- 1Division of Endocrine and Kidney Disease Research, Department of Chronic Disease Convergence, Korea National Institute of Health, Cheongju-si, Chungbuk, Republic of Korea
- 2Department of Surgery, School of Medicine, Inha University, Incheon, Republic of Korea
- 3Department of Surgery, Seoul National University Bundang Hospital, Seongnam, Republic of Korea
- 4Department of Surgery, Seoul National University Hospital, Seoul, Republic of Korea
Background: Bariatric surgery (BS) has a superior effect on reducing body weight and fat in patients with morbid obesity. As a result, BS mitigates obesity-related complications such as type 2 diabetes (T2D). However, few studies have shown the mechanism underlying diabetes remission after surgery. This study aimed to investigate the differences in serum hormone and inflammatory cytokine levels related to diabetes before surgery and during 12 months of follow-up in Korean patients with obesity.
Methods: The study participants were patients with morbid obesity (n=63) who underwent sleeve gastrectomy (SG) or Roux-en-Y gastric bypass (RYGB) between 2016 – 2017 at seven tertiary hospitals in Korea. The patients were followed for 1 year after surgery.
Results: Sixty-three patients had significant weight loss after surgery and showed improvements in clinical parameters and hormonal and inflammatory profiles. Among them, 23 patients who were diabetic preoperatively showed different remission after surgery. The levels of inflammation-related clinical parameters changed significantly in the remission group, and serum inflammatory cytokine and hormones significantly decreased at certain points and showed an overall decreasing trend.
Conclusions: Our study found postoperative changes of factors in blood samples, and the changes in hormones secreted from the three major metabolic tissue (pancreas, adipose, and gut) along with the differences in multi-origin inflammatory cytokines between remission and non-remission groups provide a path for understanding how the effect of BS in improving glucose metabolism is mediated.
1 Introduction
Compared to pharmacotherapy in the treatment of obesity (1), bariatric surgery (BS) has a superior effect on reducing body weight and fat in patients with severe obesity. Furthermore, it mitigates type 2 diabetes, hypertension, and dyslipidemia. Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG), which change the anatomical structure of the gastrointestinal (GI) tract and hormone levels, are the most performed types of BS (2–4). Postprandial hormone responses, such as that of glucagon-like peptide 1 (GLP-1), stimulate insulin secretion and lower glucose levels (5). Obese subjects have high serum levels of GLP-1 (6), indicating that the malfunction of GLP-1 signaling can cause insulin resistance. Patients who underwent BS were also reported to have increased postprandial GLP-1 levels (7). Leptin is a hormone produced by adipocytes and leptin levels have a strong correlation with obesity severity. In these cases, hyperleptinemia is usually observed, but it decreases with decreasing body weight. Thus, leptin levels can also be affected by BS. However, GI hormones are not the only ones affected by BS since it also modulates inflammatory cytokines.
Chronic inflammation in obese patients plays an important role in the development of metabolic diseases and increases the risk of complications such as hypertension, insulin resistance, atherosclerosis, chronic kidney disease, and aging-related diseases such as sarcopenia (8–10). Cytokines and hormones originating from different sites contribute to the development of tissue inflammation (11, 12). For example, interleukin-1β (IL-1β) and monocyte chemoattractant protein-1 (MCP-1) increase, and MCP-1 promotes macrophage infiltration in metabolic tissues (13).
Although BS has shown promising effects in patients with severe obesity and type 2 diabetes mellitus (T2D), not all patients who undergo BS show improvements in glucose regulation. A proportion of patients (approximately 10-67%) with BS still experience dysregulation of glucose metabolism (14–16), even though their body weights were significantly lower. The reasons why some patients experience diabetes remission while others do not after BS remain unclear. Few studies have been conducted on the effects of BS in Korean patients.
Recently, owing to its acceptance by the Korean population and coverage by medical insurance, more patients have been undergoing BS. However, few studies have been conducted on Korean patients. There are several important differences in terms of ethnic background: i) the rates of body fat content over body mass are higher in Asian populations than in Western populations (17), ii) an improvement of diabetes condition has advanced to substantial reduction of body weight (18, 19), and iii) beta cell dysfunction is more severe in Asian populations including Korean than in Western populations with the same BMI (20, 21). Therefore, it is important to establish data on Korean patients. Many researchers and clinicians have attempted to divide patients into subtypes including ethnic backgrounds for a more effective and personalized approach to therapy.
In this study, we assessed hormone and inflammatory cytokine levels up to 12 months after RYGB or SG in Korean patients. We further divided the patients into diabetes remission and non-remission groups and monitored changes in hormone and inflammatory cytokine levels.
2 Materials and methods
2.1 Study participants
The study participants and study design were previously described in the Korean Obesity Surgical Treatment Study (KOBESS) (1) and registered at ClinicalTrials.gov (NCT03100292). Briefly, this was a prospective, multicenter, single-arm, observational cohort study of obese Korean patients with obesity. Sixty-three participants were recruited between 2016 – 2017 at seven tertiary hospitals in Korea. Sleeve gastrectomy (SG) or Roux-en-Y gastric bypass (RYGB) surgical methods were conducted on the participants, and they were followed for 1 year after surgery. All consecutive patients aged 20 – 65 years with a body mass index (BMI) of ≥ 35 kg/m2 or a BMI of ≥ 30 kg/m2 with comorbid conditions, including hypertension, glucose intolerance, dyslipidemia, or obstructive sleep apnea (OSA) were included. The study was approved by the Korea Disease Control and Prevention Agency (KDCA) and Inha University Hospital Institutional Review Board (2021-10-08-P-A and 2019-10-025-002, respectively). All participants provided written informed consent.
2.2 Type 2 diabetes remission subgroup
The complete remission of T2D was defined as a glycated hemoglobin (HbA1c) level <6% and a fasting blood sugar (FBS) level <100 mg/dL for 1 year; partial remission was defined as HbA1c<6.5% and FBS 100–125 mg/dL, without anti-diabetes medication use for 1 year after the surgery. Diabetes medication was stopped in all patients postoperatively. However, if the HbA1C exceeded 7% during the follow up period, anti-diabetes medication was restarted. Non-remission group included partial remission group as well as the patients who did not achieve any remission criteria (22). The study for remission of T2D subject, participants (remission n = 11, non-remission n =12) were collected from KOBESS study as previously described (1). Clinical and serum parameters were analyzed up to 12 months after surgery.
2.3 Serum profiling
Firstly, we selected specific time points for measuring hormone levels: before the surgery and at 1, 6, and 12 months after the procedure, in order to examine the immediate changes following surgery (at 1 month) and track the progress every 6 months thereafter. Subsequently, considering the observed changes in hormone levels, we conducted additional investigations into the levels of inflammatory factors. This analysis aimed to determine if cytokine changes occurred prior to hormonal changes within the 6-month period following surgery. We examined the levels of inflammatory factors at 3 months post-surgery, opting for this time point instead of the standard 6 months. Serum samples were analyzed with a Luminex instrument (Luminex, Austin, TX) using MILLIPLEX® multiplex panels (Millipore Corporation, Billerica, MA, USA) and performed by LAS Inc, South Korea (https://www.las.kr) according to the manufacturer’s instructions. All panels available for the analysis of human hormones or inflammatory factors were provided by Millipore, including HMHEMAG-34K (GLP-1, GIP, insulin, leptin, glucagon, and amylin), HADK1MAG-61K (adiponectin and resistin), and HCYTOMAG-60K (IL-1β, IL-Ra, IL-4, IL-6, IL-17A, MCP1, tumor necrosis factor (TNF)α, TNFβ, transforming growth factor (TGF)α, TNFβ, Granulocyte colony-stimulating factor (G-CSF) and Platelet-Derived Growth Factor (PDGF) AA). The concentration of hormones or inflammatory factors was calculated using a 5-parameter logistic or cube spline standard curve and expressed as pg/mL or ng/mL.
2.4 Statistics analysis
Statistical analyses were conducted using SAS statistical software package (version 9.4; SAS Institute, Inc., Cary, NC, USA) or Prism 8.3.0 (GraphPad Software, San Diego, CA, USA). The values are expressed as means ± standard deviation (SD) for continuous variables and numbers (%) for categorical variables. The significant differences between baseline and postoperative time points were determined using one-way analysis of variance (ANOVA, mixed-effect analysis) followed by a paired t-test with Dunnett's multiple comparisons tests. For all analyses, p-values of < 0.05 were considered statistically significant.
