
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol. , 16 May 2023
Sec. Clinical Diabetes
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1144422
This article is part of the Research Topic Advanced Approaches in the Diagnosis and Treatment of Diabetes Mellitus and Secondary Complications View all 46 articles
 Xiao Xie1,2,3†
Xiao Xie1,2,3† Chao Lian2,3†
Chao Lian2,3† Zhiping Zhang4†
Zhiping Zhang4† Meng Feng5
Meng Feng5 Wenqi Wang1,2,3
Wenqi Wang1,2,3 Xiaomeng Yuan2,3
Xiaomeng Yuan2,3 Yanmei Shi1,2,3
Yanmei Shi1,2,3 Tingting Liu2,3*
Tingting Liu2,3*Purpose: This meta-analysis compared the long-term (12 months or 24 months) efficacy and safety of intravitreal aflibercept injection (IAI) for diabetic macular edema (DME) and proliferative diabetic retinopathy (PDR).
Methods: We selected 16 randomized controlled trials (RCTs) performed after 2015 that had a minimum of 12 months and up to 24 months of treatment and conducted a meta-analysis with Review Manager version 5.3. Visual acuity (VA), central subfield thickness (CST) and adverse events were the outcomes selected for evaluation from the eligible studies.
Results: Based on 16 RCTs, we evaluated a total of 7125 patients. For PDR and severe DME with poor baseline vision, after a minimum of 12 months and up to 24 months of treatment, the aflibercept treatment group obtained better VA improvement than the focal/grid laser photocoagulation treatment group (MD=13.30; 95%CI: 13.01~13.58; P<0.001) or other treatments (ranibizumab, focal/grid laser photocoagulation, PRP, et al.) group (MD=1.10; 95%CI: 1.05~1.16; P<0.001). In addition, the aflibercept treatment group got higher CST reduction than the focal/grid laser photocoagulation treatment (MD=-33.76; 95%CI: -45.53 ~ -21.99; P<0.001) or other treatments (ranibizumab, focal/grid laser photocoagulation, et al.) group (MD=-33.76; 95%CI: -45.53 ~ -21.99; P<0.001). There was no significant difference in the overall incidence of ocular and non-ocular adverse events in each treatment group.
Conclusions: This meta-analysis showed that the advantages of IAI are obvious in the management of DME and PDR with poor baseline vision for long-term observation (a minimum of 12 months and up to 24 months) with both VA improvement and CST reduction. Applied IAI separately trended to be more effective than panretinal photocoagulation separately in VA improvement for PDR. More parameters should be required to assess functional and anatomic outcomes.
Diabetic retinopathy (DR) has become an increasingly common microvascular complication of diabetes that affects visual health. Proliferative diabetic retinopathy (PDR) and severe diabetic macular edema (DME), especially center-involved DME (CI-DME), have become the most common causes of visual loss among working population (1–3).
PDR and DME have been managed by panretinal laser photocoagulation (PRP) and focal/grid laser photocoagulation for the past 40 years (4, 5). Vascular endothelial growth factor (VEGF), as a “real killer” that seriously threatens vision, plays an important role in the improvement of diabetic retinal vascular permeability and is an important contributor to both vascular leakage and new blood vessel growth (6, 7). To date, many researchers have conducted systematic reviews and standard meta-analyses of anti-VEGF treatments which have been recognized as novel approaches for visual impairment of DR (8, 9). However, these existing meta-analysis did not include direct and indirect comparisons of the long-term observation (a minimum of 12 months and up to 24 months) of aflibercept and focal/grid laser photocoagulation or other treatments respectively in patients with DME, or afliberceptand panretinal photocoagulation (PRP) in patients with PDR. Therefore, we conducted a meta-analysis that included randomized controlled trials (RCTs) reported after 2015. This report compared the therapeutic effects of intravitreal injections of aflibercept with that of focal/grid laser photocoagulation (patients with DME), PRP (patients with PDR) or other treatments, evaluated the efficacy and safety of intravitreal aflibercept injection (IAI) in the management of DME and PDR for long-term observation (a minimum of 12 months and up to 24 months).
We systematically searched and identified relevant trials and literature from the PubMed, Embase, and Web of Science databases, as well as the Cochrane Central Register of Controlled Trials, and the publication time is until January 2023. The scope of the search was restricted to both English languages. The following key search points and medical keywords were used: diabetic retinopathy, randomized controlled trials, aflibercept, anti-vascular endothelial growth factor, focal/grid laser photocoagulation, panretinal photocoagulation, diabetic macular edema, and proliferative diabetic retinopathy. Retrospective research, reviews,case reports, letters and surveys were excluded. Visual acuity (VA), central subfield thickness (CST), and adverse events were the focus of our meta-analysis. Only anonymous online public data were used in the research, without the active participation of patients and informed consent.
