-342, and miR-192 (10, 11). According to the current literature, miR-192 is one of the most investigated miRNAs with aberrant expression in DN. Mounting clinical studies demonstrated that abnormal miR-192 expression level was detected in the majority of patients with DN. In addition, the molecular mechanisms of miR-192 action are found to be complex and multidirectional in the development of DN.
Since the pivotal role of miR-192 in DN has received increasing attention from the investigators, it is, therefore, necessary to summarize all the current evidence on this topic through a comprehensive review, which may help to develop a better understanding of the prognostic and predictive role of miR-192 in DN.
MiRNAs are small noncoding RNAs that inhibit messenger RNAs by binding to their 3’-UTRs (12). MiR-192 is a conserved miRNA that is profoundly expressed in various mammalian cell types. Human miR-192 derives from a coding gene located on chromosome 11. It produces two mature transcripts, including miR-192 (miR-192-5p) and miR-192* (miR-192-3p) (13). miR-192 is found to involve in the regulation of different physiological and pathological processes, including epigenetics, differentiation, proliferation, apoptosis, epithelial-mesenchymal transition (EMT), angiogenesis, metabolism, inflammatory responses, oxidative stress, and drug resistance (14, 15). These biological functions are derived from the inhibition of the miR-192-targeted genes, such as mRNA degradation and repression of protein translation. Given its key role in cellular processes, dysregulation of miR-192 is considered to contribute to the genesis of multiple human diseases, including respiratory system diseases (i.e., asthma, nasopharyngeal carcinoma, lung cancer), digestive system diseases (i.e., hepatic disorders, esophageal, colorectal, and gastric cancers), circulatory system diseases (i.e., myocardial fibrosis, myocardial infarction, cardiac injury), urinary system diseases (i.e., bladder cancer, kidney injury), reproductive system diseases (i.e., breast, cervical, ovarian, and prostate cancer), endocrine system (i.e., diabetes, hyperglycemia, and insulin resistance), and nervous system diseases (i.e., Alzheimer’s disease, amyotrophic lateral sclerosis, tuberous sclerosis, and peripheral nerve injury) (14–19). Functionally, miR-192 induces post-transcriptional gene silencing by binding to the 3’-UTR to regulate its targeted genes. miR-192’s conserved enhancer elements contain the different binding sites for multiple targeted genes, i.e., CCNB1, Nidogen-1, RAB1A, and ACVR2B (20–23). According to the software “TargetScan Human” (https://www.targetscan.org/), there are 3,483 transcripts with sites available, indicating more than 3,000 predicted targets have been found. However, we should know that relatively few targeted genes for miR-192 were experimentally validated.
The expression levels of miRNAs can be regulated by other non-coding RNAs, such as long non-coding RNAs (lncRNAs) and circular RNAs (circRNAs). According to the current evidence, both lncRNAs and circRNAs can act as upstream regulators that modulate the expression of miR-192. The discovered lncRNAs interacted with miR-192 include KCNQ1OT1, FTX, WAC-AS1, WAC-AS1, PTTG3P, and STEAP3-AS1 (15, 24–26). The discovered circRNAs include circ_0000189, circKIF5B, circ-SWT1, and circHIPK3 (27–30). These lncRNAs and circRNAs exert their biological functions by sponging miR-192 and regulating miR-192-targeted genes.
Currently, no relevant review article has been published for summarizing the clinical implications and the molecular mechanisms of miR-192 in DN. As a result, we conducted this comprehensive review based on the current evidence.
To identify the relevant studies investigating the role of miR-192 in DN, a literature review was conducted on each of the six commonly used databases, including. MEDLINE (PubMed), EMBASE, Cochrane Library, Google Scholar, Web of Science, and the PsychINFO, The searching strategy in MEDLINE by using the keywords was: ((((((((((((((((((“Diabetic Nephropathies”[Mesh]) OR (Nephropathies, Diabetic)) OR (Nephropathy, Diabetic)) OR (Diabetic Nephropathy)) OR (Diabetic Kidney Disease)) OR (Diabetic Kidney Diseases)) OR (Kidney Disease, Diabetic)) OR (Kidney Diseases, Diabetic)) OR (Diabetic Glomerulosclerosis)) OR (Glomerulosclerosis, Diabetic)) OR (Intracapillary Glomerulosclerosis)) OR (Nodular Glomerulosclerosis)) OR (Glomerulosclerosis, Nodular)) OR (Kimmelstiel-Wilson Syndrome)) OR (Kimmelstiel Wilson Syndrome)) OR (Syndrome, Kimmelstiel-Wilson)) OR (Kimmelstiel-Wilson Disease)) OR (Kimmelstiel Wilson Disease)) AND (((((“MIRN192 microRNA”) OR (miR-192)) OR (microRNA-192)) OR (hsa-mir-192)) OR (miR-192-5p)). Furthermore, a review of the reference list was conducted to identify more relevant studies.
Finally, twenty-eight studies (31–57) (58) published in 2007-2022 were included in this review for further analysis. A routine data collection form was used to collect relevant information from the included studies, e.g., the first author name, article publication year, research subject (patients, cells, or animals), expression of miR-192 (up-regulation or down-regulation), involved molecular mechanisms, target genes, and the main findings within each eligible study. The study design for the 28 included studies was either clinical trials (10 studies) or experimental studies (18 studies). The sample size of the clinical trials ranged from 6 to 602 participants. Diverse clinical specimens were applied in the clinical studies, including serum, urine, and kidney tissues. According to the experimental studies, miR-192 was found to interact with various direct targeted proteins (i.e., ZEB1, ZEB2, SIP1, GLP1R, and Egr1) and signaling cascades (i.e., SMAD/TGF-β and PTEN/PI3K/AKT), together contributed to the pathogenesis of DN through epithelial-to-mesenchymal transition (EMT), extracellular matrix deposition, and fibrosis formation. The characteristics of eligible studies reporting miR-192 in DN were listed in Tables 1, 2. Figure 1 showed the main molecular mechanisms of miR-192 expression in the development of DN.
