
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Endocrinol., 21 March 2023
Sec. Cellular Endocrinology
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1146017
This article is part of the Research TopicImmunocellular Mechanisms and Endocrine OrgansView all 8 articles
 Damiano Chiari1,2*
Damiano Chiari1,2* Barbara Pirali3*
Barbara Pirali3* Vittoria Perano1
Vittoria Perano1 Roberto Leone4
Roberto Leone4 Alberto Mantovani1,4,5
Alberto Mantovani1,4,5 Barbara Bottazzi4
Barbara Bottazzi4Thyroid is at the crossroads of immune dysregulation, tissue remodeling and oncogenesis. Autoimmune disorders, nodular disease and cancer of the thyroid affect a large amount of general population, mainly women. We wondered if there could be a common factor behind three processes (immune dysregulation, tissue remodeling and oncogenesis) that frequently affect, sometimes coexisting, the thyroid gland. The long pentraxin 3 (PTX3) is an essential component of the humoral arm of the innate immune system acting as soluble pattern recognition molecule. The protein is found expressed in a variety of cell types during tissue injury and stress. In addition, PTX3 is produced by neutrophils during maturation in the bone-marrow and is stored in lactoferrin-granules. PTX3 is a regulator of the complement cascade and orchestrates tissue remodeling and repair. Preclinical data and studies in human tumors indicate that PTX3 can act both as an extrinsic oncosuppressor by modulating complement-dependent tumor-promoting inflammation, or as a tumor-promoter molecule, regulating cell invasion and proliferation and epithelial to mesenchymal transition, thus suggesting that this molecule may have different functions on carcinogenesis. The involvement of PTX3 in the regulation of immune responses, tissue remodeling and oncosuppressive processes led us to explore its potential role in the development of thyroid disorders. In this review, we aimed to highlight what is known, at the state of the art, regarding the connection between the long pentraxin 3 and the main thyroid diseases i.e., nodular thyroid disease, thyroid cancer and autoimmune thyroid disorders.
Thyroid gland is at the crossroads of immune dysregulation, tissue remodeling and oncogenesis (1). Thyroid problems are among the most widespread illness affecting the global population: autoimmune disorders, nodular disease and cancer of the thyroid affect 2 - 5% of the population, with higher incidence in women, and are the most prevalent organ-specific autoimmune diseases (2). The prevalence of thyroid nodules detected with ultrasound is 19-35% (3). In the world the incidence rates of new cases of thyroid cancer were about 10·1 and 3·1 per 100.000 women and men, respectively (4). Many people are affected by mild thyroid dysfunction and only a small part of these have a severe evolution. The identification of novel tools for the diagnosis, screening and monitoring of patients affected by thyroid illness could improve patient management by reducing unnecessary diagnostic tests and interventions.
The long pentraxin 3 (PTX3) is a soluble pattern recognition molecule and an essential mediator of the innate immune response, expressed by a variety of cell types of hematopoietic or stromal origin (i.e. macrophages, endothelial cells, fibroblasts, etc.) during tissue injury and stress or upon stimulation with pro-inflammatory signals (5–7). PTX3 is also produced by neutrophils during the maturation process in the bone marrow and it is stored in specific granules (8). The molecule exerts a role in tissue repair and remodeling, and acts as regulator of complement activation and leukocytes recruitment (9–15). In addition, PTX3 levels rapidly increase during inflammatory conditions (infectious diseases, sepsis, acute respiratory distress syndrome, cardiovascular diseases), correlating with severity and predicting the risk of mortality (16–21). Increased levels of PTX3 were also observed in different autoimmune disorders, such as small-vessel vasculitis and rheumatoid arthritis (22–27).
Several tumors express PTX3, including lung cancer, glioma, ovarian cancer, myxoid liposarcoma, prostate carcinoma, esophageal squamous cell carcinoma and pancreatic cancer (28–32). The role of PTX3 in tumorigenesis is still poorly defined (33, 34). In preclinical models, Ptx3 deficiency was associated to higher susceptibility to chemical carcinogenesis (9), while in human prostate cancer PTX3 expression is progressively reduced in high-grade prostatic intraepithelial neoplasia and invasive tumor areas (35). In addition, epigenetic studies showed promoter hypermethilation and silencing of PTX3 expression in selected human tumors, such as colorectal cancer and esophageal squamous cell carcinoma (9, 30, 36–38). However, in other contexts (i.e. glioma, head and neck cancers, hepatocellular carcinoma) PTX3 overexpression has a pro-tumorigenic effect, promoting cell invasion or proliferation and epithelial to mesenchymal transition (39–43). PTX3 is therefore playing a complex and still undefined role in the regulation of immune response, tissue remodeling and oncosuppressive processes.
In this review we will explore the role of PTX3 in the development of thyroid diseases, analyzing studies including patients with Graves’ disease (GD), thyroid nodules and thyroid cancer supported also by results obtained by our group.
