- 1School of Medicine, Chung Shan Medical University, Taichung, Taiwan
- 2Department of Medical Education, Linkou Chang-Gung Memorial Hospital, Taoyuan City, Taiwan
- 3Department of Pharmacology, Chung Shan Medical University, Taichung, Taiwan
- 4Department of Pharmacy, Chung Shan Medical University Hospital, Taichung, Taiwan
- 5Department of Obstetrics and Gynecology, E-Da Hospital, I-Shou University, Kaohsiung, Taiwan
- 6Department of Health Services Administration, China Medical University, Taichung, Taiwan
Background: Osteoporosis and fractures increase morbidity and mortality rates after solid organ transplantation (SOT), but few studies have analyzed the risk of osteoporosis and related fractures after SOT. In this retrospective cohort study, we investigated the risk of osteoporosis and fractures in different SOT recipients.
Methods: This study was a retrospective cohort study using a nationally representative database in Taiwan. We collected the data of SOT recipients and used the propensity score matching method to obtain a comparison cohort. To reduce bias, we excluded patients who had been diagnosed with osteoporosis or fracture before inclusion. All participants were followed up until the date of diagnosis as having a pathological fracture, death, or the end of 2018, whichever occurred first. The Cox proportional hazards model was used to investigate the risk of osteoporosis and pathological fracture in SOT recipients.
Results: After adjustment for the aforementioned variables, SOT recipients were observed to have a higher risk of osteoporosis (hazard ratio (HR) = 1.46, 95% confidence interval (CI): 1.29–1.65) and fracture (HR: 1.19, 95% CI: 1.01–1.39) than the general individuals. Among the different SOT recipients, the highest risk of fractures was noted in heart or lung transplant recipients, with a HR of 4.62 (95% CI: 2.05–10.44). Among the age groups, patients aged >61 years had the highest HRs for osteoporosis (HR: 11.51; 95% CI, 9.10–14.56) and fracture (HR: 11.75, 95% CI: 8.97–15.40).
Conclusion: SOT recipients had a higher risk of osteoporosis and related fractures than the general population, with the highest risks observed in patients receiving heart or lung transplants, older patients, and patients with CCI scores of >3.
Introduction
Solid organ transplantation (SOT) has been an established treatment for end-stage organ failure (1), including that of the kidney, liver, heart, and lung, over the past few decades (2). Compared with the healthy population, SOT recipients have a high risk of osteoporosis and fracture (3, 4).
Pretransplant bone mineral density (BMD) is considered an important determinant of subsequent osteoporosis development (5). Fractures are very common in patients referred for lung transplantation. Patients referred for lung transplantation have a high pretransplant prevalence of low BMD and osteoporotic fracture, further increasing their posttransplant fracture risk (6). BMD decline primarily occurs at the lumbar spine and femoral neck during the first year after transplantation (7). Typically, BMD decreases rapidly within the first 6 to 12 months after transplantation, accompanied by a marked increase in bone resorption (2). Posttransplantation bone disease is therefore a common and serious complication (8), with osteoporosis occurring in up to half of transplant recipients and vertebral fractures occurring in almost a third (9). Fractures commonly occur after SOT (7). Osteoporosis and fragility fractures significantly affect the quality of life and survival of SOT recipients (10–12).
Osteoporosis and fractures have been associated with considerable disability and are an important cause of morbidity and mortality after SOT. However, the risk of osteoporosis and fracture following SOT has remained unclear. In particular, large-scale epidemiological studies have examined osteoporosis and fracture risk among SOT recipients, especially based on a nationwide database (13). In this retrospective cohort study, we investigated the risk of osteoporosis and related fractures in different recipients of SOT from the National Health Insurance Research Database (NHIRD) in Taiwan from 2001 to 2018.
Materials and methods
Data sources
We analyzed the data from the NHIRD, maintained by the Health and Welfare Data Science Center (HWDC), Ministry of Health and Welfare (MOHW), Taiwan. The NHIRD includes details of beneficiaries enrolled in Taiwan’s National Health Insurance (NHI) program from 2001 to 2018, including NHI enrollment files and medical service data (diagnoses, prescription drugs, and examinations). The NHI program is a compulsory single-payer healthcare system providing comprehensive healthcare for >99% of the residents of Taiwan. The diagnostic data were coded using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) before 2016 and ICD-10-CM after 2016. The NHIRD can serve as a foundation for the procurement of real-world evidence to support clinical decisions and healthcare policy-making (14–17).
Ethics approval
This study was conducted in accordance with the Declaration of Helsinki. This study protocol was approved by the Central Regional Research Ethics Committee of China Medical University, Taiwan (No. CSMUH CS2-21134). The data used in the present study are anonymous to protect the privacy of beneficiaries. Informed consent was not required given that the database in this study contains only de-identified data.
