- 1Department of Obstetrics and Gynaecology, Faculty of Medicine, Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia
- 2Department of Pharmacology, Faculty of Medicine, Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia
- 3Department of Physiology, Faculty of Medicine, Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia
- 4Basic Medical Science Department, Kulliyyah of Medicine, International Islamic University Malaysia, Kuantan, Malaysia
- 5Department of Clinical Pharmacology, Faculty of Medicine, Menoufia University, Shebin El-Kom, Egypt
Introduction: Stress and infertility form a complex relationship. In line with this, various stress-related biological markers have been investigated in infertility.
Methods: This systematic review was performed using PRISMA guidelines (i) to report whether cortisol is highly present in infertile patients compared to fertile control; (ii) to report whether there is any significant difference in the cortisol level in infertile subjects that conceive and those that didn’t at the end of assisted reproduction treatments. Original articles involving human (male and female) as subjects were extracted from four electronic databases, including the list of references from the published papers. Sixteen original full-length articles involving male (4), female (11), and both genders (1) were included.
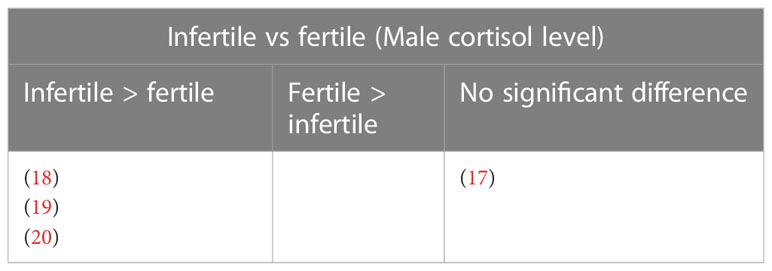
Results: Findings from studies that compared the cortisol level between infertile and fertile subjects indicate that (i) Male: three studies reported elevated cortisol level in infertile patients and one found no significant difference; (ii) Female: four studies reported increased cortisol level in infertile subjects and three studies found no significant difference. Findings from studies that measured the cortisol level from infertile patients that conceived and those that didn’t indicate that (i) Male: one study reported no significant difference; (ii) Female: one study reported elevated cortisol in infertile patients that conceived, whereas two studies reported increased cortisol in infertile patients that was unable to conceive. Five studies found no significant difference between the groups.
Discussion: In the present review we only included the cortisol value that was measured prior to stimulation or IVF treatment or during natural or spontaneous cycles, despite this, there are still variations in the sampling period, assessment techniques and patients’ characteristics. Hence, at present, we are still unable to conclude that cortisol is significantly elevated in infertile patients. We warrant future studies to standardize the time of biological sample collection and other limitations that were addressed in the review to negate the unwanted influencing factors.
1 Introduction
Infertility is defined as a disease characterized by failure to conceive after one year or more of regular, unprotected sexual intercourse due to an impaired male or female reproductive system (1). Infertility can be categorized into primary and secondary. Primary infertility is applicable for a woman that has never been diagnosed with a clinical pregnancy and fulfills the criteria for being classified as infertile, whereas a male who was not able to initiate a pregnancy with his female partner and meets the criteria for having infertility is diagnosed to have primary infertility. A woman that unable to conceive once again after previously being diagnosed with a clinical pregnancy and successfully giving birth is known to have secondary infertility. A similar classification is also applicable to males not being able to initiate pregnancy with their female partners but having done so previously (1, 2).
Based on data-driven from 195 countries in the span of 17 years (1990 to 2017), the global burden of infertility has been on the rise, with the age-standardized prevalence rate of infertility increased by 0.37% per annum for females, and 0.29% per annum for males (3). The same study also reported that the age group 35-39 had the highest prevalence rate (3). A wide range of factors has been associated with male and female infertilities including physical problems, lifestyle issues, genetic makeup, psychological problems, and hormonal disorders due to idiopathic reasons (4, 5). The impact of stress-induced psychoendocrinological changes on human reproductive function has been heavily studied over the decades (6, 7), resulting in the discovery of the role of various endogenous hormones including cortisol, catecholamines, vasopressin, gonadotrophins, thyroids, growth hormone, prolactin, and insulin in stress mechanisms (8, 9). Changes in some of these hormones were reported in infertility (10–14). This has led to research questions about whether (i) stress hormones are significantly elevated in infertility (i) causal relationship between elevated stress hormones and infertility, (ii) stress hormones as potential markers of infertility-related risk factors, and (iii) stress hormones as markers of ART outcome prediction.
Cortisol, the primary stress hormone released through the activation of the hypothalamus-pituitary-adrenal (HPA) axis was reported to affect human reproductive function through immunosuppression (15). The effect of cortisol on in vitro fertilization (IVF) treatment outcomes was systematically reviewed in the past, and the results were conflicting, with three studies reporting favorable IVF treatment outcomes in the presence of high cortisol levels and five studies reporting low cortisol to positively influence successful outcomes (16). Different treatment cycles during IVF treatment may directly influence the level of cortisol, for instance, hormonal stimulation and invasive procedures-induced stress as well. In the present review, our main aim is to determine the changes in cortisol levels in both male and female infertility in the absence of interference from assisted reproductive technology (ART) treatment procedures. Therefore, for studies that compared the cortisol level between female infertile subjects that conceived and those that didn’t at the end of ART, we only included the cortisol level that was assessed prior to stimulation or induction.
2 Methods
2.1 Search strategy
The studies were obtained from four online databases including SCOPUS, Web of Science, PubMed, and Ovid MEDLINE from 1946 until September 2022. The last search was formed on 22nd September 2022. The search strategy involved the combination (“AND”) of the following keywords: 1) corti* (cortisol, corticosteroid) OR hydrocorti* (hydrocortisone) OR glucocorti* (glucocorticoid); 2) infertil* (infertility, infertile) OR subfertil* (subfertility, subfertile). In addition, the references of all retrieved articles were reviewed for relevant citations.
2.2 Inclusion criteria
All full-length original research articles published in the English language and using humans (male or female or both) as subjects that investigated cortisol and infertility were included. For studies involving female subjects, only studies that recruited infertile subjects, and their biological specimens taken prior to a stimulated cycle or during unstimulated or spontaneous cycles were included. Among these, only studies that compared the differences in cortisol levels between fertile and infertile groups, and the pregnancy outcomes of the infertile subjects were included.
2.3 Exclusion criteria
Case series, case studies, books, reviews, letters to the editors, animal studies, cell culture studies, and conference abstracts were excluded. Human studies that specifically looked into infertile subjects with neurological or psychiatric comorbidities were excluded.
2.4 Article selection
The articles retrieved from the four databases were independently reviewed by 2 authors (BVK and JK). Any disagreement in the selection process was resolved through discussion to reach a consensus. In general, the articles were screened through three stages. First, articles that did not meet the inclusion criteria were rejected based on their titles. Second, articles that were irrelevant to infertility and cortisol were eliminated based on the abstracts. Third, the remaining articles’ methods, and results were carefully reviewed, and the articles that did not meet the inclusion criteria were eliminated. Reasons for exclusion included (i) if it was not clearly mentioned whether the biological samples to measure cortisol level was taken prior to or after the hormonal stimulation during the infertility treatment, (ii) if the participants were not diagnosed with infertility, (iii) for pre- and post-treatment studies, cortisol level was not assessed prior to treatment, (iv) if the infertile subjects were diagnosed with mood disorders, (v) if the biological samples were taken after induced ovulation, (vi) if the subjects have undergone surgical procedures (varicocelectomy), (vii) infertility patients who were at a stage prior to, during, or after their intrauterine insemination or IVF treatment, (viii) if the biological samples were collected after the embryo transfer, (ix) not reporting absolute cortisol levels.
3 Results
Initially, we identified 10,886 articles from four online databases including Ovid MEDLINE (6,843), SCOPUS (2,021), Web of Science (954), and PubMed (1,068). From this, we identified 280 articles through title screening (Ovid MEDLINE: 63, SCOPUS: 75, Web of Science: 70, and PubMeD: 72). Following the removal of duplicates, we found 143 articles. These articles’ abstracts, methods, and results were reviewed based on the inclusion criteria, and this was followed by the rejection of 127 articles. In the end, we included 16 full-length original articles involving males (4), females (11), and both genders (1) as subjects in this systematic review (Figure 1).
