- Department of Endocrinology and Metabolism, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China
Aims: Growth differentiation factor-15 (GDF-15) and adiponectin are adipokines that regulate metabolism. This study aimed to evaluate the roles of GDF-15, adiponectin, and GDF-15/adiponectin ratio (G/A ratio) as biomarkers for detecting metabolic syndrome (MS).
Materials and methods: This cross-sectional study included 676 participants aged 20–70 years in Jurong, China. The participants were divided into four groups based on sex and age (<40 and ≥40 years). MS was defined according to the modified National Cholesterol Education Program Adult Treatment Panel III criteria. Receiver operating characteristic curves were used to evaluate the performance of GDF-15, adiponectin, and the G/A ratio in predicting MS.
Results: The prevalence of MS was 22.0% (149/676). Logistic regression analysis indicated that the G/A ratio and adiponectin levels, but not GDF-15 levels, were correlated with MS [odds ratio; 95% CI 1.010 (1.006–1.013) and 0.798 (0.735–0.865), respectively] after adjusting for confounding factors. The G/A ratio displayed a significant relationship with MS in each subgroup and with each MS component in both men and women; however, adiponectin concentrations were significantly associated with MS and all its components only in men (all P <0.05). The area under the curve (AUC) of the G/A ratio and the adiponectin level for MS was 0.758 and 0.748, respectively. The highest AUC was 0.757 for the adiponectin level in men and 0.724 for the G/A ratio in women.
Conclusions: This study suggests that the G/A ratio and adiponectin are potential biomarkers for detecting MS in women and men, respectively.
1 Introduction
Metabolic syndrome (MS) is a cluster of coexisting metabolic risk factors, including central obesity, hyperglycemia, hypertension, and dyslipidemia, imposing significant burdens on global health (1). Therefore, there is an urgent need for appropriate and effective biomarkers to identify individuals at high risk of MS.
Growth differentiation factor 15 (GDF-15) belongs to the transforming growth factor (TGF)-β superfamily and is characterized by wide tissue distribution, anti-inflammatory effects, and its role in cellular responses to stress signals (2). Adipose tissue secretes GDF-15, which acts as an adipokine and may play a paracrine role in modulating adipose tissue function and body mass (3). Increased GDF-15 levels have been reported to be correlated with many metabolic diseases, including obesity, cardiovascular disease (CVD), type 2 diabetes mellitus (T2DM), and metabolic-associated fatty liver disease (4). A few studies have found that MS is associated with elevated GDF-15 levels among older adults (5–7). Therefore, both GDF-15 levels and MS prevalence are strongly affected by age (6, 8); however, the exact relationship between the two has not yet been determined.
Adiponectin is an adipocyte-specific factor that acts as a crucial bridge between adipose tissues and other metabolic organs (9). In addition, adiponectin reduces oxidative stress and inflammatory cytokines, thereby improving insulin resistance (IR) (10, 11). Previous cross-sectional studies have reported that hypoadiponectinemia is closely associated with MS and its components (12, 13); however, a meta-analysis has indicated that increased adiponectin level is an independent protector against the development of MS, especially in men (14). Moreover, the relationship between adiponectin and MS varies with sex (15) and race (16).
GDF-15 and adiponectin have been identified as metabolic coordinators, playing roles in improving lipolysis and IR through anti-inflammatory and antioxidative effects (2, 11). Higher GDF-15 concentrations and lower adiponectin levels are correlated with MS. Wu et al. first used the index of GDF-15/adiponectin ratio (G/A ratio), whose increment was independently associated with the risk of T2DM for all study populations, compared to GDF-15 or adiponectin alone, suggesting the combination of these two adipokines might have an “enhancing effect” on predicting the risk of T2DM (17). In this study, we aimed to evaluate the roles of GDF-15, adiponectin, and the G/A ratio as biomarkers for detecting MS.
2 Materials and methods
2.1 Study population
A total of 853 individuals aged 20–70 years living in Jurong City were recruited for this study. All subjects were ethnic Han Chinese. The final analysis was performed on 676 participants after excluding 107 participants treated with antidiabetic, antihypertensive, or lipid-lowering medications; 36 participants without GDF-15 measurements; 16 participants with CVD (e.g., myocardial infarction, stroke, or heart failure), autoimmune diseases, or cancers; and 18 participants with incomplete data. Based on age (40 years, which is the criterion used to divide young and middle-aged individuals) and sex, the cohort was divided into four groups: group 1 (age <40 years, women, n=185), group 2 (age <40 years, men, n=175), group 3 (age ≥40 years, women, n=113), and group 4 (age ≥40 years, men, n=203). All participants provided written informed consent, and the study was approved by the institutional review board of the First Affiliated Hospital of Nanjing Medical University (2021-SR-298).
2.2 Data collection and measurements
The participants completed a detailed health and lifestyle questionnaire, which included questions on age, sex, smoking and drinking status, physical activity, education, disease history, and medication information. Smoking status was divided into three categories: current, former, and never-smoker. Current drinking was referred to as “alcohol consumption at least once per week for the previous 6 months”. Regular exercise was defined as engaging in 30 min of leisure-time activities at least three times a week, including jogging, swimming, cycling, playing ball, dancing, and mountain climbing. Education level was classified as primary school or below, middle or high school, or college or above.
After a 12–h overnight fasting, the participants underwent anthropometric evaluation and blood collection. The separated plasma was stored at −80°C before analysis. Measurements included height, weight, and waist circumference (WC) according to a standard protocol. Body mass index (BMI) was calculated as the weight (kg)/height squared (m2). Blood pressure (BP) was measured twice with 1–2 min intervals to obtain average readings after at least 5 min rest.
Serum GDF-15 concentrations were measured using a Human Quantikine ELISA Kit (R&D Systems, Minneapolis, MN, USA). Adiponectin and insulin levels were detected using chemiluminescence detection kits (YHLO Biotech, Shenzhen, China). Fasting plasma glucose (FPG) levels were measured using the hexokinase method (AU5400; Olympus, Tokyo, Japan). Glycated hemoglobin (HbA1c) levels were examined using capillary blood samples (Variant II; Bio-Rad, Hercules, USA). Levels of triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and total cholesterol (TC) were measured using a chemiluminescence autoanalyzer (Modular E170; Roche, Basel, Switzerland). HOMA-IR was calculated as follows: FPG (mmol/L) × fasting insulin (mIU/L)/22.5 (18).
