
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol. , 24 May 2023
Sec. Pediatric Endocrinology
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1155925
This article is part of the Research Topic Exploring Obesity Risk, Prevention, and Research Innovation in the First 2000 Days of Life, volume II View all 5 articles
In the context of the childhood obesity epidemic, this narrative review aims to explore opportunities to promote physical activity (PA) between birth and age 5 years as well as the health outcomes associated with PA in early childhood. Although early childhood is an ideal time to promote healthy habits, guidelines for PA have often ignored early childhood given the limited evidence for children <5 years old. Herein we discuss and highlight infant, toddler and preschool age interventions to promote PA and prevent obesity both in the short and long-term. We describe novel and modified interventions to promote improved early childhood health outcomes, encompassing cardiorespiratory, muscle, and bone strengthening components necessary for short-term motor development and long-term health. We call for new research aimed at developing and testing innovative early childhood interventions that may be performed in home or childcare settings, monitored by parents or caregivers.
The high prevalence of obesity in all age groups is a major public health concern. Alarmingly, recent worldwide data suggests that an estimated 39 million children under the age of 5 were classified as having obesity in 2020 and that number is expected to rise to 40 million by 2030 (WHO 2021). Primary prevention is likely the best solution, as recent simulation data in the New England Journal of Medicine suggest that “a 2-year-old who is obese is more likely to be obese at 35 years of age than an overweight 19-year-old.” (1)
Physical activity (PA), a multi-faceted behavior typically associated with increased energy expenditure and load-bearing forces, has long been endorsed as a means of health promotion and disease prevention across the lifecourse (2, 3), although comparatively, very little work has conducted interventions in the first few years after birth. Infancy (0-12 months), toddlerhood (12-36 months) and the preschool period (36-60 months) are unique periods of robust developmental plasticity where health behaviors such as PA can have lasting metabolic and behavioral consequences. Obesity-prevention interventions to increase PA during this time period promote improvements in health outcomes, including weight, that persist into childhood (4–6) and early adulthood years (7). Despite these promising results, there are few specific recommendations for PA in infants (8–10) and toddlers (9, 10) with slightly more recommendations for preschoolers (10, 11). In 2011 the Institute of Medicine acknowledged the dearth of research and lack of published consensus on recommendations related to infant PA (12). Although in more recent years, several countries and the World Health Organization (WHO) have published 24-hr movement guidelines for children under 5 years old that include specific recommendations for time spent in active play, specific to each year (13–15).
The challenges related to increasing PA in early childhood, which we define as birth to age 5 years, are unique and dependent upon child development within that period. To our knowledge, no review of early childhood PA interventions has considered these unique difficulties in conjunction with proposed novel solutions that may work to address these challenges. Therefore, with this review, we aim to 1) summarize evidence and identify gaps related to PA based on the developmental timeline of infancy, toddler and preschool; 2) highlight the impact of interventions on health outcomes (such as body composition and bone health), and 3) propose novel PA interventions specifically designed to address these identified gaps and improve health outcomes.
This was designed as a narrative review, to include a search of the following electronic databases through January 2023: EBSCO (CINAHL); Cochrane Library (Central) and OVID (EMBASE, MEDLINE, PsycINFO) and Web of Science (all). Key term used in the search included infant, toddler, early obesity; physical activity; exercise, floor play, bone health, body composition, adiposity. Reference bibliographies were also searched to identify relevant studies as were additional publications or reviewers identified as relevant by study authors.
Studies were included if the following criteria were met: 1) reported results from a longitudinal, experimental, or cross-sectional study, 2) reported any movement behavior to include physical activity or exercise (acute or chronic interventions of any intensity or duration, supervised or unsupervised). Studies were considered eligible if published in English included participants with or without obesity aged 0-5. Studies were excluded if only animal data was reported. Study design, sample size, publication year, age of participants, type of participants, description of PA or exercise intervention, and associated health outcomes were extracted from each identified publication.
Within the last decade there have been promising trials done to promote healthy behaviors during infancy (5, 16, 17) that may extend into early childhood (18) (19). However, since the recommendation by the American Academy of Pediatrics (AAP) to include tummy time as part of an infant’s routine in the 1990s, there have been very few, if any, novel recommendations for providers to promote PA prior to age 1 year (20). This is unfortunate, as pediatricians can be effective promotors of PA to their patients (21) with frequent points of contact during the first several years (22). Recognizing this unique opportunity, a newly published report from the AAP encourages pediatricians to provide a clearly documented PA “prescription” that will allow for other providers, parents, and caregivers to “administer” an accurate dose of PA (20). As with any successful medication prescription and regimen, consideration must be given to timing, dose, and even individual factors such as adherence. Unfortunately, very little physiologically based research exists today that can inform PA prescriptions for infants.
Little is known regarding the exact mechanisms by which infant motor development, PA, and rapid weight gain early in life are related. Some speculate that it relates to critical periods of infant development where rapid cell development and growth occur, leaving infants vulnerable for increased risk of inappropriate weight gain (23–25). Number of adipocytes, a major predictor of fat mass in adulthood, is determined early in life (26) potentially explaining the positive association between rapid infant weight gain and persistence of obesity into both childhood and adulthood (25). Increased infant weight gain has also been shown to promote epigenetic modifications that regulate gene expression associated with persistent weight gain into childhood (27).
Infant positioning is one of the earliest activities to promote PA. Beginning on a child’s first day home from the hospital, the AAP recommends short periods of awake time in the prone position, playing and interacting with their environment in a meaningful way. Time should slowly be increased as the child begins to exhibit activity enjoyment with the end goal of accumulating 30 minutes daily. Known as “tummy time” (28) this recommendation has remained constant as one of the few recommendations for promoting infant PA (29). Tummy time is supported by several studies showing delayed gross motor development in infants who did not meet the recommended time spent in prone position (30, 31). This delay in motor development may contribute to accelerated infant weight gain by limiting the amount of PA (Figure 1) (32, 33). A recent systematic review found that tummy time was inversely related to BMI z-score in the first year, and positively related to gross and total motor development, as well as the ability to move while prone, supine or crawling (29). Additional data have shown that increased unrestricted movement during infancy has been associated with decreased waist circumference z-score and improved weight‐for‐length z-scores between ages 9 and 24 months (34). Most of the reports regarding tummy time and unrestricted movement are observational and lack objectively measured data with tools such as accelerometers (29). However, recent promising data has shown that higher levels of infant PA measured by ankle-worn accelerometers are associated with lower central adiposity (35). Data are lacking regarding associations between tummy time and infant unrestricted movement with other outcomes such as muscle strength and bone health (29, 31, 36).
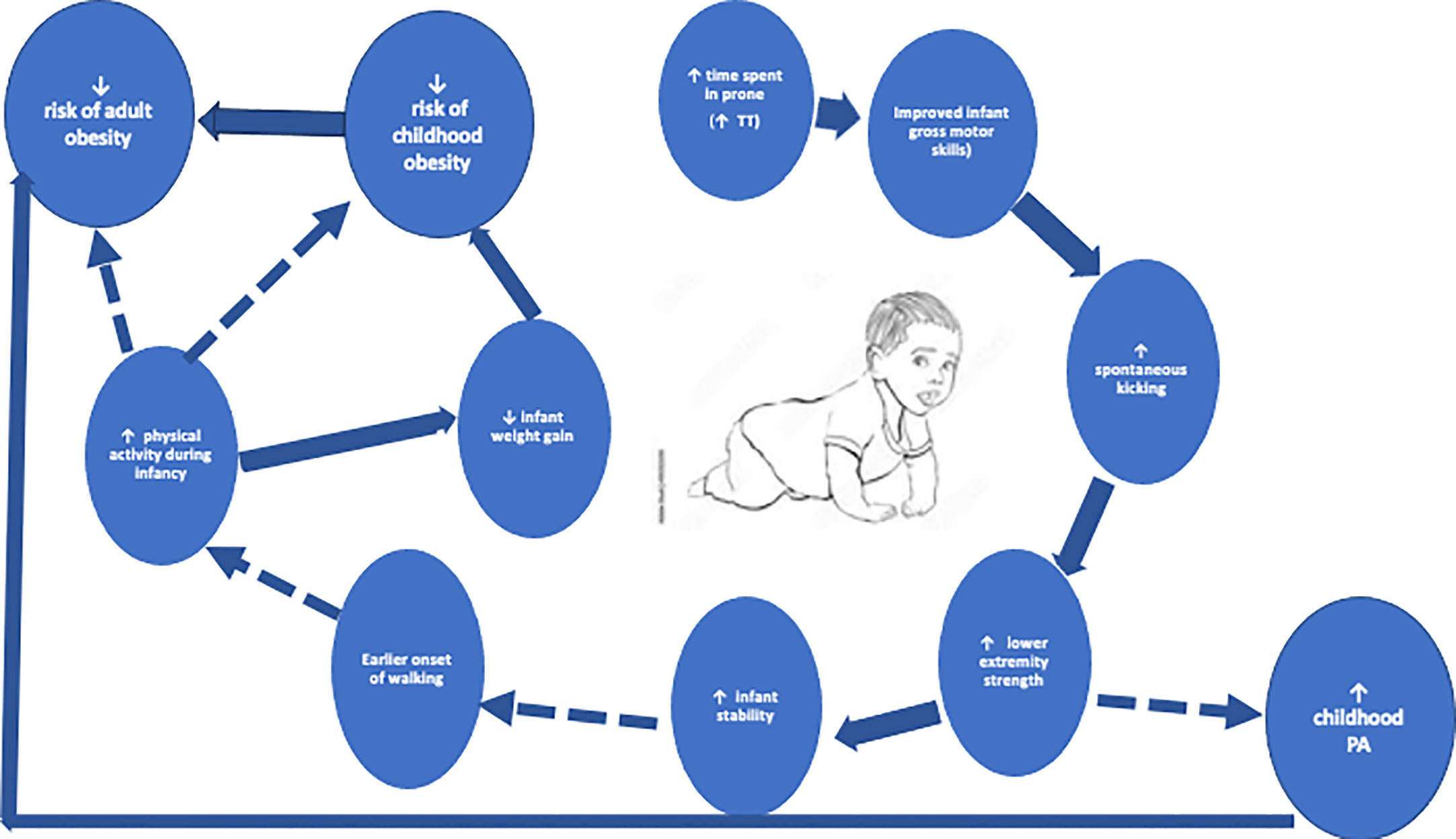
Figure 1 The reinforcing loop of increased tummy time, physical activity, and decreased obesity. Solid lines are supported references within the text. Dashed lines are opportunities for investigation.
