- 1Department of Renal Medicine, Monash University Eastern Health Clinical School, Box Hill, VIC, Australia
- 2Department of Renal Medicine, Eastern Health, Box Hill, VIC, Australia
With increasing life expectancy, the related disorders of bone loss, metabolic dysregulation and sarcopenia have become major health threats to the elderly. Each of these conditions is prevalent in patients with chronic kidney disease (CKD), particularly in more advanced stages. Our current understanding of the bone-muscle interaction is beyond mechanical coupling, where bone and muscle have been identified as interrelated secretory organs, and regulation of both bone and muscle metabolism occurs through osteokines and myokines via autocrine, paracrine and endocrine systems. This review appraises the current knowledge regarding biochemical crosstalk between bone and muscle, and considers recent progress related to the role of osteokines and myokines in CKD, including modulatory effects of physical exercise and potential therapeutic targets to improve musculoskeletal health in CKD patients.
Introduction
Chronic kidney disease (CKD) has a complex relationship with ageing, where the CKD phenotype provides an accelerated model of ageing through various mechanisms while ageing also hastens the progression of kidney disease. Patients affected by CKD experience profound musculoskeletal functional decline at a younger age, which is compounded by concurrent losses in bone and skeletal muscle mass, leading to reduced mobility and excessively high rates of falls and fractures, the effects of which are often life-limiting.
Disturbances in mineral and bone metabolism in CKD are conventionally jointly referred to as CKD-Mineral Bone Disorder (CKD-MBD), and comprises abnormalities in the homeostasis of calcium, phosphorus, vitamin D and parathyroid hormone (PTH); abnormalities of bone turnover, mineralisation or volume; and vascular or soft tissue calcification (1). Despite earlier research and vigorous exploration of therapeutic strategies in managing skeletal health focusing on bone and mineral abnormalities, a disturbing limitation in patient care persists, particularly in those with advanced CKD and/or who are dialysis-dependent. Meaningful clinical and biological targets are lacking in this population, resulting in management uncertainty for the prevention of bone loss and fractures.
Furthermore, it is increasingly recognised that sarcopenia plays a detrimental role in musculoskeletal health in CKD. Sarcopenia is a condition characterised by a reduction in muscle mass, strength and/or performance that accrues over many years and is associated with ageing (2). However, it is now recognised that sarcopenia begins earlier in life with many contributing factors beyond ageing alone. CKD patients suffer severe skeletal muscle wasting, and again this is particularly evident in its advanced stages. There is no pharmacological treatment available at present to reverse or halt the progression of muscle atrophy, although aerobic and resistance-training exercise and nutritional interventions have been shown to be of some benefit (3–5).
Our understanding of the interaction between bone and skeletal muscle now exceeds the concept of purely mechanical coupling, with evidence that these tissues communicate at a biomolecular level (6). Bone and muscle have been shown to be interrelated secretory organs, which produce osteokines (bone-derived factors) and myokines (muscle-derived factors) respectively. Each of these is important in regulating bone and muscle metabolism through autocrine, paracrine and endocrine signalling. The growing knowledge about the crosstalk between bone and muscle, and the likely influence of exercise on both tissues has important implications for clinical practice and introduces potential therapeutic targets to improve bone and muscle parameters, and consequently, the patient’s overall health and wellbeing. However, the biochemical relationship between bone and muscle in tandem is less well understood in the uraemic milieu.
This review presents an in-depth discussion about the known endocrine and other crosstalk between bone and muscle, the modulatory effects of physical exercise on these tissues, potential therapeutic targets and, lastly, important research questions in this field.
Bone fragility in patients with chronic kidney disease
In the general population, osteoporosis is defined as a reduction in bone density; while bone disorders in CKD are more complex. The diagnosis and management of bone disorders in CKD is challenging for several reasons: (i) heterogeneous changes in bone tissue other than osteoporosis that could compromise bone strength such as osteomalacia, osteitis fibrosa cystica, adynamic bone disease (with inadequate bone turnover) and mixed bone lesions; (ii) the inability of dual-energy X-ray absorptiometry to provide meaningful details regarding underlying bone mineral density; (iii) inaccuracy of serum-derived bone turnover markers due to reduced renal clearance and (iv) infrequent use of bone biopsy, the gold standard determinant of bone pathology, due to its restricted availability, invasive nature, and limited interpretative expertise (7). Dialysis-dependent patients have a 4- to 14-fold higher risk of developing fractures than the healthy general population, a risk which extends to those with an estimated glomerular filtration rate (eGFR) between 15 to 60 mL/min/1.73m2 (8–12).
Despite early research efforts to identify better management strategies, the incidence of fractures has continued to rise in recent years, with no therapeutic agent yet approved for patients with kidney-related bone disease. Agents that have been targeted include vitamin D analogues and calcimimetics to suppress PTH and improve bone remodelling, thus potentially reducing fracture risk, and antiresorptive and osteoanabolic agents. The latter are approved for osteoporosis in the general population and have been administered off-label in CKD Stage 3B-5 high-risk patients. However, their use has been limited due to the lack of large-scale clinical trials and concern regarding their contribution to further kidney dysfunction and adynamic bone disease, which has now evolved as the predominant form of renal osteodystrophy associated with poor outcomes (13, 14).
Muscle health in patients with chronic kidney disease
Skeletal muscle is the largest tissue in the human body, accounting for about 40-50% of body mass (15). It is imperative for gait and posture and also functions as an endocrine organ. Maximal muscle mass is achieved during young adulthood but after the age of 50, muscle loss occurs at a rate of ~1-2% per annum (16). Sarcopenia, a recently recognised disease entity, is common in older-aged adults but can also occur earlier in life from systemic illnesses, particularly conditions that trigger an inflammatory response such as CKD and malignancy. Sarcopenia is thus prevalent in the CKD population and is associated with an increased risk of hospitalisation and mortality in both dialysis and non-dialysis dependent patients. Low skeletal muscle mass (determined by radiological measures) is associated both with a higher waitlist mortality among kidney transplant candidates and an increase hospital readmission rate within the first 30 days after kidney transplant discharge (17, 18).
Several risk factors have been proposed to contribute to the development of sarcopenia in CKD including ageing, chronic inflammation, hormonal changes/resistance, metabolic acidosis, a more sedentary lifestyle and poor nutritional status leading to an imbalance between protein synthesis and degradation. We recently reported that the differentially expressed genes and proteins in skeletal muscle of CKD subjects belong to 8 major biological and signalling pathways, namely apoptosis, autophagy, inflammation, insulin/insulin-like growth factor 1 (IGF1) signalling, lipid metabolism, mitochondrial function, muscle cell growth and differentiation, and protein turnover (19).
Bone metabolism and remodelling
The overall composition of bone tissue is altered in CKD due to abnormal systemic mineral metabolism and bone remodelling. Cells within bone include osteocytes (90-95%), osteoblasts (5%), and osteoclasts (1%) (20). It is a dynamic tissue and its structural integrity is maintained by bone remodelling, consisting of coordinated actions of the three cell types in a process tightly regulated by both local and systemic factors (21). The presence of the myogenic interleukin-6 (IL-6) activates the secretion of osteoblast and osteocyte-induced receptor activator of nuclear factor-κB (RANK), which drives osteoclastogenesis (22). This also results in an increased expression of RANKL, which binds to its receptor and triggers a cascade of signalling events that induce osteoclast differentiation, activation and survival. By contrast, osteoprotegerin (OPG), a soluble decoy receptor, binds RANKL to prevent the latter binding to RANK, thereby inhibiting osteoclastogenesis (23) and restraining bone loss. Dysregulation of the RANK-RANKL-OPG axis can lead to osteoporosis. Studies investigating RANKL levels in CKD patients have demonstrated conflicting findings (24–26), whereas OPG concentrations have consistently been reported to be higher in haemodialysis patients (24, 27, 28), which could reflect a compensatory protective mechanism to moderate bone remodelling.
