- Department of Endocrinology, Beijing Chao-yang Hospital, Capital Medical University, Beijing, China
Background: The level of serum interleukin-27 (IL-27) was significantly decreased in the obesity group. After injection of IL-27, obese mice showed significant weight loss,reduced fat accumulation, improved insulin resistance and hepatic steatosis.IL-27 plays a key role in the regulation of metabolic processes, but there are scarce data on circulating IL-27 levels in hypothyroidism. The purpose of this study was to assess the serum levels of IL-27 in patients with hypothyroidism and its relationship with NAFLD.
Methods: 185 participants were included in this cross-sectional survey. According to thyroid function, the subjects were classified into three groups: euthyroidism (n = 55), subclinical hypothyroidism (n = 53), and hypothyroidism (n = 77). Serum IL-27 concentrations were measured by ELISA.
Results: Serum IL27 levels were significantly higher in subclinical hypothyroidism and hypothyroidism groups than in the euthyroidism group. Serum IL27 levels had a negative correlation with HOMA-IR,FBG,TG, subcutaneous fat,and visceral fat, and had a positive correlation with HDL-C (P< 0.05). Furthermore, logistic regression analysis indicated that IL-27 levels, HOMA-IR, and visceral fat showed significant associations with NAFLD after complete adjustment (P< 0.05). ROC curves showed that theoptimal cut-off value of serum IL-27 for discriminating NAFLD was 95.87pg/mL. The area under the ROC curve was 77.3% (95% CI = 0.694-0.851, p < 0.001).
Conclusions: Serum IL-27 levels demonstrated a compensatory increase in patients with subclinical hypothyroidism or hypothyroidism and showed an independent association with NAFLD. Circulating IL-27 levels could predict the occurrence of NAFLD in hypothyroidism. These results suggested that altering the circulating levels of IL-27 may be a potential therapeutic target for NAFLD.
Introduction
Hypothyroidism has an association with an increased risk of developing metabolic syndromecomponents, including obesity, insulin resistance and nonalcoholic fatty liver disease, which is widely prevalent in the common population (1). Clinical and subclinical hypothyroidism is an independent risk factor for NAFLD (2). The underlying mechanisms between NAFLD and hypothyroidism include oxidative stress, insulin resistance,dyslipidemia,metabolic syndrome, and direct action of TSH on hepatocytes (2, 3). Thyroid hormones play a key role in hepatic lipid metabolism, stimulating hepatic lipogenesis and causing hepatic fat accumulation through the thyroid hormone β receptor (4). Certain adipocytokines, such as interleukin-1, tumor necrosis factor-α (TNF-α), visfatin and leptin, and increased oxidative stress that occurs in some cases of hypothyroidism, may coincide with the development of insulin resistance (5). However,the biological mechanisms underlying the development and progression of NAFLD in patients with hypothyroidism are not fully understood (6).
NAFLD is a continuous process from simple steatosis to NASH and eventually to cirrhosis, where there is no effective treatment other than lifestyle modification and regular physical activity (7, 8). In overweight/obese NAFLD, a 7–10% weight loss is the target of most lifestyle interventions, and results in improvement of liver enzymes and histology. Dietary recommendations should consider energy restriction and exclusion of NAFLD-promoting components, macronutrient composition should be adjusted according to the Mediterranean diet, Both aerobic exercise and resistance training effectively reduce liver fat (9). Therefore, early identification of patients at a high risk of NAFLD is extremely important.Liver biopsy is the gold standard for the diagnosis of NADLD, but it has not been widely accepted due to its aggressiveness and high cost (10). At present,non invasive testing is used as a risk assessment tool for steatosis and fibrosis,clinicians mainly use ultrasound to detect fatty liver. Nevertheless, it depends on the experience and skill level of the operator (11). In addition, magnetic resonance spectroscopy (MRS) and magnetic resonance imaging (MRI) accurately determine the content of liver fat and the degree of fibrosis, but they have not been widely used in clinical practice due to the high cost (12). Therefore,noninvasive methods are urgently needed to identify NAFLD, monitor treatment effects, andtrack disease processes (13).
As a member of the IL-6/IL-12 cytokine family, IL-27 consists of two subunits, EBI3 and P28 (14, 15). IL-6 production has an association with decreased energy expenditure and increased body fat mass (16). The IL-27-IL-27Rα signaling pathway plays a key role in enhancing insulin resistance, promoting thermogenesis, and combating diet-induced obesity, which is a promising target for anti-obesity immunotherapy (17). IL-27 may be a novel target for the clinical treatment of metabolic diseases in the future. Currently, there are few inconsistent data on circulating IL-27 levels in patients with hypothyroidism. Hence, the purpose of this study was to assess the serum levels of IL-27 in patients with hypothyroidism and its relationship with NAFLD. Furthermore, the diagnostic accuracy of IL-27 was measured in the detection of NAFLD in hypothyroidism.
Materials and methods
Study group
A total of 185 patients (20-70 years) were included in this cross-sectional study. 77 patients were newly or previously diagnosed with clinical hypothyroidism, and 53 patients were newly or previously diagnosed with subclinical hypothyroidism, but had not received thyroid hormone replacement therapy within 3 months. 55 patients had normal thyroid functions. Exclusion criteria were: acute or chronic virus hepatitis, hepatolenticular degeneration, total parenteral nutrition, drug-induced liver disease, previous chronic heavy drinking, liver or kidney disease, heart failure,myocardial infarction breastfeeding or pregnancy, chronic inflammation, acute infection, and other specific diseases that cause fatty liver. Participants were recruited from the Endocrine Clinic at Beijing Chaoyang Hospital, Affiliated to Capital Medical University. All patients signed the informed consent, andthis study was approved by the Ethics Committee (2019-science-363) of Beijing Chaoyang Hospital, Capital Medical University.