The nodular development of the thyroid results from multifocal monoclonal or polyclonal proliferation of thyrocytes producing new follicles or structures similar to follicles considerably heterogeneous from functional and morphological points of view (44). More than 90% of detected nodules in adults are noncancerous, and thyroid stimulating hormone (TSH) is the main mitotic factor in nodule formation (45). Radioactive sources, many habits (iodine intake, smoking, etc.), harmful chemicals, metabolic syndrome and genetic mutations may cause chronic inflammation, contributing to nodule and cancer formation (46, 47). Destek et al. investigated the relationship of some inflammatory and autoimmune markers, among which PTX3, with thyroid nodules characteristics (48). They did not observe differences of PTX3 plasmatic levels between nodular goiter group and control group, nor correlations between PTX3 levels and nodule characteristics.
We recently evaluated the plasmatic levels of PTX3 in a group of 53 patients undergoing thyroidectomy for both benign and malignant nodules. A preliminary analysis revealed that preoperative plasmatic PTX3 levels were significantly higher than normal in patients with thyroid disease (p<0.05). Plasmatic PTX3 mean value was 4.54 ng/ml (range 1.06 – 8.63 ng/ml), when normal value is considered 2 ng/ml with 1 ng/ml of standard deviation. Preoperative PTX3 levels were not significantly different between benign and malignant nodules. At 45 days follow-up PTX3 mean value was reduce to 3.40 ng/ml (range 0.89 – 9.21 ng/ml); this reduction was statistically significant (p<0.05) (49). Moreover, we observed PTX3 expression in thyroid tissue by immune infiltrate, extracellular matrix and follicular cells (Figures 1, 2).
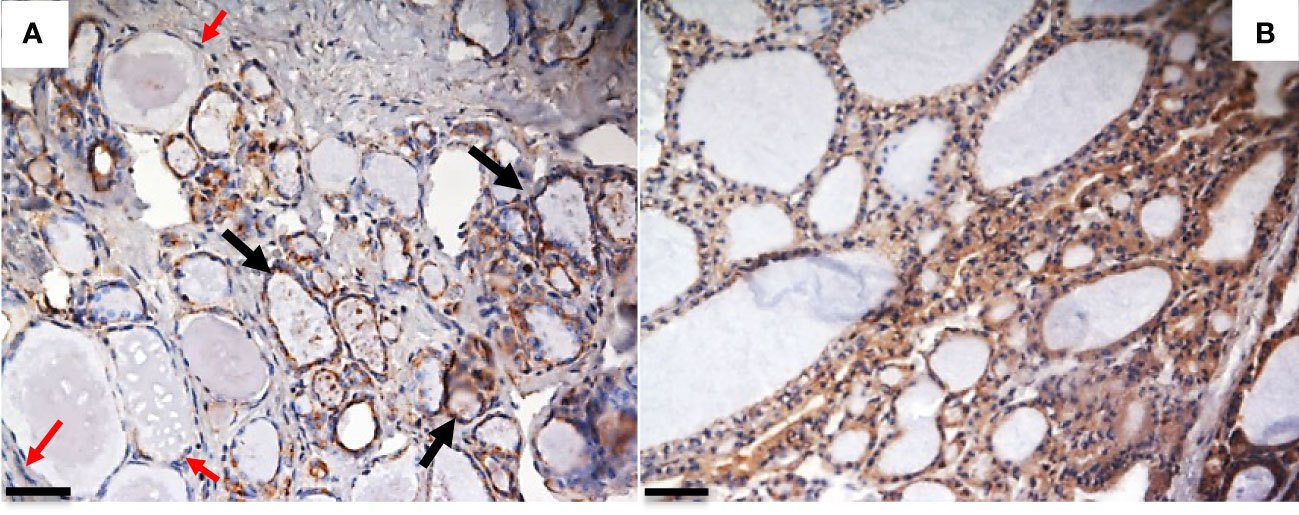
Figure 1 PTX3 immunolocalization in tissue samples of remodeling thyroid tissue. Goiter and GD specimens were obtained after surgical removal of the thyroid, frozen in liquid N2-cooled isopentane, and stored at -80°C until immunohistochemical analysis. Sections were stained with rabbit anti-human PTX3 and analyzed with Nikon Eclipse 55i microscope. Images were captured with a Digital Sight DS-5 M digital camera (Nikon) using Lucia G software (Laboratory Imaging). (A) Goiter: PTX3 staining (brown) is evident in epithelial follicular cells of some thyroid follicles (black arrow), while some follicles do not express PTX3 (red arrows); (B) GD: a diffuse PTX3 positive staining is evident for all the follicles (immunoperoxidase staining; magnification: (A) 20x; (B) 20x; scale bar, 100μm). The analysis was conducted in accordance with local ethical guidelines. The patient has provided informed clinical consent.