Study participants
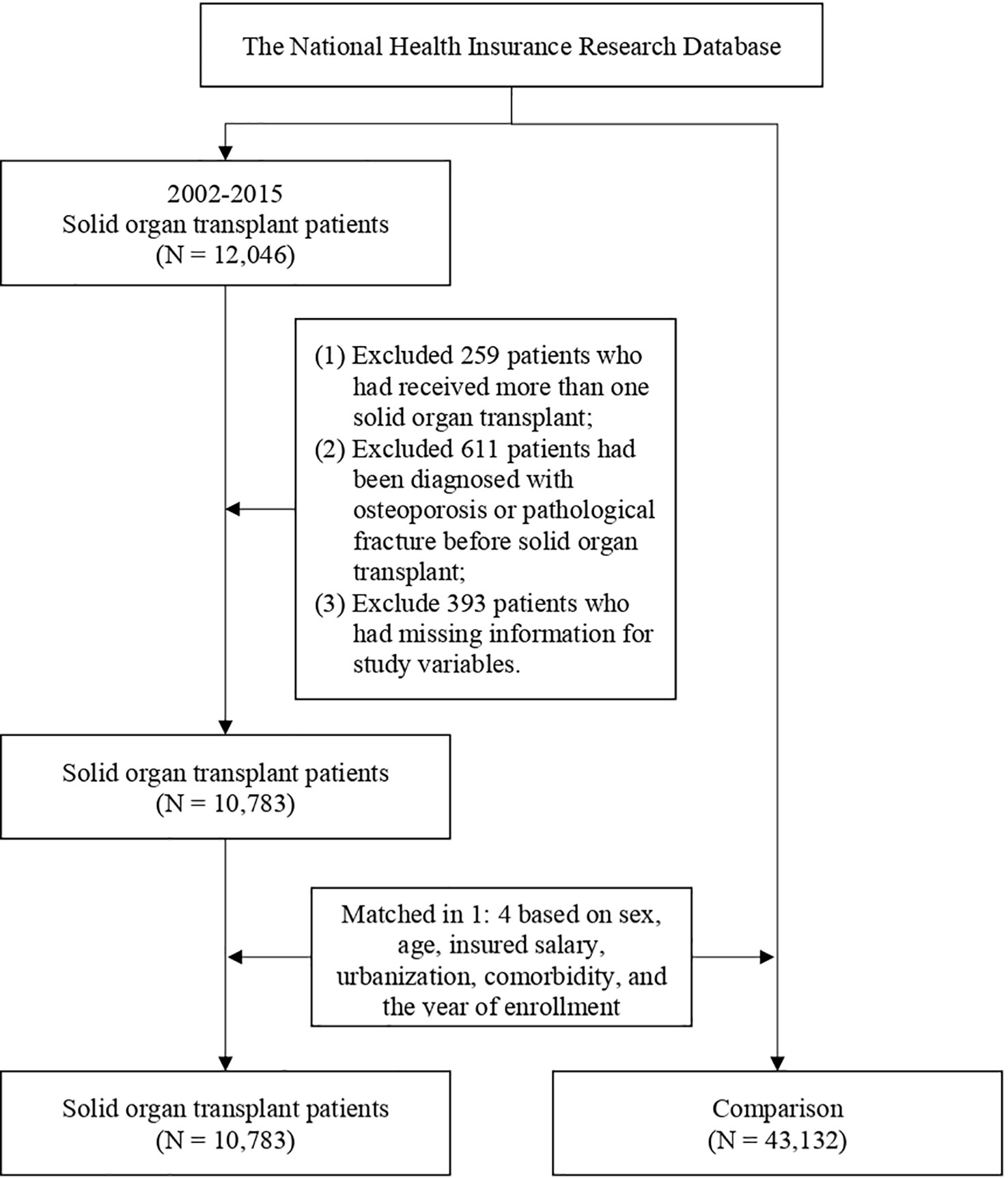
We included the data of SOT recipients between 2002 and 2015, including those patients with renal (ICD-9-CM V42.0), liver (ICD-9-CM V42.7), heart (ICD-9-CM V37.51), or lung (ICD-9-CM: V42.6) transplantation. We excluded patients who had received more than one SOT, had been diagnosed as having osteoporosis or pathological fracture before SOT, and had missing information for study variables. Osteoporosis was identified using the following codes: ICD-9-CM 733.0 and ICD-10-CM M810, M816, and M818. The pathological fracture was identified using the following codes: ICD-9-CM 733.1 and ICD-10-CM M485, M800, M808, and M843–M846. The comparison cohort was made up of general patients who had no diagnosis of osteoporosis or pathological fracture before inclusion. To reduce selection bias, we used 1:4 propensity score (PS) matching for each patient with SOT to obtain a comparison cohort matched for sex, age, insured salary, urbanization, Charlson comorbidity index (CCI), and year of inclusion in the study. After matching, 10,783 SOT recipients and 43,132 controls were included in the study. The patient selection flowchart is presented in Figure 1.
Study design
This study was a retrospective cohort study to examine the risk of osteoporosis and fracture in SOT recipients. The date of SOT was defined as the observation start date for SOT recipients. In terms of investigating the risk of osteoporosis, all participants were followed up until the date of diagnosis as having osteoporosis, death, or the end of 2018, whichever occurred first. In terms of investigating the risk of pathological fracture, all participants were followed up until the date of diagnosis as having a pathological fracture, death, or the end of 2018, whichever occurred first. The comorbidities in the study were defined with the outpatient department visits and hospital admission database in the past 2 years before the observation date. The comorbidities included hypertension (HTN) (ICD-9-CM 401-405; ICD-10-CM I13 and I15), left ventricular hypertrophy (LVH) (ICD-9-CM 429.3; ICD-10-CM I51.7), atrial fibrillation (AF) (ICD-9-CM 427.31; ICD-10-CM I48.0, I48.2, I48.91), hyperlipidemia (HPL) (ICD-9-CM 272; ICD-10-CM E78.4 and E78.5), rheumatoid arthritis (RA) (ICD-9-CM 714; ICD-10-CM M05-M06 and M45), depression (ICD-9-CM 311; ICD-10-CM F32.9), sleep disturbance (ICD-9-CM 307.4, 780; ICD-10-CM G47.8 and G47.9), gout (ICD-9-CM 274.9; ICD-10-CM M10.9), chronic obstructive pulmonary disease (COPD) (ICD-9-CM 490–492 and 494–496; ICD-10-CM J40–J44 and J47), hyperthyroidism (ICD-9-CM 242.9; ICD-10-CM E05.90), chronic kidney disease (CKD) (ICD-9-CM 585; ICD-10-CM N18), and diabetes mellitus (DM) (ICD-9-CM 250; ICD-10-CM E08-E13).
Statistical analysis
All statistical analyses in the study were conducted using SAS version 9.4. The difference in baseline characteristics between SOT recipients and the comparison cohort was analyzed via the Chi-square test and Fisher’s exact test. The Cox proportional hazards model was used to investigate the risk of osteoporosis and pathological fracture in SOT recipients with adjustment of the relevant variables, and the results are presented as hazard ratios (HRs) with 95% CIs. p <.05 was set as statistically significant.