Biochemical and anthropometric measurements
Body weight, height, and waist were measured by trained personnel. Subcutaneous fat and visceral fat were measured by bioelectrical impedance analysis (OMRON, HDS-2000 DUALSCAN). Blood samples were taken from all subjects the next morning after fasting. BMI was calculated as weight(kg)/height(m)2. HDL-C, LDL-C, TG,TC, and FBG were tested using an autoanalyzer (Hitachi 747, Germany). Hemoglobin A1c (HbA1c) was detected using an HLC-723G7 analyzer (Tokyo, Japan) through high-performance liquid chromatography. Fasting insulin(FINS) was detected using chemiluminescence. Serum IL-27 levels were evaluated using an ELISA kit (R&D Systems, Minneapolis, MN, USA). Homeostasis model assessment–insulin resistance (HOMA-IR) was calculated as FINS (mIU/L) × FBG (mmol/L)/22.5 (18).
Definition
According to thyroid function, all participants were classified into three groups: euthyroidism, subclinical hypothyroidism,TSH (>4.78uIU/mL) levels with normal FT4 and FT3 levels, and hypothyroidism, TSH (>4.78uIU/mL) levels with decreased FT4 levels. Reference values for thyroid function tests were as follows: TT4, 4.5-10.9ug/dL;FT4, 0.89-1.76ng/dL; TSH, 0.55-4.78uIU/mL.NAFLD was defined as no history of drinking or drinking< 210g/week (<140g/week for women), and ultrasound imaging outcomes were consistent with diffuse fatty liver disease (19).
Statistical analysis
Data were analyzed with SPSS 26.0(IBM Corporation, New York) and GraphPad Prism9.0(Inc,CA,USA). The normal distribution of data was represented by the mean ± standard. Non-normally distributed data were represented bythe median and interquartile range. One-way ANOVA or Kruskal-Wallis test and Bonferroni post hoc test were used for comparison between groups. In addition, covariance (ANCOVA) and Bonferroni post hoc tests were used to compare the potential confounders of the adjusted differences between groups. The association between serum IL-27 levels and NAFLD was investigated using Pearson analysis. To further explore the association between serum IL-27 levels and dyslipidemia, linear regression was conducted.ROC curves were used to define the cut-off value of IL-27. The significance was indicated as P < 0.05, and all tests were two-tailed.
Results
Characteristics of study groups
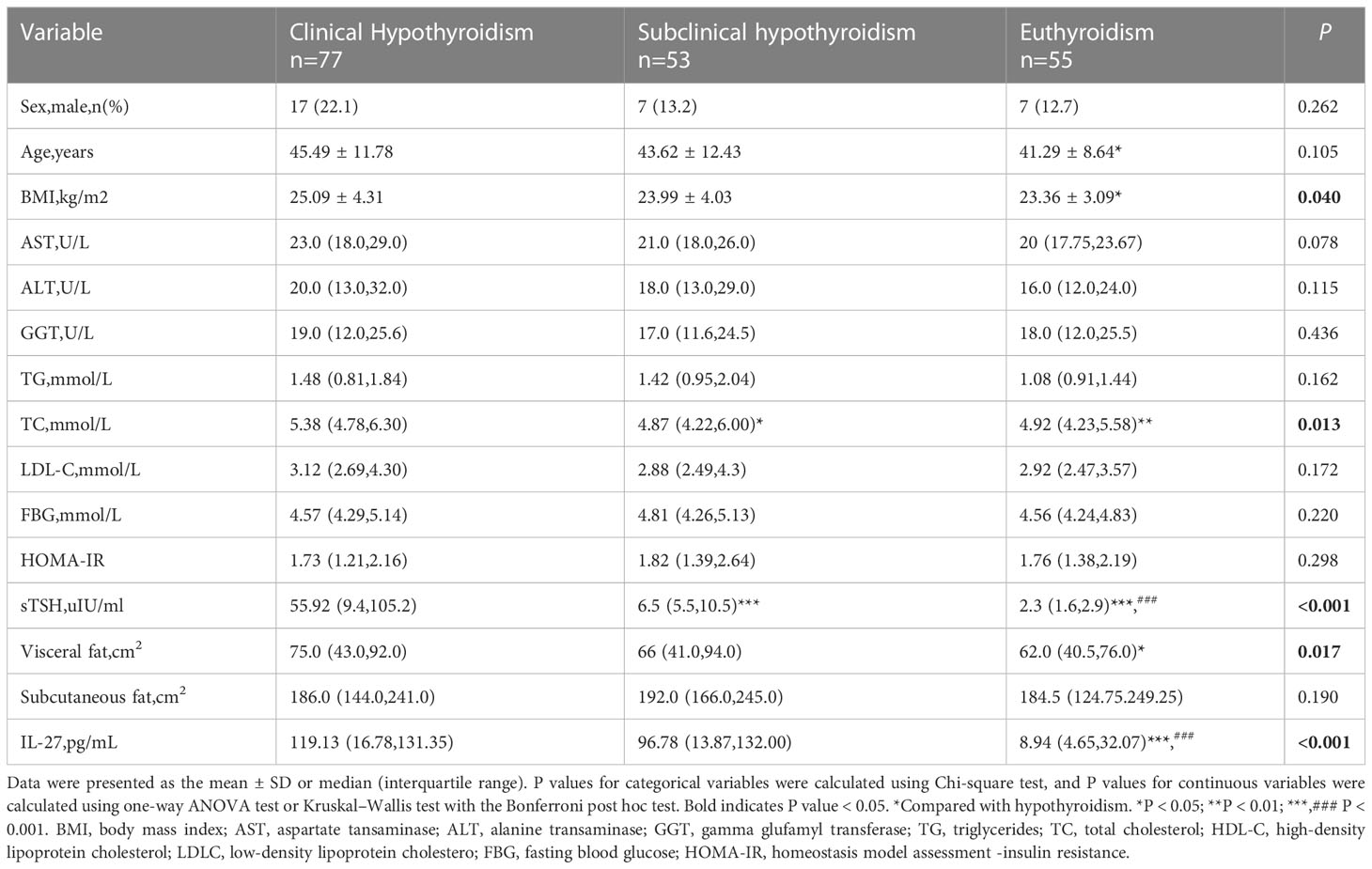
The clinical characteristics of all the participants are shown in Table 1. The subjects were classified into three groups. Among the three groups, sex, age, ALT, AST, GGT, TG, LDL-C, HOMA-IR, fasting glucose, and subcutaneous fat showed no significant differences. Compared with the euthyroidism group,patients with hypothyroidism tended to have higher levels of BMI, TC, and visceral fat (P <0.05). The cytokine level of IL-27showed a higher serum concentration in patients with hypothyroidism compared to the euthyroidism group at 119.13(16.78,131.35) versus 8.94(4.65,32.07) pg/mL. Serum IL-27 levels were higher in the subclinical hypothyroidismgroup than in the euthyroidism group. There was no statistically significant difference in IL-27 between hypothyroidism and subclinical hypothyroidism patients (Figure 1).