Targeting parenting practices through educational programs is one of the most well-documented interventions to assess both short- and long-term impact on infant PA (4, 6, 16, 18, 37). Interventions to date typically delivered for the purposes of obesity prevention have varied in focus and depth, with topics ranging from responsive parenting techniques (5), appropriate breastfeeding and sleep habits (6) as well as anticipatory guidance on infant diet and PA (16). The findings have been mixed in terms of impact on weight-related outcomes though none were designed to directly assess impact on PA as their primary outcome nor was PA during infancy objectively measured.
Novel treadmill-based interventions have been studied in children with developmental disabilities to improve attainment of neuromotor skills that might otherwise be delayed (38). The utilization of partial body weight supported treadmill training in infants as young as 4 months decreases the delay in onset of walking, and improves walking gait in infants with and without a disability (38–40). These studies have found no impairment of such interventions on child growth or development. The authors speculate that increasing the onset of walking, in conjunction with improved stability during locomotion, promotes increased PA throughout infancy (41). Given the research highlighting the association between motor development in childhood and PA engagement in adolescence (42, 43), these patterns may promote PA engagement across the life course. To date, however, no extensive work has been done utilizing treadmill interventions in normally developing infants as young as 4 months. Ulrich et al. (39) highlight that these interventions can be done safely while positively impacting infant motor and muscle development.
No recommendations exist for “strength training” i.e., muscle and motor development, in infancy. Although the idea of “strength training” during infancy may seem premature, appropriate skeletal muscle development during this time can have long-term consequences on body composition (44) and willingness to engage in activity throughout the lifespan (45), key mediators of body composition in adulthood. Genetics do play a major predeterminant of muscle fiber composition (46), however, environmental factors likely play an important role given well-known trials highlighting “fiber reprogramming” in response to exercise training (47). Interestingly, some report increases in fibers related to enhanced fat metabolism in infants (Type 1 and Type 2a) vs. increases in fibers related to impaired fat metabolism (Type 2b) in adults (48). This holds clinical relevance as type 2b fibers have been associated with obesity and insulin resistance in adolescents and adults (48). It is unknown if these fibers can be maintained or reprogrammed in infancy to promote an improved metabolic profile later in life. Animal models have shown significant increases in Type 1 and Type 2a fibers following a unilateral leg kicking program (49). Implementation of a similar program in infancy might promote a favorable muscle fiber profile, mitigating increases in Type 2b fiber types related to obesity and insulin resistance later in adolescence and adulthood.
The above intervention is relevant, as spontaneous, rhythmical kicking in infancy typically seen around 1 month (50) can promote strength improvements through flexion and extension of the knee joint, presenting a unique way to improve strength early in development. As a certain amount of strength in the lower extremities is necessary for walking skills, such as pull-to-stand, promoting kicking during infancy can lead to earlier onset of walking in children (51). This can become a self-reinforcing loop, with those children walking earlier becoming more stable and subsequently engaging in more PA throughout infancy and childhood (Figure 1). Very recent work in infants 6-7 months highlights this relationship in a multivariate analysis of infant PA counts with more advanced motor development (52)
Very little work has assessed interventions designed to promote increased strength or muscle development in infants, though there have been efforts to promote proper neurodevelopment in children born prematurely using sensorized toys (53). These toys were designed to encourage spontaneous behaviors such as reaching, kicking, and grasping, thereby promoting muscle and strength development. Like treadmill interventions described above, these tools were primarily developed for at-risk preterm, low birth weight infants to improve motor skills and improve body composition development (53–55). However, the known overlap between motor development and improved weight status in youth (56, 57) (Figure 1) highlights the utility of such interventions in high birthweight infants, another “at-risk” group. Given the toy’s unique ability to measure forces generated by the infant (54), this would provide novel quantitative insight into tracking and development of anaerobic power and muscular strength during infancy, with the goal of better understanding the role “strength training” might play in mitigating weight gain throughout the lifespan.
As with other metabolic and body composition outcomes, bone mass accrual during infancy is accelerated and occurs at one of the most rapid rates an individual will experience during their lifetime. Bone mineral content and density is increased in response to repetitive and varying load-bearing activities via increased force and strain. Unfortunately, despite this period of significant potential for bone accrual, many infants do not engage in load-bearing activities that would elicit the necessary stimulus to prompt remodeling changes in the bone. Two small studies have attempted to determine if increased PA during infancy affects bone mineral density showing mixed results (58, 59).
There is significant variability in infant gross motor development limiting the reliability of PA measurement, especially within the first 3 months after birth (60). Additionally, there is a wide range of approaches for PA measurement, ranging from physiologic to behavioral (61). Direct accurate measurements of energy expenditure in infants using methods such as indirect calorimetry (62) or doubly labeled water (63) can be done, although lack of accessibility and increased cost limits its utilization in large population studies. Other more readily available means of assessment include questionnaires, observation, and accelerometers. The limitations of questionnaire and observation are well-known and documented elsewhere (64) with major drawbacks including lack of quantifiable data that directly measures PA. Accelerometers are the “gold standard” of PA assessment in infancy (65) however, this too presents unique age-specific confounding factors including the influence of caregiver lifting and carrying of the infant, which some have shown accounts for up to 40-50% of measured activities in infants wearing accelerometer (66). The best method for monitoring PA in children younger than age 2 years warrants more extensive testing, to further our understanding of PA patterns in infancy. Fortunately, current research by Pate et al. is being done to investigate this question (67).
By the end of their first year, most infants have developed the motor patterns necessary for locomotion, such as cruising (7-11 months) and standing unassisted (11-14 months), although they typically appear to be unsteady (hence the name “toddler”). Beyond 18 months, the toddler gait has developed sufficiently that fast walking closely resembles running. This increased freedom in both motor skill and function promotes variability in PA options compared to infants. The impact of PA in toddlerhood versus infancy may also have differential effects. PA in toddlerhood is associated with greater improvements in bone and skeletal health (68) as opposed to improved adiposity and motor skills in infancy (69). (Figure 2) These findings highlight that context of PA is especially important when considering interventions during the toddler years, as this age group’s activity patterns tend to be more sporadic, involving short, rapid bursts of movement (70).
