- 1Division of Cardiology, Department of Internal Medicine, E-Da Hospital, I-Shou University, Kaohsiung, Taiwan
- 2School of Medicine, College of Medicine, I-Shou University, Kaohsiung, Taiwan
- 3Division of Cardiology, Department of Internal Medicine, E-Da Cancer Hospital, I-Shou University, Kaohsiung, Taiwan
- 4School of Medicine for International Students, College of Medicine, I-Shou University, Kaohsiung, Taiwan
- 5Department of Emergency, E-Da Hospital, I-Shou University, Kaohsiung, Taiwan
- 6Division of Cardiology, Department of Internal Medicine, E-Da Dachang Hospital, I-Shou University, Kaohsiung, Taiwan
- 7Division of General Neurology, Neurological Institute, Taipei Veterans General Hospital, Taipei, Taiwan
- 8Department Head, Lee’s Endocrinologic Clinic, Pingtung, Taiwan
- 9Division of Cardiology, Department of Internal Medicine, Taipei Veterans General Hospital, Yuli Branch, Hualien, Taiwan
- 10Faculty of Medicine, School of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan
Introduction: The prevalence of cardiovascular disease (CVD) and CVD-related deaths in patients with schizophrenia is high. An elevated risk of CVD has been associated with low heart rate variability (HRV). There is increasing evidence that fatty acid-binding protein (FABP)3 and FABP4 play roles in the development and progression of CVD. This study aimed to explore the association of circulating FABP3/FABP4 levels with HRV in patients with chronic schizophrenia.
Methods: We included 265 consecutive patients with chronic schizophrenia who attended a disease management program. We used an enzyme-linked immunosorbent assay for the measurement of plasma concentrations of FABP3 and FABP4. Standard HRV was recorded at baseline following a standard protocol. Mean high- and low-frequency (HF/LF) HRV values were analyzed by tertile of FABP3 and FABP4 using one-way analysis of variance, and linear regression analysis was performed to assess trends.
Results: A positive association between FABP3 and creatinine was found in multiple regression analysis. In addition, negative associations between levels of hematocrit, hemoglobin, HF HRV, and estimated glomerular filtration rate (eGFR) with FABP3 were also found. Moreover, positive associations between FABP4 with body mass index, diabetes mellitus, hypertension, systolic blood pressure, low-density lipoprotein-cholesterol, triglycerides, creatinine, and FABP3 were found. Furthermore, negative associations between levels of high-density lipoprotein-cholesterol, eGFR, and HF HRV with FABP4 were found. We also found a significant inverse association between FABP3 and HF HRV (p for trend = 0.008), and significant inverse associations between FABP4 with HF and LF HRV (p for trend = 0.007 and 0.017, respectively).
Discussion: Together, this suggests that elevated levels of FABP3 and FABP4 may be linked to health problems related to CVD in patients with chronic schizophrenia.
Introduction
The cardiac biomarker fatty acid-binding protein 3 (FABP3) has been used as a diagnostic tool for acute myocardial infarction (AMI) and non-ST-elevation myocardial infarction (1). It is low-molecular weight (15 kDa) cytosolic protein specific to cardiomyocytes, and it represents 5 to 15% of all cardiomyocyte-related cytosolic proteins (2). In the mitochondria, FABP3 has been shown to be involved in the intracellular transport of fatty acids for β-oxidation (3). Moreover, associations between FABP3 and other clinical parameters have also been reported in AMI patients, including high-sensitive troponin T, N-terminal pro-brain natriuretic peptide, ejection fraction, C-reactive protein (CRP), leucocytes, and length of hospital stay (4). FABP3 has been reported as a useful marker of both myocardial damage and also to induce inflammation along with vascular smooth muscle cell growth and migration (5). FABP4 is mostly expressed in macrophages and adipocytes, and it has been strongly associated with the development of insulin resistance and atherosclerosis in association with low-grade inflammation (6). FABP4 may act as an adipokine, and its release from adipocytes has been shown to be through a non-classical pathway related to lipolysis (7), which is mainly activated by catecholamines during activation of sympathetic nervous system. An increase in circulating FABP4 levels has been associated with hypertension, diabetes mellitus, insulin resistance, obesity, atherosclerosis, cardiovascular events and cardiac dysfunction (6, 8).
Heart rate variability (HRV) is defined as fluctuations in the time between heartbeats. HRV has been used to assess autonomic function, with low HRV indicating impaired autonomic function (9). Therefore, HRV can be a useful measure of cardiovascular health and stress (10). HRV decreases with age (11) and it has been associated with cognitive decline (12), while an increase in HRV has been associated with longevity and healthy aging (13). Consequently, HRV can be used to assess both physiological and neurological status. Patients with schizophrenia have been shown to have low HRV (14), and a low HRV has also been associated with a worse severity of symptoms and cognitive test scores, diabetes mellitus (15), ongoing subclinical inflammation (16), and higher risks of cardiovascular disease (CVD)-related morbidity and mortality (17, 18), and poorer quality of life (19, 20). Hence, investigating risk factors associated with reduced HRV in patients with schizophrenia is important.
Increasing evidence suggests that FABP3 and FABP4 may play roles in the progression and development of CVD (5, 6, 8). Furthermore, our previous studies found that plasma FABP3 could be used as a surrogate for reduced ejection fraction and abnormal corrected QT (QTc) interval, and that in patients with stable angina (21), plasma FABP4 level may also be associated with an abnormal QTc interval (22). However, few studies have investigated the associations of FABP3 and FABP4 levels with HRV. Therefore, the aim of this study was to examine whether FABP3 and FABP4 were associated with HRV in a cohort of patients with chronic schizophrenia.
Methods and materials
Participants
This cross-sectional study included 265 patients with chronic schizophrenia with stable status from inpatient wards at Taipei Veterans General Hospital, Yuli Branch between December 02, 2021 to December 01, 2022. The Diagnostic and Statistical Manual of Mental Disorders IV was used to confirm the diagnosis of schizophrenia. We excluded those with a major affective disorder (including mania, major depression, bipolar disorder and schizoaffective disorder), acute physical illnesses and fever. In addition, we excluded those who received steroid, antipyretic, antibiotic, or anti-inflammatory agent therapy. Before enrollment, associations among plasma levels of FABP3, FABP4, and CRP with sociodemographic characteristics were evaluated. All of the enrolled patients provided written informed consent. The Human Research Ethics Committee of Kaohsiung E-Da Hospital approved this study which was conducted following the Declaration of Helsinki.
Sociodemographic characteristics including sex, age, mental status, smoking, drinking, betel quid chewing, weight, height, and body mass index (BMI, kg/m2) were recorded. Each patient underwent neurological and physical examinations.
Laboratory measurements
Blood samples were collected after fasting for a minimum of 8 hours. Serum uric acid and complete blood count were measured using standard methods with an automatic biochemical analysis system (Hitachi 7170A, Tokyo, Japan) as reported previously (23). In addition, serum creatinine was measured using the Jaffe method. The same system was used for lipid measurements, [including plasma triglycerides, total cholesterol, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C)], as well as glucose and glycated hemoglobin (HbA1c). Peripheral leukocyte count was measured using an automated cell counter (XE-2100 Hematology Alpha Transportation System, Sysmex Corporation, Kobe, Japan). Renal function (estimated glomerular filtration rate, eGFR) was calculated using the CKD-EPI two-concentration race equation as reported previously (24). Enzyme-linked immunosorbent assay kits were used to measure the concentrations of plasma FABP3 and FABP4 (Invitrogen, Thermo Fisher Scientific Inc., USA and R&D Systems Inc., Minneapolis, MN, USA), with intra- and inter-assay coefficients of variation of 3.4% to 5.8% (n = 3) and 3.1% to 6.2% (n = 4), respectively, for FABP4, and 3.9% (n = 8) and 6.2% (n = 8), respectively, for FABP3. Plasma levels of high-sensitivity CRP were measured using a chemistry analyzer (IMMAGE, Beckman Coulter, Brea, CA, USA); the intra-assay coefficient of variation ranged from 4.2% to 8.7%, and the detection limit was 0.2 mg/L. All measurements were performed in duplicate in a single experiment.