Correlation between clinical parameters and serum IL-27 levels in hypothyroidism
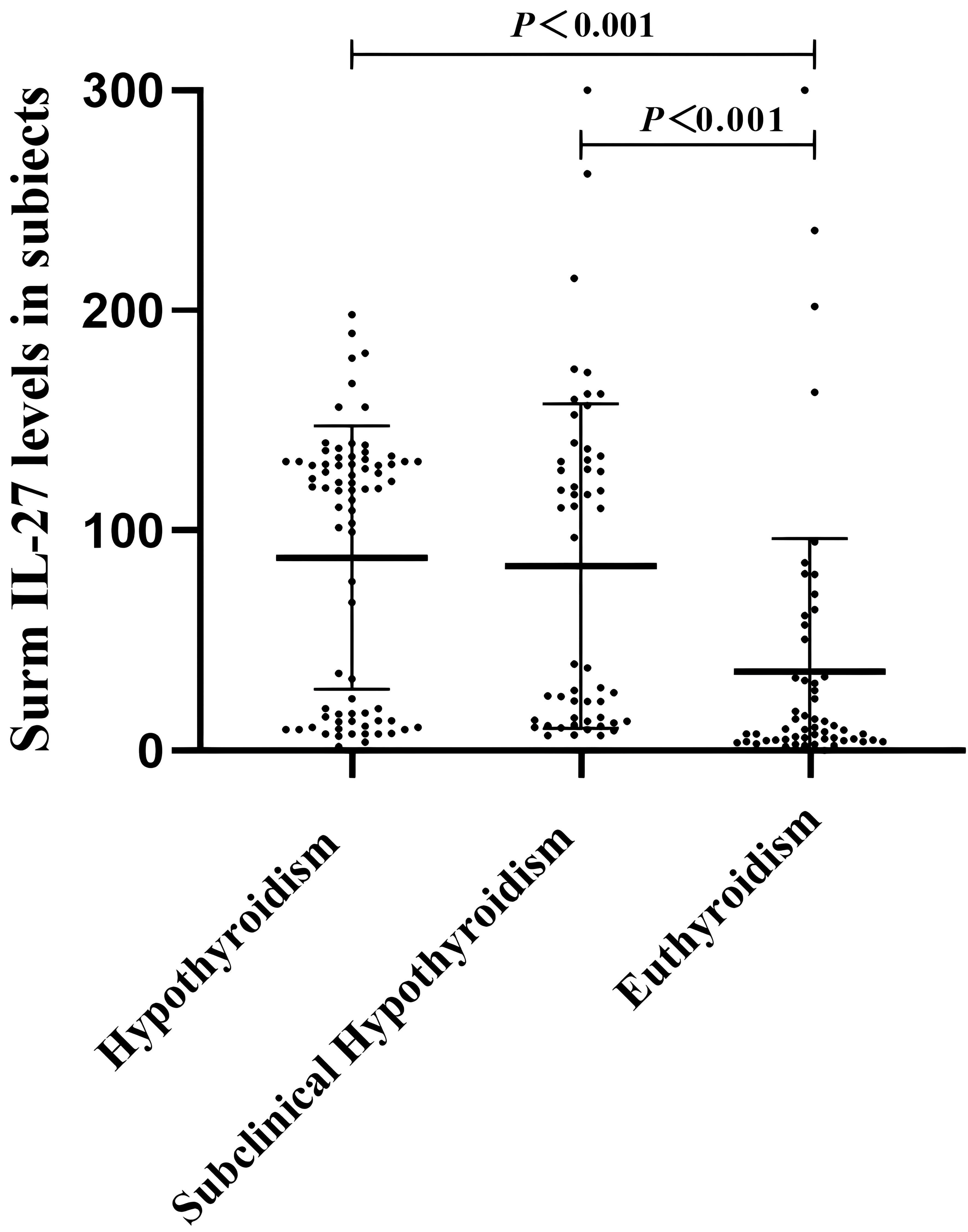
Correlation analysis was used to investigate the association between serum IL-27 levels and metabolic parameters in patients with hypothyroidism (Figure 2). The concentration of circulating IL-27 was negatively related to TG(r=-0.192,P=0.028), FBG(r=-0.269, P=0.002), subcutaneous fatr=-0.391, P<0.001), visceral fat(r=-0.569, P<0.001), HOMA-IR(r=-0.411, P<0.001), and was positively related to HDL-C(r=0.39, P<0.001).
