
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Endocrinol. , 02 June 2023
Sec. Reproduction
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1155007
 Fahimeh Ramezani Tehrani1
Fahimeh Ramezani Tehrani1 Farshad Farzadfar2
Farshad Farzadfar2 Farhad Hosseinpanah3
Farhad Hosseinpanah3 Maryam Rahmati1
Maryam Rahmati1 Faegheh Firouzi1
Faegheh Firouzi1 Mehrandokht Abedini4
Mehrandokht Abedini4 Farzad Hadaegh5
Farzad Hadaegh5 Majid Valizadeh3
Majid Valizadeh3 Farahnaz Torkestani6
Farahnaz Torkestani6 Davood Khalili5
Davood Khalili5 Masoud Solaymani-Dodaran7
Masoud Solaymani-Dodaran7 Razieh Bidhendi-Yarandi8
Razieh Bidhendi-Yarandi8 Marzieh Bakhshandeh9
Marzieh Bakhshandeh9 Afshin Ostovar10
Afshin Ostovar10 Marzieh Rostami Dovom1
Marzieh Rostami Dovom1 Mina Amiri1
Mina Amiri1 Fereidoun Azizi11
Fereidoun Azizi11 Samira Behboudi-Gandevani12*
Samira Behboudi-Gandevani12*Objectives: The aim of the study was to investigate the effect of treatment on pregnancy outcomes among women who had fasting plasma glucose (FPG) 5.1-5.6 mmol/l in the first trimester of pregnancy.
Methods: We performed a secondary-analysis of a randomized community non-inferiority trial of gestational diabetes mellitus (GDM) screening. All pregnant women with FPG values range 5.1-5.6 mmol/l in the first trimester of gestation were included in the present study (n=3297) and classified to either the (i) intervention group who received treatment for GDM along with usual prenatal care (n=1,198), (ii) control group who received usual-prenatal-care (n=2,099). Macrosomia/large for gestational age (LGA) and primary cesarean-section (C-S) were considered as primary-outcomes. A modified-Poisson-regression for binary outcome data with a log link function and robust error variance was used to RR (95%CI) for the associations between GDM status and incidence of pregnancy outcomes.
Results: The mean maternal age and BMI of pregnant women in both study groups were similar. There were no statistically significant differences in the adjusted risks of adverse pregnancy outcomes, including macrosomia, primary C-S, preterm birth, hyperbilirubinemia, preeclampsia, NICU-admission, birth trauma, and LBW both groups.
Conclusions: It is found that treating women with first-trimester FPG values of 5.1-5.6 mmol/l could not improve adverse pregnancy outcomes including macrosomia, Primary C-S, Preterm birth, hypoglycemia, hypocalcemia, preeclampsia, NICU admission, Birth trauma and LBW. Therefore, extrapolating the FPG cut-off point of the second trimester to the first –which has been proposed by the IADPSG, might therefore not be appropriate.
Clinical Trial Registration: https://www.irct.ir/trial/518, identifier IRCT138707081281N1.
Gestational diabetes (GDM) is one of the most common endocrinopathies during gestation (1, 2). Traditionally, GDM screening and diagnosis have been based on oral glucose tolerance test (OGTT) in the second trimester of pregnancy (3, 4). During this time, placenta produces diabetogenic hormones such as human placental lactogen which can lead to progressive insulin resistance and elevated blood glucose levels in individuals with insufficient insulin production to maintain euglycemia (5). Likewise, the results of the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study have confirmed a continuous association between adverse pregnancy outcomes and maternal glucose levels that are that are less severe than those in overt diabetes mellitus (DM) (6).
It is widely recognized that untreated overt DM during pregnancy is strongly associated with adverse feto-maternal and neonatal outcomes (7). Due to the increasing rate of undiagnosed DM among pregnant women in early gestation, the International Association of the Diabetes and Pregnancy Study Groups (IADPSG) and the World Health Organization (WHO) have recommended first-trimester screening to identify overt diabetes in pregnancy (ODIP) (1). Early screening also identifies women with hyperglycemia that is less than ODIP (GDM). However, with limited trial data and by extrapolating results from the HAPO study conducted during the second trimester to early gestation, IADPSG has endorsed the diagnostic criteria of GDM for fasting plasma glucose (FPG) in the range of 5.1-6.9 mmol/l (1). Nevertheless, emerging data has challenged this recommendation since many such women no longer meet the criteria for GDM when later re-screened during the second trimester of pregnancy (8–13).
Meanwhile, most cases of additional early-onset GDM were identified based on a FPG of 5.1-5.6 mmol/L during the first trimester of gestation (14, 15). These stringent criteria may potentially result in a higher prevalence of GDM, without any clear evidence to reduce the risk for adverse pregnancy outcomes. Besides, it may potentially increase the costs of care and over-medicalization of pregnancy for a large number of previously healthy pregnant women who will now be labeled as patients, especially in low- and middle-income countries with limited resources (16). Furthermore, the diagnosis and treatment of GDM may be a stressful situation accompanied by serious psychological consequences, as well as impair the quality of life for women and their families (17, 18). Therefore this study aimed to investigate the effects of treatment in women who had a fasting plasma glucose (FPG) 5.1-5.6 mmol/l in the first trimester of gestation on maternal and neonatal outcomes.
