- 1Department of General Surgery, Institutes of Biomedical Sciences, Zhongshan Hospital, Fudan University, Shanghai, China
- 2State Key Laboratory of Proteomics, Beijing Proteome Research Center, National Center for Protein Sciences, Beijing, China
Background: Surgery is the best way to cure the retroperitoneal leiomyosarcoma (RLMS), and there is currently no prediction model on RLMS after surgical resection. The objective of this study was to develop a nomogram to predict the overall survival (OS) of patients with RLMS after surgical resection.
Methods: Patients who underwent surgical resection from September 2010 to December 2020 were included. The nomogram was constructed based on the COX regression model, and the discrimination was assessed using the concordance index. The predicted OS and actual OS were evaluated with the assistance of calibration plots.
Results: 118 patients were included. The median OS for all patients was 47.8 (95% confidence interval (CI), 35.9-59.7) months. Most tumor were completely resected (n=106, 89.8%). The proportions of French National Federation of Comprehensive Cancer Centres (FNCLCC) classification were equal as grade 1, grade 2, and grade 3 (31.4%, 30.5%, and 38.1%, respectively). The tumor diameter of 73.7% (n=85) patients was greater than 5 cm, the lesions of 23.7% (n=28) were multifocal, and 55.1% (n=65) patients had more than one organ resected. The OS nomogram was constructed based on the number of resected organs, tumor diameter, FNCLCC grade, and multifocal lesions. The concordance index of the nomogram was 0.779 (95% CI, 0.659-0.898), the predicted OS and actual OS were in good fitness in calibration curves.
Conclusion: The nomogram prediction model established in this study is helpful for postoperative consultation and the selection of patients for clinical trial enrollment.
Introduction
Leiomyosarcoma (LMS) is a mesenchymal malignant tumor with distinct smooth muscle differentiation (1), accounting for about 7-10% of all soft tissue sarcomas (STS). It is the second most common subtype of retroperitoneal sarcoma (RPS) (2). Currently, surgical resection is the best way to cure, but about 70% patients suffer from recurrence within five years after the surgical resection (3). The 5-year overall survival (OS) of the LMS patient is about 50%-60% after surgical resection for primary or recurrent disease (4, 5). The postoperative outcomes after extended resection for RPS have been reported, highlighting differences by specific histologic subtype (4). In addition, a report from the French Sarcoma Group pointed out that LMS represents a heterogeneous group of tumors, and retroperitoneal leiomyosarcoma (RLMS) shows totally different clinical outcomes and molecular features than others (6). Therefore, it is key to find good means to effectively incorporate our understanding of disease biology into clinical decision-making.
Nomogram is a statistical tool to predict the prognosis of an individual patient, and it has been widely applied in RPS (5, 7–9). But the distinct patterns of local and distant recurrence of the various subtypes of RPS suggest that separate nomograms for predicting the OS of RLMS patients will be useful (9). It has been reported that the tumor size, resection margin status, and tumor grade are risk factors for the poor prognosis of RLMS (10–12). However, no nomogram is constructed currently to predict the OS of RLMS patients only.
Therefore, this study aimed to investigate the independent prognostic factors for OS of RLMS patients after surgical resection and to construct a nomogram to predict the 1-, 2-, and 5-year OS of the RLMS patients.
Methods
Patients
118 consecutive patients who underwent surgical resection from September 2010 to December 2020 at General Surgery Department, Shanghai Public Health Clinical Center, Fudan University, Shanghai, China were included. The inclusion criteria were as follows: 1) histologically confirmed LMS, 2) tumors originating in the retroperitoneum, 3) complete clinical pathological information and follow-up information. LMS is diagnosed by pathological features stained by H&E, including oval or cigar-shaped nuclei with blunt ends, variable eosinophilic cytoplasm, and uniform positive staining for α-sma, desmin, and/or h-caldesmon (6). Gastrointestinal stromal tumors were excluded. The study was approved by the Ethics Committee of Shanghai Public Health Clinical Center. All enrolled patients signed informed consent for data collection during hospitalization.
The baseline characteristics including gender, age, metastatic disease, times of surgery, complete resection (complete: R0 and R1, negative gross margins; incomplete: R2, grossly positive margins), number of resected organ, tumor diameter, French National Federation of Comprehensive Cancer Centres (FNCLCC) grade, tumor node metastasis (TNM) stage, multifocality, inferior vena cava invasion, radiation, chemotherapy, and other therapy (focused ultrasound, interventional embolization and intraperitoneal hyperthermia chemotherapy). Treatment plans for all patients were determined by a multidisciplinary team of surgeons, medical oncologists, radiologists, and pathologists. Postoperative follow-up of the patients required physical examination and enhanced CT or MRI of the chest, abdomen and pelvic. The first follow-up was 3 months after surgery, then every 3 months for 2 years; 6 months for 5 years; and every 1 year after 5 years.
Statistical analysis
The primary end-point of the study was OS (defined as the duration from date of the surgery to the date of death or follow-up) which estimated by the Kaplan–Meier method, and the differences between groups were compared with the log-rank test. Because the TNM staging system itself was a predictive tool, which already included relevant variables such as tumor size and tumor grade, it was not included in the risk factor analysis. Risk factors affecting survival were determined by COX regression. Variables with p-values <0.2 in univariate analysis were included in multivariate analysis. A nomogram was then constructed using the variables in the multivariate analysis with p-value < 0.1 to predict OS at 1, 2, and 5 years after surgery. The prediction accuracy of the nomogram was assessed by a calibration curve, and the c-index was judged the discriminative ability. Finally, the cohort was divided into a low-risk group and a high-risk group based on the values of the maximum sum of sensitivity and specificity (Youden index) (13). All the tests were two-tailed and p < 0.05 meant the difference was statistically significance.