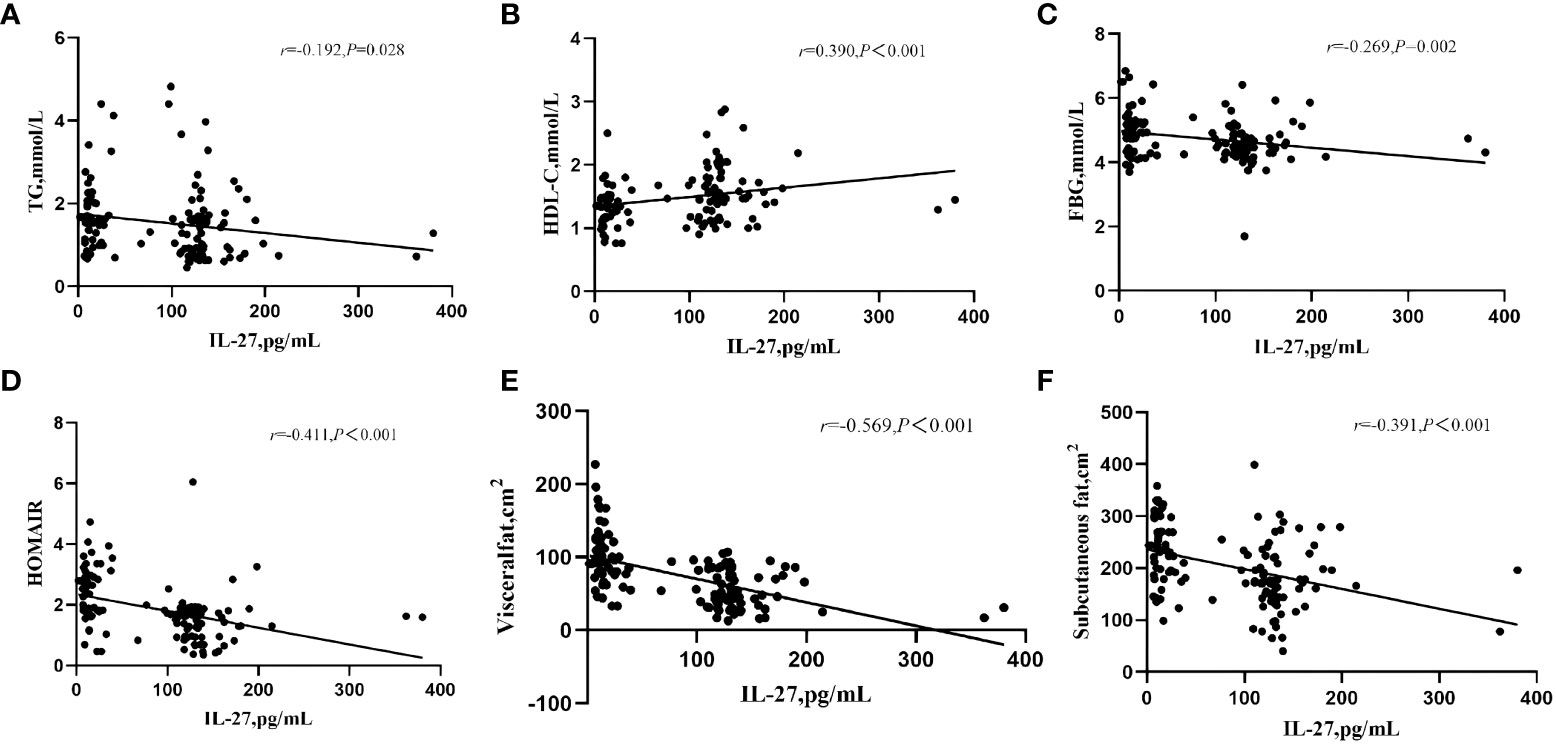
Figure 2 Correlation of serum IL-27 levels with TF (A) HDL-C (B), FBG (C), HOMIR (D), visceral fat (E), and subcutaneous fat (F) in Hypothyroidism. P value <0.05 were considered statistically significant.
Independent relationship between serum IL-27 levels and NAFLD in hypothyroidism
According to the diagnosis of NAFLD, patients with hypothyroidism or subclinical hypothyroidism were classified into two groups.There was no significant difference in TC, LDL-C, sTSH, FT3, and FT4 between the two groups. Patients with NAFLD had higher age, BMI, AST, ALT, GGT, TG, FBG, HOMA-IR, subcutaneous fat, visceral fat and lower HDL-C,IL-27 compared to those without NAFLD(Table 2). To identify whether serum