- Department of Diabetes and Endocrinology, Ipswich Hospital, East Suffolk and North East Essex NHS Foundation Trust (ESNEFT), Ipswich, United Kingdom
Diabetes sensory polyneuropathy (DSPN) is a significant complication of diabetes affecting up to 50% of patients in their lifetime and approximately 20% of patients suffer from painful diabetes neuropathic pain. DSPN – both painless and painful - leads to considerable morbidity including reduction of quality of life, increased lower limb amputations and is associated with worsening mortality. Significant progress has been made in the understanding of pathogenesis of DSPN and the last decade has seen newer techniques aimed at its earlier diagnosis. The management of painful DSPN remains a challenge despite advances made in the unravelling the pathogenesis of pain and its transmission. This article discusses the heterogenous clinical presentation of DSPN and the need to exclude key differential diagnoses. Furthermore, it reviews in detail the current diagnostic techniques involving both large and small neural fibres, their limitations and advantages and current place in the diagnosis of DSPN. Finally, the management of DSPN including newer pharmacotherapies are also discussed.
1 Introduction
The term diabetes sensorimotor polyneuropathy (DSPN) refers to a heterogeneous group of neurological disorders – either clinically evident or subclinical – that occur in the setting of diabetes mellitus and cannot be attributed to other aetiologies of peripheral neuropathy (1). It is by far the he commonest form of diabetes polyneuropathy (DPN) (Table 1) affecting up to 50% of people with diabetes, while its yearly incidence amounts to approximately 2% (3, 4). DSPN is defined as a symmetrical, length-dependent polyneuropathy attributable to not only chronic hyperglycaemia-mediated microvascular alterations but also contributed by other cardiovascular factors including dyslipidaemia, hypertension and smoking (1).

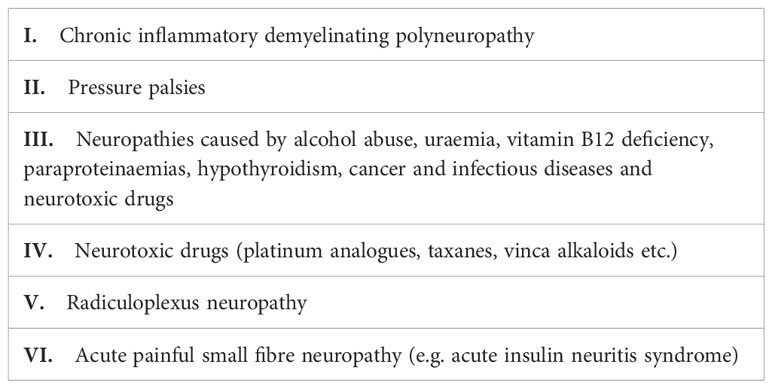
Table 1 Classification of diabetes neuropathy [adapted from Pop-Busui et al. (2)].
The classification of DSPN varies and is based on a number of factors including presence or absence of painful symptoms, pattern of neural involvement and the setting of its evaluation – clinical or research. Chronic painful DSPN is found in up to 25% of subjects with diabetes and is defined as persistent or recurrent pain lasting 3 months and caused by a lesion or disease of the somatosensory system due to diabetes and after exclusion of other causes (5, 6). (Table 2) The position statement by American Diabetes association (ADA) proposed a clinical classification of DSPN into either primarily large and small fibre or mixed based on the type of neural fibres involved (2). This differentiation will be more elaborately discussed later.
A practical definition of DSPN in the clinical setting is the presence of neuropathic symptoms as reported by the patient and/or signs of peripheral nerve dysfunction in diabetes subjects after exclusion of other causes. Historically – in busy diabetes clinics – the presence of abnormal ankle reflex, abnormal 128 Hz tuning fork sensation or insensitivity to 10-gm Semmes-Weinstein monofilament (SWMF) was considered to be valid evidences to diagnose DPN. More accurate assessments like nerve conduction studies were time consuming and resource-specific and hence reserved for atypical presentation including truncal radiculopathy, entrapment neuropathy and amyotrophy (7, 8). Whist these methods were undoubtedly useful in assessing risk of patients of foot ulceration, they are limited in their capabilities to assess mostly Aα and Aβ nerve myelinated fibres (Figure 1) which serve touch, proprioception, position sense, vibration and muscle control. Consequently, they are affected later in the natural history of diabetes (10). There is now a large body of evolving evidence that small neural fibres (thinly myelinated Aδ and unmyelinated C fibres) are affected early in the natural history of DSPN and precedes large fibre involvement (11). Hence the specific assessment of this group offers hope for earlier diagnosis of DSPN which can lead to quicker adoption of disease modifying strategies such as life style modification, glycaemic, blood pressure and lipid control or novel therapeutics to prevent onset and progression of worsening neuropathy (9).
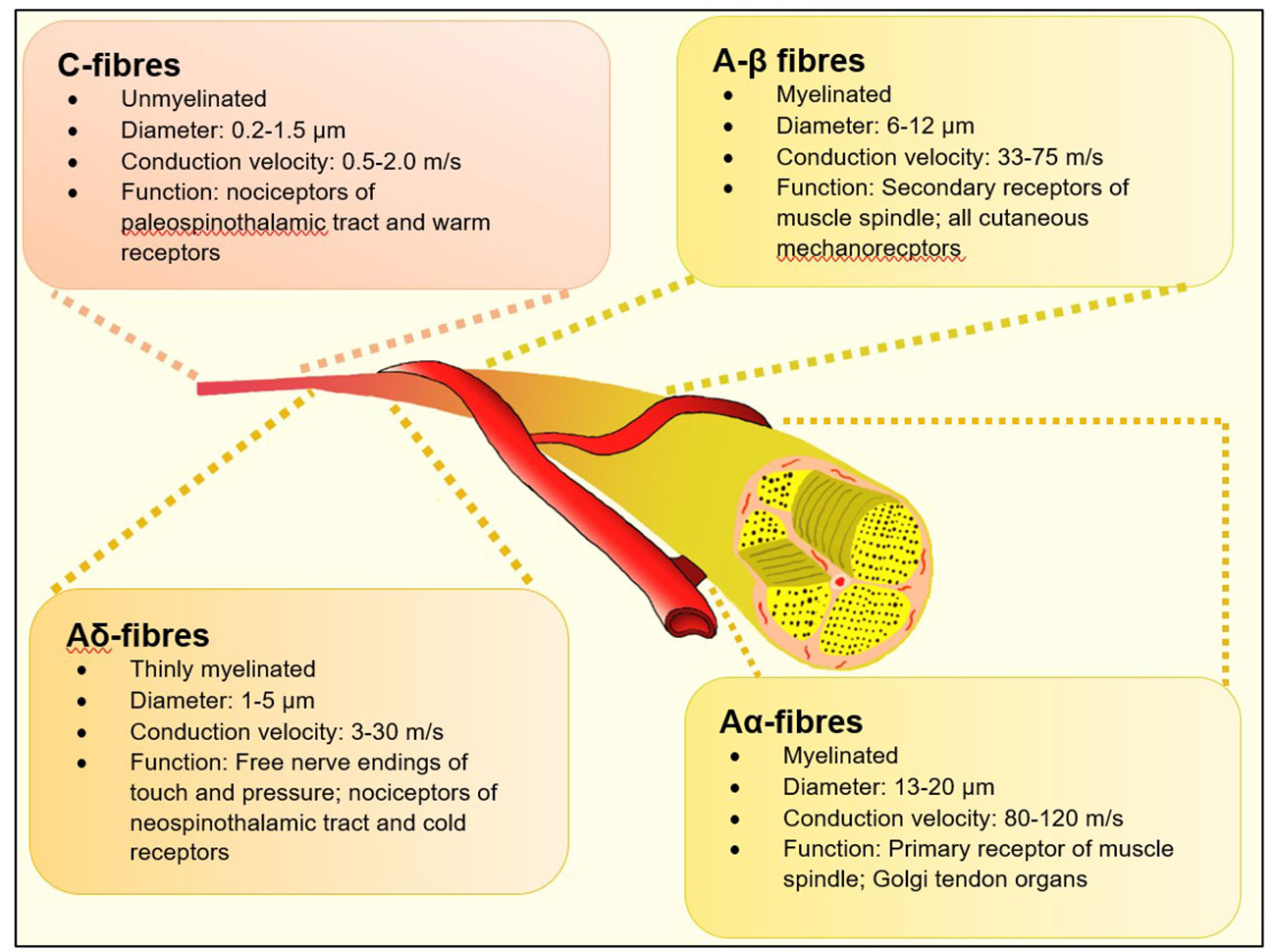
Figure 1 Types of neural fibres [adapted from Sharma S et al. (9)].