Results
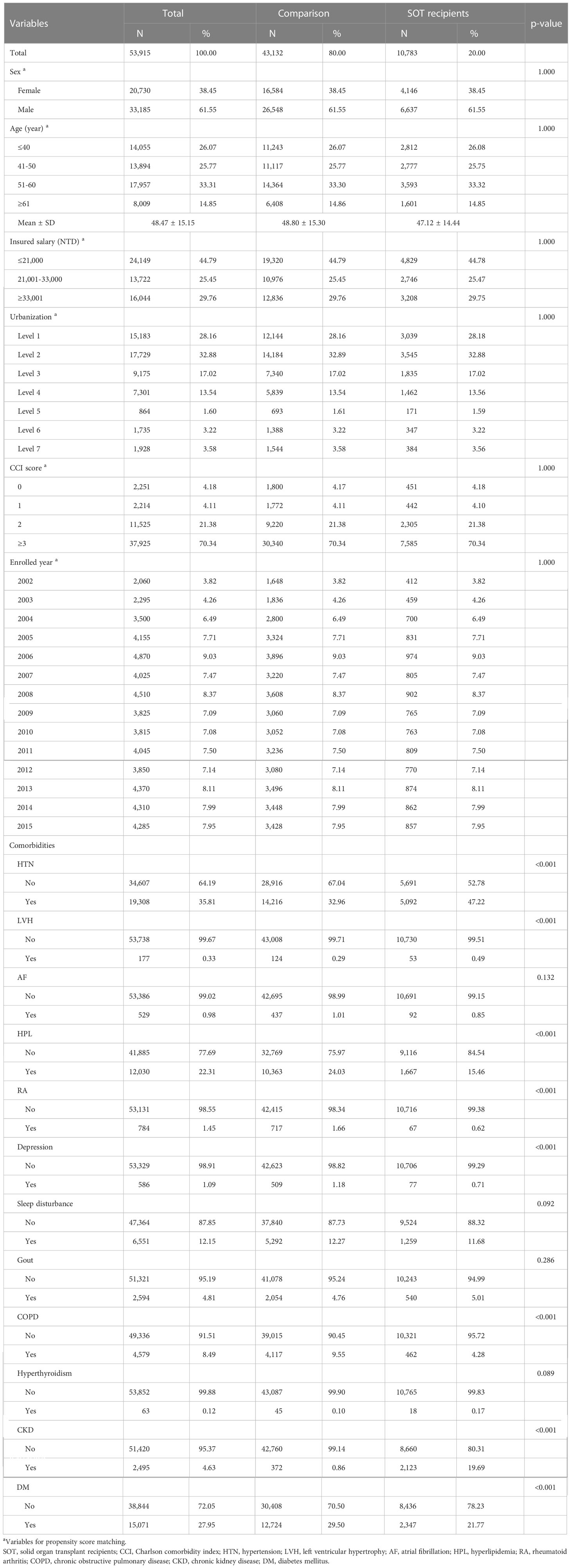
Table 1 presents the basic characteristics of SOT recipients and a comparison after matching. PS matching yielded 53,915 participants, with 43,132 (80%) men and 10,783 (20%) women. The mean age of the SOT recipients was 47.12 ± 14.44 years and that of the controls was 48.80 ± 15.30 years. After PS matching, no between-group differences were observed in sex, age, insured salary, urbanization, and CCI (all p > 0.05). Among SOT recipients, 5,092 had HTN (47.22%), 2,123 had CKD (19.69%), 1,667 had HPL (15.46%), 1,259 had sleep disturbance (11.68%), 540 had gout (5.01%), 462 had COPD (4.28%), 92 had AF (0.85%), 77 had depression (0.71%), 67 had RA (0.62%), 53 had LVH (0.49%), 18 had hyperthyroidism (0.17%), and 2,347 had DN (21.77%).
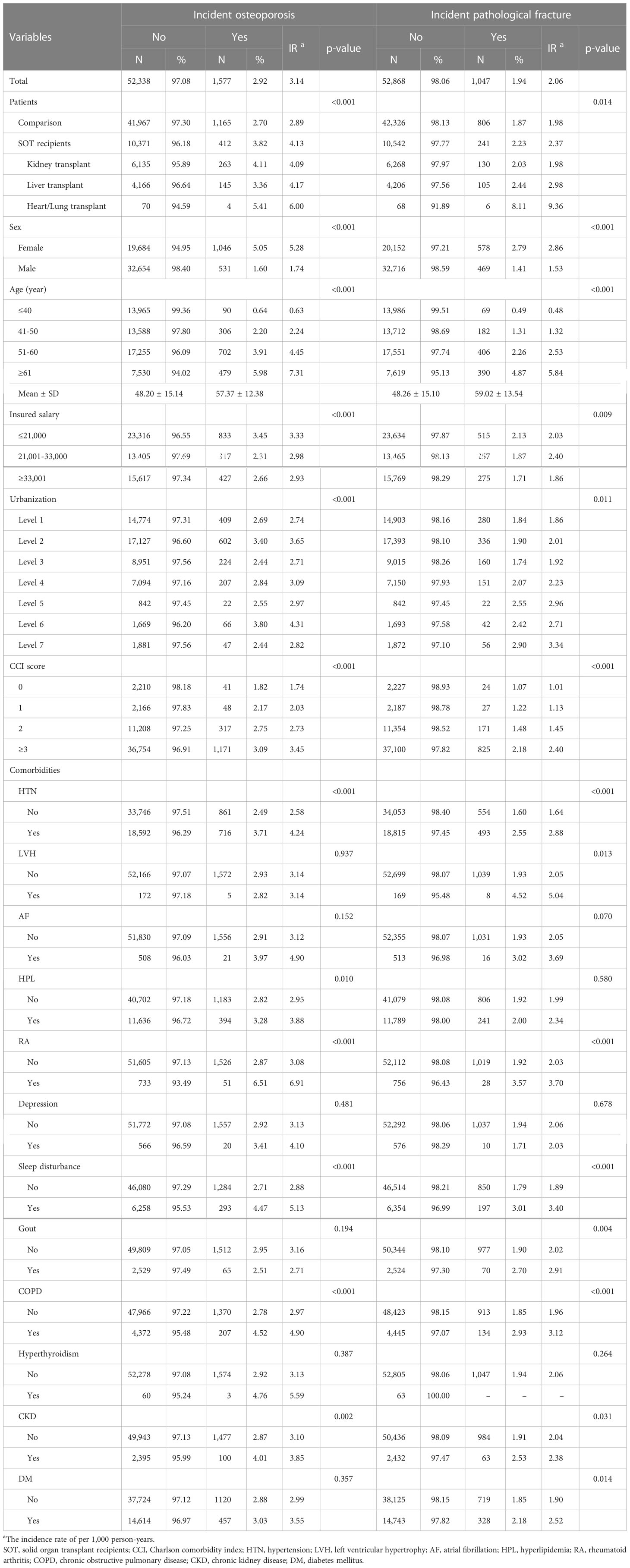
Table 2 presents the double-variable analysis for each variable and the incidence of osteoporosis and pathological fracture. Among SOT recipients, 412 (3.82%) developed osteoporosis (incidence rate: 4.13 per 1,000 person-years), and the incidence rate was significantly higher than the comparison cohort (p < 0.001). Pathological fractures occurred in 241 (2.23%) of SOT recipients (incidence rate: 2.37 per 1,000 person-years), and the incidence rate was also significantly higher than the comparison cohort (p = 0.014). Compared with patients without comorbidities, those with HTN, HPL, RA, sleep disturbance, COPD, and CKD had a higher incidence rate of osteoporosis, and those with HTN, LVH, RA, sleep disturbance, gout, COPD, CKD, and DM had a higher incidence rate of pathological fracture (all p < 0.05).
