
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 17 August 2023
Sec. Thyroid Endocrinology
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1158581
This article is part of the Research Topic Radionuclide and Molecular Probes in the Diagnosis and Treatment of Thyroid Diseases View all 6 articles
Background: The management guidelines of radioactive Iodine (RAI) therapy for distinct types of differentiated thyroid carcinoma (DTC) were the same in clinical practice. However, in distinct types DTC, differences in RAI avidity and response existed and the effect of RAI therapy could not be equated.
Methods: DTC patients’ data in SEER database were extracted to perform retrospective analysis. The differences between case group and control group were compared by chi-square tests. We used Kaplan-Meier statistics and Cox regression analyses to investigate cancer-specific survival (CSS). Propensity score–matched was performed to make 1:1 case-control matching.
Results: 105195 patients who receiving total thyroidectomy were identified in SEER database. Compared to papillary thyroid carcinoma (PTC) (52.3%), follicular thyroid carcinoma (FTC) (63.8%) and oncocytic carcinoma of thyroid (OCA) (64.4%) had higher rates of RAI therapy. In the multivariable Cox regression model, RAI therapy was independent prognosis factor in PTC but not in OCA and FTC. In subgroup analysis, RAI therapy could improve prognosis in PTC when gross extrathyroidal extension or lymph node metastases or early survival when distant metastases (DM) were presented. However, OCA and FTC patients with DM rather than regional lesions only could benefit from RAI therapy. High-risk patients receiving RAI therapy showed a better prognosis in PTC but not in OCA and FTC.
Conclusion: RAI therapy was an effective treatment for DTC and should be considered individually in PTC, OCA and FTC patients. Our results provided further guideline for treatment selection in DTC.
Thyroid cancer (TC) was the most common endocrine malignancy (1), affecting 567,000 cases worldwide, according to global cancer statistics (2). Differentiated thyroid carcinoma (DTC) originated from follicular epithelial cells and encompassed papillary thyroid carcinoma (PTC), follicular thyroid carcinoma (FTC) and oncocytic carcinoma of thyroid (OCA). PTC was the predominant type of TC accounting for 80-90% of cases, followed by FTC at 5-10%, and OCA at 3-4% (3–6). The 5th edition of the World Health Organization classification of tumors defined OCA as an invasive malignant follicular cell tumor consisting of at least 75% oncocytic cells, lacking the characteristic nuclear features of PTC or high-grade features (7). PTC had a higher incidence of regional lymph node metastases (LNM) due to its lymphatic permeation, while OCA and FTC were encapsulated tumors with capsular and/or vascular invasion that tended to spread hematogenously (7–9). Compared with PTC, OCA and FTC exhibited more aggressive clinicopathological features and worse prognosis (10–13).
Radioactive iodine (RAI) had been widely used in DTC patients after total thyroidectomy (TT) due to its targeting ability for thyroid cancer cells. American Thyroid Association (ATA) management guidelines recommended postoperative risk of recurrence risk stratification of DTC to guide RAI decision-making (1). ATA risk staging (TNM) was a relatively simple tool that contains vital clinical information on tumor size, lymph nodes and distant metastases, which could largely reflect the patient’s risk of post-operative recurrence and guide RAI therapy. However, differences in the biological behavior of PTC, FTC, and OCA had led to differences in response to RAI therapy. Most guidelines, including those of the ATA and National Comprehensive Cancer Network (NCCN) (14), incorporated PTC, OCA, and FTC into DTC for management and used the same strategy for RAI therapy. Inappropriate RAI treatment could lead to unnecessary complications, affecting quality of life and shortening survival (15). RAI avidity had been found to be independently correlated with pathological types of DTC with distant metastases (DM). In addition, there was no difference in RAI response between OCA and FTC, and both showed more disease progression after RAI compared to PTC (16). The role of RAI in FTC and OCA was still controversial with some studies suggesting it may improve survival outcomes while others had found no significant benefit (17). Previous researches revealed that RAI was not independently prognosis factor and could not improve cancer-specific survival (CSS) in OCA (18, 19). A retrospective study showed that minimally invasive FTC patients receiving RAI did not have better CSS than those without (20). Some retrospective studies considered that RAI could improve survival outcomes in OCA and FTC (21–24).
The objective of our study was to analyze the clinicopathological characteristics of three different types of DTC and compare the impact of RAI on CSS among them in order to refine the guidance for RAI in clinical practice. We conducted stratification analysis of DTC using ATA risk staging (TNM) and performed propensity score-matching (PSM) to control for potential confounders (1).
We identified patients diagnosed with PTC, OCA and FTC between 2004 and 2017 from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program. Publicly accessible data in SEER program were collected from 18 population-based central cancer registries. The third edition of International Classification of Diseases for Oncology (ICD-0–3) codes: 8050, 8260, 8290, 8330-8332, 8335, and 8339, 8340–8344, 8450 were included in our sample. The inclusion criteria were as follows: age older than 18 years and younger than 90 years, receipt of total thyroidectomy (TT), radiation exception of external radiation treatment, T stage exception of unknown and Tx, survival months greater than 0, and SEER cause-specific death classification exception of Dead (unknown or missing COD).
Variables of interest included age at diagnosed (aged <55 and ≥55 years), sex, race (White, Black, and Other [American Indian/Alaska Native, and Asian or Pacific Islander]), T stage, N stage, M stage, ATA risk staging (low-risk [T1-2, N0 and Nx, M0 and Mx], low-to-intermediate risk [T3, N0 and Nx, M0 and Mx; T1-3, N1, M0 and Mx] and high-risk [T4, any N, any M; M1, any T, any N]), RAI therapy. Outcome of interest was cancer-specific survival (CSS), defined as the date of diagnosis to the date of death from thyroid cancer.
