
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 07 June 2023
Sec. Cardiovascular Endocrinology
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1148426
This article is part of the Research Topic Modifiable Risk Factors and Interventions in Early Life for Cardiometabolic Health View all 7 articles
 Leah Gilbert1,2†
Leah Gilbert1,2† Dan Yedu Quansah1†
Dan Yedu Quansah1† Amar Arhab1
Amar Arhab1 Sybille Schenk1
Sybille Schenk1 Justine Gross1
Justine Gross1 Stefano Lanzi3
Stefano Lanzi3 Bobby Stuijfzand1
Bobby Stuijfzand1 Alain Lacroix1
Alain Lacroix1 Antje Horsch4,5*‡
Antje Horsch4,5*‡ Jardena J. Puder1*‡ and MySweetheart Research group
Jardena J. Puder1*‡ and MySweetheart Research groupIntroduction: Gestational diabetes mellitus (GDM) may negatively affect offspring outcomes. A lifestyle intervention may therefore not only improve maternal, but also offspring outcomes. The effects of lifestyle interventions on birth, anthropometric, and psychobehavioral outcomes in offspring of women with GDM need further evidence.
Design: The MySweetheart trial is a monocentric single-blind randomized controlled trial in 211 women with GDM. It tested the effect of a pre- and postpartum multidimensional interdisciplinary lifestyle and psychosocial intervention focusing on both the mothers and their infants and its effects on maternal (primary outcomes) and offspring (secondary outcomes) metabolic and psychobehavioral outcomes compared with guidelines-based usual-care. This paper focuses on offspring’s birth, anthropometric, and maternal report of psychobehavioral outcomes at singular timepoints.
Methods: Women with GDM aged ≥18 years, between 24-32 weeks of gestation, speaking French or English were included and randomly allocated to either the intervention or to an active guidelines-based usual-care group using a 1:1 allocation ratio. The intervention lasted from pregnancy until 1 year postpartum and focused on improving diet, physical activity, and mental health in the mother. For the offspring it focused on supporting breastfeeding, delaying the timing of introduction of solid foods, reducing the consumption of sweetened beverages, increasing physical activity of the family, and improving parental responsiveness to infant distress, hunger, satiety and sleeping cues, and difficult behavior.
Results: Adverse birth and neonatal outcomes rarely occurred overall. There were no differences between groups in offspring birth, neonatal, anthropometric, or psychobehavioral outcomes up to one year. After adjustments for maternal age and the offspring’s sex and age, there was a borderline significant between-group difference in birth length (β:-0.64, CI:-1.27; -0.01, p: 0.05), i.e., offspring of mothers in the intervention group were born 0.64 cm shorter compared to those in the usual-care group.
Conclusion: This is the first pre- and postpartum multidimensional interdisciplinary lifestyle and psychosocial intervention in GDM focusing on both the mother and the offspring. It did not lead to a significant improvement in most birth, anthropometric, and psychobehavioral outcomes in offspring of women with GDM. ClinicalTrials.gov Identifier: NCT02890693
Gestational diabetes mellitus (GDM) is a glucose intolerance first diagnosed during pregnancy that does not fulfil the criteria for pre-existing diabetes (1). The prevalence of GDM in Switzerland is 10.8% (2). GDM is associated with adverse offspring birth and neonatal outcomes, such as perinatal mortality, preterm delivery, and caesarean delivery (3, 4). GDM is also associated with the offspring’s anthropometry, as it leads to a higher risk of macrosomia, high birth weight, large for gestational age (LGA), accelerated weight gain after delivery, and increased risk of obesity and diabetes later in life (5–8). Breastfeeding has been associated with lower infant fat mass (9) and lower risk of obesity and better overall health in the general population (10). However, women with GDM are at greater risk of delayed breastfeeding initiation and low milk supply (11). Less social support for breastfeeding has been reported among mothers with GDM, which may partly contribute to these trends (12). Additionally, GDM-affected pregnancies carry a higher risk of obesity, cesarean delivery and neonatal hypoglycemia, which may delay breastfeeding initiation after birth (12). Furthermore, the positive effect of breastfeeding on lower fat mass has not been demonstrated in the offspring of mothers with GDM (13, 14).
GDM is also associated with worsened maternal mental health, particularly with a higher risk of maternal depression (15–17). This may also indirectly affect the offspring’s psychobehavioral outcomes. Indeed, maternal depression in women with GDM is associated with shorter sleep duration in the offspring at one year of age, which may in turn negatively impact the offspring’s development (18). In women with obesity, maternal perception of offspring difficult behavior is also more frequent and is associated with poorer offspring development, such as low mood and inadaptability up to 12 years of age; however, this still needs to be investigated in GDM populations (19, 20). In prior studies, GDM was associated with a higher risk of hyperactivity disorder in children aged six, but there is no evidence of GDM affecting earlier behavior (21). Given the risks associated with GDM, it is of utmost importance to intervene in pregnancy and the postpartum period.
Prior to the conception of the MySweetheart trial, some lifestyle intervention studies mainly focusing on diet and physical activity in the mother had investigated the effect of these interventions on adverse birth and neonatal outcomes in offspring of mothers with GDM and mainly show there is low evidence for improvements in these outcomes for the intervention group (22). Another study also investigated the impact of medication in women with GDM on the anthropometric and metabolic outcomes of children (7-9 years old) (23). Nonetheless, no prior studies in women with GDM have investigated the impact of a psychosocial and lifestyle intervention, containing an intervention conducted in both the mother and the offspring on the offspring’s birth, neonatal, anthropometric and psychobehavioral outcomes. In the general population, modifiable risk factors of adverse birth and neonatal outcomes, such as preterm delivery, include reinforcing social support and improving nutrition in mothers during pregnancy (24, 25). With regards to improvements in the offspring’s anthropometry, a study showed that intervening exclusively on maternal lifestyle behavior, including nutrition, did not favorably influence offspring adiposity outcomes up to five years of age (26). Furthermore, previous studies performed in different populations, containing interventions focusing on breastfeeding (versus formula feeding), timing of solid food introduction, sweetened beverage consumption, parental responsiveness to feeding cues and offspring distress, and maternal and offspring physical activity have shown effects on the offspring’s anthropometry (27–32). These postnatal interventions could also improve similar outcomes in offspring of women with GDM. Guidelines provided by the World Health Organization (WHO) at the time of the production of the protocol encouraged women to breastfeed exclusively for at least six months (33). European and Swiss Guidelines for the introduction of solid foods recommended that it should be initiated after at least four months of age to reduce the risk of obesity later in life (34–37). Prior parenting skills interventions have focused on anticipatory guidance, offspring cues, and distress to positively influence self-regulatory capacities to reduce the offspring’s obesity risk (31, 38, 39). One intervention study in the general population focusing on responsive parenting to feeding cues showed that when parents used responsive feeding practices, their offspring gained weight more slowly than the controls and were less likely to be overweight at 12 months (40). Responsive feeding by parents may also be an efficient strategy in ensuring healthy growth of the offspring and guidelines on how to effectively use these behaviors have been proposed in recent years (41, 42). Regarding physical activity, it is recommended that offspring under one year of age should be physically active several times a day through interactive floor-based play, as it improves measures of adiposity, motor skill development, and cognitive development (43). Finally, different parental interventions in the general population have shown positive effects on the offspring psychobehavioral outcomes, such as improvements in sleep, socioemotional development, and behavioral problems (44, 45).
