
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 14 September 2023
Sec. Thyroid Endocrinology
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1158826
This article is part of the Research Topic Predictors for the Aggressiveness of Papillary Thyroid Carcinoma View all 11 articles
Introduction: Lymph node metastasis in patients with papillary thyroid carcinoma (PTC) is associated with postoperative recurrence. Recently, most studies have focused on the evaluation of recurrence in patients with late-stage PTC, with limited data on those with early-stage PTC. We aimed to assess the relationship between lymph node ratio (LNR) and recurrence in low-to-intermediate-risk patients and validate its diagnostic efficiency in both structural (STR) and biochemical recurrence (BIR).
Methods: Clinical data of patients with PTC diagnosed at the Affiliated Hospital of Jining Medical University were retrospectively collected. The optimal LNR cut-off values for disease-free survival (DFS) were determined using X-tile software. Predictors were validated using univariate and multivariate Cox regression analyses.
Results: LNR had a higher diagnostic effectiveness than metastatic lymph nodes in patients with low-to-intermediate recurrence risk N1a PTC. The optimal LNR cutoff values for STR and BIR were 0.75 and 0.80, respectively. Multivariate Cox regression analysis showed that LNR≥0.75 and LNR≥0.80 were independent factors for STR and BIR, respectively. The 5-year DFS was 90.5% in the high LNR (≥0.75) and 96.8% in low LNR (<0.75) groups for STR. Regarding BIR, the 5-year DFS was 75.7% in the high LNR (≥0.80) and 86.9% in low LNR (<0.80) groups. The high and low LNR survival curves exhibited significant differences on the log-rank test.
Conclusion: LNR was associated with recurrence in patients with low-to-intermediate recurrence risk N1a PTC. We recommend those with LNR≥0.75 require a comprehensive evaluation of lateral neck lymphadenopathy and consideration for lateral neck dissection and RAI treatment.
Papillary thyroid carcinoma (PTC) accounts for approximately 90% of thyroid cancers and has a favorable prognosis, with a 10-year disease-specific mortality of approximately 4% (1, 2). However, the high incidence of its recurrence is nonnegligible. Although the majority of PTC recurrences are not fatal (3), they can cause significant suffering, particularly in countries such as China, where PTC is common (4).
To determine the risk of recurrence and establish a corresponding follow-up strategy and postoperative treatment plan, three risk categories have been recommended by the American Thyroid Association (ATA): low, intermediate, and high risk (5). Some studies have reported that radioactive iodine (RAI) ablation or prophylactic lateral neck dissection is required to reduce the risk of recurrence in high-risk patients with PTC (6–9). Nevertheless, patients in the low-to-intermediate-risk group also show a certain risk of recurrence (10). Patients with less than five metastatic lymph nodes (MLNs) are classified as low risk, but the number of MLNs varies according to the degree of lymph node (LN) dissection scope and pathological sampling extent (11). Some patients still exhibit a significant probability of recurrence under this system, even with fewer than five MLNs (12).
According to the American Joint Committee on Cancer (AJCC) 8th TNM classification of Differentiated Thyroid Carcinoma, N1a and N1b are categorized into the same stage (13). Therefore, the N staging of TNM classification is inadequate to assess the probability of recurrence in patients with positive LNs (14–16). Furthermore, it is unclear which patients with pN1a should undergo prophylactic lateral neck dissection to prevent lateral lymph node recurrence (17). To differentiate between “lower risk characteristics” and “higher risk characteristics” in these patients, a new risk stratification system is required in order to provide individualized therapy for each patient, reducing hazards and optimizing benefits.
The number of MLNs divided by the total number of lymph nodes (TLNs) is known as the lymph node ratio (LNR). This ratio has been utilized to assess oncological prognoses of solid tumors, including those of the lung (18), stomach (19), and colon (20). This study aimed to identify an optimal cutoff value for LNR and explore the relationship between LNR and patients with low-to-intermediate recurrence risk N1a PTC. Furthermore, we aimed to validate the value of LNR as an indicator of tumor structural recurrence (STR) and biochemical recurrence (BIR).
Data from patients with PTC who were diagnosed at the Affiliated Hospital of Jining Medical University between December 2012 and December 2017 were retrospectively collected and analyzed. Of 2861 patients who underwent surgical treatment during the study period, 617 met the inclusion criteria.
The inclusion criteria were: having undergone total thyroidectomy and central lymph node dissection; being aged at least 18 years; being classified as pathological T1-3N1aM0 according to the AJCC 8th TNM Classification of Differentiated Thyroid Carcinoma system; being classified as low-to-intermediate risk according to the 2015 ATA management guidelines; having achieved excellent responses after initial surgery (suppressed thyroglobulin (Tg) < 0.2 ng/mL or thyroid-stimulating hormone [TSH]-stimulated Tg < 1 ng/mL and negative imaging); and not having undergone radioactive iodine (RAI) ablation postoperatively.
The exclusion criteria were postoperative persistent disease; other significant malignant tumors; serious medical record deficiency or loss during follow-up; and a TLN number less than 4.
This study was conducted in compliance with the 2013 revision of the Helsinki Declaration. The Ethics Committee of the Affiliated Hospital of Jining Medical University approved the study (2022C092). All participants provided written informed consent before participation.