Osteokines and muscle metabolism
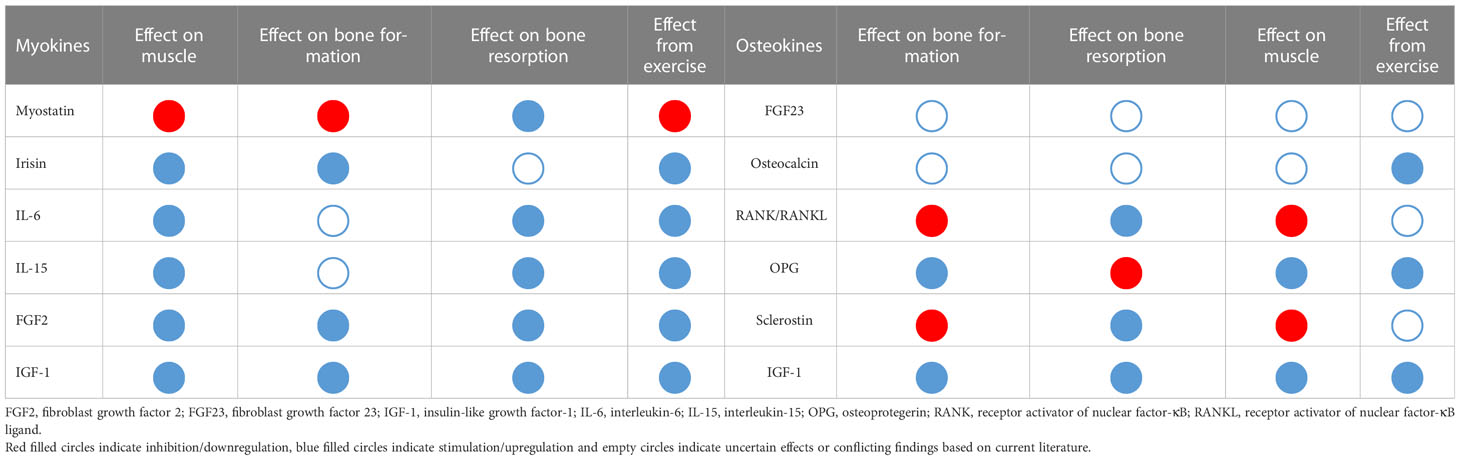
The discovery that osteocytes produced fibroblast growth factor 23 (FGF23), which circulates in different forms targeting the kidney and other organs including muscle, led to the recognition of bone as an endocrine organ. The list of osteokines has since continued to expand. Herein, we describe several osteokines that have been demonstrated to have regulatory effects on muscles (see Table 1).
Fibroblast growth factor 23
FGF23 was first discovered in 2000 as a cause of autosomal dominant hypophosphataemic rickets (29). It is part of a superfamily of 22 peptides grouped into 7 subfamilies. FGF23 is mainly secreted by osteocytes and osteoblasts. It downregulates the luminal expression of sodium/phosphorus co-transporters in the proximal renal tubules to stimulate phosphaturia (30). FGF23 also suppresses the production of 1,25(OH)2Vitamin D by inhibiting 1-alpha hydroxylase, leading to phosphate wasting (31) and consequently poor bone mineralisation. The canonical FGF23 signalling pathway requires the obligatory co-receptor alpha klotho (α-KL), a transmembrane protein with extracellular glucuronidase activity, for binding to the first of four tissue-specific fibroblast growth factor receptors (FGFR), FGFR1 (32). However, some FGF23 signalling occurs independent of α-KL and is referred to as non-canonical FGF23 signalling, through binding and activation of other receptors: FGFR3/FGFR4/calcineurin/nuclear factor of activated T-cells (33), mainly in the setting of markedly elevated circulating FGF23 levels. Effects include distinct changes in several organs. Treatment of neonatal rat ventricular myocytes for 48 hours with varying concentrations of FGF23 induced morphometric hypertrophy in a similar extent to treatment with fibroblast growth factor 2 (FGF2). Moreover, in vivo experiments using both intravenous and intramyocardial injection of FGF23 showed induction of left ventricular hypertrophy in non-CKD mice (34).
What effects FGF23 has in vivo, with or without α-KL, remains uncertain. Higher serum levels have been shown to be independently associated with pre-frailty and frailty in a large cohort of community-dwelling elderly inhabitants (35). Similarly, as part of the SPRINT trial, Jovanovich et al. reported that FGF23 was associated with increased frailty among older adults with CKD (36), suggesting that FGF23 might have more diverse negative biological effects. Both FGF23 and α-KL were previously shown to inhibit differentiation of cultured skeletal muscle cells through downregulation of insulin/IGF-1 signalling (37). FGF23 was also found to induce premature senescence of human skeletal muscle mesenchymal stem cells via the p53/p21 pathway in an α-KL-independent manner, supporting its inhibitory effects (38). However, plasma FGF23 concentrations have also been reported to be positively correlated with muscle mass indices in a small haemodialysis cohort (39). As a potential therapeutic target, exercise endurance was found to improve significantly in C57BL/6J mice following exogenous administration of recombinant FGF23 (40). However, Avin et al. found that FGF23 did not influence C2C12 myoblast proliferation and differentiation and ex-vivo FGF23 treatment did not alter soleus contractility (41). Thus, the regulatory effect of FGF23 on skeletal muscle remains unresolved and further research is required to address this question.
Osteocalcin
Osteocalcin (OCN) is the most abundant non-collagenous osteoblast-derived protein. There are two main forms of OCN in the circulation: γ−carboxylated and uncarboxylated (uOCN). Considered to be the active form, the latter has been shown to be involved in the regulation of insulin secretion and sensitivity (42), glucose metabolism (43), male fertility (44) and brain function (45). Although the data from in vitro and in vivo studies are controversial (46–49), OCN is thought to play an important role in bone remodelling by modulating osteoblast and osteoclast activity. High levels of circulating OCN and uOCN were observed in CKD patients (50–52), potentially due to increased bone metabolism, decreased renal clearance or both, with progression correlating with serum intact PTH and alkaline phosphatase (ALP) (50, 51), most probably reflecting the severity of the underlying bone disorder.
OCN was also discovered to promote muscle uptake and utilisation of glucose and fatty acids. Ocn-/- mice were found to have impaired exercise capacity, which was rescued by exogenous OCN administration (53). OCN was also shown to be a major regulator of IL-6 expression in the muscle during exercise and the rise in circulating IL-6 levels was proposed to originate from muscle, eventually forming a feed-forward axis to amplify adaptation to exercise (53). Moreover, using mice lacking OCN (Ocn-/-), its receptor in all cells (Gprc6a-/-) and specifically in myofibres (Gprc6aMck-/-), Mera et al. showed that OCN signalling is essential in maintaining muscle mass by promoting protein synthesis in myotubes (54). uOCN was later found to enhance C2C12 myoblast cell proliferation and differentiation through activation of the PI3k/Akt, p38 MAPK and GPRC6A-ERK1/2 signalling pathways (55). Taken together, these findings strongly support that OCN, especially its active form, plays an important role in regulating muscle mass and might potentially be a therapeutic target in sarcopenia. However, recent studies have found to be contrary. Using a newly generated Ocn-deficient mouse model by deleting Bglap and Bglap2, Moriishi et al. showed that OCN played no role in bone formation or resorption, glucose metabolism, testosterone synthesis, or muscle mass (56). Similarly, Diegel et al. generated Ocn-deficient mouse using a CRISPR/Cas9-mediated gene editing tool and found that these mice displayed normal bone mass, serum glucose and fertility (57). The apparent discrepancies between studies remain inexplicable and additional efforts are required to confirm these findings.