2.3 Definition of MS
MS was defined as the presence of at least three of the following abnormalities according to the modified NCEP ATP III criteria for Asians (19): (1) abdominal obesity: WC ≥90 cm in men or ≥80 cm in women; (2) hypertension: systolic BP ≥130 mmHg or diastolic BP ≥85 mmHg or drug-treated hypertension; (3) TG ≥150 mg/dL (1.70 mmol/L) or drug treatment for elevated TG; (4) HDL-C <40 mg/dL (1.03 mmol/L) for men or <50 mg/dL (1.30 mmol/L) for women or drug treatment for low HDL-C; and (5) hyperglycemia: FPG ≥110 mg/dL (6.1 mmol/L) or taking hypoglycaemic agents.
2.4 Statistical analysis
Statistical analyses were conducted using SPSS 23.0. Data are presented as the mean ± SD, number (%), or median (interquartile range). For continuous variables, Student’s t-test or Mann–Whitney U test was performed between groups. Categorical variables were compared using X2 tests. Correlations between various indicators of metabolic parameters were evaluated using Spearman’s correlation. A logistic regression model was used to determine the independent effects of GDF-15 levels, adiponectin levels, and the G/A ratio on MS and its components. Restricted cubic spline regression was used to assess dose-response relationships. Potential confounding factors were included in the model to minimize bias. Receiver operating characteristic (ROC) curves were drawn to evaluate the performance of the potential biomarkers in identifying participants with MS. A two-sided P <0.05 was considered statistically significant.
3 Results
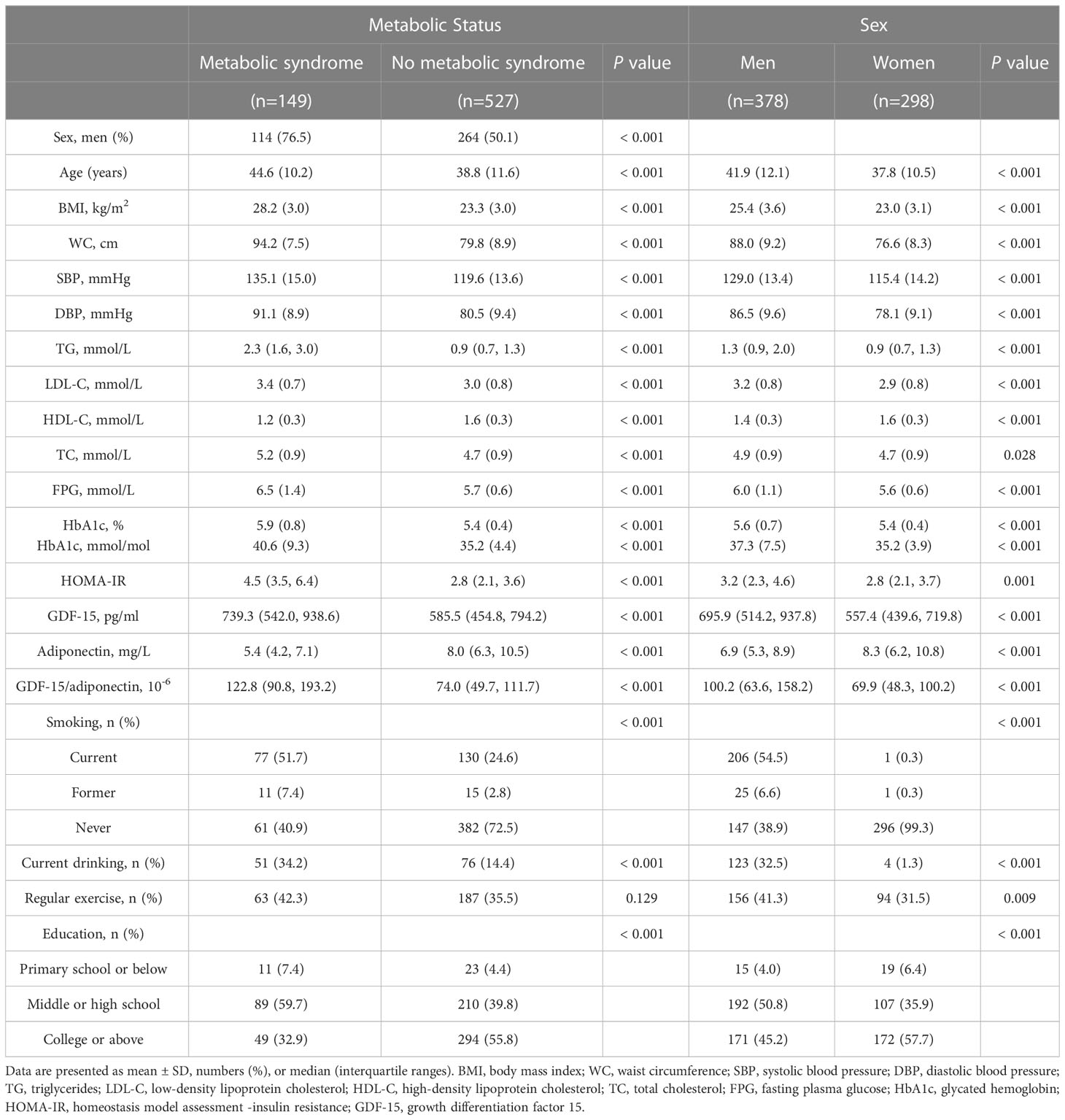
3.1 Baseline characteristics
Among the 676 individuals (378 men and 298 women) included in the final analysis, the prevalence of MS was 22.0% (149/676). Individuals with MS were mainly older, less-educated men, with a higher rate of smoking and drinking, compared with those without MS. No significant difference in regular exercise was observed between the two groups. GDF-15 levels and the G/A ratio were significantly higher in patients with MS, whereas the adiponectin level was considerably lower than that in participants without MS. Demographic and metabolic characteristics were further analyzed according to sex. These results indicated that men had higher levels of WC, BP, TG, FPG, GDF-15, and the G/A ratio but lower levels of HDL and adiponectin than women (Table 1).
