- 1Department of Endocrinology, Shenzhen Hospital, Southern Medical University, Shenzhen, China
- 2The Third School of Clinical Medicine, Southern Medical University, Guangzhou, China
- 3Department of Huiqiao Medical Centre, Nanfang Hospital, Southern Medical University, Guangzhou, China
Aims: To assess the association of rectus femoris mass index (RFMI) with diabetic peripheral neuropathy (DPN) in individuals with type 2 diabetes mellitus (T2DM).
Methods: Totally 948 T2DM cases were enrolled. Nerve conduction parameters, quantitative sensory threshold and rectus femoris cross-sectional area (RFCSA) were obtained, and rectus femoris mass index (RFMI=RFCSA/height2) was derived. The patients were assigned to four groups based on interquartile spacing of RFMI.
Results: Motor/sensory nerve amplitude and conduction velocity (CV) were significantly lower in the low-level RFMI groups (all P<0.05). RFMI was positively associated with mean motor/sensory nerve amplitude and CV (both P<0.05). T2DM duration above 10 years and RFMI below 2.37cm²/m² had significant associations with DPN (both P<0.001). Receiver operating characteristic (ROC) curve analysis demonstrated cutoffs for T2DM duration and RFMI of 7 years and 2.2 cm²/m², respectively (AUC=0.75, 95% CI: 0.72-0.79; sensitivity, 68.4%; specificity, 66.8%).
Conclusion: DPN is significantly associated with reduced RFMI in T2DM patients. Decreased muscle mass seems to be associated with motor/sensory nerve amplitude and CV. RFMI combined with T2DM duration may represent a potent tool for predicting DPN occurrence in T2DM cases.
Clinical trial registration: http://www.chictr.org.cn, identifier ChiCTR2100049150.
Introduction
Diabetic peripheral neuropathy (DPN) constitutes the commonest chronic complication of diabetes (1, 2), which affects half of patients with type 2 diabetes mellitus (T2DM) (2) and represents a major risk factor for lower limb amputation and foot ulcers (3, 4). DPN has a high incidence and insidiously onset, but its pathological severity often does not match the clinical symptoms. Totally 30-40% of patients show no clinical symptoms for a long time (5). Currently, clinical treatment strategies for DPN are limited, and efficacy is not satisfactory. Early diagnosis, screening and control of the risk factors associated with DPN is of critical clinical importance.
Sarcopenia features severely decreased skeletal muscle mass and strength (6), which is commonly found in T2DM patients. Many studies demonstrated sarcopenia prevalence is markedly elevated in T2DM cases compared with non-diabetics (7–9). DPN is associated with sarcopenia, which can result in accelerated muscle atrophy of distal lower extremity (10) and might independently predict sarcopenia (11, 12).
Measurement of muscle mass is a key factor in early screening of sarcopenia (13). Ultrasound measurement of the rectus femoris has been widely used to evaluate total body skeletal muscle mass. Mueller and collaborators demonstrated the rectus femoris muscle cross-sectional area (RFCSA) determined by ultrasound might be a quick and convenient indicator of sarcopenia (14). During muscle mass quantitation, adjustment should be made based on body shape. Height², body weight and BMI have been used (15). Height² correction of skeletal muscle mass index was first performed by Baumgartner and colleagues (16). Since then, multiple reports have utilized this index to define sarcopenia (17–20). In this study, RFCSA measured by ultrasound adjusted by height² was defined as the rectus femoris mass index (RFMI). The aim of this work was to examine the association of RFMI with DPN and to clarify whether RFMI and other indexes might predict the progression of DPN.
Materials and methods
Study design and patients
This cross-sectional study had approval from the Medical Ethics Committee, ShenZhen Hospital, Southern Medical University (NYSZYYEC20200030). Totally 948 T2DM patients admitted to the Department of Endocrinology, Shenzhen Hospital, Southern Medical University in April-August 2022 were enrolled. T2DM diagnosis was based on the 1999 WHO diagnostic criteria (21). The patients underwent DPN screening. Diagnosis of DPN was carried out as reported in a previous work (22): (1) abnormality in at least one nerve conduction index in ≥2 peripheral nerves; (2) at least two quantitative sensory threshold abnormalities. DPN was diagnosed when the above two criteria were met. Exclusion criteria were: (1) critical illness accompanied by ketoacidosis, hypertonic non-ketone coma, uremia and malignant tumors; (2) further neuropathy-associated parameters such as family-related factors, alcohol, nutrition, poisoning and drug addiction; (3) diseases that affect muscle metabolism, e.g., rheumatoid arthritis, poliomyelitis, Parkinson’s disease and cerebral infarction. (4) pregnancy. Signed informed consent was provided by each patient. This study has been registered in the Chinese Clinical Trials Register (ChiCTR2100049150).
Data collection
Medical history, gender, age, height, weight, duration of diabetes and other basic indexes were collected for all patients. Body mass index (BMI) was derived as weight (kg)/height2 (m2). Systolic (SBP) and diastolic (DBP) blood pressure measurements were carried out. Laboratory data, including serum creatinine (Scr), total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), uric acid (UA), serum cystatin C (CysC), blood urea nitrogen (BUN), fasting plasma glucose (FPG), fasting C-peptide (FCP) and glycated hemoglobin (HbA1c) levels were assessed upon fasting for 8 h. 24-h urinary albumin excretion rate (UAER) was measured and recorded.