Previous studies in GDM investigating the impact of maternal lifestyle interventions on offspring health outcomes are scarce. Importantly, there are no published intervention trials intervening in the offspring of mothers with GDM (1). The MySweetheart trial is an interdisciplinary randomized-controlled trial in mothers with GDM. It is the first pre-and postpartum complex intervention that tested the effect of a multidimensional lifestyle and psychosocial trial intervening on both the mother and their offspring on maternal (primary outcomes) and offspring (secondary outcomes) metabolic and psychobehavioral outcomes.
The aim of this paper was to focus on the secondary outcomes of the trial, i.e., on differences in the offspring’s birth, anthropometric or psychobehavioral outcomes up to one year of age between the intervention and an active guidelines-based usual-care group.
The MySweetheart trial (RCT: ClinicalTrials.gov Identifier: NCT02890693) was a monocentric single-blind randomized controlled trial (RCT) that tested the effect of a pre- and postpartum multidimensional interdisciplinary lifestyle and psychosocial intervention on metabolic and psychological outcomes in mothers with GDM and their offspring compared to an active guidelines-based control group. More details are provided in the study protocol (46). The primary aims of the trial were to improve maternal mental and metabolic health outcomes. Secondary aims were to improve offspring birth, anthropometric, and psychobehavioral outcomes. The study protocol was approved by the Human Research Ethics Committee of the Canton de Vaud (study number 2016-00745).
Women aged ≥18 years, diagnosed with GDM according to the IADPSG criteria (47), between 24-32 weeks gestational age (GA), and who understood French or English were included. We excluded women on strict bed rest, with pre-existing diabetes or if they had a current severe mental health disorder which included the presence of a current psychotic episode or acute suicidal risk. We recruited women from the diabetes and pregnancy clinic of the Lausanne University Hospital (CHUV) or women that were referred from other antenatal care clinics or obstetricians in private practices. The first patient recruitment started on September 2, 2016, and the last patient one year follow-up visit was October 25, 2021. During the COVID-19 lockdown, we suspended recruitment, testing, and follow-up of participants for three months (until 26.5.2020), and partially for an additional two months (i.e., a total of five months) due to the extension of restriction guidelines in Switzerland. To avoid unforeseen dropouts linked to the second COVID-19 wave, we recruited 11 more patients. Besides this, there were no changes to the protocol after trial commencement (46). Mothers taking part in the study provided their written informed consent for their and their child’s participation in this study.
The usual-care was an active lifestyle and guidelines-based clinical control group. All mothers and their offspring were followed-up according to the guidelines of the American Diabetes Association (ADA), the International Association of the Diabetes and Pregnancy Study Groups (IADPSG) and the Endocrine Society (1, 47, 48) and according to the NICE guidelines regarding mental health (49). They were seen at 24-32 weeks GA either by a physician, or a diabetes-specialist nurse, and followed until birth (see Horsch et al., 2018 for more details (46)). Patients received information on GDM, were counseled about lifestyle changes and optimal gestational weight gain (GWG) and were taught how to perform self-control of blood glucose (50). They had one appointment with a registered dietician to promote favorable glucose controls with individualized dietary advice. Mothers were also advised to reduce sedentary behavior and engage in physical activity according to the Endocrine Society guidelines and received general advice for breastfeeding (47). Mothers were followed up at 6-8 weeks and one year postpartum and were given advice about weight loss and lifestyle behaviors at these time-points. No other specific intervention was delivered regarding the offspring (51).
Complex interventions, such as the MySweetheart trial are characterized by several interacting components, several outcomes, a high degree of flexibility, and the possibility of tailoring the intervention. The Health Action Process Approach (HAPA) was chosen as the theoretical framework for this behavior change intervention (46). The intervention started after the first clinical visit in the diabetes and pregnancy clinic and lasted up to one year postpartum. The design of the intervention components was based on three informal focus group sessions with patients (both pregnant women with GDM and postpartum women) and on feedback from experienced clinicians. The usual-care and intervention groups of the MySweetheart trial have been previously described (46, 52).
Regarding the offspring (secondary aims), mothers received support for breastfeeding, recommendations regarding timing of solid food introduction, consumption of sweetened beverages, parental responsiveness to distress, hunger, satiety and sleeping cues and difficult behavior in the offspring, as well as his/her physical activity needs. Breastfeeding was additionally supported with the help of the midwife at birth and afterwards, as desired (51). Mothers were encouraged to maintain continuous (not necessarily exclusive) breastfeeding up to six months postpartum and were informed about its health benefits at the first interdisciplinary visit between 6-8 weeks postpartum (51). The first additional interdisciplinary visit at 4 months postpartum focused on maternal and offspring diet, breastfeeding, and introduction of solid foods, which was encouraged to take place no earlier than at four months of age, in accordance with the European Society for Paediatric Gastroenterology, Hepathology and Nutrition and Swiss guidelines at the time of protocol production (36, 37). During the second interdisciplinary visit at seven months postpartum, parental regulation of offspring distress or difficulty and self-regulation capacity were reinforced through parental education with the help of a psychologist and the lifestyle coach. This theme, particularly how to recognize and react to hunger and satiety cues and how to soothe babies when in distress, was already discussed in the peer support group workshop during pregnancy (43). During the last interdisciplinary visit at 10 months postpartum and during the second peer support group workshop in the postpartum, mothers were encouraged to increase their offspring’s physical activity (target 180 min/day) and to reduce sedentary behavior, with a particular focus on screen time (43). The peer-support group in the postpartum focused on offspring physical activity, while both encouraged peer exchanges and used different tools and brochures (53, 54)
Following these visits, the lifestyle coach followed up with the goals by summarizing them through a text message sent to the mother. Questions and concerns were discussed during the bimonthly phone calls. Furthermore, co-parents were also invited to each session to reinforce a unified approach for both parents regarding the health goals of their offspring.
At the first visit, maternal anthropometric measures (height (cm), weight (kg)) were measured and pre-pregnancy weight was self-reported. Medical information, sociodemographic variables, and social support of the mother were also collected. HbA1c in pregnancy and the requirement for maternal medical treatment were recorded at the end of the pregnancy. In addition, the type of delivery was collected at the first postpartum visit. Breastfeeding presence (yes/no) was assessed by clinicians and self-reported at 6-8 weeks and 1 year. When clinician report of breastfeeding was missing, we retrieved the information from the participant’s report.
At birth, the offspring’s baseline measures including birth and neonatal outcomes, gestational age, sex, weight, and height were assessed. At 6-8 weeks and at the one-year visits, the offspring’s weight, size, and skinfolds were measured and mothers completed additional self-report questionnaires at all visits. Data collection and outcomes were measured at the diabetes and pregnancy clinic at the Lausanne University Hospital at all time points. Data regarding birth and neonatal outcomes were extracted from medical charts and records. Secondary offspring outcomes also contained cardiometabolic laboratory outcomes in the cord blood of the offspring, but these are not presented here, as we have much fewer infants with this type of data (n=46 of the total 211 subjects) and thus the sample size would have been significantly reduced.
Adverse birth and neonatal outcomes including neonatal hospitalization to the neonatal intensive care unit, hyperbilirubinemia (total serum bilirubin levels equal or above 15 mg/dL (257 μmol/L) (55)), hypoglycemia (capillary or venous glucose value ≤2.5 mmol/l (55)), and preterm delivery (<37 GA) were extracted from the participants medical charts and records.