Statistical analyses were performed with SPSS version 22.0 (Chicago, IL, USA) and R software (The R Foundation for Statistical Computing, Vienna, Austria; version 4.1.1; http://www.r-project.org/).
Results
Baseline characteristics
Characteristics of 118 patients were provided in Table 1. Most patients were female (n=99, 83.9%) without metastasis disease (n=106, 89.8%), and the tumor was completely resected (n=106, 89.8%). Patients with initial surgery, second surgery, and more than twice accounted for 39.8%(n=47), 34.7% (n=41), and 25.4% (n=30), respectively. The proportions of FNCLCC classification were equal as grade 1, grade 2, and grade 3, which were 31.4%, 30.5%, and 38.1%, respectively. The tumor diameter of 73.7% (n=85) patients was greater than 5 cm, the lesions of 23.7% (n=28) were multifocal, and 55.1% (n=65) patients had multiple organs resected. In terms of adjuvant therapy, 22 patients received radiotherapy, 46 received chemotherapy, and 15 received other therapies.
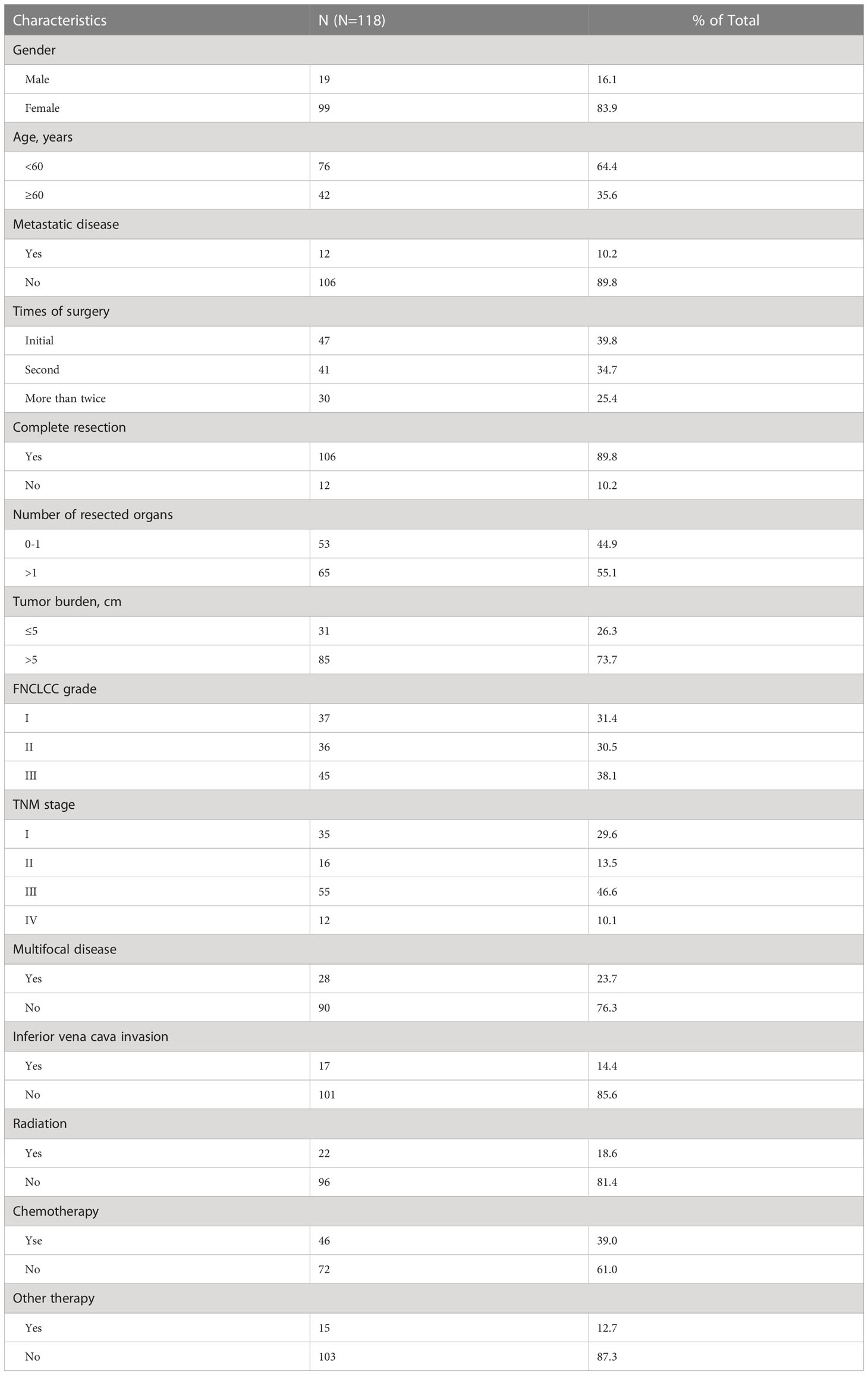
Table 1 Demographics and clinical characteristics of 118 patients with retroperitoneal leiomyosarcoma.