3.2 Spearman correlations of GDF-15, adiponectin, and G/A ratio with metabolic parameters
The components of MS (WC, BP, TG, FPG, and HDL) were significantly correlated with levels of GDF-15, adiponectin, and the G/A ratio (all P <0.01). HOMA-IR was positively correlated with the G/A ratio and negatively correlated with adiponectin concentrations, but not with GDF-15 concentrations (Supplementary Table S1).
3.3 Association between MS and GDF-15, adiponectin, and G/A ratio
Logistic regression analysis showed that MS was significantly associated with a lower level of adiponectin and a higher G/A ratio, but not with the GDF-15 level, after adjusting for age and sex. Model 2 was further adjusted for multiple factors (e.g., smoking, drinking, regular exercise, and education level), and the results showed that the G/A ratio and adiponectin levels were significantly correlated with MS with odds ratios (ORs) and a 95% confidence interval (CI) of 1.010 (1.006–1.013) and 0.798 (0.735–0.865), respectively (Table 2). Adjusted ORs for MS according to the quartiles of the G/A ratio or adiponectin levels were shown in Supplementary Table S2.
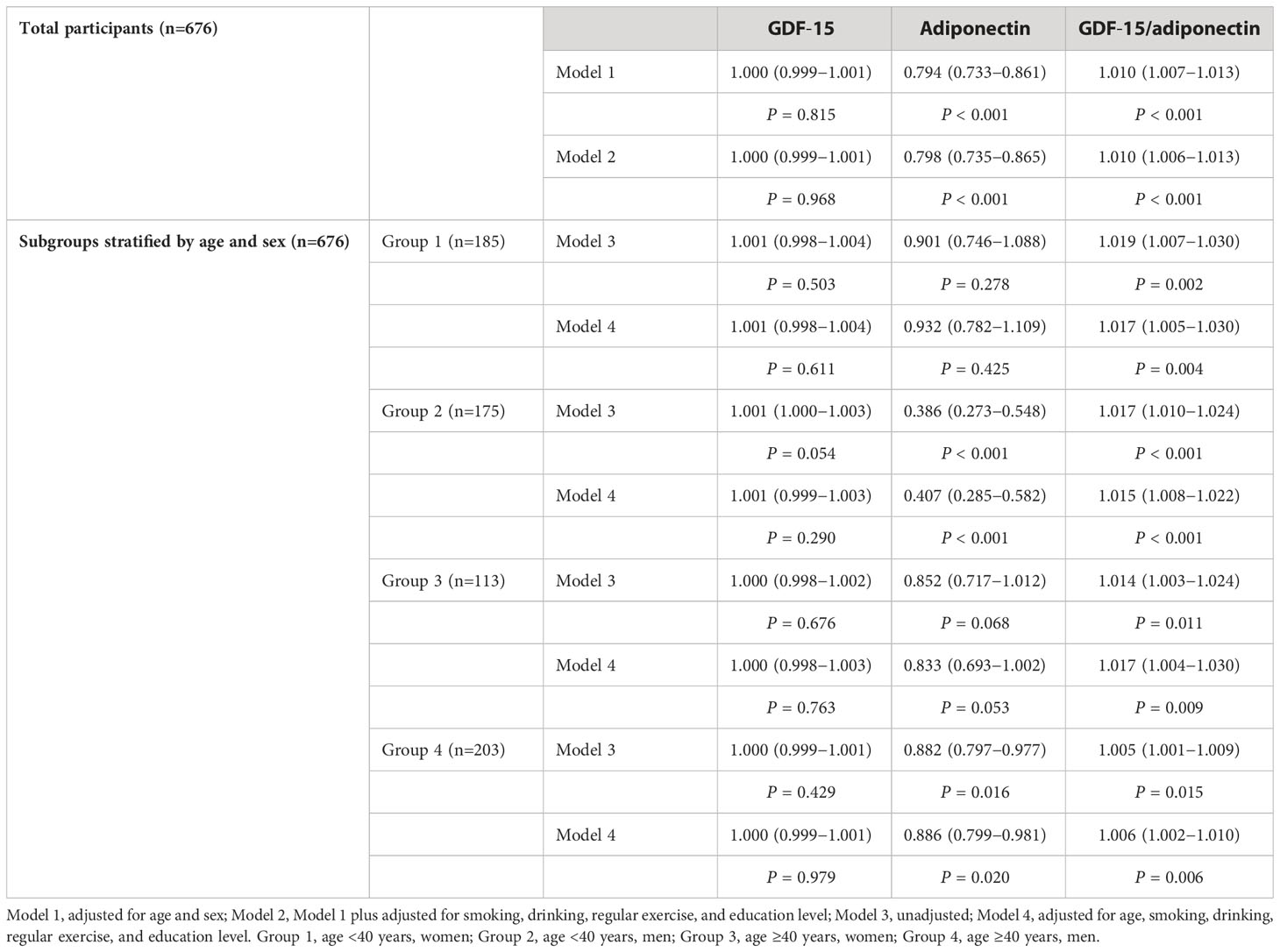
Table 2 Odds ratios for metabolic syndrome according to GDF-15, adiponectin, and GDF-15/adiponectin.
Subgroup analyses were performed according to age and sex. The prevalence of MS in groups 1, 2, 3, and 4 was 7.6%, 20.6%, 18.6%, and 38.4%, respectively. MS was significantly associated with a higher G/A ratio in all four groups; however, the relationship between MS and adiponectin levels was only significant in groups 2 and 4. Significant associations were retained after adjusting for age and other multiple factors (Table 2).
Table 3 shows the ORs for MS components based on adiponectin levels and the G/A ratio. Adiponectin levels and the G/A ratio were correlated with each MS component after adjusting for confounding factors. Sex-stratified analysis was conducted, and the results showed that the G/A ratio was associated with each MS component in both men and women; nevertheless, the adiponectin concentration was not correlated with high BP in women but had a relatively strong correlation with high TG levels in men. The GDF-15 level was not associated with any MS component (Supplementary Table S3). Additionally, we observed a dose-response relationship between MS components and the G/A ratio and adiponectin levels (Figure 1; Supplementary Figure S1).