Definitions
Patients with hypertension were classified as those with systolic/diastolic blood pressures (SBP/DBP) ≥140/90 mmHg, or prescriptions for antihypertension therapy. Patients with diabetes mellitus were classified as those with HbA1c ≥6.5% (48 mmol/mol), a fasting blood glucose level ≥126 mg/dL (7.0 mmol/L), 2-hour blood glucose ≥200 mg/dL (11.1 mmol/L) according to the American Diabetes Association 2016 Guidelines (25), or receiving antidiabetic therapy. Hyperlipidemia was defined as triglycerides >180 mg/dl, and/or LDL-C >130 mg/dl, and/or total cholesterol >200 mg/dl, and/or HDL-C level <40 mg/dl, or receiving lipid disorder treatment.
HRV assessment
The 5-minute HRV are reported according to the Reporting Articles on Psychiatry and HRV Guidelines and the procedure was based on the standard method and has been described previously (26–29). An HRV analyzer (SS101; Enjoy Research Inc., Hualien, Taiwan) was used for acquisition, storage, and processing of ECG signals. All patients are in a lying (supine) position during examination and are breathing spontaneously during ECG recording. In brief, all the signals were recorded by an 8-bit analog-to-digital converter with a sampling rate of 512 Hz. The digitized ECG signals were analyzed and stored immediately for verification. The computer algorithm identified each QRS complex and rejected each abnormal signal (such as ventricular premature complex or noise). Stationary R-R interval values were resampled and interpolated at a rate of 7.11 Hz for producing continuity in the time domain. This interpolation produced 2048 data points over 288 seconds. Through Fourier transformation, the power spectrum was then quantified into the frequency-domain measurements, which were defined in previous studies (27, 28). The frequency domain indexes included total power (TP), very low-frequency power (VLF; 0.003-0.04 Hz), low-frequency power (LF; 0.04-0.15 Hz), high-frequency power (HF; 0.15-0.40 Hz), LF/HF, and normalized LF (LF%). LF% was defined as LF/(TP − VLF) × 100. For correcting the skewed distributions, TP, VLF, LF, HF, and LF/HF were logarithmically transformed. HF is a parasympathetic index, whereas LF represent sympathetic function. Here, we focused on distributions of major heart rate oscillations in HF and LF bands according to previous studies (30–32).
Statistical analysis
Data normality was evaluated using the Kolmogorov-Smirnov test. Normally distributed continuous variables are shown as mean ± SD and compared using the unpaired Student’s t-test, and non-normally distributed continuous variables are shown as median (interquartile range). Categorical variables are shown as number (%), and compared using the chi-square test. As high-sensitivity CRP, FABP3, FABP4, and HRV had skewed distribution, logarithmically transformed values were used in the analysis. Associations were examined between plasma FABP4 and FABP3 and other variables using simple and multiple linear regression analyses. Mean HRV values for FABP3 and FABP4 tertiles were obtained using one-way analysis of variance for heterogeneity. P-values for trends were obtained from linear regression analysis. A p-value <0.05 was considered statistically significant. The analyses were performed with JMP version 7.0 for Windows (SAS Institute, Cary, NC, USA).
Results
Characteristics of the patients by sex
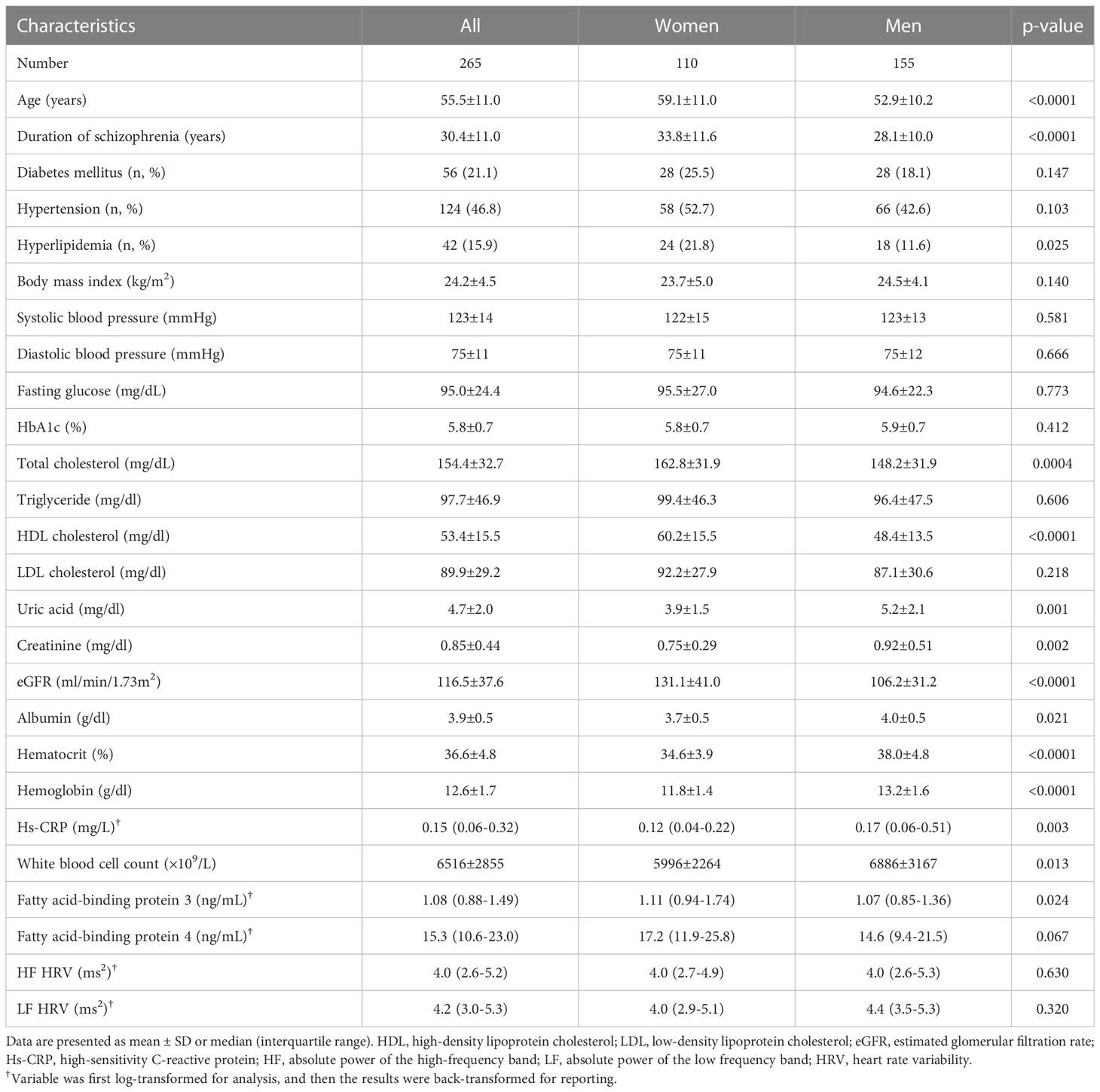
The mean (± SD) age of the patients was 55.5 ± 11.0 years (range 26-83 years), 155 were male (58.5%) and 110 were female (41.5%) (Table 1). the female patients had a longer duration of schizophrenia; higher levels of hyperlipidemia, total cholesterol, HDL-C, eGFR, and FABP3, and lower levels of uric acid, creatinine, albumin, hematocrit, hemoglobin, and high-sensitivity CRP, white blood cell count and were older than the male patients. However, HF HRV and LF HRV did not differ between female patients and male patients (Table 1). Furthermore, when we have calculated the HF HRV and LF HRV by age groups (patients >50 years and patients ≤50 years), we found that HF HRV and LF HRV did not differ between patients with >50 years and ≤50 years (3.9 ± 1.8 ms2 vs. 3.9 ± 2.2 ms2, p=0.963 for HF HRV, and 4.1 ± 1.7 ms2 vs. 4.2 ± 2.0 ms2, p=0.604 for LF HRV). Moreover, when we have calculated the HF HRV and LF HRV by with and without antihypertensive drugs, we found that HF HRV and LF HRV did not differ between patients with and without antihypertensive drugs (3.7 ± 1.7 ms2 vs. 4.1 ± 2.1 ms2, p=0.127 for HF HRV, and 4.0 ± 1.6 ms2 vs. 4.3 ± 1.9 ms2, p=0.116 for LF HRV). In addition, in the present study, there were 83 (31.3%) and 182 (68.7%) patients received typical antipsychotics and atypical antipsychotics. When calculated the HF HRV and LF HRV by antipsychotic treatment (typical antipsychotics and atypical antipsychotics), we found that HF HRV and LF HRV did not differ between patients received typical antipsychotics and atypical antipsychotics (3.7 ± 2.2 ms2 vs. 4.0 ± 1.8 ms2, p=0.348 for HF HRV, and 3.9 ± 2.0 ms2 vs. 4.3 ± 1.6 ms2, p=0.116 for LF HRV) (data not shown). These results may exclude the confounding effect of age, sex, drug of antihypertensive, and antipsychotic treatment on the HRV data of present study.