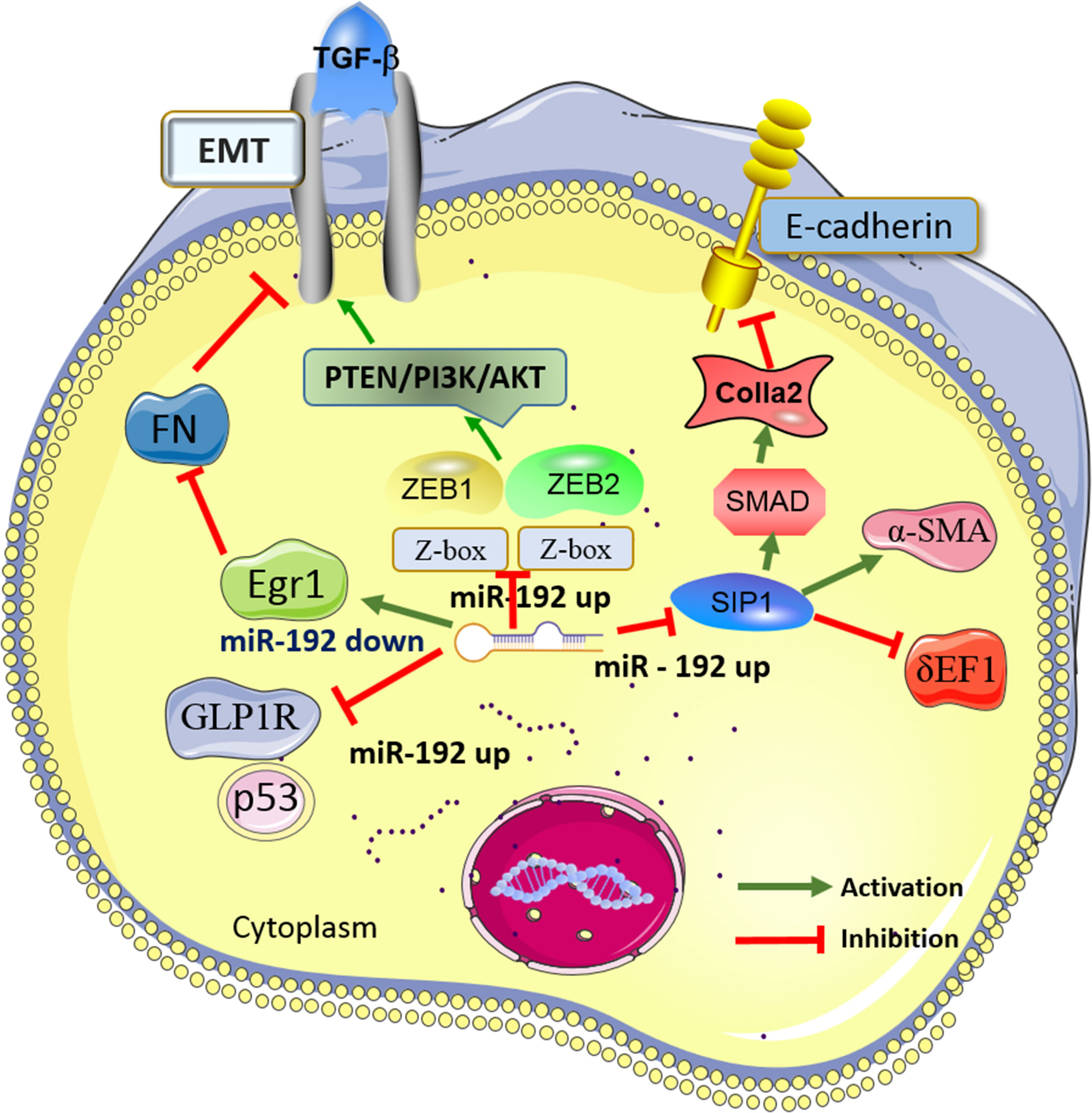
Figure 1 Main mechanisms of miR-192 in diabetic nephropathy (DN). MiR-192 exerts the biological effects in DN by interacting with its target genes (i.e., ZEB1, ZEB2, SIP1, GLP1R, and Egr1) and the signaling cascades (i.e., SMAD/TGF-β and PTEN/PI3K/AKT). DN, Diabetic nephropathy; miR-192, MicroRNA-21; PTEN, phosphatase and tensin homolog deleted in chromosome 10; TGF-β, Transforming growth factor-β; EMT, epithelial-to-mesenchymal transition; GLP-1R, Glucagon-like peptide-1 receptor; ZEB1, Zinc finger E-box-binding homeobox 1; Egr1, Early growth response factor 1; MCP-1,Monocyte chemotactic protein-1; SIP1,Smad-interacting protein 1.
Ten included clinical studies provided clinical information on miR-192 expression levels in DN. Most of these clinical trials (eight studies, 7/10, 70%) demonstrated that miR-192 was down-regulated in adult DN patients. Inconsistent with this finding, one included study (39) reported an opposite result that a high level of miR-192 in serum was found in pediatric DN patients as compared to the healthy controls. One study (31) showed that no significant difference was observed in the miR-192 expression between T2DM subjects with and without DN. Two studies reported the expression of miR-192 in urine. One study showed the results in both serum and urine.
Eight included studies investigated the expression level of miR-192 in venous blood collection (serum) of DN patients. Six out of eight studies (75%) reported the miR-192 expression was down-regulated in patients with DN.
The pathogenesis of DN causes by various factors, such as high glucose and lipid metabolism disorder, which may induce extracellular matrix (ECM) accumulation, filtration membrane damage, and renal interstitial fibrosis (59). Transforming growth factor-β1 (TGF-β1) promotes the synthesis of ECM, prevents its degradation, and accumulates ECM by promoting adhesion between cells and matrix (60). Fibronectin (FN) is one of the key components of ECM, which can be applied to assess the extent of ECM accumulation. Ma et al. (33) recruited 464 patients and found that the miR-192 level in the DN group (especially in the albuminuria group) was significantly lower than the healthy controls (P< 0.05). The expression of miR-192 was negatively correlated with TGF-β1, FN, and urine albumin creatinine ratio (UACR). The authors concluded that there was a positive association between the low level of miR-192 and nephritic fibrosis in DN.
Histopathological examination is the gold standard for identifying multiple diseases. Krupa et al. reported that a low level of miR-192 was associated with the promotion of kidney fibrosis in DN (42). However, this finding was derived from kidney tissue samples, which was hard to implement for clinical practice. Mounting evidence shows that non-invasive sampling methods (i.e., serum and urine examination) are promising for the early detection of various diseases, including DN. Yang et al. (34) investigated 283 DN patients and found that the miR-192 level in serum significantly decreased in patients with DN, whereas in urine it increased with the progression of DN. Both of the differences were statistically significant. Therefore, the authors suggested that both serum and urinary miR-192 could be a potential biomarker of DN.