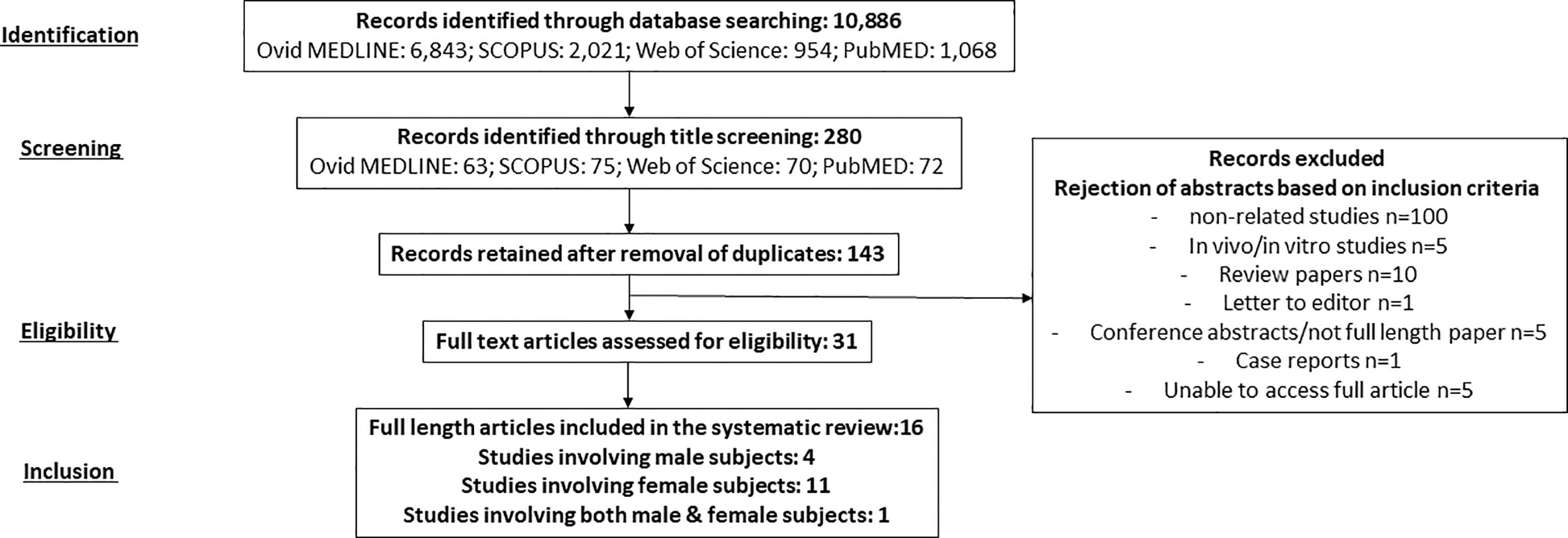
Figure 1 A summary of literature search, screening, and selection of studies based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline.
3.1 Study designs and sample characteristics
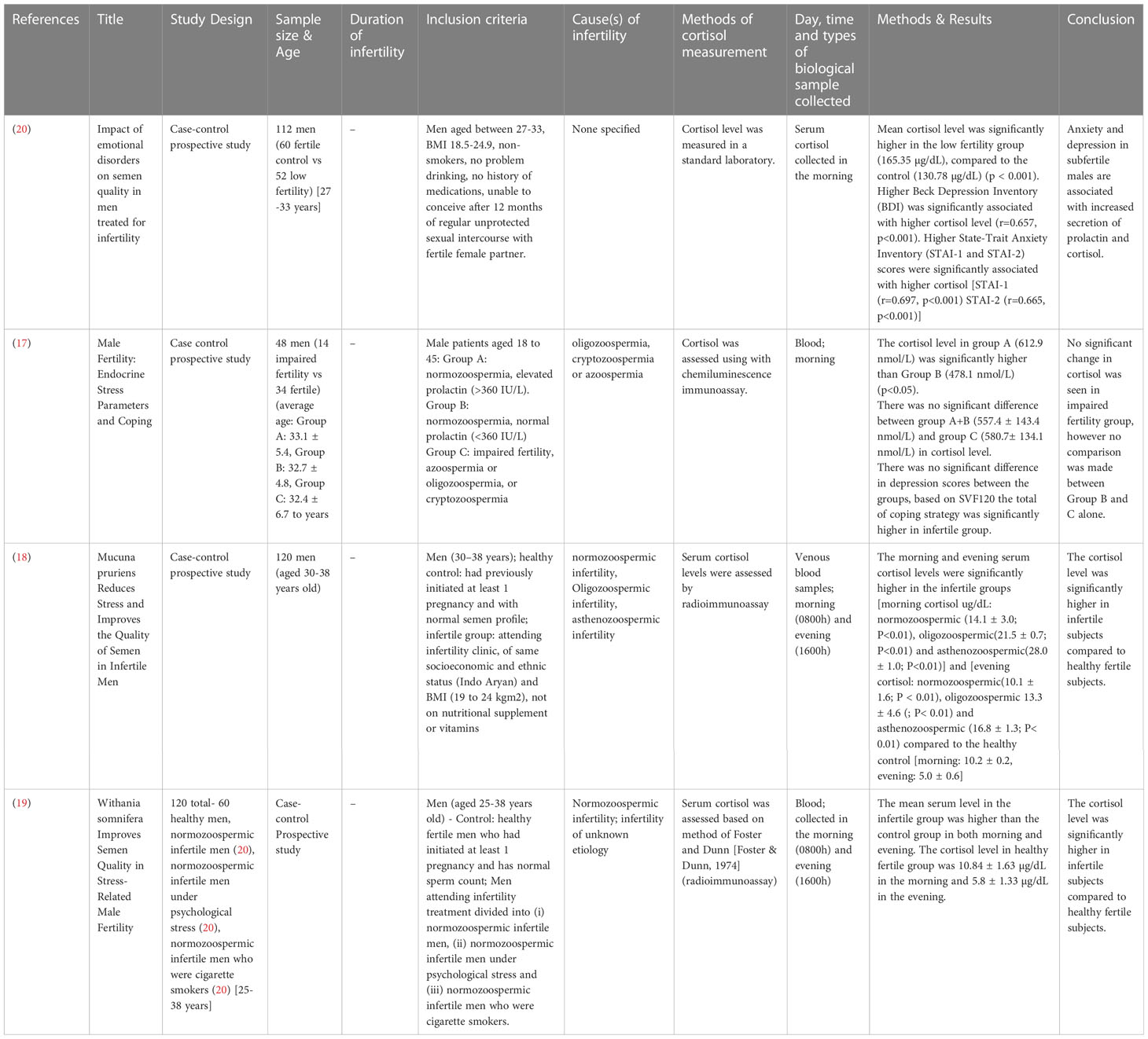
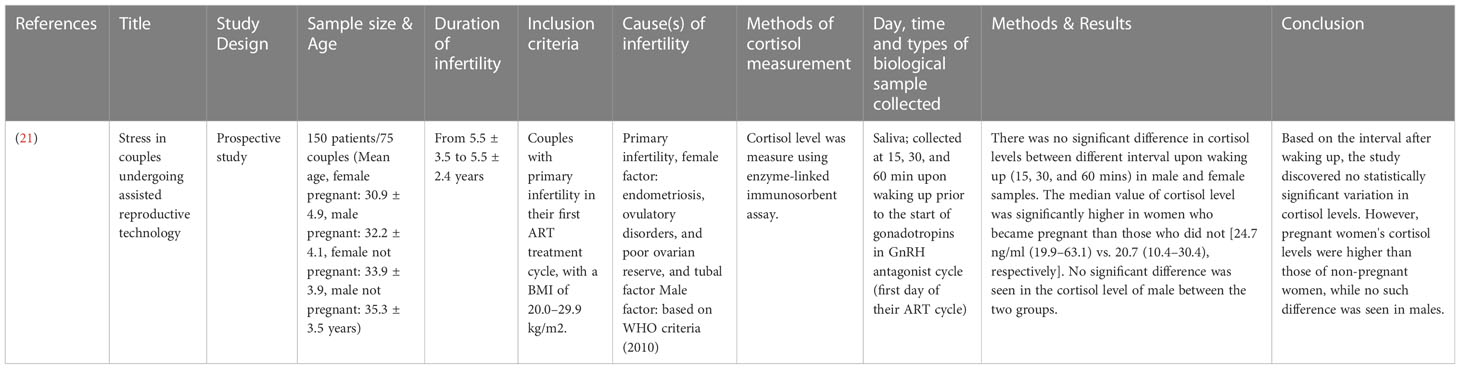
The study designs that were employed for studies involving male subjects only are case-control prospective studies (17–20), with a total of 400 subjects involving age-matched, healthy fertile controls and infertile subjects. Only one study stated the average age of the participants, infertile (32.4 ± 6.7 years) and fertile (32.7 ± 4.8 to 33.1 ± 5.4 years) (17). Three studies provided the age range of the participants, which was, in general, ranging from 25 to 38 years (18–20) (Table 1). One study recruited 150 subjects from both genders, with their age ranging from 30.9 ± 4.9 to 35.3 ± 3.5 years. The study design employed was prospective (21) (Table 2).
