- 1Changzhou Maternal and Child Health Care Hospital, Changzhou Medical Center, Nanjing Medical University, Nanjing, Jiangsu, China
- 2Department of Urology, Division of Life Sciences and Medicine, The First Affiliated Hospital of USTC, University of Science and Technology of China, Hefei, Anhui, China
Background: It is suggested that body mass index (BMI) can affect male semen quality; however, the results remain controversial. In addition, most studies have focused on the effect of obesity on semen quality. Evidence on the relationship of underweight or waist-hip ratio (WHR) with semen quality is rare. This study aimed to assess the association of BMI and WHR with semen quality.
Methods: Data, including BMI and WHR, was collected from 715.00 men who underwent a fertility evaluation. BMI (kg/m2) was categorized as <18.50 (underweight), 18.50–24.90 (normal), 25.00–27.90 (overweight), and ≥28.00 (obese) kg/m2 for analysis. WHR was categorized as <0.81 (normal) and ≥0.81 (high). Semen volume, sperm concentration, progressive motility, and total motile sperm count were detected by experienced clinical technicians.
Results: Spearman’s correlation showed that BMI was weakly associated with sperm progressive motility (r = 0.076, P < 0.05), while WHR showed no relationship with semen parameters. The azoospermia rate was significantly higher (33.33% vs. 2.10%, P < 0.001) and the sperm concentration was lower (P < 0.05) in the underweight group. The nonlinear correlation analysis showed that BMI was negatively associated with sperm concentration while BMI was more than 22.40 kg/m2 (P < 0.05), while WHR was negatively related to sperm progressive motility within 0.82 to 0.89 (P < 0.05). Furthermore, the multivariate logistic analysis showed that follicular stimulating hormone (FSH) was an independent risk factor for normal sperm concentration (odds ratio [OR]: 0.791, P = 0.001) and morphology (OR: 0.821, P = 0.002), BMI was an independent risk factor for normal sperm progressive motility, and testosterone was an independent risk factor for sperm morphology (OR: 0.908, P = 0.023).
Conclusion: BMI and WHR were significantly associated with semen parameters, while BMI was an independent risk factor for normal sperm progressive motility. Reproductive hormones, including FSH and testosterone, had a significant influence on sperm concentration and sperm morphology.
1 Introduction
Infertility is a global clinical concern, affecting 10.00–15.00% of reproductive-age couples. It is believed that 40% of cases are due to male factors (1–3). Poor semen quality is the major condition leading to male infertility. Although studies have reported several risk factors—including environmental pollutants, Mumps virus infection, and alcohol intake—that may be related to decreased semen quality, the underlying causes are still uncertain (4–6).
Recently, an increasing number of studies have explored the relationship between abnormal body mass index (BMI) and semen quality; however, the results have remained controversial. For example, Michael et al. (7) suggested that an increased BMI and waist circumference (WC) were associated with a reduction in ejaculate volume and the total sperm count, but no relationship was found between BMI and sperm concentration, sperm motility, sperm vitality, sperm morphology, or the DNA fragmentation index in the United States. Wang et al. (8) reported that an increased BMI was linked with a lower total sperm number and sperm concentration in northern China. Meanwhile, another large single-center clinical study by Ma et al. found that being underweight or overweight were both factors associated with a decreased total motile sperm count (9). On the right hand, the researchers found that being overweight was related with a reduction in semen volume and total sperm number, while no correlation with sperm concentration was observed, which was consistent with the study by Michael et al. (7, 9). In addition, Lu et al. (10) found that BMI, the waist–hip ratio (WHR), and WC cannot predict male semen quality; however, semen quality was significantly related to levels of follicular stimulating hormone (FSH) and luteinizing hormone (LH).
Taken together, the evidence on the relationship between body size (BMI or WHR) and semen quality is limited and inconclusive. In the present study, we performed a retrospective study of 715 healthy sperm donors to assess the association of BMI and WHR with semen quality.
2 Methods
2.1 Study design
A total of 715, male partners of infertile couples who could not achieve pregnancy after 12, consecutive months without contraception at the Reproductive and Genetic Hospital, Division of Life Sciences and Medicine, The First Affiliated Hospital of USTC, University of Science and Technology of China, were recruited for our study. The experimental study protocol was administrated by the Research Ethics Committee of The First Affiliated Hospital of USTC, University of Science and Technology of China, and informed consent was obtained from all participants. Semen samples from the patients with azoospermia were analyzed at least twice at an interval of 3 weeks according to the World Health Organization (WHO) Laboratory Manual for the Examination and Processing of Human Semen (5th edition). Participants were divided into four groups by different BMI values: <18.50 (underweight), 18.50–24.90 (normal), 25.00–27.90 (overweight), and ≥28.00 (obese) kg/m2 (11).WHR was categorized into two groups: <0.81 (normal) and ≥0.81 (high) (12). The patients with a history of cryptorchidism, varicocoele or testicular trauma, administration of hormones, genital infections, or other diseases during the previous 3.00 months were excluded.
2.2 Semen and hormone analysis
The semen volume was detected by the weighing method according to the WHO guidelines (2010). Computer-assisted sperm analysis was used to measure sperm concentration, progressive motility, and total motility (SAS, Beijing, China). Sperm morphology was determined through Diff-Quick staining (Anke Biotechnology, Hefei, China). The result of semen analysis including oligospermia, asthenospermia and teratospermia were undertaken according to the WHO Semen Manual, 5th edition. In detail, oligospermia was defined as the sperm concentration <15.00 * 10^6/ml, asthenospermia as P R< 32.00%, teratospermia as normal morphology of spermatozoa < 4.00% while azoospermia was defined as the absence of spermatozoa in the semen. Blood samples were obtained at 8–11 a.m. and were centrifuged for 10 min at 1800 g. The levels of testosterone, LH, and FSH were determined by radioimmunoassay (Beckman Coulter, Brea, USA).
2.3 Statistical analysis
All data were evaluated for the normal distribution by the Kolmogorov–Smirnov test. The variables departing from the normal distribution were summarized as medians and interquartile intervals. Correlations between BMI, WHR, and semen parameters were analyzed by Spearman’s correlation coefficient, as appropriate. A one-way ANOVA or Kolmogorov–Smirnov test was used to evaluate the differences among the groups. Univariate and multivariate logistic analysis was performed to seek the independent risk factors for semen parameters. Statistical analyses were performed using IBM SPSS 26 for windows and P value < 0.05 was considered statistically significant. The non-linear relationship between BMI, WHR, and semen quality were analyzed by the restricted cubic spline.