Survival analysis
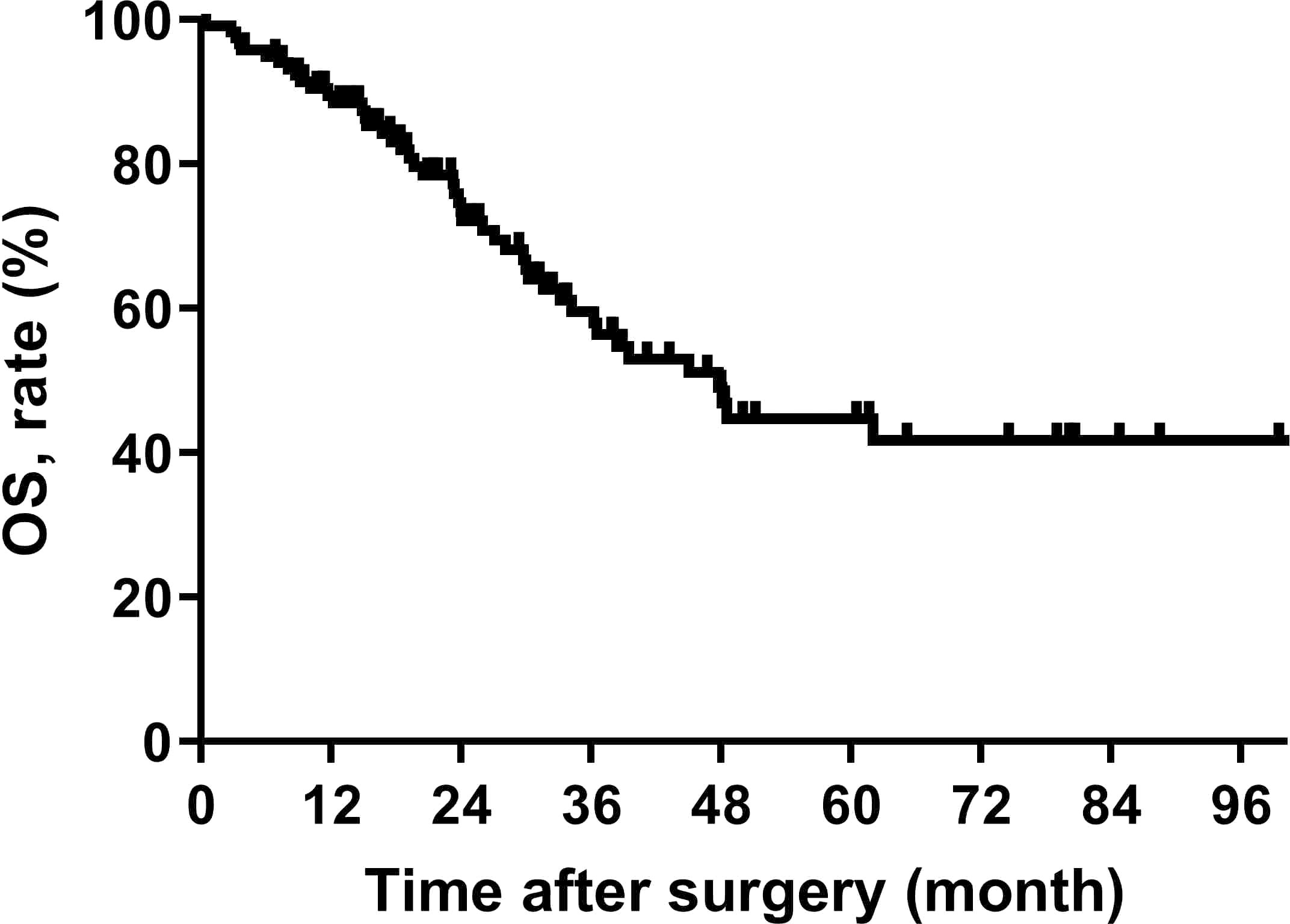
The median OS for all patients was 47.8 (95% CI, 35.9-59.7) months, the median follow-up for all survivors was 31.0 (4 ~ 113 months) months, and the 5 years OS rate was 44.7% (95% CI, 32.6%-56.8%) (Figure 1).
The univariable analysis showed that the number of resected organs (p=0.012), tumor diameter (p=0.005), and FNCLCC grade (p<0.001) were related to OS. The results of multivariate analysis suggested that FNCLCC grade (p<0.001, Grade 2 vs. Grade 1 [HR=3.320, 95% CI 1.088-10.129], Grade 3 vs. Grade 1 [HR=11.693, 95% CI 4.183-32.689]) and multifocal lesions (p=0.025, HR=2.333, 95% CI 1.114-4.887) were independent risk factors of OS of patients (Table 2).