The RCTs that meet the following criteria were considered eligible: (1) participants over 18 years of age with type 1 or 2 diabetes; (2) participants with DME or PDR; (3) published number of patients, age, gender, and intervention details; (4) treatments of interest were intravitreal injection of aflibercept 2.0mg compared with other treatment schemes, including ranibizumab 0.3mg/0.5mg, dexamethasone 0.7mg, brolucizumab 6.0 mg, faricimab 6.0 mg, focal/grid laser photocoagulation and PDR; the treatments determined by individual researchers could be proactive (fixed), reactive (pro re nata, PRN), or proactive/reactive (treat and extend, T&E); (5) the follow-up time of these study were 12 months or more; (6) studies that provided main outcomes evaluation parameters as mean ± SD: mean change in best-corrected visual acuity (BCVA) [measure in Early Treatment Diabetic Retinopathy Study (ETDRS) letters), mean change in CST, and adverse events; (7) all included studies should be compliant with the Declaration of Helsinki and written informed consent from enrolled patients; (8) if the same research subjects were reported in different publications, only the most recent and authoritative publications with available data for targeted outcomes was included.
The exclusion criteria were as follows: (1) retrospective studies and review articles; (2) unpublished data were not adopted; (3) participants only suffered from non-proliferative diabetic retinopathy; (4) no comparison was made between aflibercept and other treatment schemes; (5) RCTs with too short follow-up time.
The assessment of the full-text articles and data extraction of each study was independently conducted by two authors. In case of disagreement between two authors, the third author assessed again. Assessment contents included: publication metrics (name of the first author, year of publication, location and study design, etc.), the information of the participants (diagnosis, sample size, demographic characteristics, clinical characteristics, criteria of inclusion and exclusion), the information on intervention (options/frequency of treatment, dosage of medicine, duration of follow-up), and the main information on outcomes (BCVA, CST and adverse events).
Two authors independently assessed the risk of bias of the included RCTs, including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other bias (10). The divergences were resolved through full discussion, with the assistance of a third author if necessary.
Review Manager 5.3 was used for statistical analysis. The visual evaluation parameter was BCVA, the anatomical evaluation parameter was CST, and safety indicators included systemic or ocular adverse events during the injection treatment. The fixed effect model was used for data processing (11). Continuous outcomes were estimated using the mean difference (MD) and 95% credible intervals (CIs). Dichotomous outcomes were estimated using the risk difference (RD) and 95% CIs. Forest plots were used to summarize the weighted estimates.
We performed a preliminary literature search through all databases and retrieved 4430 articles. The literature selection process and reasons for exclusion are summarized in Figure 1. First, we excluded 2395 articles with nonconforming title and 851 articles with nonconforming abstracts, and then 1184 potential relevant articles were screened out. Next, we excluded 992 articles according to the article type, including review (n=899), case reports (n=53), letters (n=32), surveys (n=8). Then there were 192 articles qualified for full-text assessment. Moreover, 179 articles were excluded by study design, including retrospective study (n=87), irrelevant population (n=24), irrelevant intervention schemes (n=37), irrelevant comparison objects (n=9), no extracted results (n=3), incomplete published data (n=12), repetitive research (n=7). Ultimately, 13 articles (16 RCTs) were included in this meta-analysis.
Table 1 summarizes the basic characteristics of the 16 RCTs in the 13 articles included in this meta-analysis. The study sample sizes ranged from 42 to 951 patients. The characteristics of the patients with DME or PDR were similar among the trials. The follow-up duration ranged from 12 months to 24 months. The dose of aflibercept was 2.0 mg in the aflibercept treatment groups in all included studies (12–24). Other treatments include focal/grid laser photocoagulation, PRP, vitrectomy with PRP, and intravitreal injection of ranibizumab 0.3mg/0.5mg, dexamethasone 0.7mg, brolucizumab 6.0 mg, faricimab 6.0 mg.
Figure 2 showed the risk of bias graph and summary for each included study. Fifteen studies had random sequence generation and allocation concealment. Regarding blinding of participants and personnel, 12 studies were assessed as low risk and 2 studies as high risk. Regarding blinding of outcome assessment, 11 studies were assessed as low risk and one study as high risk. Regarding incomplete outcome data, 14 studies were assessed as low risk and 2 studies as high risk. Regarding selective outcome reporting, 10 studies were assessed as low risk and 5 studies as high risk. Other studies were rated as having unclear risks.
Because BCVA is the main visual index to judge the curative effect and progress, and CST is an important anatomical index to judge the degree of macular edema, we analyzed the data of BCVA and CST. Among these RCTs we have included, the baseline BCVA and CST did not exactly match. Therefore, we adopted the mean change in BCVA and CST as the primary outcome. Figures 3 and 4 showed the results of the meta-analysis of the effects of intravitreal aflibercept injection on BCVA improvement. The aflibercept treatment group had significantly better BCVA improvement than the focal/grid laser photocoagulation treatment group (MD=13.30; 95%CI: 13.01~13.58; P<0.001) or other treatments (ranibizumab, focal/grid laser photocoagulation, PRP, et al.) group (MD=1.10; 95%CI: 1.05~1.16; P<0.001).