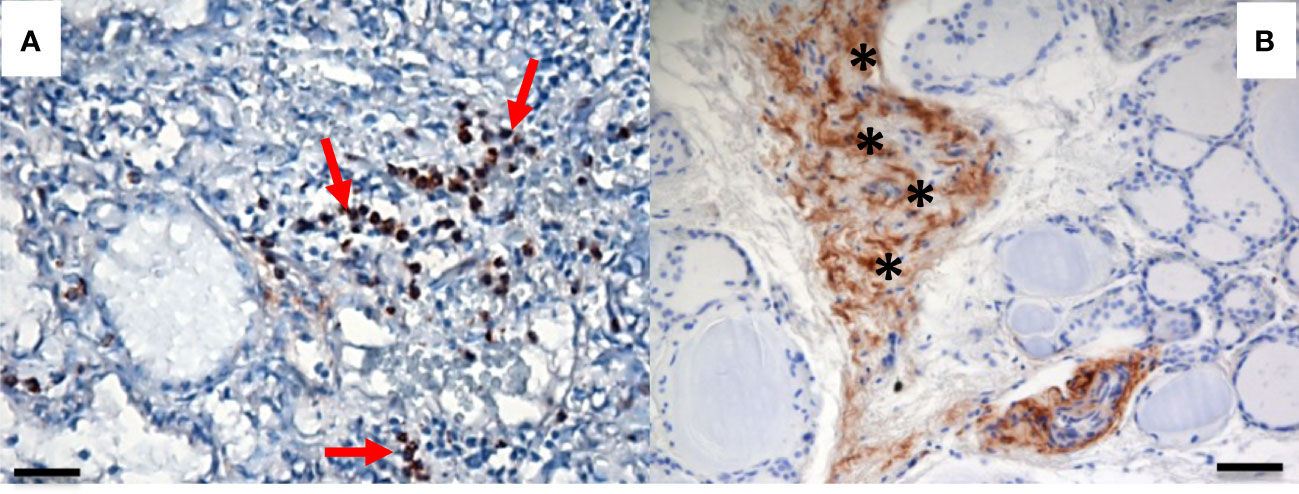
Figure 2 PTX3 immunolocalization in tissue samples of remodeling thyroid tissue. PTX3 immunostaining (brown) of thyroid tissue sections was performed as in Figure 1. (A) GD: PTX3 immunostaining of immune cells infiltrating the parenchima (red arrow). (B) Goiter: PTX3 immunostaining at the level of the extracellular matrix (asterisk). (immunoperoxidase staining; magnification: (A) 20x; (B) 20x; scale bar, 100μm).
It is well known that PTX3 modulates the cancer-related inflammation or angiogenesis involved in the carcinogenetic process of several types of cancer (33, 34). Other studies support the hypothesis that PTX3 exerts a pro-tumorigenic effect promoting macrophage infiltration and tumor cell migration and invasion. It is possible that PTX3 may have different functions on cancer development depending on the tissue and cancer type (33). The role of PTX3 as biomarker of cancer has been investigated. In patients with lung cancer, prostate cancer or colorectal carcinoma, blood PTX3 levels resulted elevated compared respectively to healthy subjects, patients with prostatic inflammation or colorectal polyps (50–52).
Ninety to 95% of thyroid cancers are categorized as well-differentiated tumors arising from the follicular cells. Papillary and follicular carcinomas are included in this category. Papillary carcinoma is the most common of the thyroid neoplasms (53) and is usually associated with an excellent prognosis. Follicular carcinoma is the second category of well-differentiated thyroid cancer and is a disease of older population. Anaplastic thyroid carcinoma (ATC) represents less than 1% of thyroid malignancies and it is the most aggressive form of thyroid cancers. Medullary thyroid carcinoma arises from the parafollicular cells (or C cells) and accounts for 5% to 10% of thyroid cancers.
A recent study identified a four genes signature including PTX3, 3′-phosphoadenosine 5′-phosphosulfate synthase 2 (PAPSS2), procollagen C-endopeptidase enhancer 2 (PCOLCE2) and transforming growth factor beta receptor 3 (TGFBR3) for papillary carcinoma as marker for risk stratification and survival prediction (54). PTX3 expression was significantly lower in patients surviving with tumors than in tumor free patients, revealing a potential correlation between PTX3 and tumor recurrence.
Another study found that PTX3, Collectin Subfamily Member 12 (COLEC12) and Platelet Derived Growth Factor receptor alfa (PDGFRA) could be used as biomarkers to differentiate the anaplastic thyroid carcinoma from follicular or papillary thyroid carcinomas (55). These three genes are overexpressed in ATC meanwhile under-expressed in the other two subtypes.