Receptor activator of nuclear factor-κB/Receptor activator of nuclear factor-κB ligand/Osteoprotegerin
RANK and RANKL are expressed in osteoclasts and osteoblasts respectively, as well as in skeletal muscle, and their interaction activates the nuclear factor-κB (NF-κB) signalling pathway, a key transcription factor inducing the expression of various proinflammatory genes, which can inhibit myogenic differentiation and activate the local ubiquitin proteasome system, ultimately leading to muscle atrophy (58). In addition, RANK has been shown to regulate calcium storage and muscle performance during denervation (59). Both genetic deletion of muscle RANK and short-term anti-RANKL treatment were shown to improve muscle integrity and strength of young dystrophic mdx mice (60). Hamoudi et al. demonstrated that anti-RANKL treatment inhibited NF-κB signalling and increased the proportion of M2 macrophages in dystrophic mice, thus reducing muscle inflammation and improving its mechanical properties (61). Similar findings were also observed in young dystrophic mdx mice when treated with recombinant full length OPG-Fc, a decoy receptor for RANKL (62). Furthermore, postmenopausal women who were treated with denosumab, a neutralising antibody against RANKL, for an average duration of 3 years were found to have improved appendicular lean mass and handgrip strength and these gains were absent in the bisphosphate treatment group (63). Altogether, it seems that the RANK-RANKL-OPG axis plays a pivotal role in bone and muscle metabolism. Given that coexistence of osteoporosis and sarcopenia is prevalent in the elderly population, the potential benefit of anti-RANKL treatment in possibly mitigating skeletal muscle atrophy while enhancing bone mechanical properties should be further investigated. However, any relationship between higher OPG concentrations (and OPG/RANKL ratio) and sarcopenia in CKD is yet to be determined.
Sclerostin
Sclerostin is primarily secreted by mature osteocytes (64) and is a negative regulator of bone formation via inhibition of the Wnt/β-catenin pathway through binding to Wnt coreceptors, low-density lipoprotein receptor-related proteins 5 and 6 (65). Wnt-3a was also found to promote C2C12 myoblast differentiation through upregulation of MyoD and Myogenin while sclerostin treatment inhibited the effect of Wnt-3a on the C2C12 myoblast differentiation (65). A recent cross-sectional study of 240 healthy non-diabetic Korean individuals found that serum sclerostin levels were significantly higher in the low muscle mass group (66) and similar findings were observed in haemodialysis patients with diabetes (67). Interestingly, in a breast cancer mice model with bone metastasis, treatment with anti-sclerostin antibody prevented tumour growth in bone and bone destruction, as well as improvement in muscle function (68). Romosozumab, a human monoclonal antibody directed against sclerostin, has recently been approved for treatment of osteoporosis in postmenopausal women with high fracture risk. Its effect on skeletal muscle remains to be confirmed in larger human studies. Interestingly, skeletal muscle has also been found to secrete sclerostin, which works synergistically with bone-derived sclerostin to strengthen the negative regulatory mechanism of osteogenesis (69).
Insulin-like growth factor 1
IGF1 is an anabolic hormone with about 50% structural homology with proinsulin. It is primarily synthesised in the liver, but also in extrahepatic tissues including bone and acts on skeletal muscle in a paracrine manner, primarily through the Type 1 IGF receptor (IGF1R) to stimulate cellular uptake of glucose and amino acids, enhance protein synthesis and suppress protein degradation (70). It is an important determinant of muscle mass and function. pAkt, which is a major cellular signalling effector of insulin and IGF-1, was consistently found to be reduced in the skeletal muscle of CKD individuals (19). A reduction of Akt activity induces activation of FOXO transcription factors, ultimately resulting in overexpression of genes that are involved in catabolic processes as well as autophagy (71). Moreover, reduced IGF1 concentrations in CKD patients have been associated with body composition and lower bone mineral density (72, 73). However, IGF1 therapy has not been shown to have beneficial effects on bone density, muscle strength or muscle mass in older women (74).
Myokines and bone metabolism
Muscle-derived factors are called myokines, a term first proposed by Pedersen and colleagues in 2010 (75). These molecules include but are not limited to myostatin, irisin, IL-6, IL-8, IL-15, leukaemia inhibitory factor, brain-derived neurotrophic factor, IGF-1 and FGF2 (76).
Myostatin
Myostatin was the first myokine identified in 1997 (77) and is primarily produced in skeletal muscle. It is a highly conserved member of the transforming growth factor-β superfamily and is one of the most potent negative regulators of skeletal myogenesis. It inhibits muscle cell growth and differentiation by interacting with the activin type II receptors (ActRIIA and ActRIIB), leading to upregulation of the cytokines and other signalling mediators that disrupt protein metabolism (71). Myostatin knockout results in excessive skeletal muscle hypertrophy in mice (78, 79) and notably, myostatin deficiency also increases bone mineral density (80–82), which might be attributed to both loading-associated effects and biochemical interaction between bone and muscle. Myostatin was later discovered to have a negative impact on bone remodelling by enhancing osteoclastogenesis and reducing bone formation (83). Enhancement of osteogenic differentiation was observed in bone-marrow derived mesenchymal stem cells from Mstn-/- mice as compared to wild-type mice, which was load-dependent (81). Furthermore, myostatin was shown to accelerate RANKL-mediated osteoclast formation via activation of the NFAT signalling pathway (84).
Several studies have investigated the plasma concentrations of myostatin in CKD patients, the majority of them reporting higher levels in CKD and dialysis-dependent patients compared to healthy subjects (85). A few novel myostatin-targeted agents such as LY2495655 (humanised myostatin antibody that neutralises myostatin) and bimagrumab (humanised monoclonal antibody that binds to ActRII) have been tested in Phase II clinical trials with inconsistent results; some demonstrating positive outcomes with increased lean body mass and improved handgrip strength and gait speed (86, 87), while others did not (88).
Irisin
Irisin, a cleaved product of fibronectin Type III domain containing 5 (FNDC5), is secreted from skeletal muscle in response to an increased expression of peroxisome proliferator-activated receptor-γ co-activator-1-α (PGC1α) following exercise, to promote thermogenesis by browning white fat. It is also possibly involved in glucose metabolism (89). Irisin has been shown to promote myogenesis by enhancing myoblast proliferation and differentiation, increasing protein synthesis via activation of Akt and ERK, expanding the satellite cell pool and upregulating the expression of exercise-related genes, for example IL-6 (90, 91). Its anabolic effects on bone tissue are supported by in vivo studies, where low-dose weekly irisin injections for 4 weeks in young male mice resulted in increased cortical bone mass and strength, stimulating bone formation via upregulation of osteogenic transcription factors including activating transcription factor 4, Runt-related transcription factor 2 and Sp7 transcription factor. Interestingly, a lower number of osteoclasts were also observed in mice treated with irisin, which might contribute to the increase in bone strength (92). Recent findings raise the possibility that irisin could be a potential target for treating osteoporosis/CKD-MBD and sarcopenia. There is a known negative correlation between circulating irisin levels and osteoporotic fractures in postmenopausal women (93). Furthermore, plasma irisin levels are known to be lower in CKD patients (94), and recently, reduced irisin expression in the gastrocnemius muscle of 5/6 nephrectomised mice was found to be correlated with cortical bone mineral density (95).
Interleukins
Some circulating inflammatory cytokines (e.g. IL-6, IL-7 and IL-15) are important for muscle development and growth as well as activation of muscle repair mechanisms. As mentioned previously, muscle-derived IL-6 promotes myogenesis and skeletal muscle growth via the regulation of proliferative capability of muscle stem cells (96). Apart from its glucose uptake and fatty acid oxidation, IL-6 stimulates bone resorption by inducing RANK and RANKL expression and the resorptive process has been demonstrated to be dependent on osteoblast signalling (22). IL-6-/- mice have increased bone formation and higher osteoclast numbers, but with a greater osteoclast apoptosis rate and reduction in resorption capacity (97). Its role in osteoblastogenesis remains controversial. Low grade chronic inflammation is prevalent in CKD patients and the interactions between cytokines, inflammation and muscle wasting are complex. On the one hand, systemic inflammation strongly correlates with muscle wasting, malnutrition, cardiovascular disease and mortality in patients with end-stage kidney disease (ESKD) (98–100). Higher expression of tumour necrosis factor-alpha (TNF-α) and IL-6 were observed in the muscle of CKD patients and mice compared to healthy controls and were associated with the development of muscle atrophy (19). Contrarily, both TNF-α and IL-6 have pleiotropic functions with a positive effect on muscle growth and regeneration. IL-6 was also shown to facilitate the local infiltration of macrophages and stimulate local IGF-1 production in muscle tissue of CKD mice (101). Furthermore, increased IL-6 efflux from muscle was found to correlate with increased muscle protein synthesis during haemodialysis (102). Similarly, IL-15, another anabolic factor in skeletal muscle, has also been demonstrated to have conflicting effects on osteoclast activity and bone mass (103, 104). Finally, higher circulating IL-15 levels correlate with a reduction in body fat and increased bone mineral content in mice (105).