3 Results
3.1 Patient demographics and clinical characteristics at baseline
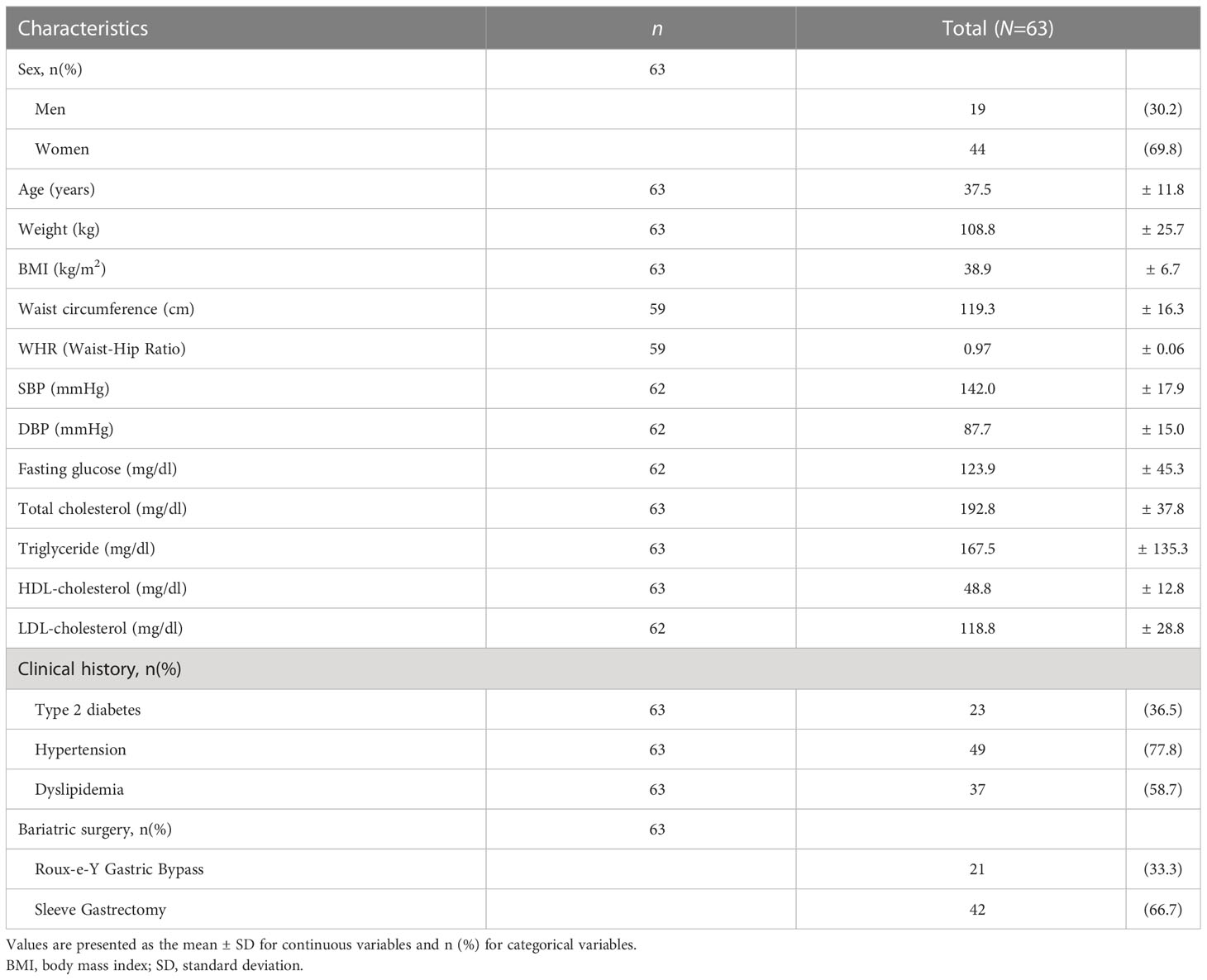
Sixty-three patients underwent BS during the study period. Twenty-one patients underwent RYGB, and 42 underwent SG. The clinical baselines and characteristics of the study participants are summarized in Table 1. The mean age for the entire cohort was 37.5 ± 11.8 years old. The mean BMI of the patients was 38.9 ± 6.7 kg/m2, indicating class II obesity, which was defined as 35 kg/m2 ≤ BMI ≤ 39.99 kg/m2 by the International Classification of Disease (ICD). Twenty-three patients (36.5%) had diabetes, and 49 (77.8%) and 37 (58.7%) had hypertension and dyslipidemia, respectively.
3.2 Post-surgery changes during 12 months of follow-up
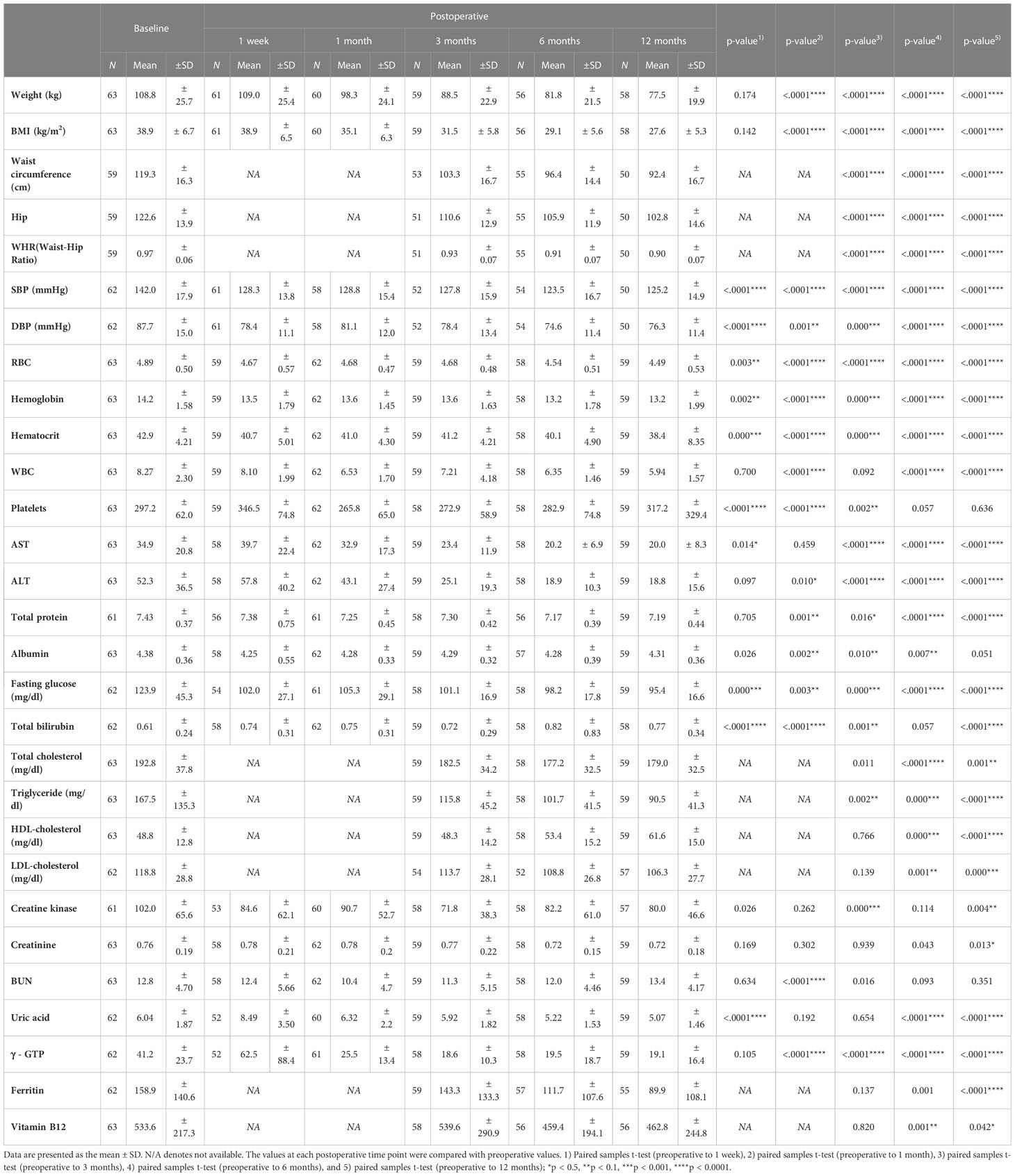
Bariatric surgery induced a mean weight loss of 10.5 ± 4.5 kg over 1 month and 31.3 ± 4.2 kg over 12 months. Likewise, a significant reduction in BMI was observed starting 1 month after surgery (Table 2).