Muscle ultrasound examination
RFCSA was measured on both sides by ultrasound (Philips Ultrasound, WA, USA). All measurements were taken by a single ultrasound physician. Supine-positioned patients were placed with the upper body raised by 30°, legs straight and muscles relaxed. Totally 60% of the distance from the anterior superior iliac spine to the patella’s upper edge was determined (14). An ultrasound probe was positioned perpendicularly to the thigh plane along this point to obtain a transverse image of the radio frequency. RFCSA was measured by the planar measurement technology provided by the vendor. The mean of the left and right RFCSAs was considered the final RFCSA and used for statistical analysis. RFMI (cm²/m²) was derived as RFCSA (cm²)/height² (m²).
Nerve conduction studies (NCS)
NCS were carried out with Viking Quest. Amplitudes and conduction velocities (CVs) for the median, ulnar, tibial, and peroneal complex muscle action potential (CMAP), and sensory nerve action potential (SNAP) for the median, ulnar, common peroneal, and sural nerves were measured. Mean motor nerve amplitude was derived as: Amplitude motor nerve = (Amplitude median nerve M + Amplitude ulnar nerve M + Amplitude tibial nerve M + Amplitude peroneal nerve M)/4. Mean values for motor CV, sensory amplitude and sensory CV were determined similarly.
Quantitative sensory tests (QST)
QST include the inspection of vibration perception threshold (VPT), warm threshold (WT) and cold threshold (CT). VPT, WT and CT were tested by the limit method in a standardized fashion (23). Tests were performed thrice and values were averaged. The highest value in either limb was further examined. Data were compared to the available references.
All measurements of NCS and QST parameters were performed by a single neurological technician.
Statistical analysis
Data were analyzed with SPSS 24.0 (SPSS, USA). Normally and skewedly distributed continuous data were presented by mean ± standard deviation (SD) and median with interquartile range (IQR), respectively. Between-group comparisons utilized the Student’s t test. Multiple group comparisons applied ANOVA and the Kruskal–Wallis test for data with normal and skewed distributions, respectively. Categorical data were analyzed by χ2 test. Pearson’s correlation analysis was carried out to analyze the associations of RFMI with nerve conduction parameters (mean motor or sensory nerve amplitude and CV). Multivariable logistic regression was used to analyze associations of clinical indicators with DPN. Receiver-operating characteristic (ROC) analysis was carried out to determine the optimal cutoffs of diabetes duration and RFMI for detecting DPN. P<0.05 indicated statistical significance.
Results
Baseline patient features
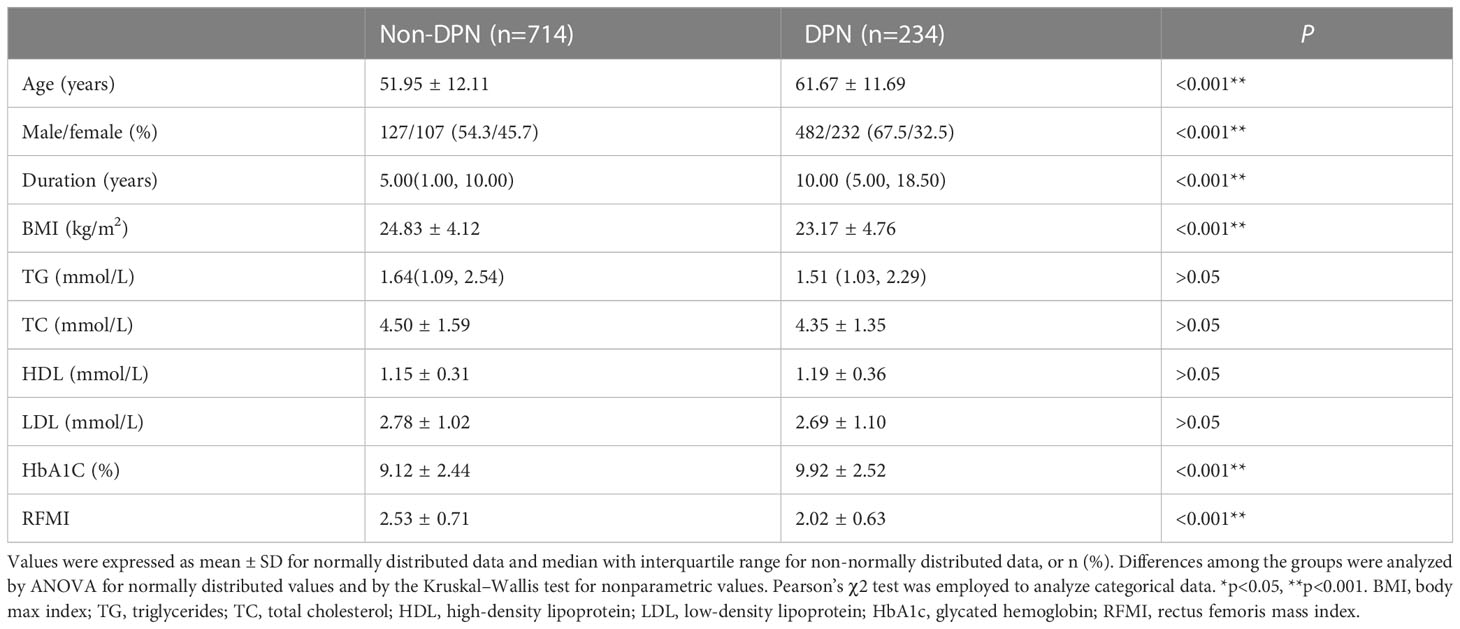
Totally 948 diabetics aged 54.35 ± 12.71 years were included and assigned to the DPN (n=234) and non-DPN (n=714) groups (Table 1). DPN cases accounted for 24.7% of the overall study population. Age, gender, diabetes duration, BMI, HbA1c and RFMI were markedly different between the DPN and non-DPN groups (all P<0.001). Compared with the non-DPN group, the DPN group had elevated age, longer diabetes duration, lower BMI, higher HbA1c and lower RFMI (all P<0.001). The proportion of male in DPN group was higher compared with non-DPN group.