All patients were followed up with and treated based on the ATA management guidelines (2009 or 2015 version) (5, 21). Outpatient reviews were recommended every 3–6 months for the first 2 years, and annually thereafter. Physical examination and thyroid ultrasonography were performed to evaluate the surgical area. Additionally, thyroid function was evaluated by testing serum thyroglobulin (Tg), anti-thyroglobulin antibodies (TgAb), and TSH levels. Data collected from medical records included age; sex; BMI; Hashimoto’s thyroiditis (HT) status; TNM staging; tumor number; tumor size; extrathyroidal extension (ETE); TLNs; MLNs; BRAF status; ultrasound and RAI scanning results; and Tg, TgAb, and TSH levels.
Based on evaluations at each follow-up visit after surgery, the disease outcomes of STR and BIR were diagnosed. STR was diagnosed based on cytological or histological proof, as well as clear results of ultrasound, computerized tomography (CT), RAI whole-body scans, or positron emission tomography-CT. BIR was defined as suppressed Tg levels of ≥1 ng/mL, TSH-stimulated Tg levels ≥10 ng/mL, or progressive rise in TgAb, without evidence of structural disease on imaging modality (22). Disease-free survival (DFS) was defined as the period without disease recurrence after initial treatment, including structural recurrence-free survival (STRFS) and biochemical recurrence-free survival (BIRFS). RAI ablation or follow-up was recommended for patients with BIR. However, RAI ablation, a second surgery, or further follow-up was recommended for patients with STR.
The Student’s t-test (normally distributed data) and Mann-Whitney test (non-normally distributed data) were used to compare continuous data. The chi-square test was used to compare categorical data. Continuous variables are presented as means ± standard deviations, whereas categorical variables are presented as numbers with percentages. The optimal cut-off values of LNR relevant to the STRFS and BIRFS were determined using X-tile software. The patients were allocated into groups according to the optimal cutoff values for LNR. The Kaplan–Meier method was used to evaluate survival rates, and the results were compared using the log-rank test. Predictors were validated using univariate and multivariate Cox regression analyses. Statistical significance was defined as a p-value of less than 0.05. X-tile and SPSS version 26.0 software were used for all statistical assessments.
This study included 617 patients (181 men and 436 women). Table 1 presents the patient and tumor characteristics. The average age was 42.94 ± 14.35 years, and the average tumor size was 1.77 ± 0.75 cm. The ETE rate was 16.21%, the Hashimoto’s thyroiditisrate was 36.47%, and BRAF mutations rate was 52.19%. In addition, the median numbers of MLNs and TLNs were 4.17 ± 1.98 and 8.08 ± 3.02 in this study, respectively.
Structural recurrence was found in 31 cases (5.02%): six in the initial thyroidectomy bed, seven in the cervical lymph nodes, and 18 in the lateral lymph nodes. Of these 31 patients, 24 were treated with secondary surgery and five with RAI ablation, while two chose to continue their follow-up (Figure 1).
Biochemical recurrence was found in 94 cases (15.24%): 37 patients were treated with RAI ablation and the others were followed up. Of these 94 patients, 12 were also diagnosed with STR. After diagnosis, the remaining 82 patients were monitored. The results showed that 16 patients developed STR in the following 1–35 months, including 10 patients who had undergone RAI. Twenty-four patients had a disease-free state at the last follow-up, 10 of whom had undergone RAI. Forty-two patients continued to have a biochemical incomplete response status, including 17 patients who had received RAI. Additional RAI ablation did not have a significant impact on the outcomes of the patients with BIR (Table 2).
The median follow-up period for the group as a whole was 69.69 ± 17.07 months (range, 6–108 months). Nine patients died during follow-up due to PTC-unrelated events. At the last follow-up, 511 (82.82%) patients were alive and disease free.
ROC curve analysis was performed to assess the diagnostic effectiveness of LNR and MLNs in STRFS and BIRFS. The results showed that the area under the curve (AUC) of the LNR was larger than that of the MLNs for both STRFS (0.669 versus 0.600) and BIRFS (0.558 versus 0.535) (Figures 2A, B). X-tile software was used to determine the ideal LNR cut-off value. The results indicated that, for STR and BIR, 0.75 and 0.80 were the optimal cut-off values, respectively. Baseline clinicopathological features were compared according to optimal LNR cut off values (Tables 3 and 4).
