
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol. , 14 April 2023
Sec. Pediatric Endocrinology
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1167351
Objective: To investigate the efficacy of monotherapy with AIs or GnRHa in improving the height of boys with idiopathic short stature (ISS).
Method: We performed a systematic search in Pubmed, The Cochrane Library, Chinese National Knowledge Infrastructure databases, and Wanfang Database for eligible studies. The network meta-analysis was conducted using STATA software.
Results: We identified a total of four studies that included 136 individuals. We used FAH/PAH as the main outcome of final height. The results revealed a statistically higher final height after treatment with AI or GnRHa in idiopathic short stature children(MD= 4.63, 95% CI[3.29,5.96]). In network meta-analysis, the direct and indirect comparison between AI and GnRHa was presented in the forest plot. Compared with control group, both AI and GnRHa were effective in increasing the final height, with the mean effect of 4.91(95%CI:1.10,8.17) and 5.55(95%CI:1.12,9.98) respectively. However, there was no statistical difference between the GnRHa and AI treatment, of which the mean effect was 0.65(95%CI: -4.30,5.60).
Conclusion: Both AIs and GnRHa monotherapy were effective in augmenting the final height of boys with idiopathic short stature when compared to placebo groups. However, there was no statistical difference between the GnRHa and AI treatments.
A common reason for consulting an endocrinologist in the clinic is concern about the final adult height (FAH) of short-statured children. It was estimated that nearly 80% of children with short stature are diagnosed with idiopathic short stature (ISS) (1). The diagnosis of idiopathic short stature (ISS) is made when an individual’s height is less than 2 standard deviations of the respective mean height for a specific age, gender, and population group, excluded from other causes such as systemic, endocrine, nutritional, or chromosomal-related diseases (2).
Aromatase inhibitors (AIs) are compounds that block the conversion of androgens to estrogens and reduce the action of estrogens at the growth plate. For this reason, AIs have been used to intervene on height outcomes in boys with GH deficiency, idiopathic short stature, constitutional growth delay, and other conditions (3–5). In ISS, AI has been found to enable ISS boys to achieve greater adult height in combination with rhGH (6, 7). As for AI monotherapy, the current studies have shown controversial results. Several studies have shown that AI monotherapy increased the growth potential of children with idiopathic short stature (8, 9). In contrast, Tero found no advantage of AI in promoting final height over placebo (10).
Gonadotropin-releasing hormone analogues (GnRHa) have been the standard treatment for central precocious puberty (CPP) since the 1980s (11). By inhibiting the secretion of gonadotrophins and gonadal steroids in children with CPP, GnRHa has been shown to decelerate bone maturation and eventually improve the FAH (12–14). However, GnRHa findings in patients with CPP are not completely consistent. GnRHa is generally thought to be effective in increasing adult height in girls younger than 6 years of age, but fails to improve final height in girls older than 8 years of age (11).In addition, in children with ISS, Khawaja’s study indicates that GnRHa monotherapy slightly increases the FAH in girls with ISS but not in boys (15). The result from Li (16)showed a positive effect of GnRHa on increasing FAH in children with ISS when compared with placebo.
Both AI and GnRHa have been used in ISS, however, there are few comparisons of these two monotherapies. The lack of direct comparison between AI and GnRHa in clinical trials makes it difficult to decide which treatment is more efficacious. In this context, a network meta-analysis provides a quantitative synthesis of the network by comparing direct and indirect evidence across clinical trials.
Therefore, we conduct a network meta-analysis to compare the effects of aromatase inhibitors and gonadotropin-releasing hormone analogue on increasing the final adult height of children with ISS. We focus on pubertal boys, since AI and GnRHa are used in pubertal children, and AI is mostly used to treat boys in clinical practice (17).
This meta-analysis was conducted and reported following the Preferred Reporting Items for Systematic reviews and Meta Analyses (PRISMA) statement (18).