IL-27 levels had an independent correlation with NAFLD, logistic regression analysis was performed among all participants (Table 3). The results indicated that lower serum IL-27 levels had a significant association with NAFLD [OR (95%CI), 0.97 (0.96-0.99)](P < 0.001). In addition,higher HOMA-IR[OR (95%CI), 16.85(2.18,130.45)] and visceral fat[OR (95%CI), 1.10(1.01,1.19)](P < 0.05) also had an independent association with NAFLD.
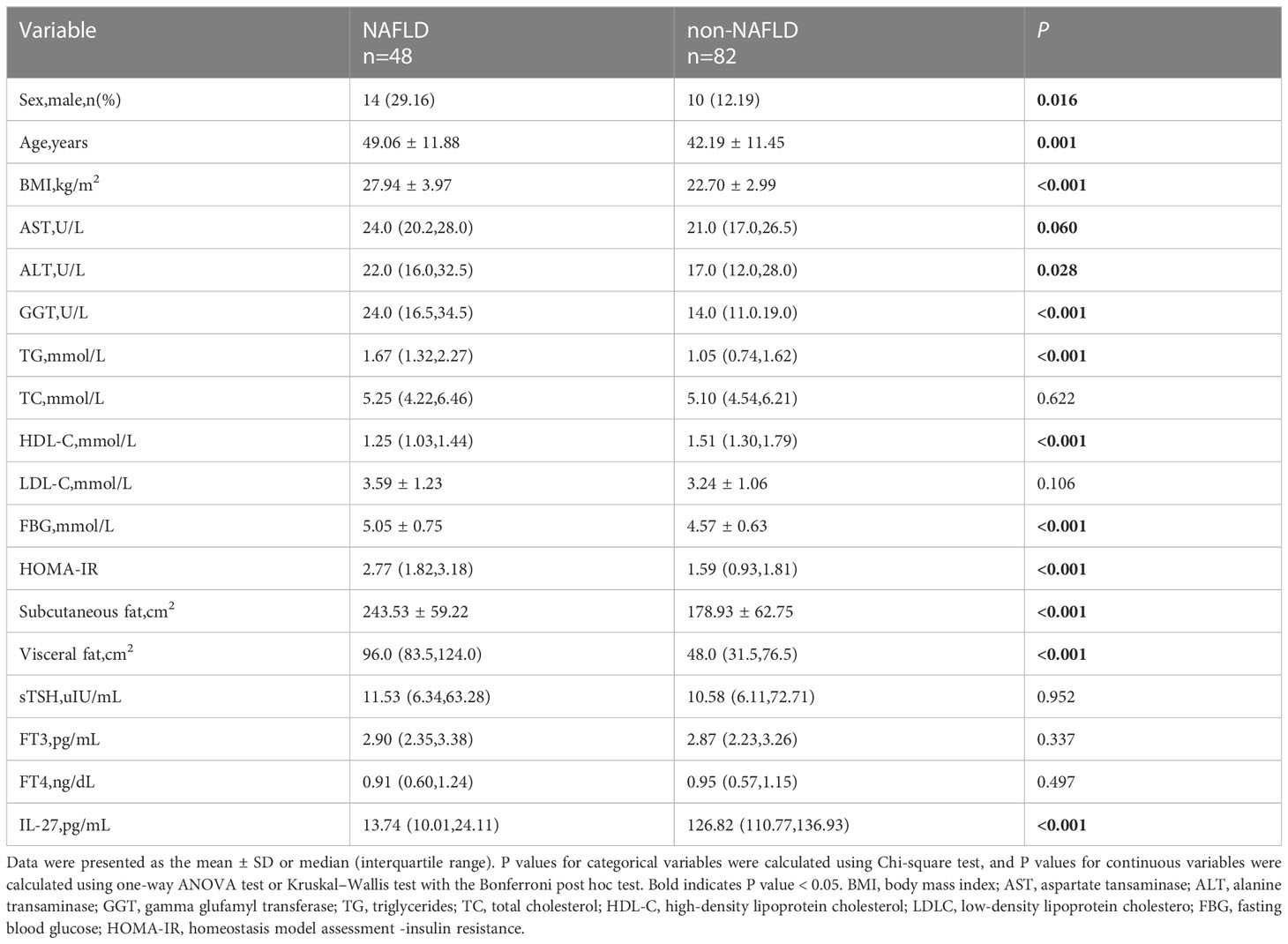
Table 2 Comparison of main metabolic related indexes between NAFLD and non-NAFLD patients with hypothyroidism.
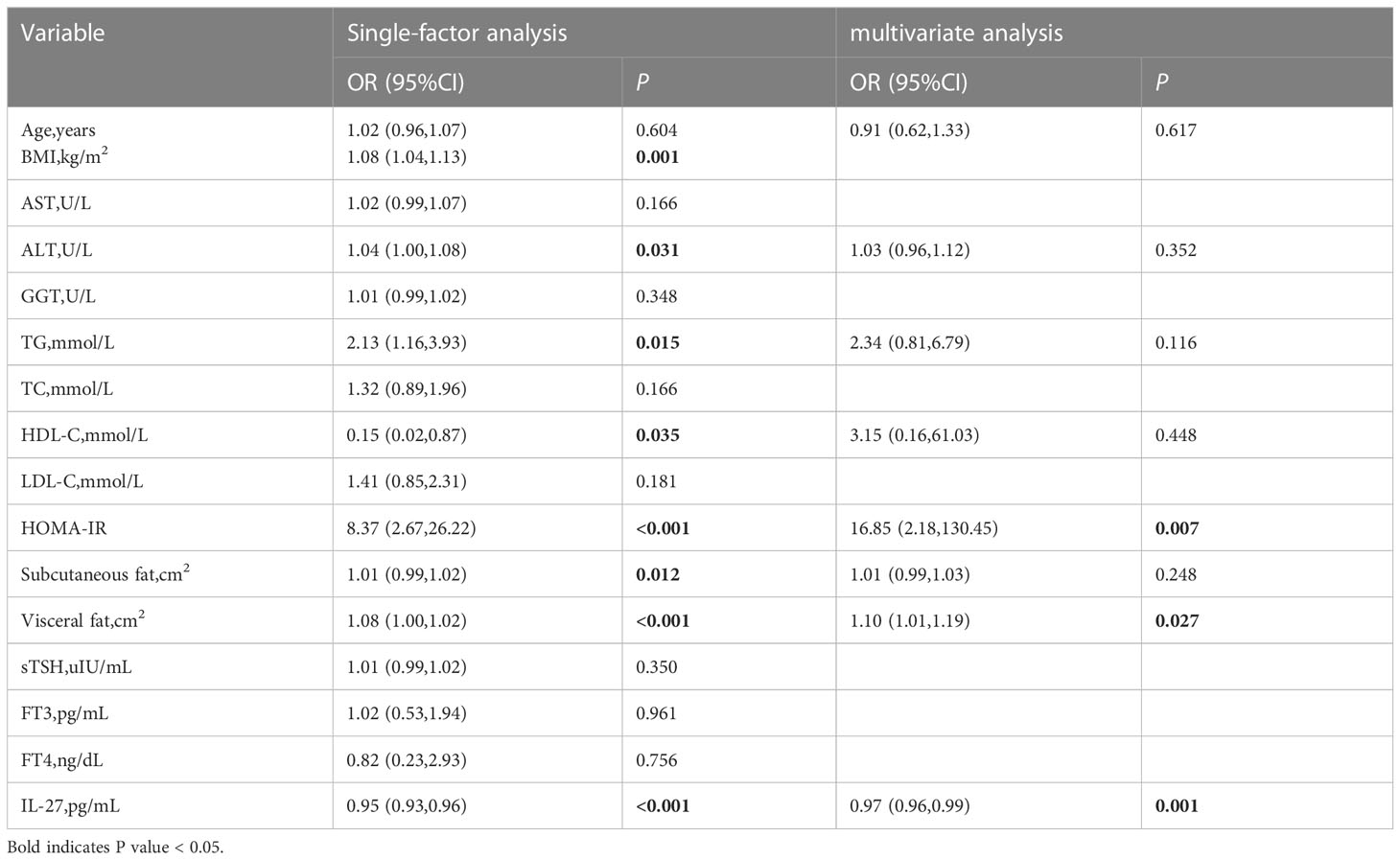
Table 3 Correlation analysis between serum IL27 concentration and non-alcoholic fatty liver disease in hypothyroidism.