This study presents a secondary analysis of a randomized community non-inferiority trial among pregnant women. Detailed description of the methods used has been reported previously (14, 19, 20). In summary, a total of 35,430 pregnant women in their first trimester were recruited from five different geographic regions of Iran across 25 selected cities. Those who were younger than 18 years, suffered from overt DM or other chronic disorders, were uncertain about their gestational age (no ultrasound estimation from 6 to 14 weeks of gestational age available and last menstrual period not certain) were excluded from the study. All pregnant women received standard prenatal care and were screened for GDM twice. The first screening was conducted in the first trimester using an FPG measurement, and the second screening was performed in the second trimester using either a one-step or a two-step screening method. Based on the GDM screening approach, participants were randomized to 5 protocols: In Protocol A, GDM was defined as a FPG between 93 mg/dL and 126 mg/dL in the first trimester, and any abnormal value using the one-step screening method in the second trimester with a 2-hour 75-gram oral glucose tolerance test (OGTT) and cutoff values of fasting 93 mg/dL, 1-hour 180 mg/dL, or 2-h 153 mg/dL. Protocol B differed from Protocol A in the definition of GDM in the first trimester, which was FPG between 100 mg/dL and 126 mg/dL, and in the second trimester, which was defined as two or more plasma glucose levels meeting or exceeding the criteria. Protocol C used the same definition for GDM in the first trimester as Protocol B (FPG between 100 mg/dL and 126mg/dL), and the same definition in the second trimester as Protocol A (any abnormal value using the one step screening method with a 2-hour, 75-gram GTT). Protocol D defined GDM in the first trimester as FPG values between 93 mg/dL and 126 mg/dL. However, in the second trimester, a two-step screening method was used, using the cut-off values of Carpenter-Coustan criteria. Protocol E differed from Protocol D in the definition of GDM in the first trimester, which was FPG between 100 mg/dL and 126 mg.
For current analysis, we restructured the original data. In this respect, those pregnant women with FPG values range 5.1-5.6 mmol/l in the first trimester of gestation were selected form original data (n=3297) and included in this secondary analysis. Then, they were classified to either the (i) intervention group who received treatment for GDM along with usual prenatal care (n=1,198), (ii) control group who received usual-prenatal-care (n=2,099). All women in the control group were re-screened for GDM between 24–28 weeks of gestation using either a one-step or a two-step screening approach. It should be noted that receiving GDM treatment in the intervention group and or usual prenatal care in controls were defined by original study randomization. On the other hand, the current study’s restructuring of the data and classification of participants into the intervention and control groups did not affect the original randomization process of the original trial. Therefore, the control group in the current study was not influenced by any new arbitrary decisions or approaches made during the secondary analysis.
All of study participants were followed-up until delivery, and their outcomes were registered in detail. The guidelines recommended by the American College of Obstetricians and Gynecologists (ACOG) 2013 (21) and the American Diabetes Association (ADA) 2016 (22) were used as the basis for the treatment of GDM, which included physical exercise, dietary intervention, and pharmacological therapy if necessary. The flowchart of the current study is presented in Figure 1.
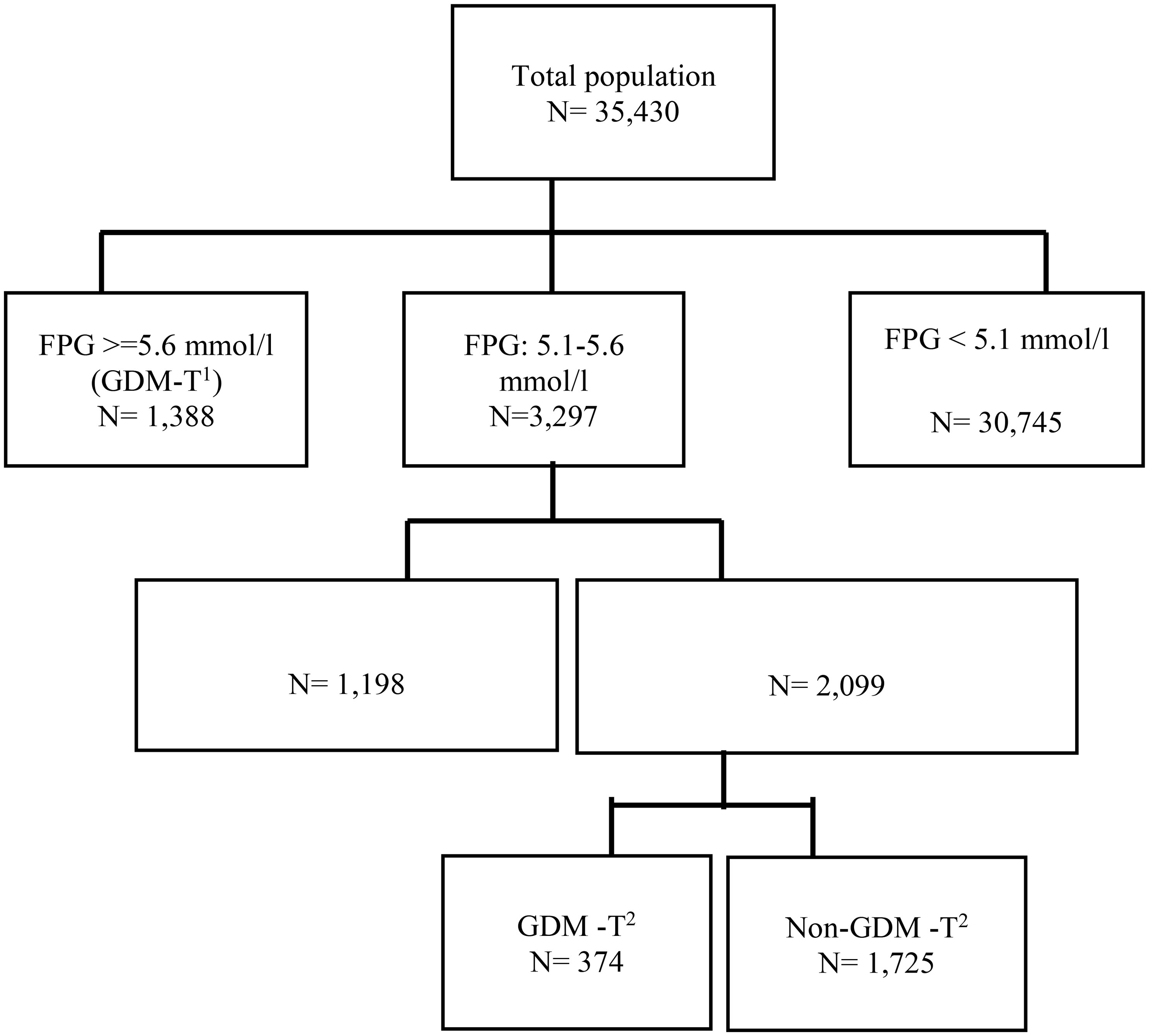
Figure 1 Flowchart of the study. T1: first trimester, T2: second trimester, FPG: fasting plasma glucose. Intervention group: those who had FPG values range 5.1-5.6 mmol/l in the first trimester of gestation and received treatment for GDM along with usual prenatal care, Control group: those who had FPG values range 5.1-5.6 mmol/l in the first trimester of gestation and received usual-prenatal-care.