3 Results
3.1 Characteristics of studied men and correlation analysis
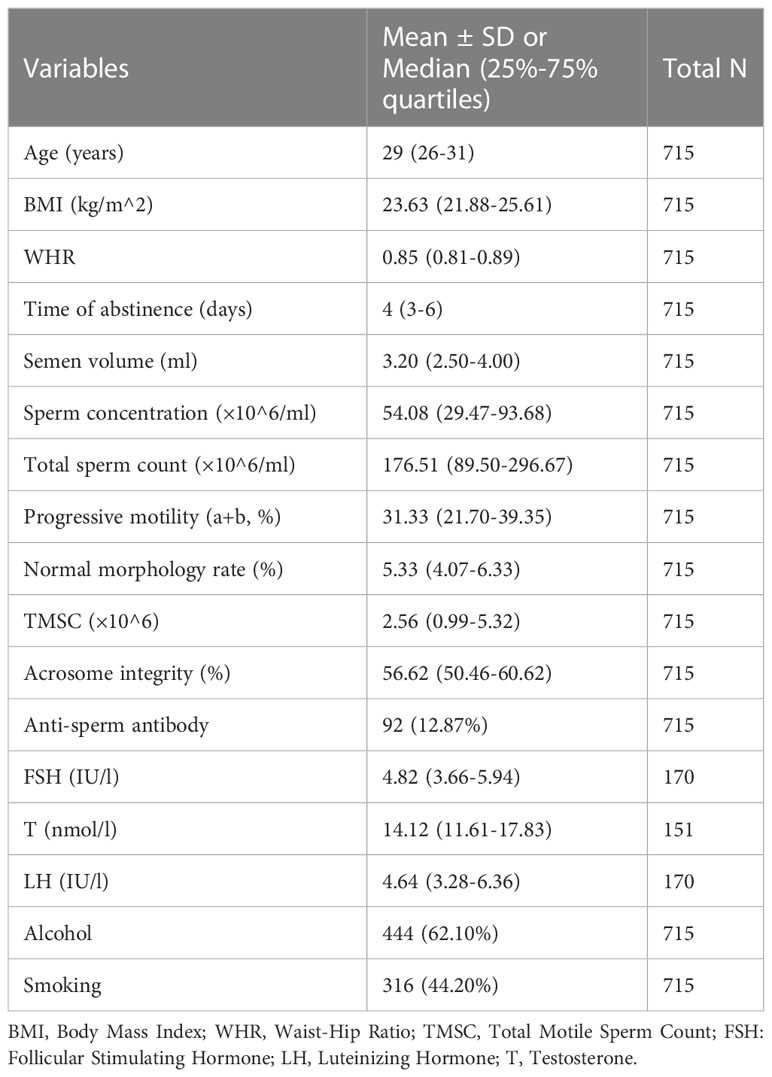
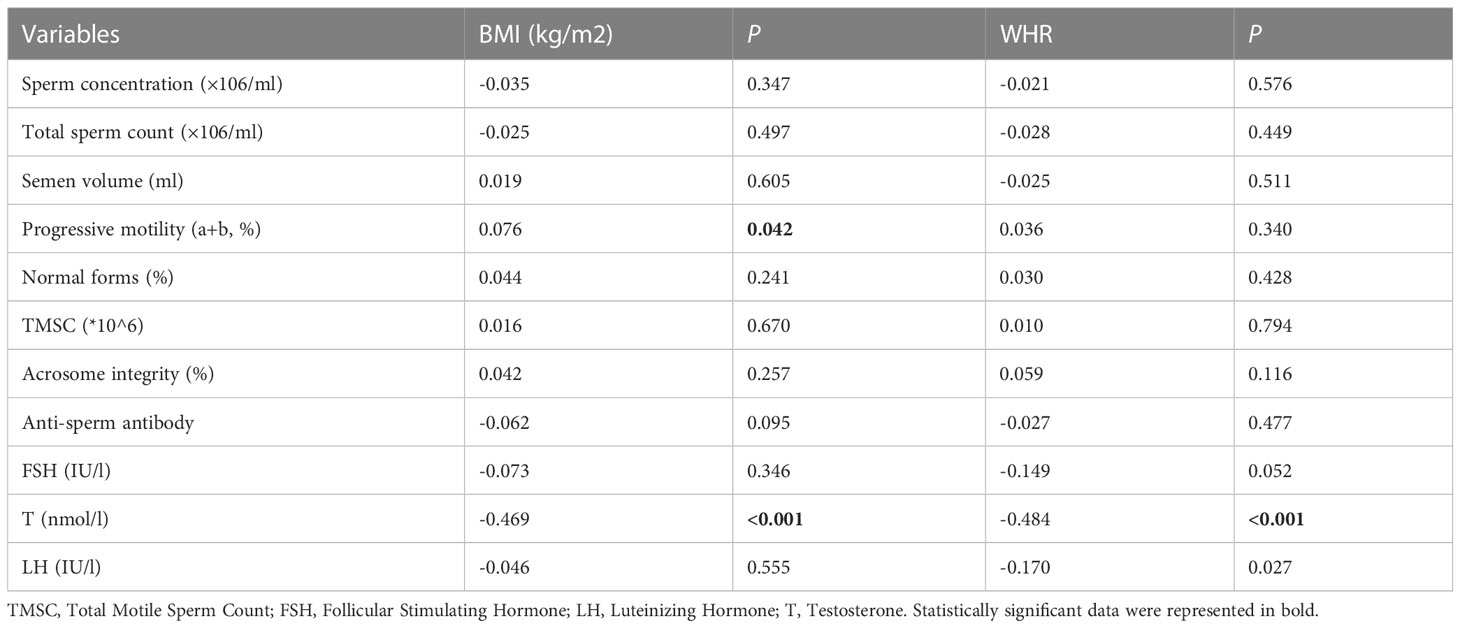
The basic data of the participants are shown in Table 1. BMI, WHR, and semen parameters were non-normally distributed, which were presented as medians and interquartile intervals. The correlations of BMI and WHR with semen parameters are shown in Table 2; a weak positive correlation was found between BMI and sperm progressive motility while both BMI and WHR were negatively correlated with serum testosterone. Other semen parameters, such as total motile sperm count (TMSC) and acrosome integrity, were not related to BMI or WHR.