Relationships among FABP3 and FABP4 with the other variables
The results of simple linear regression analysis showed that FABP3 was positively associated with age, duration of schizophrenia, hypertension, and creatinine (Table 2). In addition, sex, eGFR, hematocrit, hemoglobin, and HF HRV were negatively associated with levels of FABP3. Furthermore, FABP4 was positively associated with age, BMI, diabetes mellitus, hypertension, hyperlipidemia, SBP, LDL-C, triglycerides, creatinine, and FABP3. In addition, we found negative associations between eGFR, hematocrit, hemoglobin, and HF HRV with FABP4 level.
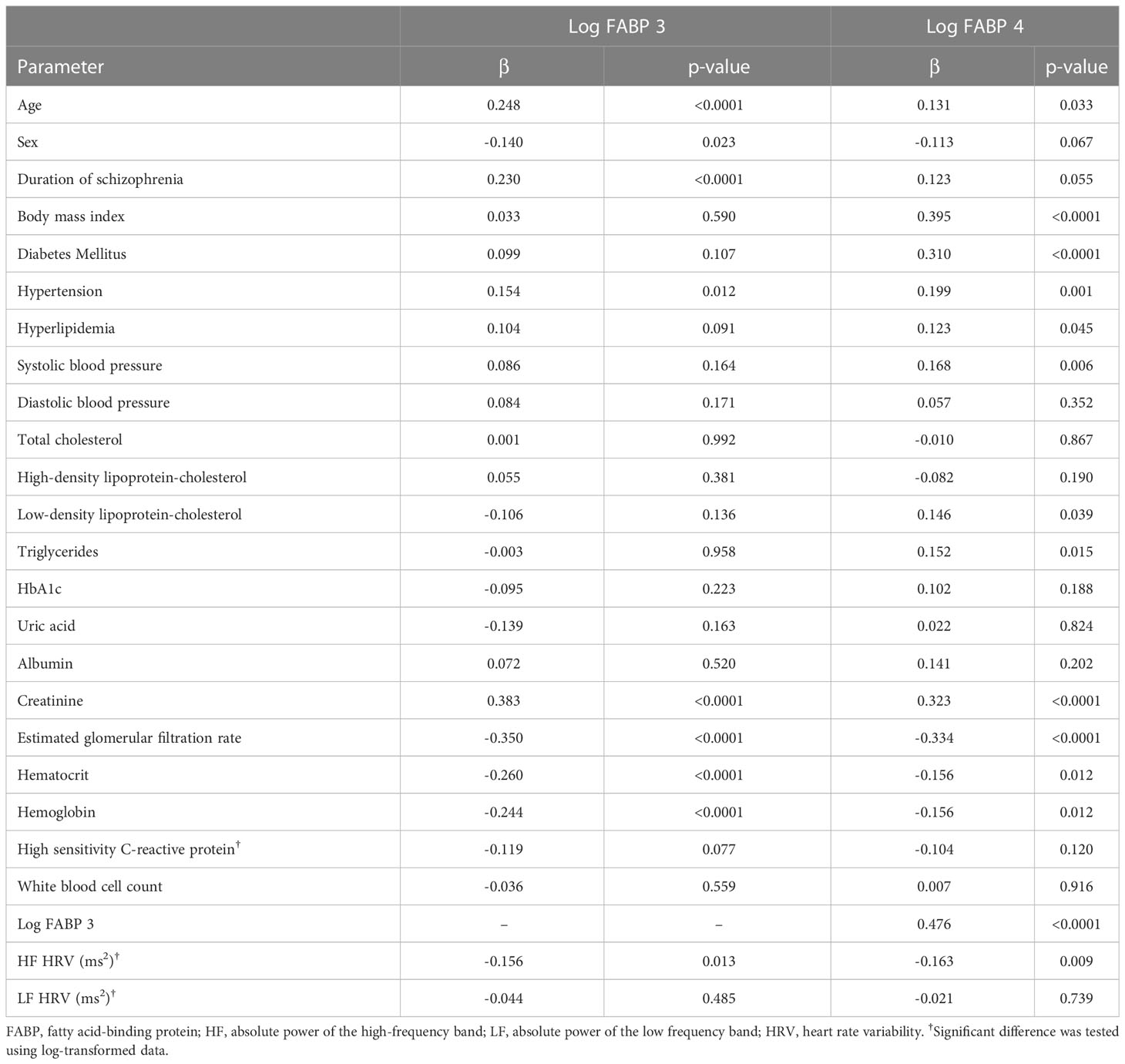
Table 2 Simple linear regression analysis between fatty acid-binding protein 3, fatty acid-binding protein 4 and other parameters.
After adjusting for age and sex, the results of multiple linear regression analysis showed a positive association between FABP3 and creatinine. In addition, levels of eGFR, hematocrit, hemoglobin, and HF HRV were negatively associated with levels of FABP3. Furthermore, a sex and age-adjusted multiple linear regression model revealed a positive association between FABP4 with BMI, diabetes mellitus, hypertension, SBP, LDL-C, triglycerides, creatinine, and FABP3. Moreover, we found negative associations between levels of HDL-C, eGFR, and HF HRV with FABP4 level (Table 3).