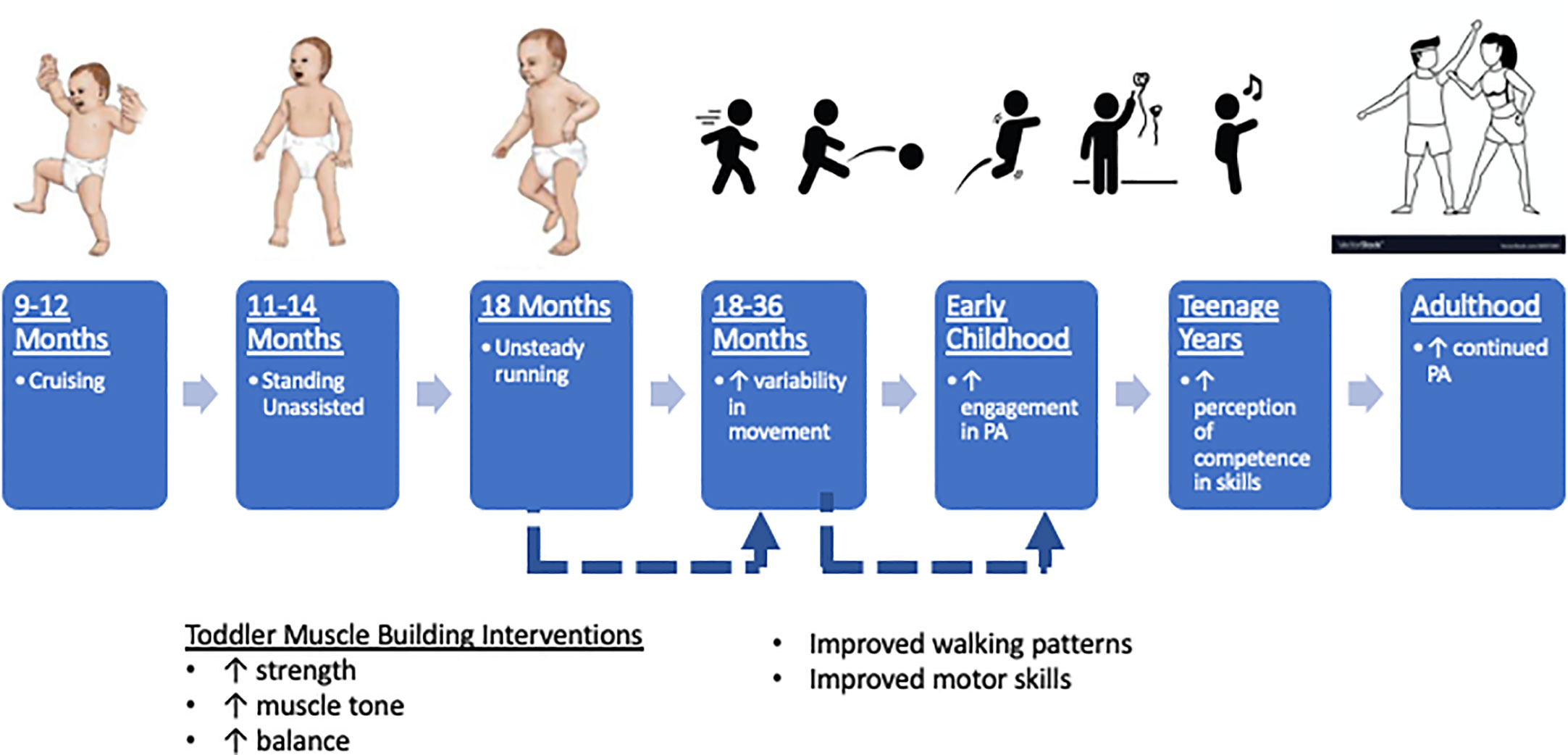
Figure 2 Toddler strength training interventions to promote continued engagement of physical activity into adulthood. Solid lines are supported references within the text. Dashed lines are opportunities for investigation.
Nearly 40% of US toddlers are cared for exclusively by a parent in the home (71). Therefore, home-based trials provide the opportunity to engage both child and caregiver in easily accessible ways. Mother-toddler diet and PA behaviors are significantly related (72, 73) since toddlers learn through modeled behaviors (74). Targeted maternal lifestyle interventions significantly increase toddler PA, although this may not influence toddler weight gain (75).
Childcare centers serve as another means of administering PA interventions. Extensive research has been done (76) in these settings to estimate not only the amount of PA toddlers engage in throughout the day, but also, the intensity levels of this PA, stratifying data based on light to moderate-to-vigorous physical activity (MVPA). A recent meta-analysis highlights that although toddlers tend to engage in >180 minutes of PA/day (exceeding the recommendation by the WHO), very little of this time is spent in activities quantified by moderate or high intensity, which may have long-term health consequences (69). In response to this concern, some have advocated for modifying the center environment with portable play equipment (77) or increased scheduled time spent in a non-restrictive outdoor environment (78) as nearly 15% more MVPA has been reported outdoors than indoors in childcare settings (79).
Other interventions to promote PA in toddlers enrolled in childcare settings include modification of center policies to promote increased active play with verbal reminders from staff trained in PA (80, 81) and limited screen time during recreational periods (82). Unfortunately, most centers in the US do not observe recommendations for PA promotion; nearly 90% of childcare services provide less than two hours of active play per day (83). With these considerations in mind, multi-component interventions addressing both childcare center practice and policies have been developed and implemented with modest results (76). Most importantly, these trials did not assess the direct impact of the intervention on PA in the cohorts of toddlers. Interventions to directly increase toddler PA across settings lacks specific and targeted goals informed by previous research; effective interventions most likely target multiple components (i.e. environment, caregiver etc.)
Similar to interventions in infancy, most strength programs done in toddlerhood to promote muscle building have been in toddlers with either intellectual or physical disabilities (84–86). Although a recent systematic review by Pate et al. noted a significant relationship between higher levels of physical activity with bone health in healthy children (87). In a very small cohort of toddlers with Down Syndrome (n=5), Sayers et al. found that an individualized, sequential at-home 8-week pediatric strength intervention designed to improve strength, tone and balance saw improved walking patterns (84). This program was designed using the theoretical basis of progressive and interactive facilitation, which combines both proprioceptive stimuli and neurodevelopmental patterning delivered through exercise. Initially, there were significant concerns that parents would not feel confident in administering this type of intervention in the home. This study provided proof-of-concept, as parents who were trained as part of this program believed they could successfully implement this intervention. Parents also believed that the intervention was effective and efficient for improving their child’s motor control (88). To our knowledge, no current interventions have implemented a theoretically- based pediatric strength intervention for children without disabilities.
Swimming, or aquatic therapy, is another full-body aerobic and motor-development intervention utilized as a conjunctive therapy for children with developmental delays or disabilities. The benefits of swimming have been well-documented in these children and include improved strength, increased active and passive range of motion, improved postural control and increased quality of life (89–91). Swimming also serves a practical purpose as the American Academy of Pediatrics recommends that all children 1 year of age or older learn swimming to prevent drowning (92). Additionally, the buoyancy of water provides a unique environment through which the toddler can develop postural control and engage in partial or full range of motion activities utilizing multiple large muscle, promoting increases in strength and motor development. Once again, despite effective swimming trials being conducted in toddlers with disabilities, no studies have been done in toddlers without a disability to determine if motor strength, range of motion or motor control would be improved similarly. This is relevant, as improvements in motor control during these early years could have long-term implications for motor development beyond childhood. A small study (n=19) showed that “baby swimming” improves hand-eye coordination and balance at a 4-year follow-up compared to children (93) (94). Some speculate that enhancements in motor development during this early period not only promote better motor skills, but also increase the child’s perception of their sports ability, reinforcing their continued engagement in PA throughout their life (94).
High intensity interval training (HIIT) interventions might be used to promote both cardiometabolic and bone health in toddlers. HIIT consists of short bursts of vigorous activity followed by recovery bouts of moderate activity and has been used to encourage individuals (primarily adults) to gain the benefits of PA when perceived lack of time is a barrier. Compared to traditional exercise training, HIIT has been shown to significantly improve cardiometabolic outcomes such as systolic blood pressure and VO2 max (a measure of cardiorespiratory fitness) in youth with obesity (95). Importantly, children perceive it to be more enjoyable (95). Given that the pattern of HIIT is very similar to how toddlers engage in active play, interventions utilizing this program remain highly applicable in this population. Unfortunately, recommendations for incorporation of HIIT into toddler programs remain vague due to the lack of current literature and methodological limitations of the data that does exist (96).
In addition to improving cardiometabolic outcomes (95) incorporation of HIIT interventions into toddler research may also improve skeletal health. Bursts of bone-loading activity with only short bouts of recovery accumulating in 2-3 minutes is a potent stimulus that has significant osteogenic effects (97). These benefits may occur in a dose-response, as MVPA has strong associations with improved bone and skeletal health in toddlers, with higher intensity of PA associated with even greater improvements in these outcomes (68). Children who engaged in higher amounts of MVPA between 2-3 years had increased bone mineral content and density at 5 years (18). Although not explicitly assessed, these interventions most likely also targeted muscle development and strength, as growing muscle is an essential component of increasing the load on a child’s growing bone, known as the muscle-bone unit. Given this relationship, we hypothesize that HIIT interventions in this cohort would also positively influence muscle development, in addition to cardiometabolic and bone health, leading to improved weight outcomes.
