
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol., 28 March 2023
Sec. Cancer Endocrinology
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1143261
This article is part of the Research TopicGnRH System Contribution in Cancer Regulation and Clinical TreatmentView all 5 articles
For many years, luteinizing hormone-releasing hormone or gonadotropin-releasing hormone (GnRH) analogs have been used to treat androgen or estrogen-dependent tumors. However, emerging evidence shows that the GnRH receptor (GnRH-R) is overexpressed in several cancer cells, including ovarian, endometrial, and prostate cancer cells, suggesting that GnRH analogs could exert direct antitumoral actions in tumoral tissues that express GnRH-R. Another recent approach based on this knowledge was the use of GnRH peptides for developing specific targeted therapies, improving the delivery and accumulation of drugs in tumoral cells, and decreasing most side effects of current treatments. In this review, we discuss the conventional uses of GnRH analogs, together with the recent advances in GnRH-based drug delivery for ovarian, breast, and prostatic cancer cells.
The luteinizing hormone-releasing hormone, herein called gonadotropin-releasing hormone (GnRH), is a peptide hormone synthesized and released in a pulsatile fashion by hypothalamic neurons. GnRH stimulates the synthesis and secretion of the gonadotropins follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from the pituitary gland (1); therefore, it displays a critical role in reproductive physiology. Their synthesis is regulated by the feedback of circulating levels of gonadal hormones to maintain the homeostasis of reproductive function.
The use of antagonists or long-acting analogs of GnRH disrupts de endocrine axis and decreases the endocrine function of gonads, producing medical castration. This treatment is widely used in some neoplasms that express androgen or estrogen receptors and whose growth is encouraged by circulating gonadal hormones (2, 3). For instance, in the case of breast cancer tissue, the immunodetection of estrogen receptors (ER) and progesterone receptors (PR) in biopsies is currently included as part of the clinical routine. Abundant evidence relates the expression of these receptors with patients’ prognosis and the response to endocrine therapy (4). In a similar manner, prostatic tissue expresses androgen receptors, and 30-50% of prostatic cancers show amplification of the androgen receptor gene, producing its overexpression (5). This knowledge has promoted the use of GnRH agonists and antagonists to induce castration in patients with breast (6) and prostatic cancer (7) (Figure 1).
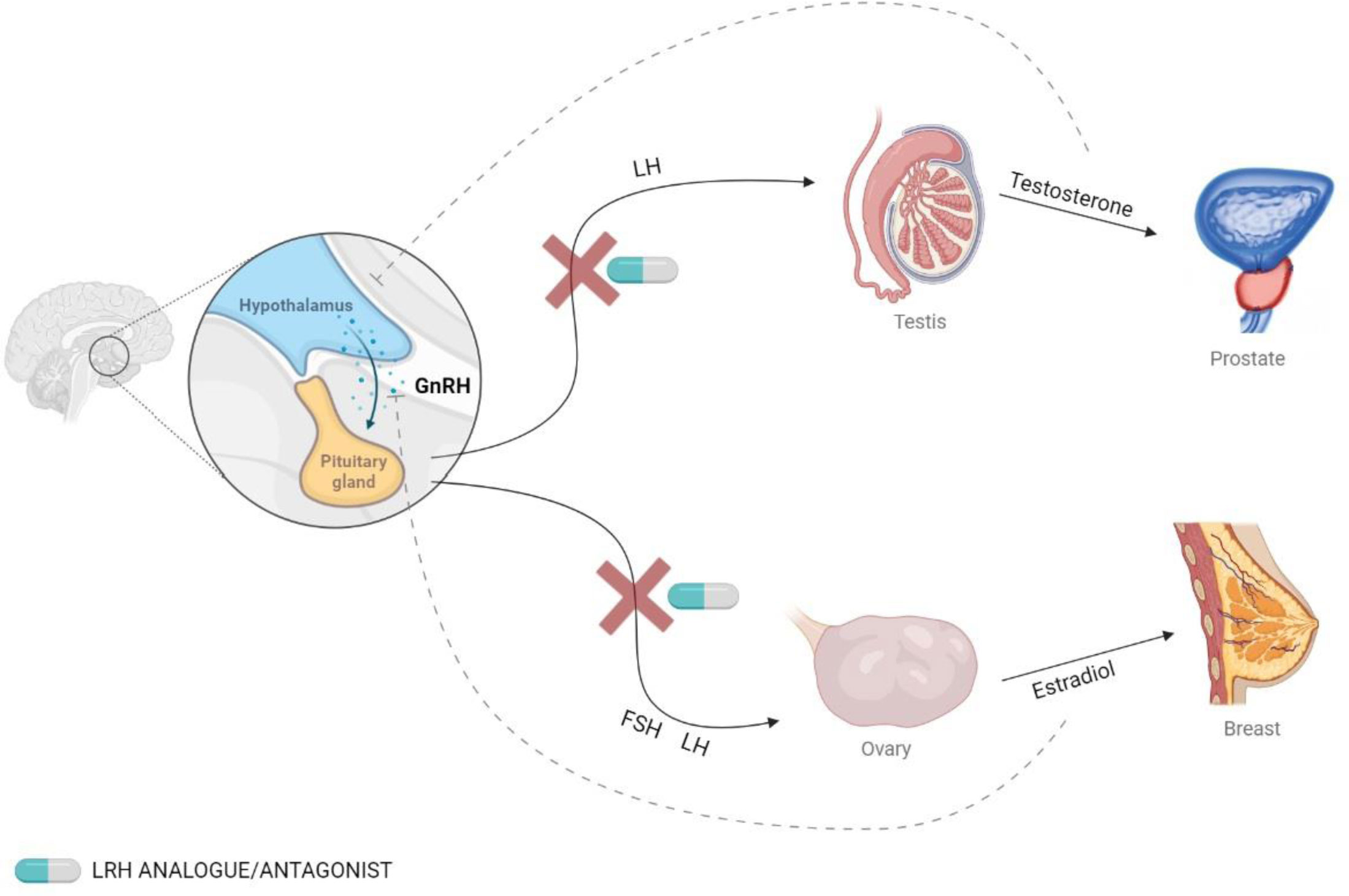
Figure 1 Secretion of GnRH and the effects on hormonal levels. GnRH is secreted by the hypothalamus and binds to its receptor in the pituitary gland, which stimulates the release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH). This effect can be blocked using GnRH analogs.
GnRH agonists produce an increase in the secretion of gonadotropin hormones, but continuous use leads to a downregulation of the receptors, which ultimately causes a decrease in gonadal hormone levels. On the other hand, GnRH antagonists produce an immediate inhibition of gonadotropin secretion (8). The most commonly used GnRH agonists are leuprolide, triptorelin, and goserelin, and the most common antagonists are degarelix and relugolix (9, 10).
Moreover, GnRH could exert direct effects in non-pituitary tissues, involving the activation of GnRH receptors (GnRH-R) (see Figure 2). The expression of GnRH and/or GnRH-R has been reported in the liver, heart, skeletal muscle, kidney, breast, and reproductive tissues, as well as malignant tumors of the breast, gonads, and urogenital tract (11–21). We will review diverse experiments using cell lines from breast, ovary, endometrium, and prostatic cancers, which have shown that GnRH analogs produce anti-tumoral effects, mainly reducing cell proliferation, tumoral size, and metastasis in vitro and in vivo.