Others
Each of muscle-derived IGF1 and FGF2 exert anabolic effects on bone metabolism by promoting osteoblast proliferation and hastening bone formation. IGF1 regulates bone anabolism as a response to enhanced osteoblast survival and proliferation, whereas FGF2 has been proposed to be secreted following disruption of the plasma membrane in response to injury or mechanical muscle contraction, rather than by exocytosis (106). Moreover, FGF2 was found to reduce glucocorticoid-mediated bone resorption via inhibition of sclerostin signalling, reinforcing its anabolic effects on bone metabolism (107). Circulating FGF2 levels have been reported to be lower in patients with more advanced CKD (108, 109), though its role in the development of sarcopenia in CKD is yet to be determined.
Effects of exercise on osteokines and myokines in chronic kidney disease
Observational studies have shown that patients with advanced CKD, particularly those on maintenance haemodialysis often have a sedentary lifestyle (110, 111) and about 45% of end-stage kidney disease (ESKD) patients report not performing any exercise at all (112). The health benefits of regular physical activity include a reduced risk of non-communicable diseases (e.g. heart disease, stroke and diabetes), better blood pressure control and improved mental health as well as overall quality of life. Physical activity improves physical function and reduces both pain and fall risk among adults with arthritis (113). In the CKD population, a highly active treatment group had a 25% risk reduction of all-cause mortality in comparison to inactive patients, even when factored for the presence of ESKD (114). Physical exercise also has a beneficial impact on bone mass, through promotion of bone formation and inhibition of bone resorption (115–117).
Several osteokines and myokines such as uOCN, OPG, irisin, IL-7, IL-15 and IL-6 are released in response to exercise training, exerting favourable physiological and metabolic effects in skeletal muscle and bone, in conjunction with a systemic anti-inflammatory effect (118, 119). In addition, both plasma and muscle myostatin levels were shown to decrease following aerobic and resistive training (120, 121). However, Gomes et al. investigated the effect of aerobic exercise on bone metabolism biomarkers (OCN, uOCN, sclerostin, PTH and total ALP) in non-dialysis CKD patients and found no differences in these biomarkers following a 24-week period of low-moderate intensity aerobic training except the total ALP (122). Similarly, both irisin and OCN levels were unaffected by resistance exercise in haemodialysis patients, though an increase in OPG was observed (123, 124). Yet, Zhou et al. reported higher plasma myostatin levels following 12 months of exercise training in a cohort of 151 non-dialysis-dependent CKD patients (125). These contrasting results underline the need for further studies in determining the effects of exercise (aerobic, resistance or alternative forms) on these bone-muscle biomarkers in CKD. Notwithstanding inconsistent findings from previous studies, a recent systematic review investigating the effects of physical activity in CKD patients reported beneficial effects of resistance exercise on bone health (126).
Conclusions and directions
Over the last thirty years, studies in CKD patients have mostly focused either on bone or on muscle separately without recognition of an interplay between the two organs. This oversight has perhaps driven the bias and associated interpretative limitations of mechanical coupling. A better understanding of the secretory crosstalk and associated biochemical coupling between these two organs represents a subject of great interest, with conceivable potential in identifying novel therapeutic targets and ultimately addressing the major unmet needs in managing renal osteodystrophy and sarcopenia in CKD population.
That said, the well-documented beneficial effects of exercise on bone and skeletal muscle in CKD cannot be understated. Although many dialysis-dependent patients might be too frail to engage in vigorous exercise, a less intense regimen may still be valuable. It is of note that the incorporation of exercise programs into standard clinical care has been slow, possibly because of feasibility concerns (127). However, in line with the World Health Organisation 2018-2030 Global Action Plan to promote physical activity for each according to their ability across the life course, health professionals play an essential role in improving access and quality of health care in the CKD population. There are also potential opportunities for digital innovations to promote and support participation in physical activity to improve the health and well-being of our patients.
To finish, several key questions remain unanswered: (1) Is there a connection between osteoporosis, renal osteodystrophy and sarcopenia? (2) Does one condition precede or metabolically influence the other? (3) Is the phenotypic loss of muscle tissue simply related to comparable changes in bone over the course of CKD progression, each profoundly influenced by uraemia and a profoundly sedentary lifestyle? (4) Are other tissues, notably adipocytes, involved in the observed deleterious changes to bone and muscle in CKD?
Author contributions
LW contributed to conception and design of the article. LW wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
LW is supported by the Royal Australasian College of Physicians Jacquot Establishment Fellowship 2021 (2021REF00021) and 2022 (2022REF00084). The aim of the Jacquot Research Establishment Fellowship is to assist establishment of a career in nephrology research for a nephrologist who has completed a research higher degree in an area of relevance. The Fellowship is supported by the Estate of the late Don and Lorraine Jacquot and co-administered with the Australian and New Zealand Society of Nephrology Fellowship.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Moe S, Drueke T, Cunningham J, Goodman W, Martin K, Olgaard K, et al. Definition, evaluation, and classification of renal osteodystrophy: A position statement from kidney disease: Improving global outcomes (KDIGO). Kidney Int (2006) 69(11):1945–53. doi: 10.1038/sj.ki.5000414
2. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing (2019) 48(1):16–31. doi: 10.1093/ageing/afy169
3. Cheema BS, Singh MA. Exercise training in patients receiving maintenance hemodialysis: A systematic review of clinical trials. Am J Nephrol (2005) 25(4):352–64. doi: 10.1159/000087184
4. Heiwe S, Jacobson SH. Exercise training for adults with chronic kidney disease. Cochrane Database Syst Rev (2011) 10:CD003236. doi: 10.1002/14651858.CD003236.pub2
5. Morley JE, Argiles JM, Evans WJ, Bhasin S, Cella D, Deutz NE, et al. Nutritional recommendations for the management of sarcopenia. J Am Med Dir Assoc (2010) 11(6):391–6. doi: 10.1016/j.jamda.2010.04.014
6. Brotto M, Johnson ML. Endocrine crosstalk between muscle and bone. Curr Osteoporos Rep (2014) 12(2):135–41. doi: 10.1007/s11914-014-0209-0
7. Iseri K, Dai L, Chen Z, Qureshi AR, Brismar TB, Stenvinkel P, et al. Bone mineral density and mortality in end-stage renal disease patients. Clin Kidney J (2020) 13(3):307–21. doi: 10.1093/ckj/sfaa089
8. Alem AM, Sherrard DJ, Gillen DL, Weiss NS, Beresford SA, Heckbert SR, et al. Increased risk of hip fracture among patients with end-stage renal disease. Kidney Int (2000) 58(1):396–9. doi: 10.1046/j.1523-1755.2000.00178.x
9. Coco M, Rush H. Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. Am J Kidney Dis (2000) 36(6):1115–21. doi: 10.1053/ajkd.2000.19812
10. Dooley AC, Weiss NS, Kestenbaum B. Increased risk of hip fracture among men with CKD. Am J Kidney Dis (2008) 51(1):38–44. doi: 10.1053/j.ajkd.2007.08.019
11. Jadoul M, Albert JM, Akiba T, Akizawa T, Arab L, Bragg-Gresham JL, et al. Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the dialysis outcomes and practice patterns study. Kidney Int (2006) 70(7):1358–66. doi: 10.1038/sj.ki.5001754
12. Nickolas TL, McMahon DJ, Shane E. Relationship between moderate to severe kidney disease and hip fracture in the united states. J Am Soc Nephrol (2006) 17(11):3223–32. doi: 10.1681/ASN.2005111194
13. Malluche HH, Mawad HW, Monier-Faugere MC. Renal osteodystrophy in the first decade of the new millennium: Analysis of 630 bone biopsies in black and white patients. J Bone Miner Res (2011) 26(6):1368–76. doi: 10.1002/jbmr.309
14. Sprague SM, Bellorin-Font E, Jorgetti V, Carvalho AB, Malluche HH, Ferreira A, et al. Diagnostic accuracy of bone turnover markers and bone histology in patients with CKD treated by dialysis. Am J Kidney Dis (2016) 67(4):559–66. doi: 10.1053/j.ajkd.2015.06.023
15. Griffiths RD. Muscle mass, survival, and the elderly ICU patient. Nutrition (1996) 12(6):456–8. doi: 10.1016/S0899-9007(96)00141-4
16. von Haehling S, Morley JE, Anker SD. From muscle wasting to sarcopenia and myopenia: update 2012. J Cachexia Sarcopenia Muscle (2012) 3(4):213–7. doi: 10.1007/s13539-012-0089-z
17. Wong L, Kent AB, Lee D, Roberts MA, McMahon LP. Low muscle mass and early hospital readmission post-kidney transplantation. Int Urol Nephrol (2022) 54(8):1977–86. doi: 10.1007/s11255-021-03085-1
18. Locke JE, Carr JJ, Nair S, Terry JG, Reed RD, Smith GD, et al. Abdominal lean muscle is associated with lower mortality among kidney waitlist candidates. Clin Transplant (2017) 31(3). doi: 10.1111/ctr.12911
19. Wong L, Kenny R, Howard JL, McMahon LP. Molecular mechanisms underpinning muscle atrophy in CKD (TH-PO828). J Am Soc Nephrol (2022) 33:285.