Though microalbuminuria was found to be an early sign of DN, some researchers wonder about its ability to precisely detect the disease progression of DN. So, it is necessary to find a diagnostic and predictive marker for DN. Given that circulating miR-192 might represent a potential novel source of non-invasive biomarkers for DN, more and more were designed for validating this finding. KAFAJI et al. (35) assessed 85 DN patients in Manama and found that lower miR-192 expression was observed in DN patients compared with healthy controls (P< 0.05). As a result, blood-based miR-192 was considered to serve as an effective biomarker for the early detection of DN. In line with this finding, Tayel et al. (36) evaluated the miR-192 expression in 229 patients and found that significantly lower levels of miR-192 were observed in patients with DN (particularly in the macroalbuminuria group) than in the controls (P< 0.05). In addition, marked downregulation of miR-126 was also detected in DN patients compared to in the control group (P< 0.05). Although effective, miR-192 had higher sensitivity (91%), specificity (94%), and area under the curve (0.967) values than that of miR-126. Tayel et al. (36) pointed out the potential role of miR-192 and miR-126 in the progression of DN and their prognostic value in the prevention of worsened progression to end-stage renal disease (ESRD). Consistently, a recent study conducted by Ren et al. (40) also reported the downregulation of miR-192 in patients with DN. Simultaneously, the expression of TGF-β1 was found to be up-regulated in DN patients. miR-192 interacted with some other miRNAs, their target genes mainly revolve around PTEN, PI3K/Akt, and MAPK signaling pathways. This transcriptional regulation mediated by miR-192 and TGF-β1 might participate in the fibrosis process of DN.
Since multiple microRNAs are associated with the development and progression of DN, Akpınar et al. (37) investigated the expression of ten microRNAs (miR-192-5p, miR-126-3p, miR-129-1-3p, miR-21-3p, miR-137, miR-29a-3p, miR-29b-3p, miR-29c-3p, miR-212-3p, and miR-320c) in 150 participants. The authors demonstrated that the expression of miR-192-5p in serum was significantly lower in the DN group than in the controls (P=0.027). Besides, the area under curve value was 0.717 for miR-192-5p for distinguishing the DN group from the control group, suggesting that this miRNA might involve in the development of DN.
Inconsistent with the above findings, Gong et al. (39) reported the opposite results which showed that the miR-192 level was increased in the serum samples of the DN patients (n=79). Different from the above included studies, the study population in Gong et al. was pediatric patients. The authors revealed that miR-192 expression negatively correlated with Alpha-Klotho (KL) levels in pediatric patients with prolonged duration of diabetes. KL was found to decrease by -246.8 pg/ml per each 1-unit increase in miR-192 relative ratio. KL, a known anti-aging protein, expresses in multiple tissues, with the highest expression in the kidneys (61). miR-192 might be an upstream regulator of KL (direct target gene) due to overexpression of miR-192 could significantly inhibit the KL expression in HK2 cells. The subsequent result showed that miR-192 targeted KL through its 3’UTR. Both miR-192 and KL together contributed to oxidative stress, inflammation, and senescence in DN development. In addition, they found that miR -192 expressions were stable among patients with shorter diabetes duration. In those with 12 years of diabetes, miR-192 levels elevated with time. Unlike the results suggesting miR-192 was significantly increased in adult DN patients or decreased in pediatric DN patients, Chien et al. demonstrated that no significant difference in serum was found regarding miR-192 expression in patients with or without DN. However, the authors found that serological miR-192 differed between microalbuminuria and overt proteinuria groups (P=0.0138), indicating that miR-192 might predict late DN progression.
Among the ten included clinical studies, three of them investigated the expression of miR-192 in the urine of DN patients. Multiple miRNAs are found to enter the body fluid and blood-stream circulation (62). Thus, the detection of miRNAs in serum or body fluids (i.e., urine) plays an important role in the early diagnosis of various diseases, including DN (63). Similar to the findings of miR-192 expression in serum, its expression level in urine was also controversial among different studies. Two clinical studies showed that miR-192 expression was higher in the serum of the DN patients than in the controls, while a decreased level was found in the other study. Jia et al. (32) developed a small sample size with 80 participants and observed that the urine extracellular vesicle level of miR-192 was significantly elevated in patients with DN, especially in those with albuminuria. They also found that TGF-β1 levels were significantly correlated with miR-192 expression (r = 0.356, P = 0.005). Thus, both urinary extracellular vesicles miR-192 and TGF-β1 might serve as promising biomarkers of the early stage of DN. Consistent with Jia et al.’s findings, Yang et al. (34) reported that the miR-192 level in urine was increased, whereas in serum it decreased with the progression of DN (n=283). They further indicated that a combination with high levels of urine miR-192 and low levels of serum miR-192 had a higher specificity and lower misdiagnosis rate. However, Smith et al. (38) observed an opposite result that low miR-192 expression might be associated with the progression of DN. The authors detected that miR-192 expression fell from a 1.54-fold change in the control cohort in DN patients. MiR-192 was found to be specifically expressed in renal cortical tissue, and stored and excreted in urine as microvesicles (64). Therefore, kidney injury caused by DN might induce more microvesicles containing miR-192 through the urine. Nevertheless, since it is still being debated on the expression level of urinary miR-192 in DN patients, its diagnostic and predictive effects on DN still require further exploration.
The aforementioned clinical studies suggested a causal association between miR-192 expression and DN, exploring the biological function of miR-192 and its potential mechanisms in DN might be profound for the researchers. MiR-192 was down-regulated in the serum of DN patients, which was reported in the majority of the clinical studies (6/8, 75%). However, the vast majority of experimental studies (14/18, 78%) demonstrated that miR-192 was up-regulated in a cell or animal model of DN (high glucose). Therefore, whether miR-192 played a protective or pathogenic role in the development of DN was still controversial among different studies. Since miRNAs function by interacting with their target genes, we summarized the 18 included in vitro and in vivo studies employing the different miR-192-targeted genes.
An essential role for EMT exists in renal interstitial fibrosis in DN (65). The formation and deposition of EMT and ECM in renal tubular epithelial cells attribute to the pathogenesis of DN. The physiological function of miR-192 was found to be closely correlated to EMT. It is known that Zinc finger E-box-binding homeobox (ZEB) plays a key role in EMT, which is closely related to many aspects of life (66). ZEB family mainly comprises two proteins ZEB1 and ZEB2. Two zinc finger clusters in ZEB1 bind to DNA sequences specific to its function. There are mainly binding sites in the zinc finger region of the N-terminal zinc finger or the zinc finger region of the C-terminal zinc finger. In the downstream region of the zinc finger of ZEB1, phosphorylated receptor-activated SMAD is bound, so ZEB1 could regulate the signaling pathway of TGFβ (67). ZEB1 interacts with various miRNAs (i.e., miR-200c and miR-205), and together mediate the corresponding signaling pathways, such as TGFβ, hippo pathway, and wingless/integrated (Wnt). It is known that EMT plays a key role in the development of fibrosis, while ZEB1 is an important transcription factor of EMT. During the process of EMT, ZEB1 effectively promotes cell proliferation, migration, collagen synthesis, and fibrosis formation (68).