3.2 Comparison of semen parameters in different groups divided by BMI and WHR
Firstly, the prevalence of oligospermia, asthenospermia, and teratospermia in each group was compared by the chi-square test. There was a reduction of the normal sperm concentration rate in the underweight group compared with other groups (Figure 1A). Interestingly, the prevalence of asthenospermia was higher in the normal BMI group and a higher rate of teratospermia was found in the normal WHR group (Figures 1B, F). However, no difference was found in the rate of teratospermia in different BMI groups while both the rate of oligospermia and asthenospermia showed no statistically significant differences in the different WHR groups (Figures 1C–E). Furthermore, the Kolmogorov–Smirnov test was used to evaluate the differences among each group. The sperm concentration in the underweight group was significantly lower than the normal BMI group and overweight group. No difference was found in any other group (Figure 2A). Furthermore, the level of serum testosterone did not show a consistent change; serum testosterone in the overweight group and obese group were significantly higher than in the normal BMI group (Figure 2B). In addition, we found that the BMI level in the azoospermia group was lower compared with the oligospermia group and normal sperm concentration group (Figure 2C). The BMI was lower in the asthenospermia group than the normal sperm motility group (Figure 2D).
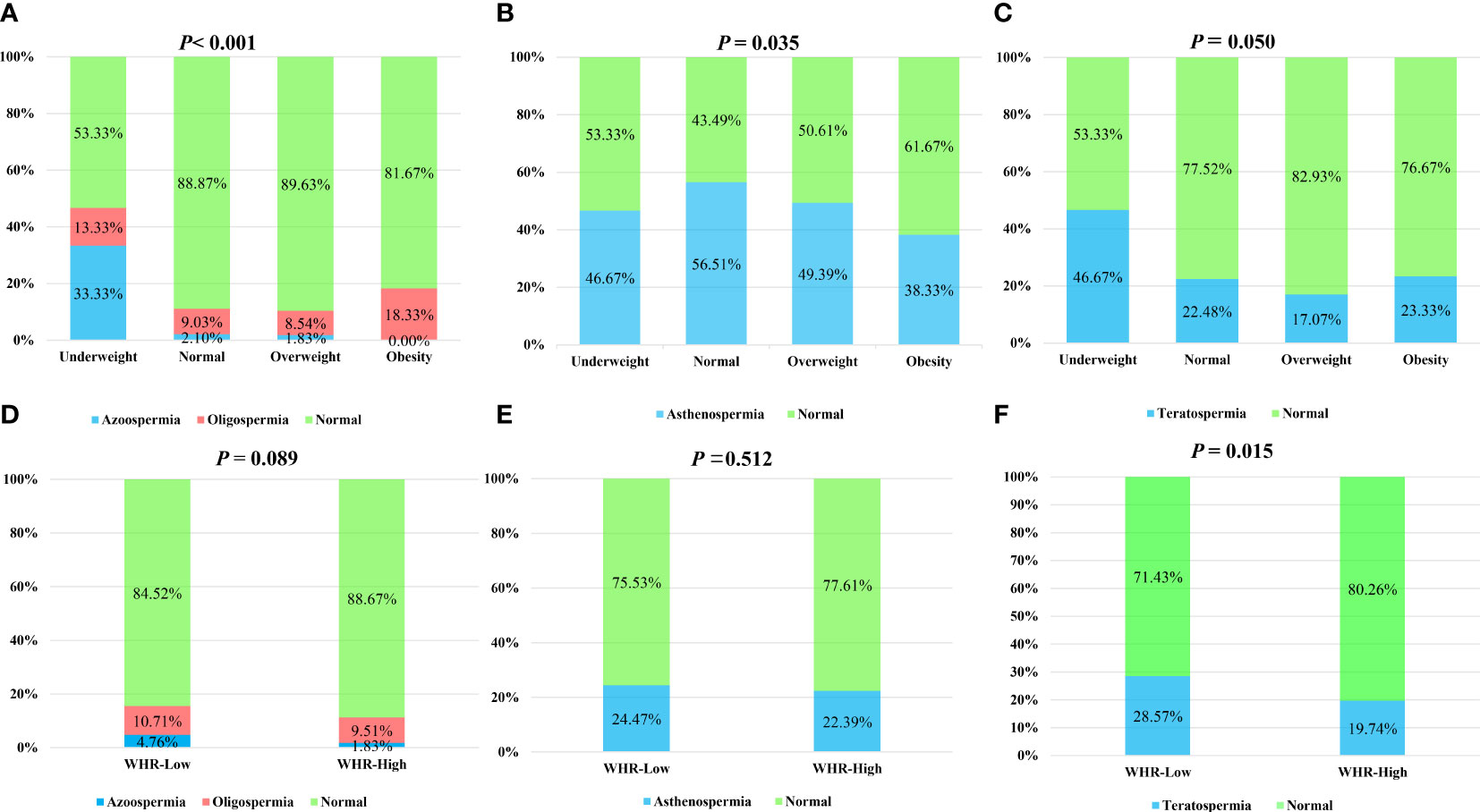
Figure 1 Chi-square test to analyze the influence of body mass index (BMI) and waist–hip ratio (WHR) on the sperm concentration, sperm progressive motility, and sperm morphology. BMI was categorized as: underweight (<18.0 kg/m2), normal weight (18.0–24.9kg/m2), overweight (25–27.9 kg/m2), and obese (≥28 kg/m2). WHR was categorized into two groups: <0.81 (normal) and ≥0.81 (high). (A) The rate of azoospermia, oligospermia, and normal sperm concentration in the different BMI groups. (B) The rate of asthenospermia and normal sperm progressive motility in the different BMI groups. (C) The rate of teratospermia and normal sperm morphology in the different BMI groups. (D) The rate of azoospermia, oligospermia, and normal sperm concentration in the different WHR groups. (E) The rate of asthenospermia and normal sperm progressive motility in the different WHR groups. (F) The rate of teratospermia and normal sperm morphology in the different WHR groups.