Birth weight (g) and length (cm) were recorded at birth using calibrated electronic offspring scales (Seca®). Macrosomia was defined as birthweight ≥4000 g. Large-for-gestational-age (LGA) and small-for-gestational-age (SGA) were defined as sex-and GA–specific birth weight >90th and <10th centile, respectively, according to the International Fetal and Newborn Growth Consortium for the 21st Century (INTERGROWTH-21st) guidelines (56). We calculated percentiles and z-scores for length and weight variables at birth according to the Intergrowth 21st newborn size guidelines (56). We measured offspring length (cm) and weight (kg) at 6-8 weeks and at one year and calculated their BMI, and the respective z-scores according to the WHO offspring growth standards anthropometric tool at both time points (57). We used offspring BMI z-scores to classify them as underweight (lower than −2 SD), normal (from −2 SD to < 1 SD), overweight (1 SD to <2 SD) or obese (≥ 2 SD) (58, 59). We measured offspring skinfolds using the Harpenden calipers, on the biceps, the triceps, the subscapular and the iliac crest at 6-8 weeks and one year (60, 61) Each skinfold was measured up to three times at each anatomical site and the mean of all measures was used. Skinfold thickness was calculated as the sum of the mean of the four skinfold measures for the 6-8 postpartum and one-year visits. Total fat mass and fat free mass were estimated from bioimpedance analysis (BIA) (Akern BIA 101) at one year using the formula of Butte et al. (62).
At one year postpartum, mothers completed two questionnaires assessing psychobehavioral outcomes of their offspring. Firstly, the Difficult Child subscale of the Parenting Stress Index short-form assessed the mother’s perception of offspring’s difficulties with self-regulation with 12 items on a 5-point Likert scale ranging from 1 ‘totally agree’ to 5 ‘totally disagree’ (46, 63). Secondly, the Brief Infant Sleep Questionnaire evaluated offspring’s nighttime sleep duration, the number of night wakings, and whether the mother perceived her offspring’s sleep as a problem was assessed with 3 of the 14 items (64).
We estimated the sample size based on the expected differences in primary outcomes of the MySweetheart trial, i.e., differences in maternal weight and depression symptoms between the usual-care and intervention groups (see (46)). Of the n=211 included mothers, n=106 mothers and their offspring were randomized to the usual-care group and n=105 to the intervention group. Details on the number of participants per time point can be found in Figure 1 below and more details on the total number of individuals we have per variable can be found in the first column of Tables 1 and 2, and this is also described in the flow-chart legend. For twin pregnancies (n=3) we decided to compare the data of the first twin to all other offspring outcomes and to assess if there were any significant differences. As there were none, we used the data for the first twin in all pregnancies.
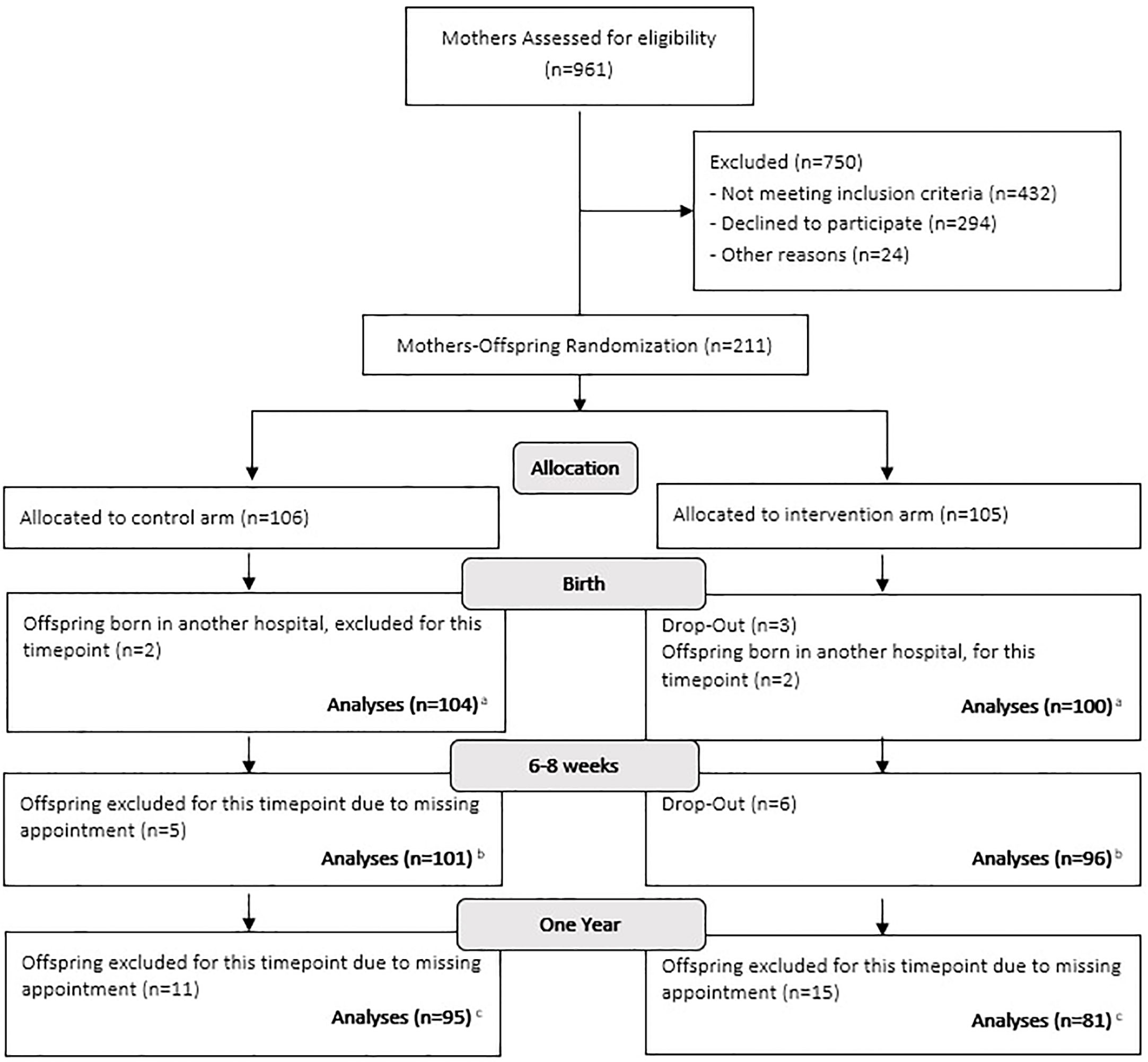
Figure 1 Flow-chart. (A) For the outcomes: hospitalization at birth, hyperbilirubinemia and hypoglycaemia, we were able to retrieve information from the other hospitals to analyse the data on 211 individuals. We were also able to retrieve birth length for 4 individuals, thus length analyses was made on 208 individuals. (B) For two separate individuals one length and one size was missing, bringing the numbers down from 197 to 196 for weight and length and to 195 for BMI and z-scores. Also, we were unable to conduct skinfolds on 7 individuals. (C) One individual did not have his weight and length measured, thus why there are 175 individuals for these measures. We did not measure 13 individuals for their skinfolds and BIA, mostly due to maternal choice which left us with 163 individuals for both measures. Finally, at one year only 153 mothers filled out the Difficult Child Indicator Scale and only 161 mothers filled out the Brief Infant Sleep Questionnaire, in which one mother omitted to answer the total nighttime sleep duration and the number of night wakings.