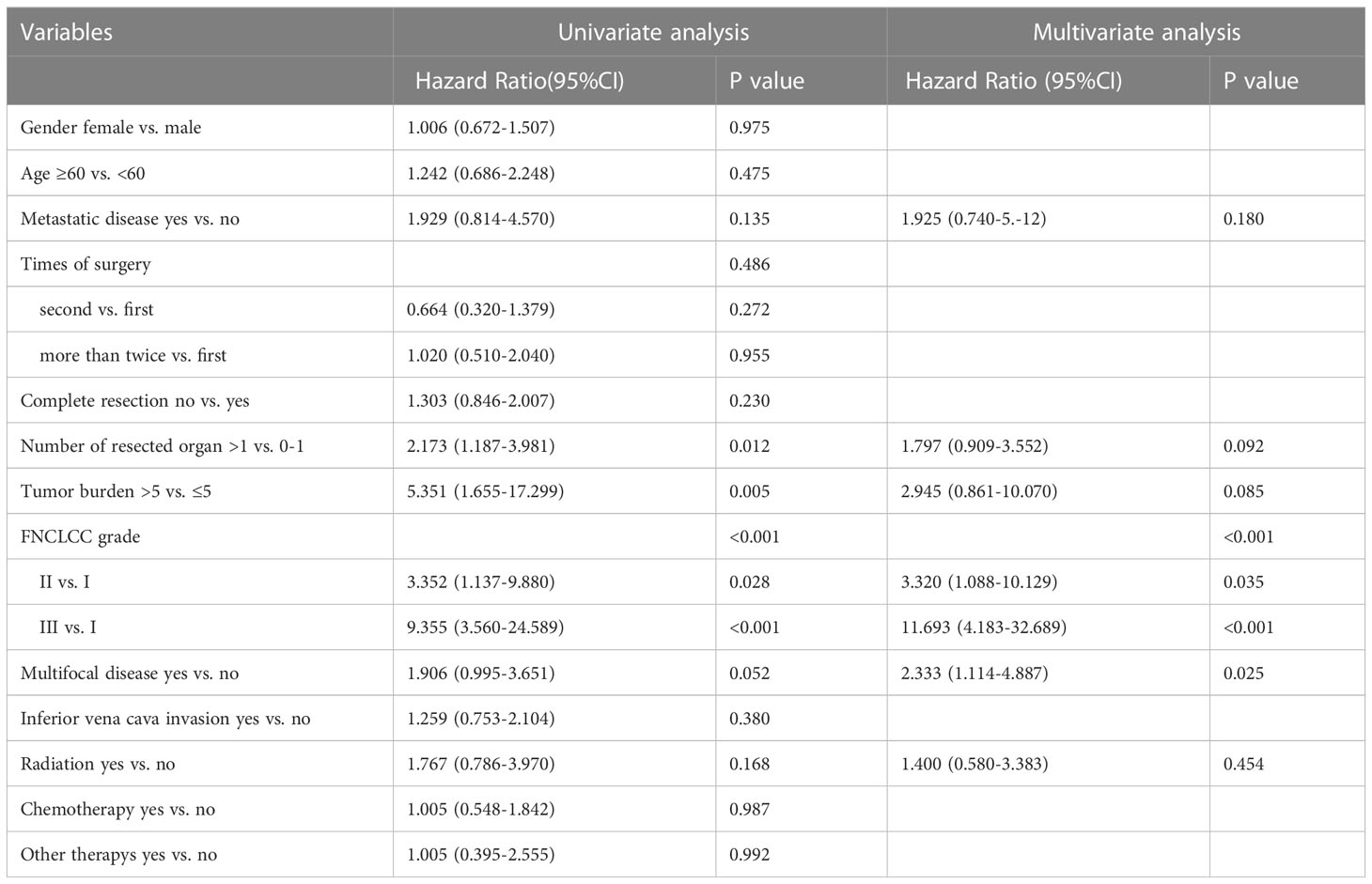
Table 2 Univariable and multivariable analyses to determine independent predictors of overall survival of retroperitoneal leiomyosarcoma.
Nomogram development and validation
Variables with p<0.1 in multivariate analysis (the number of resected organs, tumor diameter, FNCLCC grade, and multifocal lesion) were referred to construct the nomogram to predict the OS of patients with RLMS who had the abdominal lesions resected (Figure 2).
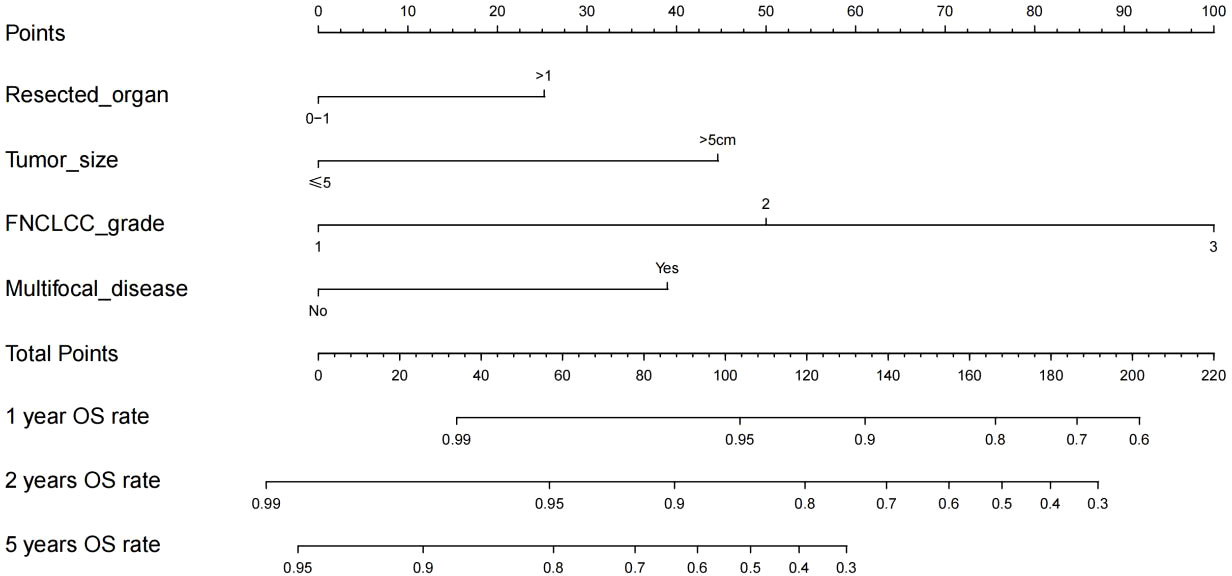
Figure 2 Nomogram for 1-year, 2-year and 5-year overall survival in patients with retroperitoneal leiomyosarcoma.
The RLMS nomogram had an internal validation c-index of 0.779 (95% CI, 0.659–0.898), which was significantly better than the TNM staging system (c-index=0.697). And the calibration curve showed that the predicted value of OS was in good agreement with the actual value (Figure 3).
