
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Endocrinol., 21 April 2023
Sec. Neuroendocrine Science
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1163482
This article is part of the Research TopicGlucocorticoids and Cognition: Recent Advances in Understanding Their Interaction, With a Particular Focus on Clinical Applicability for the Treating EndocrinologistView all 5 articles
 Eleni Papakokkinou1,2*
Eleni Papakokkinou1,2* Oskar Ragnarsson1,2,3
Oskar Ragnarsson1,2,3Cognitive impairment and affective disorders are common in patients with Cushing’s syndrome (CS). In fact, as an effect of prolonged cortisol excess on the brain, patients with CS often have memory problems, concentration difficulties, impaired attention and executive function, that are not always reversible following successful treatment. Neuroimaging is essential for understanding the deleterious effects of hypercortisolism on the brain. In CS, structural alterations have been observed, including reduction of hippocampal volume, amygdala and the prefrontal cortex. The aim of this article is to summarize results from studies that have used functional magnetic resonance imaging (fMRI) to study functional brain alterations in patients with CS. In these studies, alterations in brain areas and networks essential for cognitive function, emotional processing, and executive function have been observed, both in patients with active CS as well as following treatment. Nevertheless, longitudinal studies with a comprehensive evaluation of functional brain alterations and neurocognitive evaluation are still needed to determine whether the apparent deleterious effects of hypercortisolism on the brain are reversible or not.
Cushing’s syndrome (CS) is caused by chronic and excessive exposure to cortisol (1). The most common causes of endogenous CS are Cushing’s disease (CD), i.e., adrenocorticotropic (ACTH)-producing pituitary adenoma, cortisol-producing adrenal adenoma and ectopic ACTH-producing tumors (2). Obesity, arterial hypertension, diabetes mellitus, myopathy, fractures, and depression are common and typical features of CS (3). Consequently, CS is associated with severely impaired quality of life, increased morbidity and mortality, not only during the stage of active hypercortisolism, but also during long-term follow-up after successful treatment (4–7).
Affective disorders and cognitive dysfunction are common manifestations of CS and have a great impact on quality of life in the patients (8). In fact, memory and concentration difficulties were among the earliest symptoms described in CS (9, 10). Recently, a more comprehensive cognitive dysfunction has been revealed in patients with active CS, including impaired attention, visuospatial processing, processing speed and executive functioning. Unfortunately, despite treatment, patients with CS still have impaired memory and executive function, in comparison to healthy controls, as well as when compared with patients treated for nonfunctioning pituitary macroadenomas (11). Also, patients with CS in long-term remission have impaired attention, working memory, verbal fluency, reading speed (12) and difficulties in decision making (13). Thus, it seems likely that the brain abnormalities are only partially reversible after successful treatment (14).
Glucocorticoid receptors are expressed globally throughout the whole brain (15). Hence, the whole brain is susceptible to excessive cortisol exposure in patients with CS. Mineralocorticoid receptors are also expressed in the brain, although distributed more specifically in the limbic system and the prefrontal cortex. However, since cortisol in high concentrations also binds to the mineralocorticoid receptors, the limbic system and prefrontal cortex, i.e., regions important for cognitive function, are especially vulnerable for hypercortisolism. Consequently, widespread alterations in white matter integrity have been observed in patients with CS (16) as well as structural changes in the hippocampus, amygdala and the prefrontal cortex (14, 17).
Functional magnetic resonance imaging (fMRI) has become an important method in investigating the detrimental effects of hypercortisolism on the brain. In this review, we focus on recent studies investigating brain alterations in patients with CS that have used fMRI during cognitive task performance or during a resting state (rsfMRI).