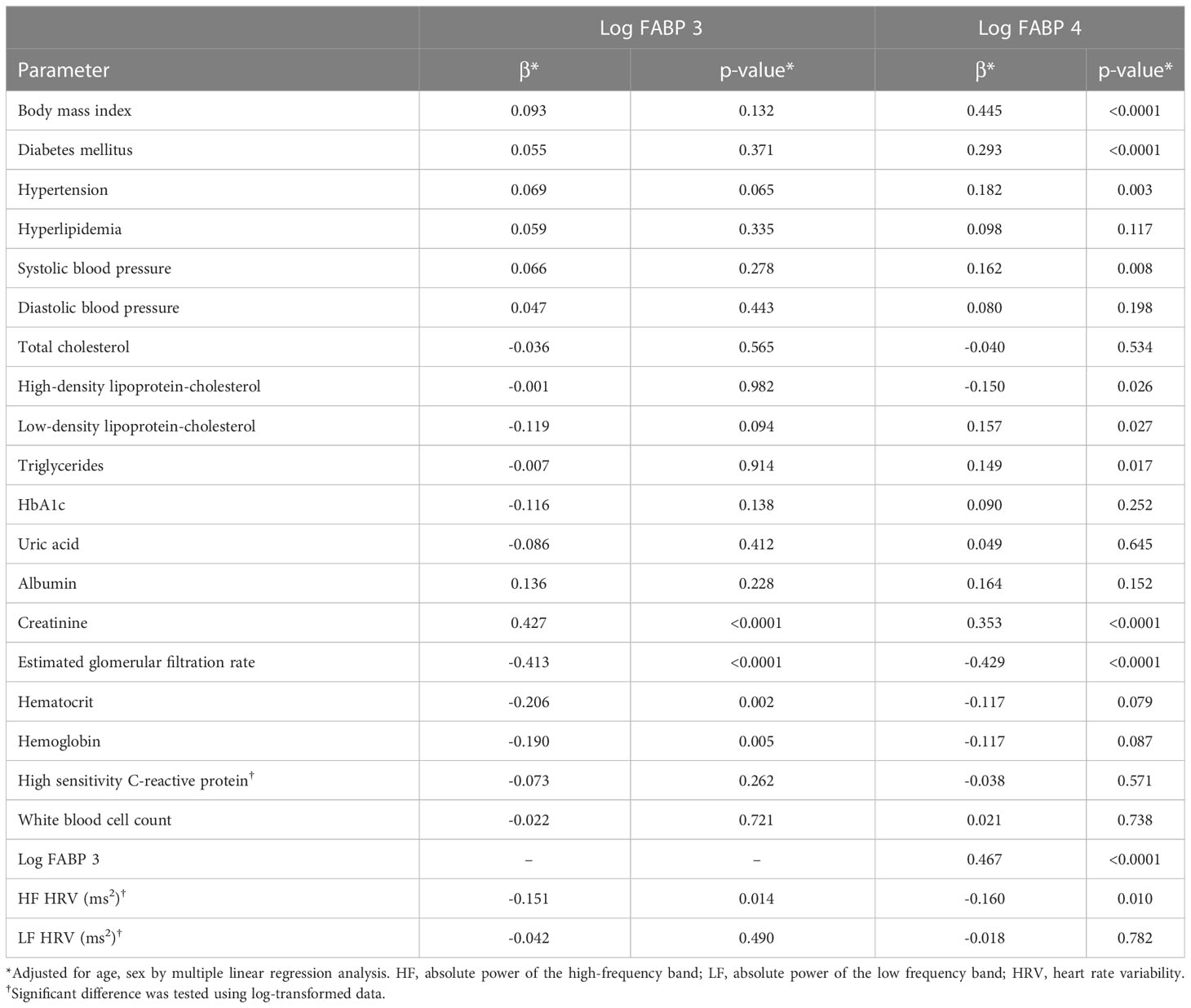
Table 3 Multiple linear regression analysis for fatty acid-binding protein 3, fatty acid-binding protein 4, serum biomarkers, and heart rate variability parameters.
HRV across tertiles of FABP3 and FABP4
In Table 4, the mean values and standard deviation of both LF and HF HRV are compared in the FABP3 and FABP4 tertiles. A significant and inverse association was found between FABP3 with HF HRV (p for trend = 0.008), and significant and inverse associations were found between FABP4 with both HF and LF HRV (p for trend = 0.007 and 0.017, respectively).
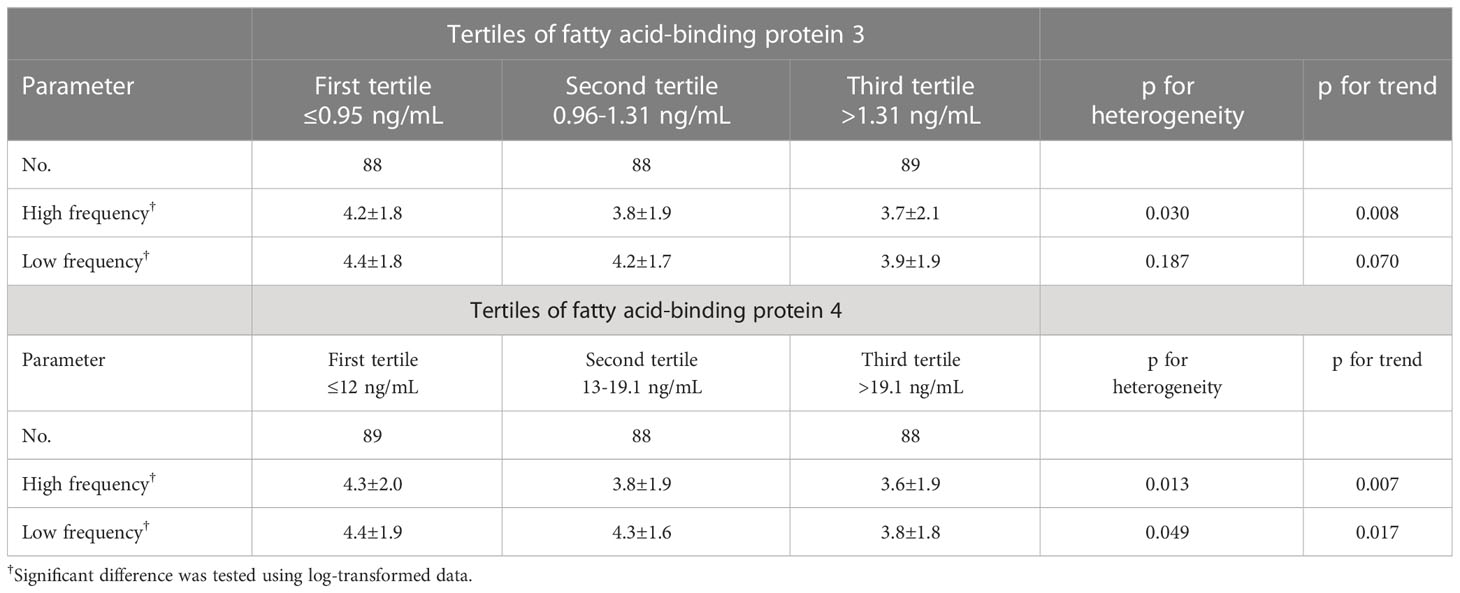
Table 4 Mean values and standard Deviation (SD) of heart rate variability across tertiles of fatty acid-binding protein 3 and fatty acid-binding protein 4.