Macrosomia/large for gestational age (LGA) and primary cesarean section (C-S) were considered primary outcomes. Secondary outcomes were preterm birth before 37 weeks of gestation, admission to the neonatal intensive care unit (NICU), neonatal hypoglycemia, neonatal hypocalcemia, neonatal hyperbilirubinemia, preeclampsia, birth trauma, and low birth weight (LBW).
Macrosomia was defined as birth weight> 4000 g (23) and primary cesarean section was defined as the cesarean deliveries out of all births to women who had not had a previous cesarean. Definition of other outcomes had been published elsewhere (14, 19, 24–26).
The present study had a power of 95% to detect 40% increased risk in macrosomia outcome based on their mild GDM treatment status in the first of pregnancy, with a two-sided 5% significance level, and a sample size of 3,297 participants. Continuous variables were checked for normality using the Shapiro–Wilk test. Characteristics of participants were compared using the independent t-test or Pearson’s Chi-squared test for continuous and categorical data, and also Mann–Whitney U test for variables with skewed distribution. A modified Poisson regression for binary outcome data with a log link function and robust error variance was used to estimate relative risks (RRs) and 95% confidence intervals (CIs) for the associations between mild GDM treatment status at the first trimester (treated mild GDM and non- treated mild GDM) and incidence of pregnancy outcomes; For comparison of first trimester treated mild GDM with those subgroup of first trimester non- treated mild GDM who developed GDM in the second trimester, adjusted risk ratios and 95% CI were reported. Adjusted variables were gestational ages at entrance and delivery, maternal BMI, gestational weight gain, method of GDM screening in the second trimester, and type of delivery. Treatment modality was also adjusted to compare those subgroups of GDM diagnosed in the second and first trimester of pregnancy treated mild GDM. Moreover, we adjust type of test in the model for comparing subgroup of first trimester non- treated mild GDM who developed GDM in the 2nd trimester with those first trimester treated mild GDM. Both unadjusted and adjusted models were fitted. In all analyses related to primary cesarean section outcomes, those with a previous history of cesarean section were excluded. Penalized maximum likelihood estimation was applied in the case of sparse data. Since the study was a cluster randomized trial, the cluster effect was considered in the analysis and the significance level of the test was set as 0.025 for considering subgroup analyses (27). Finally, the plot of the relative risk was depicted for all pregnancy outcomes by GDM treatment status. Statistical analysis was performed using STATA (version 13; STATA Inc., College station, TX, USA).
Table 1 shows the characteristics of study participants according to study groups. The mean (SD) pregnancy week for the first prenatal visit in intervention and control groups were 8.2 (3.3) and 9.1 (3.3) weeks, respectively. The mean maternal age and BMI of pregnant women in intervention and control groups were similar (31.1 (6.1) vs. 30.7 (5.8) years, P-value = 0.082) and (26.9 (4.8) vs. 26.7 (4.8) kg/m2, P-value = 0.264), respectively. Compared to the control group, women with treated GDM in intervention group had a significantly lower gestational age at enrollment (8.2 (3.3) vs. 9.1 (3.3) weeks, P-value = 0.001), a slightly lower gestational age at delivery (38.6 (1.7) vs. 38.8 (1.7) weeks, P-value = 0.025), a significantly lower number of miscarriages (0.3 (0.6) vs. 0.5 (0.7), P-value = 0.001), and a slightly higher systolic blood pressure (102.9 (9.7) vs. 101.8 (9.5) mmHg, P-value = 0.004), although those differences may not carry clinical significance.
The prevalence and Risk ratio (95% CI) of maternal and neonatal outcomes in pregnant women based on their GDM treatment status in the first of pregnancy are presented in Table 2. There was no statistically significant difference between groups in the frequency of the adverse pregnancy outcomes of macrosomia, primary C-S, preterm birth, neonatal hyperbilirubinemia, neonatal hypoglycemia, neonatal hypocalcemia, preeclampsia, NICU admission, birth trauma, and LBW considering multiplicity adjustment. There were no statistically significant differences in the adjusted risks of adverse pregnancy outcomes in intervention group compared to controls considering multiplicity adjustment (Figure 2 and Table 2).