Task-related fMRI is used to examine functional alterations during performance of specific tasks, including cognitive tasks. Currently, four studies have evaluated brain activity in patients with CS with task-related fMRI (Table 1 and Fact box). The first study was performed in twelve adolescent patients with active CS and showed increased activation in left amygdala and right anterior hippocampus during face encoding task, compared to healthy controls (28). These functional alterations were, however, not associated with affective disorders or memory impairment. Similar findings were found in adult patients with active CS, who demonstrated higher activation in the prefrontal cortex, hippocampus, thalamus and amygdala during identification of facial expressions (27). Of note, elevated activation in left middle frontal and lateral posterior/pulvinar areas, were associated with emotional processing, possibly indicating a compensatory activation (27). Likewise, these patients made more errors in identification of facial expressions and showed decreased activation in left anterior, superior temporal gyrus, a key area for facial emotional processing (27).
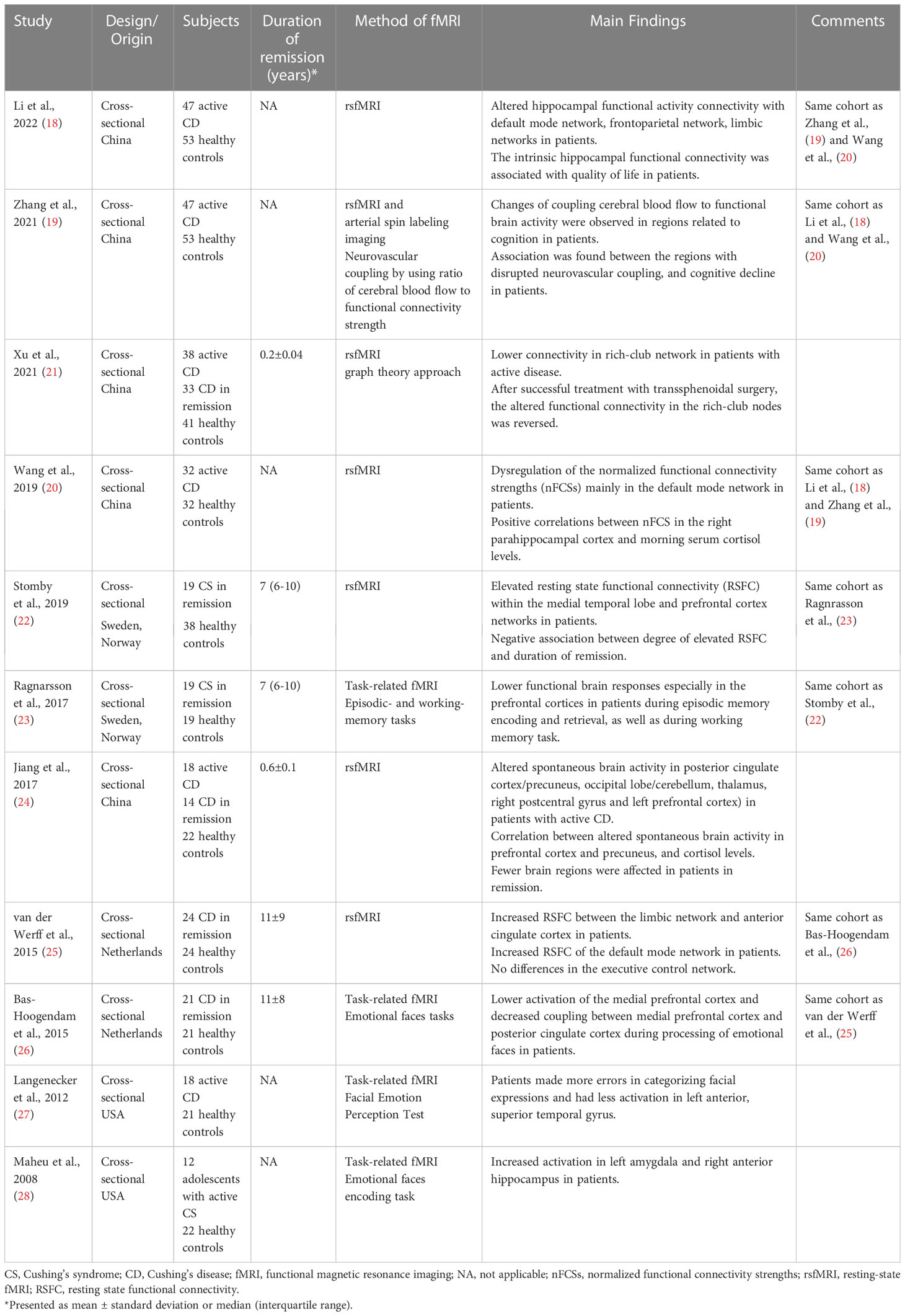
Table 1 Summary of studies in patients with Cushing’s syndrome using functional magnetic resonance imaging (fMRI).