We performed a systematic search in Pubmed, The Cochrane Library, Chinese National Knowledge Infrastructure databases, and Wanfang Database for studies until December 4,2022. The following key words and their corresponding synonyms were used to conduct the search: “idiopathic short stature”, “aromatase inhibitor”, and “gonadotropin-releasing hormone analogue”. The search strategy in Pubmed was: ((((((((GnRHa[Title/Abstract]) OR (GnRH analogues[Title/Abstract])) OR (GnRH-a[Title/Abstract] OR gonadotropin-releasing hormone analogue[Title/Abstract])) OR (gonadotropin-releasing hormone analogs[Title/Abstract])) OR (Triptorelin[Title/Abstract])) OR (Leuprorelin[Title/Abstract])) OR (Buserelin[Title/Abstract])) OR ((((Aromatase Inhibitors[Title/Abstract]) OR (Letrozole[Title/Abstract])) OR (Anastrozole[Title/Abstract])) OR (Testolactone[Title/Abstract]))) AND (((Short Stature[Title/Abstract]) OR (idiopathic short stature[Title/Abstract])) OR (ISS[Title/Abstract])). Additionally, the reference lists of selected articles were also scanned for any relevant study.
Articles that met the following criteria would be included: (i)Study design: clinical trials, including randomized controlled trails (RCTs) and non-randomized clinical controlled trials (CCTs); (ii)Participants: boys with idiopathic short stature; (iii)Intervention: Aromatase Inhibitors or Gonadotropin-releasing hormone analogue; (iv)Comparison: other treatments or no treatment control; and (v)Outcome: final height, including final adult height (FAH) and final predicted adult height(FPAH).
Based on the search strategy and inclusion criteria above, two authors searched articles and screened the titles and abstracts of them independently. Next, the two authors reviewed the full text to assess the eligibility of the selected articles. Different selections were discussed by the two authors or a third senior author was consulted to meet a consensus.
Data extraction from the included articles was finished by two authors independently. The following items were collected: first author, year of publication, sample size in each group, participants’ characteristics, treatment, and clinical outcomes. Clinical outcomes include predicted adult height (PAH), target height (TH), and final adult height (FAH). FAH was considered to be attained when growth was less than 1cm in a year, and/or the bone age was over 15 years (19).
The quality of included studies was assessed by two authors independently. The evaluation of the risk of bias was conducted with the tool provided in the Cochrane handbook (20).
Any disagreement during the data collection and quality assessment between the two authors was discussed or another author was consulted to find a conclusion.
The network meta-analysis was conducted using STATA software (version 17.0, Stata Corporation, TX, USA). The network graph and rank ordering graph of treatments were drawn by STATA. As continuous variables, the outcomes were presented as mean differences(MDs) and 95% confidence interval (CI). Heterogeneity among the studies was assessed with Chi-square and I2 tests. When the test result was p < 0.1 or I2 > 50%, the data were considered as high heterogeneity and a random effects model was used for statistics. Otherwise, a fixed effects model was adopted. In this network meta-analysis, the consistence assumption was assessed by a local inconsistence test and loop-specific test. Local inconsistency was evaluated by the node-splitting method.
In total, 216 studies were initially identified in our electronic search and 146 studies were obtained after removing duplicate studies. Following screening by title and abstract, 29 studies were obtained to evaluate in full texts. Finally, four studies met the criteria and were included in this review. The searching and selecting process is shown in Figure 1.
The main characteristics of the included studies of this meta-analysis are presented in Table 1. The included studies were published between 2005 and 2020, covering three countries (Finland, Jordan, and China). In total, 146 male participants were included. The mean ages of these participants ranged from 10.9 to 13.8 years.
The quality of the included literature was evaluated using the Cochrane bias risk assessment tool. There are three levels of bias risk assessment: Low risk, Unclear risk, and high risk.These are shown in Figure 2.
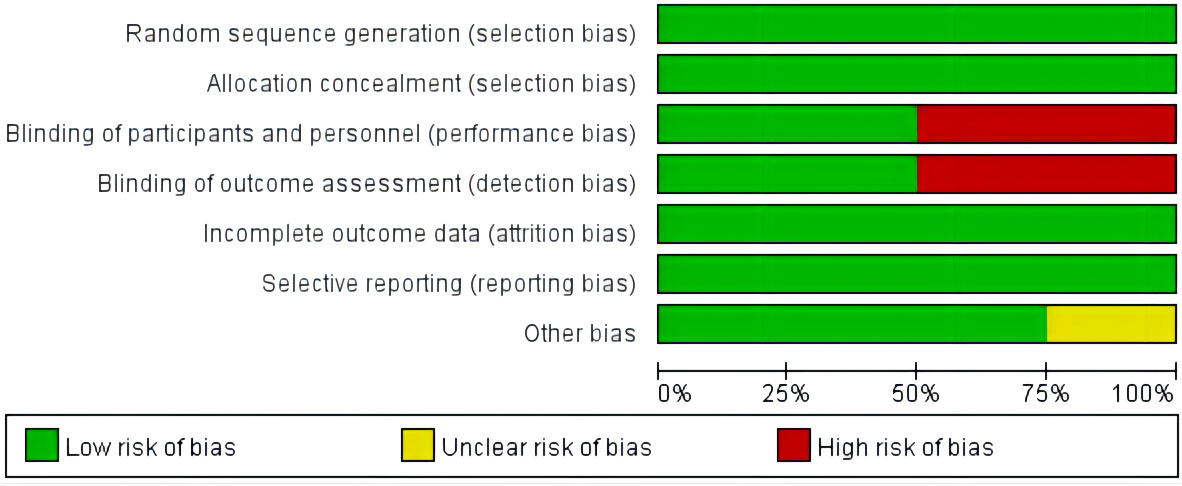
Figure 2 Assessment of the risk of bias in the analyzed studies. Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies.