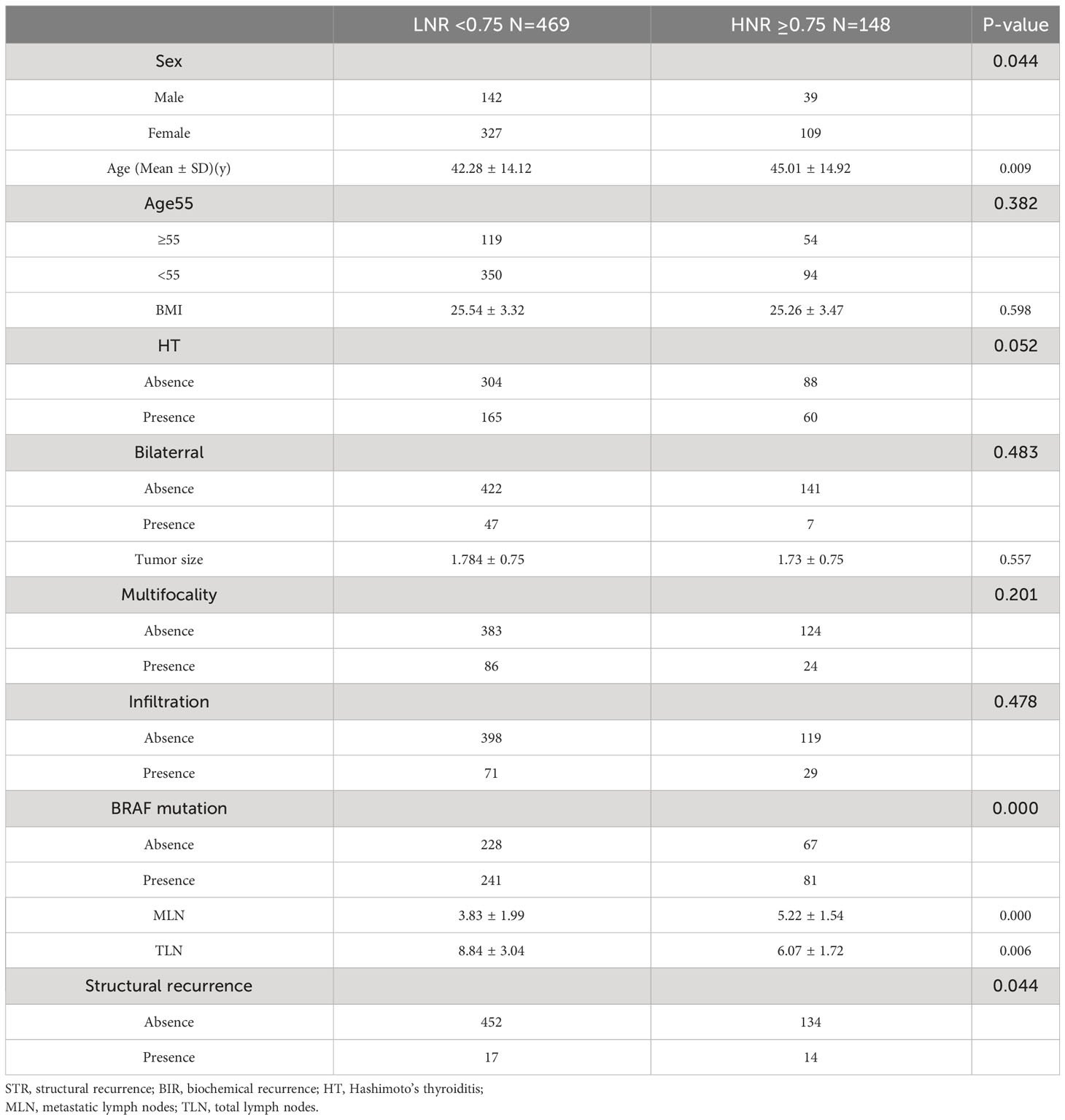
Table 3 Comparison of demographics and tumor characteristics according to optimal LNR cut off values of structural recurrence.
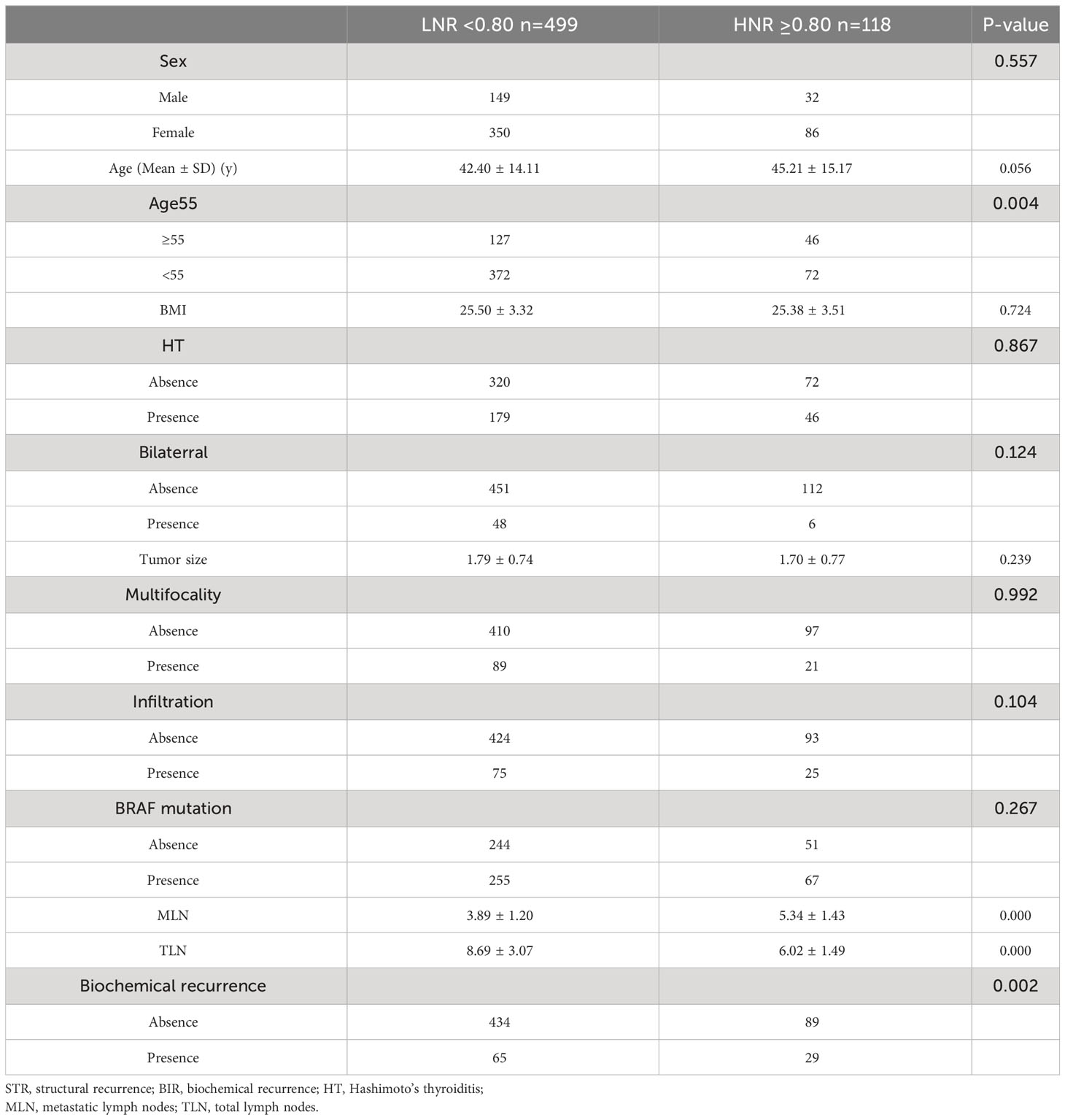
Table 4 Comparison of demographics and tumor characteristics according to optimal LNR cut off values of biochemical recurrence.
Considering STR, the high LNR (≥0.75) group had a higher recurrence rate than did the low LNR (<0.75) group (9.46% versus 3.63%, p=0.044). BIR was greater in the high LNR (≥0.80) group than in the low LNR (<0.80) group (24.58% versus 13.03%, p=0.002) group.
Univariate analyses revealed that old age (≥55 years), tumor size, ETE, BRAF mutation, MLN number, and high LNR (≥0.75) were the main factors that influenced STRFS (p<0.10). Multivariate analyses showed that tumor size, ETE, BRAF mutation, MLN number, and high LNR (≥0.75) were independent influencing factors for STRFS (p < 0.05) (Table 5).
As regards BIRFS, univariate analyses demonstrated that old age (≥ 55 years), tumor size, multifocality, ETE, and high LNR (≥0.80) were relative influencing factors (p<0.10). Multivariate analyses showed that tumor size, ETE, and high LNR (≥0.80) were independent influencing factors (p<0.05) (Table 6).