Finally, the diagnostic value of IL-27 for NAFLD was analyzed by ROC curves (Figure 3). The optimal cut-off value of serum IL-27 for discrimination of NAFLD was 95.87pg/mL (AUC = 0.773, P < 0.001).
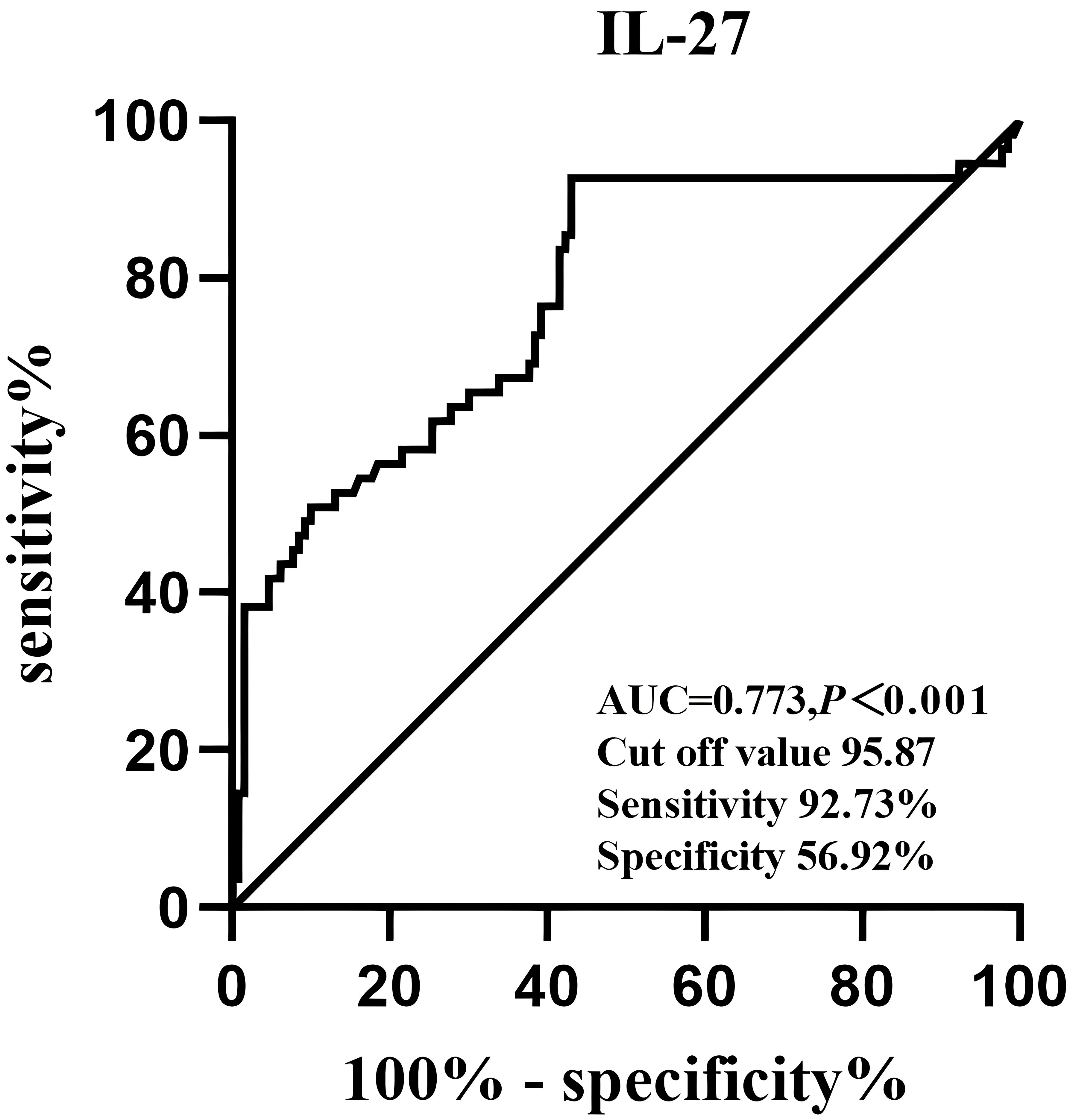
Figure 3 Receiver operative characteristics curves and cut off values of serum IL-27 levels for NAFLD.
Discussion
In this study, a compensatory increase in the concentration of serum IL-27 was observed in patients with hypothyroidism or subclinical hypothyroidism. Serum IL-27 levels were negatively associated with FBG, HOMAIR, subcutaneous fat, visceral fat, and TG, and positively associated with HDL-C in patients with hypothyroidism. More notably, lower circulating IL-27 levels in patients with hypothyroidism were independently related to NAFLD. These results suggested that IL-27 could be a promising therapeutic target for NAFLD in patients with hypothyroidism.
Hypothyroidism is a common endocrine disorder characterized by increased sensitivity to cold and unexpected weight gain, suggesting a change in response to cold and energy expenditure (20). Thyroid hormones play an important role in the normal function and cold-induced thermogenesis of brown adipose tissues in rodents (21, 22). Wang et al. reported that IL-27Rα-deficient mice were cold-intolerant because of impaired adaptive thermogenesis, and IL-27 improved thermogenesis and directly targeted adipocytes to counteract obesity (17). Other studies have shown that IL-27 exerts its beneficial effects by the upregulation of adaptive thermogenesis in brown adipose tissues (23). Consistent with their findings, a compensatory increase in the concentration of serum IL-27 was observed in patients with hypothyroidism or subclinical hypothyroidism. IL-27 may combat hypothermia in patients with hypothyroidism through its febrile effects.
NAFLD is a worldwide health problem and has an increased risk of diabetes, CVD and CKD (24). NAFLD is a complex and heterogeneous disease that is inaccurately diagnosed by liver biopsy (25). Current therapeutic strategies mainly focus on lifestyle interventions, and there are still limited appropriate drugs specifically available for NAFLD (26). Therefore, the discovery of non-invasive detection, novel therapeutic targets and strategies shall be required. Patients with hypothyroidism are at an increased risk of NAFLD. Hypothyroidism plays an important role in the development and progression of NAFLD (27).