2 Clinical presentation of DSPN
The commonest clinical presentation of DSPN is a distal symmetrical type of progressive sensorimotor polyneuropathy which is length dependent i.e., the lower limbs are affected earlier than upper extremities and with progression of the condition, there is distal to proximal axonal degeneration; hence many patients present with the typical “stocking and glove” distribution of symptoms (2). Up to 50% of subjects remain asymptomatic and hence the need for at least annual review of their feet sensations to prevent ulceration. Symptoms at presentation can be either “negative” (loss of sensations including touch, vibration, position or deficits including ataxia) or “positive” (pain, paraesthesia, allodynia). Not uncommonly patients report the subjective symptoms of “numbness” which could indicate either of the above group of symptoms and it is up to the clinicians to make this differentiation to enable treatment (12). It is important to note that autonomic dysfunction is common in patients with DSPN but is largely undetected unless heralded by symptoms as mentioned in Table 1 (2).
Common determinants for progression of DSPN include patient characteristics (advancing age, height and obesity), clinical parameters (presence of hypertension and uncontrolled dyslipidaemia) and adverse glycaemic matrices (poor glycaemic control, longer duration of diabetes, beta cell insufficiency) (1, 3, 13). Whilst it is beyond the scope of this article to examine each of these determinants individually, research into early diabetes neuropathy using methods of small fibre function have shown that the impact of glycaemic control in the progression of microangiopathy is much more important in type 1 diabetes whilst in type 2 diabetes, the impact is more multifactorial (14, 15).
3 Assessment of DSPN
3.1 Bedside foot screening
The traditional and still mostly prevalent screening tool assessment of diabetes feet in busy clinical environments remains the use of a 10gm-SWMF or 128-Hz tuning form (detects large fibres) and pin-prick sensation (detects small fibres) (2). It must be highlighted that these bedside techniques are useful for assessing diabetes feet at risk of future ulceration and are not sensitive for early detection of DSPN (16). More recently, the Ipswich Touch test has been shown to be an equally sensitive method for evaluating at-risk feet with a strong concordance with the 10-gm monofilament (17, 18).
The diagnosis of painful DSPN is based on presence of painful neuropathic symptoms and a clinical diagnosis of DSPN after exclusion of other neuropathies as shown in Table 2.
3.2 Use of Scored clinical assessments
Over the years, many scored clinical assessments have been proposed as standardized objective and quantitative measures for screening and grading of severity of DSPN. Their utility is particularly useful for epidemiological studies looking at prevalence of DSPN in larger populations. Of these, the Michigan Neuropathy Screening Instrument (MNSI) (2, 19), Toronto Clinical Neuropathy Score (20), modified Toronto Neuropathy Score (21), Neuropathy Disability Score (16), Neurological Disability Score (22), Neuropathy Symptom Score (22) and Utah Early Neuropathy (23) are commonly used. It is beyond the scope of this article to examine each score individually but their usage is limited by the time required in busy clinical settings. Recently, machine-learning severity prediction tools based on the MNSI have been proposed to save time (24).
For painful DSPN, useful scoring tools include the Neuropathy Total Symptom Score-6 (25), DN4 (26), PainDETECT (27) and Neuropathic Pain Symptom Inventory (28).
3.3 Rapid point-of care devices
In order to overcome the shortcomings of screening tools and to reduce the time taken by scoring systems in clinical practice, a number of point-of-care devices have evolved in the past few years which have the ability to reduce bias and provide more accurate results. The Vibratip™ for objective assessment of vibration sense is one such device (29). Another device is DPNCheck™ which a hand-held device providing sural nerve conduction velocity and amplitude in under five minutes correlates well with Neuropathy Disability score (30, 31). Similarly the SUDOSCAN™ is another well-established device which by assessing electrochemical skin conductance as a measure sudomotor function has been shown to show early small fibre changes (autonomic system) in patients at risk of DSPN (32, 33).
3.4 Large fibre methodologies
i) The neurothesiometer is a battery-operated device that provides mechanical vibration with a fixed frequency of approximately 100 Hz while the vibration amplitude is controlled manually using a rotatory control knob. The knob is used to adjust the voltage applied and ranges from 0 to 50 V (0-250 µm in amplitude). The operator applies the handheld probe to the pulp of the great toe and the vibration stimulus gradually increased, until the subject feels the vibration sensation. The voltage displayed on the neurothesiometer is the measured vibration perception threshold (VPT). It has been shown to more consistent than vibration sense and hence reflective of peripheral nerve function; hence it is commonly used in clinical research trials (34). The major drawback of such a device is its manual observer-dependent operability and its limited vibration intensity (35, 36).
ii) Nerve conduction studies (NCS) measure properties of transmission of electrical current along nerve and muscle fibre membranes. The nerves tested in diabetes for DSPN include both lower and upper limb peripheral nerves which include motor and sensory nerve. The first NCS abnormality observed in DSPN is a reduction of sural nerve amplitude and as the condition progresses, the distal sensory latency increases and finally there is reduction of conduction velocity (37).
One of the major criticisms of NCS in DSPN is that only large fibre activity is measured which is known to be affected in later stages (9). Furthermore, the normative ranges for NCS parameters overlap with abnormal values in DSPN limiting the sensitivity and accuracy of testing. Also, NCS must be corrected for anthropomorphic factors such as gender and body mass index to improve their accuracy (38). Despite these concerns, NCS remains an important objective measure of neuropathy and is still used to differentiate DSPN from other atypical aetiologies.
iii) Electromyography in DSPN is supplementary and exploratory to NCS above. It is of value when there are atypical presentations like radiculopathy, pure motor myopathy or inflammatory myopathy. Percutaneous needle insertion into muscles in required; a procedure that can be uncomfortable and hence not well suited for serial studies (37).