Five included studies demonstrated that ZEB1/ZEB2 might be a direct target of miR-192. Deshpande et al. (45) conducted an experimental study in cells and animals and subsequently validated it in human kidney tissues. They found an increased expression level of miR-192 and p53 and a decreased level of ZEB2 (the direct target for miR-192). TGF-b could promote the transcriptional activation of p53 through miR-192. This study showed that the TGF-b1–induced feedback amplification circuit between p53 and miR-192/ZEB2 contributed greatly to the pathogenesis of DN. Similar to this finding, Chen et al. (53) observed that miR-192 was increased in glomerular mesangial cells cultured with high glucose. High glucose levels could regulate both ZEB1 and monocyte chemotactic protein-1 (MCP-1) expression by upregulating the level of miR-192. Though lacking a luciferase reporter gene examination, the expression of miR-192 significantly increased after the targeting silencing of ZEB1, indicating ZEB1 might be the target of miR-192. miR-192/ZEB1 was considered to involve in the occurrence of the inflammatory reaction in DN development. Consistently, Putta et al. (44) also detected a high level of miR-192 in the mice and cell models of DN. Zeb1/2 (miR-192 targeted genes) were decreased by means of the TGF-β signaling. They further found that locked nucleic acid (LNA)–modified inhibitor of miR-192 dramatically increased Zeb1/2 expression and reduced the level of collagen, TGF-b, and fibronectin, therefore alleviating DN. Consistently, Yu et al. (54) reported that miR-192 elevated in a mouse model of DN. They next found that metformin or alcohol extract of Coreopsis tinctoria Nutt (AC) significantly decreased the expression of miR-192, which could alleviate the degree of renal fibrosis. The protective effect of AC on DN might be associated with the reduction of the expression level of miR-192 and upregulation of miR-192-targeted gene ZEB2. This protective effect may be attributed to the indirect modulation of the activity of the PTEN/PI3K/AKT pathway. The above four studies confirmed that miR-192 was up-regulated under high glucose conditions. Contrary to the above three included studies, Krupa et al. (42) reported that miR-192 was low in both patient tissue and HK-2 cells of the DN model. The author observed that upregulated ZEB1/ZEB2 (potential targeted genes for miR-192) and TGF-β and decreased levels of E-cadherin in the pathogenesis of DN. The downregulated miR-192 level could increase fibrosis and decline GFR in DN, which might induce by enhancing TGF-β–mediated downregulation of E-cadherin in proximal tubular cells. The above evidence indicated that ZEB1/ZEB2-associated EMT might contribute to the development of miR-21-mediated DN and the progression of miR-21-mediated DN.
Smad-interacting protein 1 (SIP1), one of the two-handed zinc-finger proteins and the transcriptional regulators for E-cadherin expression, can interact with activated SMAD transcriptional cofactors (69, 70). Smads are the primary mediators of TGFβ signaling, which modulates the activity of SIP1 as a transcriptional repressor. In the present review, three experimental studies identified SIP1 might be the direct target for miR-192. All three studies demonstrated that miR-192 was up-regulated in numerous in-vivo and in vitro DN models. A previous study developed by Kato et al. (41) showed that SIP1 and δEF1 were down-regulated and Col1a2 was increased in DN mouse and mesangial cells with high glucose. In this study, the authors found that TGF-β led to the down-regulation of SIP1 (via miR-192) and δEF1 could cooperate to reinforce Col1a2 expression via derepression at E-box elements. δEF1 is an important inhibitor of E-cadherin. The cross-talk between E-box repressors (δEF1 and SIP1) contributes to the TGF-β-mediated collagen regulation in the pathogenesis of DN. Mao et al. (52) reported that astragaloside IV decreased miR−192, TGF-β1, Smad3, α-SMA, and collagen type 1, and elevated Smad7 in rats and RMCs cells model of DN. The therapeutic effect of Astragaloside IV on DN might be associated with the repression of excessive mesangial proliferation and renal fibrosis via the TGF-β1/Smad/miR-192 pathway. A more recent study (57) showed that TGF-β and miR-192 expression significantly elevated and SIP1 decreased in the DN group. Interestingly, the administration of troxerutin and insulin significantly reversed this tendency, indicating the renal-protective effects derived from these agents might be attributed to the inhibition of miR-192 expression and the elevation of SIP1 expression.
Glucagon-like peptide-1 receptor (GLP1R) exists in various organs, including the liver, brain, pancreas, gut, and hypothalamus (71). In addition, GLP1R was also found to be highly expressed in the kidney, especially in renal tubular epithelial cells (72). Mounting evidence demonstrates that GLP1R is a pivotal pharmacological target for T2DM (73). Thus, GLP1R was believed to regulate both non-diabetic and diabetic renal fibrosis (74). Jia et al. (49) conducted an in-vitro study on HK-2 cells in the high glucose condition. The authors indicated the level of miR-192 was down-regulated and p53 expression was up-regulated. They found that exendin-4 downregulated cellular and secreted miR-192, therefore increasing the expression of GLP1R in a p53-dependent manner, which might ameliorate renal fibrosis. A subsequent in-vitro (HK-2) and in-vivo (rats) study (55) also suggested that miR-192-5p was elevated and its targeted gene GLP-1R was decreased in high-glucose-incubated human renal tubular epithelial cells and rat renal fibroblasts. The researchers next found that Icariin and dihydrotestosterone improved diabetic renal tubulointerstitial fibrosis by restoring autophagy via the miR-192-5p/GLP-1R pathway.
Early growth response factor 1 (Egr1), a transcription factor binding to DNA, has been found to abnormally express in diabetic mice (75). Besides, Egr1 is associated with the development of renal fibrosis (76). Multiple mechanisms are involved in the actions of Egr1 in diabetes mellitus-associated renal fibrosis, including elevating the expression of TGF-β, promoting the proliferation of mesangial cells, and accelerating the EMT process of renal tubular epithelial cells (77, 78). Thus, targeting with Egr1 might have promising effects for treating DN. Liu et al. (50) reported that miR-192 was down-regulated in both HK-2 cell and rat models of DN. Subsequently, the authors observed a tendency of high levels of Egr1 and low levels of TGF-β1 and FN in both cell and animal models. The biological effects exerted by miR-192 that could inhibit the progression of DN and protect DN rats from renal interstitial fibrosis might be mediated by decreasing TGF-β1 and FN. This study indicated that miR-192 could serve as an innovative and prospective therapeutic target for DN.