As highlighted above, the current research assessing strength and motor development programs has consisted of small patient cohorts or specific patient populations, such as children with disabilities, limiting interpretation of the data available. The new AAP recommendations for swimming classes for those ages 1-4 years offers an opportunity for research on health benefits of swimming for toddlers (92). As with the treadmill interventions for infants, findings among children with disabilities provides proof of concept that these trials can be implemented safely and effectively when done under proper supervision.
In comparison to infancy and toddlerhood, more recommendations exist for preschool children aged 36-60 months such as a goal of 3 hours of PA/day (9–11, 13) though less than half of children meet those recommendations (98). More specific recommendations provided by the CDC encourage active play “everyday throughout the day” that should include aerobic, muscle-strengthening, and bone-strengthening activities (11). These guidelines are much more specific in comparison to the recommendations currently made in infancy and toddlers, so as expected, more trials have been completed (81, 99–101). Despite the number of interventions that have been done to increase children’s PA, findings have been inconsistent and typically lack objective measures of PA (79, 98, 101–103) which may, in part, explain discrepancies amongst the data (Figure 3).

Figure 3 Exercise snacks to promote physical activity in preschool. Solid lines are supported references within the text. Dashed lines are opportunities for investigation.
Like toddlers, overweight preschoolers are approximately three to six times more likely to have at least one parent with obesity in comparison to children who are not overweight (101). Interestingly, Trost et al. reported no differences between these two groups in factors previously thought to mitigate risk for obesity, such as parental modeling of PA, parental support for PA, or the number of toys/equipment available at home for active play (104). Other PA factors in this age group to consider include personality (101) and peer group (105).
As more than half of preschool aged children in the US attend childcare (106), interventions conducted in preschool or childcare settings provide access to a large cohort of children who engage in mostly sedentary behaviors during that time (65, 107). Unfortunately, children’s PA levels are highly variable among preschools, suggesting that policies and practices significantly influence the day-to-day behaviors of its students (108). As in toddlers (74, 76, 77, 80), strategic modifications of the school environment on the playground can increase PA (102). More specifically, preschoolers are more active during the first minutes of recess when compared with the minutes prior to reconvening to go back inside (100) highlighting the strength of incorporation of multiple, shorter periods of recess as opposed to one long recess bout (109).
Given that increasing the amount of time spent in PA in preschool programs can be challenging and has had little success in the past, interventions should consider focusing on the intensity of activity in this age cohort. Preschool teachers can be trained to promote MVPA throughout the day (110, 111) which can have a meaningful impact. Higher levels of daily MVPA correlates with improved body weight in a large cohort of preschool children (112). Given that higher levels of MVPA are typically associated with higher levels of overall childhood PA (Figure 2) and that higher levels of childhood PA are associated with improved body composition in adulthood (Figure 1), the long-term effects of increasing intensity of exercise during the early childhood years must be highlighted in future trials.
For many years, there have been extensive debates surrounding the safety and feasibility of initiating “strength training” in youth (i.e. muscle building activities) (113). When done correctly under the supervision of appropriately trained personnel, such interventions can be safely administered (114). Very recently, a 10-week exercise program administered 3x/week in preschool and kindergarten classrooms significantly increased jump performance and muscle power (115). The program consisted of progressive repetitions of musculoskeletal loading activities such as lunges, ankle hops, lateral jumps, squats, and other exercises that could easily be done in a classroom or daycare setting. The proven feasibility of this intervention is timely, as there have been health benefits of brief “exercise snacks” in other age groups consisting of scheduled, short bouts (15-30 seconds) of vigorous activity (e.g. stair climbing) designed to break up prolonged periods of sedentary time. These snacks improve cardiometabolic outcomes such as insulin sensitivity in adults with obesity (116), though effects of “exercise snacks” in youth are unknown. However, these findings highlight the potential impact a time-efficient and easily administered novel intervention can have in childcare settings particularly those that promote anaerobic power and strength in the early childhood years, given the many known health benefits independent of aerobic fitness (117). These interventions must be easily implemented either at home or in a daycare or school setting, while not being overly complicated or increasing the amount of burden on caregivers or teachers.
One novel intervention we propose is the incorporation of the “exercise snacks” (116) described above with the muscle-building activities described by Wick et al. (115) but modified to be a very brief bout of activity. This requires little time, basic training, and could easily be incorporated into busy childcare and classroom settings with meaningful impact. The exact “dose” or amount of time or “snacks” per day necessary to elicit meaningful muscular changes is unknown, however, the utility of such an intervention should not be overlooked to break up sedentary time and promote muscular fitness. Additionally, these snacks could provide benefits beyond physical health, as Wick et al. report teachers noted improved psychosocial behavior after these exercise breaks, especially in the younger children (≤4 years) (115). The ease of administration in conjunction with the physical and psychological benefits of “exercise snacks” warrants significant attention in future PA and early childhood research.
Jumping is known to improve both hip and lumbar bone mass in prepubertal children (118), and exercise interventions for bone health in premenstrual girls are more effective than post menarche (119). Eight months of regular jumping in place for only 10 minutes, twice weekly during the school day can improve bone mass in older children as well (120). Even less of a stimulus can have a significant impact, as McKay et al. found that only 3 minutes/day of counter jump movements had a positive impact on bone health in elementary school aged children (121).
Despite more research on PA among preschoolers, there have been well-documented limitations (81, 82, 98, 122) though sex differences may play a significant role in why previous work has been inconclusive with regards to PA and health outcomes. Some have found certain interventions effective only for improving PA in preschool girls (111, 123) while others have found a greater impact on boys (124, 125). These significant differences when stratified by sex suggest that the previous trials which saw no intervention effect, may have in fact been “washed out” by this variable and unable to mediate the confounder due to small sample sizes (107). In addition to sex, other influential factors to consider include socioeconomic status, as a recent large cohort analysis found associations with socioeconomic status and measures of musculoskeletal strength in preschool children (126).
It is important to acknowledge that there are other limitations throughout infancy, toddlerhood and early childhood that are not discussed within this text, but highlighted in a recent systematic review (127). Within this section, however, we will highlight several factors that should be considered. Temperament, an early form of personality, is a behavioral style that is easily assessed in childhood as an encompassment of reaction to food, soothability, attention span, activity, sociability and emotionality (128). Even when controlling for other factors, childhood temperament is a robust predictor of adult BMI and influence choice in behaviors such as intensity of PA (129). In fact, Buss et al. found that several interpersonal attributes and personality descriptors were related to objectively measured PA levels in preschool children (99), although activity data collected during the school day has been shown to have no correlation with temperament in preschool children (130). Other individual-level factors to consider include sex (60), sleep (131), child interests (132), socioeconomic status (73), genetics (133), or epigenetics (134). Continuing research in the field should consider these factors as a means of identifying strategies to promote individualized and targeted behavior changes in infants, toddlers, and early childhood.
The childhood obesity epidemic requires the scientific community to better understand and address factors that mitigate weight gain, including the role of PA. Although infancy and the toddler years present unique and effective times to promote healthy habits, most interventions and guidelines for PA have focused on school age children and adolescents, with only modest success. Within this narrative review, we have highlighted the paucity of PA trials in early childhood. As a result, there is limited evidence to serve as the basis for PA guidelines for children <5 years old. Recognizing that the issue of obesity is extremely complex, we have proposed novel and modified interventions to promote improved health outcomes necessary for short-term motor development and health across the life course. Currently, no specific or easily accessible infant through early childhood PA recommendations exist for healthcare providers. This leaves them poorly equipped to promote PA in the office during a period in the child’s life where there are frequent provider/patient contacts and improved ability to promote behavior change. With this paper, we hope to prompt more research designed to develop and test innovative early childhood interventions that will equip providers and parents with effective approaches to combat childhood obesity and promote long-term health that persists into adulthood. Novel interventions at the individual and population level will allow for development of specific and applicable recommendations that healthcare providers can feel confident in prescribing to patients and their parents. Targeting PA during infancy through early childhood provides a distinctive lens through which researchers and clinicians in the world of pediatrics can work together to promote interventions and policies that will ensure healthy future generations of children and adults.