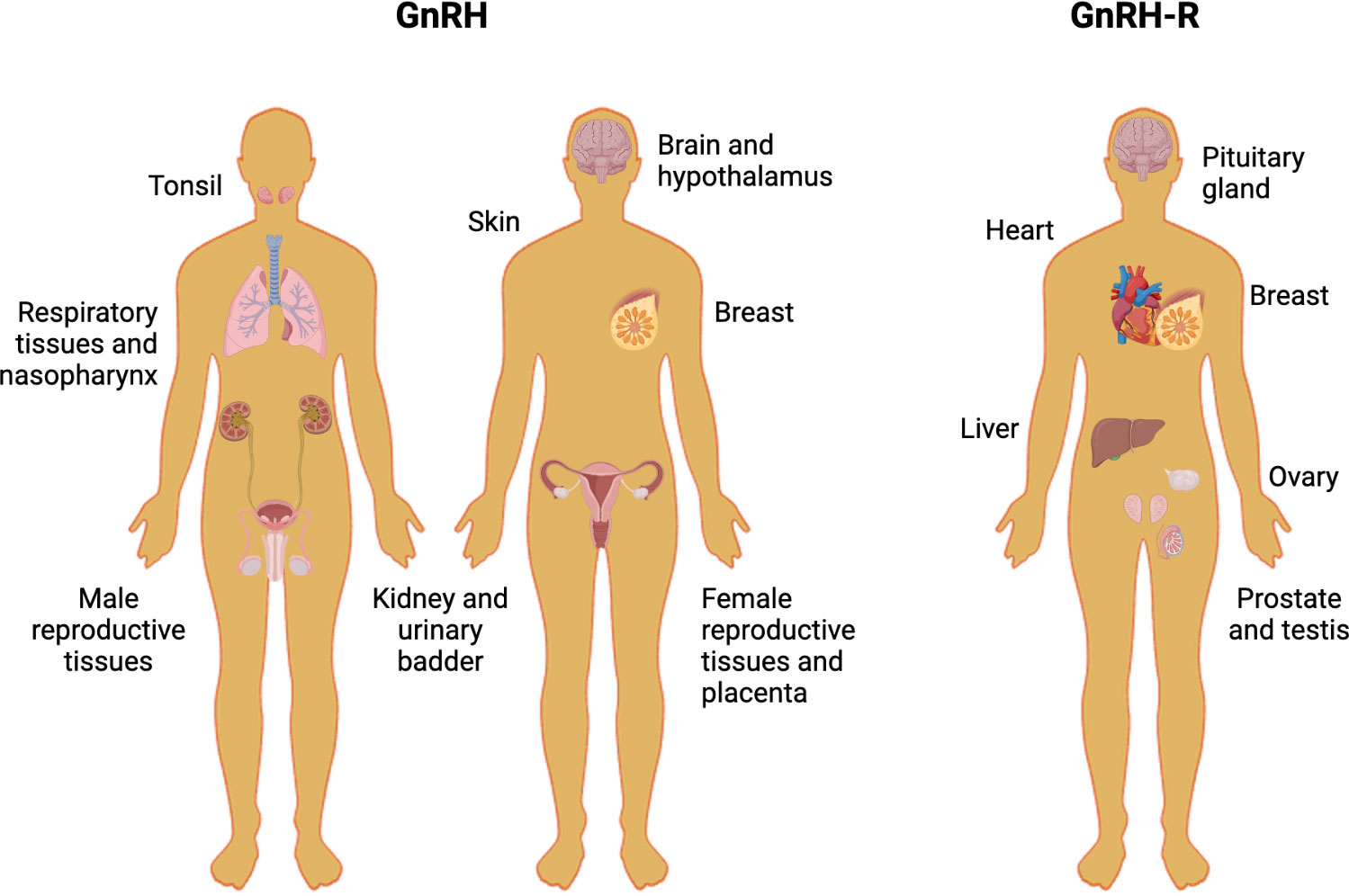
Figure 2 Human tissues that express GnRH and GnRH-R. The picture shows different human non-tumoral tissues in which the protein levels of GnRH and GnRH-R have been assessed.
Besides the direct and indirect anti-tumoral effects of GnRH, another potential therapeutic approach is their use in targeting therapy. As indicated below, GnRH-drug conjugates or nanoparticle complexes conjugated with GnRH and chemotherapeutical agents have been developed to treat tumors that express GnRH-R. These formulations allow selective delivery and exhibit several advantages, such as the improvement of drug internalization and accumulation of chemotherapeutics in cancer cells, thus minimizing side effects.
Prostate cancer is the most common cancer in men and the second one in respect of mortality (22). Risk factors include advanced age, ethnicity, and family history. Most cases are detected when the cancer is localized, and the 5-year survival rate is around 83% (23). The treatment for localized prostate cancer includes surgery and radiation, while metastatic prostate cancer is treated with chemotherapy and androgen deprivation therapy (ADT) (24).
The growth of almost 70% of prostate cancer cases is testosterone-dependent (25). Hence, GnRH agonists have been used since 1980 to treat prostatic cancer (26, 27) and currently, they are the first line of treatment. In advanced stages of the disease, combined androgen blockade (CAB) is recommended; this includes the use of chemical castration plus an anti-androgen. The use of CAB in the early stages of cancer is still under debate (10, 28, 29). GnRH agonists could be administered subcutaneously daily, even though there are slow-release formulations, such as microcapsules and implants that can release GnRH analogs for up to three months (25).
In its initial stage, prostatic cancer is largely dependent on androgens; however, some patients can develop over time an androgen-independent cancer. Antecedents have shown that FSH promotes the progression of this androgen-independent cancer, being capable of stimulating proliferation and decreasing apoptosis in an in vitro model (30, 31). Importantly, FSH levels are reduced ten times with the use of GnRH antagonists (32), in contrast with patients that undergo surgery and whose levels of FSH remain high. When cancer becomes androgen-independent, there is no consensus on whether to continue the GnRH therapy in these patients, and the decision is made considering the patient’s quality of life (33). The withdrawal of the GnRH therapy in some of these patients might contribute to the progression of androgen-independent prostate cancer.
To delay the progression of prostatic cancer to an androgen-independent phenotype, a new protocol of intermittent androgen suppression has been proposed. It is based on the adaptative mechanism of survival of the cancer cells in an environment without androgens, which can be postponed by giving the patient time off therapy to recover normal testosterone levels. The results of this protocol were not conclusive regarding the patient’s survival or cancer progression, but there is evidence that this protocol enhances their quality of life, with fewer side effects and a better sexual life, along with economic benefits (34, 35).
The adverse effects of GnRH therapy usually are impotence, osteoporosis, and dyslipidemia (36). ADT has also been associated with an increased risk of diabetes (37). These side effects should be treated preventively to improve the life quality of the patients (38).
Breast cancer is the most common cancer in women, especially in middle-aged and older women, and the leading cause of mortality due to cancer in this group (22). The risk factors include: age, gender, family history, use of hormonal contraceptives, and hormonal replacement therapy (39). Around 62% of the cases are diagnosed in the early stages, in which approximately 90% of the cases have a 5-year survival rate (40, 41). Breast cancer therapy consists of surgery, radiation, chemotherapy, and endocrine-based therapy, which includes GnRH analogs (42). 70% of women and over 80% of men with breast cancer are estrogen receptor-positive, and they are treated with tamoxifen, which blocks the estrogen receptor from binding to its ligand (43–45). Tamoxifen is used by both premenopausal and postmenopausal women, but postmenopausal women are mostly treated with aromatase inhibitors, which decrease the levels of estrogens by inhibiting the aromatase that produces estrogen (46, 47).
Around 80% of breast cancer in women and over 80% of breast cancer in men are hormone receptor-positive (48–50). As is known, estrogen and progesterone exert an important role in the progression of this cancer due to the effect of sex hormones on the proliferation of cancer cells (51). GnRH therapy is used as an adjuvant treatment in breast cancer to prevent recurrence and prolong patient survival (6). In rare cases, some patients have contraindications for tamoxifen, in which case GnRH therapy is used together with an aromatase inhibitor (42). GnRH by itself has been successfully used in premenopausal women, with a response in up to 63% of the patients, but only in 22% of postmenopausal women (52). In addition, tamoxifen and GnRH therapy can improve the overall survival of patients with breast cancer (53).
In the case of breast cancer cells, the interaction of FSH and LH with their receptors produces changes in the expression of genes related to adhesion, motility, and invasion (54). Therefore, GnRH therapy could be relevant to decrease FSH and LH levels in postmenopausal women, who have higher levels of gonadotropins. In this context, GnRH treatment would be considered adequate in the case of triple-negative breast cancer (TNBC), because half of these tumors upregulate GnRH-R (55). TNBC is estrogen-receptor (ER) negative, progesterone-receptor (PR) negative, and HER2 negative (56).