20. Lu W, Xiao W, Xie W, Fu X, Pan L, Jin H, et al. The role of osteokines in sarcopenia: Therapeutic directions and application prospects. Front Cell Dev Biol (2021) 9:735374. doi: 10.3389/fcell.2021.735374
21. Kular J, Tickner J, Chim SM, Xu J. An overview of the regulation of bone remodelling at the cellular level. Clin Biochem (2012) 45(12):863–73. doi: 10.1016/j.clinbiochem.2012.03.021
22. Udagawa N, Takahashi N, Katagiri T, Tamura T, Wada S, Findlay DM, et al. Interleukin (IL)-6 induction of osteoclast differentiation depends on IL-6 receptors expressed on osteoblastic cells but not on osteoclast progenitors. J Exp Med (1995) 182(5):1461–8. doi: 10.1084/jem.182.5.1461
23. Mizuno A, Amizuka N, Irie K, Murakami A, Fujise N, Kanno T, et al. Severe osteoporosis in mice lacking osteoclastogenesis inhibitory factor/osteoprotegerin. Biochem Biophys Res Commun (1998) 247(3):610–5. doi: 10.1006/bbrc.1998.8697
24. Doumouchtsis KK, Kostakis AI, Doumouchtsis SK, Tziamalis MP, Tsigris C, Kostaki MA, et al. sRANKL/osteoprotegerin complex and biochemical markers in a cohort of male and female hemodialysis patients. J Endocrinol Invest (2007) 30(9):762–6. doi: 10.1007/BF03350814
25. Avbersek-Luznik I, Balon BP, Rus I, Marc J. Increased bone resorption in HD patients: is it caused by elevated RANKL synthesis? Nephrol Dial Transplant (2005) 20(3):566–70. doi: 10.1093/ndt/gfh672
26. Albalate M, de la Piedra C, Fernandez C, Lefort M, Santana H, Hernando P, et al. Association between phosphate removal and markers of bone turnover in haemodialysis patients. Nephrol Dial Transplant (2006) 21(6):1626–32. doi: 10.1093/ndt/gfl034
27. Avbersek-Luznik I, Malesic I, Rus I, Marc J. Increased levels of osteoprotegerin in hemodialysis patients. Clin Chem Lab Med (2002) 40(10):1019–23. doi: 10.1515/CCLM.2002.177
28. Kazama JJ, Shigematsu T, Yano K, Tsuda E, Miura M, Iwasaki Y, et al. Increased circulating levels of osteoclastogenesis inhibitory factor (osteoprotegerin) in patients with chronic renal failure. Am J Kidney Dis (2002) 39(3):525–32. doi: 10.1053/ajkd.2002.31402
29. ADHR Consortium. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet (2000) 26(3):345–8. doi: 10.1038/81664
30. Shimada T, Urakawa I, Yamazaki Y, Hasegawa H, Hino R, Yoneya T, et al. FGF-23 transgenic mice demonstrate hypophosphatemic rickets with reduced expression of sodium phosphate cotransporter type IIa. Biochem Biophys Res Commun (2004) 314(2):409–14. doi: 10.1016/j.bbrc.2003.12.102
31. Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, et al. FGF-23 is a potent regulator of vitamin d metabolism and phosphate homeostasis. J Bone Miner Res (2004) 19(3):429–35. doi: 10.1359/JBMR.0301264
32. Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature (2006) 444(7120):770–4. doi: 10.1038/nature05315
33. Ho BB, Bergwitz C. FGF23 signalling and physiology. J Mol Endocrinol (2021) 66(2):R23–32. doi: 10.1530/JME-20-0178
34. Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest (2011) 121(11):4393–408. doi: 10.1172/JCI46122
35. Beben T, Ix JH, Shlipak MG, Sarnak MJ, Fried LF, Hoofnagle AN, et al. Fibroblast growth factor-23 and frailty in elderly community-dwelling individuals: The cardiovascular health study. J Am Geriatr Soc (2016) 64(2):270–6. doi: 10.1111/jgs.13951
36. Jovanovich A, Ginsberg C, You Z, Katz R, Ambrosius WT, Berlowitz D, et al. FGF23, frailty, and falls in SPRINT. J Am Geriatr Soc (2021) 69(2):467–73. doi: 10.1111/jgs.16895
37. Kido S, Hashimoto Y, Segawa H, Tatsumi S, Miyamoto K. Muscle atrophy in patients with CKD results from FGF23/klotho-mediated suppression of insulin/IGF-1 signalling. Kidney Res Clin Pract (2012) 31:A44.