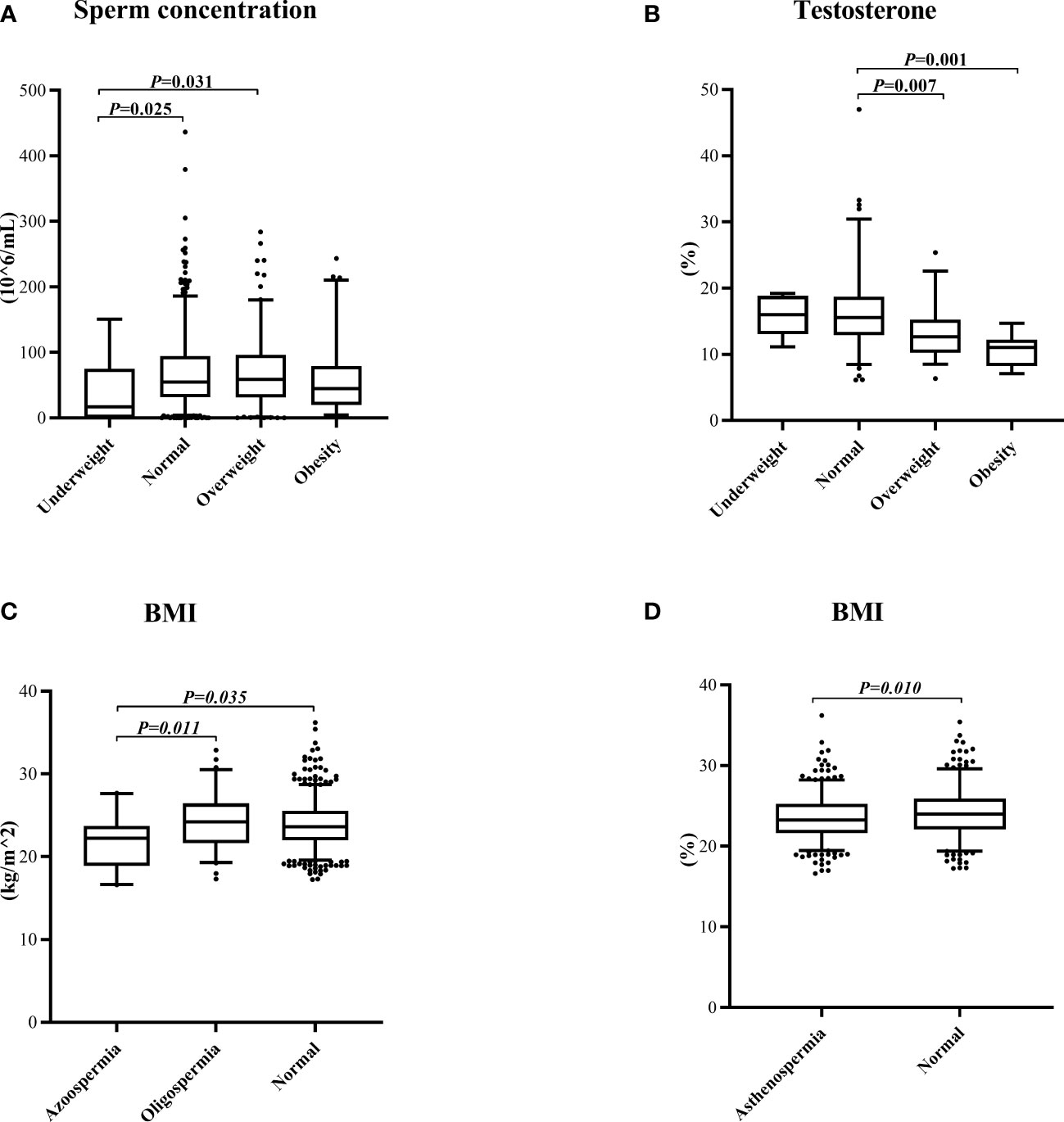
Figure 2 Kolmogorov–Smirnov test to evaluate the differences among the groups. (A) The sperm concentration in the different body mass index (BMI) groups. (B) The testosterone concentration in the different BMI groups. (C) The BMI in the different sperm concentration groups. (D) The BMI in different sperm progressive motility groups.
3.3 Restricted cubic spline analysis of the nonlinear correlation of BMI and WHR with sperm concentration, sperm morphology, and sperm progressive motility
The nonlinear correlation between BMI and semen quality parameters was expressed in Figures 3A–C, while Figures 3D–F shows the nonlinear correlation between WHR and semen quality. The result indicated that when the BMI was lower than the median BMI in normal weight subjects (22.40 kg/m2), the sperm concentration appeared to increase monotonically with increasing BMI. The sperm concentration was inversely associated with increasing BMI While BMI was more than 22.4 kg/m2. No significant relationships were observed between BMI and sperm progressive motility or sperm morphology. Interestingly, the sperm progressive motility was positively associated with the WHR when <0.82 or >0.89 and negatively associated with the WHR within 0.82 to 0.89. No relationship was observed between the WHR and sperm concentration or sperm morphology.