Among the subtypes of breast carcinoma, TNBC has a poor prognosis and shows the worst clinical outcomes. Unfortunately, due to a lack of molecular targets, the treatment for TNBC requires new therapeutic alternatives. A systematic revision of 4 investigations performed by Corona et al. (57) showed that the use of GnRH analogs could increase the overall survival of TNBC patients, in comparison to the control arm, although the difference was not statistically significant. However, most of the trials evaluated in the analysis were designed to test the efficacy of GnRH analogs to prevent premature ovarian failure in premenopausal women during adjuvant chemotherapy, which may represent a limitation. The study suggests that GnRH analogs could be useful as a targeted therapy in TNBC; therefore, clinical trials are needed to evaluate this alternative.
On the other hand, GnRH analogs could be an interesting alternative for the treatment of other cancer types, such as ovarian cancer. Despite its low incidence, ovarian cancer is the second cause of death due to gynecological cancer and over two-thirds of the cases are diagnosed in women of ages 55 or older (22). Among the risk factors are: hormonal replacement therapy, uninterrupted ovulation cycles, and family history (58). The treatment consists of cytoreductive surgery and chemotherapy (59) and this neoplasm is usually diagnosed in the late stages, when the 5-year survival rate is around 47% among all ages (60, 61).
Most ovarian cancer tissues express GnRH-R, as well as the receptors for FSH, LH, and estradiol. The interaction of these ligands and their receptors increases cell proliferation of ovarian cells (62–65). In contrast, the use of GnRH analogs decreases the proliferation of ovarian cancer cells in vitro (13).
Currently, GnRH analogs are not clinically used to treat ovarian cancer. Their use has been evaluated in several clinical trials, showing modest efficacy (66). In patients with platinum-resistant ovarian cancer, the GnRH analog, Leuprolide, and the antagonist Cetrorelix have been tested. While 9% and 18% of patients had partial remission, 26% and 35% of patients showed disease stabilization, respectively (67, 68). In vivo studies in ovarian carcinoma resistant to platinum chemotherapy showed that the use of both GnRH analogs and chemotherapy produces cytotoxic effects in ovarian cancer xenografts, with a significant reduction in the volume of ovarian tumors (14, 69).
In the long term, GnRH therapy can produce symptoms of menopause, fertility impairment, blood pressure changes, osteoporosis, and increase the risk of coronary heart disease (6, 70). However, in general, GnRH analogs have less severe side effects than chemotherapy and can be more specific in targeting hormone-dependent cancers. However, there is a need to improve the delivery methods to target a specific organ and thus, minimize the adverse effects.
Several studies have been performed to improve current anti-tumoral therapies, but with modest significant advances in cancer treatment. Most drugs used in conventional therapeutic strategies have low solubility, high metabolism, and are hydrophobic, and these features make these drugs biologically unavailable and can lead to systemic toxicity (71). Furthermore, standard chemotherapeutic treatments are limited in their selectivity toward tumor sites, and produce multiple drug resistance in tumoral cells (71). Another common problem related to cancer chemotherapy is drug toxicity and side effects, since they are designed to rapidly destroy dividing cells, including those found in healthy tissues (72).To overcome these problems, the development of drug-targeted therapies could increase drug efficacy and decrease the side effects of anticancer drugs (73).
In this context, carrier-based drug delivery systems (polymer conjugates, liposomes, micelles, dendrimers, nanogels, inorganic or other solid particles, and others) are being widely investigated to overcome the limitations of conventional drug chemotherapy and improve its overall safety and patient convenience (74). Numerous preclinical and clinical studies employing delivery systems have shown a better therapeutic effect and reduced overall toxicity, attributed mainly to a controlled drug release profile (75). Table 1 summarizes the studies that have tested nanoformulations in breast, prostate, and ovarian cancer.
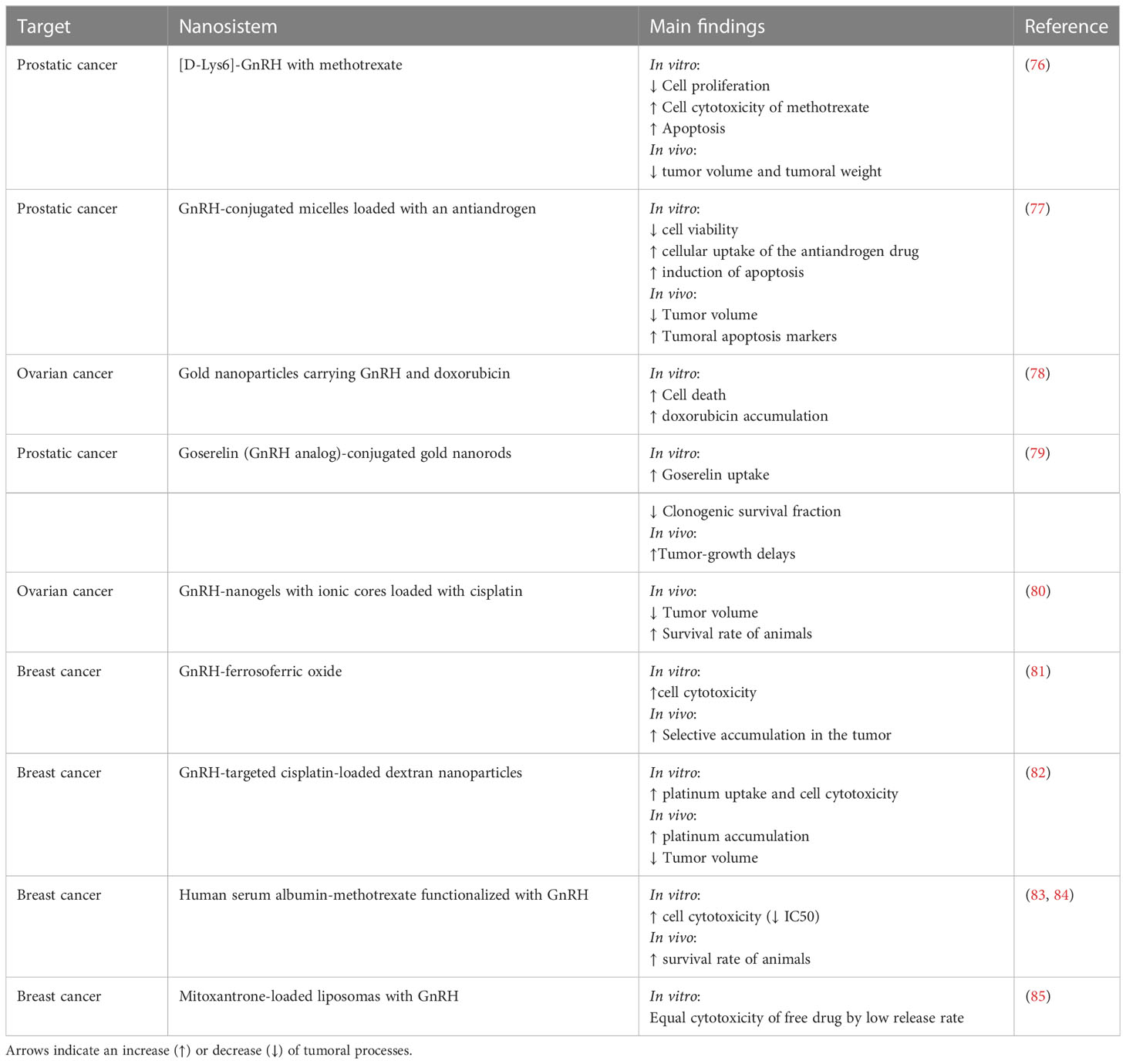
Table 1 Summary of different nanosystems that use GnRH analogs to increase the selective delivery of drugs to in vivo and in vitro models of ovarian, prostatic, and breast cancer.
Receptors that are primarily expressed in cancer cells represent attractive molecular targets for selective drug delivery. The binding of GnRH to GnRH-R appears to lead to receptor microaggregation and internalization of the peptide (86) (see Figure 3). The GnRH-R is overexpressed in most cancers, but its expression in healthy tissues, excluding pituitary cells, is limited (87–89). Accordingly, recent studies have indicated that GnRH peptides could be used as an efficient guide of anticancer compounds and imaging agents, which can selectively target tumor cells, increase the amount of these substances in tumor tissue, and prevent normal cells from unnecessary exposure. Active targeting of cancer cells is a strategy based on the modification of anticancer agents and/or drug-loaded nanoparticles with targeting ligands that specifically bind to the receptors preferentially expressed or highly overexpressed in cancer cells (90–93).