The inconsistency model was utilized to determine the inconsistency of network meta-analysis. With the result of Chi2 (2)=2.32,P= 0.3128(>0.05),it showed that the inconsistency was not significant. Thus, the fixed effects model was adopted for network meta-analysis.
Moreover, the consistence assumption was assessed by a local inconsistence test using the node-splitting method. No local inconsistency was found (p value>0.05). Next, the loop-specific test was conducted, indicating that the loop inconsistency was not significant and there was a closed loop in the network.Generally, the network was consistent with the principles of coherency, transitivity, and consistency.
The ranking probability of all treatments is shown in Table 2 and Figure 3. The surface under the cumulative ranking curve values (SUCRA values) are used to represent the ranking probability of each treatment. The intervention with a larger SUCRA value was considered to be the more effective treatment (21).The SUCRA of placebo, AI, and GnRHa treatment are 0.6%, 70.1%, and 79.3% respectively. Covering the largest surface, GnRHa had the highest probability of being the most effective treatment.
We used FAH as the main outcome of final height. One of the included studies(Hero,2005 (8)) did not provide FAH, so PAH was used for statistic analysis instead. The results revealed a statistically higher final height after treatment with AI or GnRHa in idiopathic short stature children. (MD= 4.63, 95% CI[3.29,5.96])(Figure 4).
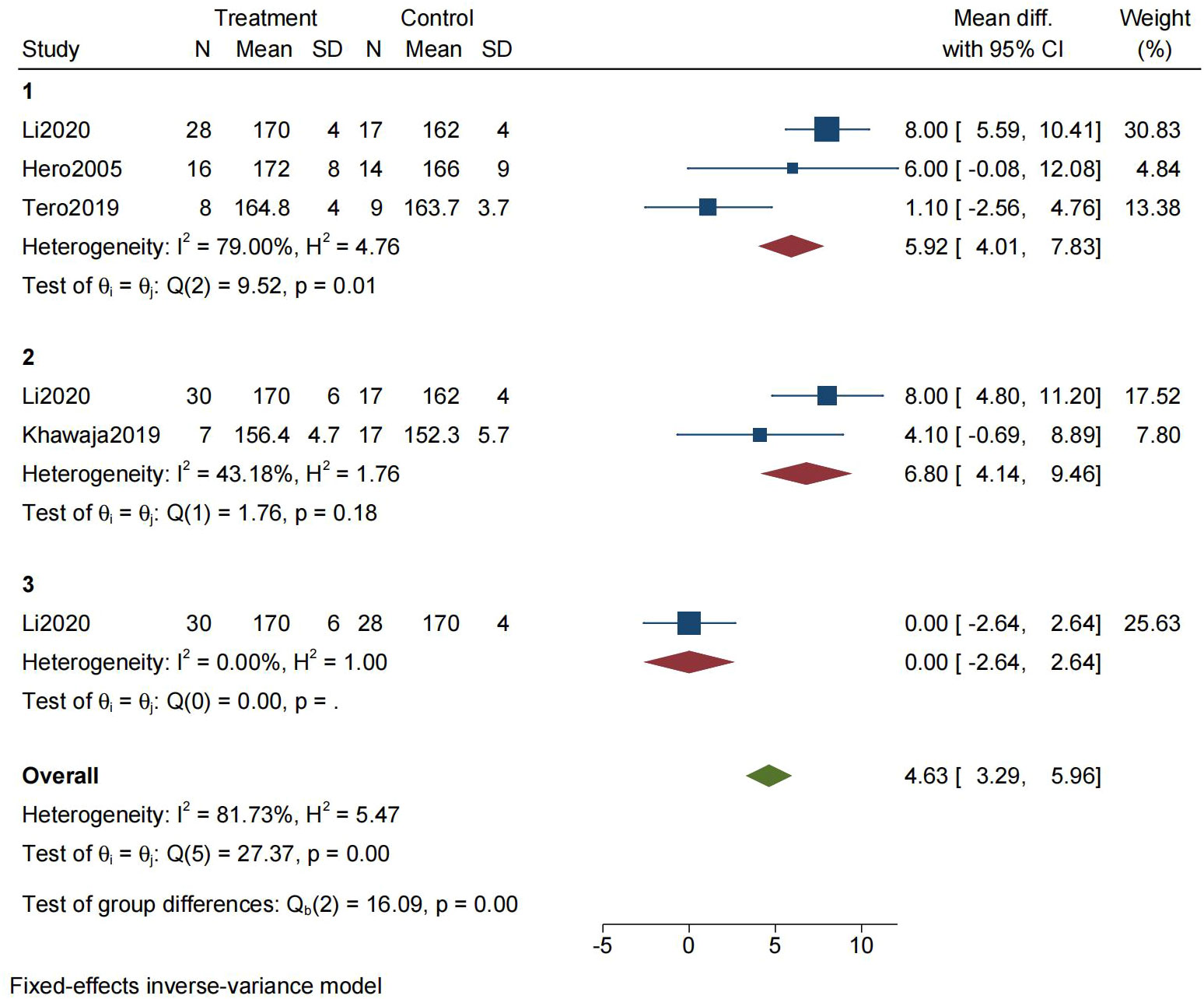
Figure 4 Forest plot for the meta-analysis of final height (1=AI vs No treatment, 2=GnRHa vs No treatment, 3=GnRHa vs AI).
In network meta-analysis, the direct and indirect comparison between AI and GnRHa was presented in the forest plot (Figure 5; Table 3). Compared with the control group, both AI and GnRHa were effective in increasing the final height, with the mean effect of 4.91(95%CI:1.10,8.17) and 5.55(95%CI:1.12,9.98) respectively. However, there was no statistical difference between the GnRHa and AI treatment, of which the mean effect was 0.65(95%CI: -4.30,5.60).
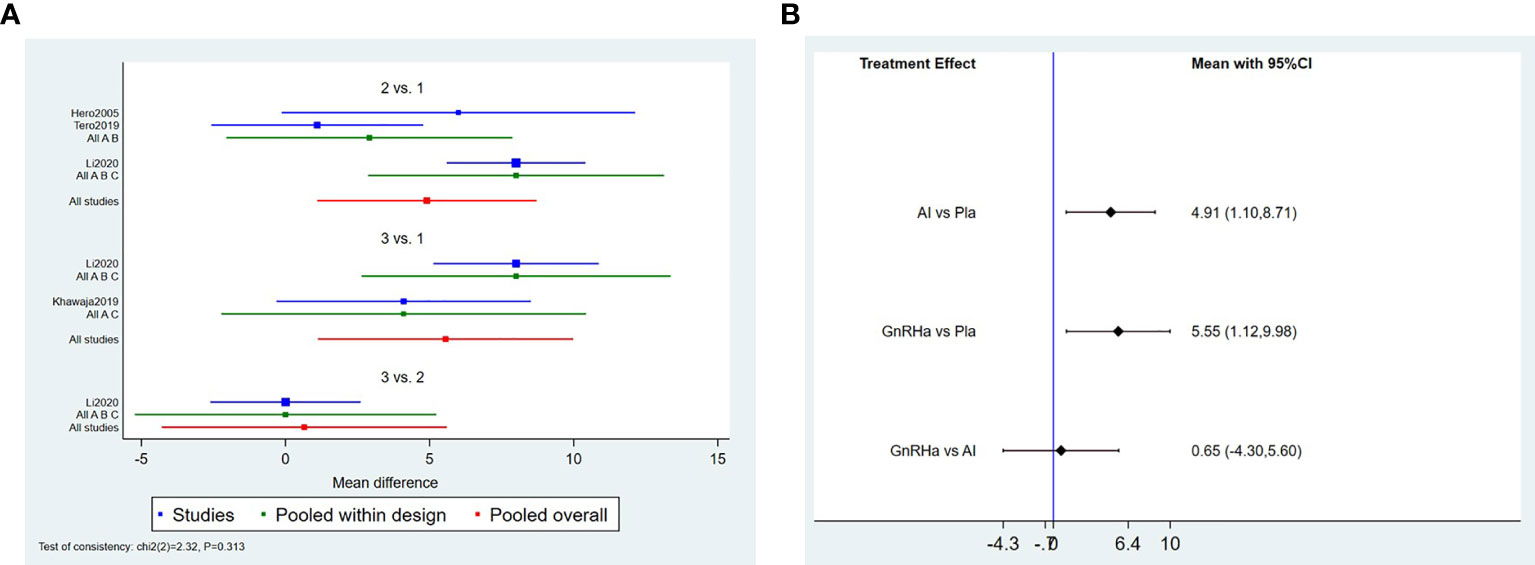
Figure 5 Forest plot of comparisons among treatments.(A) Forest plot for the network meta-analysis of treatment with AI and GnRHa. (B) Forest plot for the pairwise comparsion of the different treatments.