3.5 Small fibre methodologies
Small fibre neuropathy (SFN) is a sub-type of peripheral neuropathy affecting the thinly myelinated Aδ or the non-myelinated C fibres (Figure 1). These fibres constitute 79.6% to 91.4% (39, 40), of peripheral nerve fibres and mediate several key functions including temperature and pain perception, sweating, and tissue blood flow - all of which, when impaired are pathophysiologically related to adverse outcomes associated with foot ulcerations in people with diabetes
Small fibre methods can be broadly classified into methods for assessing small fibre function and small fibre structure. Table 3 provides a summary of methods currently available.
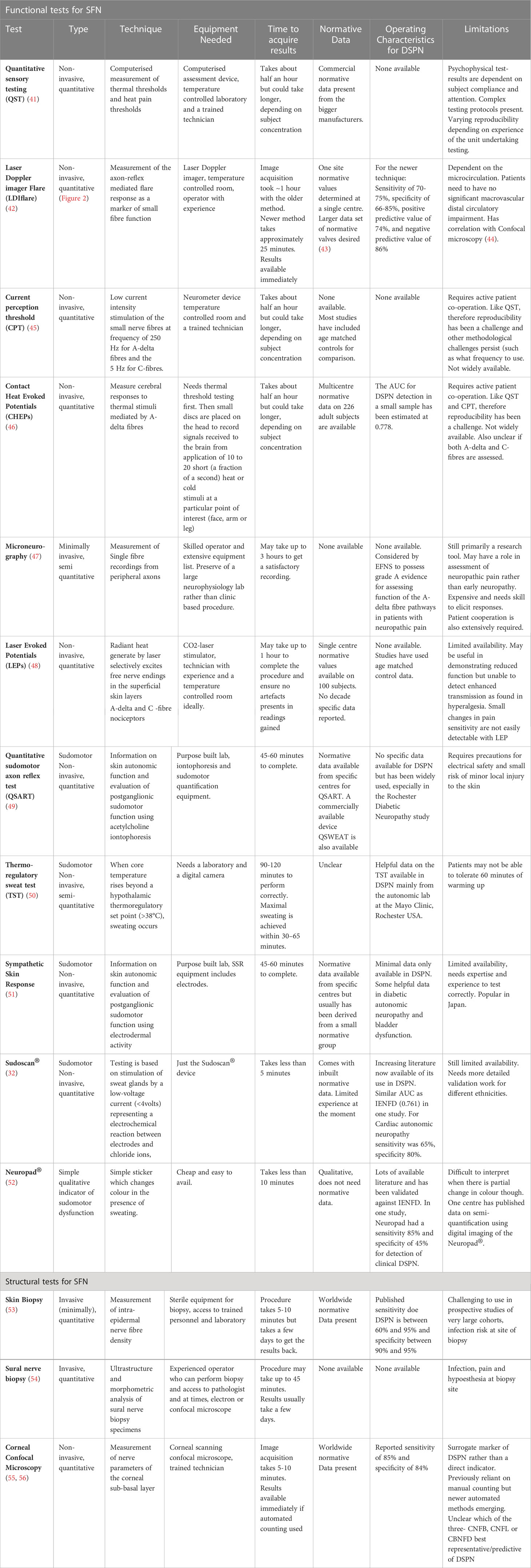
Table 3 Overview of the current methodologies for testing small fibres in DSPN [adapted from Sharma S et al. (9)].
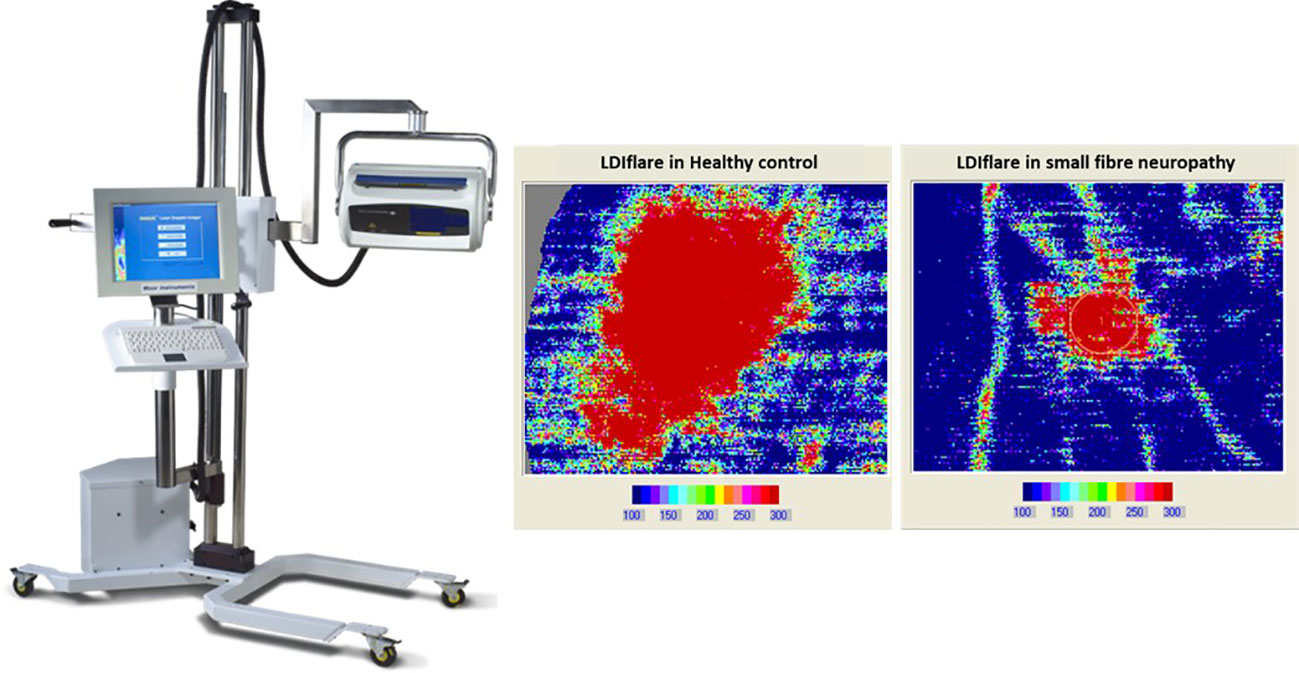
Figure 2 The laser doppler imager (LDIflare) is a non-invasive technique of measuring C fibre-mediated cutaneous vasodilatation in the foot skin in response to thermal heating. The heating is done by a 1cm2 probe and the area of induced hyperaemia measured by a 610nm laser probe. The scan image on left is a 11cm2 flare area in a healthy control while on the right is 1.1cm2 flare in a subject with small fibre neuropathy.
3.6 Tests for autonomic neuropathy
Autonomic dysfunction is common in up to 50% of subjects with DSPN but remains either asymptomatic or undiagnosed. The presenting features include impairment of cardiovascular, gastrointestinal, urogenital, thermoregulatory, sudomotor, and pupillomotor function. The accurate diagnosis of the autonomic neuropathies has been enhanced by the availability of physiological tests that measure autonomic function, and more recently, structural studies of the autonomic cutaneous innervation (57). Presence of cardiovascular autonomic neuropathy (CAN) in the presence of DSPN is an independent risk factor increased mortality and morbidity (58). The Composite Autonomic Symptom Score (COMPASS) 31 score is a validated, easy-to-use, quantitative assessment tool for autonomic symptoms in diabetic neuropathy, with a fair diagnostic accuracy for both cardiovascular autonomic neuropathy and diabetic polyneuropathy (59). A detailed description for autonomic neuropathy tests is beyond the scope of this article where the focus in mainly on DSPN.