Dyslipidemia related to hypothyroidism leads to intrahepatic fat accumulation, resulting in NAFLD and thus leading to the development of hepatic insulin resistance (1). Thyroid hormones increase the expression of HMG-CoA reductase in the liver to increase cholesterol synthesis (28). Plasma cholesteryl ester transfer proteins (CETPs) are decreased in a hypothyroid state, which shift cholesterol from HDL-C to LDL-C and VLDL (29). Consistently, data showed that NAFLD patients with hypothyroidism had higher BMI, LDL-C, FBG, HOMA-IR, subcutaneous fat, visceral fat, and lower HLD-C than non-NAFLD patients. It was found that IL-27 exerted a protective effect against diabetes by ameliorating STZ-induced hyperglycemia and islet inflammation. The hypertrophy of chronic inflammation and white adipocytes in white adipose tissues was blocked and HFD-induced liver steatosis was suppressed by Il-27 gene transfer (23). This study showed that serum IL-27 levels were negatively associated with FBG, HOMAIR, subcutaneous fat, visceral fat, and TG, and positively associated with HDL-C. Therefore, the findings support previous observations that IL-27 could improve insulin resistance and reduce fat accumulation. Meanwhile, data suggested that IL-27 could be involved in lipid metabolism. Thus, it was hypothesized that IL-27 may improve fatty liver by improving insulin resistance and reducing blood lipids. Besides, NAFLD patients with hypothyroidism had lower IL-27 compared to those without NAFLD. It can be speculated that the increase of IL-27 content may improve NAFLD. IL-27 levels may be a potential therapeutic target for dyslipidemia and NAFLD. The role of circulating IL-27 in improving NAFLD in hypothyroidism needs further investigation.
Multiple studies have demonstrated variables for predicting NAFLD (30). The noninvasive screening mode facilitates the timely identification and intervention of NAFLD and its complications in high-risk patients (31). Baseline HOMA-IR and weight gain were used as predictors of NAFLD incidence in a 7-year prospective study (32). Logistic regression analysis displayed that circulating IL-27 levels,HOMA-IR, and visceral fat were independently related to an increased risk of NAFLD. It was demonstrated that IL-27 could also predict the occurrence of NAFLD. In this study,IL-27 could predict NAFLD with an AUC of 0.897 (95% CI 0.757-0.859). In conclusion, the exact mechanism by which IL-27 regulates NAFLD metabolism needs to be further studied.
This study had a number of limitations.First, the sample size was small and the cross-sectional study failed to construct a causal relationship between IL-27 and disease. Second, other potential confounders were not eliminated, particularly cold exposure and exercise. Finally, only the Chinese population was included in this study, thus making the generalizability of the findings an issue.
In summary, serum IL-27 levels had a compensatory increase in patients with hypothyroidism or subclinical hypothyroidism and had an independent association with NAFLD. This study manifests that altering circulating IL-27 levels could predict the occurrence of NAFLD in hypothyroidism.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Beijing Chaoyang Hospital, Capital Medical University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by YW, HZ, XG, NY, and ZC. The first draft of the manuscript was written by YW. The paper was revised by JL and GW. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1173826/full#supplementary-material
References
1. Mavromati M, Jornayvaz FR. Hypothyroidism-associated dyslipidemia: potential molecular mechanisms leading to nafld. Int J Mol Sci (2021) 22(23):12797. doi: 10.3390/ijms222312797
2. Lugari S, Mantovani A, Nascimbeni F, Lonardo A. Hypothyroidism and nonalcoholic fatty liver disease - a chance association? Hormone Mol Biol Clin Invest (2018) 41(1). doi: 10.1515/hmbci-2018-0047
3. Sinha RA, Singh BK, Yen PM. Direct effects of thyroid hormones on hepatic lipid metabolism. Nat Rev Endocrinol (2018) 14(5):259–69. doi: 10.1038/nrendo.2018.10
4. Sinha RA, Bruinstroop E, Singh BK, Yen PM. Nonalcoholic fatty liver disease and hypercholesterolemia: roles of thyroid hormones, metabolites, and agonists. Thyroid (2019) 29(9):1173–91. doi: 10.1089/thy.2018.0664
5. Lonardo A, Ballestri S, Mantovani A, Nascimbeni F, Lugari S, Targher G. Pathogenesis of hypothyroidism-induced nafld: evidence for a distinct disease entity? Digestive liver Dis Off J Ital Soc Gastroenterol Ital Assoc Study Liver (2019) 51(4):462–70. doi: 10.1016/j.dld.2018.12.014
6. Lonardo A, Mantovani A, Lugari S, Targher G. Nafld in some common endocrine diseases: prevalence, pathophysiology, and principles of diagnosis and management. Int J Mol Sci (2019) 20(11):2841. doi: 10.3390/ijms20112841
7. Soon G, Wee A. Updates in the quantitative assessment of liver fibrosis for nonalcoholic fatty liver disease: histological perspective. Clin Mol Hepatol (2021) 27(1):44–57. doi: 10.3350/cmh.2020.0181
8. Calzadilla Bertot L, Adams L. The natural course of non-alcoholic fatty liver disease. Int J Mol Sci (2016) 17(5):774. doi: 10.3390/ijms17050774
9. Marchesini M, Day CP, Dufour J-F, Canbay A, Nobili V, Ratziu V. Easl–Easd–Easo clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol (2016) 64(6):1388–402. doi: 10.1016/j.jhep.2015.11.004