Discussion
In this study, we found a positive association between plasma FABP3 with creatinine, and negative associations between plasma FABP3 with eGFR, hematocrit, hemoglobin, and HF HRV after adjusting for sex and age. Moreover, we found positive associations between plasma FABP4 with BMI, diabetes mellitus, hypertension, SBP, LDL-C, triglycerides, creatinine, and FABP3, and negative associations between plasma FABP4 with HDL-C, eGFR, and HF HRV. Furthermore, a significant and inverse association was found between an increased concentration of plasma FABP3 with HF HRV (p for trend = 0.008), and significant and inverse associations were found between an increased concentration of plasma FABP4 with both HF and LF HRV (p for trend = 0.007 and 0.017, respectively). To the best of our knowledge, no previous investigation has reported relationships among FABP3 and FABP4 with HRV in patients with chronic schizophrenia. In the brain, FABP3 is expressed in neural stem/progenitor cells, neural progenitor cells and mature neurons (33). A previous study demonstrated that changes in the level of FABP3 in the brain may represent a disease mechanism in some patients with schizophrenia and autism spectrum disorder (34). Furthermore, as FABP3 binds and transports fatty acids, changes in FABP3 level could have an effect on various biological processes (35). Moreover, Maekawa et al. found that FABP4 knockout mice had changes in fatty acid composition in the cortex, and suggested that an ‘adipo-brain axis’ may underlie the pathophysiology of autism spectrum disorder, and that FABP4 could potentially be used as a biomarker (36). However, the biological mechanisms by which FABP3 and FABP4 are involved in the pathogenesis of low HRV are not well understood.
We previously found independent associations between a high level of FABP3 with plasma visfatin, high-sensitivity CRP, and white blood cell count, suggesting that the role of FABP3 in the pathophysiology of reduced ejection fraction in patients with stable angina may be through inflammatory processes (21). Furthermore, Hotamisligil et al. showed that FABP4 plays a role in many biological processes in addition to lipid metabolism regulation, including inflammation (37). FABP4 has been demonstrated to play a role in the pathogenesis of diseases mediated by inflammation, and FABP4 downregulation has been shown to reduce inflammation, oxidative stress and apoptosis (6, 38–40). In this study, we found that FABP3 and FABP4 were inversely associated with both HF and LF HRV (Table 4). Cooper et al. reported inverse associations between LF HRV with CRP, fibrinogen, and interleukin-6 (IL6), and inverse associations between HF HRV with CRP and fibrinogen, supporting the presence of a vagal anti-inflammation pathway (41). Furthermore, Alen et al. reported that HF HRV was strongly inversely associated with CRP, IL6 and fibrinogen, and that LF HRV was strongly inversely associated with IL6 and CRP, with or without adjustments for covariates. These findings are consistent with a cholinergic anti-inflammatory pathway, and suggest that parasympathetic modulation of inflammation through vagus nerves may affect certain inflammatory molecules more than others (42). In addition, Haensel et al. reported an inverse correlation between HRV with inflammatory markers in both healthy individuals and those with CVDs (16). These results may explain why high plasma FABP3 and FABP4 levels were inversely associated with HRV in our patients with chronic schizophrenia.
HRV can be used to noninvasively assess autonomic nervous system activity (43). A low HRV indicates a reduction in parasympathetic cardiac control, which has been linked to diabetes mellitus (44), sleep problems, and problems regulating emotional responses (45). Hence, HRV could be considered as a biomarker of general health and stress (46). FABP3 has been shown to be able to reliably diagnose AMI soon after symptom onset (47), and also to confirm or exclude the diagnoses of acute coronary syndromes. In addition, previous studies have shown that FABP3 may be useful to detect other conditions such as pulmonary thromboembolism, sepsis and congestive cardiac failure (48–50). In addition, increased serum levels of FABP3 have been reported in patients with metabolic syndrome and non-alcoholic fatty liver disease (51, 52), suggesting that FABP3 may serve as a biomarker of insulin resistance and subclinical myocardial damage. These findings highlight the role of FABP3 in regulating the autonomic nervous system with regards to cardiac and adipose tissue. Our results support (48–52) that high plasma FABP3 may be altered HRV through the autonomic nervous system regulation.
With regards to FABP4, evaluated levels of circulating FABP4 have been associated with arterial hypertension, type 2 diabetes mellitus, insulin resistance, obesity, atherosclerosis, cardiovascular disorders, abnormal QTc interval, kidney damage, cardiac dysfunction, and fatty liver disease (8, 22, 53–55). Our results showed significant and inverse associations between an increased concentration of plasma FABP4 with both HF and LF HRV (p for trend = 0.007 and 0.017, respectively); furthermore, in multiple linear regression model, we found that FABP4 was positively associated with BMI, diabetes mellitus, hypertension, SBP, LDL-C, triglycerides, creatinine, and FABP3. Moreover, we found negative associations between levels of eGFR, HDL-C and HF HRV with FABP4. Previous studies have reported increased concentrations of FABP4 in obese individuals, as well as positive correlations with insulin resistance, blood pressure, and waist circumference (56). Other studies have reported inverse associations between obesity and weight gain with changes in HRV (57–59). Various factors can influence HRV in obese individuals, including comorbidities, genetics, diet, emotional stress, and physical activity (60–63). Furthermore, type 2 diabetes mellitus has been associated with an overall decrease in HRV. Decreases in parasympathetic and sympathetic activity could be explained by changes in glucose metabolism having an adverse effect on HRV, resulting in cardiac autonomic neuropathy (64). Moreover, Liao et al. found that metabolic syndrome was associated with lower HRV (65). In addition, Chou et al. reported that the prevalence of autonomic dysfunction as assessed by HRV differed among patients with different stages of chronic kidney disease was different. Most patients with advanced chronic kidney disease have lower HRV (66). Drawz et al. also reported an association between lower HRV with a lack of exercise, heart failure, low eGFR, older age, and elevated phosphorus and HbA1c (67). Therefore, it is possible that FABP4 may be associated with obesity, chronic kidney disease, diabetes mellitus and metabolic syndrome, thereby contributing to lower HRV in patients with chronic schizophrenia.
Limitations
There are several limitations in our study. First, concerning the effect of existence of peripheral neuropathy in diabetics that would reduce HRV, as our study patients with chronic schizophrenia may be unable to communicate well during the sensory nerve function examination, we did not determine the relationship between the diabetic sensory and motor neuropathy with HRV. Although diabetics peripheral neuropathy of sensory nerve always noted in older diabetic patients and might relative the oscillation changes of heart rate (68). The median age of the patients were 56 years (interquartile range, 48-64 years) in the present study, and previous study showed the change of autonomic function is earlier than the impaired of diabetic sensory and motor function (68). Hence, we believe the diabetics peripheral neuropathy might not be significant confounder to affect the HRV data of present study. Second, we did not compare our results with healthy population is because the antipsychotic or psychotherapy theoretically will influence HRV measurement. It is hard to eliminate these interventional effects while compared the HRV result of patient with schizophrenia to with normal population. However, this limit the applicability of our results to normal healthy population and other groups. Third, HRV is also strongly influenced by stress and emotional change. To eliminate the influence of the schizophrenia symptom to our result as possible as we can, all patients in this study are selected from the chronic psychiatric ward with relative stable status of schizophrenia, they are in relative calm condition with no significant negative or positive symptom, and more willing to receive the HRV examination. We believe this population is a good candidate to investigate the risk factor associated with cardiovascular disease in patients with schizophrenia. Fourth, the posture and breathing of examination also influences the HRV result. Although there are several protocols available in HRV measurements, in the present study, HRV were measured by using the short-term assessment of HRV. We performed the test at resting supine position with normal spontaneous breathing in this study. Because this is the only way, we could ensure all of our patients performed the test in same condition. Previous study showed that short-term HRV measurement was more reliable at rest than at tilt or pharmacological stimulation (69). In the other hand, HRV showed overall more reliable during spontaneous breathing especially in chronic obstructive pulmonary disease population (70), which similar with patients with schizophrenia, easy influenced by stress and emotional change. Other limitations of this study also include the cross-sectional design, which hinders the ability to examine causal relationships between increased plasma FABP3/FABP4 levels with lower HRV. Long-term follow-up studies are warranted to investigate the association of FABP3 and FABP4 with lower HRV further. In addition, the study cohort was relatively small. Cross-sectional studies are useful in determining prevalence, however they do not permit robust comparisons. Further studies with larger multi-ethnic cohorts should be conducted to explore the associations found in this study. Finally, further studies are needed to assess whether elevated FABP3 and FABP4 levels are associated with lower HRV.