All statistical analyses were performed with R (65). Descriptive statistics were computed as either number of individuals, means, and standard deviation or as the number of individuals and percentages (%) (Tables 1 and 2). In Table 3, we used linear regressions to evaluate between-group differences (usual-care versus intervention) in the offspring’s anthropometric and psychobehavioral outcomes. We used logistic regressions when evaluating between-group differences in the rates of the offspring’s adverse birth and neonatal outcomes (all), anthropometric binary outcomes, such as presence of macrosomia, SGA, LGA, and maternal perception of the offspring’s sleep as being “a very serious problem”. Finally, we used ordinal logistic regressions to assess between-group differences in the offspring’s BMI z-score category (being under or normal weight versus overweight or obese). We did not correct for multiple testing in any of the analyses due to the multifactorial nature of the intervention. No data was imputed. In the first basic model we did not adjust for any covariates, except for maternal age at first appointment as there was a significant difference between groups for this variable. In the second model, we additionally adjusted for offspring sex and age at the timing of the measure, where appropriate. When these covariates were not appropriate (e.g. no adjustment for age for the outcome: prematurity), we made note of it in the legend of the tables. We also tested, as covariates, breastfeeding at 6-8 weeks (yes/no) for outcomes at 6-8 weeks and breastfeeding at 6-8 weeks (yes/no) and 1 year for outcomes at 1 year. As these analyses revealed no differences in comparison to analyses made without these covariates we removed these covariates and kept our simpler model. A post hoc power analysis was conducted to see whether or not there was sufficient power to detect differences between the usual-care and intervention groups for our most important outcome: offspring weight. The post hoc power analysis revealed a power of 1, 0.99 and 1 to detect differences between groups for weight at birth, 6-8 weeks, and one year respectively, when using model 2. All statistical significance tests were accepted at p<0.05.
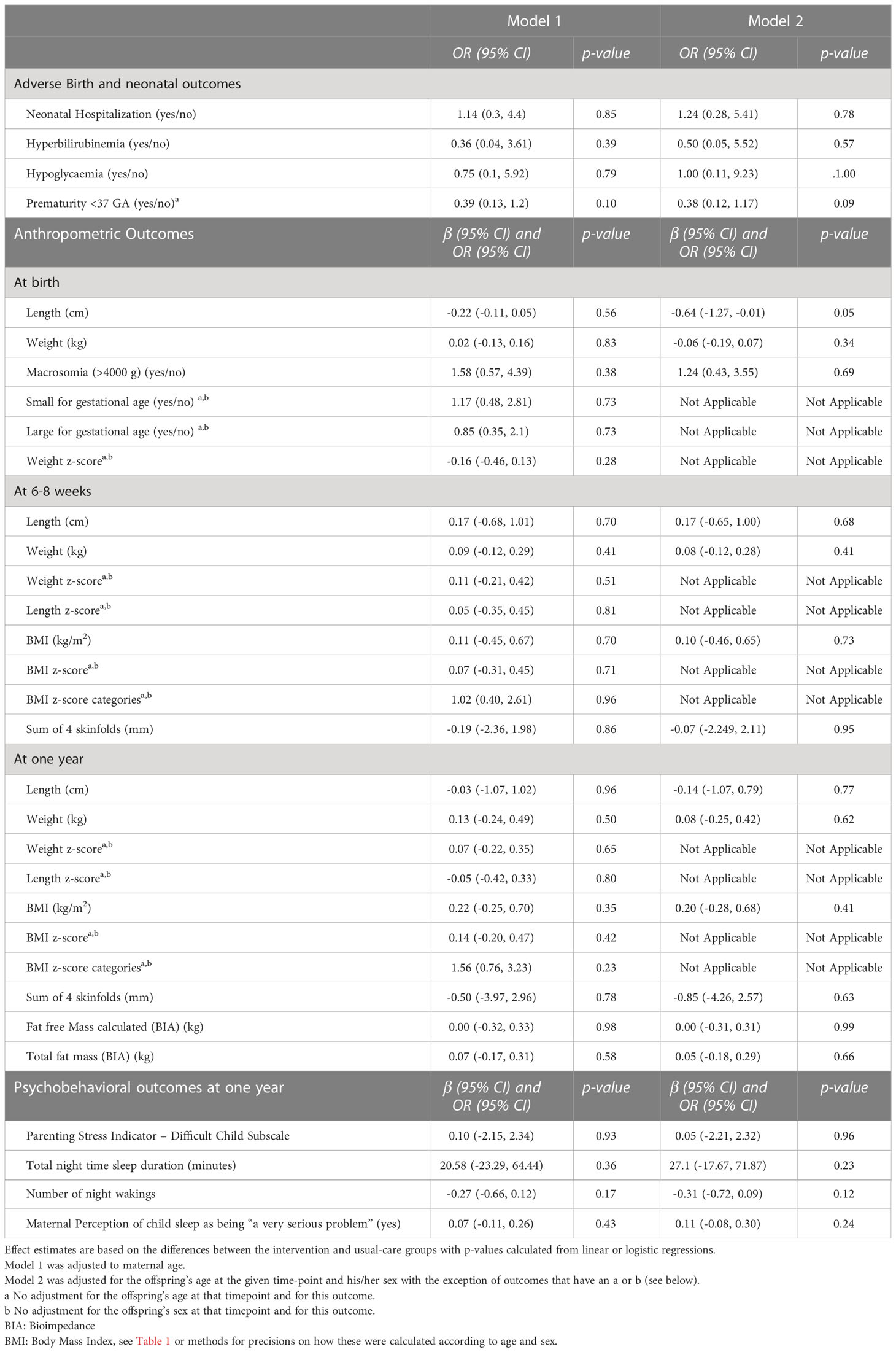
Table 3 Offspring’s between-group differences in birth, anthropometric, and psychobehavioral outcomes.
Baseline maternal characteristics were similar between both groups (Table 1), except for maternal age, which was higher in the intervention group (34.48 and 32.79 years in the intervention and usual-care groups, respectively). Baseline offspring characteristics show there were no between-group differences in infant sex or age at the different time points (Table 2).
The rate of adverse perinatal outcomes was 5% for neonatal hospitalizations, 2% for hyperbilirubinemia and hypoglycemia and 8% for prematurity for all offspring together (Table 2). There were no between-group differences in the rate of neonatal hospitalizations, hyperbilirubinemia or hypoglycemia occurrences (all p ≥0.05, Table 3). Compared to usual-care, the rate of prematurity tended to be lower in the intervention group (p=0.10 in model 1 and 0.09 in model 2).
The prevalence of LGA and SGA in the entire population were both 11%. The weight- and BMI z-scores were close to 0 in both the intervention and usual-care group at birth, 6-8 weeks and 1 year (Table 2). There were no between-group differences in the offspring’s anthropometry at birth, 6-8 weeks or one year (all p≥0.41, Table 3). Specifically, there were no differences in the occurrence of SGA and LGA, at birth, and no differences in weight, BMI, BMI z-scores or measures of adiposity (sum of four skinfold and/or BIA) at birth, 6-8 weeks or one year, except for birth length, which was lower in the intervention group compared to the usual-care group, but only in the adjusted analyses did it reach borderline significance levels (p=0.05).
In the overall offspring population, the mean Parenting Stress Index was 22.38, the mean night-time sleep duration was 639 minutes/night with a mean of 1.22 awakenings/night and 5% of mothers perceived their child’s sleep as “a very serious problem” (Table 2). There were no significant between-group differences in the offspring’s psychobehavioral outcomes (all p ≥0.24, Table 3). Specifically, there were no differences in maternal perception of difficulty with self-regulation (as measured by the Parenting Stress Index), total nighttime sleep duration, number of night wakings and in the rates of maternal perception of the child’s sleep.