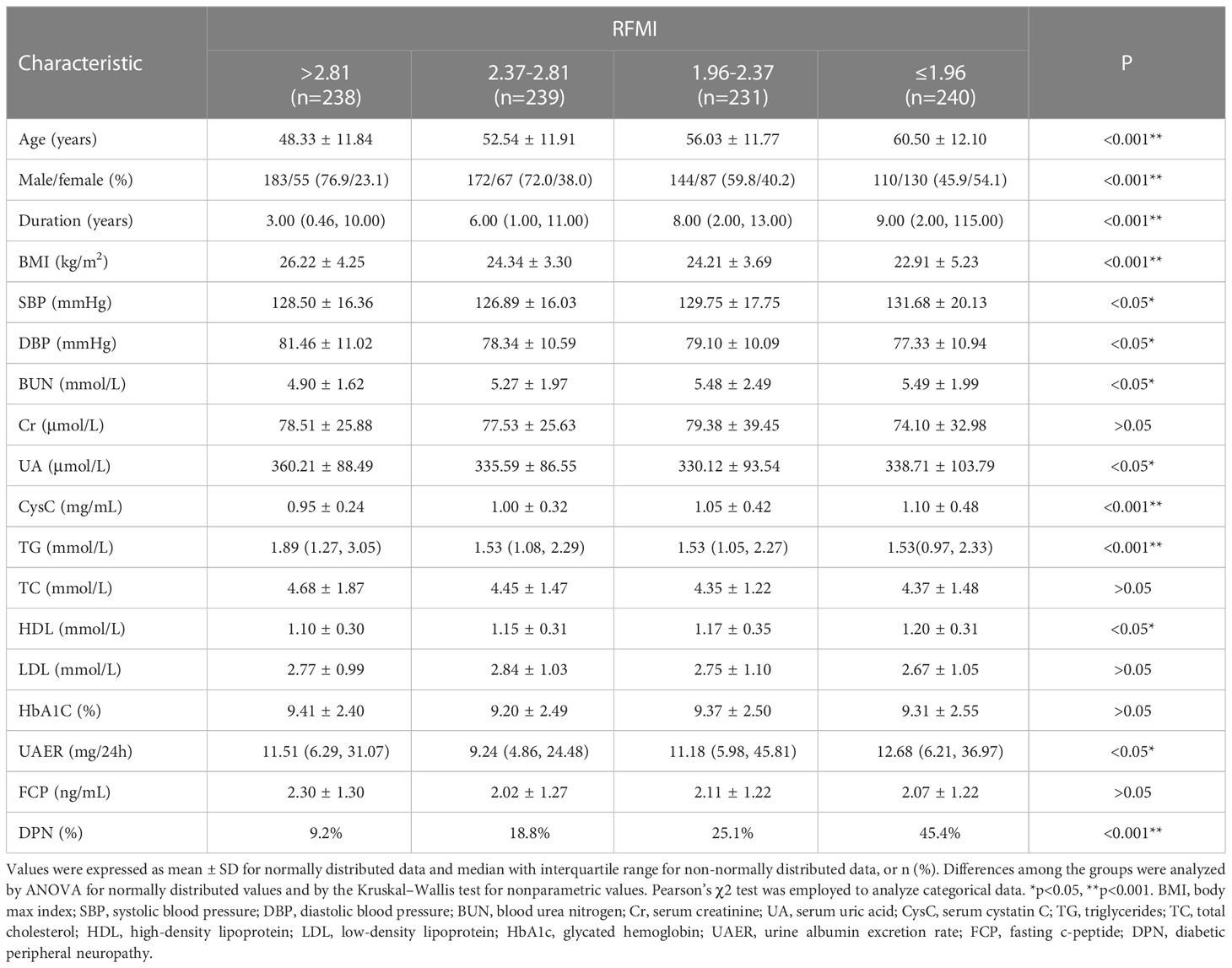
In addition, patients were categorized according to RFMI by interquartile range at the cut-off points of 1.96, 2.37 and 2.81 cm²/m². Patient features in various groups are shown in Table 2. There were significant differences in age (P<0.001), gender(P<0.001), diabetes duration (P<0.001), BMI (P<0.001), SBP (P<0.05), DBP (P<0.05), BUN (P<0.05), UA (P<0.05), CysC (P<0.001), TG (P<0.001), HDL (P<0.05), UAER (P<0.05) and DPN (P<0.001) among the groups. Age, diabetes duration, BUN, CysC, HDL and DPN increased with decreasing RFMI(P<0.05). BMI and TG declined with decreasing RFMI (both P<0.001).