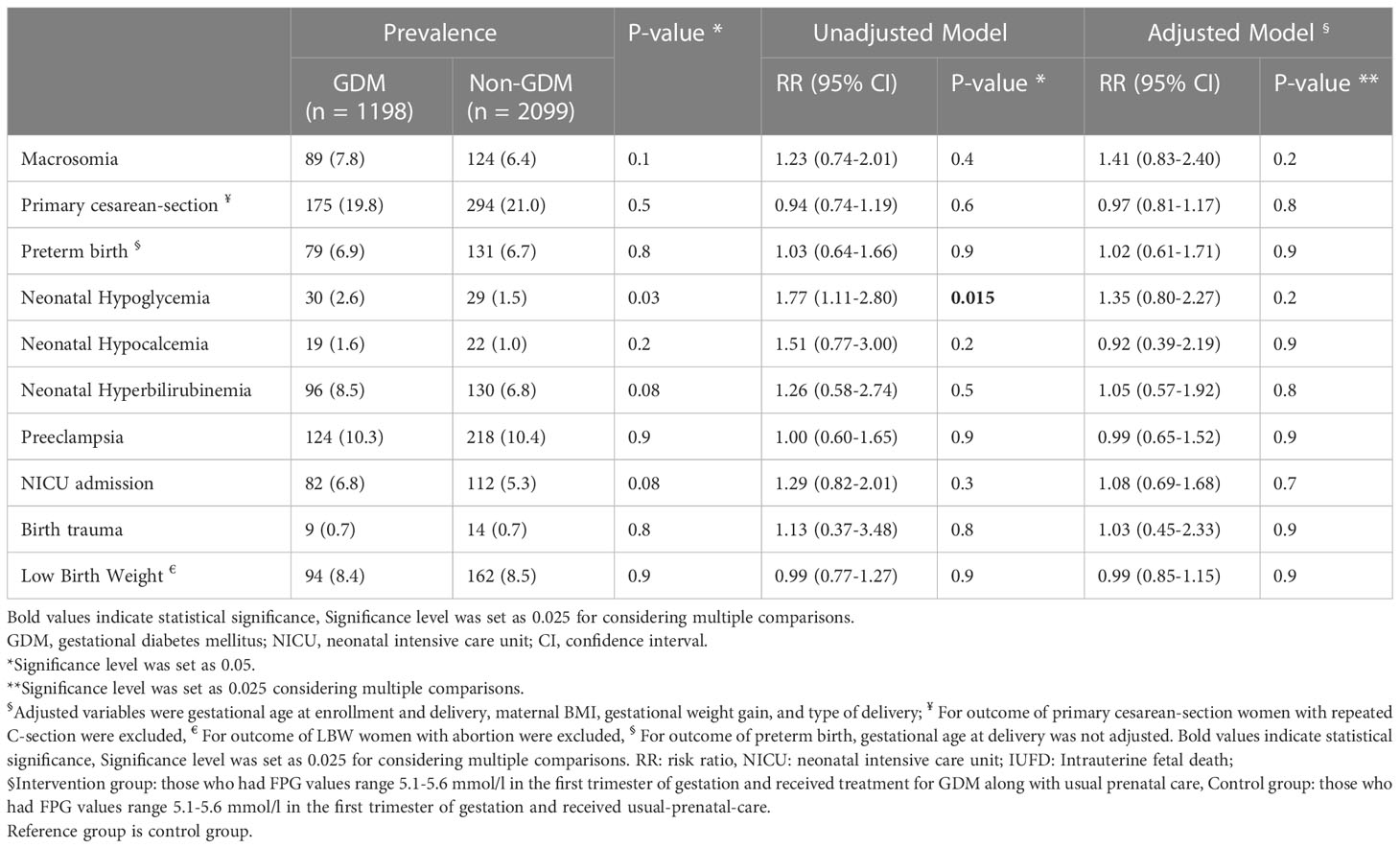
Table 2 Prevalence and Risk ratio and 95% confidence interval of adverse pregnancy outcomes in participants based on study groups§.
The prevalence and Risk ratio (95% CI) of maternal and neonatal outcomes in pregnant women with first trimester treated GDM in intervention group (n=1198) compared to those subgroup of control group who developed GDM in the second trimester (n=374) are presented in Supplementary Table 1 (Table S1). The prevalence of maternal and neonatal outcomes, except for neonatal hypoglycemia and hypocalcemia, were similar in intervention group compared to those subgroup of controls who developed GDM in the second trimester; the frequency of neonatal hypoglycemia and hypocalcemia in the latter group were significantly higher than first trimester intervention group (6.8% vs. 2.6%, P-value < 0.001 and 4.5% vs. 1.6%, P-value = 0.001, respectively). There were no statistically significant differences in the adjusted risks of adverse pregnancy outcomes in those groups, considering multiplicity adjustment, except for hypocalcemia (RR = 2.05; 95% CI: (1.12, 3.75); P = 0.02) (Table S1 and Figure S1).
In this secondary analysis of a randomized community non-inferiority trial involving pregnant women with FPG level between 5.1-5.6 mmol/l in the first trimester of pregnancy, we found that GDM treatment at the first trimester of pregnancy was not associated with a reduced risk of adverse maternal and neonatal outcomes, including macrosomia, LBW, primary C-S, preterm birth, neonatal hypoglycemia, neonatal hypocalcemia, hyperbilirubinemia, preeclampsia, NICU admission, and birth trauma. Moreover, the adjusted risks of adverse pregnancy outcomes in pregnant women with FPG between 5.1-5.6 mmol/l who did not receive GDM care but developed GDM in the second trimester were not significantly different from those with FPG between 5.1-5.6 mmol/l at first trimester who received GDM cares.