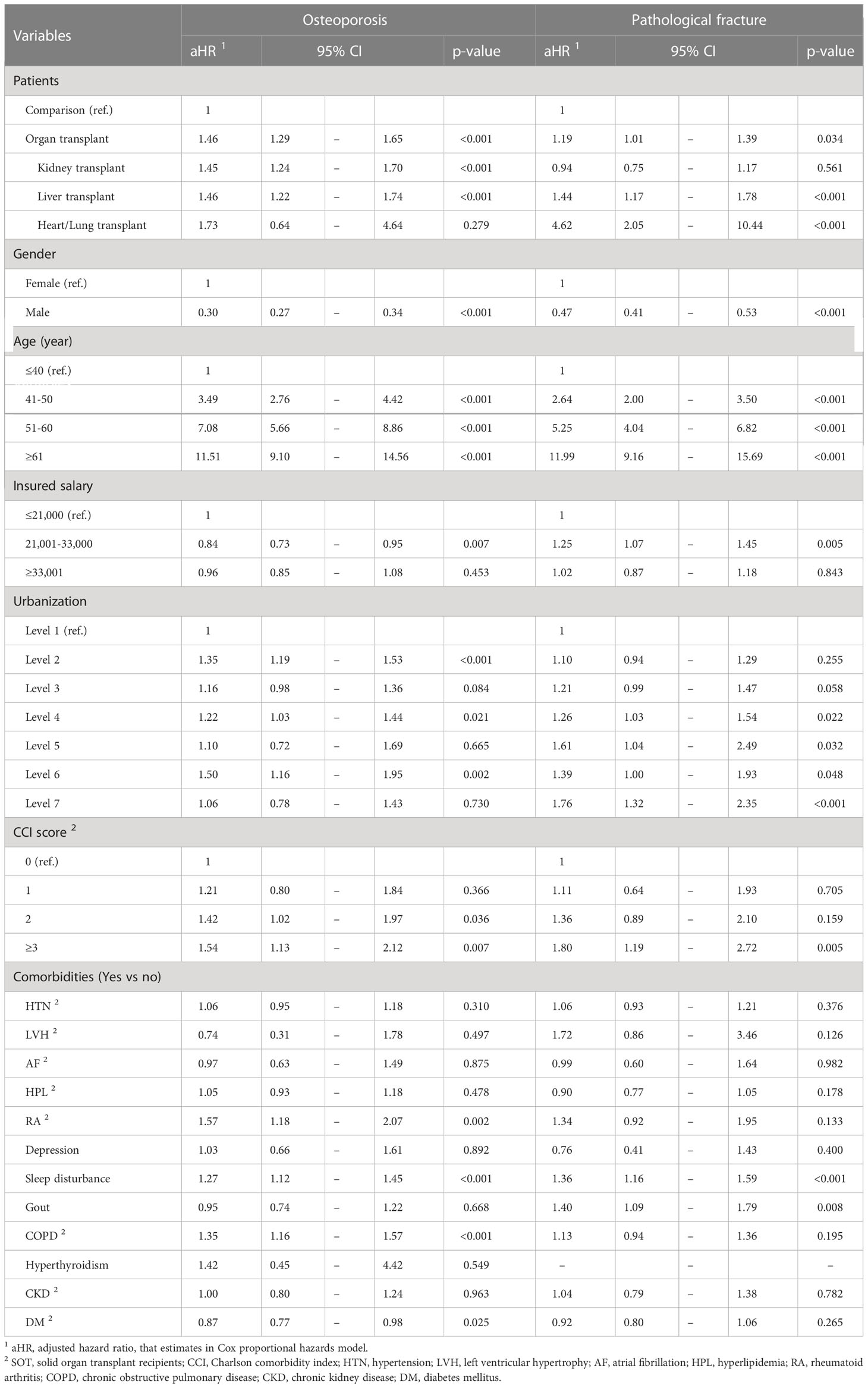
After other relevant influencing factors were controlled for, SOT recipients had a higher risk of osteoporosis (HR: 1.46, 95% CI: 1.29–1.65) than controls (Table 3). Compared with patients <40 years old, the risk of osteoporosis was higher in those aged 41 to 50 years (HR: 3.49, 95% CI: 2.76–4.42), 51 to 60 years (HR: 7.08, 95% CI: 5.66–8.86), and ≥60 years (HR: 11.51, 95% CI: 9.10–14.56). Patients with a CCI score of >3 had a higher risk of osteoporosis than those with a CCI score of 0. Compared with patients without comorbidities, patients with RA (HR: 1.57, 95% CI: 1.18–2.07), sleep disturbance (HR: 1.27, 95% CI: 1.12–1.45), and COPD (HR: 1.35, 95% CI: 1.16–1.57) had a higher risk of osteoporosis. In comparison, patients with DM had a lower risk of osteoporosis (HR: 0.87, 95% CI: 0.77–0.98). We observed that kidney and liver transplant recipients had a higher risk of osteoporosis (HR: 1.45, 95% CI: 1.24–1.70 and HR: 1.46, 95% CI: 1.22–1.74, respectively).
SOT recipients had a higher risk of pathological fracture HR of 1.19 (95% CI: 1.01–1.39) than controls. Compared with patients <40 years old, the risk of pathological fracture was higher in those aged 41 to 50 years (HR: 2.64, 95% CI: 2.00–3.50), 51 to 60 years (HR: 5.25, 95% CI: 4.04–6.82), and ≥60 years (HR: 11.99, 95% CI: 9.16–15.69). Patients with a CCI score of >3 had a higher risk of pathological fracture than those with a CCI score of 0. Patients with sleep disturbance had a higher risk of pathological fracture (HR: 1.36, 95% CI: 1.16–1.59). Patients with gout had a higher risk of pathological fracture (HR: 1.40, 95% CI: 1.09–1.79). We observed that liver and heart or lung transplant recipients had a higher risk of pathological fracture (HR: 1.44, 95% CI: 1.17–1.78 and HR: 4.62, 95% CI: 2.05–10.44, respectively).