In this review, conflicting results were identified among the included studies about the expression of miR-192 among serum, urine, renal tissues samples. Based on the 28 eligible studies, the level of miR-192 tend towards decrease in serum but increase in urine and renal tissues under the disease condition of diabetic nephropathy. One of the probable explanations was that miR-192 might release from other tissue types thus changing the actual level. Numerous studies (79, 80) indicated that serum miR-192 was downregulated in patients or animal models with diabetes mellitus. Consistently, we also found that serum miR-192 was decreased in diabetic nephropathy. It is known that the blood travel almost solely through the renal system. Therefore, the small molecules contained in the blood, like miR-192, may accumulate in the kidneys. Therefore, there is constant kidney accumulation of miR-192, leading to the high level of miR-192 in renal tissues and subsequently excrete with the urine. As a result, the expression of miR-192 may elevate in both kidney and the urine. On the other hand, miR-192 was found to be abundant in the renal glomerular and tubulointerstitial fibrosis. We speculated that renal fibrosis might encapsulate the small molecule “miR-192”, leading to an accumulation of miR-192 in the renal tissues. As aforementioned, serum miR-192 was downregulated in patients or animal models with diabetes mellitus, while diabetic nephropathy was considered to the vascular complication of diabetes mellitus. Thus, low serum miR-192 may be the early manifestation of diabetic nephropathy. Gradually, however, an increase of miR-192 level in the renal tissues and urine was detected with increased loading of circulating miR-192. According to this assumption, higher expression of miR-192 in both renal tissues and urine may indicate the progression of diabetic nephropathy. According to the above hypotheses, low serum miR-192 might be helpful for early diagnosis of DN, while higher miR-192 level in renal tissues and urine may suggest the progression of DN.
The challenges with the current knowledge about miR-192 in DN may be the inconsistent results of the expression of miR-192 in serum, urine, and renal tissues samples among the different included studies. However, these conflicting results might cause by the different stages of DN. Low serum miR-192 could be applied for the early prediction of DN (the early stage of DN), while the high miR-192 level in renal tissues and urine may imply the progression of DN (the late stage of DN). This hypothesis should be investigated in near future, especially for the well-designed clinical trials.
To the best of our knowledge, this is the first review to summarize all the current evidence on the topic of the roles of miR-192 in DN. According to the 28 included studies, most of the clinical trials indicated miR-192 might be a protective factor for DN development and progression, while the majority of experimental studies suggested miR-192 might be a pathogenic factor for DN. Mechanistically, miR-192 interacts with various direct targeted proteins (i.e., ZEB1, ZEB2, SIP1, GLP1R, and Egr1) and signaling cascades, together contributing to the pathogenesis of DN. Since the results are conflicting and controversial between clinical trials and experimental studies, further investigations are still warranted to illustrate this inconsistent phenomenon. A clear understanding of the clinicopathological features and the molecular mechanisms may provide new insights into the therapeutic applications of miR-192 in predicting and treating DN. This review highlights the dual role of miR-192 in the pathogenesis of DN, which still needs more studies to verify its role in serving as a biomarker.
XW, JL, and JC contributed to conceive and design the study. SZ performed the systematic searching. JL and HL extracted the data. XW and JC wrote the manuscript. XW and CC supervised the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Shahin DHH, Sultana R, Farooq J, Taj T, Khaiser UF, Alanazi N, et al. Insights into the uses of traditional plants for diabetes nephropathy: a review. Curr Issues Mol Biol (2022) 44:2887–902. doi: 10.3390/cimb44070199
2. Wang L, Gao P, Zhang M, Huang Z, Zhang D, Deng Q, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA (2017) 317:2515–23. doi: 10.1001/jama.2017.7596
3. Zhang XX, Kong J, Yun K. Prevalence of diabetic nephropathy among patients with type 2 diabetes mellitus in China: a meta-analysis of observational studies. J Diabetes Res (2020) 2020:2315607. doi: 10.1155/2020/2315607
4. Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, et al. Chronic kidney disease: global dimension and perspectives. Lancet (2013) 382:260–72. doi: 10.1016/S0140-6736(13)60687-X
5. Parente EB, Harjutsalo V, Forsblom C, Groop PH. The impact of central obesity on the risk of hospitalization or death due to heart failure in type 1 diabetes: a 16-year cohort study. Cardiovasc Diabetol (2021) 20:153. doi: 10.1186/s12933-021-01340-4
6. Wheeler DC, Stefansson BV, Jongs N, Chertow GM, Greene T, Hou FF, et al. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol (2021) 9:22–31. doi: 10.1016/S2213-8587(20)30369-7
7. Umanath K, Lewis JB. Update on diabetic nephropathy: core curriculum 2018. Am J Kidney Dis (2018) 71:884–95. doi: 10.1053/j.ajkd.2017.10.026
8. Martinez-Hernandez R, Marazuela M. MicroRNAs in autoimmune thyroid diseases and their role as biomarkers. Best Pract Res Clin Endocrinol Metab (2023) 37:101741. doi: 10.1016/j.beem.2023.101741
9. Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol (2014) 15:509–24. doi: 10.1038/nrm3838