Twelve months after surgery, liver function was also improved in terms of aspartate aminotransferase (AST), alanine transaminase (ALT), and gamma-glutamyl transferase (γGTP) levels. Lipoprotein profiles were also improved after BS, showing lower triglyceride (p<0.001), total cholesterol (p<0.001), and low-density lipoprotein (LDL) cholesterol (p=0.000) and increased high-density lipoprotein (HDL) cholesterol levels (p<0.001) at the 12-month follow-up compared to baseline. These significant weight losses also led to a continued decrease in fasting glucose levels.
Hormones secreted by adipose tissue (adiponectin, leptin, and resistin) showed significant changes postoperatively (Figure 1). Adiponectin levels were increased significantly at the 12-month follow-up compared to baseline (40.51 ± 15.61 ng/ml mean increment, p=0.041). Leptin levels were significantly decreased from 1 month after surgery. A decreasing pattern of resistin was seen, and the difference compared to baseline finally became significant at 12 months (23.55 ± 4.66 ng/ml mean reduction, p<0.0001). Pancreatic hormone levels showed continuous reductions over 12 months. Compared to baseline levels, surgery was associated with significantly decreased amylin levels as early as 1 month post-surgery (7.44 ± 2.79 ng/ml mean reduction, p=0.031) which was maintained up to 12 months (10.72 ± 2.69 ng/ml, p=0.001). Insulin and glucagon levels were also greatly reduced at 6 months postoperatively. As previously reported (23), BS markedly lowered the levels of gut-derived incretin hormones GLP-1 at 6 and 12 months and GIP at 12 months.
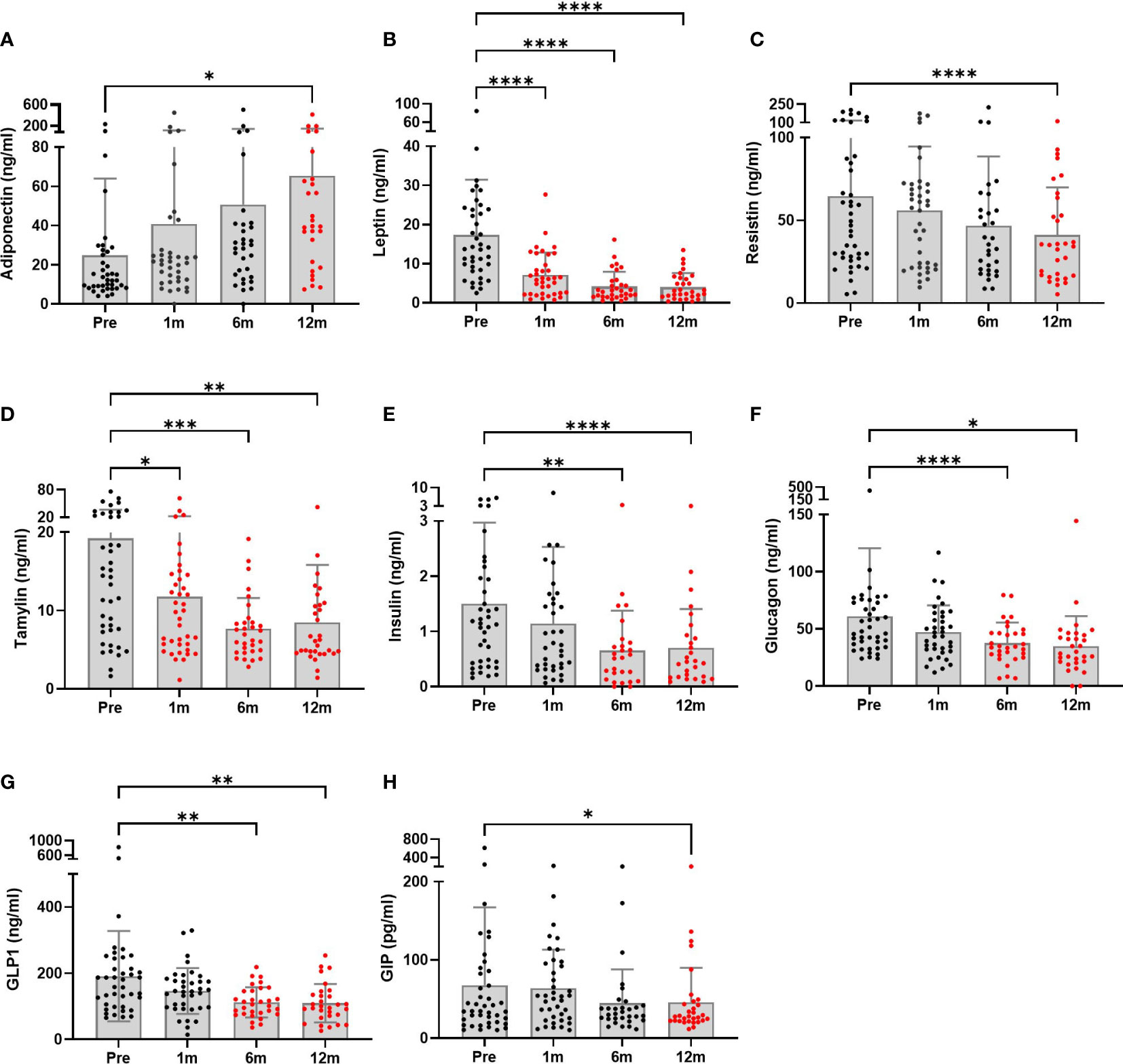
Figure 1 Metabolic hormone profiles of patients undergoing bariatric surgery (n = 28 to 40) were monitored at baseline and postoperative time points. (A–C) Adipose tissue hormones, (D–F) pancreatic hormones, and (G, H) gastrointestinal hormones. The graphs are presented showing means and SDs. Significant differences between baseline and each postoperative time point were determined; *p < 0.5, **p < 0.1, ***p < 0.001, ****p < 0.0001. Pre, pre-surgery; 1m, 1month post-surgery; 3m, 3 months post-surgery; 12m, 12 months post-surgery.
Figure 2 shows the changes in serum inflammatory cytokines at follow-up. IL-1β, IL-17A, and MCP-1 levels were significantly decreased at the 12-month follow-up compared to baseline (p=0.037, p=0.011 and p=0.050, respectively). An inconsistency was noted in cytokine levels. IL-4 levels were significantly decreased at the first 1-month (p=0.0001) and last 12-month follow-up (p=0.003). However, improvements in TNF-β and PDGF levels that occurred during the first month after surgery were not sustained. Lastly, IL-1Rα and G-CSF levels were much lower, starting 3 months postoperatively.