Discussion
Our main findings were that SOT recipients had a higher risk of osteoporosis and associated pathological fractures than the general population. To compare the risk of different recipients of SOT, the highest risk of fractures was noted in patients receiving heart or lung transplants. We also found that SOT recipients comorbid with RA, sleep disturbances, gout, and COPD had a higher risk of osteoporosis or fractures. In addition, SOT recipients who scored high on the CCI had a high HR for developing osteoporosis and fractures, and women had a higher risk than men.
Osteoporosis is a frequent and devastating complication after SOT (18) and is caused by multiple pathophysiologic mechanisms (7). Increased risk of posttransplant fracture in SOT recipients is mainly due to posttransplant bone remodeling due to reduced bone formation and continued bone erosion resulting in bone mineralization (19). Posttransplantation osteoporosis and fracture are associated with alterations in the receptor activator of the nuclear factor B ligand (RANKL)/osteoprotegerin (OPG) system (18) (20),. OPG is an antiresorptive cytokine that competes with RANKL for binding to RANK and inhibits osteoclast differentiation. OPG plays a central role in the development of transplantation osteoporosis and fracture (21).
In our study, SOT recipients had a higher risk of osteoporosis and related fractures than the general population. The loss of bone mass is particularly prominent during the first 3 to 6 months after heart, lung, and liver transplantation and during the first 6 to 18 months after kidney transplantation (22). The reduction in BMD varies between 2% and 12% in the first posttransplant year in all transplantations (23). A wide range of osteoporosis (11%–57%) and fracture rates (14%–65%) have been reported during the first posttransplant year, depending on the transplant organ and follow-up duration (24–26).
Some previous studies have shown that kidney transplant recipients have a higher risk of fracture than the general population (11, 27, 28). However, in our cohort study, kidney transplant patients had a higher risk of osteoporosis than the general population but not fracture risk. A Canadian cohort study using healthcare databases also demonstrated that the cumulative incidence of fracture in kidney transplant recipients was lower than in previously reported literature (29). This study suggested that despite the metabolic bone changes and use of steroids following kidney transplantation, recipients may not be a high-risk group for fracture. Explanations for the lower-than-expected fracture incidence may be associated with changes in maintenance immunosuppressive regimens (29). There has also been a trend toward decreasing corticosteroid doses after kidney transplantation. Corticosteroids are well known to be associated with a decrease in bone mineral density (30).
Posttransplantation bone disease is a major cause of morbidity among kidney transplant recipients, with a significantly higher risk of subsequent fractures, which are associated with a greater increase in mortality rates (31). Within the first 5 years after transplantation, an estimated 22.5% of kidney transplant recipients experience a fracture, an incidence that was four times higher in the general population (32). This risk remains significantly elevated in transplant recipients even after 10 years, suggesting that biochemical abnormalities of disordered mineral metabolism are common in recipients with kidney failure and may persist after a successful renal transplant (31).
We can also find that liver transplant recipients had a higher risk of osteoporosis and pathological fractures than the general population. Studies have concluded that most liver transplant recipients already have abnormal BMD at the time of transplantation or have had fractures before the transplant (33, 34). BMD may improve mainly in the second year after surgery, but approximately one-third of liver transplant recipients’ BMD remains under the fracture threshold (35). Low BMD before transplantation is a major risk factor for posttransplant fractures (36). The reason for BMD reduction in the early posttransplant period may be due to the pre- and posttransplant changes in bone turnover state. A meta-analysis indicated that liver transplant recipients have a fivefold increased risk of both osteoporosis and fractures compared with non-LT recipients (37). The main risk factors for postliver transplant fractures include the presence of pretransplant fractures, decreased BMD, posttransplant glucocorticoid dose, and primary biliary cholangitis (38).
Among the recipients of different SOTs, the highest risk of fractures was noted in patients receiving heart or lung transplants. Bone mass loss and fragility fractures are important complications after a heart transplant. Significant bone loss occurs after heart transplantation, and is associated with an increased risk of fracture-related morbidity and mortality rates (1). In heart transplant recipients, the incidence of fractures was highest in the first year after transplantation (39). Lung transplant recipients have a higher risk of osteoporosis and pathologic fractures than kidney, liver, and heart transplant recipients (13). This may be because lung transplant recipients are required more intensive administration of high doses of immunosuppressants to improve patient survival (40).
Our study revealed that patients with higher scores on the CCI were also at higher risk, especially those with CCI scores of >3. In addition, women had a higher risk than men. Many factors contribute to the development of bone disease and osteoporosis after SOT, including immunosuppressive agents and lifestyle risk factors (7). Typically, older adults, women, and recipients of dialysis have an increased risk of fracture (28). CCI is associated with increased mortality in fragility fracture patients (41). The CCI score may be applicable for predicting fracture risk.
We found that patients comorbid with RA, sleep disturbances, gout, and COPD had a higher risk of developing osteoporosis or fracture. The prevalence of osteoporosis in RA is approximately 30% (up to 50% in postmenopausal women), which might be a twofold increase over the general population (42, 43).
A cross-sectional study indicated that bone density is associated with sleep quality and duration (44). Sleep disorders are associated with an increased risk of osteoporosis, especially in women and older adults (45). A population-based epidemiologic study indicated that gout modestly increases the risk of osteoporosis (46). Bone disorders can develop before any organ transplantation; however, they seem to be pronounced in patients with chronic lung diseases (47, 48). COPD is associated with an increased risk of osteoporosis, regardless of the exposure period (49). Osteoporosis is highly prevalent in patients referred for lung transplantation, especially among patients with COPD (4). Evidence regarding vertebral fracture (VF) risk in patients with T2DM has been less conclusive, providing results ranging from lower risk to no association with higher prevalent or incident VF to increased risk (50–55). However, our study discovered that patients comorbid with T2DM had a lower risk of developing osteoporosis but no association with fracture. In a sample of 37,292 patients with T2DM, there was a lower risk of prevalent VF (OR: 0.84, 95% CI: 0.74–0.95) without evidence of heterogeneity across studies. In a sample of 738,018 patients with T2DM, there was an increased risk of incident VF (OR: 1.35, 95% CI: 1.27–1.44) with no evidence of heterogeneity across studies (56).