Two additional studies showed that alterations in brain function are still present in patients with CS in long-term remission. In a study including twenty-one patients with CD in remission for a mean duration of eleven years, lower activation was shown in the medial prefrontal cortex during processing of facial expressions, as compared to controls (26). On the contrary, no alterations in amygdala activation were found during emotional processing. Interestingly, decreased functional coupling between the medial prefrontal cortex and the posterior cingulate cortex during emotional performance task was demonstrated (26), areas that are part of the default mode network which is important for episodic memory, conceptual processing, and self-referential processing (29). Finally, during working and episodic memory-task fMRI, decreased functional brain responses were found in prefrontal cortices in female patients with CS in long-term remission, compared to healthy controls matched for gender, age and education (23) (Figure 1).
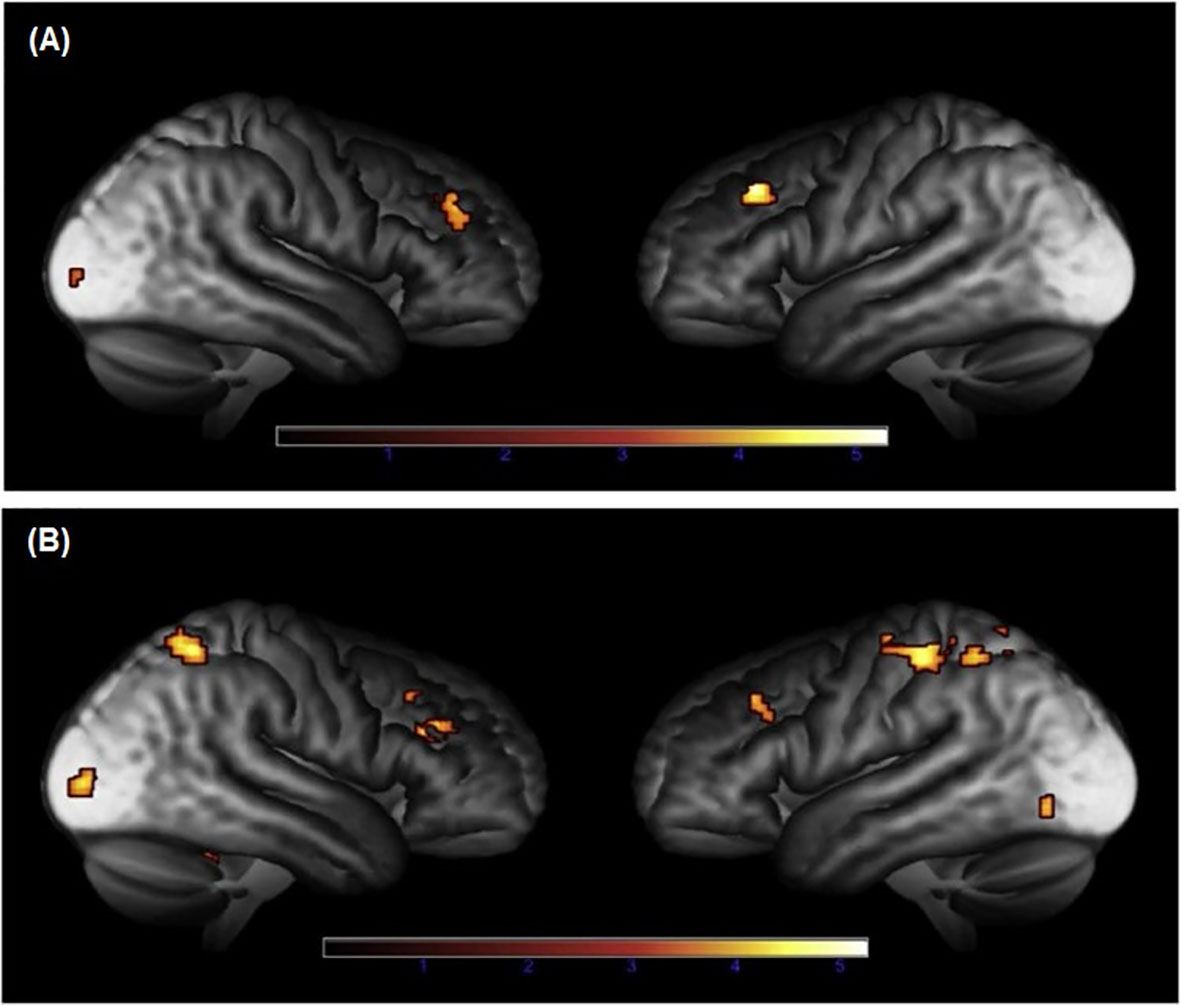
Figure 1 Brain areas with reduced functional brain responses during episodic memory during (A) encoding and (B) retrieval in patients with Cushing’s syndrome, compared to controls (23). Adapted from Ragnarsson O. et al. Psychoneuroendocrinology, 2017; 82:117-25, with permission from Elsevier.