The data extracted from SEER program were analyzed by SPSS statistical software, version 26.0 (IBM Corp, Armonk, NY). The differences between the case group (RAI) and the control group (no-RAI) were compared using x2 test or Fisher’s exact tests. Multivariate COX regression models were performed to estimate prognostic factors for CSS. Hazard ratios (HRs) and 95% confidence index (CI) were displayed. P values less than 0.05 were considered statistically significant. CSS curves were evaluated according to Kaplan-Meier analysis and log-rank test. PSM was performed to reduce the differences in patient baseline characteristics and selection bias. In the cohort, age, sex, race, T stage, N stage, and M stage were included as covariates for PSM. PTC, OCA, and FTC patients were performed 1:1 nearest neighbor matching with a matching tolerance of 0.002, 0.005, and 0.001, respectively, to obtain matched pairs.
Table 1 showed patients characteristics in our study and 105195 DTC patients (including 98288 PTC, 2153 OCA and 4754 FTC) receiving TT were finally included in our cohort. The differences in the demographic and clinical characteristics between case group (RAI) and control group (no-RAI) were exhibited in Table 2. In three types of DTC, patients undergoing RAI had higher T stage, M stage, and ATA risk staging compared with patients without. PTC (P<0.001) and FTC (P=0.039) patients receiving RAI exhibited higher N stage compared to those without, but not OCA (P=0.237). Supplementary Table 1 displayed the differences in the clinicopathological features and treatment of the three pathological types. The rates of RAI therapy in PTC, OCA, and FTC were 52.3%, 64.4%, and 63.8% (P<0.001), respectively. The PSM cohort was presented in Supplementary Table 2 and all P value > 0.05.
Univariate analysis in distinct types of DTC was presented in Supplementary Table 3. Table 3 showed the prognostic factors for CSS in three types of DTC by multivariate analyses before and after PSM. The median follow-up time in PTC, OCA, and FTC was 86 months (range from 1 to 191), 94 months (range from 1 to 191), and 91 months (range from 1 to 191), respectively. Table 4 exhibited 5-years, 10-years, and 15-years CSS rate for DTC according to pathological types in the entire cohort. PTC had the best CSS rate, FTC the second and OCA the worst (P<0.001). The prognostic factors associated with CSS in PTC included age, sex, and T stage, and N stage, and M stage, and RAI in entire cohort and PSM cohort. With regard to OCA and FTC, age, T stage, and N stage, and M stage were independent risk factors related to CSS in entire cohort and PSM cohort. Inconsistent with PTC, RAI was no prognostic factor after PSM whether in OCA (Yes as reference, HRs=1.128; 95% CI=0.699-1.82; P=0.622) or FTC (Yes as reference, HRs=1.492; 95% CI=0.958-2.325; P=0.077).
Kaplan-Meier curves were performed to analyze the effect of RAI in three types of DTC when LNM or gross extrathyroidal extension (T4 stage) or DM were presented. Survival analysis of three types of DTC with LNM were showed in Figure 1 (Figure 1). Only PTC patients with LNM could improving CSS when receiving RAI in entire cohort (Figure 1A, P<0.001) and PSM cohort (Figure 1B, P=0.001). RAI could not significantly improve CSS of OCA and FTC patients when LNM were presented either in entire cohort (OCA: Figure 1C, P=0.222; FTC: Figure 1E, P=0.795) or PSM cohort (OCA: Figure 1D, P=0.056; FTC: Figure 1F, P=0.978).
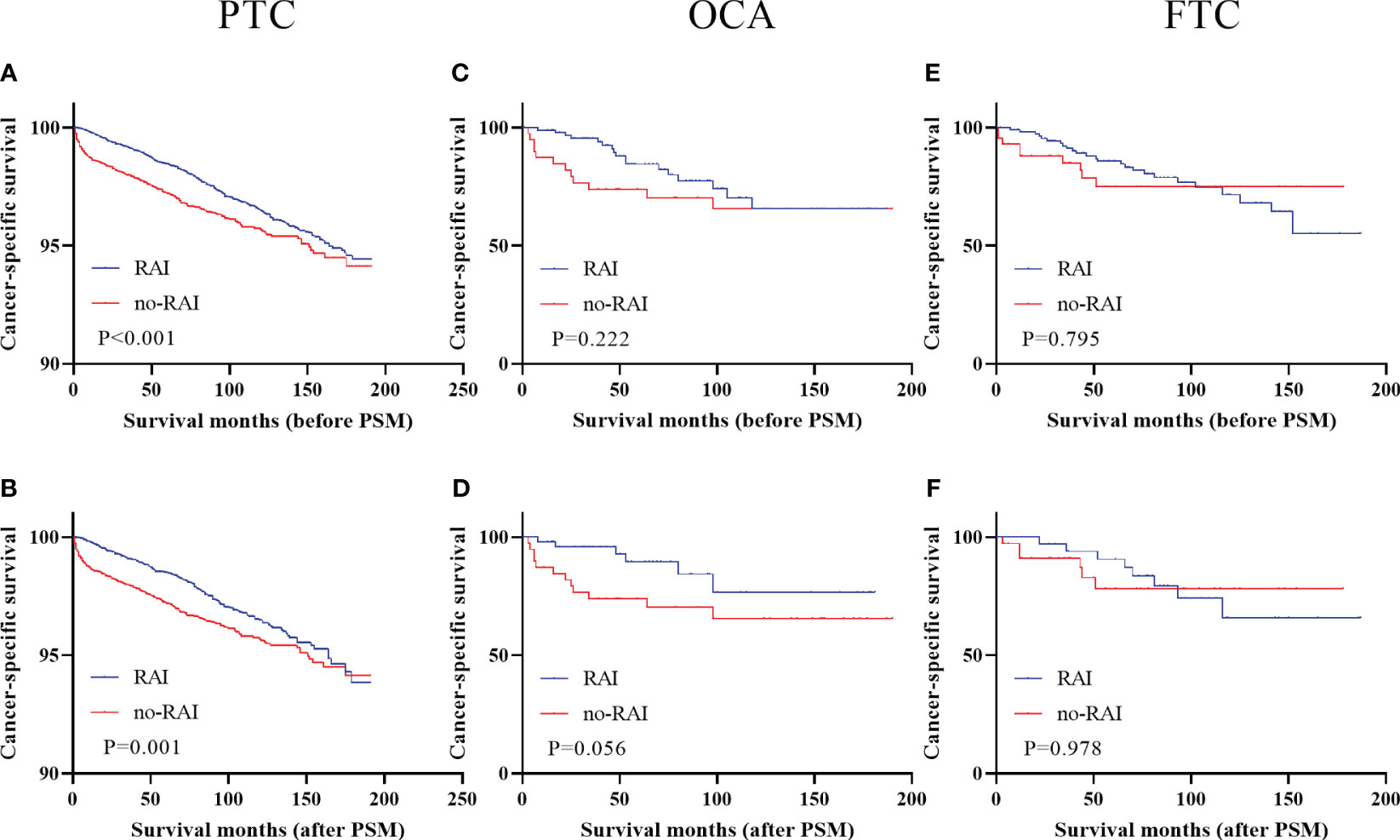
Figure 1 Cancer-specific survival for distinct types of DTC with lymph node metastases in the entire cohort and PSM cohort (A) PTC before PSM (B) PTC after PSM (C) OCA before PSM (D) OCA after PSM (E) FTC before PSM (F) FTC after PSM. DTC, differentiated thyroid carcinoma; PSM, propensity score–matched; PTC, papillary thyroid carcinoma; OCA, oncocytic carcinoma of thyroid; FTC, Follicular thyroid carcinoma; RAI, Radioactive iodine.