Figures 3 and 4 show Kaplan–Meier curves grouped by the optimal LNR cut-off value. The high and low LNR survival curves exhibited significant differences in the log-rank test for both STRFS and BIRFS (p=0.003 and p=0.001, respectively). With regard to structural recurrence, the 5-year STRFS was 90.5% in the high LNR (≥0.75) and 96.8% in low LNR (<0.75) groups. However, in terms of biochemical recurrence, the 5-year BIRFS was 75.7% in the high LNR (≥0.80) and 86.9% in low LNR (<0.80) groups.
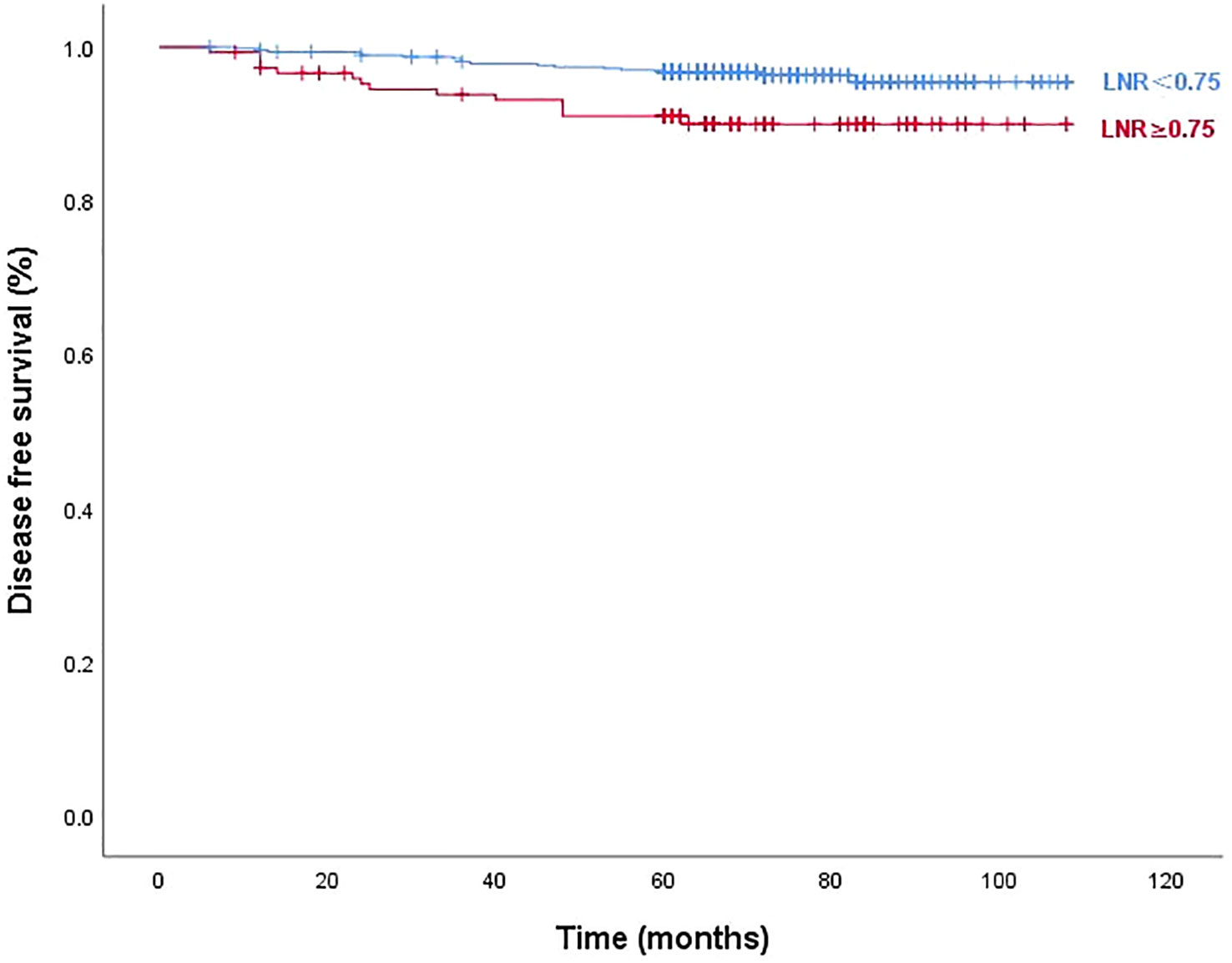
Figure 3 Disease-free survival curves according to optimal LNR cut off values of structural recurrence (log-rank p=0.003).
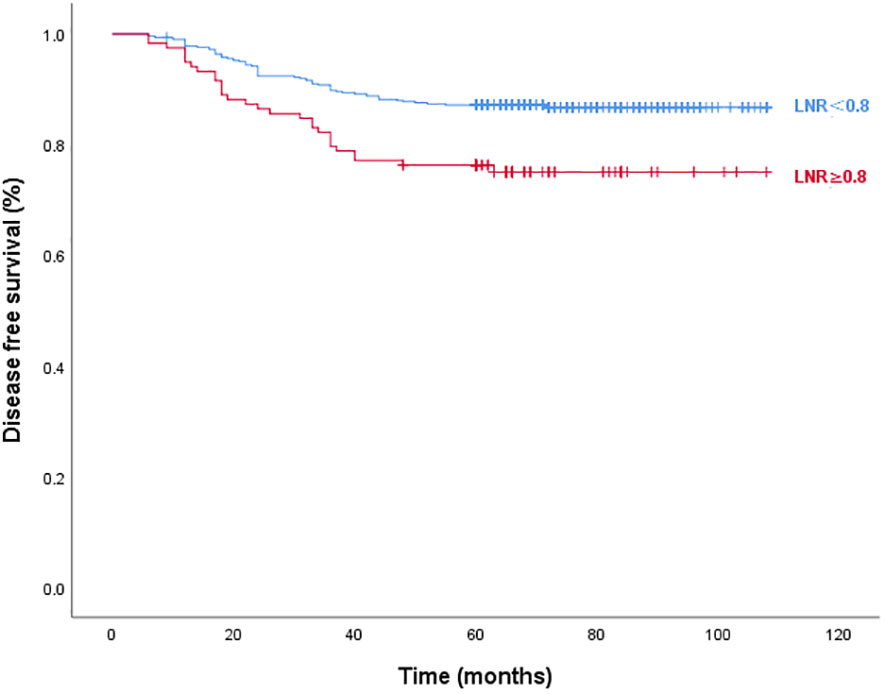
Figure 4 Disease-free survival curves according to optimal LNR cut off values of biochemical recurrence (log-rank p=0.001).
PTC has a rather favorable prognosis, with a 10-year disease-specific mortality rate of approximately 4%. However, the high incidence of recurrence is nonnegligible. Although the majority of PTC recurrences are not deadly, they can be quite painful for patients.
Age, tumor size, pathological subtype, MLNs, ETE, and multifocality are risk variables that have been established as independent predictors of PTC recurrence. Various methods have been developed to determine the likelihood of recurrence based on these factors (23–25).
The 2015 ATA management guidelines classified recurrence risk as low, intermediate, and high, according to pathological features. These features included degree of residual lesion, tumor size, pathological subtype, envelope infiltration, vascular invasion, lymph node metastasis features, molecular pathological features, stimulated Tg level, and post-treatment whole-body scan (5). Local recurrence has been observed in 3–13% of patients with low-risk tumors, 21–36% of those with intermediate-risk tumors, and 68% of those with high-risk tumours (5). In this study, the median follow-up period was 69.69 ± 17.07 months, and total structural recurrence rate was 5.02%.