NCS parameters in various groups based on RFMI
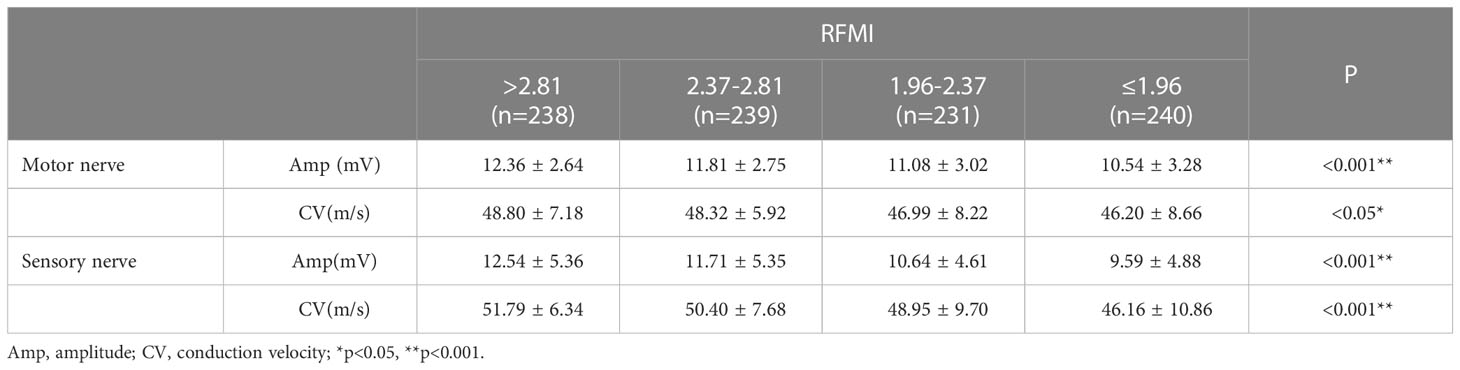
NCS indexes in various groups based on RFMI are shown in Table 3. Individuals with lower RFMI had significantly impaired NCS indexes, e.g., mean motor nerve amplitude (P<0.001), motor nerve CV (P<0.05), sensory nerve amplitude (P<0.001) and sensory nerve CV (P<0.001).
Associations of NCS indexes with RFMI in T2DM
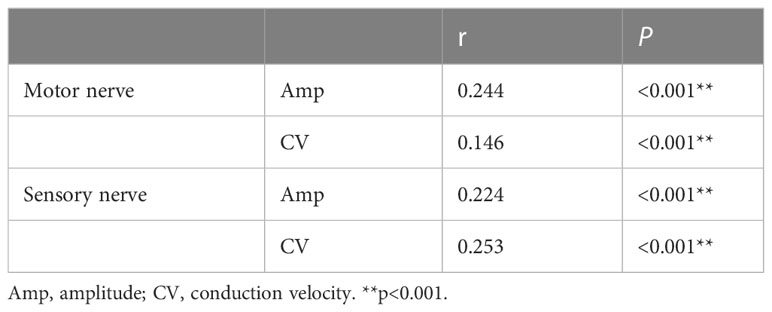
Pearson’s correlation analysis of RFMI and mean amplitudes/CVs of motor/sensory nerves was carried out, respectively. RFMI had significant positive correlations with mean motor nerve amplitude (r=0.244), motor nerve CV (r=0.146), sensory nerve amplitude (r=0.224) and sensory nerve CV (r=0.253), as shown in Table 4 (all P<0.001).
Risk factors for DPN
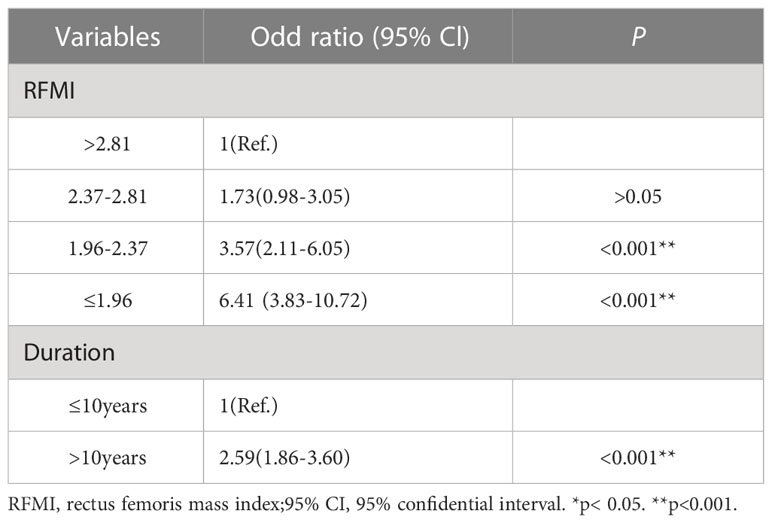
For logistic regression analysis, continuous variables underwent transformation into hierarchical variates based on the following intervals: duration of diabetes (≤10 years and > 10 years), BMI (≤24 kg/m2, 24–28 kg/m2 and >28 kg/m2), HbA1c (≤7% and >7%), RFMI (>2.81 cm²/m², 2.37-2.81 cm²/m², 1.96-2.37 cm²/m² and ≤1.96 cm²/m²).