Figure 2 showed the survival curves of DTC patients with T4 stage before and after PSM. Only PTC patients with T4 stage had better survival outcomes when receiving RAI in entire cohort (Figure 2A, P<0.001) and PSM cohort (Figure 2B, P<0.001). OCA and FTC had no significant improvements when undergoing RAI (entire cohort: OCA, Figure 2C, P=0.776; FTC, Figure 2E, P=0.119; PSM cohort: OCA, Figure 2D, P=0.495; FTC, Figure 2F, P=0.965).
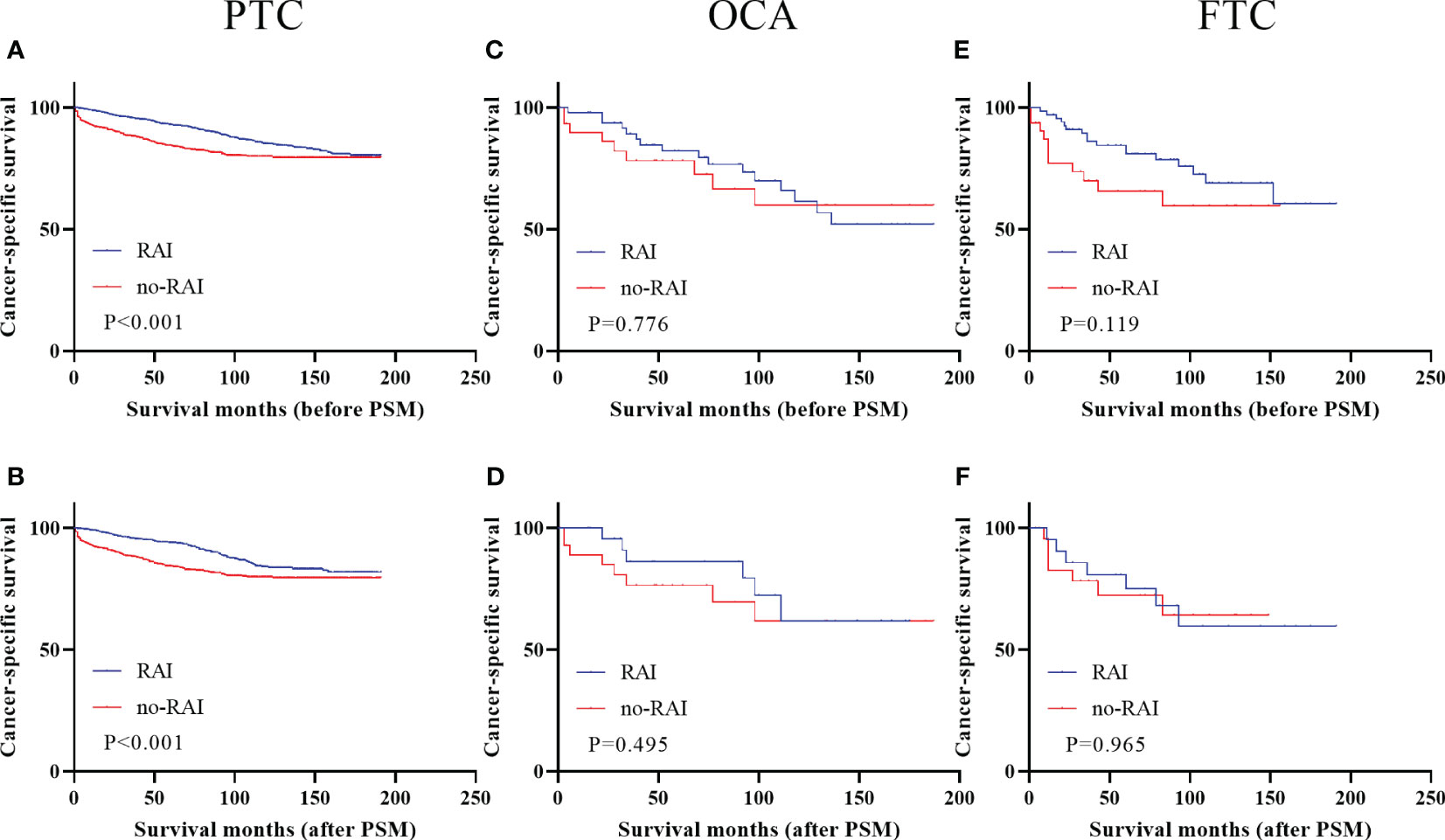
Figure 2 Cancer-specific survival for distinct types of DTC with T4 stage in the entire cohort and PSM cohort (A) PTC before PSM (B) PTC after PSM (C) OCA before PSM (D) OCA after PSM (E) FTC before PSM (F) FTC after PSM. DTC, differentiated thyroid carcinoma; PSM, propensity score–matched; PTC, papillary thyroid carcinoma; OCA, oncocytic carcinoma of thyroid; FTC, Follicular thyroid carcinoma; RAI, Radioactive iodine.