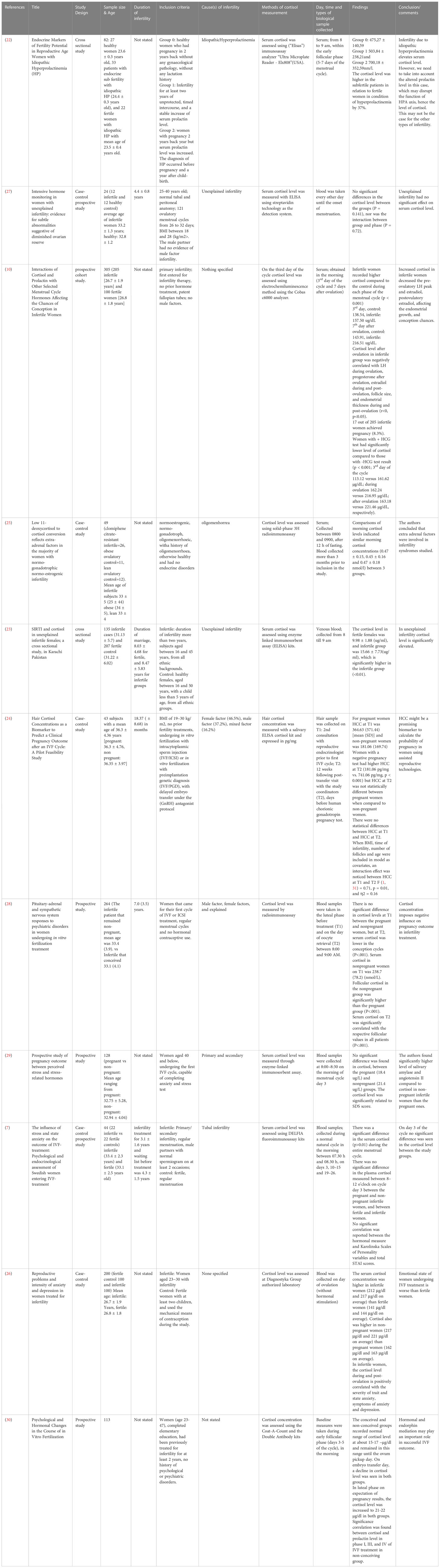
Studies involving female subjects employed designs such as cross-sectional (22, 23), prospective cohort (10), case-control study (24–26), case-control prospective study (7, 27), and prospective study (28–30). The total number of participants recruited was 1102. In general, ten studies reported the average age of the separate groups of patients recruited, such as fertile control (23.5 ± 0.4 to 34 ± 5 years) and infertile group (24.4 ± 0.3 to 33.4 ± 2.3 years) and infertile/pregnant (32.75 ± 5.78 to 36.3 ± 4.76 years) and infertile/non-pregnant (32.94 ± 4.04 to 36.35 ± 3.97 years) (7, 10, 22–29). One study provided the age range of the participants recruited (23-47 years) (30) (Table 3).
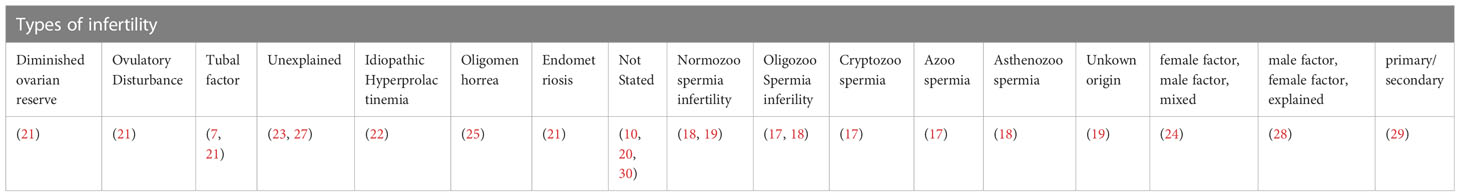
The diagnosis or types of infertility specified were diminished ovarian reserve (21), ovulatory disturbance (21), tubal factor (7, 21), unexplained (23, 27), idiopathic hyperprolactinemia (22), oligomenorrhea (25), and endometriosis (21). Some of the studies reported the types of infertility as female factor, male factor, and mixed (24), male factor, female factor, and unexplained (28), primary and secondary (29), normozoospermic infertility (18, 19), oligozoospermia (17, 18), cryptozoospermia (17), azoospermia infertility (17), asthenozoospermia infertility (18), and unknown origin (19). Whereas some studies did not report the diagnosis or types of infertility involved (10, 20, 30) (Table 4).
In general, the infertile females’ mean duration of infertility was ranging from 18.37 months to 8.47 ± 5.83 years. Some studies did not report the duration of infertility (10, 17–20, 22, 25, 29, 30). One of the studies reported the subjects’ duration of the marriage, which was ranging from 8.03 ± 4.68 to 8.47 ± 5.83 years (23). Another study reported the duration of infertility treatment, 3.1 ± 1.6 years, and the period of waiting before the treatment, 4.3 ± 1.5 years (7) (Table 5).
Some studies recruited newly diagnosed infertile patients (10, 17, 20, 21, 23–25). In some studies, infertile patients have already been exposed to infertility treatment in the past (7, 30). Whereas some studies did not report whether the recruited infertile patients were novices or have prior infertility treatment experience (18, 19, 22, 25–27, 29) (Table 6).
3.2 Cortisol measurement
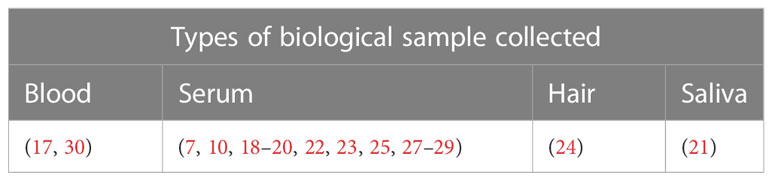
Various types of biological samples were collected to assess the cortisol level in the study subjects including serum (7, 10, 18–20, 22, 23, 25–29), saliva (21), and hair (24). Two studies used blood samples but did not specify whether plasma or serum was used for cortisol assessment (17, 30) (Table 7).