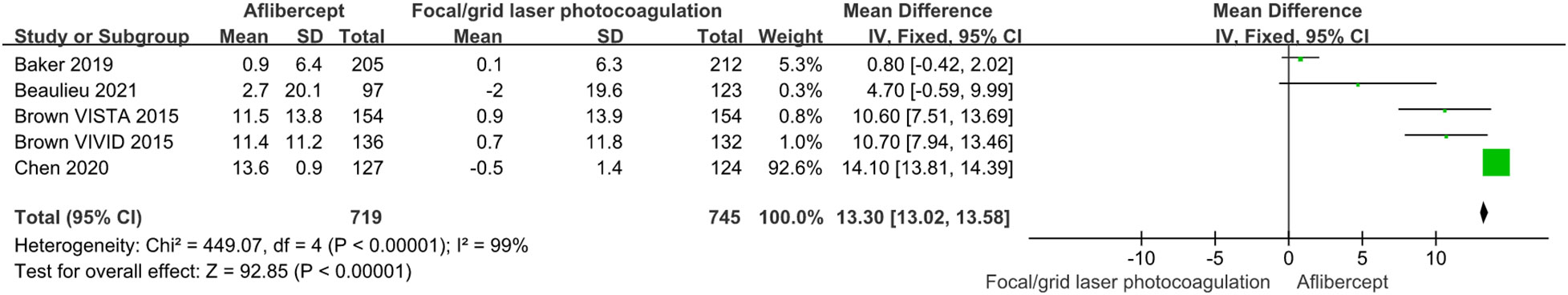
Figure 3 The mean changes from baseline in BCVA in the aflibercept group and focal/grid laser photocoagulation treatment group.
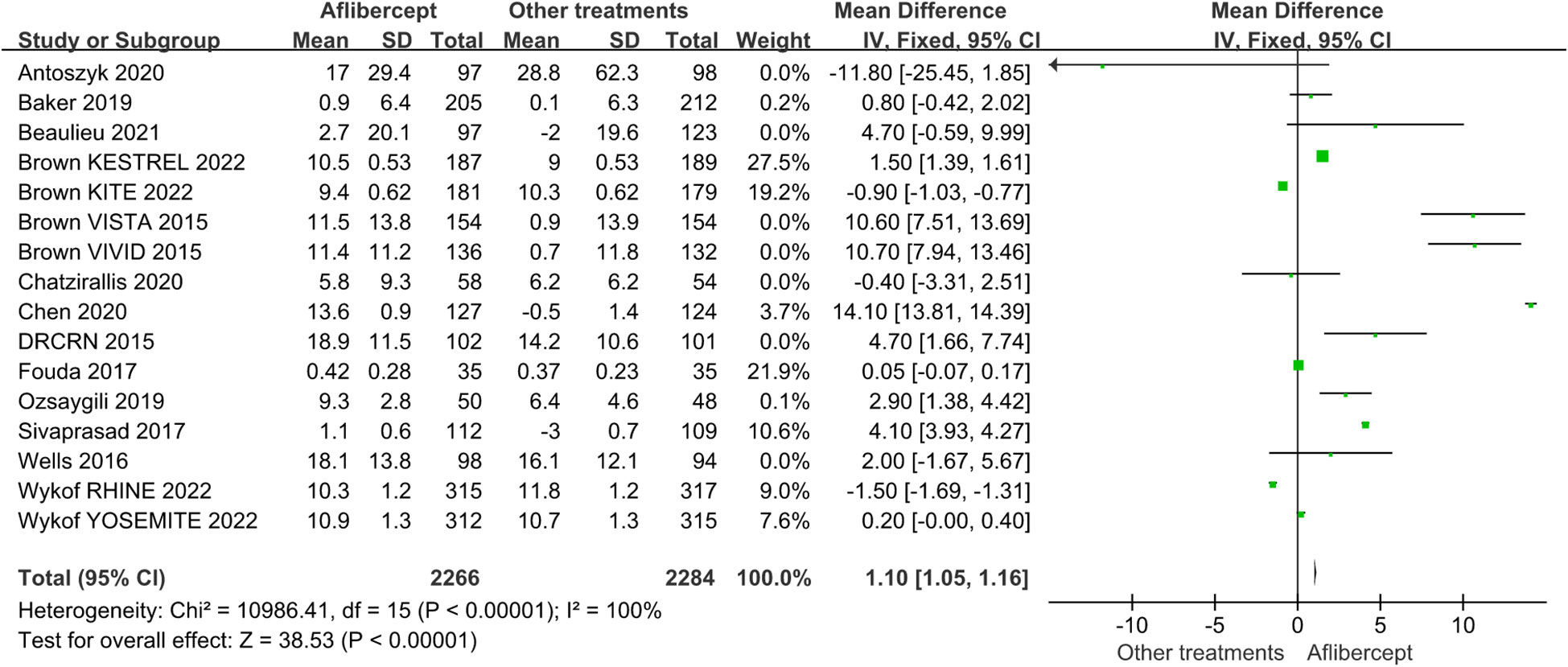
Figure 4 The mean changes from baseline in BCVA in the aflibercept group and other treatments group.
The effects of IAI in CST are shown in Figures 5 and 6. The aflibercept treatment group had higher CST reduction than the focal/grid laser photocoagulation treatment group (MD=-33.76; 95%CI: -45.53 ~ -21.99; P<0.001) or other treatments (ranibizumab, focal/grid laser photocoagulation, et al.) group (MD=-33.76; 95%CI: -45.53 ~ -21.99; P<0.001).