3.7 Newer research methodologies
Both the brain and the spinal cord have been implicated in the genesis of painful DSPN and broadly involves a shift towards excitation and reduced inhibition of transmission of pain signals (60). The brain network involved in such chronic pain states is partially distinct from those involved in acute pain. Magnetic resonance spectroscopy has been used to determine the neuronal function in the thalamus and primary somatosensory cortex in patients with and without painful DSPN. Early results have shown thalamic neuronal dysfunction was found in only advanced painless DSPN but preserved in both subclinical and painful DSPN. These methods show promise in improving our understanding of painful DSPN and might have a role in future targeted pharmacotherapy (61).
4 The conflicting evidences supporting early use of small fibre methodologies for diagnosis of DSPN
The pillars of diabetes management include early diagnosis of complications and steps to prevent their worsening. For other microangiopathic complications, advances have been made for their early detection e.g., retinal screening for diabetes retinopathy and urinary microalbuminuria for diabetes nephropathy (62). Yet in contrast, The current ADA position statement on DPN endorses the following: “Assessment for distal symmetric polyneuropathy should include a careful history and assessment of either temperature or pinprick sensation (small-fibre function) and vibration sensation using a 128-Hz tuning fork (for large-fibre function). All patients should have annual 10-g monofilament testing to identify feet at risk for ulceration and amputation” (2, 4). It should be emphasized that the ADA’s position is to “identify feet at risk for ulceration and amputation” rather than early diagnosis of neuropathy; in essence the major focus of this guidance is the prevention of disabling diabetes lower limb complications.
A comprehensive review of various methodologies for the assessment of DSPN has been done but it is to be noted that none of them, except the screening bedside tools have found their way to any established guidance for detection of DPN. There are many reasons for this: firstly, the evidences available are mostly cross-sectional and only 6 studies have been performed longitudinally – the longest being at 5 years (63). Secondly, the lack of globally agreed-upon normative data for these tests make them less practical for routine use. So far, only confocal microscopy (CCM) and intra-epidermal nerve fibre density (IEFND) have acceptable normative values (64, 65). Thirdly, age-related decline in neural function and structure is well recognised. Hence normative data should also be age-linked and only the LDIFLARE technique has shown such age-linked reduction of small fibre function (43).
Furthermore, it well known that with duration of diabetes, there is progression of other microangiopathic complications including diabetes kidney disease and it is well known that progressive uraemia affects neural health (66). Hence normative values should also be linked to renal health i.e. linked to measures of creatinine and eGFR. Finally, the methodologies of various techniques are still not uniform with various centers using their own adaptions to improve performance. Hence in view of considerable heterogeneity involved with various techniques, it is unlikely that they will appear in guidelines soon unless a much-needed consensus is agreed upon by investigators and equally importantly accepted by legislators.
5 Management of DSPN
The pillars of management of DSPN can be categorized as follows;
5.1 Reduction of risk factors contributing to progression of DSPN
Adoption of healthy lifestyle measures and weight loss remain the cornerstone of non-pharmacological management of DSPN. Whilst there are epidemiological studies which have shown the prevalence of less neuropathic symptoms in type 2 diabetes subjects with lower body weight (67). Interestingly, in a recent prospective cohort study of 131 obese participants attending a medical weight-management program, improvements were observed on the MNSI questionnaire, two Quality of Life in Neurological Disorders subdomains, and quantitative sensory testing cold thresholds whilst IENFD remained stable (68).
The impact of intensive glycaemic control in retarding the progression of DSPN has been well demonstrated in type 1 diabetes in the DCCT/EPIC study (69) but specific evidence to suggest the same is lacking in type 2 diabetes (70, 71). The reason for the latter could be due to the difference of pathogenesis of neuropathy in both types of diabetes and the effect of metabolic syndrome in type 2 diabetes. Nevertheless, it is well accepted that improved glycaemic control reduces the onset and progression of DSPN.
The EURODIAB IDDM study (13) of 3250 type 1 diabetes subjects showed significant correlations between the presence of diabetic peripheral neuropathy with age, duration of diabetes, quality of metabolic control, height, the presence of background or proliferative diabetic retinopathy, smoking, dyslipidaemia and the presence of cardiovascular disease. However prospective studies are lacking to indicate that control of any of the above factors are helpful in retarding the progression of DSPN although it is widely believed that hypertension and dyslipidaemia need to be aggressively managed to limit microangiopathic complications (72, 73).
5.2 Specific pharmacotherapy
Several compounds are available which target major pathways implicated in the pathogenesis of DSPN including polyol pathway, hexosamine pathway, protein kinase C (PKC) activity, and advanced glycation end products (AGEs) pathway (29). The AGE inhibitor Benfotiamine and antioxidant A-lipoic acid are licensed as drugs for treatment of DSPN in many countries (74, 75). In the NATHAN 1 trial, neuropathic deficits improved after 4 years in patients with mild to moderate largely asymptomatic DSPN (76). Actovegin, a poly(ADP-ribose) polymerase (PARP) inhibitor, is authorized in mainly eastern European countries, while the aldose reductase inhibitor Epalrestat is being used in India and Japan for DPN (77, 78).
Similarly for painful DSPN, several pharmacotherapies have been shown to reduce painful symptoms by their mechanisms of action on the pathways mentioned above. Treatment with A-lipoic acid 600mg twice daily for 6 months has been shown to reduce symptoms of pain, paresthesia and numbness in symptomatic DSPN (79). A-lipoic acid infusions over 3 weeks have also been shown to significantly reduce neuropathic symptoms (75). Larger randomized controlled trials are needed to study their efficacy in multicentered population subsets to strengthen the rationale of use in DSPN.
5.3 Symptomatic pharmacological treatment of painful DSPN
Painful DSPN can often be a crippling symptom leading to significant reduction of quality of life for patients, many of whom also have neuropathic deficits leading to complications. The management of painful DSPN is often challenging due to difference in efficacy between individual agents, balance between side-effects and working patterns and drug interactions. Monotherapy is usually not tolerated in maximal dosages and recent evidences from the OPTION-DM study showed that combination treatment was well tolerated and led to improved pain relief in patients with suboptimal pain control with a monotherapy (80, 81). The broad group of mediations in this class are shown in Table 4.