The purpose of this meta-analysis was to evaluate the efficacy of monotherapy with AIs or GnRHa in improving the height of boys with ISS. The crucial findings of this study were that both AIs and GnRHa monotherapy were effective in augmenting the final height of boys with idiopathic short stature when compared to placebo groups. We further compared the effectiveness of AIs and GnRHa; the results showed that the boys who were treated with AIs had similar final heights with those who received GnRHa.
To delay skeletal maturation and gain extra height, the use of AIs falls into two main groups of conditions: precocious puberty and idiopathic short stature (22). Previous studies have shown AI therapy can effectively improve final height in idiopathic short children. In a randomized controlled trial, the authors found that PAH changed significantly in the AI-treated group compared to the placebo and untreated group (The increase was on average 5.1 cm) (5). However, no FAH data were reported. In Hero’s study, the boys with constitutional delay of growth and puberty (CDGP) treated with testolactone and AI reached a higher mean near-final adult height than did boys on testolactone and placebo (175.8cm vs. 169.1cm; P = 0·04) (23). The first study in evaluating the effect of AI (letrozole) on FAH in boys with CDGP showed that the FAH for the AI group were significantly higher than that of the control group (-171 ± 4.5cm vs. 168.8 ± 4.1cm; P=0.04) (24). Another case report on the 5-year AI impact of ISS boys demonstrated that FAH was 15cm higher than PAH before treatment (25). In contrast, Tero’s study found that AI monotherapy did not improve the patients’ FAH (10). The results of our meta-analysis are consistent with most studies. Compared with the control group, AI was effective in increasing the final height, with the mean effect of 4.91(95%CI:1.10,8.17), which indicates that AI may improve FAH of ISS in boys. One possible explanation for the inconsistency in these research findings is insufficient sample size. Thus, larger placebo-controlled RCTs, targeted to idiopathic short children, are needed to assess the efficacy of AI therapy on improving adult height.
The efficacy of gonadotropin-releasing hormone agonist (GnRHa) is undisputed in improving adult stature in girls presenting with CPP up to age 6, but its efficacy is not yet clear for older age groups and boys (11). At present, controversial literature exists concerning the effectiveness of GnRHa on treating ISS children. A clinical historical cohort study was carried out on 28 children with ISS who received GnRHa treatment (21 girls and seven boys) and 31 controls with ISS (14 girls and 17 boys). The results revealed that GnRHa therapy has a modest effect in improving FAH in girls with ISS (151.3 ± 5.1 vs. 146.8 ± 3.8 cm; p = 0.01), but not in boys (156.4 ± 4.7cm vs. 152.3 ± 5.7cm; p = 0.111) (15). In addition, three other studies showed no effect in girls (26–28). Unfortunately, the research in boys is limited to these studies. In Li’s study, adolescent male children with ISS were found to improve their adult height with GnRHa treatment. Compared to the untreated group, long-term treatment with GnRHa on 30 boys effectively slowed bone age growth, resulting in an improvement in FAH (170 ± 6cm vs. 162 ± 4cm; p=0.01) (16). In our meta-analysis, a significant increase in FAH was observed in boys treated with GnRHa monotherapy. GnRHa was effective in increasing the final height with a mean effect of 5.55(95%CI:1.12,9.98). This finding was not consistent with findings from some studies on girls with ISS, which may indicate that gender is an important factor influencing efficacy.