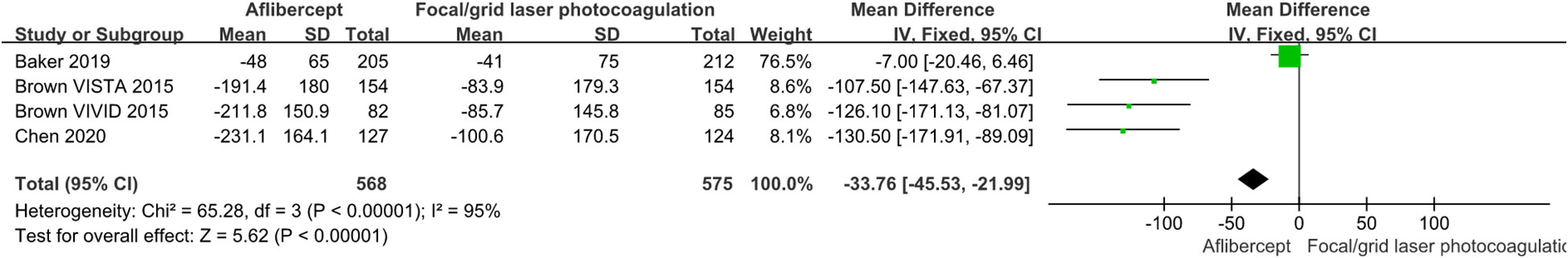
Figure 5 The mean changes from baseline in CST in the aflibercept group and focal/grid laser photocoagulation treatment group.
Of the 16 RCTs involved, the overall incidence rates of ocular and non-ocular adverse events were similar across the treatment groups (Figure 7). Regarding the frequency or pattern of serious ocular adverse events, there was no significant difference between the aflibercept group and other treatments (ranibizumab, focal/grid laser photocoagulation, et al.) group (RD=-0.02; 95%CI: -0.06 ~0.01; P=0.41).
In early 1976, the Diabetic Retinopathy Study group adopted PRP as the gold standard for the treatment of high-risk PDR eyes (25, 26). Since the ETDRS was first published in 1985, focal/grid laser photocoagulation has become the gold standard for the treatment of DME (27). Research on the Diabetic Retinopathy Clinical Research Network (DRCRnet) has confirmed that anti-VEGF drugs are not only effective alternative for PRP in patients with PDR but also as the first-line treatment for DME (28). It is very important for ophthalmologists and policymakers to compare the relative efficacy of DME or PDR treatment with the most reliable method.
Optical coherence tomography (OCT) is a noninvasive and easy-to-perform imaging tool that provides reliable and high-resolution imaging for the observation of retinal anatomy and quantification of the CST (29–31). With the help of OCT and the emergence of anti-VEGF drugs for patients with DR, clinical data suggested that anti-VEGF therapy can reduce macular edema and exudation, thus improving VA, reducing CST, and preventing further vision decline. Our findings are similar to those of previous studies from the viewpoint that anti-VEGF therapy is effective in patients with DME (32, 33). The therapeutic effects of these drugs are obviously superior to those of focal/grid laser photocoagulation separately.
To date, there are few meta-analyses comparing the expected clinical effects of aflibercept with other treatments (ranibizumab, focal/grid laser photocoagulation, PRP, pars plana vitrectomy, et al.) in the management of DME and PDR, especially the research on PDR.Based on this situation, we conducted a meta-analysis to compare the therapeutic effects of drug A with other treatment schemes. We evaluated16 RCTs published after 2015 in this meta-analysis, including 7125 patients who followed up 12 or 24 months. As reported in previous studies, among patients with visual impairment caused by DME, anti-VEGF monotherapy and combined focal/grid laser photocoagulation therapy provided better VA gain than focal/grid laser photocoagulation therapy separately (34). Although the short-term benefit of focal/grid laser photocoagulation combined with anti-VEGF therapy for DME patients was tiny in the DRCR.net Protocol I study, more than one-third of DME patients receiving anti-VEGF therapy delayed focal/grid laser photocoagulation therapy (35, 36). PRP has been the standard of care in the treatment of PDR for decades according to the DRS and the ETDRS (26). Both anti-VEGF therapy and focal/grid laser photocoagulation and PRP achieved remarkable anatomical and functional improvements during early treatment of DME and PDR respectively (37, 38). However, according to our results, for DME with long-term observation (a minimum of 12 months and up to 24 months), IAI had significantly better BCVA improvement than the focal/grid laser photocoagulation treatment (MD=13.30; 95%CI: 13.01~13.58; P<0.001) or other treatments(ranibizumab, focal/grid laser photocoagulation, PRP, et al.) (MD=1.10; 95%CI: 1.05~1.16; P<0.001). The visual improvements with IAI were primarily driven by patients with with poor baseline BCVA. In addition, IAI had significantly higher CST reduction than the focal/grid laser photocoagulation treatment (MD=-33.76; 95%CI: -45.53 ~ -21.99; P<0.001) or other treatments (ranibizumab, focal/grid laser photocoagulation, et al.) (MD=-33.76; 95%CI: -45.53 ~ -21.99; P<0.001). The increased response of patients with intractable DME or PDR may reflect the special pharmacological characteristics of aflibercept. This may be due to the fact that only aflibercept can inhibit both VEGF and placental growth factor (PGF), which are key factors leading to the pathogenesis of DME or PDR (39). More importantly, aflibercept has a faster association rate and a higher binding affinity for VEGF-A, VEGF-B, PlGF-1 and PlGF-2, resulting in accelerating a doubling of response rate (40).