In summary, we found an inverse association between FABP3 with HF HRV, and inverse associations between FABP4 with both LF and HF HRV in patients with chronic schizophrenia. Increased FABP3 and FABP4 levels may be associated with CVD-related health problems in patients with chronic schizophrenia. Our results may imply that regular monitoring of HRV is warranted in patients with chronic schizophrenia to identify the distribution of the major heart rate oscillations (the LF and HF bands), and that reduced HRV is associated with an increased risk of CVD morbidity and mortality. The benefits of improving low HRV in patients with chronic schizophrenia may also extend to improving cardiovascular health.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by The Human Research Ethics Committee of Kaohsiung E-Da Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
All authors contributed to this study. W-CH and W-HT conceived and designed the study. W-CH, Y-JL, and W-HT provided the methodology. F-MC performed the formal analysis, and project administration. W-CH and W-HT validated the data. T-HY, C-CW, T-LL, I-TT, C-FH, C-YC, and W-HT performed the investigation, resources, and data curation. T-HY, C-CW, T-LL, I-TT, C-FH, C-YC, and W-HT prepared the manuscript. W-CH, T-HY, C-CW, T-LL, I-TT, C-FH, C-YC, Y-JL, and W-HT reviewed and edited the manuscript. W-CH, Y-JL, and W-HT performed the visualization. W-HT performed the supervision and funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from Taipei Veterans General Hospital, Yuli Branch and E-Da Hospital of the Republic of China, Taiwan (contract no. VHYL111-002 and EDAHI111001).
Acknowledgments
We thank the staff and members of the psychiatry care teams for their assistance in various measurements and other organizational aspects of this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gerede DM, Güleç S, Kiliçkap M, Kaya CT, Vurgun VK, Özcan ÖU, et al. Comparison of a qualitative measurement of heart-type fatty acid-binding protein with other cardiac markers as an early diagnostic marker in the diagnosis of non-ST-segment elevation myocardial infarction. Cardiovasc J Afr. (2015) 26:204–9. doi: 10.5830/CVJA-2015-028
2. Furuhashi M, Hotamisligil GS. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discovery (2008) 7:489–503. doi: 10.1038/nrd2589
3. Schaap FG, Binas B, Danneberg H, van der Vusse GJ, Glatz JF. Impaired long-chain fatty acid utilization by cardiac myocytes isolated from mice lacking the heart-type fatty acid binding protein gene. Circ Res (1999) 85:329–37. doi: 10.1161/01.RES.85.4.329
4. Schernthaner C, Lichtenauer M, Wernly B, Paar V, Pistulli R, Rohm I, et al. Multibiomarker analysis in patients with acute myocardial infarction. Eur J Clin Invest. (2017) 47:638–48. doi: 10.1111/eci.12785
5. Chen K, Chen QJ, Wang LJ, Liu ZH, Zhang Q, Yang K, et al. Increment of HFABP level in coronary artery in-stent restenosis segments in diabetic and nondiabetic minipigs: HFABP overexpression promotes multiple pathway-related inflammation, growth and migration in human vascular smooth muscle cells. J Vasc Res (2016) 53:27–38. doi: 10.1159/000446652
6. Furuhashi M. Fatty acid-binding protein 4 in cardiovascular and metabolic diseases. J Atheroscler Thromb (2019) 26:216–32. doi: 10.5551/jat.48710
7. Spiegelman BM, Frank M, Green H. Molecular cloning of mRNA from 3T3 adipocytes. regulation of mRNA content for glycerophosphate dehydrogenase and other differentiation-dependent proteins during adipocyte development. J Biol Chem (1983) 258:10083–9. doi: 10.1016/S0021-9258(17)44608-4
8. Furuhashi M, Saitoh S, Shimamoto K, Miura T. Fatty acid-binding protein 4 (FABP4): pathophysiological insights and potent clinical biomarker of metabolic and cardiovascular diseases. Clin Med Insights Cardiol (2015) 8(Suppl 3):23–33. doi: 10.4137/CMC.S17067
9. Pomeranz B, Macaulay RJ, Caudill MA, Kutz I, Adam D, Gordon D, et al. Assessment of autonomic function in humans by heart rate spectral analysis. Am J Physiol Circ Physiol (1985) 248:H151–H3. doi: 10.1152/ajpheart.1985.248.1.H151
10. de Looff PC, Cornet LJM, Embregts PJCM, Nijman HLI, Didden HCM. Associations of sympathetic and parasympathetic activity in job stress and burnout: a systematic review. PloS One (2018) 13:e0205741. doi: 10.1371/journal.pone.0205741
11. De Meersman RE, Stein PK. Vagal modulation and aging. Biol Psychol (2007) 74:165–73. doi: 10.1016/j.biopsycho.2006.04.008
12. Mahinrad S, Jukema JW, van Heemst D, Macfarlane PW, Clark EN, de Craen AJM, et al. 10-second heart rate variability and cognitive function in old age. Neurology (2016) 86:1120–7. doi: 10.1212/WNL.0000000000002499
13. Zulfiqar U, Jurivich DA, Gao W, Singer DH. Relation of high heart rate variability to healthy longevity. Am J Cardiol (2010) 105:1181–5. doi: 10.1016/j.amjcard.2009.12.022
14. Montaquila JM, Trachik BJ, Bedwell JS. Heart rate variability and vagal tone in schizophrenia: a review. J Psychiatr Res (2015) 69:57–66. doi: 10.1016/j.jpsychires.2015.07.025
15. Malpas SC, Maling TJ. Heart-rate variability and cardiac autonomic function in diabetes. Diabetes (1990) 39:1177–81. doi: 10.2337/diab.39.10.1177
16. Haensel A, Mills PJ, Nelesen RA, Ziegler MG, Dimsdale JE. The relationship between heart rate variability and inflammatory markers in cardiovascular diseases. Psychoneuroendocrinology (2008) 33:1305–12. doi: 10.1016/j.psyneuen.2008.08.007
17. Fang SC, Wu YL, Tsai PS. Heart rate variability and risk of all-cause death and cardiovascular events in patients with cardiovascular disease: a meta-analysis of cohort studies. Biol Res Nurs. (2020) 22:45–56. doi: 10.1177/1099800419877442
18. Sessa F, Anna V, Messina G, Cibelli G, Monda V, Marsala G, et al. Heart rate variability as predictive factor for sudden cardiac death. Aging (Albany NY). (2018) 10:166–77. doi: 10.18632/aging.101386
19. Stogios N, Gdanski A, Gerretsen P, Chintoh AF, Graff-Guerrero A, Rajji TK, et al. Autonomic nervous system dysfunction in schizophrenia: impact on cognitive and metabolic health. NPJ Schizophr. (2021) 7:22. doi: 10.1038/s41537-021-00151-6