Lymph node metastasis in patients with PTC is associated with high recurrence rate. The number of MLNs varies with the degree of LNs dissection scope and pathological sampling ability. Some patients with fewer MLNs still have a high risk of recurrence. Are there other diagnostic indicators that, in combination with the existing ATA criteria, would be good predictors of recurrence in patients. According to the AJCC 8th TNM classification, N1a and N1b were categorized into the same stage. Therefore, the N staging of TNM classification is inadequate for assessing recurrence risk (26). Furthermore, it is unclear which patients with pN1a should undergo RAI ablation and which, prophylactic lateral neck dissection, to prevent recurrence. To differentiate between “lower risk characteristics” and “higher risk characteristics” in these patients, a new risk stratification strategy is required to provide individualized therapy for each patient, reducing hazards and optimizing benefits.
LNR has been widely used to assess oncological prognosis such as lungs, stomach, and colon cancer. In terms of PTC, after collecting data from 10, 955 patients, Schneider et al. (27) showed that LNR was associated with disease-related mortality. Parvathareddy et al. (28), in a retrospective study of 1407 cases, reported that TNM classification in combination with LNR had a stronger diagnostic capability for recurrence than did TNM alone. Lee et al. (29) claimed that the 2015 ATA risk stratification and 8th AJCC staging system provided greater predictive value for recurrence in patients with PTC once integrated with LNR. Kang et al. (30) reported that LNR was more accurate in predicting recurrence than N stage, after an analysis of a group of 307 patients with N1b PTC. Despite numerous findings on the use of LNR to assess PTC prognosis, no studies have assessed the clinical significance of LNR in patients with low-to-intermediate recurrence risk N1a PTC.
Our results showed that the diagnostic efficiency of LNR was higher than that of MLNs for STRFS (AUC of 0.669 versus 0.600). X-tile analysis showed that 0.75 was the optimal cut-off value for STR. Patients with LNRs of ≥0.75 had a significantly greater incidence of STR than did those with LNRs of <0.75 (9.46% versus 3.63%, p=0.044). Regarding STRFS, multivariate analyses showed that tumor size, ETE, BRAF mutation, MLN number, and high LNR (≥0.75) were independent influencing factors (p<0.05). Significant differences between the survival curves for high and low LNR were observed using the log-rank test (p=0.003). Five-year STRFS was 90.5% in the high and 96.8% in the low LNR groups. It is worth mentioning that the best cut-off value of LNR for predicting STR in this study was 0.8, which is higher than that reported in the relevant reports. We speculate that this is related to the different inclusion criteria, the patients included in this study were all low-to-intermediate risk patients (T1-3N1aM0), who had a better prognosis and a higher LNR cut-off value for the STR.
The majority of research evaluating long-term results frequently focuses on STR rather than BIR. A biochemical incomplete response is diagnosed based on increased serum levels of Tg or TgAb without any signs of structural abnormalities. In addition, if a patient achieved an excellent response after primary total thyroidectomy, this kind of biochemical incomplete response may be labelled BIR. According to the ATA, BIR is seen in 11–19% of low-risk, 2–22% of intermediate-risk, and 16–18% of high-risk patients (31, 32). However, the BIR rate of patients with N1a has not been assessed thus far. Our assessment revealed a 15.24% chance of BIR in patients with low-to-intermediate N1a. Since the long-term prognosis in these patients is not well understood, and some may develop structural illness, close follow-up is required. In this study, we also found that 0.80 was the optimal cutoff value for BIR. Patients with LNRs of ≥0.80 had significantly greater BIR incidences than did those with LNRs of <0.80 (9.46% vs. 3.63%, p=0.044). Multivariate analyses showed that tumor size, ETE, and high LNR (≥0.80) were independent factors influencing BIRFS (p<0.05). Significant differences between the survival curves for the high and low LNR groups were observed using the log-rank test (p=0.001). Five-year BIRFS was 75.7% for high LNR and 86.9% for low LNR. It is notable that, unlike the findings of other studies, performing RAI ablation after BIR had no significant effect on the progression to STR in our trial, which may have result from a high lymph node recurrence rate of STR in our cohort (33).
This retrospective study has several limitations that should be considered. Due to the possibility of selection bias in single-center studies, our findings may not be applicable to a larger population. Since we only included patients with pathologic N1a PTC, it might be challenging to extrapolate our findings to all patients with PTC. Furthermore, although certain patient data, such as histological subtypes, diameters of the biggest MLNs, extra-nodal extension and micrometastases of MLNs, did not appear in pathology reports 10 years ago, they were reported to have prognostic value (34–36). To overcome these restrictions, we intend to conduct prospective research in the future that also takes LN-related parameters into consideration. Our study also has a number of advantages. Every patient was diagnosed, treated, and followed up by the same medical team, in accordance with the same standardized procedure. In addition, our study had a longer follow-up period compared to that in similar studies. We also assessed factors influencing biochemical recurrence.
According to our findings, LNR was associated with recurrence in patients with N1a PTC. LNR≥0.75 and LNR≥0.80 were independent predictors of STRFS and BIRFS, respectively. We recommend those low-risk and intermediate-risk patients with a high LNR (≥0.75), even with limited MLNs, require a comprehensive evaluation of lateral neck lymphadenopathy and consideration for lateral neck dissection and RAI treatment. These findings may help physicians identify patients who are at risk and aid them in choosing the best postsurgical therapy and monitoring. The results of this research may help develop more effective staging standards.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The experimental protocol was established, according to the ethical guidelines of the Helsinki Declaration and was approved by the Human Ethics Committee of Affiliated Hospital of Jining Medical University (2022C092).