Kaplan-Meier curves of DTC patients with DM were presented in Figure 3. Metastatic OCA and FTC patients could benefit from RAI in entire cohort (OCA: Figure 3C, P=0.018; FTC: Figure 3D, P<0.001) and PSM cohort (OCA: Figure 3E, P=0.014; FTC: Figure 3F, P=0.012). Metastatic PTC patients had better survival outcomes in entire cohort (Figure 3A, P<0.001), but not in PSM cohort (Figure 3B, P=0.183). We further analyzed the 50 months, 100 months and 150 months CSS in PTC patients with DM (Table 5). In entire cohort, RAI therapy improved the 50 months (P<0.001), 100 months (P=0.003) and 150 months (P=0.008) CSS of metastatic PTC patients. In PSM cohort, Patients receiving RAI only showed significant differences in 50 months CSS.
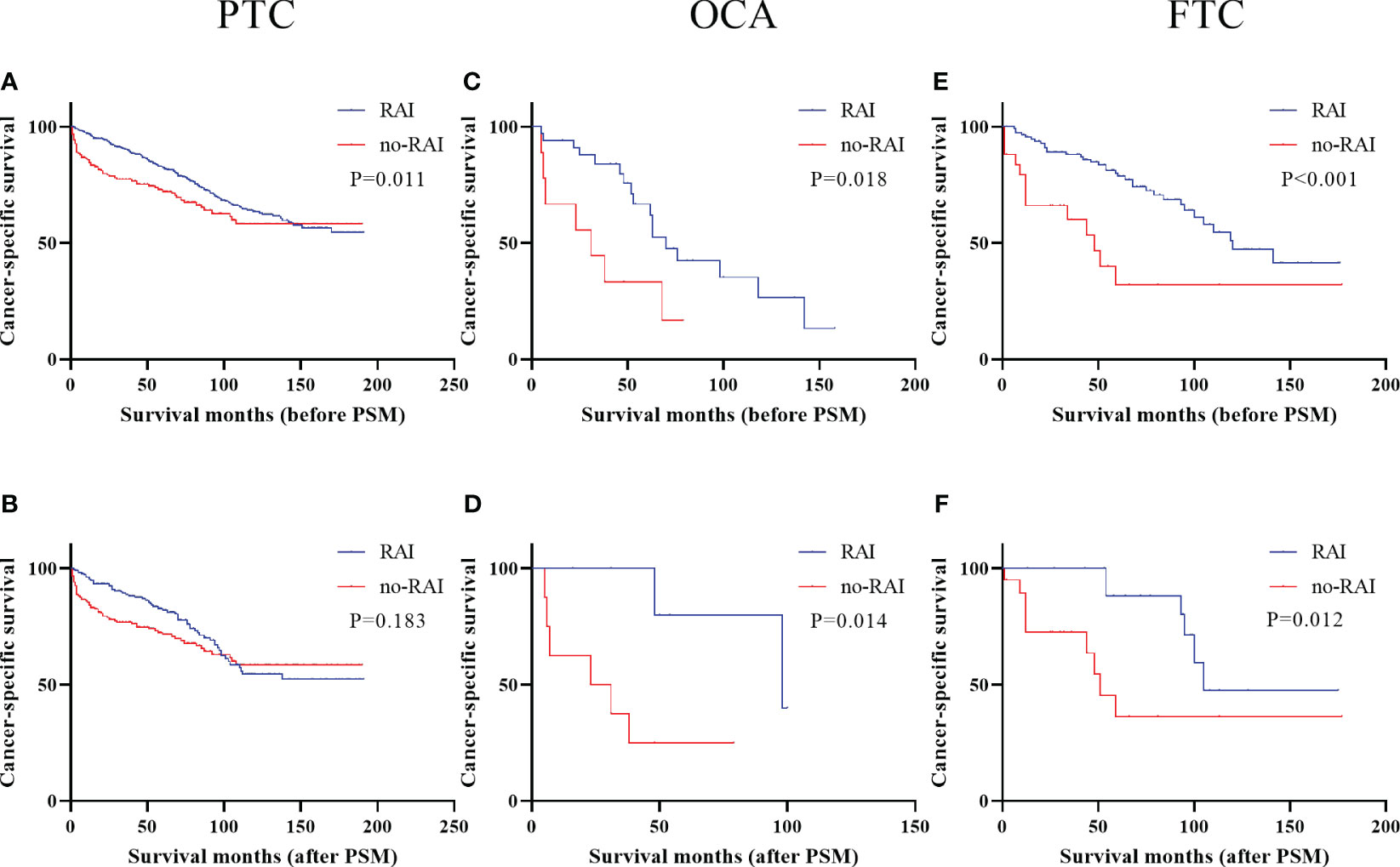
Figure 3 Cancer-specific survival for distinct types of DTC with distant metastases in the entire cohort and PSM cohort (A) PTC before PSM (B) PTC after PSM (C) OCA before PSM (D) OCA after PSM (E) FTC before PSM (F) FTC after PSM. DTC, differentiated thyroid carcinoma; PSM, propensity score–matched; PTC, papillary thyroid carcinoma; OCA, oncocytic carcinoma of thyroid; FTC, Follicular thyroid carcinoma; RAI, Radioactive iodine.