Seven studies have used rsMRI to study functional connectivity in CS (Table 1). During rsfMRI, the participants are asked to close their eyes and stay awake without performing any cognitive task. In active CD, widespread altered spontaneous brain activity was observed (24). More specifically, five brain regions were affected, including the posterior cingulate cortex/precuneus, occipital lobe/cerebellum, thalamus, right postcentral gyrus, and left prefrontal cortex. A significant correlation was shown between cortisol concentrations and altered spontaneous activity in prefrontal cortex/precuneus and the occipital lobe (24). Also, in patients with active CD, dysregulation of functional connectivity strength was found in the default mode network, including parahippocampal cortices, posterior cingulate cortices, lateral parietal cortices and right prefrontal cortex (20) (Figure 2). In the same cohort, altered hippocampal functional connectivity within the default mode network, frontoparietal and limbic network was found. The hippocampal functional activity correlated positively with quality of life (18). Moreover, disrupted connection between neurons and their vascular supply, measured as changes of coupling cerebral blood flow and functional connectivity strength, were observed in several brain regions related to cognitive function, suggesting an impact of hypercortisolism on the cerebral microenvironmental regulation (19). This disrupted neurovascular coupling was associated with cognitive impairment (19). Impaired functional connectivity was also found within the rich-club network, which consists of nodes with more dense interconnections, e.g., precuneus, cingulum and inferior temporal regions (21). After successful transsphenoidal surgery, the altered functional connectivity in the rich-club network was reversed (21).