Figure 3 Input mechanism of GnRH-based nanoparticles for cancer cells. Nanoparticles coated with GnRH interact with the GnRH-R, promoting the entrance into cancer cells via endosome formation. Then, the nanoparticle content is released inside the cell.
Some studies have evaluated the use of GnRH peptide agonist and antagonist analogs in cancer tissues expressing GnRH-R (12–14). These studies employed GnRH-R-targeted dendrimers (94), nanoparticles (83), and liposomes (95), among others, to substantially increase the intra-tumor accumulation of anti-tumoral substances and therefore, enhance their anticancer efficacy. Importantly, GnRH-R-mediated targeting is independent of the nano-carrier architecture, composition, size, and molecular mass (95). Some of these studies used the [D-Lys6]-GnRH analog because it is resistant to degradation and is selectively accumulated in the nucleus of human GnRH receptor-positive breast, ovarian, and endometrial cancer cell lines (83, 96).
Nano-formulations have also been used for the detection of tumors and/or metastases through imaging techniques and to evaluate the treatment of different cancer types that have GnRH receptors. For instance, some studies employed GnRH peptides to achieve targeted delivery of radio-nucleotides as imaging agents (which attach to GnRH using chelating compounds) for their use in positron emission tomography (PET) and single photon emission computed tomography (SPECT) (95, 97). A GnRH conjugate that demonstrated rapid accumulation in both breast and prostate tumors, and specific binding to the GnRH receptor was developed and provided an efficient visualization of cancer lesions by SPECT (95, 98).
Studies suggest that GnRH peptides could act as local regulators of tumor growth (62, 99, 100). GnRH-R overexpression has been detected in hormone-dependent cancer tissues, such as breast (101), endometrial (102), ovarian (88, 103, 104), and prostate cancer (105); and also in hormone-independent tissues, such as pancreatic cancer (106, 107), lung cancer (108), melanoma (109), and glioblastoma (110). Moreover, GnRH-R expression levels are increased in various tumoral tissues. For example, GnRH-R is expressed in about 86% of prostate cancer, 80% of human endometrial and ovarian cancers, 80% of renal cancer, 50% of breast cancer, and 32–50% of pancreatic cancer cases (89, 111–114).
Currently, androgen ablation is a commonly prescribed treatment for localized prostatic cancer. However, this treatment has a limited scope, especially for hormone-refractory prostate cancers (115). Since prostate cancer tumor cells express the GnRH-R, some nano-formulations systems have been developed to deliver chemotherapy agents, producing less toxicity, and limiting nonspecific activity.
An interesting study performed in in vivo and in vitro prostatic cancer models tested the use of [D-Lys6]-GnRH with methotrexate ([D-Lys6]-GnRH-MTX). These results showed that prostatic cell growth was inhibited more by [D-Lys6]-GnRH-MTX than MTX alone, and that [D-Lys6]-GnRH-MTX also decreased tumor volume (74% vs 62% of MTX alone) and tumoral weight (74% vs 63% respectively) (76). On the other hand, Wen and coworkers (77) reported that GnRH-conjugated micelles loaded with the antiandrogen C4-2 cells exhibited a higher cellular uptake, promoting increased cell cytotoxicity and apoptosis, and efficient inhibition of prostatic cancer cell proliferation in vitro (approximately 80% of inhibition in C4-2 cells) and tumor growth in vivo (33% compared to only CBDIV17 micelles) after treatment (77).
On the other hand, peptides such as GnRH have been widely used for targeting nanoparticles to tumor cells in gynecological cancers, such as ovarian cancer. Due to the high expression of GnRH-R in ovarian cancer (compared with normal ovaries), nanoparticles containing GnRH can interact with its receptor, which leads to an endocytic process that facilitates cell internalization. The use of nanoparticles with GnRH has been developed with a focus on drug delivery and therapy in cancer treatment. Moreover, gold nanoparticles (GNPs) can be functionalized with molecules to achieve selective delivery to tumor cells, including a GnRH analog (78). Additionally, a potent radiosensitization of prostate cancers in vitro and in vivo using goserelin-conjugated gold nanorods has been reported (79). In this context, the study shows that treatment with goserelin-conjugated gold nanorods plus radiotherapy delayed tumor regrowth of a mouse xenograft by 17 ± 1 days compared to radiotherapy alone.
In the context of ovarian cancer diagnosis, gold nanoformulations could be used not only as a selective drug delivery agent but also as a diagnostic tool for imaging technologies, promoting non-invasive and real-time monitoring. For instance, a recent work tested GnRH-conjugated gold nanoparticles in a mouse model of ovarian cancer to assess their use in multi-energy spectral photon-counting computed tomography (116). The authors evidenced a preferential uptake of GnRH-gold nanoparticles in organs of the abdominal cavity, suggesting that this technology has potential use for imaging of ovarian cancer.
Another type of nanoformulation, a GnRH-cisplatin nanogel, was designed by Nukolova et al (80) to increase cell specificity and drug accumulation in ovarian cancer cell lines. This study showed that the cisplatin accumulation was specific for the GnRH-receptor-positive cells, more effective, and less toxic than equimolar doses of free cisplatin. Therefore, GnRH-targeted cisplatin enhanced the anti-tumoral effect of this drug in an animal model of ovarian cancer, decreasing the size of the tumor xenografts (approximately 40% less volume at day 25, compared to nanogels with only cisplatin) and increasing the survival rate of the animals (60% vs 15% respectively) (80).
Some GnRH analogs have been tested in patients; for instance, a conjugate of doxorubicin-GnRH agonist (AEZS-108) was tested in a phase 2 study performed in patients with metastatic hormone-resistant prostate cancer (NCT01240629) and demonstrated clinical benefit in 56% of the patients (progression-free survival at 12 weeks with no dose-limiting toxicities that require treatment cessation) (117). The same drug was tested in patients with chemotherapy-refractory triple negative breast cancer (NCT01698281), but the clinical trial was terminated due to poor recruitment.
As mentioned before, the incidence of breast cancer has increased over the years, and even though there are various therapeutic strategies, the mortality rate has not decreased, particularly in TNBC (22). GnRH-R is a possible target of TNBC cells, and its expression and receptor kinetics have been well characterized (118, 119). These studies showed that the binding of GnRH to GnRH-R is increased in TNBC cells, indicating the existence of interactions between the overexpressed GnRH receptors and their ligands.
Both thermodynamics and kinetic models, and also in vitro experiments showed that GnRH conjugated with polyethylene glycol (PEG)-coated magnetite nanoparticles (GnRH-MNPs) can interact with TNBC and non-tumoral breast cells. This study suggested that GnRH-MNPs preferentially enter into TNBC cells via the receptor-mediated endocytosis pathway, with a significant GnRH-MNP uptake after 3 h (119). The same group also determined that the entrance of GnRH-MNPs to TBNC cells depends on its high efficiency to bind to the GnRH-R (119), suggesting that GnRH-MNPs can be used for the specific targeting of TNBC cells for both cancer detection and treatment. Similar conclusions were found by Nian et al (81), who synthetized GnRH-ferrosoferric oxide (GnRH-Fe3O4) nanoparticles. This formulation showed higher concentrations in the tumor (under the effect of a magnetic field in vivo, and most importantly, without evidence of heart, liver, or lung toxicity (81). This study concluded that GnRH-Fe3O4 nanoparticles could be useful for targeting contrast agents or targeted imaging, and for the treatment of cancers with high GnRH-R expression, such as breast cancer.
Another type of nanoformulation was designed by Li et al. (82), who tested GnRH-targeted cisplatin-loaded dextran nanoparticles in a model of metastatic breast cancer. These GnRH-based nanoparticles significantly increased the accumulation of cisplatin in the primary and metastatic tumors (twice as much as with cisplatin alone), reduced drug delivery to kidneys, and improved its anticancer activity; decreasing tumor volume by 49% compared to free cisplatin (82).