The ability to achieve significant visual improvement with less frequent intravitreal injections and visits will be a valuable strategy for managing DME. In the VISTA and VIVID study, it was reported that the less frequent intravitreal injections schemes of aflibercept 2mg every 8 weeks (2q8) and 2mg every 4 weeks (2q4) can achieve similar effects in visual and anatomical results (12). In clinical practice, IAI 2q8 is also a good choice for working-age patients who have to miss work because of frequent visits. However, further studies are needed to determine the frequency of administration in patients with PDR.
Although anti-VEGF therapy can improve the visual and anatomical functions of patients with DR, focal/grid laser photocoagulation or PRP may still play an important role as an adjuvant therapy (41–43). PRP treatment was not a “one and done” procedure, and the addition of anti-VEGF drugs prevent DR progression and provide a “window period” for PRP. On the other hand, PRP can improve retinal oxygenation and decrease the drive for VEGF production by the retina, thus reducing the number of injections required and the burden of treatment. The combination of PRP and anti-VEGF therapy is acceptable in the real world. What’s more, according to the research of Protocol W, although there was no short-term vision benefit, early treatment with aflibercept can positively restore the anatomical structure of NPDR and reduced the risk of PDR or CI-DME with vision loss development in eyes with moderate to severe NPDR (44). Aflibercept may play a certain advantage in the management of DR with different severity.
Refractory vitreous hemorrhage and tractional retinal detachment may occur when PDR develops uncontrollably into advanced pathologies (45–47). For patients with recalcitrant DME, pars plana vitrectomy (PPV) can improve ocular anatomy (48, 49). Under these circumstances, PPV still plays a key role in the treatment of DR. Some studies have shown that anti-VEGF treatment before PPV can reduce intraoperative and postoperative hemorrhage and improve postoperative VA (50). On the positive side, in some cases requiring PPV, the combination of anti-VEGF therapy has a better curative effect. However, whether PPV has a wider effect than continuous anti-VEGF treatment has not yet been confirmed in RCTs.
The main unchangeable determinant of the development of diabetic retinopathy is the duration of diabetes (51). According to the standards for medical care for diabetes published by the American Diabetes Association in 2021, patients with type 1 diabetes should have a comprehensive ophthalmological examination within 5 years after diagnosis, and patients with type 2 diabetes should have their first fundus examination as soon as possible after diagnosis (52). A large-scale real-world research conducted by Chawla et al. found that the duration of diabetes was a strong predictor of the occurrence and development of DR. When the duration of type 2 diabetes reaches 9.4 ± 6.0 (mean ± SD) years, patients may have retinopathy. Therefore, we should always emphasize the early diagnosis and treatment of diabetic retinopathy in diabetic patients (53). It is worth noting that most people with diabetes in the RCTs we included are middle-aged and elderly people or working people. This kind of people are weak in physical examination consciousness or busy with work, and patients may delay the diagnosis of diabetes for various reasons, and their body are in a state of persistent hyperglycemia without knowing it. As a results, many patients with DR started their disease managements very late, or the medical treatment processes were irregular. Therefore, the scientific and standardized management of national health examination and disease prevention and control can not be ignored.
Studies have shown that the duration of action of anti-VEGF drugs varies among individual patients (32, 49). Moreover, even with appropriate treatment, repeated injections can increase the risk of infection, endophthalmitis, ocular inflammation, stroke, or myocardial infarction. The overall incidence of ocular and non-ocular adverse events in each treatment group was similar, and there was no significant difference between the aflibercept group and the other treatments group. It is a public knowledge that patients with diabetes have a high risk of cardiovascular comorbidities. In addition to diabetes, they are vulnerable to systemic complications. One study suggested that the increase in potential cerebrovascular accidents two years after treatment may be related to pro-epidermal growth factor therapy (54). Anti-VEGF therapy should be used cautiously in patients with myocardial infarction and stroke. Therefore, drug selection, injection frequency and interval, and necessary treatments should be adjusted according to the patient’s individual function and anatomical structure.
There are several limitations in this research. First, there may be deviations in the data collection, and only a small number of RCTs were included. In addition, with the rapid development of multi-mode imaging technology, methods for evaluating the prognosis of DR are more diversified. For example, with the addition of swept-source OCT, quantitative evaluation indices are more extensive, which will also affect our results. In future work, we will incorporate more indicators to quantitatively evaluate nonproliferative diabetic retinopathy and PDR. Additional OCTA parameters will be included in subsequent meta-analyses to improve the accuracy and robustness of the above conclusions and to provide better clinical guidance.
This meta-analysis showed that the advantages of IAI are obvious in the management of DME and PDR with poor baseline vision for long-term observation (a minimum of 12 months and up to 24 months). Applied IAI separately trended to be more effective than PRP separately with VA improvement for long-term observation. More parameters should be required to assess functional and anatomic outcomes.
TL, XX, and CL designed the study and conducted data extraction and quality evaluation. XX and ZZ drafted and revised the manuscript. ZZ and MF conducted software analysis and data verification. WW and XX sorted data. XY generated the graphics and tables. YS searched literature. All authors contributed to the article and approved the submitted version.