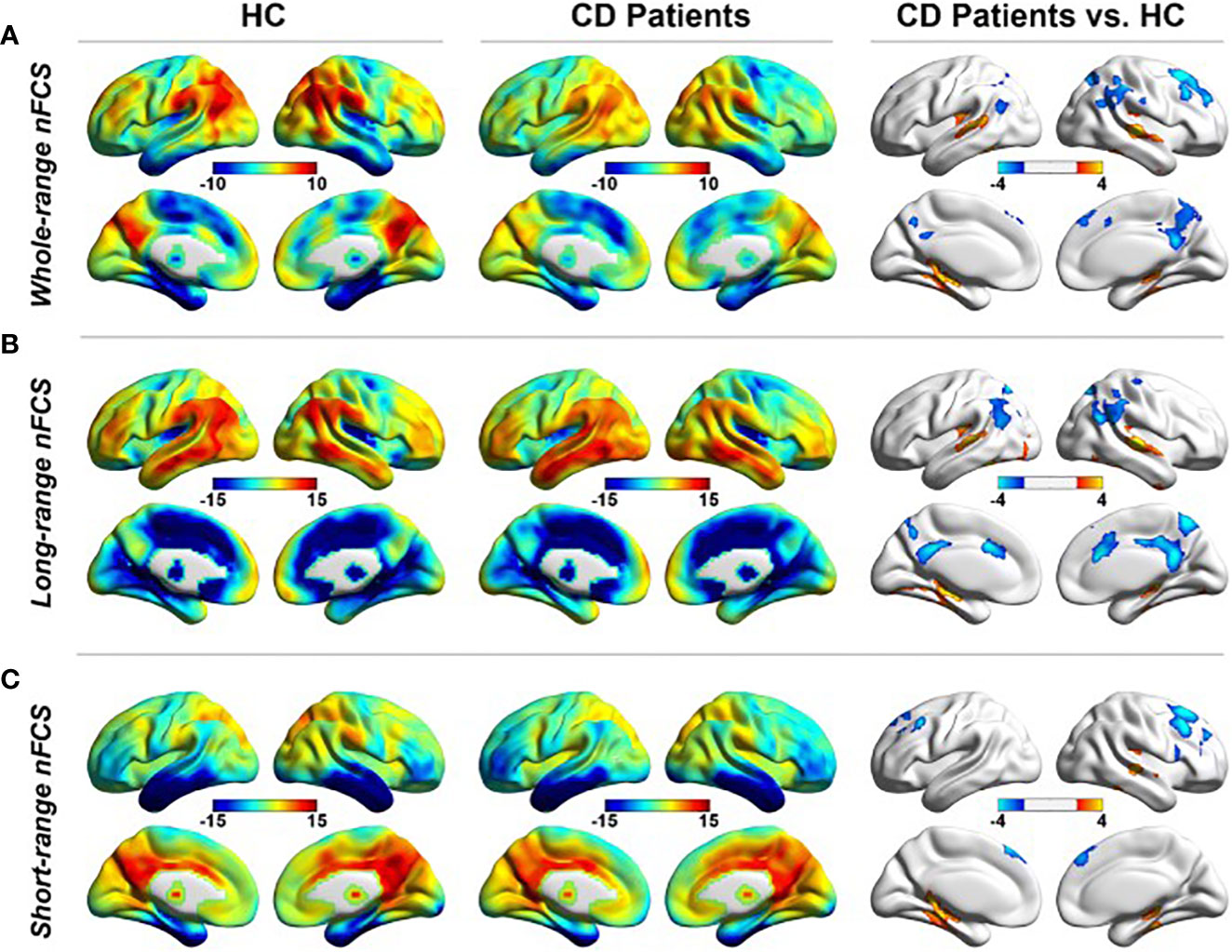
Figure 2 rsfMRI showing altered functional connectivity in the default mode network during (A) whole-range normalized functional connectivity strength (nFCS), (B) long-range nFCS, and (C) short-range nFCS, in patients with Cushing’s disease (CD) compared to healthy controls (HC) (20). Reproduced from Wang X. et al. Neuroradiology, 2019; 61 (8): 911-20, with permission from Springer Nature.
Two studies have applied rsfMRI to explore brain functional networks in patients with CS in remission. The first study by van der Werff et al., included 24 patients with CD in long-term remission, and demonstrated increased functional connectivity between the limbic network and the anterior cingulate cortex, compared to matched healthy controls (25). In addition, increased resting-state functional connectivity was found in the default mode network in the left lateral occipital cortex (25). Nevertheless, these functional alterations were not associated with affective symptoms and no differences in the executive control network were observed, probably due to subtle cognitive impairment in the patients, and the absence of cognitive demands during sfMRI (25).