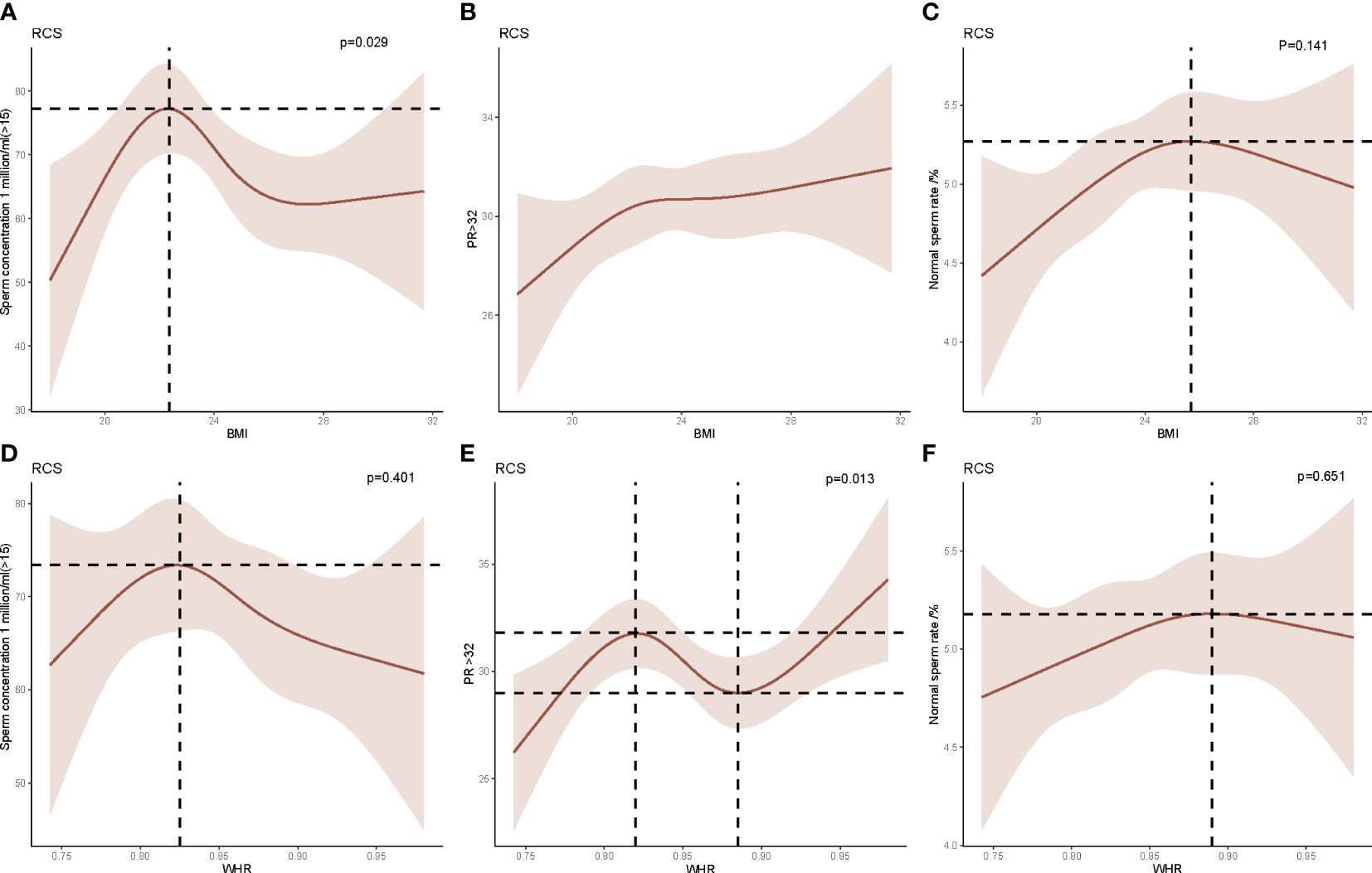
Figure 3 Restricted cubic spline (RCS) analysis of the relationship between body mass index (BMI), waist–hip ratio (WHR), and sperm concentration, sperm progressive motility, and sperm morphology. (A) The relationship between BMI and sperm concentration. (B) The relationship between BMI and sperm progressive motility. (C) The relationship between BMI and sperm morphology. (D) The relationship between WHR and sperm concentration. (E) The relationship between WHR and sperm progressive motility. (F) The relationship between WHR and sperm morphology.
3.4 Multivariate logistic analysis of the independent risk factors for sperm concentration, sperm progressive motility, and sperm morphology
The odds ratios (ORs), 95% confidence interval (CIs), and P values of the sperm concentration, sperm morphology, and sperm progressive motility are summarized in Tables 3–5. The results of the ordinal multivariate logistic analysis revealed that age (OR: 1.184, 95% CI: 1.015–1.381, P = 0.031) and FSH (OR: 0.791, 95% CI: 0.692–0.904, P =0.001) were independent risk factors of male sperm concentration; while BMI, WHR, serum testosterone, LH, alcohol and smoke intake were not. Interestingly, referring to sperm motility, the results of the multivariate logistic analysis showed that BMI (OR: 1.072, 95% CI: 1.019–1.128, P = 0.007) and abstinence time (OR: 0.913, 95% CI: 0.853–0.978, P = 0.009) were the only independent risk factors among the clinical parameters counted. Finally, the multivariate logistic analysis suggested that FSH (OR: 0.821, 95% CI: 0.727–0.927, P = 0.002) and testosterone (OR: 0.908, 95% CI: 0.835–0.987, P = 0.023) were the independent risk factors for sperm morphology, while BMI or WHR were not found to be associated with it.
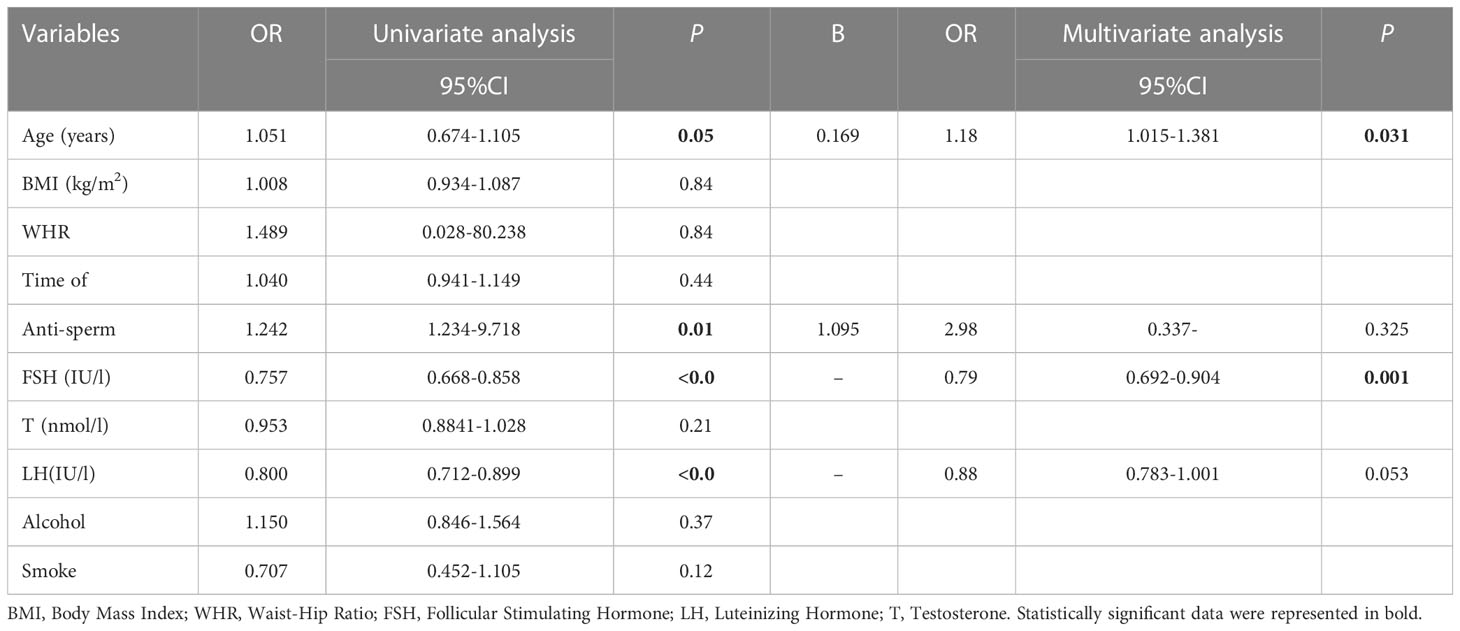
Table 3 Univariate and multivariate ordinal logistic analysis for screening the independent factors of sperm concentration.