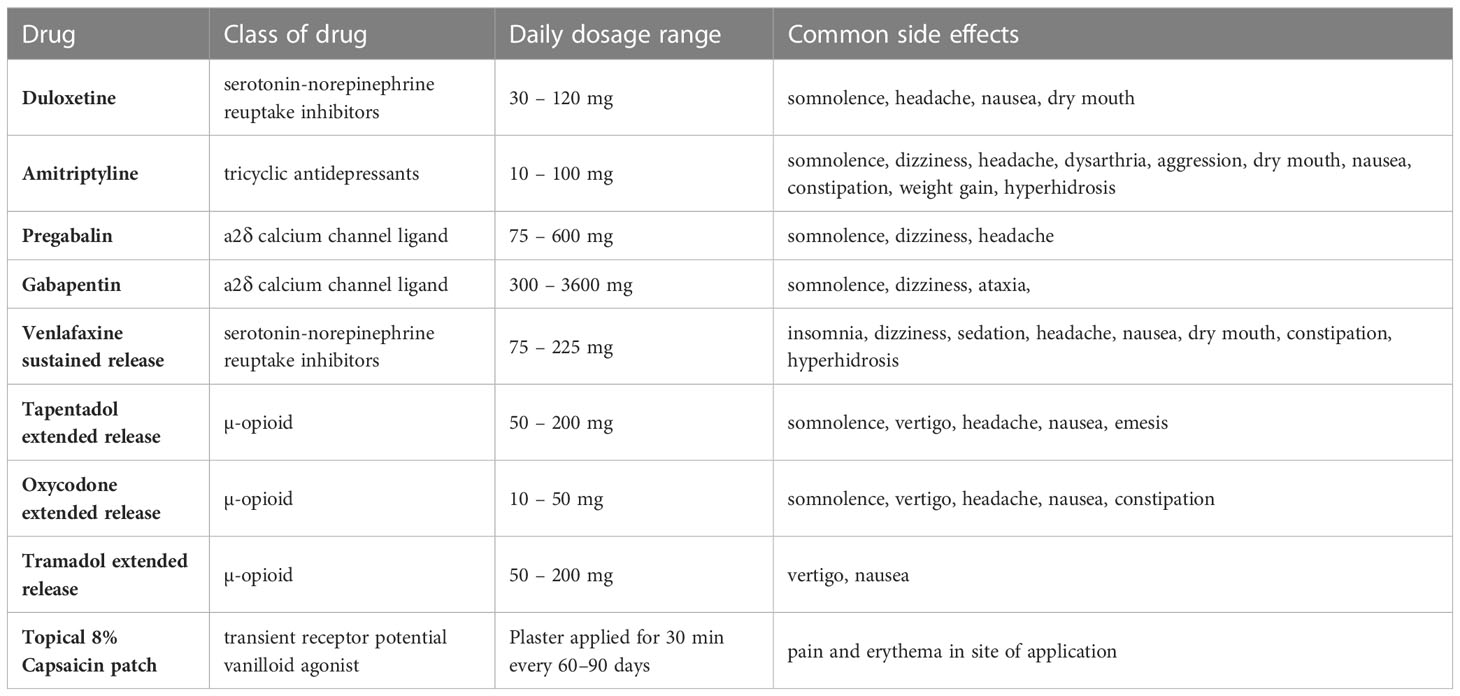
Table 4 Dosages and adverse events and pharmacotherapies used in the management of DSPN in clinical practice (82–86).
5.4 Non-pharmacological treatment of painful DSPN
In diabetes subjects with refractory pain, spinal cord stimulation has been shown to significant pain relief and improved quality of life. It is an expensive invasive procedure needing insertion of percutaneous leads placed epidurally and connected to an implantable pulse generator typically placed in the low back. Whilst the mechanism of action is yet to fully understand, early results are promising but long-term results are awaited (87, 88). Other methods such as cognitive behavioral therapy, transcutaneous electrical nerve stimulation and acupuncture have a low level of evidence but can be successful in individual subjects (89).
6 Conclusion
DSPN is a major complication of diabetes. By the time a diabetes subject develops foot ulceration, the cardiovascular risk for death is increased by 50% and their 5-year mortality is worse than most cancers. With the worldwide prevalence of diabetes increasing to pandemic standards, it is anticipated that the consequences of DSPN in diabetes will continue to be on rise leading to serious challenges in both personal wellbeing and global health expenditure. Whilst on one hand, considerable advances in the understanding of the mechanisms of DSPN have been made, as discussed in this article, its early diagnosis and effective management remains uncertain and lacks uniformity. Whilst medications like the glucagon-like-peptide 1 receptor agonists and sodium-glucose co-transporters 2 have shown significant benefit in reducing cardiovascular mortality, heart failure and chronic kidney disease associated with diabetes, there is still a substantial unmet need in the therapeutics governing DSPN and other aspects of diabetes polyneuropathy. It is our combined responsibility to pool our resources, invest in uniform management algorithms and convince legislators to embrace newer diagnostic techniques so that burden and sequalae of DSPN can be lessened.
Author contributions
SS and GR researched, wrote and reviewed all parts of this manuscript.
Acknowledgments
The authors would like to thank the colleagues of the Diabetes Trials unit and Diabetes & Endocrine centre at Ipswich hospital for their continued support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tesfaye S, Boulton AJM, Dyck PJ, Freeman R, Horowitz M, Kempler P, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care (2010) 33:2285–93. doi: 10.2337/dc10-1303
2. Pop-Busui R, Boulton AJ, Feldman EL, Bril V, Freeman R, Malik RA, et al. Diabetic neuropathy: a position statement by the American diabetes association. Diabetes Care (2017) 40:136–54. doi: 10.2337/dc16-2042
3. Ziegler D, Papanas N, Vinik AI, Shaw JE. Epidemiology of polyneuropathy in diabetes and prediabetes. Handb Clin Neurol (2014) 126:3–22. doi: 10.1016/B978-0-444-53480-4.00001-1
4. American Diabetes Association. 11. Microvascular complications and foot care: standards of medical care in diabetes–2019. Diabetes Care (2019) 42:S124–38. doi: 10.2337/dc19-S011
5. Scholz J, Finnerup NB, Attal N, Aziz Q, Baron R, Bennett MI, et al. The IASP classification of chronic pain for ICD-11: chronic neuropathic pain. Pain (2019) 160:53–9. doi: 10.1097/j.pain.0000000000001365
6. Tesfaye S, Vileikyte L, Rayman G, Sindrup S, Perkins B, Baconja M, et al. Consensus recommendations on diagnosis, assessment and management. Diabetes Metab Res Rev (2011) 27(7):629–38. doi: 10.1002/dmrr.1225
7. Albers JW, Pop-Busui R. Diabetic neuropathy: mechanisms, emerging treatments, and subtypes. Curr Neurol Neurosci Rep (2014) 14:473. doi: 10.1007/s11910-014-0473-5
8. Callaghan BC, Xia R, Reynolds E, Banerjee M, Burant C, Rothberg A, et al. Better diagnostic accuracy of neuropathy in obesity: a new challenge for neurologists. Clin Neurophysiol (2018) 129:654–62. doi: 10.1016/j.clinph.2018.01.003
9. Sharma S, Vas P, Rayman G. Small fiber neuropathy in diabetes polyneuropathy: is it time to change? J Diabetes Sci Technol (2022) 16:321–31. doi: 10.1177/1932296821996434
10. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. New Engl J Med (2017) 376:2367–75. doi: 10.1056/NEJMra1615439
11. Burgess J, Frank B, Marshall A, Khalil RS, Ponirakis G, Petropoulos IN, et al. Early detection of diabetic peripheral neuropathy: a focus on small nerve fibres. Diagn (Basel) (2021) 11(2):165. doi: 10.3390/diagnostics11020165
12. Apfel SC, Asbury AK, Bril V, Burns TM, Campbell JN, Chalk CH, et al. Positive neuropathic sensory symptoms as endpoints in diabetic neuropathy trials. J Neurol Sci (2001) 189:3–5. doi: 10.1016/S0022-510X(01)00584-6
13. Tesfaye S, Stevens LK, Stephenson JM, Fuller JH, Plater M, Ionescu-Tirgoviste C, et al. Prevalence of diabetic peripheral neuropathy and its relation to glycaemic control and potential risk factors: the EURODIAB IDDM complications study. Diabetologia (1996) 39:1377–84. doi: 10.1007/s001250050586
14. Vas PRJ, Green AQ, Rayman G. Small fibre dysfunction, microvascular complications and glycaemic control in type 1 diabetes: a case-control study. Diabetologia (2012) 55:795–800. doi: 10.1007/s00125-011-2417-9