In recent years, growing numbers of studies have indicated that abnormal expression of PTX3 may play a role in the pathogenesis and development of several autoimmune diseases (rheumatoid arthritis, systemic lupus erythematosus, etc.) (56). Hashimoto’s thyroiditis is the most common cause of hypothyroidism in iodine-sufficient areas. It is characterized clinically by gradual thyroid failure, due to lymphocytic infiltration and autoimmune-mediated destruction of the thyroid. Extremely scarce data can be found in literature regarding the relationship between this disorder and PTX3. An abstract reported that there is not a significant difference in PTX3 levels between patients with autoimmune thyroiditis and control group (57). Possibly, the lack of PTX3 overexpression in Hashimoto’s thyroiditis could be due to the inability of lymphocytes, mainly involved in this pathology, to produce PTX3, neither constitutively nor after appropriate stimulation (7). Therefore, we focused on Graves’ disease and PTX3. GD is an organ-specific autoimmune disease in which thyroid-stimulating immunoglobulins (TSI) activate the TSH receptor (TSHR) expressed on thyroid epithelial cells causing an overproduction of thyroid hormones (58). As a consequence, GD typically causes hyperthyroidism. Other possible clinical manifestations of GD are thyroid enlargement, protruding eyes, and pretibial myxedema. Cheng et al. showed that PTX3 levels are higher in GD (59) and we observed that PTX3 is expressed in thyroid epithelial follicular cells in a cohort of 14 patients that underwent total thyroidectomy for GD (Figure 1B). GD frequently manifests with a thyroid-associated ophthalmopathy (TAO), characterized by inflammation and remodeling of orbital connective tissues and extraocular muscles (60). After a symptomatic active phase, the inflammation and congestion signs may alleviate, and the disease gradually transits to the inactive phase (chronic fibrosis). As observed by Zhang et al. (61), PTX3 can form a complex with hyaluronan and it is possible that is involved in the tissue remodeling process in case of TAO. Expression of PTX3 in orbital connective tissues in active TAO was reported in earlier publications (62). Orbital fibrocytes are the leading actor of TAO and seem to derive from CD34+ fibrocytes (bone marrow–derived monocyte progenitor cells) (63). The expression of functional TSHR and other “thyroid-specific” proteins is a common characteristic between fibrocytes and CD34+ orbital fibroblast. Fibrocytes express several cytokines and other inflammatory genes, including PTX3, when activated through TSHR. According to Wang et al., at a pre-translational level TSH induces PTX3 expression by orbital fibroblasts; in addition, basal levels of PTX3 in GD orbital fibroblasts seem to be higher than those in healthy-orbital fibroblasts (64). Mou et al. (65) demonstrated higher PTX3 mRNA expression in the orbital adipose-connective tissue from TAO patients compared to healthy subjects. They also observed higher serum PTX3 concentration in patients compared to the control group, but not among active and inactive TAO. Other authors observed that serum levels of PTX3 and other cytokines were higher in active GD and that were associated with thyroid-stimulating hormone receptor antibodies (TRAbs) levels (59). These findings diverge from Diao et al. (66) who observed that basal levels of PTX3 mRNA in TAO-orbital fibroblasts did not seem to be different from those in healthy-orbital fibroblasts, but they had enrolled only patients with stable and inactive TAO. The current literature suggests that PTX3 could be a potential therapeutic molecule of an antifibrotic treatment (5). Diao et al. demonstrated in vitro that TGF-β1 inhibits PTX3 expression in human orbital fibroblasts with TAO (66). In addition, dexamethasone and IGF-1 receptor–blocking antibodies (teprotumumab and 1H7) attenuate the TSH-mediated induction of PTX3 in vitro (64).
Thyroid gland is at the crossroads of immune dysregulation, tissue remodeling and oncogenesis. We hypothesized the existence of a common mechanism underlying autoimmune disorders, nodular disease and cancer of the thyroid. PTX3 is a key component of the humoral arm of innate immunity regulating immune responses, tissue remodeling and oncosuppressive processes, all potentially involved in the development of thyroid diseases.
From histological point of view, PTX3 resulted expressed in the thyroid gland by different cells: infiltrating immune cells, extracellular matrix and follicular cells. Different patterns of expression could represent different mechanism of actions of PTX3 in the etiopathogenesis of thyroid disorders.
Chronic inflammation is considered a possible cause of nodule development. Destek et al. showed that plasmatic PTX3 levels were not different between patients with thyroid nodules compared to controls (48). On the other side, we observed significantly higher plasmatic levels of PTX3 in a cohort of patients who underwent thyroidectomy for nodular goiter (49). The indication to surgery reflects a situation of remodeling of the gland in terms of dimensional increase or hyperfunction. Therefore, we can hypothesize that PTX3 overexpression is associated with active phases of nodular remodeling.
Thyroid carcinoma, with the four subtypes papillary follicular, anaplastic and medullary, is the most common endocrine malignancy. Two different gene signatures including PTX3 were identified for the subtypes arising from the follicular cells: papillary, follicular and anaplastic. These new evidences suggest that the molecule could be an independent prognostic factor for most of the thyroid carcinomas (54), and a diagnostic tool to differentiate the anaplastic thyroid carcinoma from the other two subtypes (55).
PTX3 is also involved in GD. Several authors observed higher PTX3 levels in the orbital fibroblasts of patients with TAO. Published data suggest that PTX3 is overexpressed during the active phases of the disease and it returns to baseline levels once TAO reaches a stable phase (59, 61, 62, 64, 65).