Figure 4 showed the effect of RAI in high-risk DTC patients. Only PTC patients had better CSS when receiving RAI in entire cohort (Figure 4A, P<0.001) and PSM cohort (Figure 4B, P<0.001). In OCA and FTC patients, RAI had no significant improvement in CSS whether in entire cohort (OCA: Figure 4C, P=0.424; FTC: Figure 4E, P=0.101) or PSM cohort (OCA: Figure 4D, P=0.095; FTC: Figure 4F, P=0.391). Survival analysis of low-risk patients and low-to-intermediate-risk patients were displayed in Supplementary Figure 1 before and after PSM and there were no significant improvements in all three types of DTC.
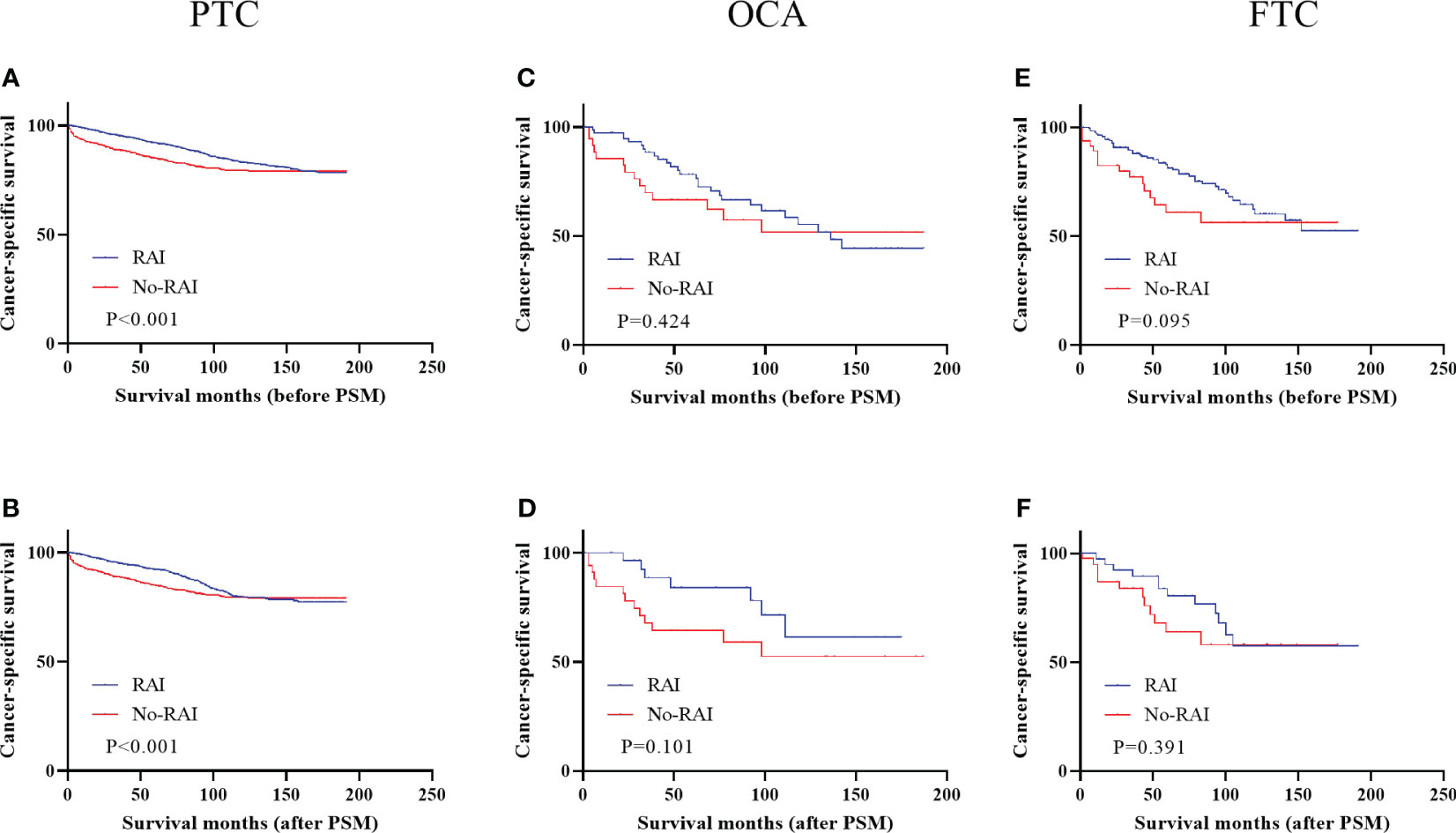
Figure 4 Cancer-specific survival for distinct types of DTC with high-risk staging in the entire cohort and PSM cohort (A) PTC before PSM (B) PTC after PSM (C) OCA before PSM (D) OCA after PSM (E) FTC before PSM (F) FTC after PSM. DTC, differentiated thyroid carcinoma; PSM, propensity score–matched; PTC, papillary thyroid carcinoma; OCA, oncocytic carcinoma of thyroid; FTC, Follicular thyroid carcinoma; RAI, Radioactive iodine.
TC was a relatively indolent cancer with a high 5-year survival rate of 98%, but it still affected a significant number of individuals each year, with an incidence rate of 14.6 per 100,000 men and women (25, 26). While thyroidectomy was the main treatment for DTC, postoperative therapies, including thyroid stimulating hormone suppression therapy, RAI therapy and tyrosine kinase inhibitors (TKIs) therapy, were also available (27). TKIs were primarily used for iodine-refractory TC and currently tested in clinical trials (28). However, RAI therapy remained a primary treatment for high-risk DTC after TT, aside from drug therapy. Despite its effectiveness, RAI therapy could have adverse effects, including dysfunction of salivary gland and lacrimal gland, testicular dysfunction, transient female gonadal dysfunction and second primary malignancies (15, 29, 30). Inappropriate RAI therapy could have an impact on quality of life and shorten survival time. Therefore, we aimed to refine RAI therapy strategies for DTCs in clinical practice, balancing the complications of RAI therapy.