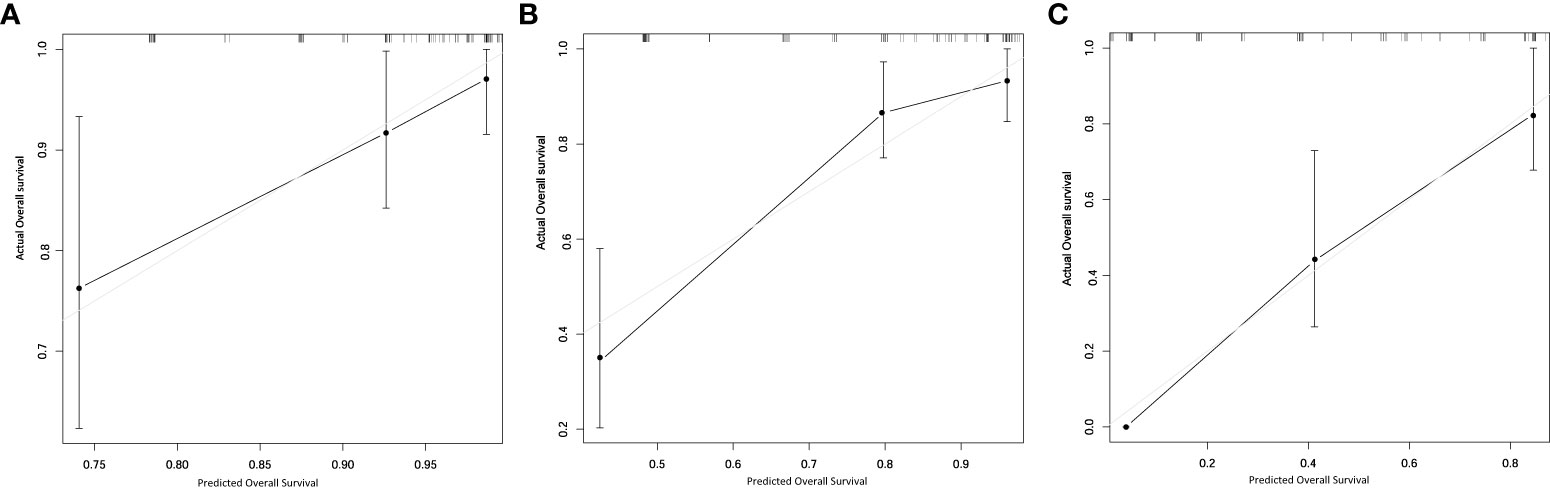
Figure 3 Calibration plots for internal validation of (A) 1-, (B) 2- and (C) 5-year overall survival nomogram.
The nomogram gave a specific value to quantify each variable. According to the total score, the Youden index was 0.572 with the cut off score of 126. 72 patients were included in the low-risk group (<126) and 46 were enrolled in the high-risk group (≥126), and their median OS was 116.5 (95% CI, NA) and 24.0 (95% CI, 22.9-25.0) months, respectively, showing a statistically significant difference (p<0.001) (Figure 4).
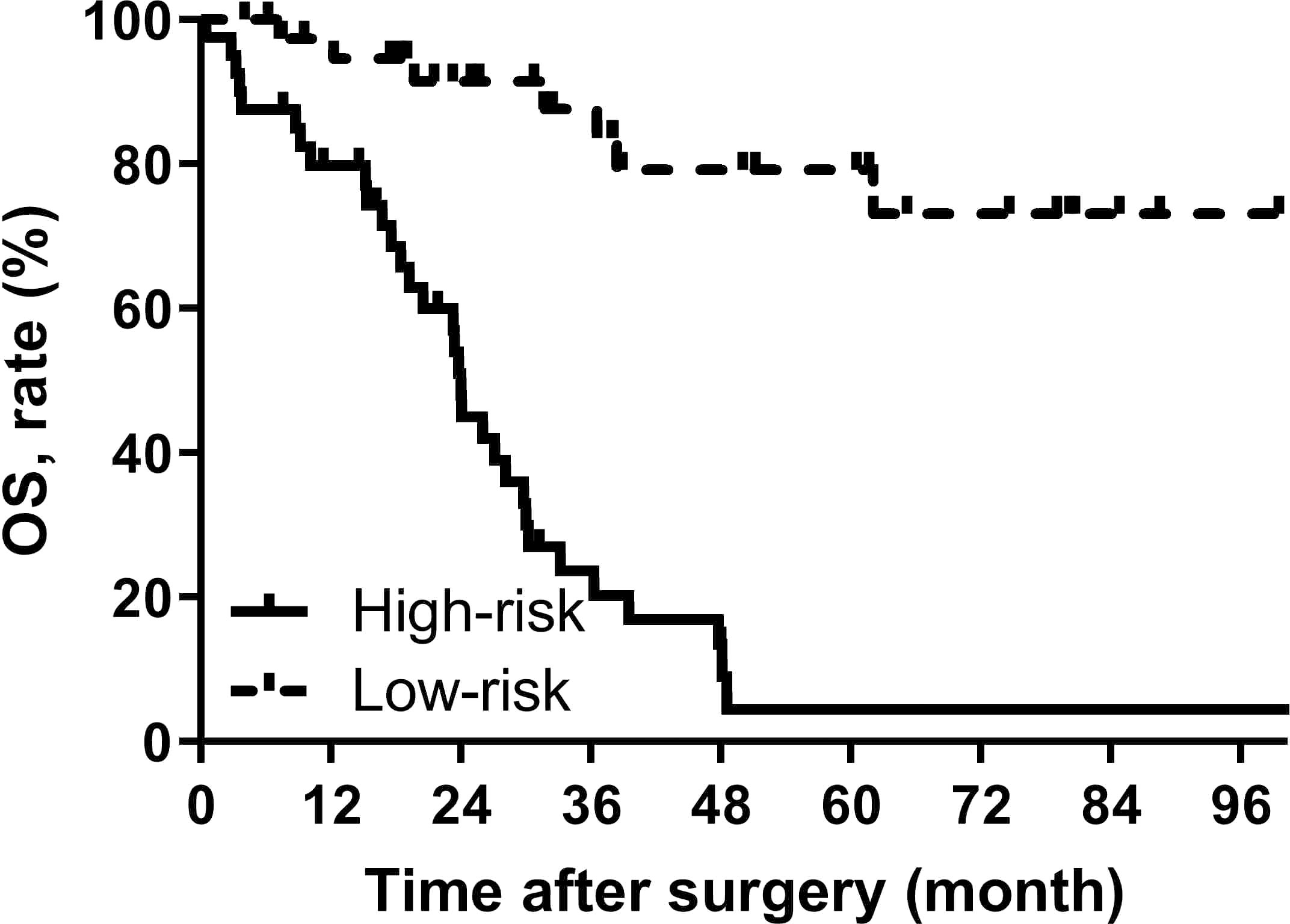
Figure 4 OS curves stratified by the score calculated by the nomogram and was stratified according to the risk score as follows: low-risk group (<100) and high-risk group (≥100).