Multivariable logistic regression analysis was carried out to determine risk factors for DPN. Patient features such as diabetes duration, BMI, HbA1c and RFMI were transformed into qualitative data and used as independent variables together with gender.
Diabetes duration above 10 years (OR=2.59, 95% CI1.86-3.60; P<0.001) independently predicted DPN. Additionally, low RFMI values of 1.96-2.37 cm²/m² (OR=3.57, 95% CI2.11-6.05; P<0.001) and ≤1.96 cm²/m² (OR=6.41, 95% CI3.83-10.72; P<0.001) increased the risk of DPN compared with higher levels (>2.81 cm²/m²). These data are summarized in Table 5.
Thus, ROC analysis was performed to assess the optimal cutoffs of T2DM duration and RFMI used jointly to detect DPN. Another multivariable logistic regression analysis was carried out with DPN as a dependent variate and T2DM duration and RFMI as independent variates. This approach enabled the assessment of the T2DM duration/RFMI combination for its predictive potential in DPN.
The cutoffs for T2DM duration and RFMI were 7 years and 2.2 cm²/m², respectively; based on these values an AUC of 0.75 (95% CI: 0.72–0.79, P<0.001) was obtained, and sensitivity and specificity of 68.4% and 66.8%, respectively (Figure 1).
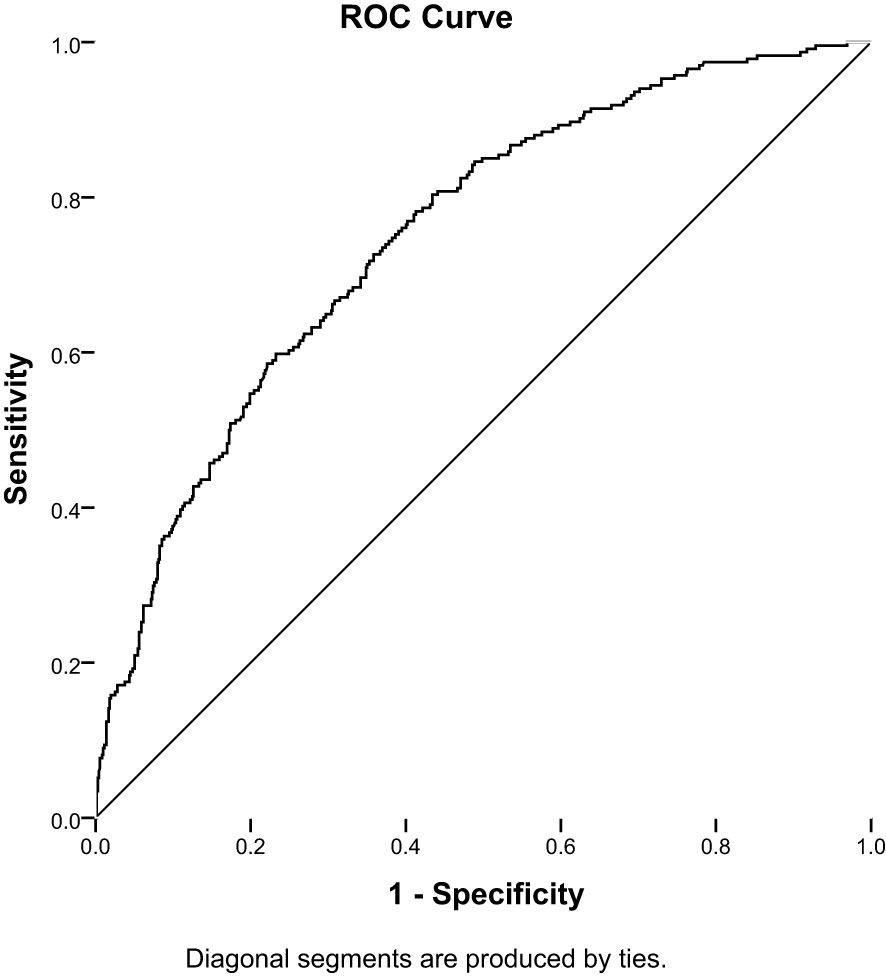
Figure 1 Receiver-operating characteristic (ROC) analysis of the duration of diabetes combined RFMI to predict DPN in T2DM patients (AUC = 0.75; 95% CI: 0.72–0.79; sensitivity,68.4%; specificity, 66.8%, P< 0.001).