The present study has several strengths. First, we included the entire Taiwanese population in this study; thus, the sample size is large and highly representative of the general population, thus increasing statistical power. The combination of NHIRD with multiple data sources can be a powerful research tool. The large-scale nationwide data include comprehensive demographic data and long-term follow-up duration. Second, we also investigated related risk factors for comorbidities and the risk of incident osteoporosis and fracture following transplantation.
Our study also had certain limitations. First, dual-energy X-ray absorptiometry (DXA) is currently considered the gold standard for the assessment of BMD and, thus, osteoporosis diagnosis. However, the NHIRD does not contain data on bone density Z-scores or T-scores, precluding the confirmation of osteoporosis diagnosis. Second, the NHIRD does not contain detailed information regarding family history of systemic diseases and socioeconomic status, which may be risk factors for osteoporosis or pathologic fracture. Finally, the NHIRD also lacks information on other risk factors for osteoporosis and osteoporotic fractures, such as body mass index, smoking, vitamin D intake, calcium intake, diet supplementation, and physical activity.
Conclusion
SOT recipients had higher incidence rates and risks of osteoporosis and fractures than the general population. Among different recipients of SOT, the highest risk of fractures was noted in patients receiving heart or lung transplants. In addition, patients who scored high on the CCI had a higher risk. Patients who were comorbid with RA, sleep disturbance, gout, and COPD had a higher risk of osteoporosis or fractures.
Data availability statement
The National Health Insurance Database used to support the findings of this study was provided by the Health and Welfare Data Science Center, Ministry of Health and Welfare (HWDC, MOHW) under license and cannot be made freely available. Requests for access to these data should be made to HWDC (https://dep.mohw.gov.tw/dos/np-2497-113.html).
Ethics statement
In the present study, we conducted secondary data analysisusing the Longitudinal Health Insurance Database (LHID) released by the Health and Welfare Data Science Center (HWDC) at the Ministry of Health and Welfare in Taiwan. The LHID is anonymous, and the HWDC provides scrambled random identification numbers for insured patients to protect their privacy. The requirement for informed consent was waived. This study protocol was approved as an ethical review by the Central Regional Research Ethics Committee of China Medical University, Taiwan (No. CRREC-109-011).
Author contributions
Study conception and design: K-HH, HC, Y-RL, YY, S-YG, C-YH, T-HT, and C-YL. Data acquisition: K-HH and C-YL. Data analysis and demonstration: K-HH and C-YL. Original draft preparation: K-HH, Y-RL, YY, HC, S-YG, T-HT, C-YH, and C-YL. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the Chung Shan Medical University Hospital, Taiwan (CSH-2023-C-002), the China Medical University, Taiwan (CMU111-MF-111), and the National Science and Technology Council, Taiwan (MOST 110-2410-H-040-002).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Anastasilakis AD, Tsourdi E, Makras P, Polyzos SA, Meier C, McCloskey EV, et al. Bone disease following solid organ transplantation: a narrative review and recommendations for management from the European calcified tissue society. Bone (2019) 127:401–18. doi: 10.1016/j.bone.2019.07.006
2. Lan GB, Xie XB, Peng LK, Liu L, Song L, Dai HL. Current status of research on osteoporosis after solid organ transplantation: pathogenesis and management. BioMed Res Int (2015) 2015:413169. doi: 10.1155/2015/413169
3. Delmas PD. Osteoporosis in patients with organ transplants: a neglected problem. Lancet (2001) 357(9253):325–6. doi: 10.1016/S0140-6736(00)03632-1
4. Jastrzebski D, Lutogniewska W, Ochman M, Margas A, Kowalski K, Wyrwol J, et al. Osteoporosis in patients referred for lung transplantation. Eur J Med Res (2010) 15 Suppl 2:68–71. doi: 10.1186/2047-783X-15-S2-68
5. Wang TK, O'Sullivan S, Gamble GD, Ruygrok PN. Bone density in heart or lung transplant recipients–a longitudinal study. Transplant Proc (2013) 45(6):2357–65. doi: 10.1016/j.transproceed.2012.09.117
6. Cahill BC, O'Rourke MK, Parker S, Stringham JC, Karwande SV, Knecht TP. Prevention of bone loss and fracture after lung transplantation: a pilot study. Transplantation (2001) 72(7):1251–5. doi: 10.1097/00007890-200110150-00012
7. Maalouf NM, Shane E. Osteoporosis after solid organ transplantation. J Clin Endocrinol Metab (2005) 90(4):2456–65. doi: 10.1210/jc.2004-1978
8. Stein E, Ebeling P, Shane E. Post-transplantation osteoporosis. Endocrinol Metab Clin North Am (2007) 36(4):937–963; viii. doi: 10.1016/j.ecl.2007.07.008
9. Cohen A, Shane E. Osteoporosis after solid organ and bone marrow transplantation. Osteoporos Int (2003) 14(8):617–30. doi: 10.1007/s00198-003-1426-z
10. Compston JE. Osteoporosis after liver transplantation. Liver Transpl (2003) 9(4):321–30. doi: 10.1053/jlts.2003.50044
11. Ramsey-Goldman R, Dunn JE, Dunlop DD, Stuart FP, Abecassis MM, Kaufman DB, et al. Increased risk of fracture in patients receiving solid organ transplants. J Bone Miner Res (1999) 14(3):456–63. doi: 10.1359/jbmr.1999.14.3.456