This work was supported by the Bethune Langmu Young Scholars Research Fund Project (BJ-LM2021007J) and New Ophthalmology Technology Incubation Fund Project.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer MC declared a shared affiliation with the authors to the handling editor at the time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Heng LZ, Comyn O, Peto T, Tadros C, Ng E, Sivaprasad S, et al. Diabetic retinopathy: pathogenesis, clinical grading, management and future developments. Diabetes Med (2013) 30(6):640–50. doi: 10.1111/dme.12089
2. Hou Y, Cai Y, Jia Z, Shi S. Risk factors and prevalence of diabetic retinopathy: A protocol for meta-analysis. Med (Baltimore) (2020) 99(42):e22695. doi: 10.1097/MD.0000000000022695
3. Varma R, Bressler NM, Doan QV, Gleeson M, Danese M, Bower JK, et al. Prevalence of and risk factors for diabetic macular edema in the united states. JAMA Ophthalmol (2014) 132(11):1334–40. doi: 10.1001/jamaophthalmol.2014.2854
4. Chauhan MZ, Rather PA, Samarah SM, Elhusseiny AM, Sallam AB. Current and novel therapeutic approaches for treatment of diabetic macular edema. Cells (2022) 11(12):1950. doi: 10.3390/cells11121950
5. Yonekawa Y, Modi YS, Kim LA, Skondra D, Kim JE, Wykoff CC. American society of retina specialists clinical practice guidelines on the management of nonproliferative and proliferative diabetic retinopathy without diabetic macular edema. J Vitreoretin Dis (2020) 4(2):125–35. doi: 10.1177/2474126419893829
6. Das A, McGuire PG, Rangasamy S. Diabetic macular edema: Pathophysiology and novel therapeutic targets. Ophthalmology (2015) 122(7):1375–94. doi: 10.1016/j.ophtha.2015.03.024
7. Romero-Aroca P, Baget-Bernaldiz M, Pareja-Rios A, Lopez-Galvez M, Navarro-Gil R, Verges R. Diabetic macular edema pathophysiology: Vasogenic versus inflammatory. J Diabetes Res (2016) 2016:2156273. doi: 10.1155/2016/2156273
8. He Y, Ren XJ, Hu BJ, Lam WC, Li XR. A meta-analysis of the effect of a dexamethasone intravitreal implant versus intravitreal anti-vascular endothelial growth factor treatment for diabetic macular edema. BMC Ophthalmol (2018) 18(1):121. doi: 10.1186/s12886-018-0779-1
9. Virgili G, Parravano M, Evans JR, Gordon I, Lucenteforte E. Anti-vascular endothelial growth factor for diabetic macular oedema: a network meta-analysis. Cochrane Database Syst Rev (2017) 6(6):CD007419. doi: 10.1002/14651858
10. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. Cochrane bias methods group; cochrane statistical methods group. the cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ (2011) 343:d5928. doi: 10.1136/bmj.d5928
11. Kanters S. Fixed- and random-effects models. Methods Mol Biol (2022) 2345:41–65. doi: 10.1007/978-1-0716-1566-9_3
12. Brown DM, Schmidt-Erfurth U, Do DV, Holz FG, Boyer DS, Midena E, et al. Intravitreal aflibercept for diabetic macular edema: 100-week results from the VISTA and VIVID studies. Ophthalmology (2015) 122(10):2044–52. doi: 10.1016/j.ophtha.2015.06.017
13. Diabetic Retinopathy Clinical Research Network, Wells JA, Glassman AR, Ayala AR, Jampol LM, Aiello LP, et al. Aflibercept, bevacizumab, or ranibizumab fordiabetic macular edema. N Engl J Med (2015) 372(13):1193–203. doi: 10.1056/NEJMoa1414264
14. Wells JA, Glassman AR, Ayala AR, Jampol LM, Bressler NM, Bressler SB, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: Two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology (2016) 123(6):1351–9. doi: 10.1016/j.ophtha.2016.02.022
15. Sivaprasad S, Prevost AT, Vasconcelos JC, Riddell A, Murphy C, Kelly J, et al. Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): a multicentre, single-blinded, randomised, controlled, phase 2b, non-inferiority trial. Lancet (2017) 389(10085):2193–203. doi: 10.1016/S0140-6736(17)31193-5
16. Fouda SM, Bahgat AM. Intravitreal aflibercept versus intravitreal ranibizumab for the treatment of diabeticmacular edema. Clin Ophthalmol (2017) 11:567–71. doi: 10.2147/OPTH.S131381
17. Baker CW, Glassman AR, Beaulieu WT, Antoszyk AN, Browning DJ, Chalam KV, et al. Effect of initial management with aflibercept vs laser photocoagulation vs observation on vision loss among patients with diabetic macular edema involving the center of the macula and good visual acuity: A randomized clinical trial. JAMA (2019) 321(19):1880–94. doi: 10.1001/jama.2019.5790
18. Ozsaygili C, Duru N. Comparison of intravitreal dexamethasone implant and aflibercept in patients with treatment-naive diabetic macular edema with serous retinal detachment. Retina (2020) 40(6):1044–52. doi: 10.1097/IAE.0000000000002537