TM designed the study, analyzed the data and commented on the manuscript at all stages. WS and LW collected the data. JC,PS, XZ, ML revised the manuscript. YS provided the research direction. All authors contributed to the article and approved the submitted version.
Academician He Lin Research Foundation of Affiliated Hospital of Jining Medical University (JYHL2022FMS10).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Megwalu UC, Moon PK. Thyroid cancer incidence and mortality trends in the United States: 2000-2018. Thyroid (2022) 32(5):560–70. doi: 10.1089/thy.2021.0662
2. La Vecchia C, Malvezzi M, Bosetti C, Garavello W, Bertuccio P, Levi F, et al. Thyroid cancer mortality and incidence: a global overview. Int J Cancer (2015) 136(9):2187–95. doi: 10.1002/ijc.29251
3. Conzo G, Mauriello C, Docimo G, Gambardella C, Thomas G, Cavallo F, et al. Clinicopathological pattern of lymph node recurrence of papillary thyroid cancer. Implications for surgery. Int J Surg (2014) 12 Suppl 1:S194–7. doi: 10.1016/j.ijsu.2014.05.010
4. Wang J, Yu F, Shang Y, Ping Z, Liu L. Thyroid cancer: incidence and mortality trends in China, 2005-2015. Endocrine (2020) 68(1):163–73. doi: 10.1007/s12020-020-02207-6
5. Haugen BR. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the american thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid (2016) 26(1):1–133. doi: 10.1089/thy.2015.0020
6. Marti JL, Morris L, Ho AS. Selective use of radioactive iodine (RAI) in thyroid cancer: No longer “one size fits all”. Eur J Surg Oncol (2018) 44(3):348–56. doi: 10.1016/j.ejso.2017.04.002
7. Zambeli-Ljepovic A, Wang F, Dinan MA, Hyslop T, Roman SA, Sosa JA, et al. Low-risk thyroid cancer in elderly: total thyroidectomy/RAI predominates but lacks survival advantage. J Surg Res (2019) 243:189–97. doi: 10.1016/j.jss.2019.05.029
8. Yang P, Li J, Jing H, Chen Q, Song X, Qian L. Effect of prophylactic central lymph node dissection on locoregional recurrence in patients with papillary thyroid microcarcinoma. Int J Endocrinol (2021) 2021:8270622. doi: 10.1155/2021/8270622
9. Sterpetti AV. Optimization of staging of the neck with prophylactic central and lateral neck dissection for papillary thyroid carcinoma. Ann Surg (2015) 261(1):e30. doi: 10.1097/SLA.0000000000000510
10. Shen FC, Hsieh CJ, Huang IC, Chang YH, Wang PW. Dynamic risk estimates of outcome in Chinese patients with well-differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation. Thyroid (2017) 27(4):531–6. doi: 10.1089/thy.2016.0479
11. Yu ST, Ge JN, Sun BH, Wei ZG, Xiao ZZ, Zhang ZC, et al. Lymph node yield in the initial central neck dissection (CND) associated with the risk of recurrence in papillary thyroid cancer: A reoperative CND cohort study. Oral Oncol (2021) 123:105567. doi: 10.1016/j.oraloncology.2021.105567
12. Feng JW, Ye J, Wu WX, Qu Z, Qin A-C, Jiang Y. Management of cN0 papillary thyroid microcarcinoma patients according to risk-scoring model for central lymph node metastasis and predictors of recurrence. J Endocrinol Invest (2020) 43(12):1807–17. doi: 10.1007/s40618-020-01326-1
13. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin (2017) 67(2):93–9. doi: 10.3322/caac.21388
14. Shaha AR. TNM classification of thyroid carcinoma. World J Surg (2007) 31(5):879–87. doi: 10.1007/s00268-006-0864-0
15. Xiang J, Wang Z, Sun W, Zhang H. A relook at the 8th edition of the AJCC TNM staging system of anaplastic thyroid carcinoma: A SEER-based study. Clin Endocrinol (Oxf) (2021) 94(4):700–10. doi: 10.1111/cen.14371
16. Kim TH, Kim YN, Kim HI, Park SY, Choe JH, Kim JH, et al. Prognostic value of the eighth edition AJCC TNM classification for differentiated thyroid carcinoma. Oral Oncol (2017) 71:81–6. doi: 10.1016/j.oraloncology.2017.06.004
17. Lim YC, Liu L, Chang JW, Koo BS. Lateral lymph node recurrence after total thyroidectomy and central neck dissection in patients with papillary thyroid cancer without clinical evidence of lateral neck metastasis. Oral Oncol (2016) 62:109–13. doi: 10.1016/j.oraloncology.2016.10.010
18. Chiappetta M, Leuzzi G, Sperduti I, Bria E, Mucilli F, Lococo F, et al. Lymph-node ratio predicts survival among the different stages of non-small-cell lung cancer: a multicentre analysisdagger. Eur J Cardiothorac Surg (2019) 55(3):405–12. doi: 10.1093/ejcts/ezy311
19. Wang H, Qi H, Liu X, Gao Z, Hidasa I, Aikebaier A, et al. Positive lymph node ratio is an index in predicting prognosis for remnant gastric cancer with insufficient retrieved lymph node in R0 resection. Sci Rep (2021) 11(1):2022. doi: 10.1038/s41598-021-81663-0