20. Chang LR, Lin YH, Kuo TB, Wu Chang HC, Liu CM, Liu CC, et al. Autonomic modulation and health-related quality of life among schizophrenic patients treated with non-intensive case management. PloS One (2011) 6:e26378. doi: 10.1371/journal.pone.0026378
21. Lu YC, Lee TL, Hsuan CF, Hung WC, Wu CC, Wang CP, et al. Elevated plasma fatty acid-binding protein 3 is related to prolonged corrected QT interval and reduced ejection fraction in patients with stable angina. Int J Med Sci (2021) 18:2076–85. doi: 10.7150/ijms.54508
22. Wang CP, Hsu CC, Hung WC, Yu TH, Wu CC, Tsai IT, et al. Plasma fatty acid-binding protein 4 (FABP4) level is associated with abnormal QTc interval in patients with stable angina and chronic kidney disease. BMC Cardiovasc Disord (2019) 19:153. doi: 10.1186/s12872-019-1134-z
23. Tang WH, Hung WC, Wang CP, Wu CC, Hsuan CF, Yu TH, et al. The lower limit of reference of urinary Albumin/Creatinine ratio and the risk of chronic kidney disease progression in patients with type 2 diabetes mellitus. Front Endocrinol (Lausanne). (2022) 13:858267. doi: 10.3389/fendo.2022.858267
24. Kong X, Ma Y, Chen J, Luo Q, Yu X, Li Y, et al. Evaluation of the chronic kidney disease epidemiology collaboration equation for estimating glomerular filtration rate in the Chinese population. Nephrol Dial Transpl. (2013) 28:641–51. doi: 10.1093/ndt/gfs491
25. American Diabetes association. 2. classification and diagnosis of diabetes. Diabetes Care (2016) 39(Suppl.1):S13–22. doi: 10.2337/dc16-S005
26. Huang WL, Hwang BT, Lai CT, Li JY, Kuo TB, Yang CC. Is heart rate variability related to season of birth? Clin Cardiol (2015) 38:407–12. doi: 10.1002/clc.22410
27. Heart rate variability: standards of measurement, physiological interpretation and clinical use. task force of the European society of cardiology and the north American society of pacing and electrophysiology. Circulation (1996) 93:1043–65. doi: 10.1161/01.CIR.93.5.1043
28. Kuo TB, Lin T, Yang CC, Li CL, Chen CF, Chou P. Effect of aging on gender differences in neural control of heart rate. Am J Physiol (1999) 277:H2233–9. doi: 10.1152/ajpheart.1999.277.6.H2233
29. Quintana DS. Statistical considerations for reporting and planning heart rate variability case-control studies. Psychophysiology (2017) 54:344–9. doi: 10.1111/psyp.12798
30. Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health (2017) 5:258. doi: 10.3389/fpubh.2017.00258
31. Yang AC, Hong CJ, Tsai SJ Heart rate variability in psychiatric disorders. Taiwanese J Psychiatry (2010) 24:99–109. Available at: http://www.psynetresearch.org›uploads›hear...PDF.
32. Benjamin BR, Valstad M, Elvsashagen T, Jönsson EG, Moberget T, Winterton A, et al. Heart rate variability is associated with disease severity in psychosis spectrum disorders. Prog Neuropsychopharmacol Biol Psychiatry (2021) 111:110108. doi: 10.1016/j.pnpbp.2020.110108
33. Veerkamp JH, Zimmerman AW. Fatty acid-binding proteins of nervous tissue. J Mol Neurosci (2001) 16:133–42. doi: 10.1385/JMN:16:2-3:133
34. Shimamoto C, Ohnishi T, Maekawa M, Watanabe A, Ohba H, Arai R, et al. Functional characterization of FABP3, 5 and 7 gene variants identified in schizophrenia and autism spectrum disorder and mouse behavioral studies. Hum Mol Genet (2015) 24:2409. doi: 10.1093/hmg/ddv011
35. Maekawa M, Iwayama Y, Arai R, Nakamura K, Ohnishi T, Toyota T, et al. Polymorphism screening of brain-expressed FABP7, 5 and 3 genes and association studies in autism and schizophrenia in Japanese subjects. J Hum Genet (2010) 55:127–30. doi: 10.1038/jhg.2009.133
36. Maekawa M, Ohnishi T, Toyoshima M, Shimamoto-Mitsuyama C, Hamazaki K, Balan S, et al. A potential role of fatty acid binding protein 4 in the pathophysiology of autism spectrum disorder. Brain Commun (2020) 2:fcaa145. doi: 10.1093/braincomms/fcaa145
37. Hotamisligil GS, Bernlohr DA. Metabolic functions of FABPs - mechanisms and therapeutic implications. Nat Rev Endocrinol (2015) 11:592−605. doi: 10.1038/nrendo.2015.122
38. Yao F, Jiang DD, Guo WH, Guo LS, Gao MM, Bai Y, et al. FABP4 inhibitor attenuates inflammation and endoplasmic reticulum stress of islet in leptin receptor knockout rats. Eur Rev Med Pharmacol Sci (2020) 24:12808−20. doi: 10.26355/eurrev_202012_24182
39. Ge XN, Bastan I, Dileepan M, Greenberg Y, Ha SG, Steen KA, et al. FABP4 regulates eosinophil recruitment and activation in allergic airway inflammation. Am J Physiol Lung Cell Mol Physiol (2018) 315:L227−L40. doi: 10.1152/ajplung.00429.2017
40. Gong Y, Yu Z, Gao Y, Deng L, Wang M, Chen Y, et al. FABP4 inhibitors suppress inflammation and oxidative stress in murine and cell models of acute lung injury. Biochem Biophys Res Commun (2018) 496:1115−21. doi: 10.1016/j.bbrc.2018.01.150
41. Cooper TM, McKinley PS, Seeman TE, Choo TH, Lee S, Sloan RP. Heart rate variability predicts levels of inflammatory markers: evidence for the vagal anti-inflammatory pathway. Brain Behav Immun (2015) 49:94–100. doi: 10.1016/j.bbi.2014.12.017
42. Alen NV, Parenteau AM, Sloan RP, Hostinar CE. Heart rate variability and circulating inflammatory markers in midlife. Brain Behav Immun Health (2021) 15:100273. doi: 10.1016/j.bbih.2021.100273
43. Berntson GG, Bigger JT Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, et al. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology (1997) 34:623–48. doi: 10.1111/j.1469-8986.1997.tb02140.x
44. Ziegler D, Laude D, Akila F, Elghozi JL. Time- and frequency-domain estimation of early diabetic cardiovascular autonomic neuropathy. Clin Auton Res (2001) 11:369–76. doi: 10.1007/BF02292769
45. Appelhans BM, Luecken LJ. Heart rate variability as an index of regulated emotional responding. Rev Gen Psychol (2006) 10:229–40. doi: 10.1037/1089-2680.10.3.229
46. Thayer JF, Ahs F, Fredrikson M, Sollers JJ 3rd, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci Biobehav Rev (2012) 36:747–56. doi: 10.1016/j.neubiorev.2011.11.009
47. Moon MG, Yoon CH, Lee K, Kang SH, Youn TJ, Chae IH. Evaluation of heart-type fatty acid-binding protein in early diagnosis of acute myocardial infarction. J Korean Med Sci (2021) 36:e61. doi: 10.3346/jkms.2021.36.e61
48. Dellas C, Puls M, Lankeit M, Schäfer K, Cuny M, Berner M, et al. Elevated heart-type fatty acid-binding protein levels on admission predict an adverse outcome in normotensive patients with acute pulmonary embolism. J Am Coll Cardiol (2010) 55:2150–7. doi: 10.1016/j.jacc.2009.10.078