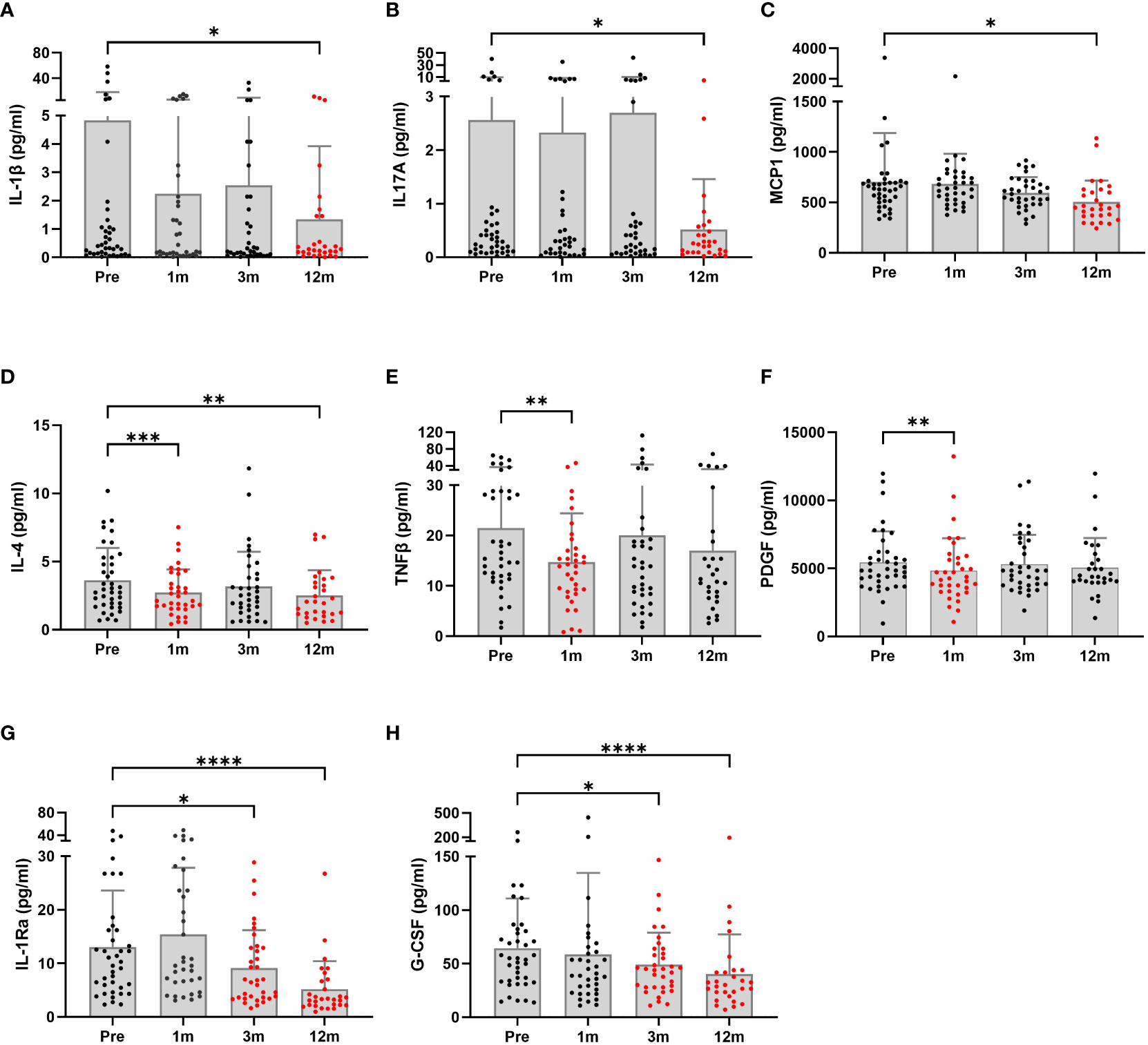
Figure 2 Serum inflammatory profiles of patients undergoing bariatric surgery (n = 29 to 40) were monitored at baseline and postoperative time points. The graphs are presented as the means and SDs. Significant differences between baseline and postoperative time points were determined; *p < 0.5, **p < 0.1, ***p < 0.001, ****p < 0.0001. Pre, pre-surgery; 1m, 1month post-surgery; 3m, 3 months post-surgery; 12m, 12 months post-surgery.
3.3 Diabetic patients show differences in type 2 diabetes remission 12 months after bariatric surgery
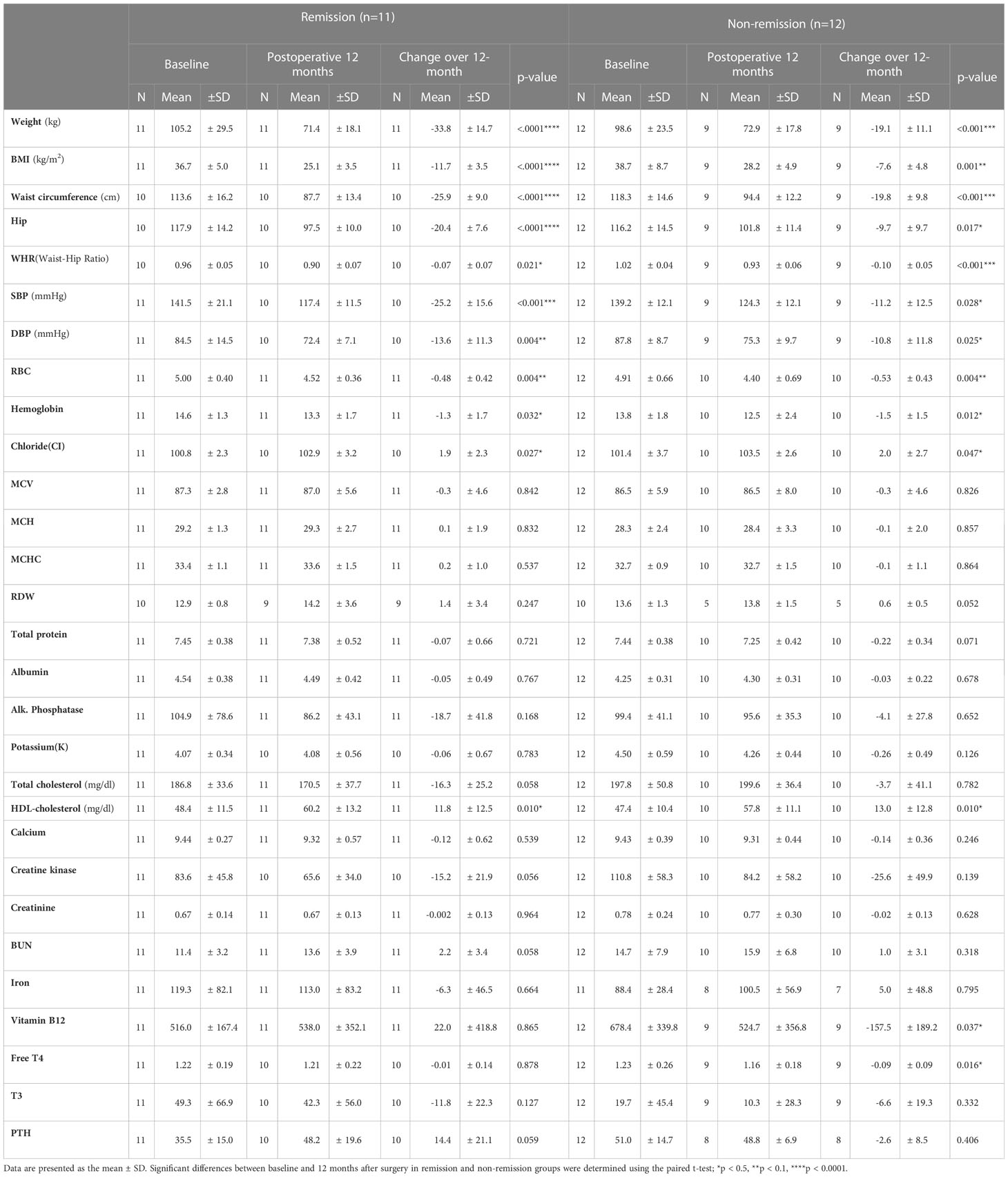
Of the 63 patients who underwent bariatric surgery, 23 patients were clinically diagnosed with diabetes at baseline. The demographics and clinical characteristics of the remission and the non-remission groups at baseline are shown in Table 3. There were significant differences in age and waist-hip ratio (WHR) between the two groups, based on only the t-test (p = 0.014 and 0.011, respectively).