38. Sato C, Iso Y, Mizukami T, Otabe K, Sasai M, Kurata M, et al. Fibroblast growth factor-23 induces cellular senescence in human mesenchymal stem cells from skeletal muscle. Biochem Biophys Res Commun (2016) 470(3):657–62. doi: 10.1016/j.bbrc.2016.01.086
39. Fukasawa H, Ishigaki S, Kinoshita-Katahashi N, Niwa H, Yasuda H, Kumagai H, et al. Plasma levels of fibroblast growth factor-23 are associated with muscle mass in haemodialysis patients. Nephrol (Carlton) (2014) 19(12):784–90. doi: 10.1111/nep.12333
40. Li DJ, Fu H, Zhao T, Ni M, Shen FM. Exercise-stimulated FGF23 promotes exercise performance via controlling the excess reactive oxygen species production and enhancing mitochondrial function in skeletal muscle. Metabolism (2016) 65(5):747–56. doi: 10.1016/j.metabol.2016.02.009
41. Avin KG, Vallejo JA, Chen NX, Wang K, Touchberry CD, Brotto M, et al. Fibroblast growth factor 23 does not directly influence skeletal muscle cell proliferation and differentiation or ex vivo muscle contractility. Am J Physiol Endocrinol Metab (2018) 315(4):E594–604. doi: 10.1152/ajpendo.00343.2017
42. Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, et al. Endocrine regulation of energy metabolism by the skeleton. Cell (2007) 130(3):456–69. doi: 10.1016/j.cell.2007.05.047
43. Ferron M, McKee MD, Levine RL, Ducy P, Karsenty G. Intermittent injections of osteocalcin improve glucose metabolism and prevent type 2 diabetes in mice. Bone (2012) 50(2):568–75. doi: 10.1016/j.bone.2011.04.017
44. Oury F, Sumara G, Sumara O, Ferron M, Chang H, Smith CE, et al. Endocrine regulation of male fertility by the skeleton. Cell (2011) 144(5):796–809. doi: 10.1016/j.cell.2011.02.004
45. Puig J, Blasco G, Daunis-i-Estadella J, Moreno M, Molina X, Alberich-Bayarri A, et al. Lower serum osteocalcin concentrations are associated with brain microstructural changes and worse cognitive performance. Clin Endocrinol (Oxf) (2016) 84(5):756–63. doi: 10.1111/cen.12954
46. Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, et al. Increased bone formation in osteocalcin-deficient mice. Nature (1996) 382(6590):448–52. doi: 10.1038/382448a0
47. Chenu C, Colucci S, Grano M, Zigrino P, Barattolo R, Zambonin G, et al. Osteocalcin induces chemotaxis, secretion of matrix proteins, and calcium-mediated intracellular signaling in human osteoclast-like cells. J Cell Biol (1994) 127(4):1149–58. doi: 10.1083/jcb.127.4.1149
48. Boskey AL, Gadaleta S, Gundberg C, Doty SB, Ducy P, Karsenty G. Fourier Transform infrared microspectroscopic analysis of bones of osteocalcin-deficient mice provides insight into the function of osteocalcin. Bone (1998) 23(3):187–96. doi: 10.1016/S8756-3282(98)00092-1
49. Bodine PV, Komm BS. Evidence that conditionally immortalized human osteoblasts express an osteocalcin receptor. Bone (1999) 25(5):535–43. doi: 10.1016/S8756-3282(99)00213-6
50. Delmas PD, Wilson DM, Mann KG, Riggs BL. Effect of renal function on plasma levels of bone gla-protein. J Clin Endocrinol Metab (1983) 57(5):1028–30. doi: 10.1210/jcem-57-5-1028
51. Alpdemir M, Fidanci V, Alpdemir MF, Azak A, Saydam G, Duranay M, et al. Serum undercarboxylated osteocalcin levels are related to bone disease in hemodialysis and peritoneal dialysis patients. Eur Res J (2021) 7(3):225–34. doi: 10.18621/eurj.734216
52. Millar SA, John SG, McIntyre CW, Ralevic V, Anderson SI, O’Sullivan SE. An investigation into the role of osteocalcin in human arterial smooth muscle cell calcification. Front Endocrinol (Lausanne) (2020) 11:369. doi: 10.3389/fendo.2020.00369
53. Mera P, Laue K, Ferron M, Confavreux C, Wei J, Galan-Diez M, et al. Osteocalcin signaling in myofibers is necessary and sufficient for optimum adaptation to exercise. Cell Metab (2016) 23(6):1078–92. doi: 10.1016/j.cmet.2016.05.004
54. Mera P, Laue K, Wei J, Berger JM, Karsenty G. Osteocalcin is necessary and sufficient to maintain muscle mass in older mice. Mol Metab (2016) 5(10):1042–7. doi: 10.1016/j.molmet.2016.07.002
55. Liu S, Gao F, Wen L, Ouyang M, Wang Y, Wang Q, et al. Osteocalcin induces proliferation via positive activation of the PI3K/Akt, P38 MAPK pathways and promotes differentiation through activation of the GPRC6A-ERK1/2 pathway in C2C12 myoblast cells. Cell Physiol Biochem (2017) 43(3):1100–12. doi: 10.1159/000481752
56. Moriishi T, Ozasa R, Ishimoto T, Nakano T, Hasegawa T, Miyazaki T, et al. Osteocalcin is necessary for the alignment of apatite crystallites, but not glucose metabolism, testosterone synthesis, or muscle mass. PloS Genet (2020) 16(5):e1008586. doi: 10.1371/journal.pgen.1008586
57. Diegel CR, Hann S, Ayturk UM, Hu JCW, Lim KE, Droscha CJ, et al. An osteocalcin-deficient mouse strain without endocrine abnormalities. PloS Genet (2020) 16(5):e1008361. doi: 10.1371/journal.pgen.1008361
58. Langen RC, Schols AM, Kelders MC, Wouters EF, Janssen-Heininger YM. Inflammatory cytokines inhibit myogenic differentiation through activation of nuclear factor-kappaB. FASEB J (2001) 15(7):1169–80. doi: 10.1096/fj.00-0463
59. Dufresne SS, Dumont NA, Boulanger-Piette A, Fajardo VA, Gamu D, Kake-Guena SA, et al. Muscle RANK is a key regulator of Ca2+ storage, SERCA activity, and function of fast-twitch skeletal muscles. Am J Physiol Cell Physiol (2016) 310(8):C663–72. doi: 10.1152/ajpcell.00285.2015
60. Dufresne SS, Boulanger-Piette A, Bosse S, Argaw A, Hamoudi D, Marcadet L, et al. Genetic deletion of muscle RANK or selective inhibition of RANKL is not as effective as full-length OPG-fc in mitigating muscular dystrophy. Acta Neuropathol Commun (2018) 6(1):31. doi: 10.1186/s40478-018-0533-1
61. Hamoudi D, Marcadet L, Piette Boulanger A, Yagita H, Bouredji Z, Argaw A, et al. An anti-RANKL treatment reduces muscle inflammation and dysfunction and strengthens bone in dystrophic mice. Hum Mol Genet (2019) 28(18):3101–12. doi: 10.1093/hmg/ddz124
62. Dufresne SS, Dumont NA, Bouchard P, Lavergne E, Penninger JM, Frenette J. Osteoprotegerin protects against muscular dystrophy. Am J Pathol (2015) 185(4):920–6. doi: 10.1016/j.ajpath.2015.01.006
63. Bonnet N, Bourgoin L, Biver E, Douni E, Ferrari S. RANKL inhibition improves muscle strength and insulin sensitivity and restores bone mass. J Clin Invest (2019) 129(8):3214–23. doi: 10.1172/JCI125915
64. Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, et al. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J (2003) 22(23):6267–76. doi: 10.1093/emboj/cdg599
65. Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, et al. Sclerostin binds to LRP5/6 and antagonizes canonical wnt signaling. J Biol Chem (2005) 280(20):19883–7. doi: 10.1074/jbc.M413274200
66. Kim JA, Roh E, Hong SH, Lee YB, Kim NH, Yoo HJ, et al. Association of serum sclerostin levels with low skeletal muscle mass: The Korean sarcopenic obesity study (KSOS). Bone (2019) 128:115053. doi: 10.1016/j.bone.2019.115053
67. Medeiros MC, Rocha N, Bandeira E, Dantas I, Chaves C, Oliveira M, et al. Serum sclerostin, body composition, and sarcopenia in hemodialysis patients with diabetes. Int J Nephrol (2020) 2020:4596920. doi: 10.1155/2020/4596920