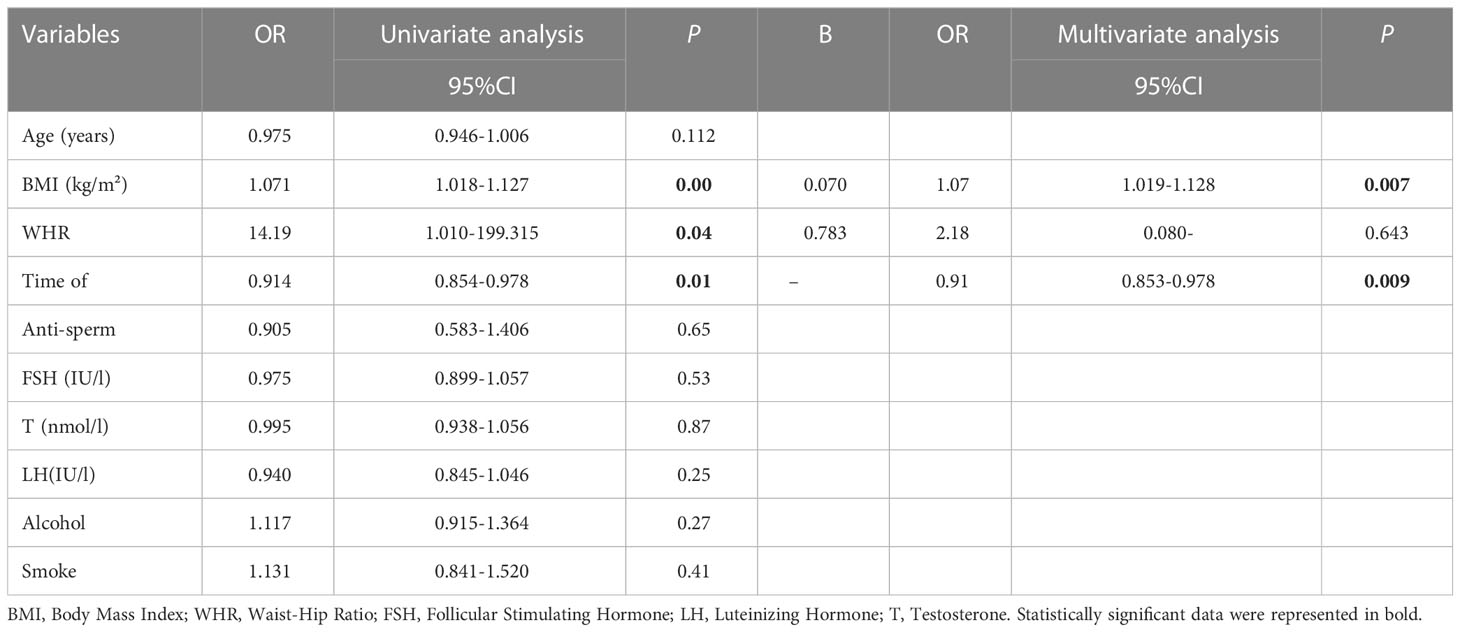
Table 4 Univariate and multivariate logistic analysis for screening the independent Influencing factors of sperm progressive motility.
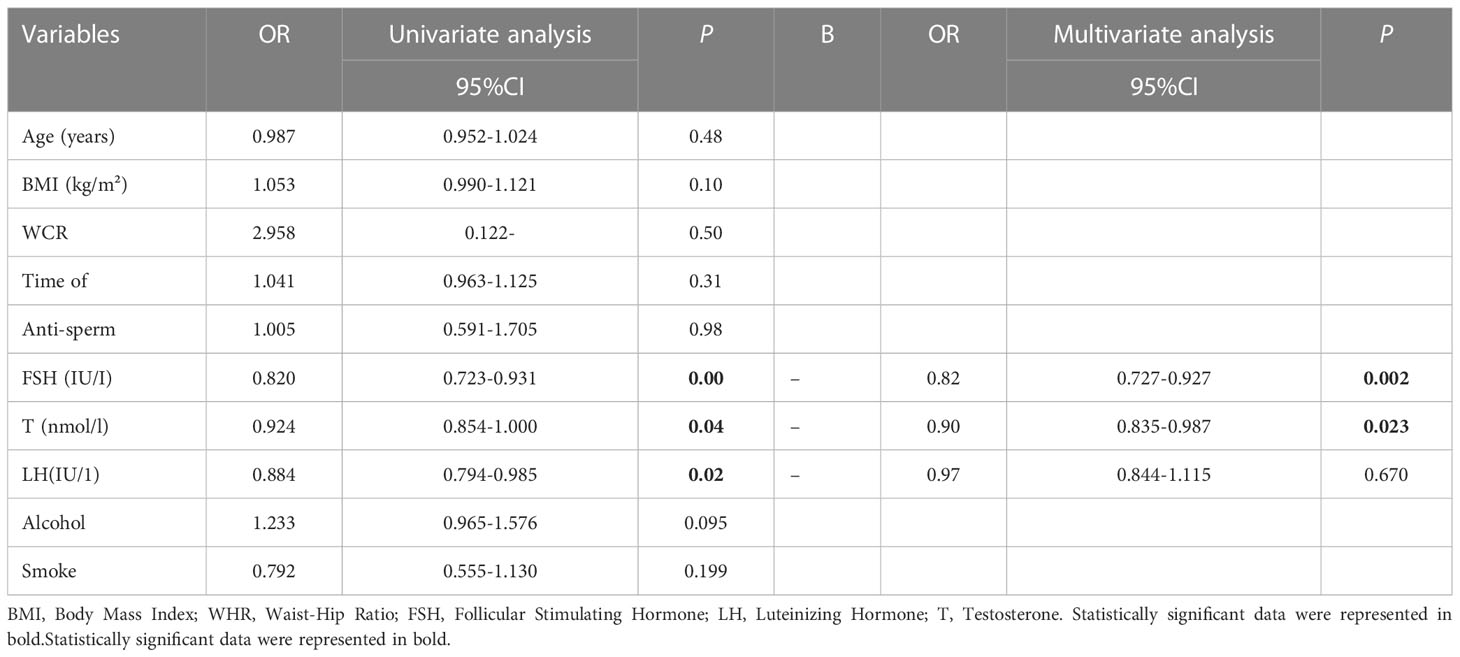
Table 5 Univariate and multivariate logistic analysis for screening the independent factors of sperm morphology.