NS, IP, and RP contributed to conception of the paper. NS and IP wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
This manuscript was supported by the Children's Miracle Network at Penn State Health Children's Hospital.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ward ZJ, MW L, SC R, CM G, AL C, Gortmaker SL. Simulation of growth trajectories of childhood obesity into adulthood. N Engl J Med (2017) 377(22):2145–53. doi: 10.1056/NEJMoa1703860
2. Goldberg JH, King AC. Physical activity and weight management across the lifespan. Annu Rev Public Health (2007) 28(1):145–70. doi: 10.1146/annurev.publhealth.28.021406.144105
3. Haskell WL, Blair SN, Hill JO. Physical activity: health outcomes and importance for public health policy. Prev Med (2009) 49(4):280–2. doi: 10.1016/j.ypmed.2009.05.002
4. Paul IM, Savage JS, Anzman-Frasca S, Marini ME, Beiler JS, Hess LB, et al. Effect of a responsive parenting educational intervention on childhood weight outcomes at 3 years of age: the INSIGHT randomized clinical trial. JAMA (2018) 320(5):461. doi: 10.1001/jama.2018.9432
5. Savage JS, Hohman EE, Marini ME, Shelly A, Paul IM, Birch LL. INSIGHT responsive parenting intervention and infant feeding practices: randomized clinical trial. Int J Behav Nutr Phys Act (2018) 15(1):64. doi: 10.1186/s12966-018-0700-6
6. Taylor BJ, Heath ALM, Galland BC, Gray AR, Lawrence JA, Sayers RM, et al. Prevention of overweight in infancy (POI.nz) study: a randomised controlled trial of sleep, food and activity interventions for preventing overweight from birth. BMC Public Health (2011) 11(1):942. doi: 10.1186/1471-2458-11-942
7. Campbell F, Conti G, Heckman JJ, Moon SH, Pinto R, Pungello E, et al. Early childhood investments substantially boost adult health. Science (2014) 343(6178):1478–85. doi: 10.1126/science.1248429
8. Clark JE, Clements RL, Guddemi M, Morgan DW, Pica R, Pivarnik JM, et al. National association for sport and physical education. In: Active start: a statement of physical activity guidelines for children birth to five years. National Association for Sport and Physical Education, Oxon Hill, MD.
9. National Health Services. Physical activity guidelines for children (under 5 years). Department of Health and Social Care, London (2019).
10. Guidelines on physical activity, sedentary behaviour and sleep for children under 5 years of age (2019). World Health Organization. Available at: http://www.ncbi.nlm.nih.gov/books/NBK541170/ (Accessed November 30, 2021).
11. Centers for Disease Control and Prevention. Physical activity guidelines for school-aged children and adolescents. US Department of Health and Human Services. Washington, DC: US (2019).
12. Burns A, Parker L, Birch LL. (Eds.) Early childhood obesity prevention policies Vol. 13124. National Academies Press, Washington D.C. (2011). doi: 10.17226/13124
13. Tremblay MS, Chaput JP, Adamo KB, Aubert S, Barnes JD, Choquette L, et al. Canadian 24-hour movement guidelines for the early years (0-4 years): an integration of physical activity, sedentary behaviour, and sleep. BMC Public Health (2017) 17(Suppl 5):874. doi: 10.1186/s12889-017-4859-6
14. World Health Organization. Guidelines on physical activity, sedentary behaviour and sleep for children under 5 years of age (2019). World Health Organization. Available at: https://apps.who.int/iris/handle/10665/311664 (Accessed April 15, 2023).
15. Cliff DP, McNeill J, Vella SA, Howard SJ, Santos R, Batterham M, et al. Adherence to 24-hour movement guidelines for the early years and associations with social-cognitive development among Australian preschool children. BMC Public Health (2017) 17(S5):857. doi: 10.1186/s12889-017-4858-7
16. Wen LM, Baur LA, Rissel C, Flood V, Simpson J M, Hayes A, et al. Healthy beginnings trial phase 2 study: follow-up and cost-effectiveness analysis. Contemp Clin Trials (2012) 33(2):396–401. doi: 10.1016/j.cct.2011.11.008
17. Hesketh KD, Campbell KJ. Interventions to prevent obesity in 0-5 year olds: an updated systematic review of the literature. Obesity (2010) 18(S1):S27–35. doi: 10.1038/oby.2009.429
18. Taylor RW, Gray AR, Heath ALM, Galland BC, Lawrence J, Sayers R, et al. Sleep, nutrition, and physical activity interventions to prevent obesity in infancy: follow-up of the prevention of overweight in infancy (POI) randomized controlled trial at ages 3.5 and 5 y. Am J Clin Nutr (2018) 108(2):228–36. doi: 10.1093/ajcn/nqy090
19. Mollborn S, Lawrence E, Krueger PM. Developing health lifestyle pathways and social inequalities across early childhood. Popul Res Policy Rev (2021) 40(5):1085–117. doi: 10.1007/s11113-020-09615-6
20. Lobelo F, Muth ND, Hanson S, Nemeth BA, COUNCIL ON SPORTS MEDICINE AND FITNESS, SECTION ON OBESITY. Physical activity assessment and counseling in pediatric clinical settings. Pediatrics (2020) 145(3):e20193992. doi: 10.1542/peds.2019-3992
21. Floriani V, Kennedy C. Promotion of physical activity in primary care for obesity treatment/prevention in children. Curr Opin Pediatr (2007) 19(1):99–103. doi: 10.1097/MOP.0b013e328013c88c
22. Sallis R, Franklin B, Joy L, Ross R, Sabgir D, Stone J. Strategies for promoting physical activity in clinical practice. Prog Cardiovasc Dis (2015) 57(4):375–86. doi: 10.1016/j.pcad.2014.10.003
23. Dietz WH. Overweight in childhood and adolescence. N Engl J Med (2004) 350(9):855–7. doi: 10.1056/NEJMp048008
24. Taveras EM, Rifas-Shiman SL, Belfort MB, Kleinman KP, Oken E, Gillman MW. Weight status in the first 6 months of life and obesity at 3 years of age. PEDIATRICS (2009) 123(4):1177–83. doi: 10.1542/peds.2008-1149
25. Zheng M, Lamb KE, Grimes C, Laws R, Bolton K, Ong KK, et al. Rapid weight gain during infancy and subsequent adiposity: a systematic review and meta-analysis of evidence: infant rapid weight gain and later adiposity. Obes Rev (2018) 19(3):321–32. doi: 10.1111/obr.12632
26. Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, et al. Dynamics of fat cell turnover in humans. Nature (2008) 453(7196):783–7. doi: 10.1038/nature06902
27. Alfano R, Robinson O, Handakas E, Nawrot TS, Vineis P, Plusquin M. Perspectives and challenges of epigenetic determinants of childhood obesity: a systematic review. Obes Rev. (2022) 23:e13389. doi: 10.1111/obr.13389
28. Chizawsky LLK, Scott-Findlay S. Tummy time! preventing unwanted effects of the “Back to sleep” campaign. AWHONN Lifelines (2005) 9(5):382–7. doi: 10.1177/1091592305283633
29. Hewitt L, Kerr E, Stanley RM, Okely AD. Tummy time and infant health outcomes: a systematic review. Pediatrics (2020) 145(6):e20192168. doi: 10.1542/peds.2019-2168
30. Dudek-Shriber L, Zelazny S. The effects of prone positioning on the quality and acquisition of developmental milestones in four-Month-Old infants. Pediatr Phys Ther (2007) 19(1):48–55. doi: 10.1097/01.pep.0000234963.72945.b1
31. Kuo YL, Liao HF, Chen PC, Hsieh WS, Hwang AW. The influence of wakeful prone positioning on motor development during the early life. J Dev Behav Pediatr (2008) 29(5):367–76. doi: 10.1097/DBP.0b013e3181856d54
32. Koren A, Kahn-D’angelo L, Reece SM, Gore R. Examining childhood obesity from infancy: the relationship between tummy time, infant BMI-z, weight gain, and motor development–an exploratory study. J Pediatr Health Care (2019) 33(1):80–91. doi: 10.1016/j.pedhc.2018.06.006
33. Li R, O’Connor L, Buckley D, Specker B. Relation of activity levels to body fat in infants 6 to 12 months of age. J Pediatr (1995) 126(3):353–7. doi: 10.1016/S0022-3476(95)70447-7
34. Sijtsma A, Sauer PJJ, Stolk RP, Corpeleijn E. Infant movement opportunities are related to early growth–GECKO drenthe cohort. Early Hum Dev (2013) 89(7):457–61. doi: 10.1016/j.earlhumdev.2013.04.002
35. Benjamin-Neelon SE, Bai J, Østbye T, Neelon B, Pate RR, Crainiceanu C. Physical activity and adiposity in a racially diverse cohort of US infants. Obes (Silver Spring) (2020) 28(3):631–7. doi: 10.1002/oby.22738
36. Gross RS, Mendelsohn AL, Yin HS, Tomopoulos S, Gross MB, Scheinmann R, et al. Randomized controlled trial of an early child obesity prevention intervention: impacts on infant tummy time. Obes (Silver Spring) (2017) 25(5):920–7. doi: 10.1002/oby.21779