Discussion
In this study, low RFMI was found to be associated with DPN in T2DM patients, corroborating previous reports. Henning Andersen and colleagues showed that diabetics have decreased lower limb muscle volume, which has a tight association with the degree of neuropathy (24). A systematic review and meta-analysis reported a significant correlation between sarcopenia and DPN (25). Christer SA and colleagues considered that impaired nerve function leads to denervation and muscle strength decrease in neuropathy cases (26). This work also showed age, gender, diabetes duration, BMI, BP control, renal function, TG and HDL were significantly different among groups categorized by RFMI. Anna Izzo and collaborators demonstrated sarcopenia has significant associations with age, gender, BMI, diabetes duration, glycemic control, microvascular/macrovascular complications, nutritional status and the intake of glucose-lowering products in T2DM patients (9).
Additionally, both the amplitude and CV of sensory/motor nerves declined with decreasing RFMI. Furthermore, RFMI and NCS indexes were positively correlated. These results showed that both demyelination and axonal degeneration of sensory/motor nerves are tightly correlated with sarcopenia in diabetics. Giorgio Orlando and colleagues showed that DPN affects the muscle by impairing motor nerve conduction (27). Andreassen and co-workers showed that DPN induces muscle atrophy and weakness via muscle denervation resulting from motor axon loss (10). Andersen and collaborators showed that muscle mass decline is associated with DPN severity and is more prominent distally (28). In the other hand, Zhang and colleagues showed elevated muscle mass improves the partial sensory/motor nerve conduction velocity (29). Nevertheless, the relationship between DPN and sarcopenia remains undefined.
Besides RFMI, risk factors for DPN also encompass diabetes duration based on logistic regression, consistent with previous findings (30, 31). The prevalence of DPN increases with diabetes duration, and studies have shown the risk of DPN shows a 7% increase with each additional year of diabetes course (32). A further study revealed DPN prevalence may reach 50% in patients with T2DM lasting more than 25 years (33). In this study, DPN cases showed elevated HbA1C compared with the non-DPN group (Table 1; P<0.001); however, in logistic regression, risk factors for DPN did not include HbA1C, which may be related to the fact that HbA1C only reflects the average blood glucose levels of patients in the first three months, which is not enough to reflect long-term blood glucose levels. The effect of gender on DPN is still unknown and most studies have not highlighted gender differences in DPN (34). Male diabetics were found to be more prone to DPN in some studies (35, 36). In the present study, the DPN group had a higher proportion of male, but in the regression analysis, there was no significant difference in gender between the two groups.
DPN incidence is elevated in diabetics, also representing a major cause of diabetes disability and even death (37). Sarcopenia also constitutes an important T2DM complication, and has recently attracted growing attention (13, 38). It increases the odds of adverse outcomes such as falls, frailty and death (39). According to available findings, DPN is correlated with muscle mass decrease, but a causal relationship between the two factors is not entirely clear. Meanwhile, no treatment slowing DPN development is currently available (40). Previous studies have shown that resistance exercise (41), endurance exercise and aerobic exercise (42) can effectively increase muscle mass in older adults. However, whether increasing muscle mass could delay the occurrence of DPN is unknown. Therefore, to avoid their interaction that may aggravate adverse effects in patients, it is recommended to conduct muscle mass measurement and DPN screening in early T2DM, and to intervene early in case of any abnormality.
In this study, ultrasound was utilized to evaluate muscle mass as a simple, non-radiative and highly operable tool, which can be easily applied in clinical and large-scale studies. Nevertheless, this study had some limitations. First, with a cross-sectional design, a causal relationship between RFMI and DPN could not be determined. Secondly, physical activity, which may be associated with muscle mass, was not assessed.
Conclusion
Overall, low RFMI and DPN are significantly associated. This suggests T2DM duration and RFMI may help predict DPN prevalence. Specifically, patients with diabetes duration greater than 10 years and RFMI below 2.2 cm²/m² are closely associated with DPN.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Medical Ethics Committee, ShenZhen Hospital, Southern Medical University (NYSZYYEC20200030). The patients/participants provided their written informed consent to participate in this study.
Author contributions
LW and XPL carried out the clinical studies, conducted the statistical analyses and wrote the first draft of the manuscript. HH and YW were involved in the interpretation of data. XXL and XZ contributed to the acquisition of data. LX designed the study, helped to review the manuscript and is the guarantor of this work. All authors edited, reviewed, and approved the final version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82270895), the Science and Technology Planning Project of Shenzhen Municipality (No. JCYJ20210324130204011 and No. JCYJ20220530154205012), the Young Scientific Talent Research Project of China Endocrinology and Metabolism (No. 2021-N-03) and the key Discipline Construction Fund of Shenzhen Hospital, Southern Medical University (No. ZDXKKYTS0025).