In line with this study, Stomby et al., showed elevated functional connectivity within the medial temporal lobe and prefrontal cortex in nineteen female patients with CS in long-term remission (22). On the contrary, reduced functional activity was found in the parietal lobe. Also, the degree of elevated functional connectivity in the medial temporal lobe was negatively associated with the duration in remission. Lack of neurocognitive evaluation of the participants did not allow further analysis of the association between altered functional activity and cognitive function.
Functional brain alterations in CS have mostly been observed in the default mode network and the limbic network. Whether these functional brain alterations are associated with cognitive deficits is still unclear. In studies where neurocognitive assessment has been performed, association between functional brain alterations and cognitive deficits or affective disorders, has not been distinctly confirmed (18, 23, 25, 26), probably due to small study populations and/or due to the use of insufficiently sensitive neurocognitive tests. Nevertheless, two studies have shown association between functional brain alterations and cognitive dysfunction. In the study by Langenecker et al., functional alterations in a region important for emotional processing were associated with worse performance in categorizing facial expressions (27). Also, Zhang et al. showed that functional alterations in areas of the executive control network were associated with cognitive decline (19). Moreover, it has previously been shown that patients with CS have impaired memory, concentration, attention as well as higher scores on the apathy scale (11, 12, 30). This is line with functional brain alterations observed in the default mode network and the limbic network.
All studies but one, were performed in adult patients and healthy controls matched at least for age and gender. In the only study including adolescents, functional brain alterations in amygdala and hippocampus were not associated with affective and memory impairments (28). Whether this reflects neural plasticity in younger patients, needs further investigation. Indeed, prospective longitudinal studies are needed to link functional brain alterations with neurocognitive deficits.
Cognitive dysfunction is one of the most important issues that impacts quality of life negatively in patients with CS, even after successful treatment. fMRI is an essential tool that can be used to explore the potential mechanisms of cortisol excess on the brain. Functional brain alterations have been illustrated in the limbic network, default mode network and executive control network, namely networks that are essential for cognitive function. These findings strongly suggest an association between hypercortisolism, functional brain alterations and cognitive impairment in patients with CS. Nevertheless, the current studies do not provide robust information on whether the functional brain alterations reflect cognitive and affective symptoms in patients with CS. Moreover, altered functional connectivity and responses to performance tasks have been demonstrated in patients in remission, indicating persistent effects of cortisol excess on the brain despite successful treatment. Yet, the available data is not sufficient to elucidate the reversibility of the functional brain abnormalities in CS due to the small cohorts and lack of longitudinal follow-up. Hence, longitudinal prospective studies are needed to enable investigation of the course of functional brain alterations in patients with CS, from active hypercortisolism to long-term remission.