4 Discussion
In this observational study, the association between BMI, WHR, and semen quality was investigated in 715, sperm donors who underwent semen examinations between 2019 and 2021 in Hefei, China. We found that BMI was weakly associated with sperm progressive motility, while WHR showed no relationship with semen parameters. The RCS showed that BMI was negatively associated with sperm concentration while BMI was more than 22.40 kg/m2 and the WHR was negatively related to sperm progressive motility within 0.82 to 0.89. Furthermore, the multivariate logistic analysis showed that BMI was an independent risk factor for normal sperm progressive motility, FSH was an independent risk factor for normal sperm concentration and morphology, and testosterone was an independent risk factor for sperm morphology. This study highlights that BMI is a more important factor in the assessment of semen quality than WHR. BMI showed a weak influence on sperm progressive motility; however, serum FSH and testosterone were the main factors affecting sperm concentration and morphology.
The relationship between BMI and semen quality remains controversial. Some studies have demonstrated that an increased BMI is significantly linked with sperm concentration, sperm motility, and sperm morphology (8, 9, 13). However, other studies reported different conclusions (7, 9, 14). In 2013, Sermondade et al. performed a meta-analysis on a large sample of data (15). They found that being overweight and obesity were significantly associated with an increased risk of azoospermia and oligozoospermia. However, our study showed that BMI was positively associated with sperm progressive motility, but not with sperm concentration or morphology. The results of the multivariate logistic analysis showing that BMI was an independent risk factor confirmed this. Therefore, an elevated BMI may be beneficial for sperm progressive motility.
Few studies have focused on the relationship between being underweight and semen quality. In 2004, Jensen et al. assessed military readiness in 1558.00 young Danish men young men between 1996 and 1998; a significant reduction in sperm concentration was observed in underweight men, which was defined as BMI <20.00 kg/m2 (16). A later study by Qin et al. (17) reported that being underweight was significantly associated with a lower sperm concentration in fertile men from the general population. Consistent with these two studies, being underweight was inversely related to sperm concentration and total sperm number, but not to sperm motility or semen volume, in Jixuan Ma’s study, which recruited 3966.00 healthy sperm donors (9). In the present study, an increased prevalence of azoospermia was observed in the underweight group and the sperm concentration was lower compared with the normal bodyweight or overweight group. This was consistent with previous research. Malnutrition may be the key factor of the association between being underweight and worse semen quality, which is known to have negative effects on male reproductive hormones (18).
It is well known that reproductive hormones, including FSH and testosterone, are closely related to male semen quality (19–21). FSH plays an important role in spermatogenesis. Clinical studies reported that FSH levels were higher in infertile patients compared with fertile controls, and that FSH was negatively correlated to sperm concentration in the population surveyed (22). However, the relationship between FSH and sperm morphology has been rarely reported. In 2020, Wei Zhao et al. (23) reported that FSH was inversely associated with sperm morphology after mutual adjustment, which was consistent with our finding. This indicated that FSH is important for sperm to maintain normal morphology. However, the mechanism remains to be studied in the future. In addition, testosterone is known to be the key factor to sustain sperm production. Therefore, testosterone may be strongly associated with sperm concentration, sperm morphology, or sperm motility. As reported, using human chorionic gonadotropin or tamoxifen citrate in infertile men with testosterone deficiency led to an improvement of the sperm concentration, sperm motility, and sperm morphology (24). Nevertheless, the results of the present study showed that testosterone was an independent risk factor for sperm morphology but was not related with sperm concentration or sperm morphology. A possible explanation is that the levels of testosterone in the serum in the present study did not reflect the true intratesticular testosterone level, which is believed to directly act in the spermatogenic process.
There are some limitations in the present research. Firstly, the sample size collected was small, especially for people who had completed sex hormone tests. Secondly, the data was acquired in the infertile population, whose age were concentrated in the reproductive age. Therefore, the result of this study cannot represent the result of all populations. Thirdly, this is a single-center retrospective research, the conclusion needs to be verified by a multi-center prospective study in the future.
In conclusion, BMI is an independent risk factor for sperm motility while the WHR did not contribute to semen parameters. Our findings highlight the important role of serum FSH and testosterone in male semen quality.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
XH and SW designed the research study. ZK, CW and PX contributed to the data acquisition. XH, BW and JX analyzed the data. SW wrote the paper. All authors approved the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (81901545,82201757), the Natural Science Foundation of Anhui Provincial of China (1908085QH315) and Changzhou Sci&Tech Program (Grant No. CJ20220115).