37. Campbell K, Hesketh K, Crawford D, Salmon J, Ball K, McCallum Z. The infant feeding activity and nutrition trial (INFANT) an early intervention to prevent childhood obesity: cluster-randomised controlled trial. BMC Public Health (2008) 8(1):103. doi: 10.1186/1471-2458-8-103
38. Angulo-Barroso RM, Wu J, Ulrich DA. Long-term effect of different treadmill interventions on gait development in new walkers with down syndrome. Gait Posture (2008) 27(2):231–8. doi: 10.1016/j.gaitpost.2007.03.014
39. Ulrich DA, Lloyd MC, Tiernan CW, Looper JE, Angulo-Barroso RM. Effects of intensity of treadmill training on developmental outcomes and stepping in infants with down syndrome: a randomized trial. Phys Ther (2008) 88(1):114–22. doi: 10.2522/ptj.20070139
40. Angulo-Barroso R, Burghardt AR, Lloyd M, Ulrich DA. Physical activity in infants with down syndrome receiving a treadmill intervention. Infant Behav Dev (2008) 31(2):255–69. doi: 10.1016/j.infbeh.2007.10.003
41. Ulrich DA, Hauck JL. Programming physical activity in young infants At-risk for early onset of obesity. Kinesiology Rev (2013) 2(4):221–32. doi: 10.1123/krj.2.4.221
42. Aaltonen S, Latvala A, Rose RJ, Pulkkinen L, Kujala UM, Kaprio J, et al. Motor development and physical activity: a longitudinal discordant twin-pair study. Med Sci Sports Exercise (2015) 47(10):2111–8. doi: 10.1249/MSS.0000000000000650
43. Vlahov E, Baghurst TM, Mwavita M. Preschool motor development predicting high school health-related physical fitness: a prospective study. Percept Mot Skills (2014) 119(1):279–91. doi: 10.2466/10.25.PMS.119c16z8
44. Ridgway CL, Ong KK, Tammelin T, Sharp SJ, Ekelund U, Jarvelin MR. Birth size, infant weight gain, and motor development influence adult physical performance. Med Sci Sports Exercise (2009) 41(6):1212–21. doi: 10.1249/MSS.0b013e31819794ab
45. van Deutekom AW, Chinapaw MJ, Vrijkotte TG, Gemke RJ. Study protocol: the relation of birth weight and infant growth trajectories with physical fitness, physical activity and sedentary behavior at 8-9 years of age - the ABCD study. BMC Pediatr (2013) 13(1):102. doi: 10.1186/1471-2431-13-102
46. Simoneau J, Lortie G, Boulay M, Marcotte M, Thibault M, Bouchard C. Inheritance of human skeletal muscle and anaerobic capacity adaptation to high-intensity intermittent training*. Int J Sports Med (1986) 07(03):167–71. doi: 10.1055/s-2008-1025756
47. Esbjörnsson M, Hellsten-Westing Y, Balsom PD, Sjödin B, Jansson E. Muscle fibre type changes with sprint training: effect of training pattern. Acta Physiol Scand (1993) 149(2):245–6. doi: 10.1111/j.1748-1716.1993.tb09618.x
48. Kriketos A, Baur L, O’Connor J, Carey D, King S, Caterson ID, et al. Muscle fibre type composition in infant and adult populations and relationships with obesity. Int J Obes (1997) 21(9):796–801. doi: 10.1038/sj.ijo.0800476
49. Song Y, Forsgren S, Liu JX, Yu JG, Stål P. Unilateral muscle overuse causes bilateral changes in muscle fiber composition and vascular supply. PloS One (2014) 9(12):e116455. doi: 10.1371/journal.pone.0116455
50. Thelen E, Bradshaw G, Ward JA. Spontaneous kicking in month-old infants: manifestation of a human central locomotor program. Behav Neural Biol (1981) 32(1):45–53. doi: 10.1016/S0163-1047(81)90257-0
51. Lloyd M, Burghardt A, Ulrich DA, Angulo-Barroso R. Physical activity and walking onset in infants with down syndrome. Adapted Phys Activity Q (2010) 27(1):1–16. doi: 10.1123/apaq.27.1.1
52. Shull ER, Dowda M, McIver KL, McLain AC, Benjamin-Neelon SE, Ulrich B, et al. Behavioral, environmental, and demographic factors associated with objectively measured physical activity in infants. Childhood Obes (2022) 18(7):466–75. doi: 10.1089/chi.2021.0197
53. Ho ES, Torres W, Prosser L, Johnson MJ. Ailu: an affordable sensorized toy for detection of neuro and motor delays in infants, in: 2019 IEEE 16th International Conference on Rehabilitation Robotics (ICORR), Toronto, ON, Canada, (2019), pp. 994–9. doi: 10.1109/ICORR.2019.8779523
54. Sgandurra G, Bartalena L, Cecchi F, Cioni G, Giampietri M, Greisen G, et al. A pilot study on early home-based intervention through an intelligent baby gym (CareToy) in preterm infants. Res Dev Disabil (2016) 53-54:32–42. doi: 10.1016/j.ridd.2016.01.013
55. Rihar A, Sgandurra G, Beani E, Cecchi F, Pašič J, Cioni G, et al. CareToy: stimulation and assessment of preterm infant’s activity using a novel sensorized system. Ann Biomed Eng (2016) 44(12):3593–605. doi: 10.1007/s10439-016-1669-4
56. Robinson LE, Stodden DF, Barnett LM, Lopes VP, Logan SW, Rodrigues LP, et al. Motor competence and its effect on positive developmental trajectories of health. Sports Med (2015) 45(9):1273–84. doi: 10.1007/s40279-015-0351-6
57. Lubans DR, Morgan PJ, Cliff DP, Barnett LM, Okely AD. Fundamental movement skills in children and adolescents. Sports Med (2010) 40(12):1019–35. doi: 10.2165/11536850-000000000-00000
58. Moyer-Mileur L, Luetkemeier M, Boomer L, Chan GM. Effect of physical activity on bone mineralization in premature infants. J Pediatr (1995) 127(4):620–5. doi: 10.1016/S0022-3476(95)70127-3
59. Specker BL, Mulligan L, Ho M. Longitudinal study of calcium intake, physical activity, and bone mineral content in infants 6-18 months of age. J Bone Miner Res (1999) 14(4):569–76. doi: 10.1359/jbmr.1999.14.4.569
60. Eaton WO, Dureski CM. Parent and actometer measures of motor activity level in the young infant. Infant Behav Dev (1986) 9(4):383–93. doi: 10.1016/0163-6383(86)90012-3
61. Loprinzi PD, Cardinal BJ. Measuring children’s physical activity and sedentary behaviors. J Exercise Sci Fitness (2011) 9(1):15–23. doi: 10.1016/S1728-869X(11)60002-6
62. Roberts SB, Savage J, Coward WA, Chew B, Lucas A. Energy expenditure and intake in infants born to lean and overweight mothers. N Engl J Med (1988) 318(8):461–6. doi: 10.1056/NEJM198802253180801
63. Jones PJH, Winthrop AL, Schoeller DA, Swyer PR, Smith J, Filler RM, et al. Validation of doubly labeled water for assessing energy expenditure in infants. Pediatr Res (1987) 21(3):242–6. doi: 10.1203/00006450-198703000-00007
64. Sallis JF, Saelens BE. Assessment of physical activity by self-report: status, limitations, and future directions. Res Q Exercise Sport (2000) 71(sup2):1–14. doi: 10.1080/02701367.2000.11082780
65. Reilly JJ, Penpraze V, Hislop J, Davies G, Grant S, Paton JY. Objective measurement of physical activity and sedentary behaviour: review with new data. Arch Dis Childhood (2008) 93(7):614–9. doi: 10.1136/adc.2007.133272
66. Tsai SY, Burr RL, Thomas KA. Effect of external motion on correspondence between infant actigraphy and maternal diary. Infant Behav Dev (2009) 32(3):340–3. doi: 10.1016/j.infbeh.2009.02.002
67. Pate RR, Frongillo EA, Cordan K, Dowda M, McLain AC, Torres ME, et al. Linking activity, nutrition, and child health (LAUNCH): protocol for a longitudinal cohort study of children as they develop from infancy to preschool age. BMC Public Health (2020) 20(1):931. doi: 10.1186/s12889-020-09023-7
68. Timmons BW, Leblanc AG, Carson V, Connor Gorber S, Dillman C, Janssen I, et al. Systematic review of physical activity and health in the early years (aged 0-4 years). Appl Physiol Nutr Metab (2012) 37(4):773–92. doi: 10.1139/h2012-070
69. Bruijns BA, Truelove S, Johnson AM, Gilliland J, Tucker P. Infants’ and toddlers’ physical activity and sedentary time as measured by accelerometry: a systematic review and meta-analysis. Int J Behav Nutr Phys Act (2020) 17(1):14. doi: 10.1186/s12966-020-0912-4
70. Cliff DP, Reilly JJ, Okely AD. Methodological considerations in using accelerometers to assess habitual physical activity in children aged 0-5 years. J Sci Med Sport (2009) 12(5):557–67. doi: 10.1016/j.jsams.2008.10.008
71. NSECE Project Team (National Opinion Research Center). National survey of early care and education (NSECE). US Department of Health and Human Services. Washington, DC: US (2012). doi: 10.3886/ICPSR35519.V14.