Previous studies found that OCA and FTC had a worse prognosis compared with PTC (12, 13). Our study was consistent with those findings, revealing that PTC had the best prognosis, followed by FTC, with OCA having the worst prognosis. The 10-year CSS rates for PTC, FTC, and OCA were 98.2%, 96%, and 94.1%, respectively. We considered that three types of DTC patients differed in RAI response. RAI therapy was an independent risk factor associated with CSS in PTC but not OCA and FTC. Specifically, In the presence of LNM or gross extrathyroidal extension, PTC patients could benefit from RAI therapy, while such benefit was not observed in OCA and FTC patients. Additionally, in OCA and FTC patients with DM, RAI therapy improved CSS. There are differences in survival analysis results of metastatic PTC patients before and after PSM. The conclusion that RAI treatment could improve early survival (50 months CSS) of patients with metastatic PTC remains consistent. However, statistical differences were observed in the analysis of 100 months and 150 months CSS in the entire cohort, but not in the PSM cohort. It was found that the sample size of patients with long-term follow-up (100 months and 150 months) in the PSM cohort was limited. Additionally, a significant amount of data for the RAI group were deleted in the PSM cohort, and even non-RAI group data were not screened in the long-term follow-up analysis, which could compromise the test efficiency. Therefore, a larger sample size is required to support the analysis of long-term survival in patients with metastatic PTC, especially in the context of RAI treatment. We further compared the differences in RAI response among DTC according to ATA risk staging (TNM). In high-risk patients, RAI therapy could improve prognosis in PTC but not in OCA and FTC. In low-risk and low-to-intermediate-risk patients, RAI therapy did not provide any benefit. Our study suggested that RAI therapy was not recommended for OCA and FTC patients with regional lesions only.
Our study was consistent with previous research, indicating that OCA and FTC could benefit from RAI. Chow et al. considered that RAI therapy could improve survival outcomes and reduce recurrence and was an effective treatment for FTC (21). A SEER-based research concluded that metastatic FTC with receiving RAI had a better overall survival than those who did not (23). A retrospective study of 251 patients showed that RAI therapy was unnecessary in minimally invasive FTC and did not improve distant-metastases-free survival or CSS (20). This conclusion about minimally invasive FTC was not contradictory to our findings that regional lesions could not benefit from RAI therapy. Jillard et al. similarly considered that RAI therapy was associated with a better prognosis in OCA with advanced TNM stage (24).
The effectiveness of RAI therapy in the three types of DTC cannot be completely equated. Our finding revealed that fewer patients could benefit from RAI therapy for OCA and FTC compared to PTC. However, the percentage of patients receiving RAI therapy in our sample was higher in OCA (64.4%) and FTC (63.8%) than in PTC (52.3%). This might be due to the more aggressive clinicopathological features and biological behaviors and poorer prognosis of OCA and FTC compared to PTC, which might lead clinicians to prefer more aggressive treatment strategies for OCA and FTC. Previous research had shown differences in RAI avidity and RAI response among the three types of DTC (16). OCA (13.6%) had the lowest RAI avidity, classical variant PTC (21.4%) had medium RAI avidity, and FTC (76.5%) and follicular variant PTC (75.6%) had highest RAI avidity. However, compared to classical and follicular variant PTC, OCA and FTC had lower RAI response and more disease progression after RAI therapy. Chindris et al. found that only 2 of 27 OCA with lung metastases showed positive RAI scans (31). A retrospective study of metastatic PTC and FTC concluded that 137 of 178 PTC patients and 156 of 245 FTC patients observed RAI avidity and 41 PTC patients and 29 FTC patients achieved remission after RAI therapy (32). The differences in pathology and genomics of PTC, OCA and FTC might contribute to the differences in RAI avidity among them. Classical variant PTC, the most common type of PTC, was characterized by papillae and nuclear changes with BRAFV600E mutation being the most frequent mutation. This mutation reduced expression of functional thyroid genes including those encoding thyroglobulin and sodium/iodide symporter (NIS) proteins through high mitogen-activated protein kinase (MAPK) pathway output, resulting in a decrease in RAI activity (33, 34). FTC was associated with mutually exclusive mutations in the RAS or PAX8-PPARG fusion oncogenes and lacked the BRAFV600E mutation, which might lead to higher RAI avidity compared to conventional PTC (35). However, in OCA, which was characterized by a high rate of mitochondrial mutations and amplifications of BRAF, rather than activating mutations were observed (36). TERT promoter mutations were observed in 22% OCA and Liu et al. found that TERT mutations were associated with loss of RAI avidity (36, 37). Current researches had yet to fully explain the low RAI avidity observed in OCA. There were pitfalls of RAI therapy that not all RAI uptake resulted in RAI response, which could be observed in poorly differentiated tumors, in older patients or in patients with a heavy tumor burden (38). One possible explanation for this phenomenon was that the heterogeneity within the tumor which could result in abnormal distribution of RAI that only killed functional tumor cells and left non-iodine taking cells (27).
Our study highlighted the importance of distinguishing the response to RAI therapy among the three types of DTC and individualizing treatment strategies accordingly. Further researches were needed to investigate the mechanisms and molecular markers associated with response to RAI treatment.
Our research had several limitations. Firstly, due to its retrospective study, there was the possibility of selection bias. Secondly, The SEER database lacked data on disease recurrence and secondary surgery, so we were unable to analyze the disease-free survival rate after RAI treatment.
RAI therapy was an effective treatment for DTC and should be considered individually in PTC, OCA and FTC patients. OCA and FTC patients with DM rather than with regional lesions only could benefit from RAI therapy. However, with regard to PTC, RAI therapy could improve survival outcomes when gross extrathyroidal extension or LNM and early survival when DM were presented. Our results provided further guideline for treatment selection in DTC.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Union Hospital, Tongji Medical College, Huazhong University of Science and Technology. The patients/participants provided their written informed consent to participate in this study.