10. Assmann TS, Recamonde-Mendoza M, de Souza BM, Bauer AC, Crispim D. MicroRNAs and diabetic kidney disease: systematic review and bioinformatic analysis. Mol Cell Endocrinol (2018) 477:90–102. doi: 10.1016/j.mce.2018.06.005
11. Asgari M, Salehi I, Ranjbar K, Khosravi M, Zarrinkalam E. Interval training and crataegus persica ameliorate diabetic nephropathy via miR-126/Nrf-2 mediated inhibition of stress oxidative in rats with diabetes after myocardial ischemia-reperfusion injury. BioMed Pharmacother (2022) 153:113411. doi: 10.1016/j.biopha.2022.113411
12. Sadakierska-Chudy A. MicroRNAs: diverse mechanisms of action and their potential applications as cancer epi-therapeutics. Biomolecules (2020) 10:1285. doi: 10.3390/biom10091285
13. Krattinger R, Bostrom A, Schioth HB, Thasler WE, Mwinyi J, Kullak-Ublick GA. microRNA-192 suppresses the expression of the farnesoid X receptor. Am J Physiol Gastrointest Liver Physiol (2016) 310:G1044–51. doi: 10.1152/ajpgi.00297.2015
14. Mishan MA, Tabari M, Parnian J, Fallahi J, Mahrooz A, Bagheri A. Functional mechanisms of miR-192 family in cancer. Genes Chromosomes Cancer (2020). doi: 10.1002/gcc.22889
15. Ren FJ, Yao Y, Cai XY, Fang GY. Emerging role of MiR-192-5p in human diseases. Front Pharmacol (2021) 12:614068. doi: 10.3389/fphar.2021.614068
16. Furuke H, Konishi H, Arita T, Kataoka S, Shibamoto J, Takabatake K, et al. Plasma microRNA-192-5p can predict the response to neoadjuvant chemotherapy and prognosis in esophageal cancer. Cancer Sci (2022) 114:1686–96. doi: 10.1111/cas.15703
17. Song J, Lin Z, Liu Q, Huang S, Han L, Fang Y, et al. MiR-192-5p/RB1/NF-kappaBp65 signaling axis promotes IL-10 secretion during gastric cancer EMT to induce treg cell differentiation in the tumour microenvironment. Clin Transl Med (2022) 12:e992. doi: 10.1002/ctm2.992
18. Park MN, Park H, Rahman MA, Kim JW, Park SS, Cho Y, et al. BK002 induces miR-192-5p-Mediated apoptosis in castration-resistant prostate cancer cells via modulation of PI3K/CHOP. Front Oncol (2022) 12:791365. doi: 10.3389/fonc.2022.791365
19. Li Y, Zu L, Wu H, Zhang F, Fan Y, Pan H, et al. MiR-192/NKRF axis confers lung cancer cell chemoresistance to cisplatin via the NF-kappaB pathway. Thorac Cancer (2022) 13:430–41. doi: 10.1111/1759-7714.14278
20. Liu J, Wen Y, Liu Z, Liu S, Xu P, Xu Y, et al. VPS33B modulates c-Myc/p53/miR-192-3p to target CCNB1 suppressing the growth of non-small cell lung cancer. Mol Ther Nucleic Acids (2021) 23:324–35. doi: 10.1016/j.omtn.2020.11.010
21. Zhu D, Xie H, Li H, Cai P, Zhu H, Xu C, et al. Nidogen-1 is a common target of microRNAs MiR-192/215 in the pathogenesis of hirschsprung's disease. J Neurochem (2015) 134:39–46. doi: 10.1111/jnc.13118
22. Zhou G, Zhang X, Wang W, Zhang W, Wang H, Xin G. Both peripheral blood and urinary miR-195-5p, miR-192-3p, miR-328-5p and their target genes PPM1A, RAB1A and BRSK1 may be potential biomarkers for membranous nephropathy. Med Sci Monit (2019) 25:1903–16. doi: 10.12659/MSM.913057
23. Senanayake U, Das S, Vesely P, Alzoughbi W, Frohlich LF, Chowdhury P, et al. miR-192, miR-194, miR-215, miR-200c and miR-141 are downregulated and their common target ACVR2B is strongly expressed in renal childhood neoplasms. Carcinogenesis (2012) 33:1014–21. doi: 10.1093/carcin/bgs126
24. Cao M, Yuan D, Jiang H, Zhou G, Chen C, Han G. Long non-coding RNA WAC antisense RNA 1 mediates hepatitis b virus replication in vitro by reinforcing miR-192-5p/ATG7-induced autophagy. Eur J Histochem (2022) 66:3438. doi: 10.4081/ejh.2022.3438
25. Dai B, Sun F, Cai X, Li C, Liu F, Shang Y. Long noncoding RNA PTTG3P/miR-192-3p/CCNB1 axis is a potential biomarker of childhood asthma. Int Immunopharmacol (2021) 101:108229. doi: 10.1016/j.intimp.2021.108229
26. Su L, Zhang J, Zhang X, Zheng L, Zhu Z. Identification of cell cycle as the critical pathway modulated by exosome-derived microRNAs in gallbladder carcinoma. Med Oncol (2021) 38:141. doi: 10.1007/s12032-021-01594-8
27. Yang J, Hou G, Chen H, Chen W, Ge J. Circ_0000189 promotes the malignancy of glioma cells via regulating miR-192-5p-ZEB2 axis. Oxid Med Cell Longev (2022) 2022:2521951. doi: 10.1155/2022/2521951
28. Fei Z, Wang Y, Gu Y, Xie R, Hao Q, Jiang Y. CircKIF5B promotes hepatocellular carcinoma progression by regulating the miR-192 Family/XIAP axis. Front Oncol (2022) 12:916246. doi: 10.3389/fonc.2022.916246
29. Chen S, Sun L, Hao M, Liu X. Circ-SWT1 ameliorates H(2)O(2)-induced apoptosis, oxidative stress and endoplasmic reticulum stress in cardiomyocytes via miR-192-5p/SOD2 axis. Cardiovasc Toxicol (2022) 22:378–89. doi: 10.1007/s12012-022-09720-2
30. Lian C, Sun J, Guan W, Zhang L, Zhang X, Yang L, et al. Circular RNA circHIPK3 activates macrophage NLRP3 inflammasome and TLR4 pathway in gouty arthritis via sponging miR-561 and miR-192. Inflammation (2021) 44:2065–77. doi: 10.1007/s10753-021-01483-2
31. Chien HY, Chen CY, Chiu YH, Lin YC, Li WC. Differential microRNA profiles predict diabetic nephropathy progression in Taiwan. Int J Med Sci (2016) 13:457–65. doi: 10.7150/ijms.15548
32. Jia Y, Guan M, Zheng Z, Zhang Q, Tang C, Xu W, et al. miRNAs in urine extracellular vesicles as predictors of early-stage diabetic nephropathy. J Diabetes Res (2016) 2016:7932765. doi: 10.1155/2016/7932765
33. Ma X, Lu C, Lv C, Wu C, Wang Q. The expression of miR-192 and its significance in diabetic nephropathy patients with different urine albumin creatinine ratio. J Diabetes Res (2016) 2016:6789402. doi: 10.1155/2016/6789402
34. Yang X, Liu S, Zhang R, Sun B, Zhou S, Chen R, et al. Microribonucleic acid-192 as a specific biomarker for the early diagnosis of diabetic kidney disease. J Diabetes Investig (2017) 9:602–9. doi: 10.1111/jdi.12753