On the other hand, GnRH has been used to functionalize endogenous proteins, such as human serum albumin (HSA). For instance, HAS-methotrexate conjugates were functionalized with GnRH to achieve better incorporation into breast cancer cells, producing a significant rise in methotrexate internalization and its antitumoral activity in GnRH-R-positive breast cancer cells (IC50 of 49.2 vs 5.8 nM for non-targeted nanoparticles and GnRH-targeted nanoparticle respectively) (84). In vivo results of this formulation show a 2-fold increase in the percentage of animal survival compared to MTX alone (83). This strategy was carried out to improve the delivery of hydrophobic drugs such as MTX, promoting its reuse after dismissing its use due to its high rate of side effects.
Another therapeutic approach that has been considered for the treatment of breast cancer is the development of GnRH-targeted liposomes and micelles. He and coworkers (85) studied the delivery of mitoxantrone using GnRH analogs modified with PEGylated (polyethylene glycol thioether bond) liposomes. In vitro studies in MCF-7 cells (a metastatic adenocarcinoma cell line) with high expression of GnRH-R revealed that targeted liposomes showed higher internalization and sustained drug release characteristics. However, the release rate of the drug was low, which depressed the action of mitoxantrone on tumor cells, suggesting that it is necessary to continue improving this type of formulations (85).
GnRH-R is overexpressed in many types of cancer and therefore, many drug delivery strategies developed for cancer-specific therapy have been used for GnRH targeting. Recent efforts are aimed at designing and developing new drug delivery systems using GnRH peptide/analogs as targeting moieties. Diverse studies also revealed positive results with many types of GnRH- nanoformulations in terms of binding, accumulation, and treatment efficacy.
Numerous studies have reported the use of the GnRH peptide or its analogs as a targeting ligand to increase the potency of chemotherapeutic drugs, demonstrating the efficiency of the specific binding between GnRH peptide/analog-based carriers and the GnRH-R in cancer cells/tumors. In vitro and in vivo experiments have reported enhanced internalization of the drugs into cancer cells and accumulation in the tumor site, confirming the effectiveness of the new GnRH-targeted delivery. One of the most attractive strategies could be the use of GnRH-targeted nanoparticles, which have shown an increase in selective drug accumulation and promising results in models of ovarian, breast, and prostatic cancer. However, up to now, only two trials have tested GnRH-targeting therapy and therefore, its clinical use is still in its early initial stages.
Writing – Original Draft Preparation: MG, AH, and CR. Writing – Review & Editing: MG, MV, EA, and CR. All authors contributed to the article and approved the submitted version.
Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT) N°1190623 (AA) and N°1220479 (CR).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Schally AV, Arimura A, Kastin AJ, Matsuo H, Baba Y, Redding TW, et al. Gonadotropin-releasing hormone: one polypeptide regulates secretion of luteinizing and follicle-stimulating hormones. Science (1971) 173(4001):1036–8. doi: 10.1126/science.173.4001.1036
2. Huerta-Reyes M, Maya-Nunez G, Perez-Solis MA, Lopez-Munoz E, Guillen N, Olivo-Marin JC, et al. Treatment of breast cancer with gonadotropin-releasing hormone analogs. Front Oncol (2019) 9:943. doi: 10.3389/fonc.2019.00943
3. Waxman JH, Wass JA, Hendry WF, Whitfield HN, Besser GM, Malpas JS, et al. Treatment with gonadotrophin releasing hormone analogue in advanced prostatic cancer. Br Med J (Clin Res Ed) (1983) 286(6374):1309–12. doi: 10.1136/bmj.286.6374.1309
4. Bulut N, Altundag K. Does estrogen receptor determination affect prognosis in early stage breast cancers? Int J Clin Exp Med (2015) 8(11):21454–9.
5. Visakorpi T, Hyytinen E, Koivisto P, Tanner M, Keinanen R, Palmberg C, et al. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat Genet (1995) 9(4):401–6. doi: 10.1038/ng0495-401
6. Goel S, Sharma R, Hamilton A, Beith J. LHRH agonists for adjuvant therapy of early breast cancer in premenopausal women. Cochrane Database Syst Rev (2009) 4):CD004562. doi: 10.1002/14651858.CD004562
7. Loblaw DA, Virgo KS, Nam R, Somerfield MR, Ben-Josef E, Mendelson DS, et al. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American society of clinical oncology practice guideline. J Clin Oncol (2007) 25(12):1596–605. doi: 10.1200/JCO.2006.10.1949
8. Harrison GS, Wierman ME, Nett TM, Glode LM. Gonadotropin-releasing hormone and its receptor in normal and malignant cells. Endocr Relat Cancer (2004) 11(4):725–48. doi: 10.1677/erc.1.00777
9. American_Cancer_Society. Hormone therapy for prostate cancer (2022). Available at: https://www.cancer.org/cancer/prostate-cancer/treating/hormone-therapy.html (Accessed December 5, 2022).
10. National_Cancer_Institute. Hormone therapy for prostate cancer (2021). Available at: https://www.cancer.gov/types/prostate/prostate-hormone-therapy-fact-sheet (Accessed December 5, 2022).
11. Kakar SS, Jennes L. Expression of gonadotropin-releasing hormone and gonadotropin-releasing hormone receptor mRNAs in various non-reproductive human tissues. Cancer Lett (1995) 98(1):57–62. doi: 10.1016/S0304-3835(06)80010-8
12. Emons G, Grundker C, Gunthert AR, Westphalen S, Kavanagh J, Verschraegen C. GnRH antagonists in the treatment of gynecological and breast cancers. Endocr Relat Cancer (2003) 10(2):291–9. doi: 10.1677/erc.0.0100291
13. Emons G, Ortmann O, Becker M, Irmer G, Springer B, Laun R, et al. High affinity binding and direct antiproliferative effects of LHRH analogues in human ovarian cancer cell lines. Cancer Res (1993) 53(22):5439–46.
14. Engel JB, Schally AV, Buchholz S, Seitz S, Emons G, Ortmann O. Targeted chemotherapy of endometrial, ovarian and breast cancers with cytotoxic analogs of luteinizing hormone-releasing hormone (LHRH). Arch Gynecol Obstet (2012) 286(2):437–42. doi: 10.1007/s00404-012-2335-1
15. Emons G, Grundker C. The role of gonadotropin-releasing hormone (GnRH) in endometrial cancer. Cells (2021) 10(2):. doi: 10.3390/cells10020292
16. Kyritsi K, Meng F, Zhou T, Wu N, Venter J, Francis H, et al. Knockdown of hepatic gonadotropin-releasing hormone by vivo-morpholino decreases liver fibrosis in multidrug resistance gene 2 knockout mice by down-regulation of miR-200b. Am J Pathol (2017) 187(7):1551–65. doi: 10.1016/j.ajpath.2017.03.013
17. Paradiso A, Pezzetta A, Cellamare G, Schittulli F, Marzullo F, Reshkin SJ. GnRH receptors in human breast cancer and its contiguous not-involved breast tissue. J Endocrinol Invest (2000) 23(2):90–6. doi: 10.1007/BF03343685
18. The_human_protein_atlas. GnRH1 - tisue. Available at: https://www.proteinatlas.org/ENSG00000147437-GNRH1/tissue (Accessed February 20, 2023).
20. Bahk JY, Kim MO, Park MS, Lee HY, Lee JH, Chung BC, et al. Gonadotropin-releasing hormone (GnRH) and GnRH receptor in bladder cancer epithelia and GnRH effect on bladder cancer cell proliferation. Urol Int (2008) 80(4):431–8. doi: 10.1159/000132703
21. Tieva A, Stattin P, Wikstrom P, Bergh A, Damber JE. Gonadotropin-releasing hormone receptor expression in the human prostate. Prostate (2001) 47(4):276–84. doi: 10.1002/pros.1072
22. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
23. Rawla P. Epidemiology of prostate cancer. World J Oncol (2019) 10(2):63–89. doi: 10.14740/wjon1191
24. Litwin MS, Tan HJ. The diagnosis and treatment of prostate cancer: A review. JAMA (2017) 317(24):2532–42. doi: 10.1001/jama.2017.7248
25. Schally AV, Block NL, Rick FG. Discovery of LHRH and development of LHRH analogs for prostate cancer treatment. Prostate (2017) 77(9):1036–54. doi: 10.1002/pros.23360
26. Falcone T, Falutz G, Tolis G. LH-RH-endocrine manipulation in cancer of the prostate. BioMed Pharmacother (1982) 36(8-9):344–8.