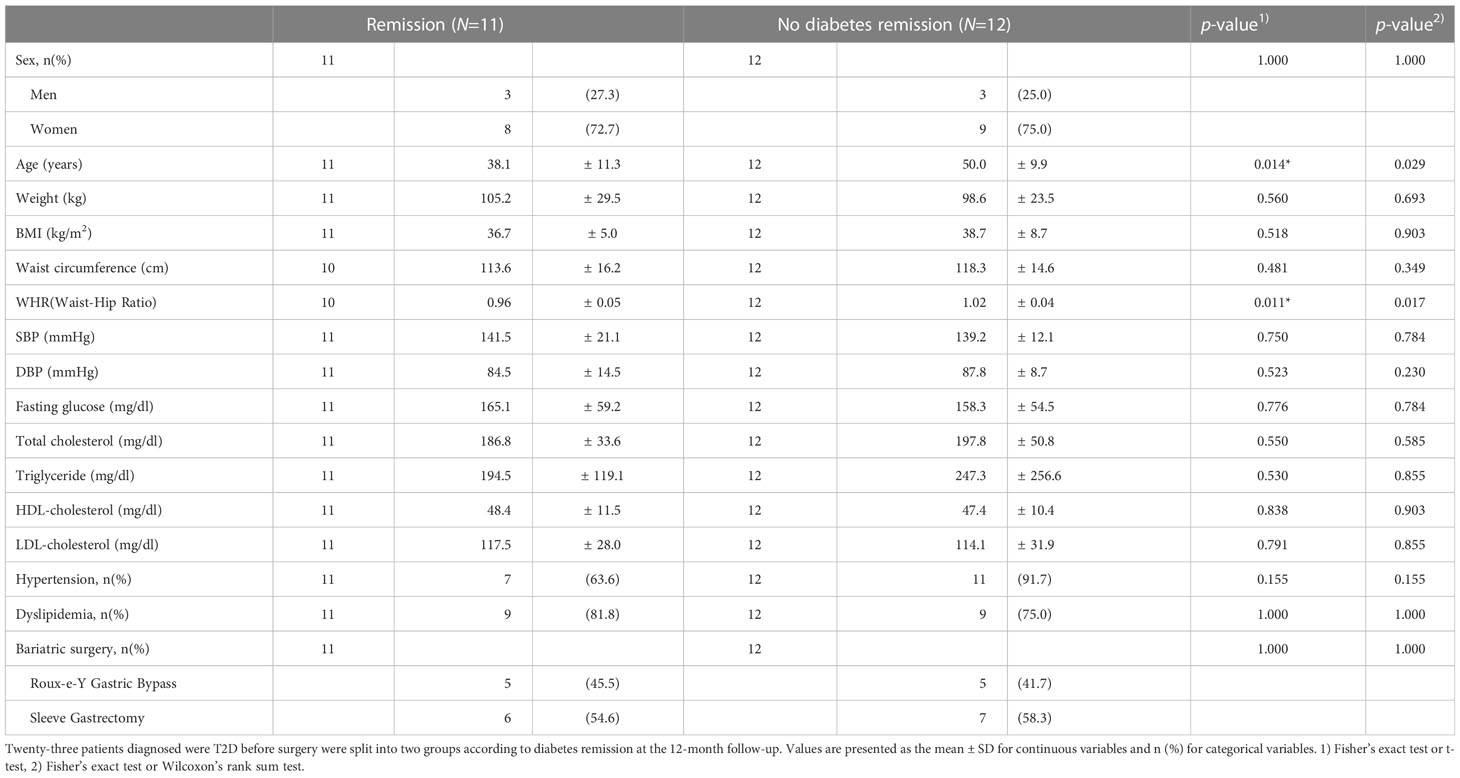
Table 3 Baseline characteristics of patients with and without type 2 diabetes remission 12-months after bariatric surgery.
Demographics and clinical characteristics were compared between baseline and the 12-month follow-up point in each remission and non-remission group to investigate the effect of BS on T2D improvement (Tables 4, 5). In both remission and non-remission groups, the parameters with significant and non-significant changes are shown in Table 4. Notably, vitamin B12 showed a significant reduction 12 months postoperatively only in the non-remission group. Twelve-month postoperative white blood cell and platelet counts, and fasting glucose, AST, ALT, TG, LDL, uric acid, γGTP, and thyroid-stimulating hormone (TSH) levels were also significantly reduced from the preoperative values only in the remission group (Table 5). Total bilirubin levels were slightly increased in both groups, but statistical significance before and after surgery was found only in the remission group.
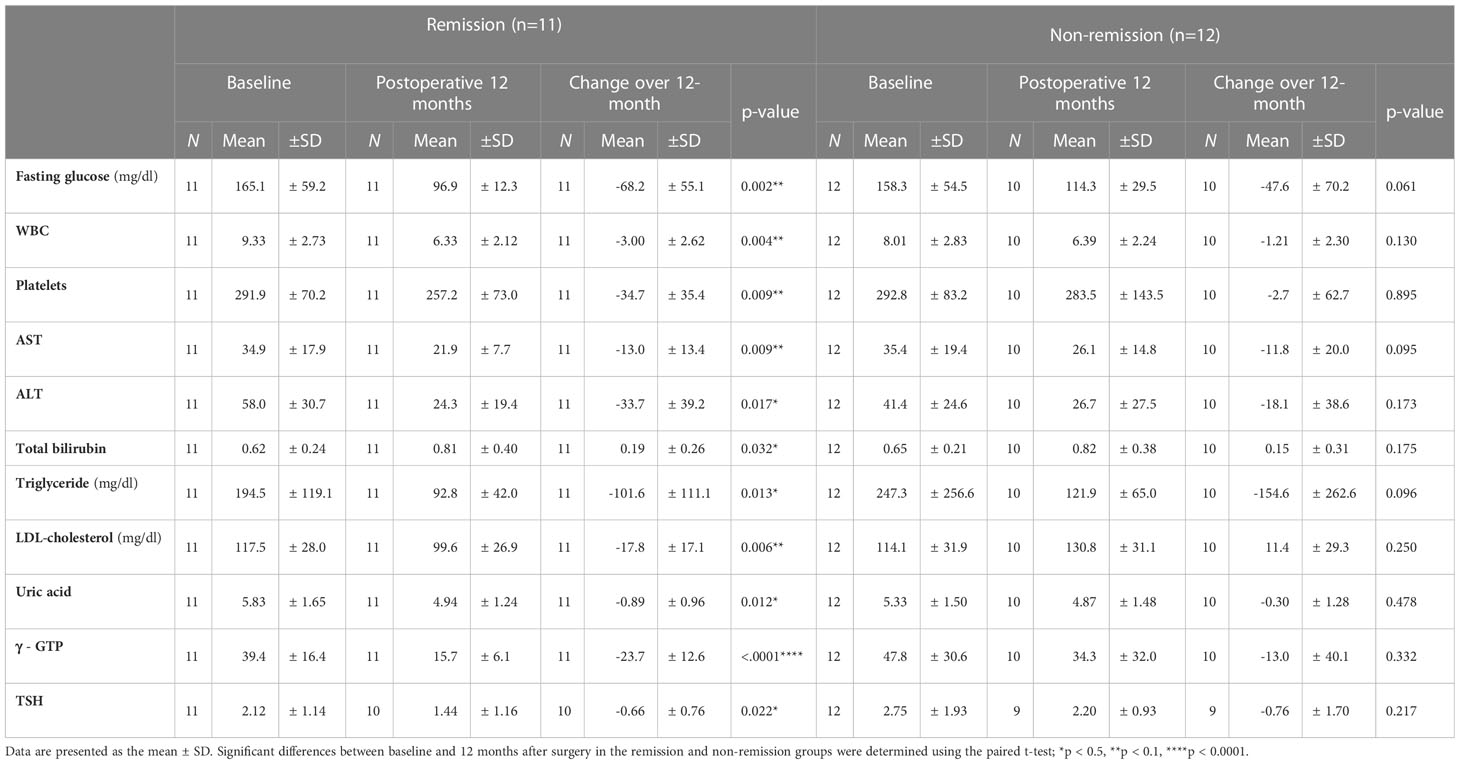
Table 5 Impact of bariatric surgery on diabetes remission at the 12th month after bariatric surgery.
Changes in inflammatory cytokine levels were analyzed in both groups at follow-ups after surgery (Figure 3). Statistical significances for postoperative time points were only seen in the remission group. At 12 months, for example, G-CSF, MCP-1, and TGFα levels were markedly decreased compared to baseline. Significant reductions in IL-6 and TNFβ levels occurred the first month after surgery and were not sustained. Hormone changes were also assessed over time after surgery. The remission group showed significant changes in insulin, amylin, and GLP-1 levels (Figure 4). Insulin and amylin showed continuously decreasing patterns after surgery, and statistical significance was found at 12 months. Although GLP-1 continued to decrease over time in both groups, a significant reduction was only found in the remission group at 6 months after surgery.