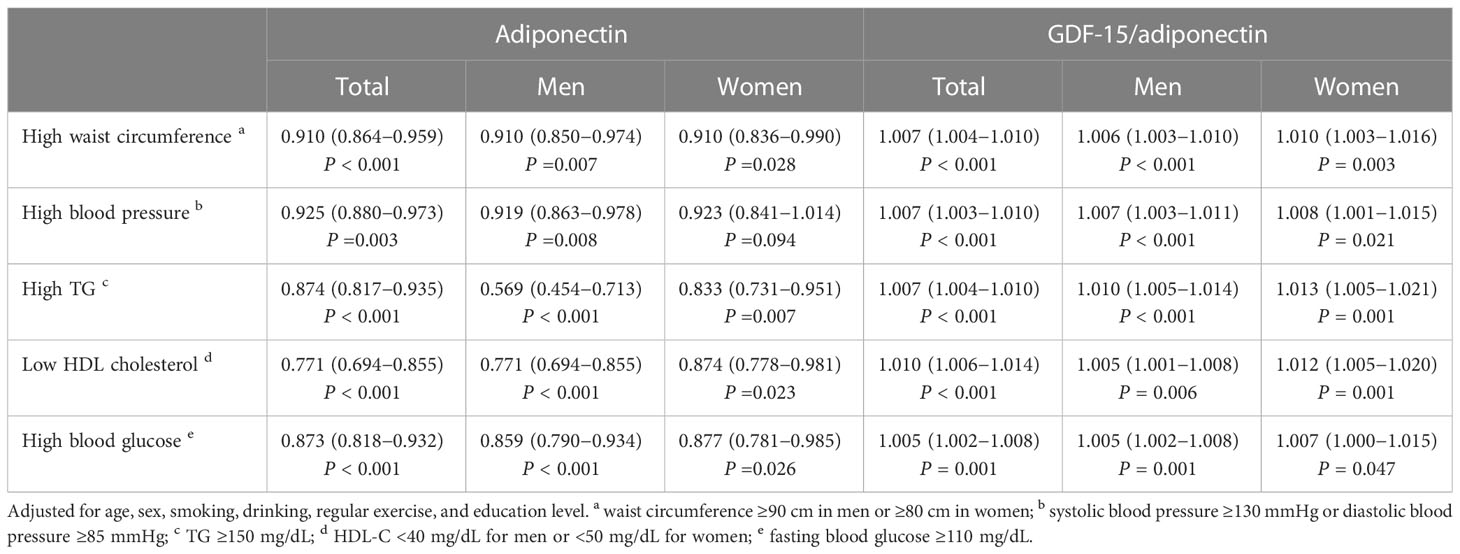
Table 3 Adjusted odds ratios for the components of metabolic syndrome according to adiponectin and GDF-15/adiponectin.
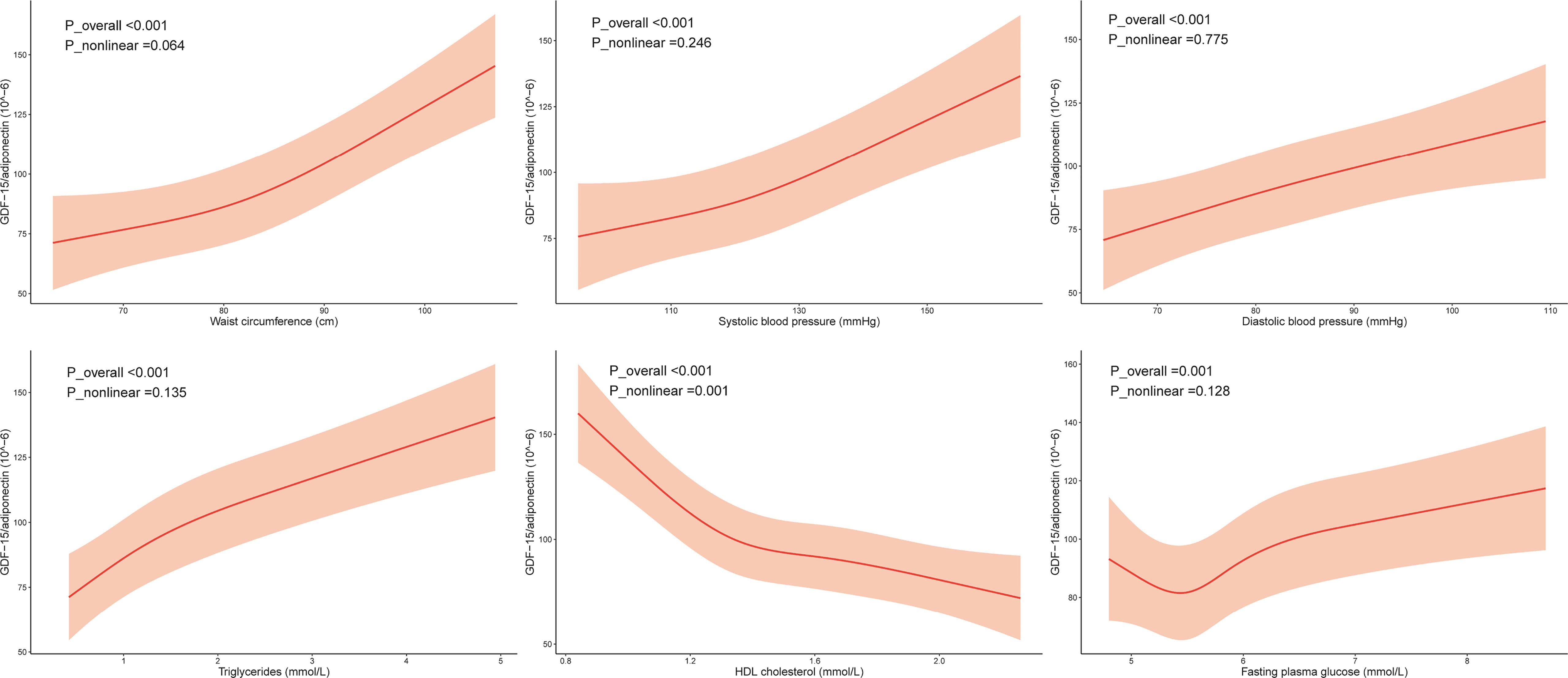
Figure 1 Association of the components of metabolic syndrome with GDF-15/adiponectin. Restricted cubic splines (RCS) were used, and the minimum AIC value was chosen as the optimal number of knots. The model was adjusted for age, sex, smoking, drinking, regular exercise, and education level. The RCS knots were located at 75.0–82.0–90.3 cm for waist circumference, 112.5–121.5–132.0 mm Hg for systolic blood pressure, 75.5–82.0–89.5 mm Hg for diastolic blood pressure, 0.7–1.1–1.6 mmol/L for triglyceride levels, 1.2–1.4–1.6–1.8 for HDL cholesterol levels, and 5.3–5.6–5.8–6.2 mmol/L for fasting plasma glucose.