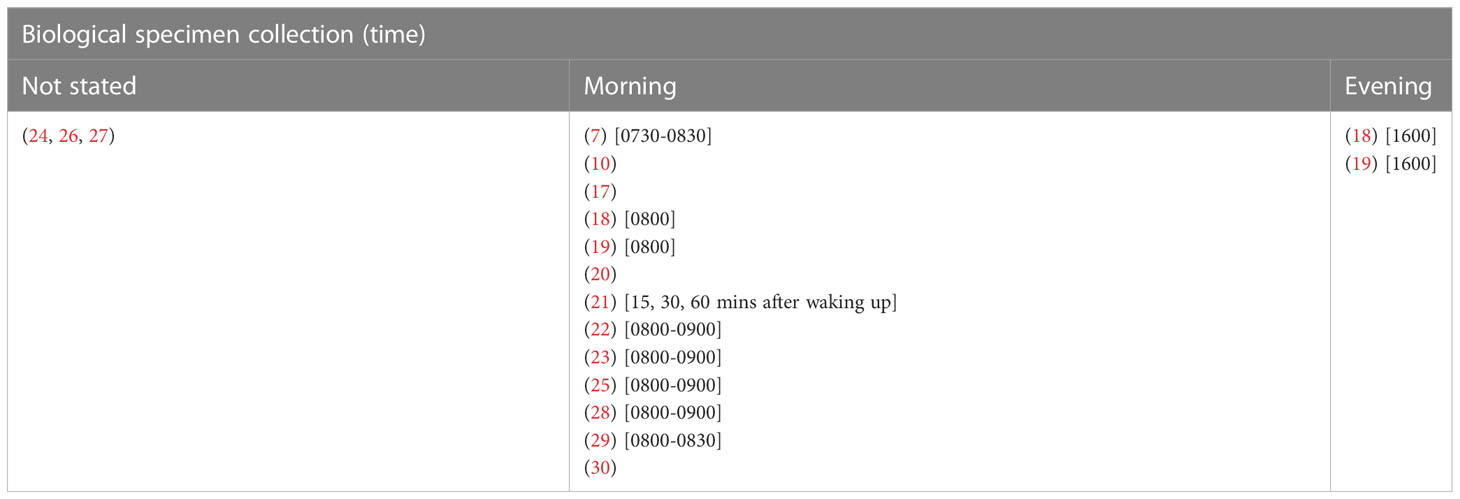
Some studies collected the biological specimens in the morning only (7, 10, 17–22, 25, 28, 29), around 0730-0900, upon waking up, and some studies collected samples on multiple time points, such as 15, 30, and 60 minutes after waking up (21). Two studies collected the biological specimens in both morning and evening (18, 19). Whereas some studies did not report the time of biological specimen collection (24, 26, 27) (Table 8).
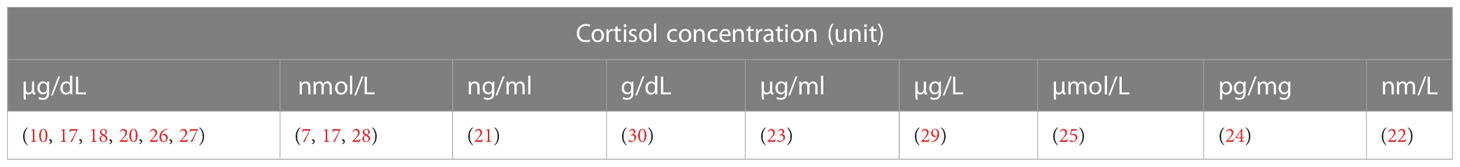
The results of cortisol levels were reported in numerous units due to differences in the measuring techniques. Some have reported their results in µg/dL (10, 18–20, 26, 27), nmol/L (7, 17, 28), ng/mL (21), g/dL (30), µg/mL (23), µg/L (29), µmol/L (25), pg/mg (24), and nm/L (22) (Table 9).
Techniques employed in the studies to measure the cortisol level were chemiluminescence immunoassay (10, 17), radioimmunoassay (18, 19, 25, 28, 30), enzyme-linked immunosorbent assay (ELISA) (21, 22, 24, 27), liquid chromatography-mass chromatography (23, 29), and dissociation-enhanced lanthanide fluoroimmunoassay (DELFIA) (7). Two studies did not specify the techniques (20, 26) (Table 10).
Some studies specified the time of female biological specimen collection as early follicular phase or luteal phase or ovulation phase or prior to the stimulation day (7, 10, 22, 26–30). Some reported the specimens were taken during the time of recruitment or while on the waiting list for treatment or prior to the beginning of treatment (21, 23–25) (Table 11).
3.3 Cortisol levels in infertility: male and both genders
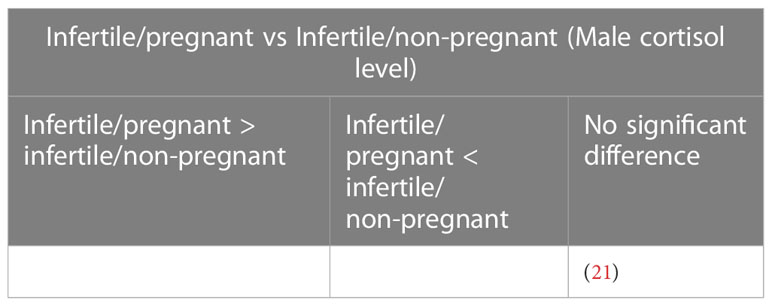
A study that assessed the cortisol levels in both male and female patients found no significant difference in the levels of cortisol among males with conceived and non-conceived female partners following the IVF treatment (21) (Table 12). Four studies evaluated the cortisol levels in infertile male patients only. Among these, three studies reported elevated cortisol levels among infertile males compared to healthy controls (18–20). One study found no significant difference between infertile and healthy controls (17) (Table 13).
3.4 Cortisol levels in infertility: female
Among the studies that recruited female subjects, seven studies compared the cortisol levels between the infertile and fertile female subjects. Out of these, four studies reported elevated cortisol levels in infertile female subjects compared to the fertile group (10, 22, 23, 26). Three studies found no significant difference in the cortisol levels between the fertile and infertile subjects (7, 25, 27) (Table 14). Nine studies measured the cortisol levels prior to treatment or stimulation in infertile subjects that conceived at the end of IVF treatment and those that did not. Two studies reported elevated cortisol levels in infertile female subjects that could not conceive following IVF compared to those who could (10, 26). However, five studies found no significant difference in the cortisol levels between the conceived, infertile female subjects and those did not (7, 24, 28–30). In contrast, one study reported that the median value of cortisol was higher among females that conceived compared to those who did not (21) (Table 15).

Table 15 Cortisol level in female infertile patients (Infertile/pregnant vs infertile/non-pregnant).
4 Discussion
4.1 Male: infertile vs fertile
Four studies reported changes in blood cortisol levels in healthy fertile and infertile males, with one reporting no significant difference (17), and three found significantly higher cortisol in infertile male patients (18–20). Out of these four studies, only three studies specified the causes of infertility which included normozoospermic, oligozoospermic, cryptozoospermic, azoospermic, asthenozoospermic, and unknown infertility (17, 18). None of the studies reported a significant direct correlation between cortisol levels and infertility characteristics investigated. One study reported higher Beck Depression Inventory (BDI), and State-Trait Anxiety Inventory (STAI-1 and STAI-2) scores to be significantly associated with higher cortisol levels in infertile patients, and BDI score also significantly negatively correlated with sperm count and ejaculate volume. Lower STAI-1 and STAI-2 scores were associated with a higher percentage of sperm with progressive motility (20). A recent systematic review reported mental disorders such as depression, sleep disorders, addiction, eating disorders, and stress negatively impact fertility in males and females (6). It is not within the scope of the present review to relate stress with infertility, nevertheless perceived stress, anxiety, and depression was reported in the past to elevate cortisol level (32, 33).
Harth and Linse (17) found no significant difference in the blood cortisol levels between infertile subjects (normozoospermic, cryptozoospermic, or azoospermic) and a combined cortisol level from Group A (normozoospermic, high prolactin, and stress levels) and Group B (normozoospermic, normal stress and prolactin levels), 580.7 nmol/L (infertile) against 557.4 nmol/L (Group A+B). Nevertheless, earlier reports on prolactin showed that prolactin may directly induce adrenal steroidogenesis, thus increasing the level of cortisol (34, 35). This should be taken into consideration when interpreting the results. In the same study, the cortisol level in Group B subjects was much lower, 478.1 nmol/L, however, no description was given of the individual comparison between group B and C. Harth and Linse (17) recruited participants between the age range of 18-45 years old, a much wider range, whereas the other three studies recruited between the age of 27-33 (20), 30-38 (18), and 25-38 (19) years old participants, with the lowest age being 25 and oldest 38. The average age of the participants recruited by (17) was 32.7 ± 4.8 to 33.1 ± 5.4 years old, whereas the other three studies did not report the participants’ average age. Cortisol is synthesized from cortisone by the enzyme 11β-hydroxysteroid dehydrogenase type 1 (11βBHSD1) and converted to inactive cortisone by 11β-hydroxysteroid dehydrogenase type 2 (11βBHSD2). Age alters the activity of 11βBHSD2 (36). Furthermore, an increase of 17 nmol/L in serum cortisol per year of age was reported. This accounts for approximately 32% of the difference in serum cortisol levels between patients (R2 = 0.315; 36).