10. Hu H, Han Y, Cao C, He Y. The triglyceride glucose-body mass index: a non-invasive index that identifies non-alcoholic fatty liver disease in the general Japanese population. J Trans Med (2022) 20(1):398. doi: 10.1186/s12967-022-03611-4
11. Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology (2011) 54(3):1082–90. doi: 10.1002/hep.24452
12. Buzzetti E, Lombardi R, De Luca L, Tsochatzis EA. Noninvasive assessment of fibrosis in patients with nonalcoholic fatty liver disease. Int J Endocrinol (2015) 2015:1–9. doi: 10.1155/2015/343828
13. Cai J, Zhang X-J, Li H. Progress and challenges in the prevention and control of nonalcoholic fatty liver disease. Medicinal Res Rev (2019) 39(1):328–48. doi: 10.1002/med.21515
14. Stefan Pflanz JCT, Cheung J, Rency Rosales HK. Il-27, a heterodimeric cytokine composed of Ebi3 and P28 protein, induces proliferation of naive Cd4+ T cells. Immunity (2002) 16(6):779–90. doi: 10.1016/s1074-7613(02)00324-2
15. Tait Wojno ED, Hunter CA, Stumhofer JS. The immunobiology of the interleukin-12 family: room for discovery. Immunity (2019) 50(4):851–70. doi: 10.1016/j.immuni.2019.03.011
16. Kubaszek AP, Punnonen K. The c-174g promoter polymorphism of the il-6 Gene:Affects energy expenditure and insulin sensitivity. Diabetes (2003) 52(2):558–61. doi: 10.2337/diabetes.52.2.558
17. Wang Q, Li D, Cao G, Shi Q, Zhu J, Zhang M, et al. Il-27 signalling promotes adipocyte thermogenesis and energy expenditure. Nature (2021) 600(7888):314–8. doi: 10.1038/s41586-021-04127-5
18. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia (1985) 28(7):412. doi: 10.1007/BF00280883
19. National Workshop on Fatty Liver and Alcoholic Liver Disease CSoH, Chinese Medical Association;Fatty Liver Expert Committee, Chinese Medical Doctor Association. Guidelines of prevention and treatment for nonalcoholic fatty liver Disease:A 2018 update. Zhonghua Gan Zang Bing Za Zhi (2018) 26(03):195–203. doi: 10.3760/cma.j.issn.1007-3418.2018.03.008
20. Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American association of clinical endocrinologists and the American thyroid association. Thyroid (2012) 22(12):1200–35. doi: 10.1089/thy.2012.0205
21. Mullur R, Liu Y-Y, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev (2014) 94(2):355–82. doi: 10.1152/physrev.00030.2013
22. Maushart CI, Loeliger R, Gashi G, Christ-Crain M, Betz MJ. Resolution of hypothyroidism restores cold-induced thermogenesis in humans. Thyroid (2019) 29(4):493–501. doi: 10.1089/thy.2018.0436
23. Yang Y, Liu H, Liu D. Preventing high-fat diet-induced obesity and related metabolic disorders by hydrodynamic transfer of il-27 gene. Int J Obes (2023) 47(5):413–21. doi: 10.1038/s41366-023-01293-6
24. Mantovani A, Byrne CD, Benfari G, Bonapace S, Simon TG, Targher G. Risk of heart failure in patients with nonalcoholic fatty liver disease. J Am Coll Cardiol (2022) 79(2):180–91. doi: 10.1016/j.jacc.2021.11.007
25. Mato JM, Alonso C, Noureddin M, Lu SC. Biomarkers and subtypes of deranged lipid metabolism in non-alcoholic fatty liver disease. World J Gastroenterol (2019) 25(24):3009–20. doi: 10.3748/wjg.v25.i24.3009
26. Xu X, Poulsen KL, Wu L, Liu S, Miyata T, Song Q, et al. Targeted therapeutics and novel signaling pathways in non-Alcohol-Associated fatty Liver/Steatohepatitis (Nafl/Nash). Signal Transduction Targeted Ther (2022) 7(1):287. doi: 10.1038/s41392-022-01119-3
27. Zeng X, Li B, Zou Y. The relationship between non-alcoholic fatty liver disease and hypothyroidism. Medicine (2021) 100(17):e25738. doi: 10.1097/md.0000000000025738
28. Choi JW, Choi HS. The regulatory effects of thyroid hormone on the activity of 3-Hydroxy-3-Methylglutaryl coenzyme a reductase. Endocr Res (2000) 26(1):1–21. doi: 10.1080/07435800009040142
29. Tan KC, Shiu SW, Kung AW. Plasma cholesteryl ester transfer protein activity in hyper- and hypothyroidism. J Clin Endocrinol Metab (1998) 83(1):140–3. doi: 10.1210/jc.83.1.140
30. Sripongpun P, Kim WR, Mannalithara A, Charu V, Vidovszky A, Asch S, et al. The steatosis-associated fibrosis estimator (Safe) score: a tool to detect low-risk nafld in primary care. Hepatology (2022) 77(1):256–267. doi: 10.1002/hep.32545
31. Hassen G, Singh A, Belete G, Jain N, de la Hoz I, Camacho-Leon GP, et al. Nonalcoholic fatty liver disease: an emerging modern-day risk factor for cardiovascular disease. Cureus (2022) 14(5):e25495. doi: 10.7759/cureus.25495
Keywords: hypothyroidism, IL-27, NAFLD, dyslipidemia, lipid metabolism
Citation: Wen Y, Zhang H, Yang N, Gao X, Chen Z, Liu J and Wang G (2023) Serum IL-27 levels increase in subjects with hypothyroidism and are negatively correlated with the occurrence of nonalcoholic fatty liver disease. Front. Endocrinol. 14:1173826. doi: 10.3389/fendo.2023.1173826
Received: 25 February 2023; Accepted: 19 June 2023;
Published: 02 August 2023.
Edited by:
Silvia Martina Ferrari, University of Pisa, ItalyReviewed by:
Zoran Gluvic, University of Belgrade, SerbiaRuilian You, Peking Union Medical College Hospital (CAMS), China
Copyright © 2023 Wen, Zhang, Yang, Gao, Chen, Liu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guang Wang, ZHJ3ZzY4NjhAMTI2LmNvbQ==; Jia Liu, bGl1amlhMDExNkAxMjYuY29t
†These authors have contributed equally to this work
 Yahui Wen
Yahui Wen Heng Zhang
Heng Zhang Ning Yang
Ning Yang