There is ongoing debate regarding the benefits and risks of screening for and treating hyperglycemia in the first trimester of pregnancy (3, 28) Most current guidelines for GDM screening are based on studies initiating diagnosis and subsequent treatment in the third trimester (6, 29, 30). The evidence available is insufficient to determine the balance of benefits and harms of early screening for and treating GDM (4). Accordingly, studies that focus on the potential benefits of early GDM screening have yielded mixed and conflicting results (31–34). Whereas some studies have reported an association between GDM and maternal and neonatal morbidity (35–37), it has also been demonstrated that early treatment for GDM does not substantially improve these potential adverse pregnancy outcomes (13, 38, 39). Additionally, recent data have failed to demonstrate a clear association between FPG levels in early pregnancy and later GDM at 24–28 weeks of gestation (8, 9). It is important to note that most of the available data were derived from retrospective studies, and there is a lack of randomized controlled trials, which could potentially provide more robust evidence.
Our community randomized trial results showed that early diagnosis and treatment of GDM did not improve pregnancy outcomes. However, there are few randomized trial to compare early versus routine screening for GDM. In agreement with our findings, Osmundson et al. (2016), conducted an RCT, to assess the effect of early GDM treatment prior to 14 weeks gestation in women with a hemoglobin A1c (Hb A1c) levels between 5.7–6.4%. The study randomly assigned 42 pregnant women to receive GDM treatment and 41 to receive usual prenatal care. The results showed that early treatment for women with prediabetes-range A1C levels in the first-trimester did not significantly reduce the risk of GDM diagnosis by the second trimester (40).
In another RCT, Roeder et al. (2019) assessed whether treating women with mild glucose intolerance earlier in pregnancy would be beneficial in the reduction of adverse perinatal outcomes. Pregnant women with hyperglycemia, including Hb A1c ≥5.7% and/or FPG ≥5.1 mmol/l in early pregnancy were randomized to early pregnancy or third-trimester treatment of GDM. Results of the study showed that treatment in early pregnancy did not significantly improve maternal or neonatal outcomes, including macrosomia, weight for length percentile at birth, maternal gestational weight gain, fat mass, and cord blood C-peptide > 90th percentile (41). In a recently published RCT, Harper et al. (2020) conducted an RCT to assess whether early screening for GDM improves perinatal outcomes in a high-risk group of obese pregnant women with BMI ≥ 30 kg/m2. The study involved 922 pregnant women randomized to early (n= 459) and routine (n= 463) GDM screening. The researchers reported that early screening for GDM in obese women did not reduce the composite perinatal outcome that included any of the following outcomes of macrosomia, primary cesarean delivery, pregnancy-induced hypertension, shoulder dystocia, neonatal hypoglycemia and neonatal hyperbilirubinemia (38). In a secondary analysis of data from the lifestyle in pregnancy study, Vinter et al. (2018), assessed a lifestyle intervention among obese women fulfilling the GDM WHO-2013 diagnostic criteria in early gestation. In this trial, 36 participants received lifestyle intervention and 54 received standard care. The researchers found that all metabolic parameters and obstetric outcomes were similar in both groups. However, there were more planned C-S in the lifestyle intervention group (42). Finally, preliminary results of a randomized controlled trial in Australia (TOBOGM study) among singleton high-risk pregnant women showed that treatment for GDM in early gestation might have both benefits and harms (13). The study found that early GDM treatment is associated with a reduced LGA rate but an increased NICU admission rate mainly due to an increase in small for gestational age (SGA) that may be resulted from fetal undernutrition as a consequence of overtreatment, or insufficient gestational weight gain, with putative long term consequences.
Our study revealed that an FPG of 5.1 mmol/l may not be considered an optimal glycemic target early in pregnancy; it was not associated with an improvement in pregnancy outcomes, and 82% (1725/2099) those pregnant women who had FPG values 5.1-5.6 mmol/l and did not receive treatment for GDM in the first trimester, did not meet the GDM criteria at second trimester. These results are consistent with those of a study conducted among Chinese subjects, which reported a 37% incidence of GDM for women with FPG values between 5.1 and 5.6 mmol/l at the first prenatal visit (8). Adopting this threshold for GDM diagnosis may overload the healthcare system and create stress and psychological burden for pregnant women impacting their quality of life and overall well-being during pregnancy (17). Labeling pregnant women with a cut-off value of 5.1 mmol/l for FPG early in pregnancy as GDM did not result in any improvements in maternal or neonatal outcomes; Instead, it has led to the over-medicalization of pregnancy, and healthcare costs in many hitherto healthy pregnant women that will be labeled as GDM. While the first-trimester FPG value is important for the diagnosis of pregestational overt diabetes, a value of 5.1 mmol/l is a poor predictor for GDM early in pregnancy and may lead to false-positive results. Therefore, treatment of this group of pregnant women early in pregnancy does not translate into improved outcomes.
Our study has several strengths, including community-based trial design of study, large sample size, geographic distribution of the regions involved, broad inclusion criteria and using similar laboratory protocols. Unlike many previous studies, which focused on high-risk populations, our study evaluated a general population of pregnant women. We also adjusted for potential risk factors, which adds to the strength of our findings. Additionally, the early enrollment of participants, on average at eight weeks of gestation, is a further strength of our study. However, there are some limitations to our trial that should be acknowledged. According to the Iranian national guidelines for prenatal care, pregnant women with known chronic disorders should be directly referred to the second level of the healthcare system and receive their prenatal care there, rather than in a primary healthcare setting, which was the platform of our study. Evaluating the adverse feto-maternal and neonatal outcomes in these high-risk groups was beyond our research aim of study. As a result, our findings are not generalizable to those with various chronic disorders. Moreover, we did not measure HbA1c in our study. Additionally, we did not used a central reference laboratory for measurements. However, all of procedures, equipment, and supplies were homogeneous in all laboratory sites, and monthly external quality-controls were done for each laboratory. Our study was conducted among Iranian women; the results may not be applicable to other populations.