Acknowledgments
We thank all the participants in the study. We are indebted to our colleagues at the Department of Endocrinology in Shenzhen Hospital, Southern Medical University for their help in facilitating this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Albers JW, Pop-Busui R. Diabetic neuropathy: mechanisms, emerging treatments, and subtypes. Curr Neurol Neurosci Rep (2014) 14(8):473. doi: 10.1007/s11910-014-0473-5
2. Pop-Busui R, Boulton AJ, Feldman EL, Bril V, Freeman R, Malik RA, et al. Diabetic neuropathy: a position statement by the American diabetes association. Diabetes Care (2017) 40(1):136–54. doi: 10.2337/dc16-2042
3. Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet (2005) 366(9498):1719–24. doi: 10.1016/S0140-6736(05)67698-2
4. Malik RA. Which test for diagnosing early human diabetic neuropathy? Diabetes (2014) 63(7):2206–8. doi: 10.2337/db14-0492
5. Selvarajah D, Kar D, Khunti K, Davies MJ, Scott AR, Walker J, et al. Diabetic peripheral neuropathy: advances in diagnosis and strategies for screening and early intervention. Lancet Diabetes Endocrinol (2019) 7(12):938–48. doi: 10.1016/S2213-8587(19)30081-6
6. Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian working group for sarcopenia. J Am Med Dir Assoc (2014) 15(2):95–101. doi: 10.1016/j.jamda.2013.11.025
7. Veronese N, Stubbs B, Punzi L, Soysal P, Incalzi RA, Saller A, et al. Effect of nutritional supplementations on physical performance and muscle strength parameters in older people: a systematic review and meta-analysis. Ageing Res Rev (2019) 51:48–54. doi: 10.1016/j.arr.2019.02.005
8. Anagnostis P, Gkekas NK, Achilla C, Pananastasiou G, Taouxidou P, Mitsiou M, et al. Type 2 diabetes mellitus is associated with increased risk of sarcopenia: a systematic review and meta-analysis. Calcif Tissue Int (2020) 107(5):453–63. doi: 10.1007/s00223-020-00742-y
9. Izzo A, Massimino E, Riccardi G, Della Pepa G. A narrative review on sarcopenia in type 2 diabetes mellitus: prevalence and associated factors. Nutrients (2021) 13(1):183. doi: 10.3390/nu13010183
10. Andreassen CS, Jakobsen J, Ringgaard S, Ejskjaer N, Andersen H. Accelerated atrophy of lower leg and foot muscles–a follow-up study of long-term diabetic polyneuropathy using magnetic resonance imaging (MRI). Diabetologia (2009) 52(6):1182–91. doi: 10.1007/s00125-009-1320-0
11. Yang Q, Zhang Y, Zeng Q, Yang C, Shi J, Zhang C, et al. Correlation between diabetic peripheral neuropathy and sarcopenia in patients with type 2 diabetes mellitus and diabetic foot disease: a cross-sectional study. Diabetes Metab Syndr Obes (2020) 13:377–86. doi: 10.2147/DMSO.S237362
12. Oh TJ, Song Y, Moon JH, Choi SH, Jang HC. Diabetic peripheral neuropathy as a risk factor for sarcopenia. Ann Geriatr Med Res (2019) 23(4):170–5. doi: 10.4235/agmr.19.0039
13. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing (2010) 39(4):412–23. doi: 10.1093/ageing/afq034
14. Mueller N, Murthy S, Tainter CR, Lee J, Riddell K, Fintelmann FJ, et al. Can sarcopenia quantified by ultrasound of the rectus femoris muscle predict adverse outcome of surgical intensive care unit patients as well as frailty? a prospective, observational cohort study. Ann Surg (2016) 264(6):1116–24. doi: 10.1097/SLA.0000000000001546
15. Kim KM, Jang HC, Lim S. Differences among skeletal muscle mass indices derived from height-, weight-, and body mass index-adjusted models in assessing sarcopenia. Korean J Intern Med (2016) 31(4):643–50. doi: 10.3904/kjim.2016.015
16. Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in new Mexico. Am J Epidemiol (1998) 147(8):755–63. doi: 10.1093/oxfordjournals.aje.a009520
17. Sanada K, Miyachi M, Tanimoto M, Yamamoto K, Murakami H, Okumura S, et al. A cross-sectional study of sarcopenia in Japanese men and women: reference values and association with cardiovascular risk factors. Eur J Appl Physiol (2010) 110(1):57–65. doi: 10.1007/s00421-010-1473-z
18. Frisoli A Jr, Chaves PH, Ingham SJ, Fried LP. Severe osteopenia and osteoporosis, sarcopenia, and frailty status in community-dwelling older women: results from the women's health and aging study (WHAS) II. Bone (2011) 48(4):952–7. doi: 10.1016/j.bone.2010.12.025
19. Looker AC. Dysmobility syndrome and mortality risk in US men and women age 50 years and older. Osteoporos Int (2015) 26(1):93–102. doi: 10.1007/s00198-014-2904-1
20. Visser M, Kritchevsky SB, Goodpaster BH, Newman AB, Nevitt M, Stamm E, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc (2002) 50(5):897–904. doi: 10.1046/j.1532-5415.2002.50217.x
21. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabetes Med (1998) 15(7):539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539
22. Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care (2010) 33(10):2285–93. doi: 10.2337/dc10-1303