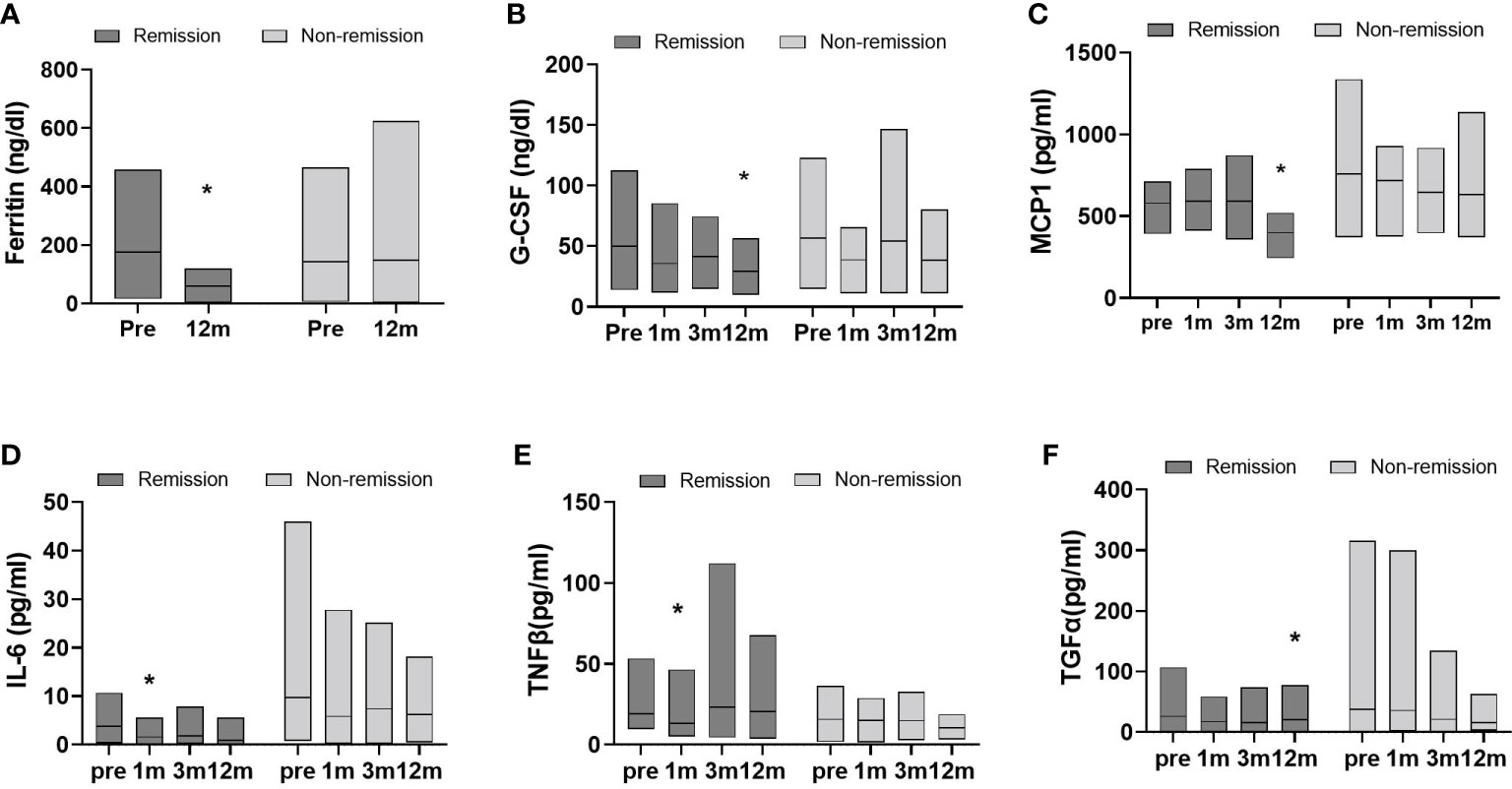
Figure 3 Data represent as a floating bar (min to max value) with a line indicating the mean of cytokine levels. Significant differences between baseline and postoperative time points within group were determined (remission group, n=11; non-remission group, n=12); *p < 0.5, Pre, pre-surgery; 1m, 1month post-surgery; 3m, 3 months post-surgery; 12m, 12 months post-surgery.

Figure 4 Data represent as a floating bar (min to max value) with a line indicating the mean of hormone levels. Significant differences between baseline and postoperative time points within group were determined (remission group, n=11; non-remission group, n=12); *p < 0.5. Pre, pre-surgery; 1m, 1month post-surgery; 6m, 6 months post-surgery; 12m, 12 months post-surgery.
4 Discussion
In this study we found that, among Korean patients with obesity, adiponectin increased and leptin, resistin, total amylin, insulin, glucagon, GLP1, and GIP decreased over 12 months following BS. These hormone changes accompany with ameliorating inflammatory cytokine levels in serum, such as IL-1β, IL-17A, and MCP1. We further divided the patients into postoperative diabetes remission and non-remission subgroups and found that remission group significantly reduced the serum levels of insulin, amylin and GLP1 hormone and several inflammatory cytokines including MCP1 and IL-6.
First, we observed a significant increase in adiponectin levels after BS in patients with obesity. It has been reported that, in patients with morbid obesity, abnormal levels and functioning of adipokines were detected. The levels of other adipokines, leptin and resistin, decreased. In obesity, increased visceral fat results in lower adiponectin and increased leptin levels (24). A large reduction in body fat through BS improves metabolic abnormalities in patients with obesity (25). High adiponectin levels were accompanied by a significant reduction in leptin levels (26). Leptin resistance is a well-known characteristic of metabolic disorders (27). Moreover, enhancing adiponectin levels improved metabolic syndrome independent of body weight (28). In our study, leptin levels dropped at 1 month postoperatively and were sustained until 12 months postoperatively, whereas adiponectin levels gradually increased and reached significance at 12 months. These changes were observed with lower insulin and glucagon levels, indicating improvement in glucose metabolism.
In a cross-sectional study using The Danish Childhood Obesity Data and Biobank data, plasma GLP1 concentrations were associated with the degree of obesity and metabolic disorders (6). GLP1 is expressed in L-cells in the distal jejunum and ileum, and gastric inhibitory polypeptide (GIP) is produced from enteroendocrine cells in the proximal gut. These hormones control glucose homeostasis and gut motility (29, 30). GLP-1 receptor signaling enhances β-cell glucose metabolism through mammalian target of rapamycin (31).
In our study, postoperative incretin hormone levels (amylin, GIP, and GLP1) were reduced with lower glucose, glucagon, and insulin levels. Postprandial GLP1 levels after RYGB and SG cannot be achieved with weight loss due to caloric restriction (32). These data suggest that BS benefits on improvement of metabolic profiles are not only due to reduction of body weight, but also changes in food intake and metabolic hormone levels.
In terms of inflammation, the production of IL-4 by lymphocytes increased serum levels in obese mice (33). Furthermore, TNFβ controls responses to diet-induced obesity (34), and it is responsible for early inflammatory reactions, cytotoxicity, and apoptosis (35–37). Severe obesity expands adipose tissue, through which PDGF-B-dependent vascular remodeling occurs (36). IL-4, TNFβ, and PDGF levels dropped in the early stages after BS, and other proinflammatory cytokines associated with obesity, such as Il-1β, IL-17A, MCP1, IL-1Ra, and G-CSF, gradually decreased over 1 year postoperatively. In addition to that, liver damage markers, ALT, AST, and γGTP were significantly ameliorated a month postoperatively. This suggests that the inflammatory status is ameliorated through sequential changes in inflammatory factors originated from different organ sites.