In conclusion, our study suggests that using the first-trimester FPG values of 5.1-5.6 mmol/l as the criteria to diagnose GDM is not recommended. Treatment of these women did not lead to improved adverse pregnancy outcomes, and they may not require the special prenatal care recommended for those with a GDM diagnosis. Therefore, we recommend that this group of pregnant women be re-screened for GDM at 24-28 weeks of gestation.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
National Institute for Medical Research Development under Grant Agreement No IR.NIMAD.REC.1394.013 has been approved and funded the study (but did not involve in the study). In addition, the national ethics committee of the National Institute for Medical Research Development approved the study protocol (Approval number: IR.NIMAD.REC.1394.013) and the Iranian Ministry of Health and Medical Education (MoHME) approved the study protocol. The pre-specified GDM modalities were made available to all those provinces as mandatory guidelines. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
FRT, SB-G, and FA conceived and designed the trial and FRT is the chief investigator. FFa, FFi, FHo, MAb, FHa, MV, FT, DK, MS-D, MB, AO, MAm, and FA contributed to the protocol and design of the study. RB-Y, MR, DK, and MS-D did the statistical analysis. FRT, SB-G, FHo, FFi, FFa and MR contributed to the preparation of the report. All authors contributed to the implementation of the study and data collection. All authors contributed to the article and approved the submitted version.
Research reported in this publication was supported by Elite Researcher Grant Committee under award number [IR.NIMAD.REC.1394.013] from the National Institute for Medical Research Development (NIMAD), Tehran, Iran.
We thank from Golestan, Bushehr, Birjand, Kurdistan and Yazd Universities of medical Sciences for their cooperation for this study and also study cooperative executive committee, including: Abbas Najari, MD (Centre for Collective Refection & Implementation of Ideas, Undersecretary for Research and Technology, Ministry of Health and Medical Education, Tehran, Iran); Abdolmohhamad Khajeian, MD (Deputy of Health, Bushehr University of Medical Sciences, Bushehr, Iran); Azita Anaraki, MD (Population, family and school health Department, Bushehr University of Medical Sciences, Bushehr, Iran); Fariba Ghazaghi, MSc (Population, family and school health Department, Birjand University of Medical Sciences, Birjand, Iran); Forouzan Lahouni, MS (Population, family and school health Group, Kurdistan University of Medical Sciences, Sanandaj, Iran); Forouzandeh Kalantari, MD (Population, family and school health Department, Yazd University of Medical Sciences, Yazd, Iran); Hossein Fallah, MSc (Nutrition Department, Ministry of Health and Medical Education, Tehran, Iran); Khadije Kordi, MD (Population, family and school health Department, Golestan University of Medical Sciences, Gorgan, Iran); Lotfollah Saed, MD (Department of Internal Medicine, Faculty of Medicine, Kurdistan University of Medical Sciences, Sanandaj, Iran); Shabahang Amirshekari, MSc (Reproductive Endocrinology Research Center, Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran); Mahsa Norooozzadeh, MSc (Reproductive Endocrinology Research Center, Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran); Maryam Farahmand, PhD (Reproductive Endocrinology Research Center, Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran); Marzieh Rostami Dovom, PhD (Reproductive Endocrinology Research Center, Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran); Mehdi Hedayati, PhD (Cellular and Molecular Research Center, Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran); Mehdi Mehdizade, MD (Deputy of Health, Birjand university of Medical science, Birjand, Iran); Mohammad Hassan Lotf, MD (Deputy of health, Kurdistan University of Medical Sciences, Sanandaj, Iran); Mohammad-Esmaeil Motlagh, MD (Department of Pediatrics, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran); Mozhgan Bashghareh, MSc (Population, family and school health Department, Golestan University of Medical Sciences, Gorgan, Iran); Nosrat Zamanipour, MSc (Population, family and school health Department, Birjand University of Medical Sciences, Birjand, Iran); Parvin Mirmiran, PhD (Nutrition and Endocrine Research Center, Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran); Saeid Sadeghian Sharif, PhD (Faculty of nutrition Science and Food Technology, Shahid Beheshti University of Medical Sciences, Tehran Iran); Saeid Shahraz, PhD (Heller School of Social Policy and Management, Brandeis University, Waltham, Massachusetts, USA); Samareh Khari, MD (Population, family and school health Department, Golestan University of Medical Sciences, Gorgan, Iran); Sedigheh Alishahi, MSc (Population, family and school health Department, Yazd University of Medical Sciences, Yazd, Iran); Shole Shahgheibi, MD (Department of Obstetrics and Gynecology, Faculty of Medicine, Kurdistan University of Medical Sciences, Sanandaj, Iran); Sima Nazarpour, PhD (Reproductive Endocrinology Research Center, Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran); Yadollah Mehrabi, PhD (Department of Epidemiology, School of Health, Shahid Beheshti University of Medical Sciences, Tehran, Iran); Zahra Ghaedmohammadi, MSc (Population, family and school health Department, Bushehr University of Medical Sciences, Bushehr, Iran).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1155007/full#supplementary-material