23. Mücke M, Cuhls H, Radbruch L, Baron R, Maier C, Tölle T, et al. Quantitative sensory testing (QST). English version. Schmerz (2021) 35(Suppl 3):153–60. doi: 10.1007/s00482-015-0093-2
24. Andersen H, Gjerstad MD, Jakobsen J. Atrophy of foot muscles: a measure of diabetic neuropathy. Diabetes Care (2004) 27(10):2382–5. doi: 10.2337/diacare.27.10.2382
25. Wannarong T, Sukpornchairak P, Naweera W, Geiger CD, Ungprasert P. Association between diabetic peripheral neuropathy and sarcopenia: a systematic review and meta-analysis. Geriatr Gerontol Int (2022) 22(9):785–9. doi: 10.1111/ggi.14462
26. Andreassen CS, Jakobsen J, Andersen H. Muscle weakness: a progressive late complication in diabetic distal symmetric polyneuropathy. Diabetes (2006) 55(3):806–12. doi: 10.2337/diabetes.55.03.06.db05-1237
27. Orlando G, Balducci S, Bazzucchi I, Pugliese G, Sacchetti M. Neuromuscular dysfunction in type 2 diabetes: underlying mechanisms and effect of resistance training. Diabetes Metab Res Rev (2016) 32(1):40–50. doi: 10.1002/dmrr.2658
28. Andersen H, Poulsen PL, Mogensen CE, Jakobsen J. Isokinetic muscle strength in long-term IDDM patients in relation to diabetic complications. Diabetes (1996) 45(4):440–5. doi: 10.2337/diab.45.4.440
29. Zhang Y, Shen X, He L, Zhao F, Yan S. Association of sarcopenia and muscle mass with both peripheral neuropathy and nerve function in patients with type 2 diabetes. Diabetes Res Clin Pract (2020) 162:108096. doi: 10.1016/j.diabres.2020.108096
30. Darivemula S, Nagoor K, Patan SK, Reddy NB, Deepthi CS, Chittooru CS. Prevalence and its associated determinants of diabetic peripheral neuropathy (DPN) in individuals having type-2 diabetes mellitus in rural south India. Indian J Community Med (2019) 44(2):88–91. doi: 10.4103/ijcm.IJCM_207_18
31. Lin X, Xu L, Zhao D, Luo Z, Pan S. Correlation between serum uric acid and diabetic peripheral neuropathy in T2DM patients. J Neurol Sci (2018) 385:78–82. doi: 10.1016/j.jns.2017.11.034
32. Li L, Chen J, Wang J, Cai D. Prevalence and risk factors of diabetic peripheral neuropathy in type 2 diabetes mellitus patients with overweight/obese in guangdong province, China. Prim Care Diabetes (2015) 9(3):191–5. doi: 10.1016/j.pcd.2014.07.006
33. Quattrini C, Jeziorska M, Malik RA. Small fiber neuropathy in diabetes: clinical consequence and assessment. Int J Low Extrem Wounds (2004) 3(1):16–21. doi: 10.1177/1534734603262483
34. Liu X, Xu Y, An M, Zeng Q. The risk factors for diabetic peripheral neuropathy: a meta-analysis. PloS One (2019) 14(2):e0212574. doi: 10.1371/journal.pone.0212574
35. Callaghan BC, Xia R, Banerjee M, de Rekeneire N, Harris TB, Newman AB, et al. Metabolic syndrome components are associated with symptomatic polyneuropathy independent of glycemic status. Diabetes Care (2016) 39(5):801–7. doi: 10.2337/dc16-0081
36. Fan B, Liu XS, Szalad A, Wang L, Zhang R, Chopp M, et al. Influence of sex on cognition and peripheral neurovascular function in diabetic mice. Front Neurosci (2018) 12:795. doi: 10.3389/fnins.2018.00795
37. Tesfaye S, Selvarajah D. Advances in the epidemiology, pathogenesis and management of diabetic peripheral neuropathy. Diabetes Metab Res Rev (2012) 28 Suppl 1:8–14. doi: 10.1002/dmrr.2239
38. Scott D, De Courten B, Ebeling PR. Sarcopenia: a potential cause and consequence of type 2 diabetes in australia's ageing population? Med J Aust (2016) 205(7):329–33. doi: 10.5694/mja16.00446
39. Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet (2019) 393(10191):2636–46. doi: 10.1016/S0140-6736(19)31138-9
40. Çakici N, Fakkel TM, Van Neck JW, Verhagen AP, Coert JH. Systematic review of treatments for diabetic peripheral neuropathy. Diabetes Med (2016) 33(11):1466–76. doi: 10.1111/dme.13083
41. Liu CJ, Latham NK. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst Rev (2009) 2009(3):Cd002759. doi: 10.1002/14651858.CD002759.pub2
Keywords: diabetic peripheral neuropathy, sarcopenia, rectus femoris cross-sectional area, rectus femoris mass index, ultrasound
Citation: Wang L, Lin X, Huang H, Wang Y, Liang X, Zheng X and Xu L (2023) Low rectus femoris mass index is closely associated with diabetic peripheral neuropathy. Front. Endocrinol. 14:1148093. doi: 10.3389/fendo.2023.1148093
Received: 19 January 2023; Accepted: 10 April 2023;
Published: 21 April 2023.
Edited by:
Habib Yaribeygi, Semnan University of Medical Sciences, IranReviewed by:
Huabing Zhang, Peking Union Medical College Hospital (CAMS), ChinaRizaldy Taslim Pinzon, Duta Wacana Christian University, Indonesia
Copyright © 2023 Wang, Lin, Huang, Wang, Liang, Zheng and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lingling Xu, bHVjeWxpbmdsQDEyNi5jb20=
†These authors have contributed equally to this work
‡ORCID: Lingling Xu, orcid.org/0000-0003-2731-4758
 Lina Wang1,2†
Lina Wang1,2† Lingling Xu
Lingling Xu