49. Chen YX, Li CS. The prognostic and risk-stratified value of heart-type fatty acid-binding protein in septic patients in the emergency department. J Crit Care (2014) 29:512–6. doi: 10.1016/j.jcrc.2014.03.026
50. Sun YP, Wei CP, Ma SC, Zhang YF, Qiao LY, Li DH, et al. Effect of carvedilol on serum heart-type fatty acid-binding protein, brain natriuretic peptide, and cardiac function in patients with chronic heart failure. J Cardiovasc Pharmacol (2015) 65:480–4. doi: 10.1097/FJC.0000000000000217
51. Akbal E, Özbek M, Günes F, Akyürek Ö, Üreten K, Delibaşı T. Serum heart type fatty acid binding protein levels in metabolic syndrome. Endocrine (2009) 36:433–7. doi: 10.1007/s12020-009-9243-6
52. Başar O, Akbal E, Köklü S, Tuna Y, Koçak E, Başar N, et al. Increased h-FABP concentrations in nonalcoholic fatty liver disease. possible marker for subclinical myocardial damage and subclinical atherosclerosis. Herz (2013) 38:417–22. doi: 10.1007/s00059-012-3714-x
53. Terra X, Quintero Y, Auguet T, Porras JA, Hernández M, Sabench F, et al. FABP 4 is associated with inflammatory markers and metabolic syndrome in morbidly obese women. Eur J Endocrinol (2011) 164:539–47. doi: 10.1530/EJE-10-1195
54. Trojnar M, Patro-Małysza J, Kimber-Trojnar Ż, Leszczyńska-Gorzelak B, Mosiewicz J. Associations between fatty acid-binding protein 4⁻A proinflammatory adipokine and insulin resistance, gestational and type 2 diabetes mellitus. Cells (2019) 8:227. doi: 10.3390/cells8030227
55. Rodríguez-Calvo R, Girona J, Alegret JM, Bosquet A, Ibarretxe D, Masana L. Role of the fatty acid-binding protein 4 in heart failure and cardiovascular disease. J Endocrinol (2017) 233:R173–R84. doi: 10.1530/JOE-17-0031
56. Xu A, Wang Y, Xu JY, Stejskal D, Tam S, Zhang J, et al. Adipocyte fatty acid-binding protein is a plasma biomarker closely associated with obesity and metabolic syndrome. Clin Chem (2006) 52:405–13. doi: 10.1373/clinchem.2005.062463
57. Phoemsapthawee J, Prasertsri P, Leelayuwat N. Heart rate variability responses to a combined exercise training program: correlation with adiposity and cardiorespiratory fitness changes in obese young men. J Exerc Rehabil. (2019) 15:114–22. doi: 10.12965/jer.1836486.243
58. Gutin B, Howe C, Johnson MH, Humphries MC, Snieder H, Barbeau P. Heart rate variability in adolescents: relations to physical activity, fitness, and adiposity. Med Sci Sports Exerc. (2005) 37:1856–63. doi: 10.1249/01.mss.0000175867.98628.27
59. Tian Y, Huang C, He Z, Hong P, Zhao J. Autonomic function responses to training: correlation with body composition changes. Physiol Behav (2015) 151:308–13. doi: 10.1016/j.physbeh.2015.07.038
60. Tiwari R, Kumar R, Malik S, Raj T, Kumar P. Analysis of heart rate variability and implication of different factors on heart rate variability. Curr Cardiol Rev (2021) 17:e160721189770. doi: 10.2174/1573403X16999201231203854
61. Kangas P, Tikkakoski A, Uitto M, Viik J, Bouquin H, Niemelä O, et al. Metabolic syndrome is associated with decreased heart rate variability in a sex-dependent manner: a comparison between 252 men and 249 women. Clin Physiol Funct Imag (2019) 39:160–7. doi: 10.1111/cpf.12551
62. Poon AK, Whitsel EA, Heiss G, Soliman EZ, Wagenknecht LE, Suzuki T, et al. Insulin resistance and reduced cardiac autonomic function in older adults: the atherosclerosis risk in communities study. BMC Cardiovasc Disord (2020) 20:217. doi: 10.1186/s12872-020-01496-z
63. Albarado-Ibañez A, Arroyo-Carmona RE, Sánchez-Hernández R, Ramos-Ortiz G, Frank A, García-Gudiño D, et al. The role of the autonomic nervous system on cardiac rhythm during the evolution of diabetes mellitus using heart rate variability as a biomarker. J Diabetes Res (2019) 2019:5157024. doi: 10.1155/2019/5157024
64. Benichou T, Pereira B, Mermillod M, Tauveron I, Pfabigan D, Maqdasy S, et al. Heart rate variability in type 2 diabetes mellitus: a systematic review and meta-analysis. PloS One (2018) 13:e0195166. doi: 10.1371/journal.pone.0195166
65. Liao D, Sloan RP, Cascio WE, Folsom AR, Liese AD, Evans GW, et al. Multiple metabolic syndrome is associated with lower heart rate variability. the atherosclerosis risk in communities study. Diabetes Care (1998) 21:2116–22. doi: 10.2337/diacare.21.12.2116
66. Chou YH, Huang WL, Chang CH, Yang CCH, Kuo TBJ, Lin SL, et al. Heart rate variability as a predictor of rapid renal function deterioration in chronic kidney disease patients. Nephrol (Carlton). (2019) 24:806–13. doi: 10.1111/nep.13514
67. Drawz PE, Babineau DC, Brecklin C, He J, Kallem RR, Soliman EZ, et al. CRIC study investigators. heart rate variability is a predictor of mortality in chronic kidney disease: a report from the CRIC study. Am J Nephrol. (2013) 38:517–28. doi: 10.1159/000357200
68. Vinik A, Ullal J, Parson HK, Casellini CM. Diabetic neuropathies: clinical manifestations and current treatment options. Nat Clin Pract Endocrinol Metab (2006) 2:269–81. doi: 10.1038/ncpendmet0142
69. Sandercock GR, Bromley PD, Brodie DA. The reliability of short-term measurements of heart rate variability. Int J Cardiol (2005) 103:238–47. doi: 10.1016/j.ijcard.2004.09.013
Keywords: schizophrenia, cardiovascular disease, heart rate variability, high frequency, low-frequency, fatty acid-binding protein
Citation: Hung W-C, Yu T-H, Wu C-C, Lee T-L, Tsai I-T, Hsuan C-F, Chen C-Y, Chung F-M, Lee Y-J and Tang W-H (2023) FABP3, FABP4, and heart rate variability among patients with chronic schizophrenia. Front. Endocrinol. 14:1165621. doi: 10.3389/fendo.2023.1165621
Received: 22 February 2023; Accepted: 04 May 2023;
Published: 15 May 2023.
Edited by:
Patrick Osei-Owusu, Case Western Reserve University, United StatesReviewed by:
Massimo Tusconi, University of Cagliari, ItalyLisa C. Brown, Great Scott! Consulting, LLC, United States
Copyright © 2023 Hung, Yu, Wu, Lee, Tsai, Hsuan, Chen, Chung, Lee and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei-Hua Tang, YWZyaWNhcGF1bDEyQHlhaG9vLmNvbQ==
 Wei-Chin Hung1,2
Wei-Chin Hung1,2 Chun-Yu Chen
Chun-Yu Chen Yau-Jiunn Lee
Yau-Jiunn Lee Wei-Hua Tang
Wei-Hua Tang