72. Hesketh KR, McMinn AM, Ekelund U, Sharp SJ, Collings PJ, Harvey NC, et al. Objectively measured physical activity in four-year-old British children: a cross-sectional analysis of activity patterns segmented across the day. Int J Behav Nutr Phys Act (2014) 11(1):1. doi: 10.1186/1479-5868-11-1
73. Papas MA, Hurley KM, Quigg AM, Oberlander SE, Black MM. Low-income, African American adolescent mothers and their toddlers exhibit similar dietary variety patterns. J Nutr Educ Behav (2009) 41(2):87–94. doi: 10.1016/j.jneb.2008.01.005
74. Shimpi PM, Akhtar N, Moore C. Toddlers’ imitative learning in interactive and observational contexts: the role of age and familiarity of the model. J Exp Child Psychol (2013) 116(2):309–23. doi: 10.1016/j.jecp.2013.06.008
75. Black MM, Hager ER, Wang Y, Hurley KM, Latta LW, Candelaria M, et al. Toddler obesity prevention: a two-generation randomized attention-controlled trial. Matern Child Nutr (2021) 17(1):e13075. doi: 10.1111/mcn.13075
76. Finch M, Jones J, Yoong S, Wiggers J, Wolfenden L. Effectiveness of centre-based childcare interventions in increasing child physical activity: a systematic review and meta-analysis for policymakers and practitioners: childcare physical activity trial. Obes Rev (2016) 17(5):412–28. doi: 10.1111/obr.12392
77. Bower JK, Hales DP, Tate DF, Rubin DA, Benjamin SE, Ward DS. The childcare environment and children’s physical activity. Am J Prev Med (2008) 34(1):23–9. doi: 10.1016/j.amepre.2007.09.022
78. Boldemann C, Blennow M, Dal H, Mårtensson F, Raustorp A, Yuen K, et al. Impact of preschool environment upon children’s physical activity and sun exposure. Prev Med (2006) 42(4):301–8. doi: 10.1016/j.ypmed.2005.12.006
79. Vanderloo LM, Tucker P, Johnson AM, Holmes JD. Physical activity among preschoolers during indoor and outdoor childcare play periods. Appl Physiol Nutr Metab (2013) 38(11):1173–5. doi: 10.1139/apnm-2013-0137
80. Gubbels JS, Kremers SPJ, van Kann DHH, Stafleu A, Candel MJ, Dagnelie PC, et al. Interaction between physical environment, social environment, and child characteristics in determining physical activity at child care. Health Psychol (2011) 30(1):84–90. doi: 10.1037/a0021586
81. Trost SG, Ward DS, Senso M. Effects of child care policy and environment on physical activity. Med Sci Sports Exerc (2010) 42(3):520–5. doi: 10.1249/MSS.0b013e3181cea3ef
82. Dowda M, Brown WH, McIver KL, Pfeiffer KA, O'Neill JR, Addy CL, et al. Policies and characteristics of the preschool environment and physical activity of young children. PEDIATRICS (2009) 123(2):e261–6. doi: 10.1542/peds.2008-2498
83. McWilliams C, Ball SC, Benjamin SE, Hales D, Vaughn A, Ward DS. Best-practice guidelines for physical activity at child care. PEDIATRICS (2009) 124(6):1650–9. doi: 10.1542/peds.2009-0952
84. Sayers LK, Cowden JE, Newton M, Warren B, Eason B. Qualitative analysis of a pediatric strength intervention on the developmental stepping movements of infants with down syndrome. Adapted Phys activity Q (1996) 13(3):247–68. doi: 10.1123/apaq.13.3.247
85. Prosser LA, Ohlrich LB, Curatalo LA, Alter KE, Damiano DL. Feasibility and preliminary effectiveness of a novel mobility training intervention in infants and toddlers with cerebral palsy. Dev Neurorehabilitation (2012) 15(4):259–66. doi: 10.3109/17518423.2012.687782
86. Wu J, Ulrich DA, Looper J, Tiernan CW, Angulo-Barroso RM. Strategy adoption and locomotor adjustment in obstacle clearance of newly walking toddlers with down syndrome after different treadmill interventions. Exp Brain Res (2008) 186(2):261–72. doi: 10.1007/s00221-007-1230-7
87. Pate RR, Hillman CH, Janz KF, Katzmarzyk PT, Powell KE, Torres A, et al. Physical activity and health in children younger than 6 years: a systematic review. Med Sci Sports Exercise (2019) 51(6):1282–91. doi: 10.1249/MSS.0000000000001940
88. Sayers LK, Cowden JE, Sherrill C. Parents’ perceptions of motor interventions for infants and toddlers with down syndrome. Adapted Phys Activity Q (2002) 19(2):199.
89. McManus BM, Kotelchuck M. The effect of aquatic therapy on functional mobility of infants and toddlers in early intervention. Pediatr Phys Ther (2007) 19(4):275–82. doi: 10.1097/PEP.0b013e3181575190
90. Hutzler Y, Chacham A, Bergman U, Szeinberg A. Effects of a movement and swimming program on vital capacity and water orientation skills of children with cerebral palsy. Dev Med Child Neurol (2008) 40(3):176–81. doi: 10.1111/j.1469-8749.1998.tb15443.x
91. Dimitrijević L, Aleksandrović M, Madić D, Okičić T, Radovanović D, Daly D. The effect of aquatic intervention on the gross motor function and aquatic skills in children with cerebral palsy. J Hum Kinetics (2012) 2012:32. doi: 10.2478/v10078-012-0033-5
92. Denny SA, Quan L, Gilchrist J, McCallin T, Shenoi R, Yusuf S, et al. Prevention of drowning. Pediatrics (2021) 148(2):e2021052227. doi: 10.1542/peds.2021-052227
93. Sigmundsson H, Hopkins B. Baby swimming: exploring the effects of early intervention on subsequent motor abilities. Child: Care Health Dev (2010) 36(3):428–30. doi: 10.1111/j.1365-2214.2009.00990.x
94. Stodden DF, Goodway JD, Langendorfer SJ, Roberton MA, Rudisill ME, Garcia C, et al. A developmental perspective on the role of motor skill competence in physical activity: an emergent relationship. Quest (2008) 60(2):290–306. doi: 10.1080/00336297.2008.10483582
95. García-Hermoso A, Cerrillo-Urbina AJ, Herrera-Valenzuela T, Cristi-Montero C, Saavedra JM, Martínez-Vizcaíno V. Is high-intensity interval training more effective on improving cardiometabolic risk and aerobic capacity than other forms of exercise in overweight and obese youth? a meta-analysis. Obes Rev (2016) 17(6):531–40. doi: 10.1111/obr.12395
96. Eddolls WTB, McNarry MA, Stratton G, Winn CON, Mackintosh KA. High-intensity interval training interventions in children and adolescents: a systematic review. Sports Med (2017) 47(11):2363–74. doi: 10.1007/s40279-017-0753-8
97. Robling AG, Burr DB, Turner CH. Recovery periods restore mechanosensitivity to dynamically loaded bone. J Exp Biol (2001) 204(19):3389–99. doi: 10.1242/jeb.204.19.3389
98. Pate RR, O’Neill JR, Brown WH, Pfeiffer KA, Dowda M, Addy CL. Prevalence of compliance with a new physical activity guideline for preschool-age children. Childhood Obes (2015) 11(4):415–20. doi: 10.1089/chi.2014.0143
99. Buss DM, Block JH, Block J. Preschool activity level: personality correlates and developmental implications. Child Dev (1980), 401–8. doi: 10.2307/1129273
100. McKenzie TL, Sallis JF, Elder JP, Berry CC, Hoy PL, Nader PR, et al. Physical activity levels and prompts in young children at recess: a two-year study of a bi-ethnic sample. Res Q Exerc Sport (1997) 68(3):195–202. doi: 10.1080/02701367.1997.10607998
101. Trost SG, Sirard JR, Dowda M, Pfeiffer KA, Pate RR. Physical activity in overweight and nonoverweight preschool children. Int J Obes Relat Metab Disord (2003) 27(7):834–9. doi: 10.1038/sj.ijo.0802311
102. Hannon JC, Brown BB. Increasing preschoolers’ physical activity intensities: an activity-friendly preschool playground intervention. Prev Med (2008) 46(6):532–6. doi: 10.1016/j.ypmed.2008.01.006
103. Boyle MH, Olsho LEW, Mendelson MR, Stidsen CM, Logan CW, Witt MB, et al. Physical activity opportunities in US early child care programs. Pediatrics (2022) 149(6):e2020048850. doi: 10.1542/peds.2020-048850
104. Trost SG, Sallis JF, Pate RR, Freedson PS, Taylor WC, Dowda M. Evaluating a model of parental influence on youth physical activity. Am J Prev Med (2003) 25(4):277–82. doi: 10.1016/s0749-3797(03)00217-4
105. Hinkley T, Salmon J, Okely AD, Hesketh K, Crawford D. Correlates of preschool children’s physical activity. Am J Prev Med (2012) 43(2):159–67. doi: 10.1016/j.amepre.2012.04.020
106. Federal Interagency Forum on Child, & Family Studies (US) (Eds.). Federal interagency forum on child, and family studies (US). In: America’s children: key national indicators of well-being, 2017. Government Printing Office, Washington D.C.