Acknowledgments
We are grateful to Jing-Ru Xu, Xia Wu and Wenlu Yu from the First Affiliated Hospital of USTC for their valuable contribution to the data collection and article revision.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Boivin J, Bunting L, Collins JA, Nygren KG.. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod (2007) 22(6):1506–12. doi: 10.1093/humrep/dem046
2. Agarwal A, Parekh N, Panner Selvam MK, Henkel R, Shah R, Homa ST, et al. Male Oxidative stress infertility (MOSI): proposed terminology and clinical practice guidelines for management of idiopathic Male infertility. World J Mens Health (2019) 37(3):296–312. doi: 10.5534/wjmh.190055
3. Inhorn MC, Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update (2015) 21(4):411–26. doi: 10.1093/humupd/dmv016
4. Chiu YH, Gaskins AJ, Williams PL, Mendiola J, Jørgensen N, Levine H, et al. Intake of fruits and vegetables with low-to-Moderate pesticide residues is positively associated with semen-quality parameters among young healthy men. J Nutr (2016) 146(5):1084–92. doi: 10.3945/jn.115.226563
5. Salas-Huetos A, Bullo M, Salas-Salvado J. Dietary patterns, foods and nutrients in male fertility parameters and fecundability: a systematic review of observational studies. Hum Reprod Update (2017) 23(4):371–89. doi: 10.1093/humupd/dmx006
6. Zafar MI, Yu J, Li H. Implications of RNA viruses in the Male reproductive tract: an outlook on SARS-CoV-2. Front Microbiol (2021) 12:783963. doi: 10.3389/fmicb.2021.783963
7. Eisenberg ML, Kim S, Chen Z, Sundaram R, Schisterman EF, Buck Louis GM, et al. The relationship between male BMI and waist circumference on semen quality: data from the LIFE study. Hum Reprod (2014) 29(2):193–200. doi: 10.1093/humrep/det428
8. Wang EY, Huang Y, Du QY, Yao GD, Sun YP. Body mass index effects sperm quality: a retrospective study in northern China. Asian J Androl (2017) 19(2):234–7. doi: 10.4103/1008-682X.169996
9. Ma J, Wu L, Zhou Y, Zhang H, Xiong C, Peng Z, et al. Association between BMI and semen quality: an observational study of 3966 sperm donors. Hum Reprod (2019) 34(1):155–62. doi: 10.1093/humrep/dey328
10. Lu JC, Jing J, Chen L, Ge YF, Feng RX, Liang YJ, et al. Analysis of human sperm DNA fragmentation index (DFI) related factors: a report of 1010 subfertile men in China. Reprod Biol Endocrinol (2018) 16(1):23. doi: 10.1186/s12958-018-0345-y
11. Li S, Li Y, Lv H, Jiang R, Zhao P, Zheng X, et al. The prevalence and correlates of burnout among Chinese preschool teachers. BMC Public Health (2020) 20(1):160. doi: 10.1186/s12889-020-8287-7
12. Yu D, Zheng W, Johansson M, Lan Q, Park Y, White E, et al. Overall and central obesity and risk of lung cancer: a pooled analysis. J Natl Cancer Inst (2018) 110(8):831–42. doi: 10.1093/jnci/djx286
13. Belloc S, Cohen-Bacrie M, Amar E, Izard V, Benkhalifa M, Dalléac A, et al. High body mass index has a deleterious effect on semen parameters except morphology: results from a large cohort study. Fertil Steril (2014) 102(5):1268–73. doi: 10.1016/j.fertnstert.2014.07.1212
14. Rufus O, James O, Michael A. Male Obesity and semen quality: any association? Int J Reprod BioMed (2018) 16(4):285–90. doi: 10.29252/ijrm.16.4.285
15. Sermondade N, Faure C, Fezeu L, Shayeb AG, Bonde JP, Jensen TK, et al. BMI in relation to sperm count: an updated systematic review and collaborative meta-analysis. Hum Reprod Update (2013) 19(3):221–31. doi: 10.1093/humupd/dms050
16. Jensen TK, Andersson AM, Jørgensen N, Andersen AG, Carlsen E, Petersen JH, et al. Body mass index in relation to semen quality and reproductive hormones among 1,558 Danish men. Fertil Steril (2004) 82(4):863–70. doi: 10.1016/j.fertnstert.2004.03.056
17. Qin DD, Yuan W, Zhou WJ, Cui YQ, Wu JQ, Gao ES, et al. Do reproductive hormones explain the association between body mass index and semen quality? Asian J Androl (2007) 9(6):827–34. doi: 10.1111/j.1745-7262.2007.00268.x
18. Santos AM, Ferraz MR, Teixeira CV, Sampaio FJ, da Fonte Ramos C. Effects of undernutrition on serum and testicular testosterone levels and sexual function in adult rats. Horm Metab Res (2004) 36(1):27–33. doi: 10.1055/s-2004-814198
19. Zhang X, Chen J, Cui Y, Jin Y, Wang X. FSH can improve semen parameters in patients with idiopathic oligoasthenoteratozoospermia: a systematic review and meta-analysis. Andrologia (2022) 54(11):e14596. doi: 10.1111/and.14596
20. Valenti D, La Vignera S, Condorelli RA, Rago R, Barone N, Vicari E, et al. Follicle-stimulating hormone treatment in normogonadotropic infertile men. Nat Rev Urol (2013) 10(1):55–62. doi: 10.1038/nrurol.2012.234
21. Olesen IA, et al, Joensen UN, Petersen JH, Almstrup K, Rajpert-De Meyts E, Carlsen E. Decrease in semen quality and leydig cell function in infertile men: a longitudinal study. Hum Reprod (2018) 33(11):1963–74. doi: 10.1093/humrep/dey283
22. Corinne TM, Anatole PC, Jeanne NY. Comparison of serum inhibin b and follicle-stimulating hormone (FSH) level between normal and infertile men in Yaounde. Int J Reprod Med 2020 (2020) p:4765809. doi: 10.1155/2020/4765809
23. Zhao W, Jing J, Shao Y, Zeng R, Wang C, Yao B, et al. Circulating sex hormone levels in relation to male sperm quality. BMC Urol (2020) 20(1):101. doi: 10.1186/s12894-020-00674-7
Keywords: body mass index, waist-hip ratio, semen parameters, testosterone, follicular stimulating hormone
Citation: Wang S, Wu B, Wang C, Ke Z, Xiang P, Hu X and Xiao J (2023) Influence of body mass index and waist–hip ratio on male semen parameters in infertile men in the real world: a retrospective study. Front. Endocrinol. 14:1148715. doi: 10.3389/fendo.2023.1148715
Received: 20 January 2023; Accepted: 18 May 2023;
Published: 26 June 2023.
Edited by:
Shun Bai, University of Science and Technology of China, ChinaReviewed by:
Yanping Huang, Shanghai Jiao Tong University, ChinaXiongbing Zu, Central South University, China
Yeting Hong, Hangzhou Medical College, China
Copyright © 2023 Wang, Wu, Wang, Ke, Xiang, Hu and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Xiao, eGlhb2p1bnBwQDEyNi5jb20=; Ping Xiang, eGlhbmdwaW5nMjVAMTYzLmNvbQ==; Xuechun Hu, YjA5MTIzMDAyOEAxNjMuY29t
†These authors have contributed equally to this work
 Shuxian Wang
Shuxian Wang Baorui Wu
Baorui Wu Changming Wang
Changming Wang Zongpan Ke2
Zongpan Ke2 Xuechun Hu
Xuechun Hu Jun Xiao
Jun Xiao