15. Mizokami-Stout KR, Li Z, Foster NC, Shah V, Aleppo G, McGill JB, et al. The contemporary prevalence of diabetic neuropathy in type 1 diabetes: findings from the T1D exchange. Diabetes Care (2020) 43:806–12. doi: 10.2337/dc19-1583
16. Young M, Boulton A, MacLeod A, Williams D, Sonksen P. A multicentre study of the prevalence of diabetic peripheral neuropathy in the united kingdom hospital clinic population. Diabetologia (1993) 36:150–4. doi: 10.1007/BF00400697
17. Rayman G, Vas PR, Baker N, Taylor CG, Gooday C, Alder AI, et al. The Ipswich touch test: a simple and novel method to identify inpatients with diabetes at risk of foot ulceration. Diabetes Care (2011) 34:1517–8. doi: 10.2337/dc11-0156
18. Sharma S, Kerry C, Atkins H, Rayman G. The Ipswich touch test: a simple and novel method to screen patients with diabetes at home for increased risk of foot ulceration. Diabetes Med (2014) 31:1100–3. doi: 10.1111/dme.12450
19. Martin CL, Albers J, Herman WH, Cleary P, Waberski B, Greene DA, et al. Neuropathy among the diabetes control and complications trial cohort 8 years after trial completion. Diabetes Care (2006) 29:340–4. doi: 10.2337/diacare.29.02.06.dc05-1549
20. Bril V, Perkins BA. Validation of the Toronto clinical scoring system for diabetic polyneuropathy. Diabetes Care (2002) 25:2048–52. doi: 10.2337/diacare.25.11.2048
21. Bril V, Tomioka S, Buchanan RA, Perkins BA. Reliab validity modified Toronto Clin Neuropathy Score Diabetic sensorimotor polyneuropathy. Diabetes Med (2009) 26(3):240–6. doi: 10.1111/j.1464-5491.2009.02667.x
22. Dyck PJ. Detection, characterization, and staging of polyneuropathy: assessed in diabetics. Muscle Nerve (1988) 11:21–32. doi: 10.1002/mus.880110106
23. Singleton JR, Bixby B, Russell JW, Feldman EL, Peltier A, Goldstein J, et al. The Utah early neuropathy scale: a sensitive clinical scale for early sensory predominant neuropathy. J Peripher Nerv Syst (2008) 13:218–27. doi: 10.1111/j.1529-8027.2008.00180.x
24. Haque F, Reaz MBI, Chowdhury MEH, Shapiai MIB, Malik RA, Alhatou M, et al. A machine learning-based severity prediction tool for the Michigan neuropathy screening instrument. Diagn (Basel) 13 (2023) 13(2):264. doi: 10.3390/diagnostics13020264
25. Bastyr EJ, Price 3KL, Bril V, mTCNS. Group. Development and validity testing of the neuropathy total symptom score-6: questionnaire for the study of sensory symptoms of diabetic peripheral neuropathy. Clin Ther (2005) 27:1278–94. doi: 10.1016/j.clinthera.2005.08.002
26. Spallone V, Morganti R, D’Amato C, Greco C, Cacciotti L, Marfia GA. Validation of DN4 as a screening tool for neuropathic pain in painful diabetic polyneuropathy. Diabetes Med (2012) 29:578–85. doi: 10.1111/j.1464-5491.2011.03500.x
27. Freynhagen R, Baron R, Gockel U, Tolle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin (2006) 22:1911–20. doi: 10.1185/030079906X132488
28. Bouhassira D, Attal N, Fermanian J, Alchaar H, Gautron M, Masquelier E, et al. Development and validation of the neuropathic pain symptom inventory. Pain (2004) 108:248–57. doi: 10.1016/j.pain.2003.12.024
29. Bonhof GJ, Herder C, Strom A, Papanas N, Roden M, Ziegler D. Emerging biomarkers, tools, and treatments for diabetic polyneuropathy. Endocr Rev (2019) 40:153–92. doi: 10.1210/er.2018-00107
30. Lee JA, Halpern EM, Lovblom LE, Yeung E, Bril V, Perkins BA. Reliability and validity of a point-of-Care sural nerve conduction device for identification of diabetic neuropathy. PloS One (2014) 9:e86515. doi: 10.1371/journal.pone.0086515
31. Sharma S, Vas PR, Rayman G. Assessment of diabetic neuropathy using a point-of-care nerve conduction device shows significant associations with the LDIFLARE method and clinical neuropathy scoring. J Diabetes Sci Technol (2015) 9:123–31. doi: 10.1177/1932296814551044
32. Selvarajah D, Cash T, Davies J, Sankar A, Rao G, Grieg M, et al. SUDOSCAN: a simple, rapid, and objective method with potential for screening for diabetic peripheral neuropathy. PloS One (2015) 10:e0138224. doi: 10.1371/journal.pone.0138224
33. Cabre JJ, Mur T, Costa B, Barrio F, Lopez-Moya C, Sagarra R, et al. Feasibility and effectiveness of electrochemical dermal conductance measurement for the screening of diabetic neuropathy in primary care. decoding study (Dermal electrochemical conductance in diabetic neuropathy). J Clin Med (2019) 8. doi: 10.3390/jcm8050598
34. Bril V, Kojic J, Ngo M, Clark K. Comparison of a neurothesiometer and vibration in measuring vibration perception thresholds and relationship to nerve conduction studies. Diabetes Care (1997) 20:1360–2. doi: 10.2337/diacare.20.9.1360
35. van Deursen RW, Sanchez MM, Derr JA, Becker MB, Ulbrecht JS, Cavanagh PR. Vibration perception threshold testing in patients with diabetic neuropathy: ceiling effects and reliability. Diabetes Med (2001) 18:469–75. doi: 10.1046/j.1464-5491.2001.00503.x
36. Abbott CA, Malik RA, van Ross ER, Kulkarni J, Boulton AJ. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes Care (2011) 34:2220–4. doi: 10.2337/dc11-1108
37. Perkins B, Bril A. Electrophysiologic testing in diabetes neuropathy. In: Zochodne DM, editor. Diabetes & the nervous system. New York: Elsevier (2014). p. 235–48.