35. Al-Kafaji G, Al-Muhtaresh HA. Expression of microRNA−377 and microRNA−192 and their potential as blood−based biomarkers for early detection of type 2 diabetic nephropathy. Mol Med Rep (2018) 18:1171–80. doi: 10.3892/mmr.2018.9040
36. Tayel SI, Saleh AA, El-Hefnawy SM, Elzorkany KM, Elgarawany GE, Noreldin RI. Simultaneous assessment of MicroRNAs 126 and 192 in diabetic nephropathy patients and the relation of these MicroRNAs with urinary albumin. Curr Mol Med (2020) 20:361–71. doi: 10.2174/1566524019666191019103918
37. Akpinar K, Aslan D, Fenkci SM, Caner V. miR-21-3p and miR-192-5p in patients with type 2 diabetic nephropathy. Diagnosis (Berl) (2022) 9:499–507. doi: 10.1515/dx-2022-0036
38. Smith DA, Simpson K, Lo CM, Newbury LJ, Nicholas P, Fraser DJ, et al. Detection of urinary microRNA biomarkers using diazo sulfonamide-modified screen printed carbon electrodes. RSC Adv (2021) 11:18832–9. doi: 10.1039/d0ra09874d
39. Gong Z, Banchs P, Liu Y, Fu H, Arena VC, Forno E, et al. Serum alpha-KL, a potential early marker of diabetes complications in youth with T1D, is regulated by miRNA 192. Front Endocrinol (Lausanne) (2022) 13:937093. doi: 10.3389/fendo.2022.937093
40. Ren H, Shao Y, Ma X, An L, Liu Y, Wang Q. Interaction of circulating TGFbeta regulatory miRNAs in different severity of diabetic kidney disease. Arch Physiol Biochem (2022), 1–15. doi: 10.1080/13813455.2022.2034884
41. Kato M, Zhang J, Wang M, Lanting L, Yuan H, JJ R, et al. MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-beta-induced collagen expression via inhibition of e-box repressors. Proc Natl Acad Sci USA (2007) 104:3432–7. doi: 10.1073/pnas.0611192104
42. Krupa A, Jenkins R, Luo DD, Lewis A, Phillips A, Fraser D. Loss of MicroRNA-192 promotes fibrogenesis in diabetic nephropathy. J Am Soc Nephrol (2010) 21:438–47. doi: 10.1681/ASN.2009050530
43. Chen YQ, Wang XX, Yao XM, Zhang DL, Yang XF, Tian SF, et al. Abated microRNA-195 expression protected mesangial cells from apoptosis in early diabetic renal injury in mice. J Nephrol (2012) 25:566–76. doi: 10.5301/jn.5000034
44. Putta S, Lanting L, Sun G, Lawson G, Kato M, Natarajan R. Inhibiting microRNA-192 ameliorates renal fibrosis in diabetic nephropathy. J Am Soc Nephrol (2012) 23:458–69. doi: 10.1681/ASN.2011050485
45. Deshpande SD, Putta S, Wang M, Lai JY, Bitzer M, Nelson RG, et al. Transforming growth factor-beta-induced cross talk between p53 and a microRNA in the pathogenesis of diabetic nephropathy. Diabetes (2013) 62:3151–62. doi: 10.2337/db13-0305
46. Mu J, Pang Q, Guo YH, Chen JG, Zeng W, Huang YJ, et al. Functional implications of microRNA-215 in TGF-beta1-induced phenotypic transition of mesangial cells by targeting CTNNBIP1. PloS One (2013) 8:e58622. doi: 10.1371/journal.pone.0058622
47. Kato M, Dang V, Wang M, Park JT, Deshpande S, Kadam S, et al. TGF-beta induces acetylation of chromatin and of ets-1 to alleviate repression of miR-192 in diabetic nephropathy. Sci Signal (2013) 6:ra43. doi: 10.1126/scisignal.2003389
48. Oghbaei H, Ahmadi AN, Sheikhzadeh F, Alipour MR, Khamaneh AM. The effect of regular moderate exercise on miRNA-192 expression changes in kidney of streptozotocin-induced diabetic Male rats. Adv Pharm Bull (2015) 5:127–32. doi: 10.5681/apb.2015.018
49. Jia Y, Zheng Z, Guan M, Zhang Q, Li Y, Wang L, et al. Exendin-4 ameliorates high glucose-induced fibrosis by inhibiting the secretion of miR-192 from injured renal tubular epithelial cells. Exp Mol Med (2018) 50:1–13. doi: 10.1038/s12276-018-0084-3
50. Liu F, Zhang ZP, Xin GD, Guo LH, Jiang Q, Wang ZX. miR-192 prevents renal tubulointerstitial fibrosis in diabetic nephropathy by targeting Egr1. Eur Rev Med Pharmacol Sci (2018) 22:4252–60. doi: 10.26355/eurrev_201807_15420
51. Ebadi Z, Moradi N, Kazemi FT, Balochnejadmojarrad T, Chamani E, Fadaei R, et al. Captopril and spironolactone can attenuate diabetic nephropathy in wistar rats by targeting microRNA-192 and microRNA-29a/b/c. DNA Cell Biol (2019) 38:1134–42. doi: 10.1089/dna.2019.4732
52. Mao Q, Chen C, Liang H, Zhong S, Cheng X, Li L. Astragaloside IV inhibits excessive mesangial cell proliferation and renal fibrosis caused by diabetic nephropathy via modulation of the TGF-beta1/Smad/miR-192 signaling pathway. Exp Ther Med (2019) 18:3053–61. doi: 10.3892/etm.2019.7887
53. Chen F, Wei G, Zhou Y, Ma X, Wang Q. The mechanism of miR-192 in regulating high glucose-induced MCP-1 expression in rat glomerular mesangial cells. Endocr Metab Immune Disord Drug Targets (2019) 19:1055–63. doi: 10.2174/1871530319666190301154640
54. Yu S, Zhao H, Yang W, Amat R, Peng J, Li Y, et al. The alcohol extract of coreopsis tinctoria nutt ameliorates diabetes and diabetic nephropathy in db/db mice through miR-192/miR-200b and PTEN/AKT and ZEB2/ECM pathways. BioMed Res Int (2019) 2019:5280514. doi: 10.1155/2019/5280514
55. Jia Z, Wang K, Zhang Y, Duan Y, Xiao K, Liu S, et al. Icariin ameliorates diabetic renal tubulointerstitial fibrosis by restoring autophagy via regulation of the miR-192-5p/GLP-1R pathway. Front Pharmacol (2021) 12:720387. doi: 10.3389/fphar.2021.720387
56. Mojadami S, Ahangarpour A, Mard SA, Khorsandi L. Diabetic nephropathy induced by methylglyoxal: gallic acid regulates kidney microRNAs and glyoxalase1-Nrf2 in male mice. Arch Physiol Biochem (2023) 129:655–62. doi: 10.1080/13813455.2020.1857775
57. Keyhanmanesh R, Hamidian G, Lotfi H, Zavari Z, Seyfollahzadeh M, Ghadiri A, et al. Troxerutin affects nephropathy signaling events in the kidney of type-1 diabetic male rats. Avicenna J Phytomed (2022) 12:109–15. doi: 10.22038/AJP.2021.18875
58. Rafiee Z, Orazizadeh M, Nejad DF, Neisi N, Babaahmadi-Rezaei H, Mansouri E. Mesenchymal stem cells derived from the kidney can ameliorate diabetic nephropathy through the TGF-beta/Smad signaling pathway. Environ Sci Pollut Res Int (2022) 29:53212–24. doi: 10.1007/s11356-021-17954-w