3.4 ROC curve for the detection of MS
ROC analysis was performed to compare the predictive power of GDF-15, adiponectin, and the G/A ratio for MS. In the total population, the area under the curve (AUC) of GDF-15 levels, adiponectin levels, and the G/A ratio was 0.627, 0.758, and 0.748, respectively (Figure 2A). The highest AUC was 0.757 for the adiponectin level in men and 0.724 for the G/A ratio in women (Figures 2B, C).
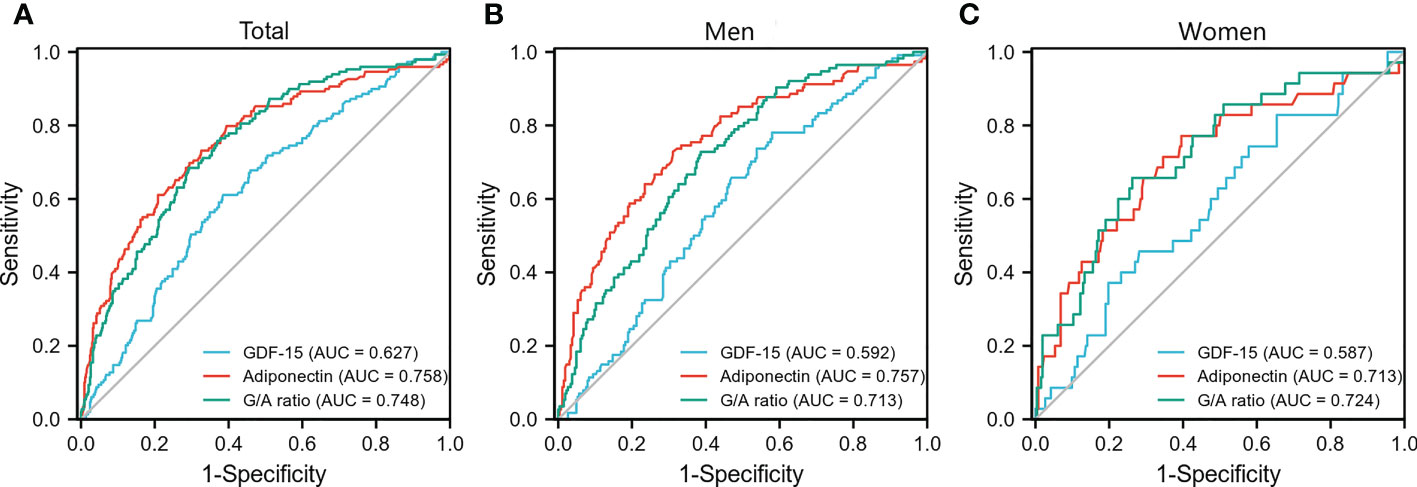
Figure 2 Receiver operating characteristic curve for the detection of metabolic syndrome. Receiver operating characteristic curves of GDF-15, adiponectin, and G/A ratio for the detection of metabolic syndrome in the total population (A), men (B), and women (C), respectively. G/A ratio, GDF-15/adiponectin ratio.
4 Discussion
In this study, we found that the G/A ratio and adiponectin, but not GDF-15, were significantly associated with MS and its components in all participants aged 20–70 years after controlling for confounding variables. Furthermore, the G/A ratio displayed a significant relationship with MS in each subgroup stratified by sex and age (40 years) and was correlated with each component of MS in both men and women. Nevertheless, adiponectin showed a better diagnostic performance in classifying men with MS. This finding implies that the G/A ratio and adiponectin are potential biomarkers for detecting MS in women and men, respectively.
Recent studies have found that GDF-15 regulates lipid and carbohydrate metabolism, reduces food intake and body mass, and improves insulin sensitivity (20, 21), suggesting its potential therapeutic applications in metabolic diseases. GDF-15 levels in ob/ob mice and obese high-fat-fed mice were significantly higher than in the control (22). Further treatment with recombinant GDF-15 in obese mice significantly reduced body weight, whereas the use of GDF-15 antibody significantly increased body weight (22, 23). In addition, GDF-15 knockout mice on high-fat diets were more prone to obesity (24). Therefore, GDF-15 could be a compensatory protective factor in obesity. Similar results to animal tests, GDF-15 levels are correlated with obesity and also positively associated with adipose tissue mass and body weight after correcting for age and sex (25, 26). However, few studies have directly explored the association between GDF-15 and MS. Adrián et al. found that MS was associated with elevated GDF-15 levels in participants aged ≥65 years. High WC, increased FPG, and low HDL-C levels were the main drivers of this association (5). Two other studies in older participants (average age: 80 and 59 years) also showed that elevated GDF-15 levels were associated with MS (6, 7). Among younger individuals, a case-control study of 40 participants with obesity (mean age: 34 years) reported higher GDF-15 levels in patients with MS than in the control group without MS; nevertheless, GDF-15 concentration was not associated with any component of MS (27). Ho et al. found GDF-15 levels were positively related to the presence of MS, after recruiting 279 subjects younger than 65 years old. When substituting MS with its components, only hyperglycemia was positively correlated with GDF-15 levels (28). In the present study, GDF-15 levels were not associated with MS and its components in all participants aged 20–70 years and subgroups stratified by age and sex. This observation could be because of age, as GDF-15 levels are highly influenced by age, or because of other factors, including differences in obesity and sample size.