38. Dyck PJ, Litchy WJ, Lehman KA, Hokanson JL, Low PA, O’Brien PC. Variables influencing neuropathic endpoints: the Rochester diabetic neuropathy study of healthy subjects. Neurology (1995) 45:1115–21. doi: 10.1212/WNL.45.6.1115
39. Said G, Baudoin D, Toyooka K. Sensory loss, pains, motor deficit and axonal regeneration in length-dependent diabetic polyneuropathy. J Neurol (2008) 255:1693–702. doi: 10.1007/s00415-008-0999-z
40. Malik RA, Tesfaye S, Newrick PG, Walker D, Rajbhandari SM, Siddique I, et al. Sural nerve pathology in diabetic patients with minimal but progressive neuropathy. Diabetologia (2005) 48:578–85. doi: 10.1007/s00125-004-1663-5
41. Chao CC, Hsieh SC, Yang WS, Lin YH, Lin WM, Tai TY, et al. Glycemic control is related to the severity of impaired thermal sensations in type 2 diabetes. Diabetes Metab Res Rev (2007) 23:612–20. doi: 10.1002/dmrr.734
42. Vas PR, Rayman G. Validation of the modified LDIFlare technique: a simple and quick method to assess c-fiber function. Muscle Nerve (2013) 47:351–6. doi: 10.1002/mus.23532
43. Sharma S, Tobin V, Vas PRJ, Malik RA, Rayman G. The influence of age, anthropometric and metabolic variables on LDIFLARE and corneal confocal microscopy in healthy individuals. PloS One (2018) 13:e0193452. doi: 10.1371/journal.pone.0193452
44. Sharma S, Tobin V, Rayman G. A prospective study of small fibre structure and function in newly diagnosed type 1 diabetes. presented at the 76th scientific sessions of the American diabetes association (New Orleans, USA. June 10-14, 2016). Diabetes (2016) 65:A101–69.
45. Inceu GV, Veresiu IA. Measurement of current perception thresholds using the Neurometer((R)) - applicability in diabetic neuropathy. Clujul Med (2015) 88:449–52. doi: 10.15386/cjmed-491
46. Ruscheweyh R, Emptmeyer K, Putzer D, Kropp P, Marziniak M. Reproducibility of contact heat evoked potentials (CHEPs) over a 6 months interval. Clin Neurophysiol (2013) 124:2242–7. doi: 10.1016/j.clinph.2013.05.003
47. Vallbo AB. Microneurography: how it started and how it works. J Neurophysiol (2018) 120:1415–27. doi: 10.1152/jn.00933.2017
48. La Cesa S, Di Stefano G, Leone C, Pepe A, Galosi E, Alu F, et al. Skin denervation does not alter cortical potentials to surface concentric electrode stimulation: a comparison with laser evoked potentials and contact heat evoked potentials. Eur J Pain (2018) 22:161–9. doi: 10.1002/ejp.1112
49. Shimada H, Kihara M, Kosaka S, Ikeda H, Kawabata K, Tsutada T, et al. Comparison of SSR and QSART in early diabetic neuropathy–the value of length-dependent pattern in QSART. Auton Neurosci (2001) 92:72–5. doi: 10.1016/S1566-0702(01)00287-9
50. Sun PC, Lin HD, Jao SH, Chan RC, Kao MJ, Cheng CK. Thermoregulatory sudomotor dysfunction and diabetic neuropathy develop in parallel in at-risk feet. Diabetes Med (2008) 25:413–8. doi: 10.1111/j.1464-5491.2008.02395.x
51. Lin X, Chen C, Liu Y, Peng Y, Chen Z, Huang H, et al. Peripheral nerve conduction and sympathetic skin response are reliable methods to detect diabetic cardiac autonomic neuropathy. Front Endocrinol (Lausanne) (2021) 12:709114. doi: 10.3389/fendo.2021.709114
52. Papanas N, Paschos P, Papazoglou D, Papatheodorou K, Paletas K, Maltezos E, et al. Accuracy of the neuropad test for the diagnosis of distal symmetric polyneuropathy in type 2 diabetes. Diabetes Care (2011) 34:1378–82. doi: 10.2337/dc10-2205
53. Lauria G, Bakkers M, Schmitz C, Lombardi R, Penza P, Devigili G, et al. Intraepidermal nerve fiber density at the distal leg: a worldwide normative reference study. J Peripher Nerv Syst (2010) 15:202–7. doi: 10.1111/j.1529-8027.2010.00271.x
55. Petropoulos IN, Ponirakis G, Khan A, Gad H, Almuhannadi H, Brines M, et al. Corneal confocal microscopy: ready for prime time. Clin Exp Optometry (2019) 103(3):265–77. doi: 10.1111/cxo.12887
56. Pritchard N, Edwards K, Russell AW, Perkins BA, Malik RA, Efron N. Corneal confocal microscopy predicts 4-year incident peripheral neuropathy in type 1 diabetes. Diabetes Care (2015) 38:671–5. doi: 10.2337/dc14-2114
57. Dineen J, Freeman R. Autonomic neuropathy. Semin Neurol (2015) 35:458–68. doi: 10.1055/s-0035-1558983
58. Spallone V. Update on the impact, diagnosis and management of cardiovascular autonomic neuropathy in diabetes: what is defined, what is new, and what is unmet. Diabetes Metab J (2019) 43:3–30. doi: 10.4093/dmj.2018.0259
59. Greco C, Di Gennaro F, D’Amato C, Morganti R, Corradini D, Sun A, et al. Validation of the composite autonomic symptom score 31 (COMPASS 31) for the assessment of symptoms of autonomic neuropathy in people with diabetes. Diabetes Med (2017) 34:834–8. doi: 10.1111/dme.13310
60. Sloan G, Alam U, Selvarajah D, Tesfaye S. The treatment of painful diabetic neuropathy. Curr Diabetes Rev (2022) 18:e070721194556. doi: 10.2174/1573399817666210707112413
61. Gandhi R, Selvarajah D, Sloan G, Greig M, Wilkinson ID, Shaw PJ, et al. Preservation of thalamic neuronal function may be a prerequisite for pain perception in diabetic neuropathy: a magnetic resonance spectroscopy study. Front Pain Res (Lausanne) (2022) 3:1086887. doi: 10.3389/fpain.2022.1086887
62. C. American Diabetes Association Professional Practice. 4. comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes-2022. Diabetes Care (2022) 45:S46–59. doi: 10.2337/dc22-S004
63. Sharma S, Cross J, Rayman G. 83-OR: the LDIFLAREMethod predicts the development of incipient diabetes polyneuropathy: results of the five-year longitudinal Ipswich NeuroDiab study. Diabetes (2022) 71. doi: 10.2337/db22-83-OR
64. Tavakoli M, Ferdousi M, Petropoulos IN, Morris J, Pritchard N, Zhivov A, et al. Normative values for corneal nerve morphology assessed using corneal confocal microscopy: a multinational normative data set. Diabetes Care (2015) 38:838–43. doi: 10.2337/dc14-2311
65. McArthur JC, Stocks EA, Hauer P, Cornblath DR, Griffin JW. Epidermal nerve fiber density: normative reference range and diagnostic efficiency. Arch Neurol (1998) 55:1513–20. doi: 10.1001/archneur.55.12.1513
66. Pop-Busui R, Roberts L, Pennathur S, Kretzler M, Brosius FC, Feldman EL. The management of diabetic neuropathy in CKD. Am J Kidney Dis (2010) 55:365–85. doi: 10.1053/j.ajkd.2009.10.050