Discussion
RLMS is a very rare mesenchymal malignant that originates in the retroperitoneal space. RLMS is fatal but rare disease, and metastasis, multifocal lesions and resection of multiple organs are usually contraindications to the surgical resection (14). Therefore, it is very important to analyze its long-term survival rate. In addition, the potential beneficial effects of surgical resection are not always obvious, resulting in very complicated decision-making. In other words, it is extremely difficult for clinically determine whether the patients with recurred RLMS could be treated by surgical resection. The nomogram established in this study is conductive for such decision or determination. For example, if the preoperative assessment shows no need for combined organ resection, the tumor diameter is smaller than 5 cm, FNCLCC shows grade 1 and single center disease, and even if it is a multiple recurrence disease, the postoperative 5-year OS rate may exceed 90%, so that patients have a high probability of benefiting from surgical resection. On the contrary, if the combined resection of multiple organs is needed, the tumor diameter is larger than 5cm, the FNCLCC showed grade 3 and multifocal disease, and the nomogram score was greater than 200 points, the postoperative 2-year OS is estimated to be less than 30%, so it has to be carefully considered to take surgical resection.
Since the prediction model can be simplified into a continuous numerical estimate tailored to individual patient conditions, the role of the nomogram prediction model has gradually become prominent with the help of precision medicine. Some nomogram prediction models have been incorporated into the staging system of the American Joint Committee on Cancer (AJCC) to improve the estimation accuracy of the prognosis for patients with subtype pathology. In the eighth edition of the AJCC manual, the nomogram by Trans-Atlantic Retroperitoneal Sarcoma Working Group for RPS (7) was included as a model that met all AJCC quality criteria. In this study, 523 patients were included to provide basis for establishing the nomogram prediction model, and 135 patients were used for foreign language validation. The c-index values for OS in the training set and validation set were 0.74 and 0.68, respectively. However, LMS only accounted for 17.6% in the training set, and its nomogram prediction model could only predict the OS of patients at the seventh year after the surgical resection, while the postoperative 5-year OS was only about 50%, showing limited practicality. In 2010, Anaya et al. established a nomogram model for predicting the postoperative 3-year and 5-year OS of patients by using the clinicopathological characteristics of 343 RPS patients, and its concordance index was 0.73. However, this model defined the pathological type of LMS as “others”, which was not listed separately (8). In 2019, Chandrajit et al. established a nomogram model for predicting the 6-year OS in RPS patients with first local recurrence based on the data of 602 patients from 22 sarcoma centers. Likewise, due to the rarity of RLMS, only 12.1% of LMS patients were included in the cohort (5).
In addition to nomogram prediction models for retroperitoneal sarcoma, there are also some LMS nomograms for the whole body. MingFeng et al. used the SEER database to establish a nomogram prediction model for predicting extremity LMS OS and cancer specific survival, with c-index of 0.776 and 0.835, respectively (15). And the LMS nomogram prediction model for the 5-year OS of urinary system was established by Oliver et al. with the c-index of 0.67 (16). Although the primary tumor site has a significant impact on the prognosis of LMS, there is currently no nomogram prediction model for LMS originating in the retroperitoneum. Therefore, this study developed and internally validated a novel RLMS-specific nomogram for predicting the 1-, 2-, and 5-year OS of patients with RLMS, and the c-index of the model was 0.779 (95% CI, 0.659-0.898). The calibration plots showed that the predicted OS rate was perfectly match with the actual value. In addition, to enhance the clinical utility, patients were further rolled into high-risk and low-risk groups based on the scores of the nomogram prediction model. The median OS of patients in the high-risk and low-risk groups was 116.5 (95% CI, NA) and 24.0 (95% CI, 22.9-25.0) months, respectively. The TNM staging system of the AJCC is the most commonly used method for staging STS, including RLMS. Staging is based on pathological findings, including tumor size, lymph node status, metastasis, and tumor grade based on the FNCLCC system (17). The RLMS nomogram in this study had a c-index of 0.779 (95% CI, 0.659-0.898) after internal validation, which was superior to the TNM staging system (c-index= 0.697). A more accurate prognosis could lead to a better postoperative counseling for patients, so it is possible to monitor the high-risk patients more appropriately.