1. International Association of Diabetes and Pregnancy Study Groups Consensus Panel, Metzger BE, Gabbe SG, Persson B, Buchanan TA, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care (2010) 33:676–82. doi: 10.2337/dc09-1848
2. Behboudi-Gandevani S, Amiri M, Bidhendi Yarandi R, Ramezani Tehrani F. The impact of diagnostic criteria for gestational diabetes on its prevalence: a systematic review and meta-analysis. Diabetol Metab Syndr (2019) 11:11. doi: 10.1186/s13098-019-0406-1
3. Simmons D. Paradigm shifts in the management of diabetes in pregnancy: the importance of type 2 diabetes and early hyperglycemia in pregnancy: the 2020 Norbert freinkel award lecture. Diabetes Care (2021) 44:1075–81. doi: 10.2337/dci20-0055
4. Su FL, Lu MC, Yu SC, Yang CP, Yang CC, Tseng ST, et al. Increasing trend in the prevalence of gestational diabetes mellitus in Taiwan. J Diabetes Investig (2021) 12:2080–8. doi: 10.1111/jdi.13595
5. Genevay M, Pontes H, Meda P. Beta cell adaptation in pregnancy: a major difference between humans and rodents? Diabetologia (2010) 53:2089–92. doi: 10.1007/s00125-010-1848-z
6. Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med (2008) 358:1991–2002. doi: 10.1056/NEJMoa0707943
7. Wong T, Ross GP, Jalaludin BB, Flack JR. The clinical significance of overt diabetes in pregnancy. Diabetes Med (2013) 30:468–74. doi: 10.1111/dme.12110
8. Zhu WW, Yang HX, Wei YM, Yan J, Wang ZL, Li XL, et al. Evaluation of the value of fasting plasma glucose in the first prenatal visit to diagnose gestational diabetes mellitus in China. Diabetes Care (2013) 36:586–90. doi: 10.2337/dc12-1157
9. Corrado F, D’Anna R, Cannata ML, Interdonato ML, Pintaudi B, Di Benedetto A. Correspondence between first-trimester fasting glycaemia, and oral glucose tolerance test in gestational diabetes diagnosis. Diabetes Metab (2012) 38:458–61. doi: 10.1016/j.diabet.2012.03.006
10. López Del Val T, Alcázar Lázaro V, García Lacalle C, Torres Moreno B, Castillo Carbajal G, Alameda Fernandez B. Fasting glucose in the first trimester: an initial approach to diagnosis of gestational diabetes. Endocrinol Diabetes Nutr (2019) 66:11–8. doi: 10.1016/j.endinu.2018.06.012
11. Benhalima K, Van Crombrugge P, Moyson C, Verhaeghe J, Vandeginste S, Verlaenen H, et al. Women with mild fasting hyperglycemia in early pregnancy have more neonatal intensive care admissions. J Clin Endocrinol Metab (2021) 106:e836–e54. doi: 10.1210/clinem/dgaa831
12. Raets L, Beunen K, Benhalima K. Screening for gestational diabetes mellitus in early pregnancy: what is the evidence? J Clin Med (2021) 10:1257. doi: 10.3390/jcm10061257
13. Simmons D, Nema J, Parton C, Vizza L, Robertson A, Rajagopal R, et al. The treatment of booking gestational diabetes mellitus (TOBOGM) pilot randomised controlled trial. BMC Pregnancy Childbirth (2018) 18:151. doi: 10.1186/s12884-018-1809-y
14. Ramezani Tehrani F, Behboudi-Gandevani S, Farzadfar F, Hosseinpanah F, Hadaegh F, Khalili D, et al. A cluster randomized non−inferiority field trial of gestational diabetes mellitus screening. J Clin Endocrinol Metab (2022) 107:e2906–20. doi: 10.1210/clinem/dgac181
15. Cosson E, Carbillon L, Valensi P. High fasting plasma glucose during early pregnancy: a review about early gestational diabetes mellitus. J Diabetes Res (2017) 2017:8921712. doi: 10.1155/2017/8921712
16. Fitria N, van Asselt ADI, Postma MJ. Cost-effectiveness of controlling gestational diabetes mellitus: a systematic review. Eur J Health Econ (2019) 20:407–17. doi: 10.1007/s10198-018-1006-y
17. Marchetti D, Carrozzino D, Fraticelli F, Fulcheri M, Vitacolonna E. Quality of life in women with gestational diabetes mellitus: a systematic review. J Diabetes Res (2017) 2017:7058082. doi: 10.1155/2017/7058082
18. Pantzartzis KA, Manolopoulos PP, Paschou SA, Kazakos K, Kotsa K, Goulis DG. Gestational diabetes mellitus and quality of life during the third trimester of pregnancy. Qual Life Res (2019) 28:1349–54. doi: 10.1007/s11136-018-2090-2
19. Ramezani Tehrani F, Gulf Study Cooperative Research Group. Cost effectiveness of different screening strategies for gestational diabetes mellitus screening: study protocol of a randomized community non-inferiority trial. Diabetol Metab Syndr (2019) 11:106. doi: 10.1186/s13098-019-0493-z
20. Ramezani Tehrani F, Rahmati M, Farzadfar F, Abedini M, Farahmand M, Hosseinpanah F, et al. One-step versus two-step screening for diagnosis of gestational diabetes mellitus in Iranian population: a randomized community trial. Front Endocrinol (2023) 13:1039643. doi: 10.3389/fendo.2022.1039643
21. Practice bulletin no. 137. gestational diabetes mellitus. Obstet Gynecol (2013) 122:406–16. doi: 10.1097/01.AOG.0000433006.09219.f1
22. Standards of medical care in diabetes-2016. summary of revisions. Diabetes Care (2016) 39:S4–5. doi: 10.2337/dc16-S003