Two studies (18, 19) reported the morning cortisol level (10.2 to 10.84 µg/dL) to be higher than the evening cortisol level (5.0 to 5.8 µg/dL) in healthy fertile subjects, which is in line with circadian cortisol rhythmicity reported in the past (37–39). Normozoospermia (18, 19), asthenozoospermia (18), and unknown origin (19) were reported as infertility-related factors in infertile males with higher cortisol. To date, very little literature is available to physiologically link asthenozoospermia to altered cortisol levels. At the preclinical level, asthenozoospermia induced via the inactivation of AMP-activated protein kinase-α1 (AMPKα1) in mice caused no significant changes in plasma cortisol level (40).
4.2 Female: infertile vs fertile and infertile/pregnant vs infertile/non-pregnant
Four studies found that the cortisol level in infertile females was significantly higher compared to fertile females (10, 22, 23, 26). Out of this, only one study reported a significant negative correlation between high cortisol (after ovulation) and LH level during ovulation, progesterone level after ovulation, estradiol level during and post-ovulation, follicle size and endometrial thickness during and post-ovulation (10). One study recruited infertile patients with idiopathic hyperprolactinemia hence elevated cortisol levels due to the direct effect of enhanced prolactin on adrenal steroidogenesis should be considered in the interpretation of results (34, 35). Three studies reported no significant difference in the cortisol level (7, 25, 27). All seven studies measured cortisol using serum samples taken in the morning, except for two studies that did not state the time of biological specimen collection (26, 27). In general, studies that reported elevated cortisol levels in infertile females recruited a larger pool of participants (929 subjects in four studies) compared to the studies that reported no significant difference in the cortisol level (117 subjects in three studies). The average age of participants recruited by studies reporting significant differences in the cortisol level (control: 23.6 ± 0.3 to 31.22 ± 6.02; infertile: 24.4 ± 0.3 to 31.13 ± 5.7 years old) is also lower compared to the studies that found no significant difference in the cortisol (control: 32.8 ± 1.2 to 34 ± 5; infertile: 33 ± 4 to 33.4 ± 2.3 years old). Higher age of the control group (in studies that found no significant difference in cortisol level) may have reduced the deficit in the cortisol value between study groups due to the aging-related effects on cortisol value (36, 41).
Two studies reported higher cortisol in infertile/non-pregnant patients (10, 26), however, none of the studies reported a significant association with pregnancy outcomes. Five studies found no significant change in the cortisol level (7, 24, 28–30). Both sets of studies recruited an almost similar pool of participants (525 versus 595 subjects). In general, the average age of participants in studies with elevated cortisol is in their 20s. Infertility factor such as idiopathic hyperprolactinemia (22) was reported in infertile females with elevated cortisol. Hyperandrogenism was reported in PCOS due to dysregulation of 11β-hydroxysteroid dehydrogenase (42, 43).
The menstrual cycle is known to affect the activity of the HPA axis which can result in variation in cortisol synthesis, such as higher cortisol levels during the luteal phase compared to the follicular phase (31). 14 out of 17 selected studies collected the biological specimen for cortisol analysis in the morning, and four studies did not specify the time of collection (10, 17, 20, 30). Physiological cortisol level is usually higher in the morning, especially 30-45 minutes after awakening and gradually reduces for the rest of the day under normal conditions (44).
Eight out of 17 selected studies did not report whether the recruited infertile patients have any prior exposure to IVF treatment procedures. Anticipatory stress was associated with higher stress reactivity for cortisol (45) and higher stress task-induced increase in cortisol (46). Contrary to this, some researchers found no significant association between cortisol awakening response and stress anticipation (47). Furthermore, reports on the effects of stress during and prior to IVF treatment on pregnancy outcomes have been conflicting (48, 49). It will be interesting to see in future studies whether there is a significant difference in stress and stress-related hormones among infertile patients that undergoing ART treatment for the first time and those with prior exposure.
Based on the data we have listed, while high cortisol may have been reported in most of the infertile subjects, this is not always the case (Tables 13–15). It is challenging to ascribe infertility to cortisol levels due to the presence of various other confounding factors. The complex interactions of multiple hormones, each of which plays a specialized role in regulating fertility, make the human reproductive system even more complex. The stress hormone cortisol interacts with this complex hormonal network and has the potential to cause disruptions (50), which is still poorly understood. Finding the precise mechanisms through which cortisol affects infertility is challenging due to the complexity of the hormonal pathways and feedback mechanisms involved in fertility regulation. Nevertheless, a majority of results have linked chronically elevated cortisol levels with disrupted reproductive endocrinology based on observational and correlational data. For instance, excessive amounts of cortisol might interfere with GnRH’s pulsatile release, which controls the menstrual cycle and ovulation. This can result in irregular or absent ovulation and, ultimately, infertility (51, 52). High cortisol levels could inhibit LH and FSH release as well, which affects ovarian function and lowers the likelihood of pregnancy. The menstrual cycle is hampered by increased secretion of cortisol and prolactin in infertile women by lowering pre-ovulatory LH peak and E2 and post-ovulatory E2 levels that alter endometrial development and thus lower the likelihood of conception (10). However, it’s crucial to remember that some people might be more resilient to the effects of cortisol on reproductive function. In line with this, it was reported that psychopathology, stress, and hair cortisol concentration scores were higher for pregnant women with poorer resilience than for those with better resilience (53). The likelihood that someone would experience infertility due to cortisol can depend on a variety of variables, including genetic differences, general health state, and stress resilience. It is difficult to establish a universal link between cortisol and infertility owing to this individual diversity.
In the present review, we noticed a large span in the age difference of the recruited study participants. The relationship between age and infertility is complex, with aging older having a significant role in both men’s and women’s declining fertility (54–56). In women, the quality and quantity of eggs decrease during aging, resulting in a decreased ovarian reserve (57), which leads to a longer time to conceive, decreased fertility, and a higher risk of pregnancy-related complications (58, 59). An increase in paternal age also has an impact on reproductive outcomes in males, affecting sperm volume, motility, and morphological changes that may result in decreased fertility (55). Individuals’ stress response systems may change as they get older (60), which could have an impact on how cortisol is regulated. Age-related changes in cortisol patterns, such as a diurnal cortisol fluctuation (61, 62), or changes in overall cortisol output (63). These alterations may be caused by aging-related effects on the hypothalamic-pituitary-adrenal (HPA) axis, the system that regulates cortisol. The relationship between cortisol and age might be bidirectional. Aging-related elements including long-term underlying illness (64), endocrinological changes (65), or psychological stressors (66) could affect cortisol levels. On the contrary, cortisol dysregulation by itself may hasten aging (67, 68), resulting in a complicated interplay between cortisol levels and age. While cortisol and age can both have an independent impact on fertility, they may also affect fertility synergistically. The delicate hormonal balance required for fertility can also be upset by altered cortisol levels linked to long-term stress or by age-related changes in cortisol regulation (10, 52). Hormonal imbalances brought on by cortisol may make the age-related decline in fertility worse and aid infertility.
Stress has long been thought to affect fertility as well as other areas of general well-being (69). While stress is associated with elevated cortisol (70), proving a direct cause-and-effect relationship between cortisol and fertility is challenging, and thus it necessitates more research. The delicate hormonal balance involved in reproductive processes is thought to be affected by prolonged exposure to elevated cortisol levels, which may affect fertility. Based on existing literature, in both genders, prolonged stress has been associated with diminished reproductive functions (71, 72). Even though cortisol is part of the stress-fertility relationship, it’s vital to understand that fertility is a complex process that governed by a myriad of factors. Stress affects fertility indirectly through psychological and physiological impacts such as change in lifestyle factors (73, 74), sleep quality (75, 76), and sexual behavior (77). Understanding the connection between cortisol and fertility presents a substantial problem in separating the precise effects of cortisol from the general stress response. Individual variation in stress reactions and coping mechanisms further complicates the interpretation of research findings. While some people can handle stress better than others, some may be more susceptible to its negative consequences (78). Furthermore, patients who are diagnosed as infertile suffer from a great deal of emotional instability as a result of their condition, increasing the risk of anxiety, depression, and other mood disorders substantially. Infertility drugs like leuprolide, gonadotropins, and clomiphene can affect the patient’s psychological well-being causing side effects such as irritability, anxiety, and depression. Hence, it is often challenging to distinguish between the psychological impacts of infertility and the adverse effects of the medications when evaluating symptoms in women receiving infertility-related pharmacotherapy (70). Therefore, it is important to note that stress can cause infertility, and vice versa, and given the complexity, pinpointing cortisol’s exact function in the relationship between stress and fertility is challenging at present.