Adiponectin levels are reduced in participants with obesity, and this reduction is proposed to play a crucial role in the pathogenesis of CVD associated with obesity and MS (29). Additionally, adiponectin is associated with MS regardless of age (13, 30); however, this association varies with sex and race. Adiponectin concentration is reported to be lower in men than in women, partly because of the ability of androgens to suppress adiponectin (31), which might contribute to severe IR in men (29). Prospective studies on the Korean population have reported that adiponectin levels improve the clinical prediction of MS in men but not in women (32, 33). However, a prospective study on Japanese-Americans suggested that low levels of total and high-molecular-weight adiponectin might be a possible predictor of MS in both men and women (34). Previous studies have shown that Asian populations have lower adiponectin concentrations than Caucasians (35, 36). Moreover, adiponectin is correlated with MS in Chinese and Caucasian populations (12, 13), but not in the Indonesian population (16). Additionally, the relationship between adiponectin and each MS component varies among participants of different races and sexes (12, 29). In the present study, an inverse association was found between adiponectin levels and MS in the total population and men, but not in women, irrespective of age. ROC curves showed that adiponectin was a preferential marker for MS in men compared to GDF-15 or the G/A ratio. Furthermore, the adiponectin level was correlated with each MS component in the total population and the sex-stratified subgroups, except for BP in women. However, the high prevalence of MS in men might amplify the effect of adiponectin in detecting MS. Therefore, the effects of sex and race on the association between adiponectin and MS and its components need further investigation.
Obesity-related IR is central to the development of MS. Both GDF-15 and adiponectin have a protective role in regulating insulin sensitivity, weight gain, and inflammation (4, 12). Although GDF-15 is expressed in various tissues, its role as an adipokine that regulates metabolism, which overlaps with the functions of adiponectin, has attracted increasing attention. In addition, GDF-15 and adiponectin can improve IR via the activation of adenosine monophosphate-activated protein kinase (AMPK), a cellular energy sensor, in the liver and skeletal muscles, respectively (37, 38). The G/A ratio is a recently proposed index whose increment was independently correlated with the risk of T2DM in all study populations aged 18–70 years and subgroups, compared to GDF-15 or adiponectin levels alone after adjusting for confounders; however, adiponectin concentrations had a stronger association with T2DM in relatively healthy men, but not in relatively healthy women and participants with metabolic disorders (17). In the present study, we used the G/A ratio to assess the ORs of MS and its components and found that the G/A ratio was associated with MS in all participants and age- and sex-stratified subgroups, correlating with each MS component in both men and women. Furthermore, a significant dose-response relationship was observed between the G/A ratio and each MS component. Our results were consistent with those of Wu et al., showing that adiponectin is a biomarker for evaluating T2DM and MS in men, whereas the G/A ratio benefited both men and women, suggesting that the combination of these two adipokines may have an “enhancing effect”. Additionally, previous studies have explored the association between GDF-15 and adiponectin. Tsai et al. found that GDF-15 treatment reduced adiposity and corrected metabolic dysfunction in high-fat diet-fed mice, accompanied by higher circulating adiponectin levels (39). Recombinant GDF-15 enhanced adiponectin release from adipocytes (3), and GDF-15-overexpressing transgenic mice showed upregulated adiponectin at the mRNA level (40), implying that GDF-15 is a positive regulator of adiponectin (3). In an obesity-related disease state, higher GDF-15 concentrations and lower adiponectin levels have been observed (41). The obesity-related disease is a chronic inflammatory status, in which the compensatory protective effect of elevated GDF-15 might be due to its anti-inflammatory role, but eventually futile (42). We speculate that a compensatory increase of GDF-15 levels improves adiponectin concentration; however, the elevated level of GDF-15 is limited, and it cannot offset the decrease in adiponectin level that accompanies the chronic low-grade inflammatory progression of the disease. The G/A ratio can potentially exclude confounding factors, such as age, sex, body composition, and several life habits that can be correlated with MS and its components. However, further studies are required to elucidate this mechanism.
Our study has several advantages. First, our analysis adjusted for several confounding factors, such as smoking, drinking, regular exercise, and education level, in addition to age and sex. We also excluded participants with hypoglycaemic, antihypertensive, and lipid-lowering drugs and some disease states, such as CVD, autoimmune diseases, and cancers, to avoid the impact on the detection of GDF-15 and adiponectin. In addition, restricted cubic splines were used to reflect the dose-response relationship between biomarkers and MS on a continuous scale. However, this study also has several limitations. First, because of the cross-sectional data, we could not determine whether the G/A ratio plays a causal role in the pathogenesis of MS. Second, accurately determining the relationship between the G/A ratio and MS was challenging, given the relatively small sample sizes. Finally, our study was conducted only in an Eastern Chinese Han population; therefore, it is unclear whether our results could be generalized to different ethnic groups.
In conclusion, our study indicates a significant and independent association between an increased G/A ratio and MS and each of its components in the total study population and all subgroups. Our results indicate that the G/A ratio and adiponectin are potential biomarkers for detecting MS in women and men, respectively. Future prospective studies should confirm these findings in a larger number of participants across different age groups, sexes, and races. In addition, the relationship between GDF-15 and adiponectin needs to be comprehensively explored.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the institutional review board of the First Affiliated Hospital of Nanjing Medical University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
SZ analyzed the data and wrote the manuscript; MShen and YQ made data analyses and performed experimental work; SL, YC, HJ, HL, DC, and RZ made data collection; XZ, MSun, and TY provided valuable advice; QF and YS designed the study and reviewed the article. All authors have read and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (82100837, 82070803, 81830023, and 81900708), Jiangsu Provincial Natural Science Fund Youth Program (BK20210959), and the China Postdoctoral Science Foundation (2021M691335).