12. Monegal A, Navasa M, Guanabens N, Peris P, Pons F, Martinez de Osaba MJ, et al. Bone disease after liver transplantation: a long-term prospective study of bone mass changes, hormonal status and histomorphometric characteristics. Osteoporos Int (2001) 12(6):484–92. doi: 10.1007/s001980170094
13. Yu TM, Lin CL, Chang SN, Sung FC, Huang ST, Kao CH. Osteoporosis and fractures after solid organ transplantation: a nationwide population-based cohort study. Mayo Clin Proc (2014) 89(7):888–95. doi: 10.1016/j.mayocp.2014.02.017
14. Hsieh CY, Su CC, Shao SC, Sung SF, Lin SJ, Kao Yang YH, et al. Taiwan's national health insurance research database: past and future. Clin Epidemiol (2019) 11:349–58. doi: 10.2147/CLEP.S196293
15. Chang SH, Chou IJ, Yeh YH, Chiou MJ, Wen MS, Kuo CT, et al. Association between use of non-vitamin K oral anticoagulants with and without concurrent medications and risk of major bleeding in nonvalvular atrial fibrillation. JAMA (2017) 318(13):1250–9. doi: 10.1001/jama.2017.13883
16. Hsieh CY, Tsao WC, Lin RT, Chao AC. Three years of the nationwide post-acute stroke care program in Taiwan. J Chin Med Assoc (2018) 81(1):87–8. doi: 10.1016/j.jcma.2017.09.003
17. Lai SW, Liao KF, Lin CL, Lin CC, Lin CH. Longitudinal data of multimorbidity and polypharmacy in older adults in Taiwan from 2000 to 2013. Biomedicine (Taipei) (2020) 10(2):1–4. doi: 10.37796/2211-8039.1013
18. Song L, Xie XB, Peng LK, Yu SJ, Peng YT. Mechanism and treatment strategy of osteoporosis after transplantation. Int J Endocrinol (2015) 2015:280164. doi: 10.1155/2015/280164
19. Molnar MZ, Naser MS, Rhee CM, Kalantar-Zadeh K, Bunnapradist S. Bone and mineral disorders after kidney transplantation: therapeutic strategies. Transplant Rev (Orlando) (2014) 28(2):56–62. doi: 10.1016/j.trre.2013.12.003
20. Fabrega E, Orive A, Garcia-Unzueta M, Amado JA, Casafont F, Pons-Romero F. Osteoprotegerin and receptor activator of nuclear factor-kappaB ligand system in the early post-operative period of liver transplantation. Clin Transplant (2006) 20(3):383–8. doi: 10.1111/j.1399-0012.2006.00497.x
21. Fahrleitner A, Prenner G, Leb G, Tscheliessnigg KH, Piswanger-Solkner C, Obermayer-Pietsch B, et al. Serum osteoprotegerin is a major determinant of bone density development and prevalent vertebral fracture status following cardiac transplantation. Bone (2003) 32(1):96–106. doi: 10.1016/S8756-3282(02)00926-2
22. Floreani A, Mega A, Tizian L, Burra P, Boccagni P, Baldo V, et al. Bone metabolism and gonad function in male patients undergoing liver transplantation: a two-year longitudinal study. Osteoporos Int (2001) 12(9):749–54. doi: 10.1007/s001980170051
23. Smets YF, de Fijter JW, Ringers J, Lemkes HH, Hamdy NA. Long-term follow-up study on bone mineral density and fractures after simultaneous pancreas-kidney transplantation. Kidney Int (2004) 66(5):2070–6. doi: 10.1111/j.1523-1755.2004.00986.x
24. Cohen A, Sambrook P, Shane E. Management of bone loss after organ transplantation. J Bone Miner Res (2004) 19(12):1919–32. doi: 10.1359/jbmr.040912
25. Ball AM, Gillen DL, Sherrard D, Weiss NS, Emerson SS, Seliger SL, et al. Risk of hip fracture among dialysis and renal transplant recipients. JAMA (2002) 288(23):3014–8. doi: 10.1001/jama.288.23.3014
26. Ebeling PR. Approach to the patient with transplantation-related bone loss. J Clin Endocrinol Metab (2009) 94(5):1483–90. doi: 10.1210/jc.2009-0205
27. Abbott KC, Oglesby RJ, Hypolite IO, Kirk AD, Ko CW, Welch PG, et al. Hospitalizations for fractures after renal transplantation in the united states. Ann Epidemiol (2001) 11(7):450–7. doi: 10.1016/S1047-2797(01)00226-5
28. Naylor KL, Li AH, Lam NN, Hodsman AB, Jamal SA, Garg AX. Fracture risk in kidney transplant recipients: a systematic review. Transplantation (2013) 95(12):1461–70. doi: 10.1097/TP.0b013e31828eead8
29. Naylor KL, Jamal SA, Zou G, McArthur E, Lam NN, Leslie WD, et al. Fracture incidence in adult kidney transplant recipients. Transplantation (2016) 100(1):167–75. doi: 10.1097/TP.0000000000000808
30. Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int (2007) 18(10):1319–28. doi: 10.1007/s00198-007-0394-0
31. Nair SS, Lenihan CR, Montez-Rath ME, Lowenberg DW, Chertow GM, Winkelmayer WC. Temporal trends in the incidence, treatment and outcomes of hip fracture after first kidney transplantation in the united states. Am J Transplant (2014) 14(4):943–51. doi: 10.1111/ajt.12652
32. Nikkel LE, Hollenbeak CS, Fox EJ, Uemura T, Ghahramani N. Risk of fractures after renal transplantation in the united states. Transplantation (2009) 87(12):1846–51. doi: 10.1097/TP.0b013e3181a6bbda
33. Ninkovic M, Love SA, Tom B, Alexander GJ, Compston JE. High prevalence of osteoporosis in patients with chronic liver disease prior to liver transplantation. Calcif Tissue Int (2001) 69(6):321–6. doi: 10.1007/s00223-001-2028-4
34. Monegal A, Navasa M, Peris P, Colmenero J, Cuervo A, Muxi A, et al. Bone disease in patients awaiting liver transplantation. has the situation improved in the last two decades? Calcif Tissue Int (2013) 93(6):571–6. doi: 10.1007/s00223-013-9797-4
35. Hamburg SM, Piers DA, van den Berg AP, Slooff MJ, Haagsma EB. Bone mineral density in the long term after liver transplantation. Osteoporos Int (2000) 11(7):600–6. doi: 10.1007/s001980070081