To our knowledge, the MySweetheart trial is the first randomized multidimensional lifestyle and psychosocial intervention trial during and after pregnancy in women with GDM also intervening on the offspring up to one year. The intervention did not lead to a significant improvement in most birth and neonatal outcomes, offspring anthropometry, or their psychobehavioral outcomes up to one year compared to an active lifestyle and guidelines-based usual-care comparator. However, adverse birth and neonatal outcomes, increased anthropometric measures (weight, BMI and body fat), and psychobehavioral problems were very low in both groups. No between group differences were found for birth and neonatal outcomes. No between-group differences were observed in weight, BMI, BMI z-scores, sum of four skinfold, fat mass (BIA) at birth, 6-8 weeks or one year, except for birth length, which was -0.64 cm lower in the intervention compared to the usual-care group and was close to reaching statistical significance. Furthermore, there were no differences in maternal perception of self-regulation difficulty, the offspring’s total nighttime sleep duration, number of night waking, or the maternal perception of their child’s sleep as being “a very serious problem”.
Regarding birth and neonatal outcomes, we had very low rates of neonatal hospitalization, hypoglycemia, hyperbilirubinemia, and prematurity in both groups. Prior intervention studies investigating the effect of lifestyle interventions on offspring birth and neonatal outcomes are scarce. A recent retrospective analysis of a cohort using a special program for women, infants, and children (“WIC” program including nutrition education, breastfeeding education and support, referral to prenatal and pediatric health care and other maternal, child health, and human service programs) in women recently diagnosed with GDM showed a reduction in the risk of prematurity in the group of women with GDM who had benefitted from this program (25). This last finding is partially in line with our results, as we did find that the treatment effect on prematurity was close to being significant.
Anthropometric outcomes at birth were close to a healthy control population in the entire GDM group with 11% LGA and 11% SGA (defined as > 90th and < 10th percentile, respectively in an international control population (57). The cause for the reduced length of a mean of 0.64 cm found in model 2 for our intervention group is not entirely clear and has not been previously reported. Potentially, stunting could play a role, but the prevalence of SGA was not increased in the intervention compared to the usual-care group. One trial (n=932) that focused on adherence to Mediterranean diet in women with GDM found higher birthweight percentiles in the intervention group, but similar length percentiles compared to the usual-care group (66). Another intervention study conducted in women with GDM, which focused on chrono-nutrition and sleep hygiene from the time of GDM diagnosis until delivery, showed no differences in offspring anthropometry, particularly LGA, compared to the usual-care group (67). The mean BMI Z-scores of the entire GDM population were close to a healthy control population of the WHO (-0.27 and 0.21 at 6-8 weeks and 1 year) with only 5% being obese (13). Thereby, we did not find any between-group differences in any of the anthropometric outcomes at these two time points. Comparing anthropometric outcomes to other studies beyond birth, a review concluded that treating obese pregnant women through lifestyle interventions consisting of diet and/or physical activity had a limited impact on offspring anthropometry during childhood (26). Other studies even showed an adverse impact of interventions on the offspring’s anthropometry. For instance, the above-mentioned retrospective analysis of the “WIC program” reported that the cohort of women with GDM in the program gave birth to larger offspring than the women not participating (25). All these studies show that intervening on maternal lifestyle behavior has a limited impact on the offspring’s anthropometry.
Regarding psychobehavioral outcomes in the offspring, the fact that we used a subscale of the Parenting Stress Index makes it difficult to compare to prior studies. However, regarding sleep, the mean night-time sleep duration was 639 minutes/night, which is lower than the Swiss mean recommended amount per night, as described in a previous paper in women with GDM (18). Our mean of 1.22 night wakings is similar to prior literature in the general population at the same age (68, 69) and the prevalence of 5% of mothers perceiving their child’s sleep as “a very serious problem” is relatively low. Another systematic review and meta-analysis in the general population demonstrated that difficulty in the offspring’s behavior and self-regulation may be reduced by any type of parenting intervention taking place from pregnancy and up to three years, as these interventions promote infant psychobehavioral development and reduce behavior problems (70).
In general, our results align with prior studies performed in other populations with and without GDM. Importantly, we had a very active guidelines-based control group. Thus, in both groups, the rates of perinatal adverse events were very low and anthropometric outcomes were close to a healthy control group despite women having GDM. Similarly, the number of night wakings were comparable to the general population. Also, rates of breastfeeding were 96% in the intervention and 97% in the usual-care group at 6-8 weeks and 71% in the intervention and 72% in the usual-care group at one year, which is much higher than in the general Swiss population. The fact that the offspring of this study had better outcomes, or outcomes comparable to the general swiss population, and the fact that there were no major improvements regarding outcomes in the offspring from the intervention group suggests that our usual care already follows high standards and allows improvements in offspring outcomes. This absence in differences could also be explained with participation bias, whereby individuals agreeing to take part in the study are individuals who may be more willing to make positive changes in their lifestyle behaviors. Finally, it could be explained by the fact that clinicians seeing these women were aware that a study was taking place and may have delivered higher quality care to women from both groups.
An interesting avenue for future studies seems to be the use of technology rather than face-to-face interventions focusing specifically on infant nutrition and health. As described above by our intervention did use text messaging to summarize behavioral goals for the mother, but less focus was placed on the offspring’s behavioral goals, as our primary aim was to reduce maternal weight and depression symptoms. Thus, the lack of group differences in our study may have been caused by the larger focus placed on maternal outcomes and may have involved too many components and behaviors to adapt in a short period. We suggest that future interventions should focus on technological interventions that target behaviours in the offspring beyond the first year of life, given their higher risk of childhood obesity (8, 71). This is especially challenging in a more high-risk and vulnerable population. Furthermore, once the offspring was born, face-to-face interventions were less frequent, and the mothers less available. Therefore, mother and child were followed up mostly by phone, which could have led to a lower adherence regarding goals for the offspring. Also, breastfeeding rates in both groups were very high and similar, which probably contributed to the favorable anthropometric and psychobehavioral outcomes observed in the offspring of both groups.
This complex lifestyle and psychosocial intervention focusing on many important factors and intervening both on the mother and the offspring in a relatively large sample is an important strength of this study. The limitations of this study first concern the fact that we had an active lifestyle and guidelines-based usual-care group that also received important advice about maternal lifestyle behaviors and breastfeeding, limiting potential intervention effects. This differs compared to other populations such as healthy control or obese women, as women with GDM are regularly followed up and current recommendations also include postpartum visits. Furthermore, the intervention group may have had too many maternal and infant health goals to be able to implement them. We did not measure adherence to the behavioral goals regarding the offspring, such as timing of introduction of solid foods or objective measures of physical activity (accelerometry), as this would have increased the participant burden. However, the mothers were regularly followed up by the coach who reviewed the adherence with the participants. Moreover, the WHO recommends exclusive breastfeeding for at least 6 months. However, in accordance with the local pediatricians and the usual practice in Switzerland, we based ourselves on European and Swiss guidelines to introduce solid foods after at least 4 months of age. This could be considered as a potential limitation. Unfortunately, we did not formally check if women were still breastfeeding at 6 months. In addition, the psychobehavioral outcomes were solely assessed via maternal self-report. As the primary power analyses focused on maternal outcomes, conclusions need to be drawn tentatively and replication of results would be needed.