5 Strength and limitations
To our knowledge, this is the first systematic review that investigated the changes in cortisol levels in infertile male and female patients. Prior to this review, Massey et al. (2014) conducted a systematic review of cortisol levels and IVF treatment outcomes and reported three studies to associate higher cortisol with favorable IVF outcomes and five studies to relate lower cortisol to IVF success. The present review aimed to report whether cortisol is highly present in infertile patients compared to fertile healthy subjects, infertile patients who conceived, and those who didn’t at the end of the ART. We only included studies that reported the cortisol level in the unstimulated/natural cycle or prior to hormonal stimulation to rule out changes in cortisol levels due to hormonal stimulation.
One of the limitations we faced in the analysis was variation in the sampling period for studies involving female subjects as the biological specimens were collected on different days of natural cycles such as early follicular phase, day of ovulation, and days after ovulation. Future studies should standardize the time of biological specimen collection.
6 Conclusion and future direction
A complex relationship exists between psychological well-being and infertility, where infertility can cause stress to patients through emotional and financial burdens, and mental health disorders such as depression and anxiety could lead to infertility. Biological markers of stress have been extensively investigated and correlated to various health-related problems, including infertility. Seven out of eleven studies that compared the cortisol level between fertile and infertile subjects found significantly higher cortisol in the infertile group. Out of this, only one study correlated cortisol levels with infertility markers. On a separate note, out of 8 studies, only 3 reported significantly higher cortisol levels in infertile subjects that could not conceive at the end of ART. Based on the evidence we gathered through this review, at present, due to variations in study designs, sampling periods, and patients’ characteristics, it is still unclear if high cortisol level causes infertility in males and females. In the present review, we only included the cortisol value that was measured prior to stimulation or IVF treatment, despite this, there are still variations in the sampling period as the collection of biological specimens was carried out during different time phases of the menstrual cycle such as follicular and luteal phases. Hence, we warrant future studies to standardize the time of biological sample collection to negate the unwanted influencing factors.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
BK, AK, and JK contributed to conception and design; BK, AK, and JK contributed to data acquisition; BK, AK, and JK were involved in the analysis, interpretation, and drafting of the manuscript; IM, AU, WM, AF and AA revise the manuscript critically for important intellectual content. The version to be published has received final approval from all authors.
Funding
This research was funded by Faculty of Medicine, Universiti Kebangsaan Malaysia, and Ministry of Higher Education (FRGS/1/2020/SKK0/UKM/03/1).
Acknowledgments
The authors would like to thank the Faculty of Medicine, Universiti Kebangsaan Malaysia.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Vander Borght M, Wyns C. Fertility and infertility: definition and epidemiology. Clin Biochem (2018) 62:2–10. doi: 10.1016/j.clinbiochem.2018.03.012
2. Zegers-Hochschild F, David Adamson G, Dyer S, Racowsky C, De Mouzon J, Sokol R, et al. The international glossary on infertility and fertility care, 2017. Hum Reprod (2017) 32(9):1786–801. doi: 10.1093/humrep/dex234
3. Sun H, Gong T-T, Jiang Y-T, Zhang S, Zhao Y-H, Wu Q-J. Global, regional, and national prevalence and disability-adjusted life-years for infertility in 195 countries and territories, 1990–2017: results from a global burden of disease study, 2017. Aging (Albany NY) (2019) 11(23):10952. doi: 10.18632/aging.102497
4. Babakhanzadeh E, Nazari M, Ghasemifar S, Khodadadian A. Some of the factors involved in male infertility: a prospective review. Int J Gen Med (2020) 13:29. doi: 10.2147/IJGM.S241099
5. Bala R, Singh V, Rajender S, Singh K. Environment, lifestyle, and female infertility. Reprod Sci (2021) 28(3):617–38. doi: 10.1007/s43032-020-00279-3
6. Szkodziak F, Krzyżanowski J, Szkodziak P. Psychological aspects of infertility. a systematic review. J Int Med Res (2020) 48(6):0300060520932403. doi: 10.1177/0300060520932403
7. Csemiczky G, Landgren B-M, Collins A. The influence of stress and state anxiety on the outcome of IVF-treatment: psychological and endocrinological assessment of Swedish women entering IVF-treatment. Acta Obstet Gynecol Scandinavica: ORIGINAL ARTICLE (2000) 79(2):113–8. doi: 10.1034/j.1600-0412.2000.079002113.x
8. Russell G, Lightman S. The human stress response. Nat Rev Endocrinol (2019) 15(9):525–34. doi: 10.1038/s41574-019-0228-0
9. Ranabir S, Reetu K. Stress and hormones. Indian J Endocrinol Metab (2011) 15(1):18. doi: 10.4103/2230-8210.77573
10. Wdowiak A, Raczkiewicz D, Janczyk P, Bojar I, Makara-Studzińska M, Wdowiak-Filip A. Interactions of cortisol and prolactin with other selected menstrual cycle hormones affecting the chances of conception in infertile women. Int J Environ Res Public Health (2020) 17(20):7537. doi: 10.3390/ijerph17207537
11. Zehravi M, Maqbool M, Ara I. Polycystic ovary syndrome and infertility: an update. Int J Adolesc Med Health (2022) 34(2):1–9. doi: 10.1515/ijamh-2021-0073
12. Kwon W-S, Park Y-J, Kim Y-H, Kim I-C, Pang M-G. Vasopressin has detrimental effect on male fertility. Biol Rep (2012) 87:354–4. doi: 10.1093/biolreprod/87.s1.354
13. Korevaar TIM, Mínguez-Alarcón L, Messerlian C, de Poortere RA, Williams PL, Broeren MA, et al. Association of thyroid function and autoimmunity with ovarian reserve in women seeking infertility care. Thyroid (2018) 28:1349–58. doi: 10.1089/thy.2017.0582
14. Albu D, Albu A. Is growth hormone administration essential for in vitro fertilization treatment of female patients with growth hormone deficiency? Syst Biol Reprod Med (2019) 65(1):71–4. doi: 10.1080/19396368.2018.1492044
15. Vedhara K, Miles JNV, Sanderman R, Ranchor AV. Psychosocial factors associated with indices of cortisol production in women with breast cancer and controls. Psychoneuroendocrinology (2006) 31(3):299–311. doi: 10.1016/j.psyneuen.2005.08.006
16. Massey AJ, Campbell B, Raine-Fenning N, Aujla N, Vedhara K. The association of physiological cortisol and IVF treatment outcomes: a systematic review. Reprod Med Biol (2014) 13(4):161–76. doi: 10.1007/s12522-014-0179-z
17. Harth W, Linse R. Male Fertility: endocrine stress parameters and coping. Dermatol Psychosomatics/Dermatol und Psychosomatik (2004) 5(1):22–9. doi: 10.1159/000078051
18. Shukla KK, Mahdi AA, Ahmad MK, Jaiswar SP, Shankwar SN, Tiwari SC. Mucuna pruriens reduces stress and improves the quality of semen in infertile men. Evidence-Based Complement Altern Med (2010) 7(1):137–44. doi: 10.1093/ecam/nem171
19. Mahdi AA, Shukla KK, Ahmad MK, Rajender S, Shankhwar SN, Singh V, et al. Withania somnifera improves semen quality in stress-related male fertility. Evidence-Based Complement Altern Med (2011) 2011. doi: 10.1093/ecam/nep138
20. Wdowiak A, Bien A, Iwanowicz-Palus G, Makara-Studzińska M, Bojar I. Impact of emotional disorders on semen quality in men treated for infertility. Neuro-endocrinol Lett (2017) 38(1).