Studies showed that AI or GnRHa monotherapy may improve the FAH in children with ISS. However, there are few studies on the differences between the efficacy of the two compounds in children with ISS. The available data suggest that aromatase inhibitors improved short‐term growth outcomes, which is better than GnRHa alone (29, 30). In order to gain an overall evaluation, we further explored the differences between the two therapies in the studies we included. It is worth noting that there was no statistical difference between the area of SUCRA of GnRHa and AI treatment, although there was a larger area under SUCRA in the GnRHa treatment group. Our study suggested that the two compounds have similar effects in improving FAH. As far as we know, this is to date the first meta-analysis to evaluate the efficacy difference between AI and GnRHa monotherapy in children with ISS.
GnRHas treatment are considered safe (29). GnRHa may affect short-term bone mineral density (BMD) (31), although this seems to be transient (32), and can cause the psychological problem of delaying puberty (33). The long-term safety of aromatase inhibitors in male patients with ISS has not been proven. Previous studies showed that Vertebral morphology was adversely affected. Transient HDL reduction, BMD reduction, and slight insulin sensitivity increase may also occur during AI treatment (17, 32). Therefore, there is more uncertainty about the long-term safety of aromatase inhibitors (34). In our study, we found that either AI or GnRHa can help improve the FAH of ISS in boys. In terms of price and convenicence, AI is preferable, whereas for long-term security, GnRHa is the better choice.
Publication bias in this study was not assessed because the number of included studies was small and the power of funnel plot asymmetry test too low to distinguish real bias. The inconsistence test was conducted, and the results indicated that the results of the direct and indirect therapy comparison for ISS patients were consistent, and PAH/FAH indexes across the included literatures were reliable.
There are several possible limitations to our meta-analysis. First, this study was aimed at the increase of final adult height. The key indices, such as the difference of final adult height SDS, the difference of final height minus PAH (FH-PAH), and the difference of final height minus TH (FH-TH), have not been comprehensively assessed (35). As a result, the conclusions were limited. Second, the population in this study consisted of boys with idiopathic short stature. Further evidence is needed to determine whether the findings can be applied to other short stature conditions and female patients. Third, the number of studies included in each therapy was limited and the sample sizes were small. Furthermore, because relevant studies were insufficient and not every trial documented final adult height, predicted adult height of Hero’s study was used instead for analysis. We have conducted an analysis exluding Hero’s study, nevertheless, and it shows that no heterogeneous source was found for those three papers. Since it is difficult to conduct statistical analysis if that study is excluded, we decided to include it in our research. The above limitation could affect the reliability of the final outcome.Therefore, more high-quality placebo-controlled RCTs and multicenter studies are required in the future to provide greater support for the clinical evidence.
Compared with no treatment, the current evidence indicates that both AI and GnRHa treatment improve the FAH of boys with ISS. However, the sample sizes were small, so more studies are needed to confirm the long-term safety of AI.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
HL: protocol development, manuscript review, and revision; KX: data analysis and manuscript revision; SW: study selection, data collection, and partial drafting; ZW: study selection, data collection, and partial drafting; YC: data analysis and partial drafting; KL: literature search and quality assessment; ZC: literature search and quality assessment; JYZ: data interpretation; JZ: data interpretation. All authors have read and approved the final manuscript.
This work was supported by a grant from the National Natural Science Foundation of China (grant numbers 81974111 to HL).
We would like to sincerely thank all authors who performed eligible studies that were included in the present network meta‐analysis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Pedicelli S, Peschiaroli E, Violi E, Cianfarani S. Controversies in the definition and treatment of idiopathic short stature (ISS). J Clin Res Pediatr Endocrinol (2009) 1(3):105–15. doi: 10.4008/jcrpe.v1i3.53
2. Ranke MB. Towards a consensus on the definition of idiopathic short stature. Horm Res (1996) 45 Suppl 2:64–6. doi: 10.1159/000184851
3. Kumar KVSH, Jayaraman M, Verma A, Modi KD. Letrozole as a booster therapy in growth hormone deficiency. Indian Pediatr (2009) 46(7):625–7.