27. Tolis G, Ackman D, Stellos A, Mehta A, Labrie F, Fazekas AT, et al. Tumor growth inhibition in patients with prostatic carcinoma treated with luteinizing hormone-releasing hormone agonists. Proc Natl Acad Sci U.S.A. (1982) 79(5):1658–62. doi: 10.1073/pnas.79.5.1658
28. Garje R, Rumble RB, Parikh RA. Systemic therapy update on (177)Lutetium-PSMA-617 for metastatic castration-resistant prostate cancer: ASCO rapid recommendation. J Clin Oncol (2022) 40(31):3664–6. doi: 10.1200/JCO.22.01865
29. Akaza H. Combined androgen blockade for prostate cancer: review of efficacy, safety and cost-effectiveness. Cancer Sci (2011) 102(1):51–6. doi: 10.1111/j.1349-7006.2010.01774.x
30. Weckermann D, Harzmann R. Hormone therapy in prostate cancer: LHRH antagonists versus LHRH analogues. Eur Urol (2004) 46(3):279–83. doi: 10.1016/j.eururo.2004.05.006
31. Dizeyi N, Trzybulska D, Al-Jebari Y, Huhtaniemi I, Lundberg Giwercman Y. Cell-based evidence regarding the role of FSH in prostate cancer. Urol Oncol (2019) 37(4):290 e1– e8. doi: 10.1016/j.urolonc.2018.12.011
32. Beer TM. Experimental use of GnRH antagonists as second-line hormonal therapy. Rev Urol (2004) 6 Suppl 7(Suppl 7):S33–8.
33. Perlmutter MA, Lepor H. Androgen deprivation therapy in the treatment of advanced prostate cancer. Rev Urol (2007) 9 Suppl 1(Suppl 1):S3–8.
34. Calais da Silva FE, Bono AV, Whelan P, Brausi M, Marques Queimadelos A, Martin JA, et al. Intermittent androgen deprivation for locally advanced and metastatic prostate cancer: results from a randomised phase 3 study of the south European uroncological group. Eur Urol (2009) 55(6):1269–77. doi: 10.1016/j.eururo.2009.02.016
35. Abrahamsson PA. Potential benefits of intermittent androgen suppression therapy in the treatment of prostate cancer: A systematic review of the literature. Eur Urol (2010) 57(1):49–59. doi: 10.1016/j.eururo.2009.07.049
36. Kumar RJ, Barqawi A, Crawford ED. Adverse events associated with hormonal therapy for prostate cancer. Rev Urol (2005) 7 Suppl 5(Suppl 5):S37–43.
37. Alibhai SM, Duong-Hua M, Sutradhar R, Fleshner NE, Warde P, Cheung AM, et al. Impact of androgen deprivation therapy on cardiovascular disease and diabetes. J Clin Oncol (2009) 27(21):3452–8. doi: 10.1200/JCO.2008.20.0923
38. Rhee H, Gunter JH, Heathcote P, Ho K, Stricker P, Corcoran NM, et al. Adverse effects of androgen-deprivation therapy in prostate cancer and their management. BJU Int (2015) 115 Suppl 5:3–13. doi: 10.1111/bju.12964
39. Momenimovahed Z, Salehiniya H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer (Dove Med Press) (2019) 11:151–64. doi: 10.2147/BCTT.S176070
40. Ahmad A. Breast cancer statistics: Recent trends. Adv Exp Med Biol (2019) 1152:1–7. doi: 10.1007/978-3-030-20301-6_1
41. Tokumaru Y, Joyce D, Takabe K. Current status and limitations of immunotherapy for breast cancer. Surgery (2020) 167(3):628–30. doi: 10.1016/j.surg.2019.09.018
42. Senkus E, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rutgers E, et al. Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2015) 26 Suppl 5:v8–30. doi: 10.1093/annonc/mdv298
43. Yadav S, Giridhar KV, Leone JP, Leon-Ferre RA, Ruddy KJ. A practical guide to endocrine therapy in the management of estrogen receptor-positive male breast cancer. Breast Cancer Manage (2021) 10(3):BMT59. doi: 10.2217/bmt-2021-0001
44. Hill DA, Friend S, Lomo L, Wiggins C, Barry M, Prossnitz E, et al. Breast cancer survival, survival disparities, and guideline-based treatment. Breast Cancer Res Treat (2018) 170(2):405–14. doi: 10.1007/s10549-018-4761-7
45. Yu F, Bender W. The mechanism of tamoxifen in breast cancer prevention. Breast Cancer Res (2001) 3(1):A74. doi: 10.1186/bcr404
46. Moo TA, Sanford R, Dang C, Morrow M. Overview of breast cancer therapy. PET Clin (2018) 13(3):339–54. doi: 10.1016/j.cpet.2018.02.006
47. Ratre P, Mishra K, Dubey A, Vyas A, Jain A, Thareja S. Aromatase inhibitors for the treatment of breast cancer: A journey from the scratch. Anticancer Agents Med Chem (2020) 20(17):1994–2004. doi: 10.2174/1871520620666200627204105
48. Joe BN. UpToDate: Clinical features, diagnosis, and staging of newly diagnosed breast cancer (2022). Available at: https://www.uptodate.com/contents/clinical-features-diagnosis-and-staging-of-newly-diagnosed-breast-cancer (Accessed December 19, 2022).
50. Visram H, Kanji F, Dent SF. Endocrine therapy for male breast cancer: rates of toxicity and adherence. Curr Oncol (2010) 17(5):17–21. doi: 10.3747/co.v17i5.631
51. Russo J, Russo IH. Breast development, hormones and cancer. Adv Exp Med Biol (2008) 630:52–6. doi: 10.1007/978-0-387-78818-0_4
52. Burger CW, Prinssen HM, Kenemans P. LHRH agonist treatment of breast cancer and gynecological malignancies: A review. Eur J Obstet Gynecol Reprod Biol (1996) 67(1):27–33. doi: 10.1016/0301-2115(96)02424-4
53. Love RR, Duc NB, Allred DC, Binh NC, Dinh NV, Kha NN, et al. Oophorectomy and tamoxifen adjuvant therapy in premenopausal Vietnamese and Chinese women with operable breast cancer. J Clin Oncol (2002) 20(10):2559–66. doi: 10.1200/JCO.2002.08.169
54. Sanchez AM, Flamini MI, Zullino S, Russo E, Giannini A, Mannella P, et al. Regulatory actions of LH and follicle-stimulating hormone on breast cancer cells and mammary tumors in rats. Front Endocrinol (Lausanne) (2018) 9:239. doi: 10.3389/fendo.2018.00239
55. Seitz S, Buchholz S, Schally AV, Weber F, Klinkhammer-Schalke M, Inwald EC, et al. Triple negative breast cancers express receptors for LHRH and are potential therapeutic targets for cytotoxic LHRH-analogs, AEZS 108 and AEZS 125. BMC Cancer (2014) 14:847. doi: 10.1186/1471-2407-14-847
56. American_Cancer_Society. Triple-negative breast cancer (2023). Available at: https://www.cancer.org/cancer/breast-cancer/about/types-of-breast-cancer/triple-negative.html (Accessed February 16, 2023).