All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Newell-Price J, Bertagna X, Grossman AB, Nieman LK. Cushing’s syndrome. Lancet (2006) 367(9522):1605–17. doi: 10.1016/S0140-6736(06)68699-6
2. Lacroix A, Feelders RA, Stratakis CA, Nieman LK. Cushing’s syndrome. Lancet (2015) 386(9996):913–27. doi: 10.1016/S0140-6736(14)61375-1
3. Valassi E, Santos A, Yaneva M, Toth M, Strasburger CJ, Chanson P, et al. The European registry on cushing’s syndrome: 2-year experience. baseline demographic and clinical characteristics. Eur J Endocrinol (2011) 165(3):383–92. doi: 10.1530/EJE-11-0272
4. Ragnarsson O, Olsson DS, Papakokkinou E, Chantzichristos D, Dahlqvist P, Segerstedt E, et al. Overall and disease-specific mortality in patients with cushing disease: A Swedish nationwide study. J Clin Endocrinol Metab (2019) 104(6):2375–84. doi: 10.1210/jc.2018-02524
5. Papakokkinou E, Olsson DS, Chantzichristos D, Dahlqvist P, Segerstedt E, Olsson T, et al. Excess morbidity persists in patients with Cushing’s disease during long-term remission: a Swedish nationwide study. J Clin Endocrinol Metab (2020) 105(8). doi: 10.1210/clinem/dgaa291
6. Dekkers OM, Horvath-Puho E, Jorgensen JO, Cannegieter SC, Ehrenstein V, Vandenbroucke JP, et al. Multisystem morbidity and mortality in cushing’s syndrome: a cohort study. J Clin Endocrinol Metab (2013) 98(6):2277–84. doi: 10.1210/jc.2012-3582
7. van Haalen FM, Broersen LH, Jorgensen JO, Pereira AM, Dekkers OM. Management of endocrine disease: mortality remains increased in Cushing’s disease despite biochemical remission: A systematic review and meta-analysis. Eur J Endocrinol (2015) 172(4):R143–9. doi: 10.1530/EJE-14-0556
8. Bride MM, Crespo I, Webb SM, Valassi E. Quality of life in cushing’s syndrome. Best Pract Res Clin Endocrinol Metab (2021) 35(1):101505. doi: 10.1016/j.beem.2021.101505
9. Cushing H. The basophil adenomas of the pituitary bodyand their clinical manifestations (Pituitary basophilism). Bull Johns Hopkins Hosp (1932) 50:137–95.
10. Starkman MN, Gebarski SS, Berent S, Schteingart DE. Hippocampal formation volume, memory dysfunction, and cortisol levels in patients with cushing’s syndrome. Biol Psychiatry (1992) 32(9):756–65. doi: 10.1016/0006-3223(92)90079-f
11. Tiemensma J, Kokshoorn NE, Biermasz NR, Keijser BJ, Wassenaar MJ, Middelkoop HA, et al. Subtle cognitive impairments in patients with long-term cure of Cushing’s disease. J Clin Endocrinol Metab (2010) 95(6):2699–714. doi: 10.1210/jc.2009-2032
12. Ragnarsson O, Berglund P, Eder DN, Johannsson G. Long-term cognitive impairments and attentional deficits in patients with Cushing’s disease and cortisol-producing adrenal adenoma in remission. J Clin Endocrinol Metab (2012) 97(9):E1640–8. doi: 10.1210/jc.2012-1945
13. Crespo I, Esther GM, Santos A, Valassi E, Yolanda VG, De Juan-Delago M, et al. Impaired decision-making and selective cortical frontal thinning in cushing’s syndrome. Clin Endocrinol (Oxf) (2014) 81(6):826–33. doi: 10.1111/cen.12564
14. Andela CD, van Haalen FM, Ragnarsson O, Papakokkinou E, Johannsson G, Santos A, et al. Mechanisms in endocrinology: Cushing’s syndrome causes irreversible effects on the human brain: a systematic review of structural and functional magnetic resonance imaging studies. Eur J Endocrinol (2015) 173(1):R1–14. doi: 10.1530/EJE-14-1101
15. de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci (2005) 6(6):463–75. doi: 10.1038/nrn1683
16. Bauduin S, van der Wee NJA, van der Werff SJA. Structural brain abnormalities in cushing’s syndrome. Curr Opin Endocrinol Diabetes Obes (2018) 25(4):285–9. doi: 10.1097/MED.0000000000000414
17. Piasecka M, Papakokkinou E, Valassi E, Santos A, Webb SM, de Vries F, et al. Psychiatric and neurocognitive consequences of endogenous hypercortisolism. J Intern Med (2020) 288(2):168–82. doi: 10.1111/joim.13056