20. Chen SL, Steele SR, Eberhardt J, Zhu K, Bilchik A, Stojadinovic A. Lymph node ratio as a quality and prognostic indicator in stage III colon cancer. Ann Surg (2011) 253(1):82–7. doi: 10.1097/SLA.0b013e3181ffa780
21. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid (2009) 19(11):1167–214. doi: 10.1089/thy.2009.0110
22. Llamas-Olier AE, Cuellar DI, Buitrago G. Intermediate-risk papillary thyroid cancer: risk factors for early recurrence in patients with excellent response to initial therapy. Thyroid (2018) 28(10):1311–7. doi: 10.1089/thy.2017.0578
23. Siddiqui S, White MG, Antic T, Grogan RH, Angelos P, Kaplan E, et al. Clinical and pathologic predictors of lymph node metastasis and recurrence in papillary thyroid microcarcinoma. Thyroid (2016) 26(6):807–15. doi: 10.1089/thy.2015.0429
24. Heng Y, Feng S, Yang Z, Cai W, Qiu W, Tao L. Features of lymph node metastasis and structural recurrence in papillary thyroid carcinoma located in the upper portion of the thyroid: A retrospective cohort study. Front Endocrinol (Lausanne) (2021) 12:793997. doi: 10.3389/fendo.2021.793997
25. Jiang LH, Yin KX, Wen QL, Chen C, Ge MH, Tan Z. Predictive risk-scoring model for central lymph node metastasis and predictors of recurrence in papillary thyroid carcinoma. Sci Rep (2020) 10(1):710. doi: 10.1038/s41598-019-55991-1
26. Tam S, Boonsripitayanon M, Amit M, Fellman BM, Li Y, Busaidy NL, et al. Survival in differentiated thyroid cancer: comparing the AJCC cancer staging seventh and eighth editions. Thyroid (2018) 28(10):1301–10. doi: 10.1089/thy.2017.0572
27. Schneider DF, Chen H, Sippel RS. Impact of lymph node ratio on survival in papillary thyroid cancer. Ann Surg Oncol (2013) 20(6):1906–11. doi: 10.1245/s10434-012-2802-8
28. Parvathareddy SK, Siraj AK, Qadri Z, Ahmed SO, DeVera F, Al-Sobhi S, et al. Lymph node ratio is superior to AJCC N stage for predicting recurrence in papillary thyroid carcinoma. Endocr Connect (2022) 11(2). doi: 10.1530/EC-21-0518
29. Lee J, Lee SG, Kim K, Yim S, Ryu H, Lee CR, et al. Clinical value of lymph node ratio integration with the 8(th) edition of the UICC TNM classification and 2015 ATA risk stratification systems for recurrence prediction in papillary thyroid cancer. Sci Rep (2019) 9(1):13361. doi: 10.1038/s41598-019-50069-4
30. Kang IK, Kim K, Park J, Bae JS, Kim JS. Central lymph node ratio predicts recurrence in patients with N1b papillary thyroid carcinoma. Cancers (Basel) (2022) 14(15). doi: 10.3390/cancers14153677
31. Tuttle RM, Tala H, Shah J, Leboeuf R, Ghossein R, Gonen M, et al. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid (2010) 20(12):1341–9. doi: 10.1089/thy.2010.0178
32. Pitoia F, Bueno F, Urciuoli C, Abelleira E, Cross G, Tuttle RM. Outcomes of patients with differentiated thyroid cancer risk-stratified according to the American thyroid association and Latin American thyroid society risk of recurrence classification systems. Thyroid (2013) 23(11):1401–7. doi: 10.1089/thy.2013.0011
33. Haddad RI, Bischoff L, Ball D, Bernet D, Blomain D, Busaidy D, et al. Thyroid carcinoma, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw (2022) 20(8):925–51. doi: 10.6004/jnccn.2022.0040
34. Sun Y, Liu X, Ouyang W, Feng H, Wu J, Chen P, et al. Lymph node characteristics for predicting locoregional recurrence of papillary thyroid cancer in adolescents and young adults. Oral Oncol (2017) 66:22–7. doi: 10.1016/j.oraloncology.2016.12.028
35. Ricarte-Filho J, Ganly I, Rivera M, Katabi N, Fu W, Shaha A, et al. Papillary thyroid carcinomas with cervical lymph node metastases can be stratified into clinically relevant prognostic categories using oncogenic BRAF, the number of nodal metastases, and extra-nodal extension. Thyroid (2012) 22(6):575–84. doi: 10.1089/thy.2011.0431
Keywords: papillary thyroid carcinoma, lymph node ratio, structural recurrence, biochemical recurrence, total thyroidectomy
Citation: Ma T, Cui J, Shi P, Liang M, Song W, Zhang X, Wang L and Shi Y (2023) Assessing the role of central lymph node ratio in predicting recurrence in N1a low-to-intermediate risk papillary thyroid carcinoma. Front. Endocrinol. 14:1158826. doi: 10.3389/fendo.2023.1158826
Received: 04 February 2023; Accepted: 23 August 2023;
Published: 14 September 2023.
Edited by:
Emese Mezosi, University of Pécs, HungaryReviewed by:
Pietro Locantore, Catholic University of the Sacred Heart, ItalyCopyright © 2023 Ma, Cui, Shi, Liang, Song, Zhang, Wang and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yafei Shi, anlzeWZAbWFpbC5qbm1jLmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.