In this study, the inclusion of radiation therapy in the multivariate analysis was based on recent reports highlighting its potential therapeutic benefits, despite having a p-value of 0.168 in the univariate analysis. The combination of radiation therapy with radical surgery has been shown to potentially be the standard of care for a subgroup of patients with RPS. Studies have demonstrated that patients treated with RT and surgery have significantly improved median overall survival and 5-year survival rates compared to those treated with surgery alone (p < 0.00001, p < 0.001). Additionally, median recurrence-free survival was significantly increased in patients treated with either preoperative or postoperative RT compared to those undergoing surgery alone (18). Another study further supports the use of radiotherapy in RPS, indicating that both preoperative and postoperative radiotherapy are significantly associated with improved overall survival compared to surgery alone (19). However, a recent randomized phase 3 study showed that the addition of preoperative radiotherapy did not improve local control rates or provide survival benefits, particularly in high-grade (G3) liposarcomas and LMS. Nevertheless, for recurrent retroperitoneal sarcomas primarily localized within the abdominal cavity, preoperative radiotherapy may help reduce the risk of local recurrence, especially in well-differentiated liposarcomas and low-grade dedifferentiated liposarcomas (20).Although radiation therapy did not demonstrate a significant association with overall survival in the further multivariate analysis of this study, considering the evidence from the mentioned high-quality studies, we still regard radiation therapy as a potential effective modality for treating leiomyosarcomas sarcoma of the retroperitoneum.
The results of survival analysis suggested that the FNCLCC grade and multifocal lesion were the independent risk factors for postoperative OS. The FNCLCC system is a commonly used histological grading system for STS, and it is one of the best indicators for predicting the metastasis-free survival and OS (21). This study was similar to a previous study reported by Qian Li et al., for RLMS patients with recurrent or metastatic disease who had a higher FNCLCC grade experienced worse prognosis (22). Consistent with previous reports on RPS, patients with multifocal lesions accounted for 23% of all subjects in this study; in addition, the mortality risk of patients with multifocal lesion increased by twice compared with non-multifocal lesion (23). Although there are some reports about the role of multifocal lesions in the prognosis of RPS, this study was the first to report it as an independent prognostic factor in RLMS.
There were some limitations for this study. First, this is a retrospective cohort study, and selection bias is inevitable. Second, the median follow-up time in this study was only 31 months, and further follow-up was needed to improve the reliability of the study. Thirdly, due to the complexity of treatments received by patients in our study cohort, there is a lack of information regarding adjuvant therapies. Finally, although the RLMS nomogram prediction model was internally validated, the cohort of this study was from an Asian tertiary hospital and was not externally validated, so its scope of use may be limited.
Conclusions
We established an RLMS-specific nomogram prediction model that can accurately predict postoperative survival in RLMS patients. Dividing patients into high and low-risk groups by nomogram can assist doctors in clinical decision-making.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Shanghai Public Health Clinical Center (B2020-338). The patients/participants provided their written informed consent to participate in this study.
Author contributions
AZ and HT developed the concept of the article. XY and AZ developed the design and methodology. AZ, XY,YZ, FH and WL contributed to the manuscript revision. AZ, XY and YZ contributed to the collection and analysis of clinical data. AZ, XY and HT contributed to the drafting of the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to thank our patients, without whom this study would not possible.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1160817/full#supplementary-material
Abbreviations
RLMS, retroperitoneal leiomyosarcoma; c-index, concordance index; LMS, leiomyosarcoma; STS, soft tissue sarcoma; FNCLCC, Federation Nationale des Centres de Lutte Contre le Cancer; OS, overall survival; RPS, retroperitoneal soft tissue sarcoma.
References
1. Anderson WJ, Doyle LA. Updates from the 2020 world health organization classification of soft tissue and bone tumours. Histopathology (2021) 78(5):644–57. doi: 10.1111/his.14265
2. Bathan AJ, Constantinidou A, Pollack SM, Jones RL. Diagnosis, prognosis, and management of leiomyosarcoma: recognition of anatomic variants. Curr Opin Oncol (2013) 25(4):384–9. doi: 10.1097/CCO.0b013e3283622c77
3. Ikoma N, Torres KE, Lin HY, Ravi V, Roland CL, Mann GN, et al. Recurrence patterns of retroperitoneal leiomyosarcoma and impact of salvage surgery. J Surg Oncol (2017) 116(3):313–9. doi: 10.1002/jso.24667
4. Gronchi A, Strauss DC, Miceli R, Bonvalot S, Swallow CJ, Hohenberger P, et al. Variability in patterns of recurrence after resection of primary retroperitoneal sarcoma (RPS): a report on 1007 patients from the multi-institutional collaborative RPS working group. Ann Surg (2016) 263(5):1002–9. doi: 10.1097/SLA.0000000000001447