Next, we divided the patients into diabetes remission and non-remission groups. The effect of BS on body weight, BMI, and hypertension significantly improved in both groups, and total cholesterol and HDL did not significantly change in both groups. It seems the difference remission or non-remission comes from how improved inflammatory factors since there was a significant difference between inflammatory factor levels. Elevated serum ferritin levels are an important indicator and predictor of the incidence of T2D and insulin resistance (38–40) and are associated with clinical inflammation (41, 42). In our study, the remission group showed significant decreases in factors associated with monocyte migration, inflammatory proteins, and insulin resistance-related cytokines, as illustrated by the changes in G-CSF, MCP1, and TNFβ (43–46). These results, accompanied by reduced insulin or incretin hormone levels, were only significant in the remission group. The data showing the lack of significant changes in the non-remission T2D group suggests that hormones, immunological factors, and inflammatory vigor play a dominant role in metabolic dysfunction in the obese subpopulation rather than body weight itself. Notably, the non-remission group had high levels of IL-6 and TGFα and had more variations in values within the group compared to the remission group, indicating the degree of reduction in these two cytokines after BS may be crucial in the remission of diabetes.
This study had several strengths and limitations. First, because few studies to date have examined the effect of BS in Korean patients, this study significantly contributes to the field. Second, this novel cohort reproduced the results of previous studies that were conducted in other groups with different ethnic and geographical backgrounds. Third, the BS group was classified into subpopulations with type 2 diabetes. However, owing to the small number of patients, they could not be divided into subgroups according to the type of surgery (Roux-en-Y and gastric sleeve) for a more detailed analysis. The effect of BS differs according to age and duration of diagnosis. Results in this study need to be confirmed in a study with a larger population.
The effects of BS in Korean patients are not well-known. Our study analyzed changes in blood parameters postoperatively. The changes in hormones secreted from the three major metabolic tissues (pancreas, adipose, and gut) and differences in multi-origin inflammatory cytokines between remission and non-remission groups provide a path for understanding how the effect of BS in improving glucose metabolism is mediated. The accumulated data will provide valuable information for the development of noninvasive anti-obesity and metabolic syndrome marker that are more appropriate for Korean patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Korea Disease Control and Prevention Agency (KDCA) Inha University Hospital Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
Author contributions
EK, JY, and SK designed the research. JY, H-JK, YH, DP, and YP contributed to acquisition of data. EK, JY, H-JK, and HP analyzed data and EK wrote the manuscript. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This study was supported by an intramural research grant from the Korea National Institute of Health (2020-NG-013-00~02).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Park YS, Park DJ, Lee JH, Lee HJ, Ha TK, Kim YJ, et al. Korean OBEsity Surgical Treatment Study (KOBESS): protocol of a prospective multicentre cohort study on obese patients undergoing laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass. BMJ Open (2017) 7(10):e018044. doi: 10.1136/bmjopen-2017-018044
2. Holst JJ, Madsbad S, Bojsen-Møller KN, Svane MS, Jørgensen NB, Dirksen C, et al. Mechanisms in bariatric surgery: Gut hormones, diabetes resolution, and weight loss. Surg Obes Relat Dis (2018) 14(5):708–14. doi: 10.1016/j.soard.2018.03.003
3. Nielsen HJ, Nedrebø BG, Fosså A, Andersen JR, Assmus J, Dagsland VH, et al. Seven-year trajectories of body weight, quality of life and comorbidities following Roux-en-Y gastric bypass and sleeve gastrectomy. Int J Obes (Lond) (2022) 46(4):739–49. doi: 10.1038/s41366-021-01028-5
4. Ashrafian H, Athanasiou T, Li JV, Bueter M, Ahmed K, Nagpal K, et al. Diabetes resolution and hyperinsulinaemia after metabolic Roux-en-Y gastric bypass. Obes Rev (2011) 12(5):e257–72. doi: 10.1111/j.1467-789X.2010.00802.x
5. Thomas MC, Coughlan MT, Cooper ME. The postprandial actions of GLP-1 receptor agonists: The missing link for cardiovascular and kidney protection in type 2 diabetes. Cell Metab (2023) 35(2):253–73. doi: 10.1016/j.cmet.2023.01.004
6. Stinson SE, Jonsson AE, Lund MAV, Frithioff-Bøjsøe C, Aas Holm L, Pedersen O, et al. Fasting plasma GLP-1 is associated with overweight/obesity and cardiometabolic risk factors in children and adolescents. J Clin Endocrinol Metab (2021) 106(6):1718–27. doi: 10.1210/clinem/dgab098
7. Jirapinyo P, Jin DX, Qazi T, Mishra N, Thompson CC. A meta-analysis of GLP-1 after Roux-En-Y gastric bypass: impact of surgical technique and measurement strategy. Obes Surg (2018) 28(3):615–26. doi: 10.1007/s11695-017-2913-1
8. Livshits G, Kalinkovich A. A cross-talk between sestrins, chronic inflammation and cellular senescence governs the development of age-associated sarcopenia and obesity. Ageing Res Rev (2023) 86:101852. doi: 10.1016/j.arr.2023.101852
9. Arabi T, Shafqat A, Sabbah BN, Fawzy NA, Shah H, Abdulkader H, et al. Obesity-related kidney disease: Beyond hypertension and insulin-resistance. Front Endocrinol (Lausanne) (2022) 13:1095211. doi: 10.3389/fendo.2022.1095211
10. Yuen MMA. Health complications of obesity: 224 obesity-associated comorbidities from a mechanistic perspective. Gastroenterol Clin North Am (2023) 52(2):363–80. doi: 10.1016/j.gtc.2023.03.006
11. Del Rosso S, Baraquet ML, Barale A, Defago MD, Tortosa F, Perovic NR, et al. Long-term effects of different exercise training modes on cytokines and adipokines in individuals with overweight/obesity and cardiometabolic diseases: A systematic review, meta-analysis, and meta-regression of randomized controlled trials. Obes Rev (2023) 24(6):e13564. doi: 10.1111/obr.13564
12. Daryabor G, Amirghofran Z, Gholijani N, Bemani P. Obesity and adipose tissue-derived cytokines in the pathogenesis of multiple sclerosis. Endocr Metab Immune Disord Drug Targets (2022) 22(12):1217–31. doi: 10.2174/1871530322666220215110041
13. Singh S, Anshita D, Ravichandiran V. MCP-1: Function, regulation, and involvement in disease. Int Immunopharmacol (2021) 101(Pt B):107598. doi: 10.1016/j.intimp.2021.107598
14. Affinati AH, Esfandiari NH, Oral EA, Kraftson AT. Bariatric surgery in the treatment of type 2 diabetes. Curr Diabetes Rep (2019) 19(12):156. doi: 10.1007/s11892-019-1269-4
15. Stenberg E, Olbers T, Cao Y, Sundbom M, Jans A, Ottosson J, et al. Factors determining chance of type 2 diabetes remission after Roux-en-Y gastric bypass surgery: a nationwide cohort study in 8057 Swedish patients. BMJ Open Diabetes Res Care (2021) 9(1):e002033. doi: 10.1136/bmjdrc-2020-002033
16. Park JY. Prediction of type 2 diabetes remission after bariatric or metabolic surgery. J Obes Metab Syndr (2018) 27(4):213–22. doi: 10.7570/jomes.2018.27.4.213