Acknowledgments
The authors wish to thank all participants and the community workers for their cooperation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1146376/full#supplementary-material
References
1. Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep (2018) 20(2):12. doi: 10.1007/s11906-018-0812-z
2. Desmedt S, Desmedt V, De Vos L, Delanghe JR, Speeckaert R, Speeckaert MM. Growth differentiation factor 15: A novel biomarker with high clinical potential. Crit Rev Clin Lab Sci (2019) 56(5):333–50. doi: 10.1080/10408363.2019.1615034
3. Ding Q, Mracek T, Gonzalez-Muniesa P, Kos K, Wilding J, Trayhurn P, et al. Identification of macrophage inhibitory cytokine-1 in adipose tissue and its secretion as an adipokine by human adipocytes. Endocrinology (2009) 150(4):1688–96. doi: 10.1210/en.2008-0952
4. Wang D, Day EA, Townsend LK, Djordjevic D, Jørgensen SB, Steinberg GR. GDF15: emerging biology and therapeutic applications for obesity and cardiometabolic disease. Nat Rev Endocrinol (2021) 17(10):592–607. doi: 10.1038/s41574-021-00529-7
5. Carballo-Casla A, García-Esquinas E, Buño-Soto A, Struijk EA, López-García E, Rodríguez-Artalejo F, et al. Metabolic syndrome and growth differentiation factor 15 in older adults. Geroscience (2022) 44(2):867–80. doi: 10.1007/s11357-021-00370-w
6. Echouffo-Tcheugui JB, Daya N, Matsushita K, Wang D, Ndumele CE, Al Rifai M, et al. Growth differentiation factor (GDF)-15 and cardiometabolic outcomes among older adults: The atherosclerosis risk in communities study. Clin Chem (2021) 67(4):653–61. doi: 10.1093/clinchem/hvaa332
7. Ho JE, Mahajan A, Chen MH, Larson MG, McCabe EL, Ghorbani A, et al. Clinical and genetic correlates of growth differentiation factor 15 in the community. Clin Chem (2012) 58(11):1582–91. doi: 10.1373/clinchem.2012.190322
8. Fujita Y, Taniguchi Y, Shinkai S, Tanaka M, Ito M. Secreted growth differentiation factor 15 as a potential biomarker for mitochondrial dysfunctions in aging and age-related disorders. Geriatr Gerontol Int (2016) 16 Suppl 1:17–29. doi: 10.1111/ggi.12724
9. Wang ZV, Scherer PE. Adiponectin, the past two decades. J Mol Cell Biol (2016) 8(2):93–100. doi: 10.1093/jmcb/mjw011
10. Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun (2004) 323(2):630–5. doi: 10.1016/j.bbrc.2004.08.145
11. Tao L, Gao E, Jiao X, Yuan Y, Li S, Christopher TA, et al. Adiponectin cardioprotection after myocardial ischemia/reperfusion involves the reduction of oxidative/nitrative stress. Circulation (2007) 115(11):1408–16. doi: 10.1161/circulationaha.106.666941
12. Ghadge AA, Khaire AA, Kuvalekar AA. Adiponectin: A potential therapeutic target for metabolic syndrome. Cytokine Growth Factor Rev (2018) 39:151–8. doi: 10.1016/j.cytogfr.2018.01.004
13. Wang J, Li H, Franco OH, Yu Z, Liu Y, Lin X. Adiponectin and metabolic syndrome in middle-aged and elderly Chinese. Obes (Silver Spring) (2008) 16(1):172–8. doi: 10.1038/oby.2007.42
14. Liu Z, Liang S, Que S, Zhou L, Zheng S, Mardinoglu A. Meta-analysis of adiponectin as a biomarker for the detection of metabolic syndrome. Front Physiol (2018) 9:1238. doi: 10.3389/fphys.2018.01238
15. Eglit T, Lember M, Ringmets I, Rajasalu T. Gender differences in serum high-molecular-weight adiponectin levels in metabolic syndrome. Eur J Endocrinol (2013) 168(3):385–91. doi: 10.1530/eje-12-0688
16. Sigit FS, Trompet S, Tahapary DL, Sartono E, Willems van Dijk K, Yazdanbakhsh M, et al. The associations of leptin and adiponectin with the metabolic syndrome in an Indonesian and a Dutch population. Nutr Metab Cardiovasc Dis (2021) 31(8):2426–35. doi: 10.1016/j.numecd.2021.05.012
17. Wu X, Xuan W, You L, Lian H, Li F, Zhang X, et al. Associations of GDF-15 and GDF-15/adiponectin ratio with odds of type 2 diabetes in the Chinese population. Endocrine (2021) 72(2):423–36. doi: 10.1007/s12020-021-02632-1
18. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia (1985) 28(7):412–9. doi: 10.1007/bf00280883
19. Tan CE, Ma S, Wai D, Chew SK, Tai ES. Can we apply the national cholesterol education program adult treatment panel definition of the metabolic syndrome to asians? Diabetes Care (2004) 27(5):1182–6. doi: 10.2337/diacare.27.5.1182
20. Patel S, Alvarez-Guaita A, Melvin A, Rimmington D, Dattilo A, Miedzybrodzka EL, et al. GDF15 provides an endocrine signal of nutritional stress in mice and humans. Cell Metab (2019) 29(3):707–718.e708. doi: 10.1016/j.cmet.2018.12.016
21. Coll AP, Chen M, Taskar P, Rimmington D, Patel S, Tadross JA, et al. GDF15 mediates the effects of metformin on body weight and energy balance. Nature (2020) 578(7795):444–8. doi: 10.1038/s41586-019-1911-y
22. Xiong Y, Walker K, Min X, Hale C, Tran T, Komorowski R, et al. Long-acting MIC-1/GDF15 molecules to treat obesity: Evidence from mice to monkeys. Sci Transl Med (2017) 9(412):eaan8732. doi: 10.1126/scitranslmed.aan8732
23. Emmerson PJ, Wang F, Du Y, Liu Q, Pickard RT, Gonciarz MD, et al. The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat Med (2017) 23(10):1215–9. doi: 10.1038/nm.4393
24. Tran T, Yang J, Gardner J, Xiong Y. GDF15 deficiency promotes high fat diet-induced obesity in mice. PloS One (2018) 13(8):e0201584. doi: 10.1371/journal.pone.0201584