4. Kreher NC, Eugster EA, Shankar RR. The use of tamoxifen to improve height potential in short pubertal boys. Pediatrics (2005) 116(6):1513–5. doi: 10.1542/peds.2005-0577
5. Wickman S, Sipilä I, Ankarberg-Lindgren C, Norjavaara E, Dunkel L. A specific aromatase inhibitor and potential increase in adult height in boys with delayed puberty: a randomised controlled trial. Lancet (2001) 357(9270):1743–8. doi: 10.1016/S0140-6736(00)04895-9
6. Rothenbuhler A, Linglart A, Bougnères P. A randomized pilot trial of growth hormone with anastrozole versus growth hormone alone, starting at the very end of puberty in adolescents with idiopathic short stature. Int J Pediatr Endocrinol (2015) 2015(1):4. doi: 10.1186/1687-9856-2015-4
7. Mauras N, Ross JL, Gagliardi P, Yu YM, Hossain J, Permuy J, et al. Randomized trial of aromatase inhibitors, growth hormone, or combination in pubertal boys with idiopathic, short stature. J Clin Endocrinol Metab (2016) 101(12):4984–93. doi: 10.1210/jc.2016-2891
8. Hero M, Norjavaara E, Dunkel L. Inhibition of estrogen biosynthesis with a potent aromatase inhibitor increases predicted adult height in boys with idiopathic short stature: a randomized controlled trial. J Clin Endocrinol Metab (2005) 90(12):6396–402. doi: 10.1210/jc.2005-1392
9. Liu J, Yin S, Luo Y, Bai X, Chen S, Yang H, et al. Treatment of short stature with aromatase inhibitors: A systematic review and meta-analysis. Horm Metab Res (2021) 53(6):391–401. doi: 10.1055/a-1492-2841
10. Varimo T, Toiviainen-Salo S, Raivio T, Kerttula L, Dunkel L, Hero M. Letrozole monotherapy in pre- and early-pubertal boys does not increase adult height. Front Endocrinol (Lausanne) (2019) 10:201. doi: 10.3389/fendo.2019.00201
11. Carel JC, Eugster EA, Rogol A, Ghizzoni L, Palmert MR, ESPE-LWPES GnRH Analogs Consensus Conference Group, et al. Consensus statement on the use of gonadotropin-releasing hormone analogs in children. Pediatrics (2009) 123(4):e752–762. doi: 10.1542/peds.2008-1783
12. Bertelloni S, Baroncelli GI, Sorrentino MC, Perri G, Saggese G. Effect of central precocious puberty and gonadotropin-releasing hormone analogue treatment on peak bone mass and final height in females. Eur J Pediatr (1998) 157(5):363–7. doi: 10.1007/s004310050831
13. Li WJ, Gong CX, Guo MJ, Xing J, Li T, Song WH, et al. Efficacy and safety of domestic leuprorelin in girls with idiopathic central precocious puberty: a multicenter, randomized, parallel, controlled trial. Chin Med J (Engl) (2015) 128(10):1314–20. doi: 10.4103/0366-6999.156773
14. Swaiss HH, Khawaja NM, Farahid OH, Batieha AM, Ajlouni KM. Effect of gonadotropin-releasing hormone analogue on final adult height among Jordanian children with precocious puberty. Saudi Med J (2017) 38(11):1101. doi: 10.15537/smj.2017.11.21187
15. Khawaja N, Owaineh H, Batieha A, Frahid O, El-Khateeb M, Ajlouni KM. The effect of gonadotropin-releasing hormone analogue on final adult height in children with idiopathic short stature. Med Princ Pract (2019) 28(6):509–16. doi: 10.1159/000499929
16. Li Y, Du M, Ma H, Chen Q, Chen H, Zhang J. Efficacy of letrozole in treatment of male adolescents with idiopathic short stature. Zhejiang Da Xue Xue Bao Yi Xue Ban (2020) 49(3):308–14. doi: 10.3785/j.issn.1008-9292.2020.04.05
17. Hero M. Aromatase inhibitors in the treatment of short stature. Advanced Therapies Pediatr Endocrinol Diabetol (2016) 30:130–40. doi: 10.1159/000439338
18. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (2009) 339:b2700. doi: 10.1136/bmj.b2700
19. Frindik JP, Baptista J. Adult height in growth hormone deficiency: historical perspective and examples from the national cooperative growth study. Pediatrics (1999) 104(4 Pt 2):1000–4. doi: 10.1542/peds.104.S5.1000
20. Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions: Cochrane book series. 1st ed. Wiley (2008). Available at: https://onlinelibrary.wiley.com/doi/book/10.1002/9781119536604.