21. Tuncay G, Yıldız S, Karaer A, Reyhani I, Özgöcer T, Ucar C, et al. Stress in couples undergoing assisted reproductive technology. Arch Gynecol Obstet (2020) 301(6):1561–7. doi: 10.1007/s00404-020-05549-8
22. Atalyan AV, Shelokhov LF, Kolesnikova LI. Endocrine markers of fertility potential in reproductive age women with idiopathic hyperprolactinemia. J Pharm Res Int (2020) 32(23):71–7. doi: 10.9734/jpri/2020/v32i2330790
23. Alam F, Khan TA, Ali R, Tariq F, Rehman R. SIRTI and cortisol in unexplained infertile females; a cross sectional study, in Karachi Pakistan. Taiwanese J Obstet Gynecol (2020) 59(2):189–94. doi: 10.1016/j.tjog.2020.01.004
24. C. Santa-Cruz D, Caparros-Gonzalez RA, Romero-Gonzalez B, Peralta-Ramirez MI, Gonzalez-Perez R, García-Velasco JA. Hair cortisol concentrations as a biomarker to predict a clinical pregnancy outcome after an IVF cycle: a pilot feasibility study. Int J Environ Res Public Health (2020) 17(9):3020. doi: 10.3390/ijerph17093020
25. Dolfing JG, Tucker KE, Lem CM, Uittenbogaart J, Verzijl JC, Schweitzer DH. Low 11-deoxycortisol to cortisol conversion reflects extra-adrenal factors in the majority of women with normo-gonadotrophic normo-estrogenic infertility. Hum Reprod (2003) 18(2):333–7. doi: 10.1093/humrep/deg082
26. Wdowiak A, Makara-Studzińska M, Raczkiewicz D, Cyranka K. Reproductive problems and intensity of anxiety and depression in women treated for infertility. Psychiatr Pol (2022) 56(1):153–70. doi: 10.12740/PP/125885
27. Leach RE, Moghissi KS, Randolph JF, Reame NE, Blacker CM, Ginsburg KA, et al. Intensive hormone monitoring in women with unexplained infertility: evidence for subtle abnormalities suggestive of diminished ovarian reserve. Fertil Steril. (1997) 68(3):413–20. doi: 10.1016/s0015-0282(97)00222-7
28. An Y, Wang Z, Ji H, Zhang Y, Wu K. Pituitary-adrenal and sympathetic nervous system responses to psychiatric disorders in women undergoing in vitro fertilization treatment. Fertil Steril. (2011) 96(2):404–8. doi: 10.1016/j.fertnstert.2011.05.092
29. Cui Y, Yu H, Meng F, Liu J, Yang F. Prospective study of pregnancy outcome between perceived stress and stress-related hormones. J Obstet Gynaecol Res (2020) 46(8):1355–63. doi: 10.1111/jog.14278
30. Merari D, Feldberg D, Elizur A, Goldman J, Modan B. Psychological and hormonal changes in the course of in vitro fertilization. J Assist Reprod Genet (1992) 9(2):161–9. doi: 10.1007/BF01203757
31. Rohleder N, Schomner NC, Hellhammer R, Engle R, Kirschbaum C. Sex differences in glucocorticoid sensitivity of proinflamatorycytokine production after psychosocial stress. Psychosom Med (2001) 63:966–72. doi: 10.1097/00006842-200111000-00016
32. Barbaresco GQ, Reis AVP, Da Rocha Lopes G, Pereira Boaventura L, Freitas Castro A, Ferreira Vilanova TC, et al. Effects of environmental noise pollution on perceived stress and cortisol levels in street vendors. J Toxicol Environ Health (2019) 82(5):331–7. doi: 10.1080/15287394.2019.1595239
33. Jia Y, Liu L, Sheng C, Cheng Z, Cui L, Li M, et al. Increased serum levels of cortisol and inflammatory cytokines in people with depression. J nervous Ment Dis (2019) 207(4):271–6. doi: 10.1097/NMD.0000000000000957
34. Glasow A, Breidert M, Haidan A, Anderegg U, Kelly PA, Bornstein SR. Functional aspects of the effect of prolactin (PRL) on adrenal steroidogenesis and distribution of the PRL receptor in the human adrenal gland. J Clin Endocrinol Metab (1996) 81(8):3103–11. doi: 10.1210/jcem.81.8.8768882
35. Levine S, Muneyyirci-Delale O. Stress-induced hyperprolactinemia: pathophysiology and clinical approach. Obstet Gynecol Int (2018) 2018:9253083. doi: 10.1155/2018/9253083
36. Campino C, Martinez-Aguayo A, Baudrand R, Carvajal CA, Aglony M, Garcia H, et al. Age-related changes in 11βhydroxysteroid dehydrogenase type 2 activity in normotensive subjects. Am J Hypertens (2013) 26(4):481–7. doi: 10.1093/ajh/hps080
37. Clow A, Hucklebridge F, Stalder T, Evans P, Thorn L. The cortisol awakening response:more than a measure of HPA axis function. Neurosci Biobehav Rev (2010) 35:97–103. doi: 10.1016/j.neubiorev.2009.12.011
38. Wilhelm I, Born J, Kudielka BM, Schlotz W, Wüst S. Is the cortisol awakening rise a response to awakening? Psychoneuroendocrinology (2007) 43(4):358–66. doi: 10.1016/j.psyneuen.2007.01.008
39. Petrowski K, Schmalbach B, Stalder T. Morning and evening type: the cortisol awakening response in a sleep laboratory. Psychoneuroendocrinology (2020) 112:104519. doi: 10.1016/j.psyneuen.2019.104519
40. Tartarin P, Guibert E, Toure A, Ouiste C, Leclerc J, Sanz N, et al. Inactivation of AMPKα1 induces asthenozoospermia and alters spermatozoa morphology. Endocrinology (2012) 153(7):3468–81. doi: 10.1210/en.2011-1911
41. Titman A, Price V, Hawcutt D, Chesters C, Ali M, Cacace G, et al. Salivary cortisol, cortisone and serum cortisol concentrations are related to age and body mass index in healthy children and young people. Clin Endocrinol (2020) 93(5):572–8. doi: 10.1111/cen.14294
42. Rodin A, Thakkar H, Taylor N, Richard C. Hyperandrogenism in polycystic ovary syndrome–evidence of dysregulation of 11β-hydroxysteroid dehydrogenase. New Engl J Med (1994) 330(7):460–5. doi: 10.1056/NEJM199402173300703
43. Tsilchorozidou T, Honour JW, Conway GS. Altered cortisol metabolism in polycystic ovary syndrome: insulin enhances 5α-reduction but not the elevated adrenal steroid production rates. J Clin Endocrinol Metab (2003) 88(12):5907–13. doi: 10.1210/jc.2003-030240
44. Wüst S, Federenko I, Hellhammer DH, Kirschbaum Genetic factors C. Perceived chronic stress, and the free cortisol response to awakening. Psychoneuroendocrinology (2000) 25:707–20. doi: 10.1016/S0306-4530(00)00021-4
45. Juster R-P, Perna A, Marin M-F, Sindi S, Lupien SJ. Timing is everything: anticipatory stress dynamics among cortisol and blood pressure reactivity and recovery in healthy adults. Stress (2012) 15(6):569–77. doi: 10.3109/10253890.2012.661494
46. Pulopulos MM, Vanderhasselt M-A, De Raedt R. Association between changes in heart rate variability during the anticipation of a stressful situation and the stress-induced cortisol response. Psychoneuroendocrinology (2018) 94:63–71. doi: 10.1016/j.psyneuen.2018.05.004
47. Powell DJ, Schlotz W. Daily life stress and the cortisol awakening response: testing the anticipation hypothesis. PloS One (2012) 7(12):e52067. doi: 10.1371/journal.pone.0052067
48. Xu H, Ouyang N, Li R, Tuo P, Mai M, Wang W. The effects of anxiety and depression on in vitro fertilization outcomes of Chinese women. Psychol Health Med (2017) 22:37–43. doi: 10.1080/13548506.2016.1218031
49. Boivin J, Griffiths E, Venetis CA. Emotional distress in infertile women and failure of assisted reproductive technologies: meta-analysis of prospective psychosocial studies. BMJ (2011) 342:d223. doi: 10.1136/bmj.d223