Knowledge regarding the importance of the transgenerational impact of metabolic diseases has increased in recent years. This is the first interdisciplinary lifestyle and psychosocial pre- and postpartum intervention in women with GDM that also focused on their offspring. It did not lead to a significant improvement in most birth and neonatal outcomes, offspring anthropometry or psychobehavioral outcomes in the offspring up to one year compared to an active lifestyle guidelines-based usual-care group, but the rates of adverse outcomes were very low in both groups and anthropometric and psychobehavioral outcomes were similar to healthy control populations. Prematurity tended to be lower in the intervention group and length at birth was reduced in the intervention group, although, these between-group difference were only close to reaching statistical significance. Also, the clinical significance of the reduction in length at birth remains to be elucidated. There were few adverse outcomes in both groups and no differences between the intervention and the guidelines-and lifestyle-based usual-care group. Thus, we could conclude that following the current guidelines in mental health and lifestyle recommendations in women with GDM stringently, following up and motivating patients regularly might be sufficient, although this should continue far beyond one year postpartum. A future trial to improve offspring outcomes in mothers with GDM might benefit from an intervention starting early in pregnancy, a stronger focus on the offspring behavior and health, as well as on novel, culturally adapted technologies and should continue far beyond one year postpartum.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Human Research Ethics Committee of the Canton de Vaud (study number 2016-00745). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
JP and AH conceived the study, designed the trial, obtained grant funding, and oversaw management of the trial. LG, DQ, JG and SL helped in designing parts of the study and participated in the implementation of the study. LG performed the data analysis with the help of AL, BS and DQ. All authors (LG, DQ, AA, AH, and JP) were involved in the interpretation of data. LG and DQ wrote the draft manuscript. Authors LG, DQ, SS, JG, AA, AH, AL, SL, BS and JP revised the manuscript for important intellectual content and gave final approval for the version to be published. JP and AH supervised all the work. All authors contributed to the article and approved the submitted version.
This study was supported by a project grant from the Swiss National Science Foundation (SNF 32003B_176119 PIs: JP & AH) and by an unrestricted educational grant from Novo Nordisk. The SNF and Novo Nordisk had no role regarding the content of the original data or analyses or in the drafting of this manuscript. AH is a board member of the COST Action CA18211.
Our sincere appreciation goes to our study participants and their children and partners for their time and participation. We thank Deborah Degen, Dominique Stulz and Isabelle Cohen-Salmon who helped with data collection. We would like to thank the clinical team and especially Magali Andrey and Olivier Le Dizès and Seyda Demircan who participated in several intervention sessions and gave helpful input.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 15. management of diabetes in pregnancy: standards of care in diabetes-2023. Diabetes Care (2023) 46(Supplement_1):S254–66. doi: 10.2337/dc23-S015
2. Ryser Rüetschi J, Jornayvaz FR, Rivest R, Huhn EA, Irion O, Boulvain M. Fasting glycaemia to simplify screening for gestational diabetes. BJOG (2016) 123(13):2219–22. doi: 10.1111/1471-0528.13857
3. Wendland EM, Torloni MR, Falavigna M, Trujillo J, Dode MA, Campos MA, et al. Gestational diabetes and pregnancy outcomes - a systematic review of the world health organization (WHO) and the international association of diabetes in pregnancy study groups (IADPSG) diagnostic criteria. BMC Pregnancy Childbirth. (2012) 12(1):23. doi: 10.1186/1471-2393-12-23
4. Xiong X, Saunders LD, Wang FL, Demianczuk NN. Gestational diabetes mellitus: prevalence, risk factors, maternal and infant outcomes. Int J Gynaecol Obstet. (2001) 75(3):221–8. doi: 10.1016/S0020-7292(01)00496-9
5. Subhan FB, Colman I, McCargar L, Bell RC, APrON Study Team. Higher pre-pregnancy BMI and excessive gestational weight gain are risk factors for rapid weight gain in infants. Matern Child Health J (2017) 21(6):1396–407. doi: 10.1007/s10995-016-2246-z
6. Santos S, Voerman E, Amiano P, Barros H, Beilin LJ, Bergström A, et al. Impact of maternal body mass index and gestational weight gain on pregnancy complications: an individual participant data meta-analysis of European, north American and Australian cohorts. BJOG (2019) 126(8):984–95. doi: 10.1111/1471-0528.15661
7. Goldstein RF, Abell SK, Ranasinha S, Misso M, Boyle JA, Black MH, et al. Association of gestational weight gain with maternal and infant outcomes: a systematic review and meta-analysis. JAMA (2017) 317(21):2207–25. doi: 10.1001/jama.2017.3635
8. Woo Baidal JA, Locks LM, Cheng ER, Blake-Lamb TL, Perkins ME, Taveras EM. Risk factors for childhood obesity in the first 1,000 days: a systematic review. Am J Prev Med (2016) 50(6):761–79. doi: 10.1016/j.amepre.2015.11.012
9. Kakaroukas A, Abrahamse-Berkeveld M, Hayes L, McNally RJQ, Berrington JE, van Elburg RM, et al. Early infancy growth, body composition and type of feeding in late and moderate preterms. Pediatr Res (2022) 2022:1–9. doi: 10.1038/s41390-022-02317-z
10. Wasilewska E, Małgorzewicz S, Szczepankiewicz A, Myśliwczyk D, Hennig M, Jassem E, et al. Are obesity and asthma in school-age children still strongly related to breastfeeding in infancy? - a real-life study. Eur Rev Med Pharmacol Sci (2022) 26(5):1658–67. doi: 10.26355/eurrev_202203_28234
11. Suwaydi MA, Wlodek ME, Lai CT, Prosser SA, Geddes DT, Perrella SL. Delayed secretory activation and low milk production in women with gestational diabetes: a case series. BMC Pregnancy Childbirth. (2022) 22(1):350. doi: 10.1186/s12884-022-04685-0
12. Doughty KN, Taylor SN. Barriers and benefits to breastfeeding with gestational diabetes. Semin Perinatol (2021) 45(2):151385. doi: 10.1016/j.semperi.2020.151385
13. de Onis M, Blossner M, World Health Organization. WHO global database on child growth and malnutrition (1997). Available at: https://apps.who.int/iris/bitstream/handle/10665/63750/WHO_NUT_97.4.pdf.
14. Stanczyk JV. Effectiveness of behavior-based counseling for weight loss maintenance: a systematic literature review [Master’s alternative plan paper, Minnesota state university, mankato]. Mankato: Cornerstone: A Collection of Scholarly and Creative Works for Minnesota State University (2021). Available at: https://cornerstone.lib.mnsu.edu/etds/1092/.