Overall, the data summarized here suggest that PTX3 could play a role both in thyroid cancer, as demonstrated by the presence of PTX3 among the gene signatures associated to the different subtypes of thyroid carcinomas, and in the nodular remodeling of the thyroid, as suggested by PTX3 overexpression from goiter patients and GD patients. Ongoing and future researches are required to better understand these aspects and, from a clinical point of view, to determine the possible role of PTX3 as a biomarker of thyroid cancer and as a possible therapeutic target in GD.
DC conceived the study; BP, VP and DC were the major contributors in writing the manuscript; RL was involved in experimental activities; AM and BB were involved in drafting the manuscript and revising it critically. All authors contributed to the article and approved the submitted version.
The 5x1000 Grant of the Fondazione Humanitas per la Ricerca covered the costs of publications.
The authors acknowledge Antonella Monno who performed immunohistochemistry and provided the figures. We are also grateful to Fondazione Humanitas per la Ricerca for the support.
AM and BB are inventors of a patent on PTX3 and obtain royalties on related reagents.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Vanderpump MP. The epidemiology of thyroid disease. Br Med Bull (2011) 99:39–51. doi: 10.1093/bmb/ldr030
2. Simmonds MJ, Gough SC. Unravelling the genetic complexity of autoimmune thyroid disease: Hla, ctla-4 and beyond. Clin Exp Immunol (2004) 136(1):1–10. doi: 10.1111/j.1365-2249.2004.02424.x
3. Dean DS, Gharib H. Epidemiology of thyroid nodules. Best Pract Res Clin Endocrinol Metab (2008) 22(6):901–11. doi: 10.1016/j.beem.2008.09.019
4. Pizzato M, Li M, Vignat J, Laversanne M, Singh D, La Vecchia C, et al. The epidemiological landscape of thyroid cancer worldwide: Globocan estimates for incidence and mortality rates in 2020. Lancet Diabetes Endocrinol (2022) 10(4):264–72. doi: 10.1016/S2213-8587(22)00035-3
5. Doni A, Mantovani A, Bottazzi B, Russo RC. Ptx3 regulation of inflammation, hemostatic response, tissue repair, and resolution of fibrosis favors a role in limiting idiopathic pulmonary fibrosis. Front Immunol (2021) 12:676702. doi: 10.3389/fimmu.2021.676702
6. Doni A, Musso T, Morone D, Bastone A, Zambelli V, Sironi M, et al. An acidic microenvironment sets the humoral pattern recognition molecule Ptx3 in a tissue repair mode. J Exp Med (2015) 212(6):905–25. doi: 10.1084/jem.20141268
7. Garlanda C, Bottazzi B, Magrini E, Inforzato A, Mantovani A. Ptx3, a humoral pattern recognition molecule, in innate immunity, tissue repair, and cancer. Physiol Rev (2018) 98(2):623–39. doi: 10.1152/physrev.00016.2017
8. Jaillon S, Peri G, Delneste Y, Fremaux I, Doni A, Moalli F, et al. The humoral pattern recognition receptor Ptx3 is stored in neutrophil granules and localizes in extracellular traps. J Exp Med (2007) 204(4):793–804. doi: 10.1084/jem.20061301
9. Bonavita E, Gentile S, Rubino M, Maina V, Papait R, Kunderfranco P, et al. Ptx3 is an extrinsic oncosuppressor regulating complement-dependent inflammation in cancer. Cell (2015) 160(4):700–14. doi: 10.1016/j.cell.2015.01.004
10. Braunschweig A, Jozsi M. Human pentraxin 3 binds to the complement regulator C4b-binding protein. PloS One (2011) 6(8):e23991. doi: 10.1371/journal.pone.0023991
11. Deban L, Castro Russo R, Sironi M, Moalli F, Scanziani M, Zambelli V, et al. Regulation of leukocyte recruitment by the long pentraxin Ptx3. Nat Immunol (2010) 11(4):328–34. doi: 10.1038/ni.1854
12. Grcevic D, Sironi M, Valentino S, Deban L, Cvija H, Inforzato A, et al. The long pentraxin 3 plays a role in bone turnover and repair. Front Immunol (2018) 9:417. doi: 10.3389/fimmu.2018.00417
13. Inforzato A, Doni A, Barajon I, Leone R, Garlanda C, Bottazzi B, et al. Ptx3 as a paradigm for the interaction of pentraxins with the complement system. Semin Immunol (2013) 25:75–85. doi: 10.1016/j.smim.2013.05.002
14. Ma YJ, Garred P. Pentraxins in complement activation and regulation. Front Immunol (2018) 9:3046. doi: 10.3389/fimmu.2018.03046