50. Galst JP. The elusive connection between stress and infertility: a research review with clinical implications. J Psychother Integration (2018) 28(1):1. doi: 10.1037/int0000081
51. Breen KM, Karsch FJ. Does cortisol inhibit pulsatile luteinizing hormone secretion at the hypothalamic or pituitary level? Endocrinology (2004) 145(2):692–8. doi: 10.1210/en.2003-1114
52. Morrison AE, Fleming S, Levy MJ. A review of the pathophysiology of functional hypothalamic amenorrhoea in women subject to psychological stress, disordered eating, excessive exercise or a combination of these factors. Clin Endocrinol (2021) 95(2):229–38. doi: 10.1111/cen.14399
53. García-León MÁ, Caparrós-González RA, Romero-González B, González-Perez R, Peralta-Ramírez I. Resilience as a protective factor in pregnancy and puerperium: its relationship with the psychological state, and with hair cortisol concentrations. Midwifery (2019) 75:138–45. doi: 10.1016/j.midw.2019.05.006
54. du Fossé NA, Marie-Louise P, van der Hoorn JMM, van Lith SleC, Lashley EELO. Advanced paternal age is associated with an increased risk of spontaneous miscarriage: a systematic review and meta-analysis. Hum Reprod Update (2020) 26(5):650–69. doi: 10.1093/humupd/dmaa010
55. Johnson SL, Dunleavy J, Gemmell NJ, Nakagawa S. Consistent age-dependent declines in human semen quality: a systematic review and meta-analysis. " Ageing Res Rev (2015) 19:22–33. doi: 10.1016/j.arr.2014.10.007
56. Pinheiro R, Lomelino ALA, Pinto AM, Donato H. Advanced maternal age: adverse outcomes of pregnancy, a meta-analysis. Acta Med portuguesa (2019) 32(3):219–26. doi: 10.20344/amp.11057
57. Amanvermez R, Tosun M. An update on ovarian aging and ovarian reserve tests. Int J fertil steril (2016) 9:411. doi: 10.22074/ijfs.2015.4591
58. Llarena N, Hine C. Reproductive longevity and aging: geroscience approaches to maintain long-term ovarian fitness. Journals Gerontol: Ser A (2021) 76(9):1551–60. doi: 10.1093/gerona/glaa204
59. Cavazos-Rehg PA, Krauss MJ, Spitznagel EL, Bommarito K, Madden T, Olsen MA, et al. Maternal age and risk of labor and delivery complications. Maternal Child Health J (2015) 19:1202–11. doi: 10.1007/s10995-014-1624-7
60. Chen K, Shen W, Zhang Z, Xiong F, Ouyang Q, Luo C. Age-dependent decline in stress response capacity revealed by proteins dynamics analysis. Sci Rep (2020) 10(1):15211. doi: 10.1038/s41598-020-72167-4
61. Agbedia OO, Varma VR, Seplaki CL, Seeman TE, Fried LP, Li L, et al. Blunted diurnal decline of cortisol among older adults with low socioeconomic status. Ann N Y Acad Sci (2011) 1231:56–64. doi: 10.1111/j.1749-6632.2011.06151.x
62. Johar H, Emeny RT, Bidlingmaier M, Reincke M, Thorand B, Peters A, et al. Blunted diurnal cortisol pattern is associated with frailty: a cross-sectional study of 745 participants aged 65 to 90 years. J Clin Endocrinol Metab (2014) 99(3):E464–8. doi: 10.1210/jc.2013-3079
63. Gonzalez Rodriguez E, Marques-Vidal P, Aubry-Rozier B, Papadakis G, Preisig M, Kuehner C, et al. Diurnal salivary cortisol in sarcopenic postmenopausal women: the OsteoLaus cohort. Calcified Tissue Int (2021) 109(5):499–509. doi: 10.1007/s00223-021-00863-y
64. Schoorlemmer RM, Peeters GM, van Schoor NM, Lips P. Relationships between cortisol level, mortality and chronic diseases in older persons. Clin Endocrinol (Oxf). (2009) 71(6):779–86. doi: 10.1111/j.1365-2265.2009.03552.x
65. Yiallouris A, Tsioutis C, Agapidaki E, Zafeiri M, Agouridis AP, Ntourakis D, et al. Adrenal aging and its implications on stress responsiveness in humans. Front Endocrinol (Lausanne). (2019) 10:54. doi: 10.3389/fendo.2019.00054
66. Zapater-Fajarí M, Crespo-Sanmiguel I, Pulopulos MM, Hidalgo V, Salvador A. Resilience and psychobiological response to stress in older people: the mediating role of coping strategies. Front Aging Neurosci (2021) 13:632141. doi: 10.3389/fnagi.2021.632141
67. McAuley MT, Kenny RA, Kirkwood TBL, Wilkinson DJ, Jones JJL, Miller VM. A mathematical model of aging-related and cortisol induced hippocampal dysfunction. BMC Neurosci (2009) 10(1):1–14. doi: 10.1186/1471-2202-10-26
68. Valbuena Perez JV, Linnenberger R, Dembek A, Bruscoli S, Riccardi C, Schulz MH, et al. Altered glucocorticoid metabolism represents a feature of macroph-aging. Aging Cell (2020) 19(6):e13156. doi: 10.1111/acel.13156
69. Rooney KL, Domar AD. The relationship between stress and infertility. Dialogues Clin Neurosci (2018) 20(1):41–7. doi: 10.31887/DCNS.2018.20.1/klrooney
70. Stalder T, Steudte-Schmiedgen S, Alexander N, Klucken T, Vater A, Wichmann S, et al. Stress-related and basic determinants of hair cortisol in humans: a meta-analysis. Psychoneuroendocrinology (2017) 77:261–74. doi: 10.1016/j.psyneuen.2016.12.017
71. Joseph DN, Whirledge S. Stress and the HPA axis: balancing homeostasis and fertility. Int J Mol Sci (2017) 18(10):2224. doi: 10.3390/ijms18102224
72. Nargund VH. Effects of psychological stress on male fertility. Nat Rev Urol (2015) 12(7):373–82. doi: 10.1038/nrurol.2015.112
73. Wright C, Milne S, Leeson H. Sperm DNA damage caused by oxidative stress: modifiable clinical, lifestyle and nutritional factors in male infertility. Reprod biomed Online (2014) 28(6):684–703. doi: 10.1016/j.rbmo.2014.02.004
74. Ilacqua A, Izzo G, Emerenziani GP, Baldari C, Aversa A. Lifestyle and fertility: the influence of stress and quality of life on male fertility. Reprod Biol Endocrinol (2018) 16:1–11. doi: 10.1186/s12958-018-0436-9
75. Kloss JD, Perlis ML, Zamzow JA, Culnan EJ, Gracia CR. Sleep, sleep disturbance, and fertility in women. Sleep Med Rev (2015) 22:78–87. doi: 10.1016/j.smrv.2014.10.005
76. Palnitkar G, Phillips CL, Hoyos CM, Marren AJ, Bowman MC, Yee BJ. Linking sleep disturbance to idiopathic male infertility. Sleep Med Rev (2018) 42:149–59. doi: 10.1016/j.smrv.2018.07.006
77. Bodenmann G, Atkins DC, Schär M, Poffet V. The association between daily stress and sexual activity. J Family Psychol (2010) 24(3):271–9. doi: 10.1037/a0019365
Keywords: cortisol, infertility, subfertility, HPA, fertility, pregnancy, stress, sterility
Citation: Karunyam BV, Abdul Karim AK, Naina Mohamed I, Ugusman A, Mohamed WMY, Faizal AM, Abu MA and Kumar J (2023) Infertility and cortisol: a systematic review. Front. Endocrinol. 14:1147306. doi: 10.3389/fendo.2023.1147306
Received: 18 January 2023; Accepted: 07 June 2023;
Published: 29 June 2023.
Edited by:
Shunfeng Cheng, Qingdao Agricultural University, ChinaReviewed by:
Rafeah Pakri Mohamed, UCSI University, MalaysiaJing-Cai Liu, Nanjing Agricultural University, China
Copyright © 2023 Karunyam, Abdul Karim, Naina Mohamed, Ugusman, Mohamed, Faizal, Abu and Kumar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jaya Kumar, jayakumar@ukm.edu.my; Abdul Kadir Abdul Karim, abdulkadir73@ppukm.ukm.edu.my
 Bheena Vyshali Karunyam1
Bheena Vyshali Karunyam1