15. Wilson CA, Newham J, Rankin J, Ismail K, Simonoff E, Reynolds RM, et al. Is there an increased risk of perinatal mental disorder in women with gestational diabetes? a systematic review and meta-analysis. Diabetic Med (2020) 37(4):602–22. doi: 10.1111/dme.14170
16. Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstetrics gynecology (2005) 106(5 Pt 1):1071–83. doi: 10.1097/01.AOG.0000183597.31630.db
17. Hinkle SN, Buck Louis GM, Rawal S, Zhu Y, Albert PS, Zhang C. A longitudinal study of depression and gestational diabetes in pregnancy and the postpartum period. Diabetologia (2016) 59(12):2594–602. doi: 10.1007/s00125-016-4086-1
18. Gilbert L, Sandoz V, Quansah DY, Puder JJ, Horsch A. Prospective associations between maternal depression and infant sleep in women with gestational diabetes mellitus. Front Psychol (2022) 13. doi: 10.3389/fpsyg.2022.926315
19. Guerin DW, Gottfried AW. Temperamental consequences of infant difficultness. Infant Behav Dev (1994) 17(4):413–21. doi: 10.1016/0163-6383(94)90033-7
20. Daraki V, Roumeliotaki T, Koutra K, Georgiou V, Kampouri M, Kyriklaki A, et al. Effect of parental obesity and gestational diabetes on child neuropsychological and behavioral development at 4 years of age: the rhea mother-child cohort, Crete, Greece. Eur Child Adolesc Psychiatry (2017) 26(6):703–14. doi: 10.1007/s00787-016-0934-2
21. Nomura Y, Marks DJ, Grossman B, Yoon M, Loudon H, Stone J, et al. Exposure to gestational diabetes mellitus and low socioeconomic status: effects on neurocognitive development and risk of attention-deficit/hyperactivity disorder in offspring. Arch Pediatr Adolesc Med (2012) 166(4):337–43. doi: 10.1001/archpediatrics.2011.784
22. Brown J, Alwan NA, West J, Brown S, McKinlay CJ, Farrar D, et al. Lifestyle interventions for the treatment of women with gestational diabetes. Cochrane Database Systematic Rev (2017) 2017(5):1–102. doi: 10.1002/14651858.CD011970.pub2
23. Rowan JA, Rush EC, Plank LD, Lu J, Obolonkin V, Coat S, et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition and metabolic outcomes at 7-9 years of age. BMJ Open Diabetes Res Care (2018) 6(1):e000456. doi: 10.1136/bmjdrc-2017-000456
24. Maldonado LE, Farzan SF, Toledo-Corral CM, Dunton GF, Habre R, Eckel SP, et al. A vegetable, oil, and fruit dietary pattern in late pregnancy is linked to reduced risks of adverse birth outcomes in a predominantly low-income Hispanic and latina pregnancy cohort. J Nutr (2022). doi: 10.1093/jn/nxac209
25. Clark GV, Powell JM, Hersh AR, Valent AM. Association of perinatal outcomes among pregnant patients with gestational diabetes receiving benefits from the special supplemental nutrition program for women, infants, and children. Am J Obstet Gynecol MFM. (2023) 5(1):100750. doi: 10.1016/j.ajogmf.2022.100750
26. Louise J, Poprzeczny AJ, Deussen AR, Vinter C, Tanvig M, Jensen DM, et al. The effects of dietary and lifestyle interventions among pregnant women with overweight or obesity on early childhood outcomes: an individual participant data meta-analysis from randomised trials. BMC Med (2021) 19(1):128. doi: 10.1186/s12916-021-01995-6
27. McDonald SM, Mouro S, Wisseman B, Isler C, DeVente J, Newton E, et al. Influence of prenatal exercise on the relationship between maternal overweight and obesity and select delivery outcomes. Sci Rep (2022) 12(1):17343. doi: 10.1038/s41598-022-22283-0
28. Wardle J, Sanderson S, Guthrie CA, Rapoport L, Plomin R. Parental feeding style and the inter-generational transmission of obesity risk. Obes Res (2002) 10(6):453–62. doi: 10.1038/oby.2002.63
29. Stifter CA, Anzman-Frasca S, Birch LL, Voegtline K. Parent use of food to soothe infant/toddler distress and child weight status. exploratory study. Appetite. (2011) 57(3):693–9. doi: 10.1016/j.appet.2011.08.013
30. Redsell SA, Edmonds B, Swift JA, Siriwardena AN, Weng S, Nathan D, et al. Systematic review of randomised controlled trials of interventions that aim to reduce the risk, either directly or indirectly, of overweight and obesity in infancy and early childhood. Matern Child Nutr (2016) 12(1):24–38. doi: 10.1111/mcn.12184
31. Birch LL, Anzman-Frasca S, Paul IM. Starting early: obesity prevention during infancy. (2012), 81–94. doi: 10.1159/000341300
32. Liu Q, Xia W, Xiong X, Li JX, Li Y, Xu SQ, et al. Associations of gestational diabetes mellitus and excessive gestational weight gain with offspring obesity risk. Curr Med Sci (2022) 42(3):520–9. doi: 10.1007/s11596-022-2547-y
33. Michael K, Ritsuko K. The optimal duration of exclusive breastfeeding a systematic review (2002). Available at: https://apps.who.int/iris/bitstream/handle/10665/67208/WHO_NHD_01.08.pdf.
34. Dugas C, Perron J, Kearney M, Mercier R, Tchernof A, Marc I, et al. Postnatal prevention of childhood obesity in offspring prenatally exposed to gestational diabetes mellitus: where are we now? Obes Facts (2017) 10(4):396–406. doi: 10.1159/000477407
35. Dugas C, Perron J, Marc I, Weisnagel SJ, Robitaille J. Association between early introduction of fruit juice during infancy and childhood consumption of sweet-tasting foods and beverages among children exposed and unexposed to gestational diabetes mellitus in utero. Appetite (2019) 132:190–5. doi: 10.1016/j.appet.2018.08.033
36. Nutrition SSF. L’alimentation du nourrisson durant la premiére année de vie. Berne (2012). Available at: https://www.sge-ssn.ch/media/feuille_d_info_alimentation_du_nourrisson_2012_4.pdf.
37. Fewtrell M, Bronsky J, Campoy C, Domellöf M, Embleton N, Fidler Mis N, et al. Complementary feeding: a position paper by the European society for paediatric gastroenterology, hepatology, and nutrition (ESPGHAN) committee on nutrition. J Pediatr Gastroenterol Nutr (2017) 64(1):119–32. doi: 10.1097/MPG.0000000000001454
38. Paul IM, Williams JS, Anzman-Frasca S, Beiler JS, Makova KD, Marini ME, et al. The intervention nurses start infants growing on healthy trajectories (INSIGHT) study. BMC Pediatr (2014) 14:184. doi: 10.1186/1471-2431-14-184
39. Paul IM, Bartok CJ, Downs DS, Stifter CA, Ventura AK, Birch LL. Opportunities for the primary prevention of obesity during infancy. Adv Pediatr (2009) 56(1):107–33. doi: 10.1016/j.yapd.2009.08.012
40. Savage JS, Birch LL, Marini M, Anzman-Frasca S, Paul IM. Effect of the INSIGHT responsive parenting intervention on rapid infant weight gain and overweight status at age 1 year. JAMA Pediatr (2016) 170(8):742. doi: 10.1001/jamapediatrics.2016.0445
41. Black MM, Aboud FE. Responsive feeding is embedded in a theoretical framework of responsive parenting. J Nutr (2011) 141(3):490–4. doi: 10.3945/jn.110.129973
42. Pérez-Escamilla R, Segura-Pérez S, Lott M. Feeding guidelines for infants and young toddlers. Nutr Today (2017) 52(5):223–31. doi: 10.1097/NT.0000000000000234
43. Tremblay MS, LeBlanc AG, Carson V, Choquette L, Connor Gorber S, Dillman C, et al. Canadian Physical activity guidelines for the early years (aged 0–4 years). Appl Physiology Nutrition Metab (2012) 37(2):345–56. doi: 10.1139/h2012-018
44. Field T. Infant sleep problems and interventions: a review. Infant Behav Dev (2017) 47:40–53. doi: 10.1016/j.infbeh.2017.02.002
45. Cook GW, Benton MG, Akerley W, Mayhew GF, Moehlenkamp C, Raterman D, et al. Structural variation and its potential impact on genome instability: novel discoveries in the EGFR landscape by long-read sequencing. PloS One (2020) 15(1):e0226340. doi: 10.1371/journal.pone.0226340
46. Horsch A, Gilbert L, Lanzi S, Gross J, Kayser B, Vial Y, et al. Improving cardiometabolic and mental health in women with gestational diabetes mellitus and their offspring: study protocol for MySweetHeart trial, a randomised controlled trial. BMJ Open (2018) 8(2):1–18. doi: 10.1136/bmjopen-2017-020462
47. Weinert LS. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care (2010) 33(7):e97–7. doi: 10.2337/dc10-0544
48. Blumer I, Hadar E, Hadden DR, Jovanovič L, Mestman JH, Murad MH, et al. Diabetes and pregnancy: an endocrine society clinical practice guideline. J Clin Endocrinol Metab (2013) 98(11):4227–49. doi: 10.1210/jc.2013-2465
49. National Institute for Health and Care Excellence. Antenatal and postnatal mental health: clinical management and service guidance (2014). Available at: https://www.nice.org.uk/guidance/cg192.