5. Raut CP, Callegaro D, Miceli R, Barretta F, Rutkowski P, Blay JY, et al. Predicting survival in patients undergoing resection for locally recurrent retroperitoneal sarcoma: a study and novel nomogram from TARPSWG. Clin Cancer Res (2019) 25(8):2664–71. doi: 10.1158/1078-0432.CCR-18-2700
6. Italiano A, Lagarde P, Brulard C, Terrier P, Lae M, Marques B, et al. Genetic profiling identifies two classes of soft-tissue leiomyosarcomas with distinct clinical characteristics. Clin Cancer Res (2013) 19(5):1190–6. doi: 10.1158/1078-0432.CCR-12-2970
7. Gronchi A, Miceli R, Shurell E, Eilber FC, Eilber FR, Anaya DA, et al. Outcome prediction in primary resected retroperitoneal soft tissue sarcoma: histology-specific overall survival and disease-free survival nomograms built on major sarcoma center data sets. J Clin Oncol (2013) 31(13):1649–55. doi: 10.1200/JCO.2012.44.3747
8. Anaya DA, Lahat G, Wang X, Xiao L, Pisters PW, Cormier JN, et al. Postoperative nomogram for survival of patients with retroperitoneal sarcoma treated with curative intent. Ann Oncol (2010) 21(2):397–402. doi: 10.1093/annonc/mdp298
9. Tan MC, Brennan MF, Kuk D, Agaram NP, Antonescu CR, Qin LX, et al. Histology-based classification predicts pattern of recurrence and improves risk stratification in primary retroperitoneal sarcoma. Ann Surg (2016) 263(3):593–600. doi: 10.1097/SLA.0000000000001149
10. Kim HJ, Cho YJ, Kim SH, Rha SY, Ahn JB, Yang WI, et al. Leiomyosarcoma: investigation of prognostic factors for risk-stratification model. Int J Clin Oncol (2015) 20(6):1226–32. doi: 10.1007/s10147-015-0847-y
11. Abraham JA, Weaver MJ, Hornick JL, Zurakowski D, Ready JE. Outcomes and prognostic factors for a consecutive case series of 115 patients with somatic leiomyosarcoma. J Bone Joint Surg Am (2012) 94(8):736–44. doi: 10.2106/JBJS.K.00460
12. Gronchi A, Miceli R, Allard MA, Callegaro D, Le Pechoux C, Fiore M, et al. Personalizing the approach to retroperitoneal soft tissue sarcoma: histology-specific patterns of failure and postrelapse outcome after primary extended resection. Ann Surg Oncol (2015) 22(5):1447–54. doi: 10.1245/s10434-014-4130-7
13. Fluss R, Faraggi D, Reiser B. Estimation of the youden index and its associated cutoff point. Biom J (2005) 47(4):458–72. doi: 10.1002/bimj.200410135
14. van Houdt WJ, Fiore M, Barretta F, Rutkowski P, Blay JY, Lahat G, et al. Patterns of recurrence and survival probability after second recurrence of retroperitoneal sarcoma: a study from TARPSWG. Cancer (2020) 126(22):4917–25. doi: 10.1002/cncr.33139
15. Xue M, Chen G, Dai J, Hu J. Development and validation of a prognostic nomogram for extremity soft tissue leiomyosarcoma. Front Oncol (2019) 9:346. doi: 10.3389/fonc.2019.00346
16. Lu YJ, Wang H, Fang LY, Wang WJ, Song W, Wang Y, et al. A nomogram for predicting overall survival in patients with uterine leiomyosarcoma: a SEER population-based study. Future Oncol (2020) 16(10):573–84. doi: 10.2217/fon-2019-0674
17. von Mehren M, Kane JM, Bui MM, Choy E, Connelly M, Dry S, et al. NCCN guidelines insights: soft tissue sarcoma, version 1.2021. J Natl Compr Canc Netw (2020) 18(12):1604–12. doi: 10.6004/jnccn.2020.0058
18. Diamantis A, Baloyiannis I, Magouliotis DE, Tolia M, Symeonidis D, Bompou E, et al. Perioperative radiotherapy versus surgery alone for retroperitoneal sarcomas: a systematic review and meta-analysis. Radiol Oncol (2020) 54(1):14–21. doi: 10.2478/raon-2020-0012
19. Nussbaum DP, Rushing CN, Lane WO, Cardona DM, Kirsch DG, Peterson BL, et al. Preoperative or postoperative radiotherapy versus surgery alone for retroperitoneal sarcoma: a case-control, propensity score-matched analysis of a nationwide clinical oncology database. Lancet Oncol (2016) 17(7):966–75. doi: 10.1016/S1470-2045(16)30050-X
20. Bonvalot S, Gronchi A, Le Pechoux C, Swallow CJ, Strauss D, Meeus P, et al. Preoperative radiotherapy plus surgery versus surgery alone for patients with primary retroperitoneal sarcoma (EORTC-62092: STRASS): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol (2020) 21(10):1366–77. doi: 10.1016/S1470-2045(20)30446-0
21. Coindre JM, Terrier P, Guillou L, Le Doussal V, Collin F, Ranchere D, et al. Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: a study of 1240 patients from the French federation of cancer centers sarcoma group. Cancer (2001) 91(10):1914–26. doi: 10.1002/1097-0142(20010515)91:10<1914::AID-CNCR1214>3.0.CO;2-3
22. Li Q, Zhuang R, Zhu J, Lu W, Hou Y, Liu J, et al. Prognostic factors in patients with recurrent or metastatic retroperitoneal leiomyosarcoma. Future Oncol (2015) 11(12):1759–66. doi: 10.2217/fon.15.54
Keywords: nomogram, retroperitoneal leiomyosarcoma, survival, prediction of RLMS after surgery, FNCLCC
Citation: Zhuang A, Yue X, Tong H, Zhang Y, He F and Lu W (2023) Nomogram predicting overall survival after surgical resection for retroperitoneal leiomyosarcoma patients. Front. Endocrinol. 14:1160817. doi: 10.3389/fendo.2023.1160817
Received: 07 February 2023; Accepted: 12 June 2023;
Published: 17 July 2023.
Edited by:
Liam Chen, University of Minnesota, United StatesReviewed by:
Wengang Li, Xiamen University, ChinaCalin Cainap, University of Medicine and Pharmacy Iuliu Hatieganu, Romania
Copyright © 2023 Zhuang, Yue, Tong, Zhang, He and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fuchu He, aGVmY0BuaWMuYm1pLmFjLmNu; Weiqi Lu, bHUuV2VpcWlAenMtaG9zcGl0YWwuc2guY24=
†These authors share senior authorship
 Aojia Zhuang
Aojia Zhuang Xuetong Yue
Xuetong Yue Hanxing Tong
Hanxing Tong Yong Zhang1
Yong Zhang1