25. Dostálová I, Roubícek T, Bártlová M, Mráz M, Lacinová Z, Haluzíková D, et al. Increased serum concentrations of macrophage inhibitory cytokine-1 in patients with obesity and type 2 diabetes mellitus: the influence of very low calorie diet. Eur J Endocrinol (2009) 161(3):397–404. doi: 10.1530/eje-09-0417
26. Vila G, Riedl M, Anderwald C, Resl M, Handisurya A, Clodi M, et al. The relationship between insulin resistance and the cardiovascular biomarker growth differentiation factor-15 in obese patients. Clin Chem (2011) 57(2):309–16. doi: 10.1373/clinchem.2010.153726
27. Shariat A, Abbasalizad Farhangi M, Zeinalian R. Association between serum levels of vascular endothelial growth factor, macrophage inhibitory cytokine and markers of oxidative stress, with the metabolic syndrome and its components in obese individuals. Nutr Clinique Metabol (2018) 32(2):95–101. doi: 10.1016/j.nupar.2018.02.003
28. Ho LC, Wu HT, Hung HC, Chou HW, Cheng KP, Lin CH, et al. Growth differentiation factor-15 is independently associated with metabolic syndrome and hyperglycemia in non-elderly subjects. Biofactors (2023) 49(1):119–26. doi: 10.1002/biof.1871
29. Yanai H, Yoshida H. Beneficial effects of adiponectin on glucose and lipid metabolism and atherosclerotic progression: Mechanisms and perspectives. Int J Mol Sci (2019) 20(5):1190. doi: 10.3390/ijms20051190
30. Kowalska I, Straczkowski M, Nikolajuk A, Adamska A, Karczewska-Kupczewska M, Otziomek E, et al. Insulin resistance, serum adiponectin, and proinflammatory markers in young subjects with the metabolic syndrome. Metabolism (2008) 57(11):1539–44. doi: 10.1016/j.metabol.2008.06.008
31. Nishizawa H, Shimomura I, Kishida K, Maeda N, Kuriyama H, Nagaretani H, et al. Androgens decrease plasma adiponectin, an insulin-sensitizing adipocyte-derived protein. Diabetes (2002) 51(9):2734–41. doi: 10.2337/diabetes.51.9.2734
32. Kim JY, Ahn SV, Yoon JH, Koh SB, Yoon J, Yoo BS, et al. Prospective study of serum adiponectin and incident metabolic syndrome: the ARIRANG study. Diabetes Care (2013) 36(6):1547–53. doi: 10.2337/dc12-0223
33. Huh JH, Yoon TW, Kang DR, Kim JY. Prospective study of sex-specific adiponectin changes and incident metabolic syndrome: The ARIRANG study. J Clin Med (2019) 8(5):599. doi: 10.3390/jcm8050599
34. Nakashima R, Yamane K, Kamei N, Nakanishi S, Kohno N. Low serum levels of total and high-molecular-weight adiponectin predict the development of metabolic syndrome in Japanese-americans. J Endocrinol Invest (2011) 34(8):615–9. doi: 10.3275/7409
35. Smith J, Al-Amri M, Sniderman A, Cianflone K. Leptin and adiponectin in relation to body fat percentage, waist to hip ratio and the apoB/apoA1 ratio in Asian Indian and Caucasian men and women. Nutr Metab (Lond) (2006) 3:18. doi: 10.1186/1743-7075-3-18
36. Mente A, Razak F, Blankenberg S, Vuksan V, Davis AD, Miller R, et al. Ethnic variation in adiponectin and leptin levels and their association with adiposity and insulin resistance. Diabetes Care (2010) 33(7):1629–34. doi: 10.2337/dc09-1392
37. Matsuda M, Shimomura I. Roles of adiponectin and oxidative stress in obesity-associated metabolic and cardiovascular diseases. Rev Endocr Metab Disord (2014) 15(1):1–10. doi: 10.1007/s11154-013-9271-7
38. Townsend LK, Weber AJ, Day EA, Shamshoum H, Shaw SJ, Perry CGR, et al. AMPK mediates energetic stress-induced liver GDF15. FASEB J (2021) 35(1):e21218. doi: 10.1096/fj.202000954R
39. Tsai VW, Zhang HP, Manandhar R, Lee-Ng KKM, Lebhar H, Marquis CP, et al. Treatment with the TGF-b superfamily cytokine MIC-1/GDF15 reduces the adiposity and corrects the metabolic dysfunction of mice with diet-induced obesity. Int J Obes (Lond) (2018) 42(3):561–71. doi: 10.1038/ijo.2017.258
40. Lertpatipanpong P, Lee J, Kim I, Eling T, Oh SY, Seong JK, et al. The anti-diabetic effects of NAG-1/GDF15 on HFD/STZ-induced mice. Sci Rep (2021) 11(1):15027. doi: 10.1038/s41598-021-94581-y
41. Ferrannini G, Manca ML, Magnoni M, Andreotti F, Andreini D, Latini R, et al. Coronary artery disease and type 2 diabetes: A proteomic study. Diabetes Care (2020) 43(4):843–51. doi: 10.2337/dc19-1902
Keywords: GDF-15, adiponectin, GDF-15/adiponectin ratio, metabolic syndrome, biomarkers
Citation: Zheng S, Shen M, Qian Y, Li S, Chen Y, Jiang H, Lv H, Chen D, Zhao R, Zheng X, Sun M, Yang T, Shi Y and Fu Q (2023) Growth differentiation factor-15/adiponectin ratio as a potential biomarker for metabolic syndrome in Han Chinese. Front. Endocrinol. 14:1146376. doi: 10.3389/fendo.2023.1146376
Received: 17 January 2023; Accepted: 03 April 2023;
Published: 19 April 2023.
Edited by:
Shuwen Qian, Fudan University, ChinaReviewed by:
Suzhen Chen, Shanghai Jiao Tong University, ChinaJan Mieszkowski, Gdansk University of Physical Education and Sport, Poland
Copyright © 2023 Zheng, Shen, Qian, Li, Chen, Jiang, Lv, Chen, Zhao, Zheng, Sun, Yang, Shi and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qi Fu, ZHJmdXFpQG5qbXUuZWR1LmNu; Yun Shi, ZHJzaGl5dW5AbmptdS5lZHUuY24=
†These authors have contributed equally to this work
 Shuai Zheng
Shuai Zheng Min Shen
Min Shen Yu Qian†
Yu Qian† Yang Chen
Yang Chen Hemin Jiang
Hemin Jiang Hui Lv
Hui Lv Xuqin Zheng
Xuqin Zheng Min Sun
Min Sun Tao Yang
Tao Yang Qi Fu
Qi Fu