36. Bai XL, Liang TB, Wu LH, Li DL, Geng L, Wang WL, et al. Elevation of intact parathyroid hormone level is a risk factor for low bone mineral density in pretransplant patients with liver diseases. Transplant Proc (2007) 39(10):3182–5. doi: 10.1016/j.transproceed.2007.06.093
37. Lim WH, Ng CH, Ow ZGW, Ho OTW, Tay PWL, Wong KL, et al. A systematic review and meta-analysis on the incidence of osteoporosis and fractures after liver transplant. Transpl Int (2021) 34(6):1032–43. doi: 10.1111/tri.13863
38. Rodriguez-Aguilar EF, Perez-Escobar J, Sanchez Herrera D, Garcia-Alanis M, Toapanta-Yanchapaxi L, Gonzalez-Flores E, et al. Bone disease and liver transplantation: a review. Transplant Proc (2021) 53(7):2346–53. doi: 10.1016/j.transproceed.2021.07.049
39. Hariman A, Alex C, Heroux A, Camacho P. Incidence of fractures after cardiac and lung transplantation: a single center experience. J Osteoporos (2014) 2014:573041. doi: 10.1155/2014/573041
40. Kotecha S, Ivulich S, Snell G. Review: immunosuppression for the lung transplant patient. J Thorac Dis (2021) 13(11):6628–44. doi: 10.21037/jtd-2021-11
41. Jiang L, Chou ACC, Nadkarni N, Ng CEQ, Chong YS, Howe TS, et al. Charlson comorbidity index predicts 5-year survivorship of surgically treated hip fracture patients. Geriatr Orthop Surg Rehabil (2018) 9:2151459318806442. doi: 10.1177/2151459318806442
42. Haugeberg G, Uhlig T, Falch JA, Halse JI, Kvien TK. Bone mineral density and frequency of osteoporosis in female patients with rheumatoid arthritis: results from 394 patients in the Oslo county rheumatoid arthritis register. Arthritis Rheum (2000) 43(3):522–30. doi: 10.1002/1529-0131(200003)43:3<522::AID-ANR7>3.0.CO;2-Y
43. Hauser B, Riches PL, Wilson JF, Horne AE, Ralston SH. Prevalence and clinical prediction of osteoporosis in a contemporary cohort of patients with rheumatoid arthritis. Rheumatol (Oxford) (2014) 53(10):1759–66. doi: 10.1093/rheumatology/keu162
44. Lee CL, Tzeng HE, Liu WJ, Tsai CH. A cross-sectional analysis of the association between sleep duration and osteoporosis risk in adults using 2005-2010 NHANES. Sci Rep (2021) 11(1):9090. doi: 10.1038/s41598-021-88739-x
45. Yen CM, Kuo CL, Lin MC, Lee CF, Lin KY, Lin CL, et al. Sleep disorders increase the risk of osteoporosis: a nationwide population-based cohort study. Sleep Med (2014) 15(11):1339–44. doi: 10.1016/j.sleep.2014.07.005
46. Kok VC, Horng JT, Wang MN, Chen ZY, Kuo JT, Hung GD. Gout as a risk factor for osteoporosis: epidemiologic evidence from a population-based longitudinal study involving 108,060 individuals. Osteoporos Int (2018) 29(4):973–85. doi: 10.1007/s00198-018-4375-2
47. Tschopp O, Boehler A, Speich R, Weder W, Seifert B, Russi EW, et al. Osteoporosis before lung transplantation: association with low body mass index, but not with underlying disease. Am J Transplant (2002) 2(2):167–72. doi: 10.1034/j.1600-6143.2002.020208.x
48. Ferrari SL, Nicod LP, Hamacher J, Spiliopoulos A, Slosman DO, Rochat T, et al. Osteoporosis in patients undergoing lung transplantation. Eur Respir J (1996) 9(11):2378–82. doi: 10.1183/09031936.96.09112378
49. Graat-Verboom L, Wouters EF, Smeenk FW, van den Borne BE, Lunde R, Spruit MA. Current status of research on osteoporosis in COPD: a systematic review. Eur Respir J (2009) 34(1):209–18. doi: 10.1183/09031936.50130408
50. Jia P, Bao L, Chen H, Yuan J, Liu W, Feng F, et al. Risk of low-energy fracture in type 2 diabetes patients: a meta-analysis of observational studies. Osteoporos Int (2017) 28(11):3113–21. doi: 10.1007/s00198-017-4183-0
51. Dytfeld J, Michalak M. Type 2 diabetes and risk of low-energy fractures in postmenopausal women: meta-analysis of observational studies. Aging Clin Exp Res (2017) 29(2):301–9. doi: 10.1007/s40520-016-0562-1
52. Wang J, You W, Jing Z, Wang R, Fu Z, Wang Y. Increased risk of vertebral fracture in patients with diabetes: a meta-analysis of cohort studies. Int Orthop (2016) 40(6):1299–307. doi: 10.1007/s00264-016-3146-y
53. Moayeri A, Mohamadpour M, Mousavi SF, Shirzadpour E, Mohamadpour S, Amraei M. Fracture risk in patients with type 2 diabetes mellitus and possible risk factors: a systematic review and meta-analysis. Ther Clin Risk Manag (2017) 13:455–68. doi: 10.2147/TCRM.S131945
54. Napoli N, Schwartz AV, Schafer AL, Vittinghoff E, Cawthon PM, Parimi N, et al. Vertebral fracture risk in diabetic elderly men: the MrOS study. J Bone Miner Res (2018) 33(1):63–9. doi: 10.1002/jbmr.3287
55. Wang H, Ba Y, Xing Q, Du JL. Diabetes mellitus and the risk of fractures at specific sites: a meta-analysis. BMJ Open (2019) 9(1):e024067. doi: 10.1136/bmjopen-2018-024067
Keywords: solid organ transplantation, hazard ratios, osteoporosis, fracture, cohort study
Citation: Chen H, Lai Y-R, Yang Y, Gau S-Y, Huang C-Y, Tsai T-H, Huang K-H and Lee C-Y (2023) High risk of osteoporosis and fracture following solid organ transplantation: a population-based study. Front. Endocrinol. 14:1167574. doi: 10.3389/fendo.2023.1167574
Received: 16 February 2023; Accepted: 04 May 2023;
Published: 23 May 2023.
Edited by:
Valeria Sordi, San Raffaele Hospital (IRCCS), ItalyCopyright © 2023 Chen, Lai, Yang, Gau, Huang, Tsai, Huang and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chien-Ying Lee, Y3NoZDAxNUBjc211LmVkdS50dw==
†These authors have contributed equally to this work
‡These authors have contributed equally to this work
 Hsin Chen
Hsin Chen Yung-Rung Lai3,4†
Yung-Rung Lai3,4† Shuo-Yan Gau
Shuo-Yan Gau Chien-Ying Lee
Chien-Ying Lee