57. Corona SP, Roviello G, Strina C, Milani M, Allevi G, Aguggini S, et al. Could gonadotropin-releasing hormone analogs be helpful in the treatment of triple-negative breast cancer? Future Oncol (2017) 13(27):2473–7. doi: 10.2217/fon-2017-0272
58. Stewart C, Ralyea C, Lockwood S. Ovarian cancer: An integrated review. Semin Oncol Nurs (2019) 35(2):151–6. doi: 10.1016/j.soncn.2019.02.001
59. Matulonis UA, Sood AK, Fallowfield L, Howitt BE, Sehouli J, Karlan BY. Ovarian cancer. Nat Rev Dis Primers (2016) 2:16061. doi: 10.1038/nrdp.2016.61
60. Momenimovahed Z, Tiznobaik A, Taheri S, Salehiniya H. Ovarian cancer in the world: epidemiology and risk factors. Int J Womens Health (2019) 11:287–99. doi: 10.2147/IJWH.S197604
61. Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin (2018) 68(4):284–96. doi: 10.3322/caac.21456
62. Emons G, Weiss S, Ortmann O, Grundker C, Schulz KD. LHRH might act as a negative autocrine regulator of proliferation of human ovarian cancer. Eur J Endocrinol (2000) 142(6):665–70. doi: 10.1530/eje.0.1420665
63. Perales-Puchalt A, Svoronos N, Rutkowski MR, Allegrezza MJ, Tesone AJ, Payne KK, et al. Follicle-stimulating hormone receptor is expressed by most ovarian cancer subtypes and is a safe and effective immunotherapeutic target. Clin Cancer Res (2017) 23(2):441–53. doi: 10.1158/1078-0432.CCR-16-0492
64. Xiong S, Mhawech-Fauceglia P, Tsao-Wei D, Roman L, Gaur RK, Epstein AL, et al. Expression of the luteinizing hormone receptor (LHR) in ovarian cancer. BMC Cancer (2019) 19(1):1114. doi: 10.1186/s12885-019-6153-8
65. Salehi F, Dunfield L, Phillips KP, Krewski D, Vanderhyden BC. Risk factors for ovarian cancer: An overview with emphasis on hormonal factors. J Toxicol Environ Health B Crit Rev (2008) 11(3-4):301–21. doi: 10.1080/10937400701876095
66. Zheng Y, Katsaros D, Shan SJ, de la Longrais IR, Porpiglia M, Scorilas A, et al. A multiparametric panel for ovarian cancer diagnosis, prognosis, and response to chemotherapy. Clin Cancer Res (2007) 13(23):6984–92. doi: 10.1158/1078-0432.CCR-07-1409
67. Engel JB, Schally AV. Drug insight: clinical use of agonists and antagonists of luteinizing-hormone-releasing hormone. Nat Clin Pract Endocrinol Metab (2007) 3(2):157–67. doi: 10.1038/ncpendmet0399
68. Verschraegen CF, Westphalen S, Hu W, Loyer E, Kudelka A, Volker P, et al. Phase II study of cetrorelix, a luteinizing hormone-releasing hormone antagonist in patients with platinum-resistant ovarian cancer. Gynecol Oncol (2003) 90(3):552–9. doi: 10.1016/s0090-8258(03)00408-6
69. Emons G, Tomov S, Harter P, Sehouli J, Wimberger P, Staehle A, et al. Phase II study of AEZS-108 (AN-152), a targeted cytotoxic LHRH analog, in patients with LHRH receptor-positive platinum resistant ovarian cancer. J Clin Oncol (2010) 28(15_suppl):5035. doi: 10.1200/jco.2010.28.15_suppl.5035
70. McLachlan RI, Healy DL, Burger HG. Clinical aspects of LHRH analogues in gynaecology: a review. Br J Obstet Gynaecol (1986) 93(5):431–54. doi: 10.1111/j.1471-0528.1986.tb08652.x
71. Wicki A, Witzigmann D, Balasubramanian V, Huwyler J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J Control Release (2015) 200:138–57. doi: 10.1016/j.jconrel.2014.12.030
72. Vriesendorp HM, Vriesendorp R, Vriesendorp FJ. Prediction of normal tissue damage induced by cancer chemotherapy. Cancer Chemother Pharmacol (1987) 19(4):273–6. doi: 10.1007/BF00261471
73. Yan L, Rosen N, Arteaga C. Targeted cancer therapies. Chin J Cancer (2011) 30(1):1–4. doi: 10.5732/cjc.010.10553
74. Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discovery (2003) 2(5):347–60. doi: 10.1038/nrd1088
75. Chien JY, Ho RJ. Drug delivery trends in clinical trials and translational medicine: Evaluation of pharmacokinetic properties in special populations. J Pharm Sci (2011) 100(1):53–8. doi: 10.1002/jps.22253
76. Zhu S, Wang Q, Jiang J, Luo Y, Sun Z. A conjugate of methotrexate and an analog of luteinizing hormone releasing hormone shows increased efficacy against prostate cancer. Sci Rep (2016) 6:33894. doi: 10.1038/srep33894
77. Wen D, Chitkara D, Wu H, Danquah M, Patil R, Miller DD, et al. LHRH-conjugated micelles for targeted delivery of antiandrogen to treat advanced prostate cancer. Pharm Res (2014) 31(10):2784–95. doi: 10.1007/s11095-014-1375-6
78. Kakar SS, Pohlgrees KM, Panaguluri SK. LHRH and doxorubicin conjugated gold nanotechnology for ovarian cancer treatment. Biol Reprod (2008) 78(Suppl_1):290. doi: 10.1093/biolreprod/78.s1.290b
79. Wolfe T, Chatterjee D, Lee J, Grant JD, Bhattarai S, Tailor R, et al. Targeted gold nanoparticles enhance sensitization of prostate tumors to megavoltage radiation therapy in vivo. Nanomedicine (2015) 11(5):1277–83. doi: 10.1016/j.nano.2014.12.016
80. Nukolova NV, Oberoi HS, Zhao Y, Chekhonin VP, Kabanov AV, Bronich TK. LHRH-targeted nanogels as a delivery system for cisplatin to ovarian cancer. Mol Pharm (2013) 10(10):3913–21. doi: 10.1021/mp4003688
81. Nian D, Shi P, Sun J, Ren L, Hao X, Han J. Application of luteinizing hormone-releasing hormone-ferrosoferric oxide nanoparticles in targeted imaging of breast tumors. J Int Med Res (2019) 47(4):1749–57. doi: 10.1177/0300060519834457
82. Li M, Tang Z, Zhang Y, Lv S, Li Q, Chen X. Targeted delivery of cisplatin by LHRH-peptide conjugated dextran nanoparticles suppresses breast cancer growth and metastasis. Acta Biomater (2015) 18:132–43. doi: 10.1016/j.actbio.2015.02.022
83. Taheri A, Dinarvand R, Ahadi F, Khorramizadeh MR, Atyabi F. The in vivo antitumor activity of LHRH targeted methotrexate-human serum albumin nanoparticles in 4T1 tumor-bearing balb/c mice. Int J Pharm (2012) 431(1-2):183–9. doi: 10.1016/j.ijpharm.2012.04.033
84. Taheri A, Dinarvand R, Atyabi F, Ahadi F, Nouri FS, Ghahremani MH, et al. Enhanced anti-tumoral activity of methotrexate-human serum albumin conjugated nanoparticles by targeting with luteinizing hormone-releasing hormone (LHRH) peptide. Int J Mol Sci (2011) 12(7):4591–608. doi: 10.3390/ijms12074591
85. He Y, Zhang L, Song C. Luteinizing hormone-releasing hormone receptor-mediated delivery of mitoxantrone using LHRH analogs modified with PEGylated liposomes. Int J Nanomed (2010) 5:697–705. doi: 10.2147/ijn.s12129
86. Schally AV, Nagy A. Chemotherapy targeted to cancers through tumoral hormone receptors. Trends Endocrinol Metab (2004) 15(7):300–10. doi: 10.1016/j.tem.2004.07.002
87. Cheung LW, Wong AS. Gonadotropin-releasing hormone: GnRH receptor signaling in extrapituitary tissues. FEBS J (2008) 275(22):5479–95. doi: 10.1111/j.1742-4658.2008.06677
88. Dharap SS, Wang Y, Chandna P, Khandare JJ, Qiu B, Gunaseelan S, et al. Tumor-specific targeting of an anticancer drug delivery system by LHRH peptide. Proc Natl Acad Sci U.S.A. (2005) 102(36):12962–7. doi: 10.1073/pnas.0504274102
89. Nagy A, Schally AV. Targeting of cytotoxic luteinizing hormone-releasing hormone analogs to breast, ovarian, endometrial, and prostate cancers. Biol Reprod (2005) 73(5):851–9. doi: 10.1095/biolreprod.105.043489
90. Bae YH, Park K. Targeted drug delivery to tumors: myths, reality and possibility. J Control Release (2011) 153(3):198–205. doi: 10.1016/j.jconrel.2011.06.001
91. Torchilin VP. Passive and active drug targeting: drug delivery to tumors as an example. Handb Exp Pharmacol (2010) 197):3–53. doi: 10.1007/978-3-642-00477-3_1
92. Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Delivery Rev (2014) 66:2–25. doi: 10.1016/j.addr.2013.11.009
93. Danhier F, Feron O, Preat V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release (2010) 148(2):135–46. doi: 10.1016/j.jconrel.2010.08.027
94. Minko T, Patil ML, Zhang M, Khandare JJ, Saad M, Chandna P, et al. LHRH-targeted nanoparticles for cancer therapeutics. Methods Mol Biol (2010) 624:281–94. doi: 10.1007/978-1-60761-609-2_19
95. Saad M, Garbuzenko OB, Ber E, Chandna P, Khandare JJ, Pozharov VP, et al. Receptor targeted polymers, dendrimers, liposomes: which nanocarrier is the most efficient for tumor-specific treatment and imaging? J Control Release (2008) 130(2):107–14. doi: 10.1016/j.jconrel.2008.05.024
96. Grundker C, Emons G. The role of gonadotropin-releasing hormone in cancer cell proliferation and metastasis. Front Endocrinol (Lausanne) (2017) 8:187. doi: 10.3389/fendo.2017.00187
97. Schottelius M, Berger S, Poethko T, Schwaiger M, Wester HJ. Development of novel 68Ga- and 18F-labeled GnRH-I analogues with high GnRHR-targeting efficiency. Bioconjug Chem (2008) 19(6):1256–68. doi: 10.1021/bc800058k
98. Guo H, Gallazzi F, Sklar LA, Miao Y. A novel indium-111-labeled gonadotropin-releasing hormone peptide for human prostate cancer imaging. Bioorg Med Chem Lett (2011) 21(18):5184–7. doi: 10.1016/j.bmcl.2011.07.055
99. Schally AV, Comaru-Schally AM. Agonists of LHRH. In: Holland-Frei cancer medicine, 6th. Hamilton, Ontario: BC Decker (2003).