19. Chen YX, Li XX, Yoon YH, Sun X, Astakhov Y, Xu G, et al. Intravitreal aflibercept versus laser photocoagulation in asian patients with diabetic macular edema: The VIVID-east study. Clin Ophthalmol (2020) 14:741–50. doi: 10.2147/OPTH.S235267
20. Chatzirallis A, Theodossiadis P, Droutsas K, Koutsandrea C, Ladas I, Moschos MM. Ranibizumab versus aflibercept for diabetic macular edema: 18-month results of a comparative, prospective, randomized study and multivariate analysis of visual outcome predictors. Cutan Ocul Toxicol (2020) 39(4):317–22. doi: 10.1080/15569527.2020.1802741
21. Antoszyk AN, Glassman AR, Beaulieu WT, Jampol LM, Jhaveri CD, Punjabi OS, et al. Effect of intravitreous aflibercept vs vitrectomy with panretinal photocoagulation on visual acuity in patients with vitreous hemorrhage from proliferative diabetic retinopathy: A randomized clinical trial. JAMA (2020) 324(23):2383–95. doi: 10.1001/jama.2020.23027
22. Beaulieu WT, Glassman AR, Baker CW, Maguire MG, Johnson CA, Melia M, et al. Effect of initial aflibercept, laser, or observation on low-contrast visual acuity in eyes with diabetic macular edema and good vision: Ancillary study within a randomized clinical trial. Transl Vis Sci Technol (2021) 10(3):3. doi: 10.1167/tvst.10.3.3
23. Brown DM, Emanuelli A, Bandello F, Barranco JJE, Figueira J, Souied E, et al. KESTREL and KITE: 52-week results from two phase III pivotal trials of brolucizumab for diabetic macular edema. Am J Ophthalmol (2022) 238:157–72. doi: 10.1016/j.ajo.2022.01.004
24. Wykoff CC, Abreu F, Adamis AP, Basu K, Eichenbaum DA, Haskova Z, et al. Efficacy, durability, and safety of intravitreal faricimab with extended dosing up to every 16 weeks in patients with diabetic macular oedema (YOSEMITE and RHINE): two randomised, double-masked, phase 3 trials. Lancet (2022) 399(10326):741–55. doi: 10.1016/S0140-6736(22)00018-6
25. The Diabetic Retinopathy Study Research Group. Preliminary report on effects of photocoagulation therapy. Am J Ophthalmol (1976) 81(4):383–96. doi: 10.1016/0002-9394(76)90292-0
26. El Rami H, Barham R, Sun JK, Silva PS. Evidence-based treatment of diabetic retinopathy. Semin Ophthalmol (2017) 32(1):67–74. doi: 10.1080/08820538.2016.1228397
27. Stitt AW, Curtis TM, Chen M, Medina RJ, McKay GJ, Jenkins A, et al. The progress in understanding and treatment of diabetic retinopathy. Prog Retin Eye Res (2016) 51:156–86. doi: 10.1016/j.preteyeres.2015.08.001
28. Sun JK, Jampol LM. The diabetic retinopathy clinical research network (DRCR.net) and its contributions to the treatment of diabetic retinopathy. Ophthalmic Res (2019) 62(4):225–30. doi: 10.1159/000502779
29. Arf S, Sayman Muslubas I, Hocaoglu M, Ersoz MG, Ozdemir H, Karacorlu M. Spectral domain optical coherence tomography classification of diabetic macular edema: a new proposal to clinical practice. Graefes Arch Clin Exp Ophthalmol (2020) 258(6):1165–72. doi: 10.1007/s00417-020-04640-9
30. Dysli M, Rückert R, Munk MR. Differentiation of underlying pathologies of macular edema using spectral domain optical coherence tomography (SD-OCT). Ocul Immunol Inflamm (2019) 27(3):474–83. doi: 10.1080/09273948.2019.1603313
31. Wang JC, Miller JB. Optical coherence tomography angiography: Review of current technical aspects and applications in chorioretinal disease. Semin Ophthalmol (2019) 34(4):211–7. doi: 10.1080/08820538.2019.1620797
32. Figueira J, Henriques J, Carneiro Â, Marques-Neves C, Flores R, Castro-Sousa JP, et al. Guidelines for the management of center-involving diabetic macular edema: Treatment options and patient monitorization. Clin Ophthalmol (2021) 15:3221–30. doi: 10.2147/OPTH.S318026
33. Kodjikian L, Bellocq D, Bandello F, Loewenstein A, Chakravarthy U, Koh A, et al. First-line treatment algorithm and guidelines in center-involving diabetic macular edema. Eur J Ophthalmol (2019) 29(6):573–84. doi: 10.1177/1120672119857511
34. Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P, Schlingemann RO, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology (2011) 118(4):615–25. doi: 10.1016/j.ophtha.2011.01.031
35. Mansour SE, Browning DJ, Wong K, Flynn HW Jr, Bhavsar AR. The evolving treatment of diabetic retinopathy. Clin Ophthalmol (2020) 14:653–78. doi: 10.2147/OPTH.S236637
36. Mehta H, Gillies MC, Fraser-Bell S. Combination of vascular endothelial growth factor inhibitors and laser therapy for diabetic macular oedema: a review. Clin Exp Ophthalmol (2016) 44(4):335–9. doi: 10.1111/ceo.12757
37. Heier JS, Korobelnik JF, Brown DM, Schmidt-Erfurth U, Do DV, Midena E, et al. Intravitreal aflibercept for diabetic macular edema: 148-week results from the VISTA and VIVID studies. Ophthalmology (2016) 123(11):2376–85. doi: 10.1016/j.ophtha.2016.07.032