68. Hesse E, Schroder S, Brandt D, Pamperin J, Saito H, Taipaleenmaki H. Sclerostin inhibition alleviates breast cancer-induced bone metastases and muscle weakness. JCI Insight (2019) 5(9). doi: 10.1172/jci.insight.125543
69. Magaro MS, Bertacchini J, Florio F, Zavatti M, Poti F, Cavani F, et al. Identification of sclerostin as a putative new myokine involved in the muscle-to-Bone crosstalk. Biomedicines (2021) 9(1). doi: 10.3390/biomedicines9010071
70. Le Roith D. Seminars in medicine of the Beth Israel deaconess medical center. insulin-like growth factors. N Engl J Med (1997) 336(9):633–40. doi: 10.1056/NEJM199702273360907
71. Wang XH, Mitch WE, Price SR. Pathophysiological mechanisms leading to muscle loss in chronic kidney disease. Nat Rev Nephrol (2022) 18(3):138–52. doi: 10.1038/s41581-021-00498-0
72. Park SH, Jia T, Qureshi AR, Barany P, Heimburger O, Larsson TE, et al. Determinants and survival implications of low bone mineral density in end-stage renal disease patients. J Nephrol (2013) 26(3):485–94. doi: 10.5301/jn.5000185
73. Jia T, Gama Axelsson T, Heimburger O, Barany P, Lindholm B, Stenvinkel P, et al. IGF-1 and survival in ESRD. Clin J Am Soc Nephrol (2014) 9(1):120–7. doi: 10.2215/CJN.02470213
74. Friedlander AL, Butterfield GE, Moynihan S, Grillo J, Pollack M, Holloway L, et al. One year of insulin-like growth factor I treatment does not affect bone density, body composition, or psychological measures in postmenopausal women. J Clin Endocrinol Metab (2001) 86(4):1496–503. doi: 10.1210/jcem.86.4.7377
75. Pedersen BK. Muscles and their myokines. J Exp Biol (2011) 214(Pt 2):337–46. doi: 10.1242/jeb.048074
76. Brotto M, Bonewald L. Bone and muscle: Interactions beyond mechanical. Bone (2015) 80:109–14. doi: 10.1016/j.bone.2015.02.010
77. Lee JH, Jun HS. Role of myokines in regulating skeletal muscle mass and function. Front Physiol (2019) 10:42. doi: 10.3389/fphys.2019.00042
78. McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature (1997) 387(6628):83–90. doi: 10.1038/387083a0
79. Lin J, Arnold HB, Della-Fera MA, Azain MJ, Hartzell DL, Baile CA. Myostatin knockout in mice increases myogenesis and decreases adipogenesis. Biochem Biophys Res Commun (2002) 291(3):701–6. doi: 10.1006/bbrc.2002.6500
80. Kellum E, Starr H, Arounleut P, Immel D, Fulzele S, Wenger K, et al. Myostatin (GDF-8) deficiency increases fracture callus size, sox-5 expression, and callus bone volume. Bone (2009) 44(1):17–23. doi: 10.1016/j.bone.2008.08.126
81. Hamrick MW, Shi X, Zhang W, Pennington C, Thakore H, Haque M, et al. Loss of myostatin (GDF8) function increases osteogenic differentiation of bone marrow-derived mesenchymal stem cells but the osteogenic effect is ablated with unloading. Bone (2007) 40(6):1544–53. doi: 10.1016/j.bone.2007.02.012
82. Hamrick MW. Increased bone mineral density in the femora of GDF8 knockout mice. Anat Rec A Discovery Mol Cell Evol Biol (2003) 272(1):388–91. doi: 10.1002/ar.a.10044
83. Elkasrawy MN, Hamrick MW. Myostatin (GDF-8) as a key factor linking muscle mass and bone structure. J Musculoskelet Neuronal Interact (2010) 10(1):56–63.
84. Dankbar B, Fennen M, Brunert D, Hayer S, Frank S, Wehmeyer C, et al. Myostatin is a direct regulator of osteoclast differentiation and its inhibition reduces inflammatory joint destruction in mice. Nat Med (2015) 21(9):1085–90. doi: 10.1038/nm.3917
85. Bataille S, Chauveau P, Fouque D, Aparicio M, Koppe L. Myostatin and muscle atrophy during chronic kidney disease. Nephrol Dial Transplant (2021) 36(11):1986–93. doi: 10.1093/ndt/gfaa129
86. Rooks D, Praestgaard J, Hariry S, Laurent D, Petricoul O, Perry RG, et al. Treatment of sarcopenia with bimagrumab: Results from a phase II, randomized, controlled, proof-of-Concept study. J Am Geriatr Soc (2017) 65(9):1988–95. doi: 10.1111/jgs.14927
87. Becker C, Lord SR, Studenski SA, Warden SJ, Fielding RA, Recknor CP, et al. Myostatin antibody (LY2495655) in older weak fallers: a proof-of-concept, randomised, phase 2 trial. Lancet Diabetes Endocrinol (2015) 3(12):948–57. doi: 10.1016/S2213-8587(15)00298-3
88. Woodhouse L, Gandhi R, Warden SJ, Poiraudeau S, Myers SL, Benson CT, et al. A phase 2 randomized study investigating the efficacy and safety of myostatin antibody LY2495655 versus placebo in patients undergoing elective total hip arthroplasty. J Frailty Aging (2016) 5(1):62–70. doi: 10.14283/jfa.2016.81
89. Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature (2012) 481(7382):463–8. doi: 10.1038/nature10777
90. Huh JY, Dincer F, Mesfum E, Mantzoros CS. Irisin stimulates muscle growth-related genes and regulates adipocyte differentiation and metabolism in humans. Int J Obes (Lond) (2014) 38(12):1538–44. doi: 10.1038/ijo.2014.42
91. Reza MM, Subramaniyam N, Sim CM, Ge X, Sathiakumar D, McFarlane C, et al. Irisin is a pro-myogenic factor that induces skeletal muscle hypertrophy and rescues denervation-induced atrophy. Nat Commun (2017) 8(1):1104. doi: 10.1038/s41467-017-01131-0
92. Colaianni G, Cuscito C, Mongelli T, Pignataro P, Buccoliero C, Liu P, et al. The myokine irisin increases cortical bone mass. Proc Natl Acad Sci U S A (2015) 112(39):12157–62. doi: 10.1073/pnas.1516622112
93. Anastasilakis AD, Polyzos SA, Makras P, Gkiomisi A, Bisbinas I, Katsarou A, et al. Circulating irisin is associated with osteoporotic fractures in postmenopausal women with low bone mass but is not affected by either teriparatide or denosumab treatment for 3 months. Osteoporos Int (2014) 25(5):1633–42. doi: 10.1007/s00198-014-2673-x
94. Wen MS, Wang CY, Lin SL, Hung KC. Decrease in irisin in patients with chronic kidney disease. PloS One (2013) 8(5):e64025. doi: 10.1371/journal.pone.0064025
95. Kawao N, Kawaguchi M, Ohira T, Ehara H, Mizukami Y, Takafuji Y, et al. Renal failure suppresses muscle irisin expression, and irisin blunts cortical bone loss in mice. J Cachexia Sarcopenia Muscle (2022) 13(1):758–71. doi: 10.1002/jcsm.12892
96. Munoz-Canoves P, Scheele C, Pedersen BK, Serrano AL. Interleukin-6 myokine signaling in skeletal muscle: a double-edged sword? FEBS J (2013) 280(17):4131–48. doi: 10.1111/febs.12338
97. Liu H, Feng W, Yimin, Cui J, Lv S, Hasegawa T, et al. Histological evidence of increased osteoclast cell number and asymmetric bone resorption activity in the tibiae of interleukin-6-Deficient mice. J Histochem Cytochem (2014) 62(8):556–64. doi: 10.1369/0022155414537830
98. Mihai S, Codrici E, Popescu ID, Enciu AM, Albulescu L, Necula LG, et al. Inflammation-related mechanisms in chronic kidney disease prediction, progression, and outcome. J Immunol Res (2018) 2018:2180373. doi: 10.1155/2018/2180373
99. Honda H, Qureshi AR, Heimburger O, Barany P, Wang K, Pecoits-Filho R, et al. Serum albumin, c-reactive protein, interleukin 6, and fetuin a as predictors of malnutrition, cardiovascular disease, and mortality in patients with ESRD. Am J Kidney Dis (2006) 47(1):139–48. doi: 10.1053/j.ajkd.2005.09.014