50. Institute of Medicine. Weight gain during pregnancy: reexamining the guidelines. Yaktine A, Rasmussen K, editors. Washington (2009).
52. Quansah DY, Schenk S, Gilbert L, Arhab A, Gross J, Marques-Vidal PM, et al. Intuitive eating behavior, diet quality and metabolic health in the postpartum in women with gestational diabetes. Nutrients (2022) 14(20):4272. doi: 10.3390/nu14204272
53. Paprica. Promotion de l’activite physique au cabinet medical. Available at: https://www.paprica.ch/category/pro_cat/formations-paprica-petite-enfance/.
54. Melissa B, Joannie P. Exercise and have fun with your grandchildren (2012). Available at: http://www.education.gouv.qc.ca/fileadmin/site_web/documents/loisir-sport/ExercisesENp.pdf.
55. Antoniou MC, Gilbert L, Gross J, Rossel JB, Fischer Fumeaux CJ, Vial Y, et al. Potentially modifiable predictors of adverse neonatal and maternal outcomes in pregnancies with gestational diabetes mellitus: can they help for future risk stratification and risk-adapted patient care? BMC Pregnancy Childbirth (2019) 19(1):469. doi: 10.1186/s12884-019-2610-2
56. Papageorghiou AT, Kennedy SH, Salomon LJ, Altman DG, Ohuma EO, Stones W, et al. The INTERGROWTH-21st fetal growth standards: toward the global integration of pregnancy and pediatric care. Am J Obstet Gynecol. (2018) 218(2S):S630–40. doi: 10.1016/j.ajog.2018.01.011
57. World Health Organisation. Child growth standards (2022). Available at: https://www.who.int/tools/child-growth-standards.
58. Woźniak D, Podgórski T, Dobrzyńska M, Przysławski J, Drzymała S, Drzymała-Czyż S. The influence of parents’ nutritional education program on their infants’ metabolic health. Nutrients (2022) 14(13):2671. doi: 10.3390/nu14132671
59. Rolland-Cachera MF. Childhood obesity: current definitions and recommendations for their use. Int J Pediatr Obes (2011) 6(5–6):325–31. doi: 10.3109/17477166.2011.607458
60. Weststrate JA, Deurenberg P. Body composition in children: proposal for a method for calculating body fat percentage from total body density or skinfold-thickness measurements. Am J Clin Nutr (1989) 50(5):1104–15. doi: 10.1093/ajcn/50.5.1104
61. Schmelzle HR, Fusch C. Body fat in neonates and young infants: validation of skinfold thickness versus dual-energy X-ray absorptiometry. Am J Clin Nutr (2002) 76(5):1096–100. doi: 10.1093/ajcn/76.5.1096
62. Butte NF, Hopkinson JM, Wong WW, Smith EO, Ellis KJ. Body composition during the first 2 years of life: an updated reference. Pediatr Res (2000) 47(5):578–85. doi: 10.1203/00006450-200005000-00004
63. Singer LT, Salvator A, Guo S, Collin M, Lilien L, Baley J. Maternal psychological distress and parenting stress after the birth of a very low-birth-weight infant. JAMA (1999) 281(9):799–805. doi: 10.1001/jama.281.9.799
64. Mindell JA, Lee C, Sadeh A. Young child and maternal sleep in the middle East. Sleep Med (2017) 32:75–82. doi: 10.1016/j.sleep.2016.11.011
65. R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing (2021). Available at: https://www.R-project.org/.
66. de la Torre NG, Assaf-Balut C, Jiménez Varas I, del Valle L, Durán A, Fuentes M, et al. Effectiveness of following Mediterranean diet recommendations in the real world in the incidence of gestational diabetes mellitus (GDM) and adverse maternal-foetal outcomes: a prospective, universal, interventional study with a single group. the St carlos study. Nutrients (2019) 11(6):1–15. doi: 10.3390/nu11061210
67. Messika A, Toledano Y, Hadar E, Shmuel E, Tauman R, Shamir R, et al. Relationship among chrononutrition, sleep, and glycemic control in women with gestational diabetes mellitus: a randomized controlled trial. Am J Obstet Gynecol MFM. (2022) 4(5):100660. doi: 10.1016/j.ajogmf.2022.100660
68. Scher A. A longitudinal study of night waking in the first year. Child Care Health Dev (1991) 17(5):295–302. doi: 10.1111/j.1365-2214.1991.tb00699.x
69. Scher A, Blumberg O. Night waking among 1-year olds: a study of maternal separation anxiety. Child Care Health Dev (1999) 25(5):323–34. doi: 10.1046/j.1365-2214.1999.00099.x
70. Jeong J, Franchett EE, Ramos de Oliveira CV, Rehmani K, Yousafzai AK. Parenting interventions to promote early child development in the first three years of life: a global systematic review and meta-analysis. PloS Med (2021) 18(5):e1003602. doi: 10.1371/journal.pmed.1003602
Keywords: sleep, temperament, weight, fat mass, skinfold, prematurity, hypoglycemia, BMI - body mass index
Citation: Gilbert L, Quansah DY, Arhab A, Schenk S, Gross J, Lanzi S, Stuijfzand B, Lacroix A, Horsch A, Puder JJ and MySweetheart Research group (2023) Effect of the MySweetheart randomized controlled trial on birth, anthropometric and psychobehavioral outcomes in offspring of women with GDM. Front. Endocrinol. 14:1148426. doi: 10.3389/fendo.2023.1148426
Received: 20 January 2023; Accepted: 22 May 2023;
Published: 07 June 2023.
Edited by:
Jian-Rong He, Guangzhou Medical University, ChinaReviewed by:
Karen L. Lindsay, University of California, Irvine, United StatesCopyright © 2023 Gilbert, Quansah, Arhab, Schenk, Gross, Lanzi, Stuijfzand, Lacroix, Horsch, Puder and MySweetheart Research group. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Antje Horsch, YW50amUuaG9yc2NoQGNodXYuY2g=; Jardena J. Puder, amFyZGVuYS5wdWRlckBjaHV2LmNo
†These authors share first authorship
‡These authors share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.