15. Salustri A, Garlanda C, Hirsch E, De Acetis M, Maccagno A, Bottazzi B, et al. Ptx3 plays a key role in the organization of the cumulus oophorus extracellular matrix and in in vivo fertilization. Development (2004) 131:1577–86. doi: 10.1242/dev.01056
16. Caironi P, Masson S, Mauri T, Bottazzi B, Leone R, Magnoli M, et al. Pentraxin 3 in patients with severe sepsis or shock: The albios trial. Eur J Clin Invest (2017) 47(1):73–83. doi: 10.1111/eci.12704
17. Cunha C, Aversa F, Lacerda JF, Busca A, Kurzai O, Grube M, et al. Genetic Ptx3 deficiency and aspergillosis in stem-cell transplantation. N Engl J Med (2014) 370(5):421–32. doi: 10.1056/NEJMoa1211161
18. Davoudian S, Piovani D, Desai A, Mapelli SN, Leone R, Sironi M, et al. A Cytokine/Ptx3 prognostic index as a predictor of mortality in sepsis. Front Immunol (2022) 13:979232. doi: 10.3389/fimmu.2022.979232
19. Jenny NS, Blumenthal RS, Kronmal RA, Rotter JI, Siscovick DS, Psaty BM. Associations of pentraxin 3 with cardiovascular disease: The multi-ethnic study of atherosclerosis. J Thromb Haemost (2014) 12(6):999–1005. doi: 10.1111/jth.12557
20. Mauri T, Bellani G, Patroniti N, Coppadoro A, Peri G, Cuccovillo I, et al. Persisting high levels of plasma pentraxin 3 over the first days after severe sepsis and septic shock onset are associated with mortality. Intensive Care Med (2010) 36(4):621–9. doi: 10.1007/s00134-010-1752-5
21. Mauri T, Coppadoro A, Bellani G, Bombino M, Patroniti N, Peri G, et al. Pentraxin 3 in acute respiratory distress syndrome: An early marker of severity. Crit Care Med (2008) 36(8):2302–8. doi: 10.1097/CCM.0b013e3181809aaf
22. Boutet MA, Nerviani A, Lliso-Ribera G, Leone R, Sironi M, Hands R, et al. Circulating and synovial pentraxin-3 (Ptx3) expression levels correlate with rheumatoid arthritis severity and tissue infiltration independently of conventional treatments response. Front Immunol (2021) 12:686795. doi: 10.3389/fimmu.2021.686795
23. Guan SY, Chen Y, Shao M, Yang H, Xu W, Shuai Z, et al. Increased circulating pentraxin 3 levels in patients with rheumatoid arthritis: A meta-analysis. Curr Pharm Des (2022) 28(27):2260–9. doi: 10.2174/1381612828666220614155037
24. Huang XL, Zhang L, Duan Y, Wang YJ, Wang J. Association of pentraxin 3 with autoimmune diseases: A systematic review and meta-analysis. Arch Med Res (2016) 47(3):223–31. doi: 10.1016/j.arcmed.2016.05.006
25. Ramirez GA, Rovere-Querini P, Blasi M, Sartorelli S, Di Chio MC, Baldini M, et al. Ptx3 intercepts vascular inflammation in systemic immune-mediated diseases. Front Immunol (2019) 10:1135. doi: 10.3389/fimmu.2019.01135
26. Simon A, Subra JF, Guilpain P, Jeannin P, Pignon P, Blanchard S, et al. Detection of anti-Pentraxin-3 autoantibodies in anca-associated vasculitis. PloS One (2016) 11(1):e0147091. doi: 10.1371/journal.pone.0147091
27. van Rossum AP, Pas HH, Fazzini F, Huitema MG, Limburg PC, Jonkman MF, et al. Abundance of the long pentraxin Ptx3 at sites of leukocytoclastic lesions in patients with small-vessel vasculitis. Arthritis Rheum (2006) 54(3):986–91. doi: 10.1002/art.21669
28. Locatelli M, Ferrero S, Martinelli Boneschi F, Boiocchi L, Zavanone M, Maria Gaini S, et al. The long pentraxin Ptx3 as a correlate of cancer-related inflammation and prognosis of malignancy in gliomas. J Neuroimmunol (2013) 260(1-2):99–106. doi: 10.1016/j.jneuroim.2013.04.009
29. Planque C, Kulasingam V, Smith CR, Reckamp K, Goodglick L, Diamandis EP. Identification of five candidate lung cancer biomarkers by proteomics analysis of conditioned media of four lung cancer cell lines. Mol Cell Proteomics (2009) 8(12):2746–58. doi: 10.1074/mcp.M900134-MCP200
30. Wang JX, He YL, Zhu ST, Yang S, Zhang ST. Aberrant methylation of the 3q25 tumor suppressor gene Ptx3 in human esophageal squamous cell carcinoma. World J Gastroenterol (2011) 17(37):4225–30. doi: 10.3748/wjg.v17.i37.4225
31. Willeke F, Assad A, Findeisen P, Schromm E, Grobholz R, von Gerstenbergk B, et al. Overexpression of a member of the pentraxin family (Ptx3) in human soft tissue liposarcoma. Eur J Cancer (2006) 42(15):2639–46. doi: 10.1016/j.ejca.2006.05.035