100. Irmer G, Burger C, Muller R, Ortmann O, Peter U, Kakar SS, et al. Expression of the messenger RNAs for luteinizing hormone-releasing hormone (LHRH) and its receptor in human ovarian epithelial carcinoma. Cancer Res (1995) 55(4):817–22.
101. group LH-aiEBCO, Cuzick J, Ambroisine L, Davidson N, Jakesz R, Kaufmann M, et al. Use of luteinising-hormone-releasing hormone agonists as adjuvant treatment in premenopausal patients with hormone-receptor-positive breast cancer: a meta-analysis of individual patient data from randomised adjuvant trials. Lancet (2007) 369(9574):1711–23. doi: 10.1016/S0140-6736(07)60778-8
102. Grundker C, Gunthert AR, Millar RP, Emons G. Expression of gonadotropin-releasing hormone II (GnRH-II) receptor in human endometrial and ovarian cancer cells and effects of GnRH-II on tumor cell proliferation. J Clin Endocrinol Metab (2002) 87(3):1427–30. doi: 10.1210/jcem.87.3.8437
103. Grundker C, Emons G. Role of gonadotropin-releasing hormone (GnRH) in ovarian cancer. Cells (2021) 10(2). doi: 10.3390/cells10020437
104. Dharap SS, Minko T. Targeted proapoptotic LHRH-BH3 peptide. Pharm Res (2003) 20(6):889–96. doi: 10.1023/a:1023839319950
105. Limonta P, Montagnani Marelli M, Mai S, Motta M, Martini L, Moretti RM. GnRH receptors in cancer: From cell biology to novel targeted therapeutic strategies. Endocr Rev (2012) 33(5):784–811. doi: 10.1210/er.2012-1014
106. Friess H, Buchler M, Kiesel L, Kruger M, Beger HG. LH-RH receptors in the human pancreas. basis for antihormonal treatment in ductal carcinoma of the pancreas. Int J Pancreatol (1991) 10(2):151–9. doi: 10.1007/BF02924118
107. Szende B, Srkalovic G, Timar J, Mulchahey JJ, Neill JD, Lapis K, et al. Localization of receptors for luteinizing hormone-releasing hormone in pancreatic and mammary cancer cells. Proc Natl Acad Sci U.S.A. (1991) 88(10):4153–6. doi: 10.1073/pnas.88.10.4153
108. Koushik K, Bandi N, Sundaram S, Kompella UB. Evidence for LHRH-receptor expression in human airway epithelial (Calu-3) cells and its role in the transport of an LHRH agonist. Pharm Res (2004) 21(6):1034–46. doi: 10.1023/b:pham.0000029294.70707.74
109. Moretti RM, Montagnani Marelli M, Van Groeninghen JC, Limonta P. Locally expressed LHRH receptors mediate the oncostatic and antimetastatic activity of LHRH agonists on melanoma cells. J Clin Endocrinol Metab (2002) 87(8):3791–7. doi: 10.1210/jcem.87.8.8755
110. Montagnani Marelli M, Moretti RM, Mai S, Muller O, Van Groeninghen JC, Limonta P. Novel insights into GnRH receptor activity: Role in the control of human glioblastoma cell proliferation. Oncol Rep (2009) 21(5):1277–82. doi: 10.3892/or_00000351
111. Grundker C, Ernst J, Reutter MD, Ghadimi BM, Emons G. Effective targeted chemotherapy using AEZS-108 (AN-152) for LHRH receptor-positive pancreatic cancers. Oncol Rep (2011) 26(3):629–35. doi: 10.3892/or.2011.1340
112. Engel J, Emons G, Pinski J, Schally AV. AEZS-108 : a targeted cytotoxic analog of LHRH for the treatment of cancers positive for LHRH receptors. Expert Opin Investig Drugs (2012) 21(6):891–9. doi: 10.1517/13543784.2012.685128
113. Grundker C, Volker P, Griesinger F, Ramaswamy A, Nagy A, Schally AV, et al. Antitumor effects of the cytotoxic luteinizing hormone-releasing hormone analog AN-152 on human endometrial and ovarian cancers xenografted into nude mice. Am J Obstet Gynecol (2002) 187(3):528–37. doi: 10.1067/mob.2002.124278
114. Straub B, Muller M, Krause H, Schrader M, Goessl C, Heicappell R, et al. Increased incidence of luteinizing hormone-releasing hormone receptor gene messenger RNA expression in hormone-refractory human prostate cancers. Clin Cancer Res (2001) 7(8):2340–3.
115. Kwon IK, Lee SC, Han B, Park K. Analysis on the current status of targeted drug delivery to tumors. J Control Release (2012) 164(2):108–14. doi: 10.1016/j.jconrel.2012.07.010
116. Kumar D, Moghiseh M, Chitcholtan K, Mutreja I, Lowe C, Kaushik A, et al. LHRH conjugated gold nanoparticles assisted efficient ovarian cancer targeting evaluated via spectral photon-counting CT imaging: a proof-of-concept research. J Mater Chem B (2023). doi: 10.1039/d2tb02416k
117. Yu S, Athreya K, Liu SV, Schally AV, Groshen SG, Tsao-Wei DD, et al. A phase II trial of zoptarelin doxorubicin in castration- and taxane-resistant prostate cancer. J Clin Oncol (2017) 35(6_suppl):210. doi: 10.1200/JCO.2017.35.6_suppl.210
118. Kwok CW, Treeck O, Buchholz S, Seitz S, Ortmann O, Engel JB. Receptors for luteinizing hormone-releasing hormone (GnRH) as therapeutic targets in triple negative breast cancers (TNBC). Target Oncol (2015) 10(3):365–73. doi: 10.1007/s11523-014-0340-y
Keywords: GnRH, targeted therapy, ovarian cancer, breast cancer, prostatic cancer
Citation: Garrido MP, Hernandez A, Vega M, Araya E and Romero C (2023) Conventional and new proposals of GnRH therapy for ovarian, breast, and prostatic cancers. Front. Endocrinol. 14:1143261. doi: 10.3389/fendo.2023.1143261
Received: 12 January 2023; Accepted: 10 March 2023;
Published: 28 March 2023.
Edited by:
Guadalupe Maya-Núñez, Mexican Social Security Institute (IMSS), MexicoReviewed by:
Rocío García-Becerra, National Autonomous University of Mexico, MexicoCopyright © 2023 Garrido, Hernandez, Vega, Araya and Romero. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carmen Romero, Y3JvbWVyb0BoY3VjaC5jbA==; Eyleen Araya, ZXlsZWVuLmFyYXlhQHVuYWIuY2w=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.