HHG: Conceptualization, Methodology, Data curation, Formal analysis, Figures and Tables creation, Writing - Original Draft, Writing - Review and Editing. NS and TH: Conceptualization, Methodology, Formal analysis, Writing - Review and Editing. NZ: Data curation, Formal analysis, Figures and Tables creation, Writing - Review and Editing. YXH and FRZ: Formal analysis, Figures and Tables creation. All authors contributed to the article and approved the submitted version.
This work is supported by Key Program of Natural Science Foundation of Hubei Province (Grant No. 2021BCA142).
The authors sincerely appreciate the invaluable support of our department members.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1158581/full#supplementary-material
Supplementary Figure 1 | Cancer-specific survival for distinct types of DTC with low-risk or low-to-intermediate-risk staging in the entire cohort and PSM cohort. Low-risk DTC: (A) PTC before PSM (B) PTC after PSM (E) OCA before PSM (F) OCA after PSM (I) FTC before PSM (J) FTC after PSM. Low-to-intermediate-risk staging: (C) PTC before PSM (D) PTC after PSM (J) OCA before PSM (H) OCA after PSM (K) FTC before PSM (L) FTC after PSM. DTC, differentiated thyroid carcinoma; PSM, propensity score–matched; PTC, papillary thyroid carcinoma; OCA, oncocytic carcinoma of thyroid; FTC, Follicular thyroid carcinoma; RAI, Radioactive iodine.
1. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the american thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid (2015) 26(1):1–133. doi: 10.1089/thy.2015.0020
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
3. Correa P, Chen VW. Endocrine gland cancer. Cancer (1995) 75(1 Suppl):338–52. doi: 10.1002/1097-0142(19950101)75:1+<338::aid-cncr2820751316>3.0.co;2-f
4. Kondo T, Ezzat S, Asa SL. Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat Rev Cancer (2006) 6(4):292–306. doi: 10.1038/nrc1836
5. Grimm D. Cell and molecular biology of thyroid disorders 2.0. Int J Mol Sci (2021) 22(4). doi: 10.3390/ijms22041990
6. DeLellis RA. Pathology and genetics of tumours of endocrine organs. World Health Organization: Lester D. R. Thompson (2004).
7. Baloch ZW, Asa SL, Barletta JA, Ghossein RA, Juhlin CC, Jung CK, et al. Overview of the 2022 WHO classification of thyroid neoplasms. Endocr Pathol (2022) 33(1):27–63. doi: 10.1007/s12022-022-09707-3
8. Carcangiu ML, Zampi G, Pupi A, Castagnoli A, Rosai J. Papillary carcinoma of the thyroid. A clinicopathologic study of 241 cases treated at the University of Florence, Italy. Cancer (1985) 55(4):805–28. doi: 10.1002/1097-0142(19850215)55:4<805::aid-cncr2820550419>3.0.co;2-z
9. Carcangiu ML, Zampi G, Rosai J. Papillary thyroid carcinoma: a study of its many morphologic expressions and clinical correlates. Pathol Annu (1985) 20 Pt 1:1–44.
10. Gulcelik MA, Gulcelik NE, Kuru B, Camlibel M, Alagol H. Prognostic factors determining survival in differentiated thyroid cancer. J Surg Oncol (2007) 96(7):598–604. doi: 10.1002/jso.20845
11. Passler C, Scheuba C, Prager G, Kaczirek K, Kaserer K, Zettinig G, et al. Prognostic factors of papillary and follicular thyroid cancer: differences in an iodine-replete endemic goiter region. Endocr Relat Cancer (2004) 11(1):131–9. doi: 10.1677/erc.0.0110131
12. Vorburger SA, Ubersax L, Schmid SW, Balli M, Candinas D, Seiler CA. Long-term follow-up after complete resection of well-differentiated cancer confined to the thyroid gland. Ann Surg Oncol (2009) 16(10):2862–74. doi: 10.1245/s10434-009-0592-4
13. Kuo EJ, ROman SA, Sosa JA. Patients with follicular and Hurthle cell microcarcinomas have compromised survival: a population level study of 22,738 patients. Surgery (2013) 154(6):1246–53. doi: 10.1016/j.surg.2013.04.033
14. Haddad RI, Bischoff L, Ball D, Bernet V, Blomain E, Busaidy NL, et al. Thyroid carcinoma, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw (2022) 20(8):925–51. doi: 10.6004/jnccn.2022.0040
15. Clement SC, Peeters RP, Ronckers CM, Links TP, van den Heuvel-Eibrink MM, Nieveen van Dijkum EJ, et al. Intermediate and long-term adverse effects of radioiodine therapy for differentiated thyroid carcinoma–a systematic review. Cancer Treat Rev (2015) 41(10):925–34. doi: 10.1016/j.ctrv.2015.09.001
16. Simoes-Pereira J, Mourinho N, Ferreira TC, Limbert E, Cavaco BM, Leite V. Avidity and outcomes of radioiodine therapy for distant metastasis of distinct types of differentiated thyroid cancer. J Clin Endocrinol Metab (2021) 106(10):e3911–e22. doi: 10.1210/clinem/dgab436
17. Grani G, Lamartina L, Durante C, Filetti S, Cooper DS. Follicular thyroid cancer and Hurthle cell carcinoma: challenges in diagnosis, treatment, and clinical management. Lancet Diabetes Endocrinol (2018) 6(6):500–14. doi: 10.1016/S2213-8587(17)30325-X