100. Avesani CM, Carrero JJ, Axelsson J, Qureshi AR, Lindholm B, Stenvinkel P. Inflammation and wasting in chronic kidney disease: Partners in crime. Kidney Int (2006) 70:S8–13. doi: 10.1038/sj.ki.5001969
101. Hu L, Klein JD, Hassounah F, Cai H, Zhang C, Xu P, et al. Low-frequency electrical stimulation attenuates muscle atrophy in CKD–a potential treatment strategy. J Am Soc Nephrol (2015) 26(3):626–35. doi: 10.1681/ASN.2014020144
102. Raj DS, Moseley P, Dominic EA, Onime A, Tzamaloukas AH, Boyd A, et al. Interleukin-6 modulates hepatic and muscle protein synthesis during hemodialysis. Kidney Int (2008) 73(9):1054–61. doi: 10.1038/ki.2008.21
103. Djaafar S, Pierroz DD, Chicheportiche R, Zheng XX, Ferrari SL, Ferrari-Lacraz S. Inhibition of T cell-dependent and RANKL-dependent osteoclastogenic processes associated with high levels of bone mass in interleukin-15 receptor-deficient mice. Arthritis Rheumatol (2010) 62(11):3300–10. doi: 10.1002/art.27645
104. Takeda H, Kikuchi T, Soboku K, Okabe I, Mizutani H, Mitani A, et al. Effect of IL-15 and natural killer cells on osteoclasts and osteoblasts in a mouse coculture. Inflammation (2014) 37(3):657–69. doi: 10.1007/s10753-013-9782-0
105. Quinn LS, Anderson BG, Strait-Bodey L, Stroud AM, Argiles JM. Oversecretion of interleukin-15 from skeletal muscle reduces adiposity. Am J Physiol Endocrinol Metab (2009) 296(1):E191–202. doi: 10.1152/ajpendo.90506.2008
106. Hamrick MW. The skeletal muscle secretome: an emerging player in muscle-bone crosstalk. Bonekey Rep (2012) 1:60. doi: 10.1038/bonekey.2012.60
107. Adhikary S, Choudhary D, Tripathi AK, Karvande A, Ahmad N, Kothari P, et al. FGF-2 targets sclerostin in bone and myostatin in skeletal muscle to mitigate the deleterious effects of glucocorticoid on musculoskeletal degradation. Life Sci (2019) 229:261–76. doi: 10.1016/j.lfs.2019.05.022
108. Bozic M, Betriu A, Bermudez-Lopez M, Ortiz A, Fernandez E, Valdivielso JM, et al. Association of FGF-2 concentrations with atheroma progression in chronic kidney disease patients. Clin J Am Soc Nephrol (2018) 13(4):577–84. doi: 10.2215/CJN.07980717
109. Velasquez-Mao AJ, Velasquez MA, Hui Z, Armas-Ayon D, Wang J, Vandsburger MH. Hemodialysis exacerbates proteolytic imbalance and pro-fibrotic platelet dysfunction. Sci Rep (2021) 11(1):11764. doi: 10.1038/s41598-021-91416-8
110. Johansen KL, Chertow GM, Ng AV, Mulligan K, Carey S, Schoenfeld PY, et al. Physical activity levels in patients on hemodialysis and healthy sedentary controls. Kidney Int (2000) 57(6):2564–70. doi: 10.1046/j.1523-1755.2000.00116.x
111. Segura-Orti E, Gordon PL, Doyle JW, Johansen KL. Correlates of physical functioning and performance across the spectrum of kidney function. Clin Nurs Res (2018) 27(5):579–96. doi: 10.1177/1054773816689282
112. Avesani CM, Trolonge S, Deleaval P, Baria F, Mafra D, Faxen-Irving G, et al. Physical activity and energy expenditure in haemodialysis patients: an international survey. Nephrol Dial Transplant (2012) 27(6):2430–4. doi: 10.1093/ndt/gfr692
113. Torres A, Tennant B, Ribeiro-Lucas I, Vaux-Bjerke A, Piercy K, Bloodgood B. Umbrella and systematic review methodology to support the 2018 physical activity guidelines advisory committee. J Phys Act Health (2018) 15(11):805–10. doi: 10.1123/jpah.2018-0372
114. Kuo CP, Tsai MT, Lee KH, Lin YP, Huang SS, Huang CC, et al. Dose-response effects of physical activity on all-cause mortality and major cardiorenal outcomes in chronic kidney disease. Eur J Prev Cardiol (2022) 29(3):452–61. doi: 10.1093/eurjpc/zwaa162
115. Alghadir AH, Gabr SA, Al-Eisa ES, Alghadir MH. Correlation between bone mineral density and serum trace elements in response to supervised aerobic training in older adults. Clin Interv Aging (2016) 11:265–73. doi: 10.2147/CIA.S100566
116. Marinho SM, Moraes C, Barbosa JE, Carraro Eduardo JC, Fouque D, Pelletier S, et al. Exercise training alters the bone mineral density of hemodialysis patients. J Strength Cond Res (2016) 30(10):2918–23. doi: 10.1519/JSC.0000000000001374
117. Adami S, Gatti D, Viapiana O, Fiore CE, Nuti R, Luisetto G, et al. Physical activity and bone turnover markers: a cross-sectional and a longitudinal study. Calcif Tissue Int (2008) 83(6):388–92. doi: 10.1007/s00223-008-9184-8
118. Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol (2012) 8(8):457–65. doi: 10.1038/nrendo.2012.49
119. Leal DV, Ferreira A, Watson EL, Wilund KR, Viana JL. Muscle-bone crosstalk in chronic kidney disease: The potential modulatory effects of exercise. Calcif Tissue Int (2021) 108(4):461–75. doi: 10.1007/s00223-020-00782-4
120. Hittel DS, Axelson M, Sarna N, Shearer J, Huffman KM, Kraus WE. Myostatin decreases with aerobic exercise and associates with insulin resistance. Med Sci Sports Exerc (2010) 42(11):2023–9. doi: 10.1249/MSS.0b013e3181e0b9a8
121. Ryan AS, Ivey FM, Prior S, Li G, Hafer-Macko C. Skeletal muscle hypertrophy and muscle myostatin reduction after resistive training in stroke survivors. Stroke (2011) 42(2):416–20. doi: 10.1161/STROKEAHA.110.602441
122. Gomes TS, Aoike DT, Baria F, Graciolli FG, Moyses RMA, Cuppari L. Effect of aerobic exercise on markers of bone metabolism of overweight and obese patients with chronic kidney disease. J Ren Nutr (2017) 27(5):364–71. doi: 10.1053/j.jrn.2017.04.009
123. Moraes C, Leal VO, Marinho SM, Barroso SG, Rocha GS, Boaventura GT, et al. Resistance exercise training does not affect plasma irisin levels of hemodialysis patients. Horm Metab Res (2013) 45(12):900–4. doi: 10.1055/s-0033-1354402
124. Marinho SM, Carraro Eduardo JC, Mafra D. Effect of a resistance exercise training program on bone markers in hemodialysis patients. Sci Sports (2017) 32:99–105. doi: 10.1016/j.scispo.2017.01.003
125. Zhou Y, Hellberg M, Hellmark T, Hoglund P, Clyne N. Muscle mass and plasma myostatin after exercise training: a substudy of renal exercise (RENEXC)-a randomized controlled trial. Nephrol Dial Transplant (2021) 36(1):95–103. doi: 10.1093/ndt/gfz210
126. Cardoso DF, Marques EA, Leal DV, Ferreira A, Baker LA, Smith AC, et al. Impact of physical activity and exercise on bone health in patients with chronic kidney disease: a systematic review of observational and experimental studies. BMC Nephrol (2020) 21(1):334. doi: 10.1186/s12882-020-01999-z
Keywords: osteokines, myokines, chronic kidney disease, crosstalk, bone, muscle
Citation: Wong L and McMahon LP (2023) Crosstalk between bone and muscle in chronic kidney disease. Front. Endocrinol. 14:1146868. doi: 10.3389/fendo.2023.1146868
Received: 18 January 2023; Accepted: 14 March 2023;
Published: 23 March 2023.
Edited by:
Andrea Galassi, Santi Paolo e Carlo Hospital, ItalyReviewed by:
Renqing Zhao, Yangzhou University, ChinaCopyright © 2023 Wong and McMahon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Limy Wong, bGlteXdvbmdAZ21haWwuY29t
†ORCID: Limy Wong, orcid.org/0000-0003-0604-0380
 Limy Wong
Limy Wong Lawrence P. McMahon1,2
Lawrence P. McMahon1,2