67. Look ARG. Effects of a long-term lifestyle modification programme on peripheral neuropathy in overweight or obese adults with type 2 diabetes: the look AHEAD study. Diabetologia (2017) 60:980–8. doi: 10.1007/s00125-017-4253-z
68. Callaghan BC, Reynolds EL, Banerjee M, Akinci G, Chant E, Villegas-Umana E, et al. Dietary weight loss in people with severe obesity stabilizes neuropathy and improves symptomatology. Obes (Silver Spring) (2021) 29:2108–18. doi: 10.1002/oby.23246
69. Martin CL, Albers JW, Pop-Busui R, D.E.R. Group. Neuropathy and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care (2014) 37:31–8. doi: 10.2337/dc13-2114
70. Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. New Engl J Med (2008) 358:580–91. doi: 10.1056/NEJMoa0706245
71. Boussageon R, Bejan-Angoulvant T, Saadatian-Elahi M, Lafont S, Bergeonneau C, Kassai B, et al. Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: meta-analysis of randomised controlled trials. BMJ 343 (2011) 343:d4169. doi: 10.1136/bmj.d4169
72. Ziegler D, Tesfaye S, Spallone V, Gurieva I, Al Kaabi J, Mankovsky B, et al. Screening, diagnosis and management of diabetic sensorimotor polyneuropathy in clinical practice: international expert consensus recommendations. Diabetes Res Clin Pract (2022) 186:109063. doi: 10.1016/j.diabres.2021.109063
73. Tesfaye S, Selvarajah D. The eurodiab study: what has this taught us about diabetic peripheral neuropathy? Curr Diabetes Rep (2009) 9:432–4. doi: 10.1007/s11892-009-0070-1
74. Balakumar P, Rohilla A, Krishan P, Solairaj P, Thangathirupathi A. The multifaceted therapeutic potential of benfotiamine. Pharmacol Res (2010) 61:482–8. doi: 10.1016/j.phrs.2010.02.008
75. Papanas N, Ziegler D. Efficacy of alpha-lipoic acid in diabetic neuropathy. Expert Opin Pharmacother (2014) 15:2721–31. doi: 10.1517/14656566.2014.972935
76. Ziegler D, Low PA, Litchy WJ, Boulton AJ, Vinik AI, Freeman R, et al. Efficacy and safety of antioxidant treatment with alpha-lipoic acid over 4 years in diabetic polyneuropathy: the NATHAN 1 trial. Diabetes Care (2011) 34:2054–60. doi: 10.2337/dc11-0503
77. Ziegler D, Movsesyan L, Mankovsky B, Gurieva I, Abylaiuly Z, Strokov I. Treatment of symptomatic polyneuropathy with actovegin in type 2 diabetic patients. Diabetes Care (2009) 32:1479–84. doi: 10.2337/dc09-0545
78. Hotta N, Akanuma Y, Kawamori R, Matsuoka K, Oka Y, Shichiri M, et al. Long-term clinical effects of epalrestat, an aldose reductase inhibitor, on diabetic peripheral neuropathy: the 3-year, multicenter, comparative aldose reductase inhibitor-diabetes complications trial. Diabetes Care (2006) 29:1538–44. doi: 10.2337/dc05-2370
79. El-Nahas MR, Elkannishy G, Abdelhafez H, Elkhamisy ET, El-Sehrawy AA. Oral alpha lipoic acid treatment for symptomatic diabetic peripheral neuropathy: a randomized double-blinded placebo-controlled study. Endocr Metab Immune Disord Drug Targets (2020) 20:1531–4. doi: 10.2174/1871530320666200506081407
80. Tesfaye S, Sloan G, Petrie J, White D, Bradburn M, Young T, et al. Optimal pharmacotherapy pathway in adults with diabetic peripheral neuropathic pain: the OPTION-DM RCT. Health Technol Assess (2022) 26:1–100. doi: 10.3310/RXUO6757
81. Tesfaye S, Wilhelm S, Lledo A, Schacht A, Tolle T, Bouhassira D, et al. Duloxetine and pregabalin: high-dose monotherapy or their combination? the “COMBO-DN study”–a multinational, randomized, double-blind, parallel-group study in patients with diabetic peripheral neuropathic pain. Pain (2013) 154:2616–25. doi: 10.1016/j.pain.2013.05.043
82. Dy SM, Bennett WL, Sharma R, Zhang A, Waldfogel JM, Nesbit SA, et al. Preventing complications and treating symptoms of diabetic peripheral neuropathy [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US). (2017). Report No.: 17-EHC005-EF.
83. Snedecor SJ, Sudharshan L, Cappelleri JC, Sadosky A, Mehta S, Botteman M. Systematic review and meta-analysis of pharmacological therapies for painful diabetic peripheral neuropathy. Pain Pract (2014) 14:167–84. doi: 10.1111/papr.12054
84. Liampas A, Rekatsina M, Vadalouca A, Paladini A, Varrassi G, Zis P. Pharmacological management of painful peripheral neuropathies: a systematic review. Pain Ther (2021) 10:55–68. doi: 10.1007/s40122-020-00210-3
85. Griebeler ML, Morey-Vargas OL, Brito JP, Tsapas A, Wang Z, Carranza Leon BG, et al. Pharmacologic interventions for painful diabetic neuropathy: an umbrella systematic review and comparative effectiveness network meta-analysis. Ann Internal Med (2014) 161:639–49. doi: 10.7326/M14-0511
86. Simpson DM, Robinson-Papp J, Van J, Stoker M, Jacobs H, Snijder RJ, et al. Capsaicin 8% patch in painful diabetic peripheral neuropathy: a randomized, double-blind, placebo-controlled study. J Pain (2017) 18:42–53. doi: 10.1016/j.jpain.2016.09.008
87. Pollard EM, Lamer TJ, Moeschler SM, Gazelka HM, Hooten WM, Bendel MA, et al. The effect of spinal cord stimulation on pain medication reduction in intractable spine and limb pain: a systematic review of randomized controlled trials and meta-analysis. J Pain Res (2019) 12:1311–24. doi: 10.2147/JPR.S186662
88. Petersen EA, Stauss TG, Scowcroft JA, Brooks ES, White JL, Sills SM, et al. Effect of high-frequency (10-kHz) spinal cord stimulation in patients with painful diabetic neuropathy: a randomized clinical trial. JAMA Neurol (2021) 78:687–98. doi: 10.1001/jamaneurol.2021.0538
Keywords: diabetes, polyneuropathy, small fibre neuropathy, large fibre neuropathy, early diagnosis diabetes neuropathy
Citation: Sharma S and Rayman G (2023) Frontiers in diagnostic and therapeutic approaches in diabetic sensorimotor neuropathy (DSPN). Front. Endocrinol. 14:1165505. doi: 10.3389/fendo.2023.1165505
Received: 14 February 2023; Accepted: 01 May 2023;
Published: 18 May 2023.
Edited by:
Lixin Guo, Peking University, ChinaReviewed by:
Carla Greco, University of Modena and Reggio Emilia, ItalyShazli Azmi, Manchester University NHS Foundation Trust (MFT), United Kingdom
Prashanth R. J. Vas, King’s College Hospital NHS Foundation Trust, United Kingdom
Copyright © 2023 Sharma and Rayman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sanjeev Sharma, c2FuamVldi5zaGFybWFAZXNuZWZ0Lm5ocy51aw==
 Sanjeev Sharma
Sanjeev Sharma Gerry Rayman
Gerry Rayman