59. Chen Y, Zou H, Lu H, Xiang H, Chen S. Research progress of endothelial-mesenchymal transition in diabetic kidney disease. J Cell Mol Med (2022) 26:3313–22. doi: 10.1111/jcmm.17356
60. Sun C, Tian X, Jia Y, Yang M, Li Y, Fernig DG. Functions of exogenous FGF signals in regulation of fibroblast to myofibroblast differentiation and extracellular matrix protein expression. Open Biol (2022) 12:210356. doi: 10.1098/rsob.210356
61. Zou D, Wu W, He Y, Ma S, Gao J. The role of klotho in chronic kidney disease. BMC Nephrol (2018) 19:285. doi: 10.1186/s12882-018-1094-z
62. Geekiyanage H, Rayatpisheh S, Wohlschlegel JA, Brown RJ, Ambros V. Extracellular microRNAs in human circulation are associated with miRISC complexes that are accessible to anti-AGO2 antibody and can bind target mimic oligonucleotides. Proc Natl Acad Sci USA (2020) 117:24213–23. doi: 10.1073/pnas.2008323117
63. Mansouri E, Orazizadeh M, Mard SA, Gorji AV, Rashno M, Fakhredini F. Therapeutic effect of kidney tubular cells-derived conditioned medium on the expression of MicroRNA-377, MicroRNA-29a, aquapurin-1, biochemical, and histopathological parameters following diabetic nephropathy injury in rats. Adv BioMed Res (2022) 11:119. doi: 10.4103/abr.abr_375_21
64. Bhatt K, Kato M, Natarajan R. Mini-review: emerging roles of microRNAs in the pathophysiology of renal diseases. Am J Physiol Renal Physiol (2016) 310:F109–18. doi: 10.1152/ajprenal.00387.2015
65. Du L, Chen Y, Shi J, Yu X, Zhou J, Wang X, et al. Inhibition of S100A8/A9 ameliorates renal interstitial fibrosis in diabetic nephropathy. Metabolism (2022), 155376. doi: 10.1016/j.metabol.2022.155376
66. Lehmann W, Mossmann D, Kleemann J, Mock K, Meisinger C, Brummer T, et al. ZEB1 turns into a transcriptional activator by interacting with YAP1 in aggressive cancer types. Nat Commun (2016) 7:10498. doi: 10.1038/ncomms10498
67. Sanchez-Tillo E, Siles L, de Barrios O, Cuatrecasas M, Vaquero EC, Castells A, et al. Expanding roles of ZEB factors in tumorigenesis and tumor progression. Am J Cancer Res (2011) 1:897–912.
68. Lee JG, Jung E, Heur M. Fibroblast growth factor 2 induces proliferation and fibrosis via SNAI1-mediated activation of CDK2 and ZEB1 in corneal endothelium. J Biol Chem (2018) 293:3758–69. doi: 10.1074/jbc.RA117.000295
69. Epifanova E, Babaev A, Newman AG, Tarabykin V. Role of Zeb2/Sip1 in neuronal development. Brain Res (2019) 1705:24–31. doi: 10.1016/j.brainres.2018.09.034
70. van Grunsven LA, Schellens A, Huylebroeck D, Verschueren K. SIP1 (Smad interacting protein 1) and deltaEF1 (delta-crystallin enhancer binding factor) are structurally similar transcriptional repressors. J Bone Joint Surg Am (2001) 83-A Suppl 1:S40–7.
71. Bullock BP, Heller RS, Habener JF. Tissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide-1 receptor. Endocrinology (1996) 137:2968–78. doi: 10.1210/endo.137.7.8770921
72. Zhao X, Liu G, Shen H, Gao B, Li X, Fu J, et al. Liraglutide inhibits autophagy and apoptosis induced by high glucose through GLP-1R in renal tubular epithelial cells. Int J Mol Med (2015) 35:684–92. doi: 10.3892/ijmm.2014.2052
73. Aroda VR. A review of GLP-1 receptor agonists: evolution and advancement, through the lens of randomised controlled trials. Diabetes Obes Metab (2018) 20 Suppl 1:22–33. doi: 10.1111/dom.13162
74. Li YK, Ma DX, Wang ZM, Hu XF, Li SL, Tian HZ, et al. The glucagon-like peptide-1 (GLP-1) analog liraglutide attenuates renal fibrosis. Pharmacol Res (2018) 131:102–11. doi: 10.1016/j.phrs.2018.03.004
75. Wang WN, Zhang WL, Zhou GY, Ma FZ, Sun T, Su SS, et al. Prediction of the molecular mechanisms and potential therapeutic targets for diabetic nephropathy by bioinformatics methods. Int J Mol Med (2016) 37:1181–8. doi: 10.3892/ijmm.2016.2527
76. Ai K, Li X, Zhang P, Pan J, Li H, He Z, et al. Genetic or siRNA inhibition of MBD2 attenuates the UUO- and I/R-induced renal fibrosis via downregulation of EGR1. Mol Ther Nucleic Acids (2022) 28:77–86. doi: 10.1016/j.omtn.2022.02.015
77. Sheng S, Zou M, Yang Y, Guan M, Ren S, Wang X, et al. miR-23a-3p regulates the inflammatory response and fibrosis in diabetic kidney disease by targeting early growth response 1. In Vitro Cell Dev Biol Anim (2021) 57:763–74. doi: 10.1007/s11626-021-00606-1
78. Yu D, Yang X, Zhu Y, Xu F, Zhang H, Qiu Z. Knockdown of plasmacytoma variant translocation 1 (PVT1) inhibits high glucose-induced proliferation and renal fibrosis in HRMCs by regulating miR-23b-3p/early growth response factor 1 (EGR1). Endocr J (2021) 68:519–29. doi: 10.1507/endocrj.EJ20-0642
79. Ye Z, Wang S, Huang X, Chen P, Deng L, Li S, et al. Plasma exosomal miRNAs associated with metabolism as early predictor of gestational diabetes mellitus. Diabetes (2022) 71:2272–83. doi: 10.2337/db21-0909
Keywords: MicroRNA-192, diabetic nephropahy, biomarker, Zeb1, mechanism
Citation: Wan X, Liao J, Lai H, Zhang S, Cui J and Chen C (2023) Roles of microRNA-192 in diabetic nephropathy: the clinical applications and mechanisms of action. Front. Endocrinol. 14:1179161. doi: 10.3389/fendo.2023.1179161
Received: 03 March 2023; Accepted: 25 May 2023;
Published: 15 June 2023.
Edited by:
Quan Hong, Chinese PLA General Hospital, ChinaReviewed by:
Wang Nan, First Affiliated Hospital, Dalian Medical University, ChinaCopyright © 2023 Wan, Liao, Lai, Zhang, Cui and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunyan Chen, Y2h1bnlhbmNoZW4yMDEzQHllYWgubmV0
†These authors have contributed equally to this work
‡ORCID: Jianling Cui, orcid.org/0009-0004-0255-2543
Chunyan Chen, orcid.org/0009-0006-2909-492X
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.