38. Virgili G, Parravano M, Menchini F, Brunetti M. Antiangiogenic therapy with anti-vascular endothelial growth factor modalities for diabetic macular oedema. Cochrane Database Syst Rev (2012) 12:CD007419. doi: 10.1002/14651858.CD007419.pub3
39. Liu Y, Cheng J, Gao Y, Qin L, Min X, Zhang M. Efficacy of switching therapy to aflibercept for patients with persistent diabetic macular edema: a systematic review and meta-analysis. Ann Transl Med (2020) 8(6):382. doi: 10.21037/atm.2020.02.04
40. Ciombor KK, Berlin J, Chan E. Aflibercept. Clin Cancer Res (2013) 19(8):1920–5. doi: 10.1158/1078-0432.CCR-12-2911
41. Schmidt-Erfurth U, Garcia-Arumi J, Bandello F, Berg K, Chakravarthy U, Gerendas BS, et al. Guidelines for the management of diabetic macular edema by the european society of retina specialists (EURETINA). Ophthalmologica (2017) 237(4):185–222. doi: 10.1159/000458539
42. Gonzalez VH, Wang PW, Ruiz CQ. Panretinal photocoagulation for diabetic retinopathy in the RIDE and RISE trials: Not "1 and done". Ophthalmology (2021) 128(10):1448–57. doi: 10.1016/j.ophtha.2019.08.010
43. Distefano LN, Garcia-Arumi J, Martinez-Castillo V, Boixadera A. Combination of anti-VEGF and laser photocoagulation for diabetic macular edema: A review. J Ophthalmol (2017) 2017:2407037. doi: 10.1155/2017/2407037
44. Maturi RK, Glassman AR, Josic K, Baker CW, Gerstenblith AT, Jampol LM, et al. Four-year visual outcomes in the protocol w randomized trial of intravitreous aflibercept for prevention of vision-threatening complications of diabetic retinopathy. JAMA (2023) 329(5):376–85. doi: 10.1001/jama.2022.25029
45. Sharma T, Fong A, Lai TY, Lee V, Das S, Lam D. Surgical treatment for diabetic vitreoretinal diseases: a review. Clin Exp Ophthalmol (2016) 44(4):340–54. doi: 10.1111/ceo.12752
46. Rice TA, Michels RG. Long-term anatomic and functional results of vitrectomy for diabetic retinopathy. Am J Ophthalmol (1980) 90(3):297–303. doi: 10.1016/s0002-9394(14)74907-4
47. Jackson TL, Johnston RL, Donachie PH, Williamson TH, Sparrow JM, Steel DH. The royal college of ophthalmologists' national ophthalmology database study of vitreoretinal surgery: Report 6, diabetic vitrectomy. JAMA Ophthalmol (2016) 134(1):79–85. doi: 10.1001/jamaophthalmol.2015.4587
48. Diabetic Retinopathy Clinical Research Network Writing Committee, Haller JA, Qin H, Apte RS, Beck RR, Bressler NM, et al. Vitrectomy outcomes in eyes with diabetic macular edema and vitreomacular traction. Ophthalmology (2010) 117(6):1087–1093.e3. doi: 10.1016/j.ophtha.2009.10.040
49. Browning DJ, Lee C, Stewart MW, Landers MB 3rd. Vitrectomy for center-involved diabetic macular edema. Clin Ophthalmol (2016) 10:735–42. doi: 10.2147/OPTH.S104906
50. Fauser S, Muether PS. Clinical correlation to differences in ranibizumab and aflibercept vascular endothelial growth factor suppression times. Br J Ophthalmol (2016) 100(11):1494–8. doi: 10.1136/bjophthalmol-2015-308264
51. Lin KY, Hsih WH, Lin YB, Wen CY, Chang TJ. Update in the epidemiology, risk factors, screening, and treatment of diabetic retinopathy. J Diabetes Investig (2021) 12(8):1322–5. doi: 10.1111/jdi.13480
52. American Diabetes Association. 11. microvascular complications and foot care: Standards of medical care in diabetes-2021. Diabetes Care (2021) 44(Suppl 1):S151–67. doi: 10.2337/dc21-S011
53. Chawla S, Trehan S, Chawla A, Jaggi S, Chawla R, Kumar V, et al. Relationship between diabetic retinopathy microalbuminuria and other modifiable risk factors. Prim Care Diabetes. (2021) 15(3):567–70. doi: 10.1016/j.pcd.2021.01.012
Keywords: diabetic macular edema, proliferative diabetic retinopathy, aflibercept, meta-analysis, anti-vascular endothelial growth factor, focal/grid laser photocoagulation, panretinal photocoagulation
Citation: Xie X, Lian C, Zhang Z, Feng M, Wang W, Yuan X, Shi Y and Liu T (2023) Aflibercept for long-term treatment of diabetic macular edema and proliferative diabetic retinopathy: a meta-analysis. Front. Endocrinol. 14:1144422. doi: 10.3389/fendo.2023.1144422
Received: 14 January 2023; Accepted: 27 April 2023;
Published: 16 May 2023.
Edited by:
Pranav Kumar Prabhakar, Lovely Professional University, IndiaReviewed by:
Zulfiqarali Abbas, Muhimbili University of Health and Allied Sciences, TanzaniaCopyright © 2023 Xie, Lian, Zhang, Feng, Wang, Yuan, Shi and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tingting Liu, dGluZ3RpbmdsaXVAdmlwLnNpbmEuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.