18. Oluic B, Paunovic I, Loncar Z, Djukic V, Diklic A, Jovanovic M, et al. Survival and prognostic factors for survival, cancer specific survival and disease free interval in 239 patients with Hurthle cell carcinoma: a single center experience. BMC Cancer (2017) 17(1):371. doi: 10.1186/s12885-017-3370-x
19. Wang X, Zheng X, Zhu J, Li Z, Wei T. Radioactive iodine therapy does not improve cancer-specific survival in hurthle cell carcinoma of the thyroid. J Clin Endocrinol Metab (2022) 107(11):3144–51. doi: 10.1210/clinem/dgac448
20. Sugino K, Kameyama K, Ito K, Nagahama M, Kitagawa W, Shibuya H, et al. Outcomes and prognostic factors of 251 patients with minimally invasive follicular thyroid carcinoma. Thyroid (2012) 22(8):798–804. doi: 10.1089/thy.2012.0051
21. Chow SM, Law SC, Mendenhall WM, Au SK, Yau S, Yuen KT, et al. Follicular thyroid carcinoma: prognostic factors and the role of radioiodine. Cancer (2002) 95(3):488–98. doi: 10.1002/cncr.10683
22. Lin JD, Chao TC, Chen ST, Huang YY, Liou MJ, Hsueh C. Operative strategy for follicular thyroid cancer in risk groups stratified by pTNM staging. Surg Oncol (2007) 16(2):107–13. doi: 10.1016/j.suronc.2007.05.004
23. Podnos YD, Smith D, Wagman LD, Ellenhorn JD. Radioactive iodine offers survival improvement in patients with follicular carcinoma of the thyroid. Surgery (2005) 138(6):1072–6. doi: 10.1016/j.surg.2005.09.021
24. Jillard CL, Youngwirth L, Scheri RP, ROman S, Sosa JA. Radioactive iodine treatment is associated with improved survival for patients with hurthle cell carcinoma. Thyroid (2016) 26(7):959–64. doi: 10.1089/thy.2016.0246
25. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin (2023) 73(1):17–48. doi: 10.3322/caac.21763
26. SEER. Cancer Stat Facts: Thyroid Cancer. National Cancer Institute. Bethesda, MD. Available at: https://seer.cancer.gov/statfacts/html/thyro.html.
27. Schlumberger M, Leboulleux S. Current practice in patients with differentiated thyroid cancer. Nat Rev Endocrinol (2021) 17(3):176–88. doi: 10.1038/s41574-020-00448-z
28. Gild ML, Tsang VHM, Clifton-Bligh RJ, Robinson BG. Multikinase inhibitors in thyroid cancer: timing of targeted therapy. Nat Rev Endocrinol (2021) 17(4):225–34. doi: 10.1038/s41574-020-00465-y
29. Wichers M, Benz E, Palmedo H, Biersack HJ, Grunwald F, Klingmuller D. Testicular function after radioiodine therapy for thyroid carcinoma. Eur J Nucl Med (2000) 27(5):503–7. doi: 10.1007/s002590050535
30. Rubino C, de Vathaire F, Dottorini ME, Hall P, Schvartz C, Couette JE, et al. Second primary Malignancies in thyroid cancer patients. Br J Cancer (2003) 89(9):1638–44. doi: 10.1038/sj.bjc.6601319
31. Chindris AM, Casler JD, Bernet VJ, Rivera M, Thomas C, Kachergus JM, et al. Clinical and molecular features of Hurthle cell carcinoma of the thyroid. J Clin Endocrinol Metab (2015) 100(1):55–62. doi: 10.1210/jc.2014-1634
32. Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab (2006) 91(8):2892–9. doi: 10.1210/jc.2005-2838
33. Durante C, Puxeddu E, Ferretti E, Morisi R, Moretti S, Bruno R, et al. BRAF mutations in papillary thyroid carcinomas inhibit genes involved in iodine metabolism. J Clin Endocrinol Metab (2007) 92(7):2840–3. doi: 10.1210/jc.2006-2707
34. Fagin JA, Wells SA Jr.Biologic and clinical perspectives on thyroid cancerN Engl J Med (2016) 375(11):1054–67 doi: 10.1056/NEJMra1501993
35. Nikiforova MN, Lynch RA, Biddinger PW, Alexander EK, Dorn GW 2nd, Tallini G, et al. RAS point mutations and PAX8-PPAR gamma rearrangement in thyroid tumors: evidence for distinct molecular pathways in thyroid follicular carcinoma. J Clin Endocrinol Metab (2003) 88(5):2318–26. doi: 10.1210/jc.2002-021907
36. Ganly I, Makarov V, Deraje S, Dong Y, Reznik E, Seshan V, et al. Integrated genomic analysis of hurthle cell cancer reveals oncogenic drivers, recurrent mitochondrial mutations, and unique chromosomal landscapes. Cancer Cell (2018) 34(2):256–70 e5. doi: 10.1016/j.ccell.2018.07.002
37. Liu J, Liu R, Shen X, Zhu G, Li B, Xing M. The genetic duet of BRAF V600E and TERT promoter mutations robustly predicts loss of radioiodine avidity in recurrent papillary thyroid cancer. J Nucl Med (2020) 61(2):177–82. doi: 10.2967/jnumed.119.227652
Keywords: radioactive iodine (131 I) treatment, thyroid cancer (TC), differentiated thyroid cancer (DTC), survival, propensity score (PS) matching (PSM)
Citation: Guo H, Zhang N, Hu Y, Zhang F, Huang T and Shen N (2023) Radioactive iodine therapy strategies for distinct types of differentiated thyroid cancer: a propensity score–matched analysis. Front. Endocrinol. 14:1158581. doi: 10.3389/fendo.2023.1158581
Received: 04 February 2023; Accepted: 24 July 2023;
Published: 17 August 2023.
Edited by:
Rui Huang, Sichuan University, ChinaReviewed by:
Pietro Locantore, Catholic University of the Sacred Heart, Rome, ItalyCopyright © 2023 Guo, Zhang, Hu, Zhang, Huang and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Na Shen, bmFzaGVuQGh1c3QuZXVkLmNu; Tao Huang, aHVhbmd0YW93aEAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.