17. Lakdawala M, Bhasker A, Asian Consensus Meeting on Metabolic S. Report: Asian consensus meeting on metabolic surgery. Recommendations for the use of bariatric and gastrointestinal metabolic surgery for treatment of obesity and type II diabetes mellitus in the Asian population: august 9th and 10th, 2008, Trivandrum, India. Obes Surg (2010) 20(7):929–36. doi: 10.1007/s11695-010-0162-7
18. Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg (1995) 222(3):339–50. doi: 10.1097/00000658-199509000-00011
19. Wickremesekera K, Miller G, Naotunne TD, Knowles G, Stubbs RS. Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obes Surg (2005) 15(4):474–81. doi: 10.1381/0960892053723402
20. Yoon KH, Lee JH, Kim JW, Cho JH, Choi YH, Ko SH, et al. Epidemic obesity and type 2 diabetes in Asia. Lancet. (2006) 368(9548):1681–8. doi: 10.1016/S0140-6736(06)69703-1
21. Kim DJ, Lee MS, Kim KW, Lee MK. Insulin secretory dysfunction and insulin resistance in the pathogenesis of korean type 2 diabetes mellitus. Metabolism. (2001) 50(5):590–3. doi: 10.1053/meta.2001.22558
22. Buse JB, Caprio S, Cefalu WT, Ceriello A, Del Prato S, Inzucchi SE, et al. How do we define cure of diabetes? Diabetes Care (2009) 32(11):2133–5. doi: 10.2337/dc09-9036
23. Ntika S, Krizhanovskii C, Tracy LM, Bringman S, Lundquist PB. Improved beta-cell function and altered plasma levels of GLP-1 during OMTT and fasting following bariatric surgery in women with NGT. Interventions Obes Diabetes (2021) 5(1):419–24. doi: 10.31031/IOD.2021.05.000604
24. Gariballa S, Alkaabi J, Yasin J, Al Essa A. Total adiponectin in overweight and obese subjects and its response to visceral fat loss. BMC Endocr Disord (2019) 19(1):55. doi: 10.1186/s12902-019-0386-z
25. Malin SK, Bena J, Abood B, Pothier CE, Bhatt DL, Nissen S, et al. Attenuated improvements in adiponectin and fat loss characterize type 2 diabetes non-remission status after bariatric surgery. Diabetes Obes Metab (2014) 16(12):1230–8. doi: 10.1111/dom.12376
26. Askarpour M, Alizadeh S, Hadi A, Symonds ME, Miraghajani M, Sheikhi A, et al. Effect of bariatric surgery on the circulating level of adiponectin, chemerin, plasminogen activator inhibitor-1, leptin, resistin, and visfatin: A systematic review and meta-analysis. Horm Metab Res (2020) 52(4):207–15. doi: 10.1055/a-1129-6785
27. Gruzdeva O, Borodkina D, Uchasova E, Dyleva Y, Barbarash O. Leptin resistance: underlying mechanisms and diagnosis. Diabetes Metab Syndr Obes (2019) 12:191–8. doi: 10.2147/DMSO.S182406
28. Sparrenberger K, Sbaraini M, Cureau FV, Teló GH, Bahia L, Schaan BD. Higher adiponectin concentrations are associated with reduced metabolic syndrome risk independently of weight status in Brazilian adolescents. Diabetol Metab Syndr (2019) 11:40. doi: 10.1186/s13098-019-0435-9
29. Jacobsen SH, Olesen SC, Dirksen C, Jørgensen NB, Bojsen-Møller KN, Kielgast U, et al. Changes in gastrointestinal hormone responses, insulin sensitivity, and beta-cell function within 2 weeks after gastric bypass in non-diabetic subjects. Obes Surg (2012) 22(7):1084–96. doi: 10.1007/s11695-012-0621-4
30. Hutch CR, Sandoval D. The role of GLP-1 in the metabolic success of bariatric surgery. Endocrinology. (2017) 158(12):4139–51. doi: 10.1210/en.2017-00564
31. Carlessi R, Chen Y, Rowlands J, Cruzat VF, Keane KN, Egan L, et al. GLP-1 receptor signalling promotes β-cell glucose metabolism via mTOR-dependent HIF-1α activation. Sci Rep (2017) 7(1):2661. doi: 10.1038/s41598-017-02838-2
32. Laferrère B, Teixeira J, McGinty J, Tran H, Egger JR, Colarusso A, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab (2008) 93(7):2479–85. doi: 10.1210/jc.2007-2851
33. Shiau MY, Chuang PH, Yang CP, Hsiao CW, Chang SW, Chang KY, et al. Mechanism of interleukin-4 reducing lipid deposit by regulating hormone-sensitive lipase. Sci Rep (2019) 9(1):11974. doi: 10.1038/s41598-019-47908-9
34. Upadhyay V, Poroyko V, Kim TJ, Devkota S, Fu S, Liu D, et al. Lymphotoxin regulates commensal responses to enable diet-induced obesity. Nat Immunol (2012) 13(10):947–53. doi: 10.1038/ni.2403
35. Etemadi N, Holien JK, Chau D, Dewson G, Murphy JM, Alexander WS, et al. Lymphotoxin alpha induces apoptosis, necroptosis and inflammatory signals with the same potency as tumour necrosis factor. FEBS J (2013) 280(21):5283–97. doi: 10.1111/febs.12419
36. Onogi Y, Wada T, Okekawa A, Matsuzawa T, Watanabe E, Ikeda K, et al. Pro-inflammatory macrophages coupled with glycolysis remodel adipose vasculature by producing platelet-derived growth factor-B in obesity. Sci Rep (2020) 10(1):670. doi: 10.1038/s41598-019-57368-w
37. Delves PJ, Roitt IM. Encyclopedia of immunology. San Diego: Academic Press (1998). Available at: https://archive.org/details/encyclopediaofim00delv.
38. Ikeda Y, Watanabe H, Shiuchi T, Hamano H, Horinouchi Y, Imanishi M, et al. Deletion of H-ferritin in macrophages alleviates obesity and diabetes induced by high-fat diet in mice. Diabetologia. (2020) 63(8):1588–602. doi: 10.1007/s00125-020-05153-0
39. Yu L, Yan J, Zhang Q, Lin H, Zhu L, Liu Q, et al. Association between Serum Ferritin and Blood Lipids: Influence of Diabetes and hs-CRP Levels. J Diabetes Res (2020) 2020:4138696. doi: 10.1155/2020/4138696
40. Shim YS, Kang MJ, Oh YJ, Baek JW, Yang S, Hwang IT. Association of serum ferritin with insulin resistance, abdominal obesity, and metabolic syndrome in Korean adolescent and adults: The Korean National Health and Nutrition Examination Survey, 2008 to 2011. Med (Baltimore) (2017) 96(8):e6179. doi: 10.1097/MD.0000000000006179
41. Sciacqua A, Ventura E, Tripepi G, Cassano V, D'Arrigo G, Roumeliotis S, et al. Ferritin modifies the relationship between inflammation and arterial stiffness in hypertensive patients with different glucose tolerance. Cardiovasc Diabetol (2020) 19(1):123. doi: 10.1186/s12933-020-01102-8
42. Mahroum N, Alghory A, Kiyak Z, Alwani A, Seida R, Alrais M, et al. Ferritin - from iron, through inflammation and autoimmunity, to COVID-19. J Autoimmun (2022) 126:102778. doi: 10.1016/j.jaut.2021.102778
43. Ordelheide AM, Gommer N, Bohm A, Hermann C, Thielker I, Machicao F, et al. Granulocyte colony-stimulating factor (G-CSF): A saturated fatty acid-induced myokine with insulin-desensitizing properties in humans. Mol Metab (2016) 5(4):305–16. doi: 10.1016/j.molmet.2016.02.001
44. Zhang Y, Zhou X, Liu P, Chen X, Zhang J, Zhang H, et al. GCSF deficiency attenuates nonalcoholic fatty liver disease through regulating GCSFR-SOCS3-JAK-STAT3 pathway and immune cells infiltration. Am J Physiol Gastrointest Liver Physiol (2021) 320(4):G531–G42. doi: 10.1152/ajpgi.00342.2020
45. Buhrmann C, Shayan P, Aggarwal BB, Shakibaei M. Evidence that TNF-beta (lymphotoxin alpha) can activate the inflammatory environment in human chondrocytes. Arthritis Res Ther (2013) 15(6):R202. doi: 10.1186/ar4393
Keywords: obesity, bariatric surgery, diabetes, hormone, inflammation
Citation: Kim ER, Yun JH, Kim H-J, Park HY, Heo Y, Park YS, Park DJ and Koo SK (2023) Evaluation of hormonal and circulating inflammatory biomarker profiles in the year following bariatric surgery. Front. Endocrinol. 14:1171675. doi: 10.3389/fendo.2023.1171675
Received: 22 February 2023; Accepted: 06 July 2023;
Published: 25 July 2023.
Edited by:
Reina Villareal, Baylor College of Medicine, United StatesReviewed by:
FNU Deepika, Baylor College of Medicine, United StatesEric Morris Bomberg, University of Minnesota, United States
Copyright © 2023 Kim, Yun, Kim, Park, Heo, Park, Park and Koo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Soo Kyung Koo, c2trb29Aa29yZWEua3I=
†These authors have contributed equally to this work
 Eun Ran Kim1†
Eun Ran Kim1† Soo Kyung Koo
Soo Kyung Koo