21. Trinquart L, Attiche N, Bafeta A, Porcher R, Ravaud P. Uncertainty in treatment rankings: Reanalysis of network meta-analyses of randomized trials. Ann Intern Med (2016) 164(10):666–73. doi: 10.7326/M15-2521
22. Wit JM, Hero M, Nunez SB. Aromatase inhibitors in pediatrics. Nat Rev Endocrinol (2011) 8(3):135–47. doi: 10.1038/nrendo.2011.161
23. Hero M, Wickman S, Dunkel L. Treatment with the aromatase inhibitor letrozole during adolescence increases near-final height in boys with constitutional delay of puberty. Clin Endocrinol (Oxf) (2006) 64(5):510–3. doi: 10.1111/j.1365-2265.2006.02499.x
24. Rohani F, Asadi R, Mirboluk AA, Soheilipour F. Letrozole effect on final height of patients with constitutional delay of growth and puberty. Med Arch (2019) 73(5):307–10. doi: 10.5455/medarh.2019.73.307-310
25. Krebs A, Moske-Eick O, Doerfer J, Roemer-Pergher C, van der Werf-Grohmann N, Schwab KO. Marked increase of final height by long-term aromatase inhibition in a boy with idiopathic short stature. J Pediatr Endocrinol Metab (2012) 25(5–6):581–5. doi: 10.1515/jpem-2011-0435
26. Carel JC, Hay F, Coutant R, Rodrigue D, Chaussain JL. Gonadotropin-releasing hormone agonist treatment of girls with constitutional short stature and normal pubertal development. J Clin Endocrinol Metab (1996) 81(9):3318–22. doi: 10.1210/jcem.81.9.8784090
27. Bouvattier C, Coste J, Rodrigue D, Teinturier C, Carel JC, Chaussain JL, et al. Lack of effect of GnRH agonists on final height in girls with advanced puberty: a randomized long-term pilot study. J Clin Endocrinol Metab (1999) 84(10):3575–8. doi: 10.1210/jcem.84.10.6032
28. Cassio A, Cacciari E, Balsamo A, Bal M, Tassinari D. Randomised trial of LHRH analogue treatment on final height in girls with onset of puberty aged 7.5-8.5 years. Arch Dis Child (1999) 81(4):329–32. doi: 10.1136/adc.81.4.329
29. Bangalore Krishna K, Fuqua JS, Rogol AD, Klein KO, Popovic J, Houk CP, et al. Use of gonadotropin-releasing hormone analogs in children: Update by an international consortium. Horm Res Paediatr (2019) 91(6):357–72. doi: 10.1159/000501336
30. McGrath N, O’Grady MJ. Aromatase inhibitors for short stature in male children and adolescents. Cochrane Database Syst Rev (2015) 2015(10):CD010888. doi: 10.1002/14651858.CD010888.pub2
31. Yanovski JA, Rose SR, Municchi G, Pescovitz OH, Hill SC, Cassorla FG, et al. Treatment with a luteinizing hormone-releasing hormone agonist in adolescents with short stature. N Engl J Med (2003) 348(10):908–17. doi: 10.1056/NEJMoa013555
32. Mericq V, Gajardo H, Eggers M, Avila A, Cassorla F. Effects of treatment with GH alone or in combination with LHRH analog on bone mineral density in pubertal GH-deficient patients. J Clin Endocrinol Metab (2002) 87(1):84–9. doi: 10.1210/jcem.87.1.8148
33. Mazur T, Clopper RR. Pubertal disorders. psychology and clinical management. Endocrinol Metab Clin North Am (1991) 20(1):211–30. doi: 10.1016/S0889-8529(18)30289-5
34. Wit JM. Should skeletal maturation be manipulated for extra height gain? Front Endocrinol (Lausanne) (2021) 12:812196. doi: 10.3389/fendo.2021.812196
Keywords: idiopathic short stature, aromatase inhibitors, gonadotropin-releasing hormone analogue, final height, network meta-analysis
Citation: Wang S, Wu Z, Chen Y, Luo K, Cui Z, Zhang J, Zheng J, Xiao K and Li H (2023) Comparative efficacy of aromatase inhibitors and gonadotropin-releasing hormone analogue in increasing final height of idiopathic short stature boys: a network meta-analysis. Front. Endocrinol. 14:1167351. doi: 10.3389/fendo.2023.1167351
Received: 16 February 2023; Accepted: 27 March 2023;
Published: 14 April 2023.
Edited by:
Maurizio Delvecchio, Giovanni XXIII Children’s Hospital, ItalyReviewed by:
Carla Bizzarri, Bambino Gesù Children’s Hospital (IRCCS), ItalyCopyright © 2023 Wang, Wu, Chen, Luo, Cui, Zhang, Zheng, Xiao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huiqing Li, bGhxaW5nNUAxMjYuY29t; Kangli Xiao, ODcyODczNjA1QHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.