32. Goulart MR, Watt J, Siddiqui I, Lawlor RT, Imrali A, Hughes C, et al. Pentraxin 3 is a stromally-derived biomarker for detection of pancreatic ductal adenocarcinoma. NPJ Precis Oncol (2021) 5(1):61. doi: 10.1038/s41698-021-00192-1
33. Bogdan M, Meca AD, Turcu-Stiolica A, Oancea CN, Kostici R, Surlin MV, et al. Insights into the relationship between pentraxin-3 and cancer. Int J Mol Sci (2022) 23(23). doi: 10.3390/ijms232315302
34. Doni A, Stravalaci M, Inforzato A, Magrini E, Mantovani A, Garlanda C, et al. The long pentraxin Ptx3 as a link between innate immunity, tissue remodeling, and cancer. Front Immunol (2019) 10:712. doi: 10.3389/fimmu.2019.00712
35. Ronca R, Alessi P, Coltrini D, Salle ED, Giacomini A, Leali D, et al. Long pentraxin-3 as an epithelial-stromal fibroblast growth factor-targeting inhibitor in prostate cancer. J Pathol (2013) 230(2):228–38. doi: 10.1002/path.4181
36. Rubino M, Kunderfranco P, Basso G, Greco CM, Pasqualini F, Serio S, et al. Epigenetic regulation of the extrinsic oncosuppressor Ptx3 gene in inflammation and cancer. Oncoimmunology (2017) 6(7):e1333215. doi: 10.1080/2162402x.2017.1333215
37. Tsuji S, Midorikawa Y, Seki M, Takayama T, Sugiyama Y, Aburatani H. Network-based analysis for identification of candidate genes for colorectal cancer progression. Biochem Biophys Res Commun (2016) 476(4):534–40. doi: 10.1016/j.bbrc.2016.05.158
38. Matarazzo S, Melocchi L, Rezzola S, Grillo E, Maccarinelli F, Giacomini A, et al. Long pentraxin-3 follows and modulates bladder cancer progression. Cancers (Basel) (2019) 11(9):1277. doi: 10.3390/cancers11091277
39. Chang WC, Wu SL, Huang WC, Hsu JY, Chan SH, Wang JM, et al. Ptx3 gene activation in egf-induced head and neck cancer cell metastasis. Oncotarget (2015) 6(10):7741–57. doi: 10.18632/oncotarget.3482
40. Choi B, Lee EJ, Park YS, Kim SM, Kim EY, Song Y, et al. Pentraxin-3 silencing suppresses gastric cancer-related inflammation by inhibiting chemotactic migration of macrophages. Anticancer Res (2015) 35(5):2663–8.
41. Ying TH, Lee CH, Chiou HL, Yang SF, Lin CL, Hung CH, et al. Knockdown of pentraxin 3 suppresses tumorigenicity and metastasis of human cervical cancer cells. Sci Rep (2016) 6:29385. doi: 10.1038/srep29385
42. Zhang H, Wang Y, Zhao Y, Liu T, Wang Z, Zhang N, et al. Ptx3 mediates the infiltration, migration, and inflammation-Resolving-Polarization of macrophages in glioblastoma. CNS Neurosci Ther (2022) 28(11):1748–66. doi: 10.1111/cns.13913
43. Song T, Wang C, Guo C, Liu Q, Zheng X. Pentraxin 3 overexpression accelerated tumor metastasis and indicated poor prognosis in hepatocellular carcinoma Via driving epithelial-mesenchymal transition. J Cancer (2018) 9(15):2650–8. doi: 10.7150/jca.25188
44. Wong R, Farrell SG, Grossmann M. Thyroid nodules: Diagnosis and management. Med J Aust (2018) 209(2):92–8. doi: 10.5694/mja17.01204
45. Popoveniuc G, Jonklaas J. Thyroid nodules. Med Clin North Am (2012) 96(2):329–49. doi: 10.1016/j.mcna.2012.02.002
46. Provatopoulou X, Georgiadou D, Sergentanis TN, Kalogera E, Spyridakis J, Gounaris A, et al. Interleukins as markers of inflammation in malignant and benign thyroid disease. Inflammation Res (2014) 63(8):667–74. doi: 10.1007/s00011-014-0739-z
47. Yildirim Simsir I, Cetinkalp S, Kabalak T. Review of factors contributing to nodular goiter and thyroid carcinoma. Med Princ Pract (2020) 29(1):1–5. doi: 10.1159/000503575
48. Destek S, Benturk B, Yapalak Y, Ozer OF. Clinical significance of erythrocyte sedimentation rate, leukocyte, fibrinogen, c-reactive protein, and pentraxin 3 values in thyroid nodules. Sisli Etfal Hastan Tip Bul (2022) 56(2):270–5. doi: 10.14744/SEMB.2021.78871
49. Chiari D, Bottazzi B, Leone R, Rodda GA, Pirali B, Branchini L, et al. Correlation between plasmatic long pentraxin Ptx3 and nodular thyroid disease: A preliminary report. Endocrine Abstract (2022) 81:EP1136. doi: 10.1530/endoabs.81.EP1136