18. Li C, Zhang Y, Wang W, Zhou T, Yu X, Tao H. Altered hippocampal volume and functional connectivity in patients with Cushing’s disease. Brain Behav (2022) 12(6):e2507. doi: 10.1002/brb3.2507
19. Zhang Y, Zhou T, Feng S, Wang W, Liu H, Wang P, et al. The chronic effect of cortisol on orchestrating cerebral blood flow and brain functional connectivity: Evidence from Cushing’s disease. Metabolism (2021) 115:154432. doi: 10.1016/j.metabol.2020.154432
20. Wang X, Zhou T, Wang P, Zhang L, Feng S, Meng X, et al. Dysregulation of resting-state functional connectivity in patients with Cushing’s disease. Neuroradiology (2019) 61(8):911–20. doi: 10.1007/s00234-019-02223-y
21. Xu CX, Jiang H, Zhao ZJ, Sun YH, Chen X, Sun BM, et al. Disruption of rich-club connectivity in cushing disease. World Neurosurg (2021) 148:e275–e81. doi: 10.1016/j.wneu.2020.12.146
22. Stomby A, Salami A, Dahlqvist P, Evang JA, Ryberg M, Bollerslev J, et al. Elevated resting-state connectivity in the medial temporal lobe and the prefrontal cortex among patients with cushing’s syndrome in remission. Eur J Endocrinol (2019) 180(5):329–38. doi: 10.1530/EJE-19-0028
23. Ragnarsson O, Stomby A, Dahlqvist P, Evang JA, Ryberg M, Olsson T, et al. Decreased prefrontal functional brain response during memory testing in women with cushing’s syndrome in remission. Psychoneuroendocrinology (2017) 82:117–25. doi: 10.1016/j.psyneuen.2017.05.010
24. Jiang H, He NY, Sun YH, Jian FF, Bian LG, Shen JK, et al. Altered spontaneous brain activity in Cushing’s disease: a resting-state functional mri study. Clin Endocrinol (Oxf) (2017) 86(3):367–76. doi: 10.1111/cen.13277
25. van der Werff SJ, Pannekoek JN, Andela CD, Meijer OC, van Buchem MA, Rombouts SA, et al. Resting-state functional connectivity in patients with long-term remission of Cushing’s disease. Neuropsychopharmacology (2015) 40(8):1888–98. doi: 10.1038/npp.2015.38
26. Bas-Hoogendam JM, Andela CD, van der Werff SJ, Pannekoek JN, van Steenbergen H, Meijer OC, et al. Altered neural processing of emotional faces in remitted Cushing’s disease. Psychoneuroendocrinology (2015) 59:134–46. doi: 10.1016/j.psyneuen.2015.05.001
27. Langenecker SA, Weisenbach SL, Giordani B, Briceno EM, Guidotti Breting LM, Schallmo MP, et al. Impact of chronic hypercortisolemia on affective processing. Neuropharmacology (2012) 62(1):217–25. doi: 10.1016/j.neuropharm.2011.07.006
28. Maheu FS, Mazzone L, Merke DP, Keil MF, Stratakis CA, Pine DS, et al. Altered amygdala and hippocampus function in adolescents with hypercortisolemia: a functional magnetic resonance imaging study of cushing syndrome. Dev Psychopathol (2008) 20(4):1177–89. doi: 10.1017/S0954579408000564
29. Smallwood J, Bernhardt BC, Leech R, Bzdok D, Jefferies E, Margulies DS. The default mode network in cognition: a topographical perspective. Nat Rev Neurosci (2021) 22(8):503–13. doi: 10.1038/s41583-021-00474-4
Keywords: Cushing’s syndrome, functional connectivity, task-related functional magnetic resonance imaging, resting-state functional magnetic resonance imaging, prefrontal cortex, hippocampus, default mode network
Citation: Papakokkinou E and Ragnarsson O (2023) Functional brain alterations in Cushing’s syndrome. Front. Endocrinol. 14:1163482. doi: 10.3389/fendo.2023.1163482
Received: 10 February 2023; Accepted: 28 March 2023;
Published: 21 April 2023.
Edited by:
Ian Ross, University of Cape Town, South AfricaReviewed by:
Malgorzata Monika Brzozowska, University of New South Wales, AustraliaCopyright © 2023 Papakokkinou and Ragnarsson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eleni Papakokkinou, ZWxlbmkucGFwYWtva2tpbm91QHZncmVnaW9uLnNl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.