107. Pate RR, Pfeiffer KA, Trost SG, Ziegler P, Dowda M. Physical activity among children attending preschools. Pediatrics (2004) 114(5):1258–63. doi: 10.1542/peds.2003-1088-L
108. Dowda M, Pate RR, Trost SG, Almeida MJCA, Sirard JR. Influences of preschool policies and practices on children’s physical activity. J Community Health (2004) 29(3):183–96. doi: 10.1023/b:johe.0000022025.77294.af
109. Boyd BA, Reichow B, Barton EE, Odom SL. (Eds) Handbook of early childhood special education. Springer Science+Business Media, Switzerland (2016).
110. De Marco AC, Zeisel S, Odom SL. An evaluation of a program to increase physical activity for young children in child care. Early Educ Dev (2015) 26(1):1–21. doi: 10.1080/10409289.2014.932237
111. Pate RR, Brown WH, Pfeiffer KA, Howie EK, Saunders RP, Addy CL, et al. An intervention to increase physical activity in children: a randomized controlled trial with 4-Year-Olds in preschools. Am J Prev Med (2016) 51(1):12–22. doi: 10.1016/j.amepre.2015.12.003
112. Raistenskis J, Sidlauskiene A, Cerkauskiene R, Burokiene S, Strukcinskiene B, Buckus R. Physical activity and sedentary screen time in obese and overweight children living in different environments. Cent Eur J Public Health (2015) 23 Suppl:S37–43. doi: 10.21101/cejph.a4184
113. Malina RM. Weight training in youth-growth, maturation, and safety: an evidence-based review. Clin J Sport Med (2006) 16(6):478–87. doi: 10.1097/01.jsm.0000248843.31874.be
114. van Sluijs EMF, Kriemler S. Reflections on physical activity intervention research in young people - dos, don’ts, and critical thoughts. Int J Behav Nutr Phys Act (2016) 13:25. doi: 10.1186/s12966-016-0348-z
115. Wick K, Kriemler S, Granacher U. Effects of a strength-dominated exercise program on physical fitness and cognitive performance in preschool children. J Strength Cond Res (2021) 35(4):983–90. doi: 10.1519/JSC.0000000000003942
116. Rafiei H, Omidian K, Myette-Côté É, Little JP. Metabolic effect of breaking up prolonged sitting with stair climbing exercise snacks. Med Sci Sports Exercise (2021) 53(1):150–8. doi: 10.1249/MSS.0000000000002431
117. Smith JJ, Eather N, Morgan PJ, Plotnikoff RC, Faigenbaum AD, Lubans DR. The health benefits of muscular fitness for children and adolescents: a systematic review and meta-analysis. Sports Med (2014) 44(9):1209–23. doi: 10.1007/s40279-014-0196-4
118. Fuchs RK, Bauer JJ, Snow CM. Jumping improves hip and lumbar spine bone mass in prepubescent children: a randomized controlled trial. J Bone Miner Res (2001) 16(1):148–56. doi: 10.1359/jbmr.2001.16.1.148
119. Kannus P, Haapasalo H, Sankelo M, Sievanen H, Pasanen M, Heinonen A, et al. Effect of starting age of physical activity on bone mass in the dominant arm of tennis and squash players. Ann Intern Med (1995) 123(1):27–31. doi: 10.7326/0003-4819-123-1-199507010-00003
120. Weeks BK, Young CM, Beck BR. Eight months of regular in-school jumping improves indices of bone strength in adolescent boys and girls: the POWER PE study. J Bone Miner Res (2008) 23(7):1002–11. doi: 10.1359/jbmr.080226
121. McKay H. Ground reaction forces associated with an effective elementary school based jumping intervention. Br J Sports Med (2005) 39(1):10–4. doi: 10.1136/bjsm.2003.008615
122. Brown WH, Pfeiffer KA, Mclver KL, Dowda M, Almeida MJCA, Pate RR. Assessing preschool children’s physical activity: the observational system for recording physical activity in children-preschool version. Res Q Exerc Sport (2006) 77(2):167–76. doi: 10.1080/02701367.2006.10599351
123. de Meij JSB, Chinapaw MJM, van Stralen MM, van der Wal MF, van Dieren L, van Mechelen W. Effectiveness of JUMP-in, a Dutch primary school-based community intervention aimed at the promotion of physical activity. Br J Sports Med (2011) 45(13):1052–7. doi: 10.1136/bjsm.2010.075531
124. Goldfield GS, Mallory R, Prud’homme D, Adamo KB. Gender differences in response to a physical activity intervention in overweight and obese children. J Phys Act Health (2008) 5(4):592–606. doi: 10.1123/jpah.5.4.592
125. Magnusson KT, Sigurgeirsson I, Sveinsson T, Johannsson E. Assessment of a two-year school-based physical activity intervention among 7-9-year-old children. Int J Behav Nutr Phys Act (2011) 8:138. doi: 10.1186/1479-5868-8-138
126. Merino-De Haro I, Mora-Gonzalez J, Cadenas-Sanchez C, Borras PA, Benito PJ, Chiva-Bartoll O, et al. Higher socioeconomic status is related to healthier levels of fatness and fitness already at 3 to 5 years of age: the PREFIT project. J Sports Sci (2019) 37(12):1327–37. doi: 10.1080/02640414.2018.1558509
127. Arts J, Drotos E, Singh AS, Chinapaw MJM, Altenburg TM, Gubbels JS. Correlates of physical activity in 0- to 5-year-olds: a systematic umbrella review and consultation of international researchers. Sports Med (2023) 53(1):215–40. doi: 10.1007/s40279-022-01761-5
128. Rowe DC, Plomin R. Temperament in early childhood. J Pers Assess (1977) 41(2):150–6. doi: 10.1207/s15327752jpa4102_5
129. Song M, Corwyn RF, Bradley RH, Lumeng JC. Temperament and physical activity in childhood. J Phys Activity Health (2017) 14(11):837–44. doi: 10.1123/jpah.2016-0633
130. Irwin JD, Johnson AM, Vanderloo LM, Burke SM, Tucker P. Temperament and objectively measured physical activity and sedentary time among Canadian preschoolers. Prev Med Rep (2015) 2:598–601. doi: 10.1016/j.pmedr.2015.07.007
131. Bucko AG, Dowda M, Frongillo EA, Torres ME, Pate RR. Nighttime sleep and physical activity in 6-7 month-old infants. Infant Behav Dev (2021) 65:101628. doi: 10.1016/j.infbeh.2021.101628
132. Strauss RS, Rodzilsky D, Burack G, Colin M. Psychosocial correlates of physical activity in healthy children. Arch Pediatr Adolesc Med (2001) 155(8):897–902. doi: 10.1001/archpedi.155.8.897
133. Lightfoot JT, Geus EJC DE, Booth FW, Bray MS, Den Hoed M, Kaprio J, et al. Biological/Genetic regulation of physical activity level: consensus from GenBioPAC. Med Sci Sports Exerc (2018) 50(4):863–73. doi: 10.1249/MSS.0000000000001499
Keywords: obesity, exercise, pediatric, body composition, motor development, infant, toddler, preschool
Citation: Eichner-Seitz N, Pate RR and Paul IM (2023) Physical activity in infancy and early childhood: a narrative review of interventions for prevention of obesity and associated health outcomes. Front. Endocrinol. 14:1155925. doi: 10.3389/fendo.2023.1155925
Received: 31 January 2023; Accepted: 10 May 2023;
Published: 24 May 2023.
Edited by:
Miaobing Jazzmin Zheng, Deakin University, AustraliaCopyright © 2023 Eichner-Seitz, Pate and Paul. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ian M. Paul, aXBhdWxAcHN1LmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.