50. Diamandis EP, Goodglick L, Planque C, Thornquist MD. Pentraxin-3 is a novel biomarker of lung carcinoma. Clin Cancer Res (2011) 17(8):2395–9. doi: 10.1158/1078-0432.CCR-10-3024
51. Stallone G, Netti GS, Cormio L, Castellano G, Infante B, Pontrelli P, et al. Modulation of complement activation by pentraxin-3 in prostate cancer. Sci Rep (2020) 10(1):18400. doi: 10.1038/s41598-020-75376-z
52. Zhang J, Wang TY, Niu XC. Increased plasma levels of pentraxin 3 are associated with poor prognosis of colorectal carcinoma patients. Tohoku J Exp Med (2016) 240(1):39–46. doi: 10.1620/tjem.240.39
53. Lundgren CI, Hall P, Dickman PW, Zedenius J. Clinically significant prognostic factors for differentiated thyroid carcinoma: A population-based, nested case-control study. Cancer (2006) 106(3):524–31. doi: 10.1002/cncr.21653
54. Luo Y, Chen R, Ning Z, Fu N, Xie M. Identification of a four-gene signature for determining the prognosis of papillary thyroid carcinoma by integrated bioinformatics analysis. Int J Gen Med (2022) 15:1147–60. doi: 10.2147/IJGM.S346058
55. Espinal-Enriquez J, Munoz-Montero S, Imaz-Rosshandler I, Huerta-Verde A, Mejia C, Hernandez-Lemus E. Genome-wide expression analysis suggests a crucial role of dysregulation of matrix metalloproteinases pathway in undifferentiated thyroid carcinoma. BMC Genomics (2015) 16(1):207. doi: 10.1186/s12864-015-1372-0
56. Wu Q, Cao F, Tao J, Li X, Zheng SG, Pan HF. Pentraxin 3: A promising therapeutic target for autoimmune diseases. Autoimmun Rev (2020) 19(12):102584. doi: 10.1016/j.autrev.2020.102584
57. Aydin U, Culha C, Aktas A, Keskin M, Ozcan M, Karaca A, et al. Pentraxin 3 levels in patients with hashimoto disease. Endocrine Abstract (2015) 37:EP972. doi: 10.1530/endoabs.37.EP972
58. Tomer Y, Huber A. The etiology of autoimmune thyroid disease: A story of genes and environment. J Autoimmun (2009) 32(3-4):231–9. doi: 10.1016/j.jaut.2009.02.007
59. Cheng CW, Fang WF, Tang KT, Lin JD. Serum interferon levels associated with the disease activity in women with overt graves' disease. Cytokine (2021) 138:155353. doi: 10.1016/j.cyto.2020.155353
61. Zhang S, Zhu YT, Chen SY, He H, Tseng SC. Constitutive expression of pentraxin 3 (Ptx3) protein by human amniotic membrane cells leads to formation of the heavy chain (Hc)-hyaluronan (Ha)-Ptx3 complex. J Biol Chem (2014) 289(19):13531–42. doi: 10.1074/jbc.M113.525287
62. Planck T, Parikh H, Brorson H, Martensson T, Asman P, Groop L, et al. Gene expression in graves' ophthalmopathy and arm lymphedema: Similarities and differences. Thyroid (2011) 21(6):663–74. doi: 10.1089/thy.2010.0217
63. Pilling D, Fan T, Huang D, Kaul B, Gomer RH. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PloS One (2009) 4(10):e7475. doi: 10.1371/journal.pone.0007475
64. Wang H, Atkins SJ, Fernando R, Wei RL, Smith TJ. Pentraxin-3 is a tsh-inducible protein in human fibrocytes and orbital fibroblasts. Endocrinology (2015) 156(11):4336–44. doi: 10.1210/en.2015-1399
65. Mou P, Chen Z, Jiang L, Cheng J, Wei R. Ptx3: A potential biomarker in thyroid associated ophthalmopathy. BioMed Res Int (2018) 2018:5961974. doi: 10.1155/2018/5961974
Keywords: PTX3, inflammation, thyroid disorders, thyroid cancer, nodular thyroid disease, Graves' disease
Citation: Chiari D, Pirali B, Perano V, Leone R, Mantovani A and Bottazzi B (2023) The crossroad between autoimmune disorder, tissue remodeling and cancer of the thyroid: The long pentraxin 3 (PTX3). Front. Endocrinol. 14:1146017. doi: 10.3389/fendo.2023.1146017
Received: 16 January 2023; Accepted: 07 March 2023;
Published: 21 March 2023.
Edited by:
Daniela Gallo, ASST dei Sette Laghi, ItalyReviewed by:
Claudio Cusini, MultiMedica Scientific and Technological Pole, Scientific Institute for Research, Hospitalization and Healthcare (IRCCS), ItalyCopyright © 2023 Chiari, Pirali, Perano, Leone, Mantovani and Bottazzi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Barbara Pirali, YmFyYmFyYS5waXJhbGkzQGdtYWlsLmNvbQ==; Damiano Chiari, ZGFtaWFuby5jaGlhcmlAaWNsb3VkLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.