23. Practice bulletin no. 173. fetal macrosomia. Obstet Gynecol (2016) 128:e195–209. doi: 10.1097/AOG.0000000000001767
24. Sweet CB, Grayson S, Polak M. Management strategies for neonatal hypoglycemia. J Pediatr Pharmacol Ther (2013) 18:199–208. doi: 10.5863/1551-6776-18.3.199
25. Mustafa R, Ahmed S, Gupta A, Venuto RC. A comprehensive review of hypertension in pregnancy. J Pregnancy (2012) 2012:105918. doi: 10.1155/2012/105918
26. Practice bulletin no. 130. prediction and prevention of preterm birth. Obstet Gynecol (2012) 120:964–73. doi: 10.1097/AOG.0b013e3182723b1b
27. Li G, Taljaard M, Van den Heuvel ER, Levine MA, Cook DJ, Wells GA, et al. An introduction to multiplicity issues in clinical trials: the what, why, when and how. Int J Epidemiol (2017) 46:746–55. doi: 10.1093/ije/dyw320
28. McIntyre HD, Sacks DA, Barbour LA, Feig DS, Catalano PM, Damm P, et al. Issues with the diagnosis and classification of hyperglycemia in early pregnancy. Diabetes Care (2016) 39(1):53–4. doi: 10.2337/dc15-1887
29. Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med (2009) 361:1339–48. doi: 10.1056/NEJMoa0902430
30. Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med (2005) 352:2477–86. doi: 10.1056/NEJMoa042973
31. Immanuel J, Simmons D. Screening and treatment for early-onset gestational diabetes mellitus: a systematic review and meta-analysis. Curr Diabetes Rep (2017) 17:115. doi: 10.1007/s11892-017-0943-7
32. Harreiter J, Simmons D, Desoye G, Corcoy R, Adelantado JM, Devlieger R, et al. IADPSG and WHO 2013 gestational diabetes mellitus criteria identify obese women with marked insulin resistance in early pregnancy. Diabetes Care (2016) 39:e90–2. doi: 10.2337/dc16-0200
33. Behboudi-Gandevani S, Bidhendi-Yarandi R, Panahi MH, Vaismoradi M. The effect of mild gestational diabetes mellitus treatment on adverse pregnancy outcomes: a systemic review and meta-analysis. Front Endocrinol (2021) 12:640004. doi: 10.3389/fendo.2021.640004
34. Bidhendi Yarandi R, Vaismoradi M, Panahi MH, Gåre Kymre I, Behboudi-Gandevani S. Mild gestational diabetes and adverse pregnancy outcome: a systemic review and meta-analysis. Front Med (2021) 8:699412. doi: 10.3389/fmed.2021.699412
35. Riskin-Mashiah S, Younes G, Damti A, Auslender R. First-trimester fasting hyperglycemia and adverse pregnancy outcomes. Diabetes Care (2009) 32:1639–43. doi: 10.2337/dc09-0688
36. Feghali MN, Abebe KZ, Comer DM, Caritis S, Catov JM, Scifres CM. Pregnancy outcomes in women with an early diagnosis of gestational diabetes mellitus. Diabetes Res Clin Pract (2018) 138:177–86. doi: 10.1016/j.diabres.2018.02.004
37. Kyozuka H, Yasuda S, Murata T, Fukuda T, Yamaguchi A, Kanno A, et al. Adverse obstetric outcomes in early-diagnosed gestational diabetes mellitus: the Japan environment and children’s study. J Diabetes Investig (2021) 12:2071–9. doi: 10.1111/jdi.13569
38. Harper LM, Jauk V, Longo S, Biggio JR, Szychowski JM, Tita AT. Early gestational diabetes screening in obese women: a randomized controlled trial. Am J Obstet Gynecol (2020) 222:495. doi: 10.1016/j.ajog.2019.12.021
39. Hong WY, Biggio JR, Tita A, Harper LM. Impact of early screening for gestational diabetes on perinatal outcomes in high-risk women. Am J Perinatol (2016) 33:758–64. doi: 10.1055/s-0036-1571317
40. Osmundson SS, Norton ME, El-Sayed YY, Carter S, Faig JC, Kitzmiller JL. Early screening and treatment of women with prediabetes: a randomized controlled trial. Am J Perinatol (2016) 33:172–9. doi: 10.1055/s-0035-1563715
41. Roeder HA, Moore TR, Wolfson MT, Gamst AC, Ramos GA. Treating hyperglycemia in early pregnancy: a randomized controlled trial. Am J Obstet Gynecol (2019) 1:33–41. doi: 10.1016/j.ajogmf.2019.03.003
42. Vinter CA, Tanvig MH, Christensen MH, Ovesen PG, Jørgensen JS, Andersen MS, et al. Lifestyle intervention in Danish obese pregnant women with early gestational diabetes mellitus according to WHO 2013 criteria does not change pregnancy outcomes: results from the LiP (Lifestyle in pregnancy) study. Diabetes Care (2018) 41:2079–85. doi: 10.2337/dc18-0808
Keywords: early screening, randomized non-inferiority field trial, gestational diabetes, first trimester of gestation, glucose values 5.1-5.6 mmol/l
Citation: Ramezani Tehrani F, Farzadfar F, Hosseinpanah F, Rahmati M, Firouzi F, Abedini M, Hadaegh F, Valizadeh M, Torkestani F, Khalili D, Solaymani-Dodaran M, Bidhendi-Yarandi R, Bakhshandeh M, Ostovar A, Dovom MR, Amiri M, Azizi F and Behboudi-Gandevani S (2023) Does fasting plasma glucose values 5.1-5.6 mmol/l in the first trimester of gestation a matter? Front. Endocrinol. 14:1155007. doi: 10.3389/fendo.2023.1155007
Received: 31 January 2023; Accepted: 13 April 2023;
Published: 02 June 2023.
Edited by:
Jan I. Olofsson, Karolinska Institutet (KI), SwedenReviewed by:
Eric Kilpatrick, Sidra Medicine, QatarCopyright © 2023 Ramezani Tehrani, Farzadfar, Hosseinpanah, Rahmati, Firouzi, Abedini, Hadaegh, Valizadeh, Torkestani, Khalili, Solaymani-Dodaran, Bidhendi-Yarandi, Bakhshandeh, Ostovar, Dovom, Amiri, Azizi and Behboudi-Gandevani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Samira Behboudi-Gandevani, c2FtaXJhLmJlaGJvdWRpLWdhbmRldmFuaUBub3JkLm5v
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.