- 1Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, New York, NY, United States
- 2Division of Endocrinology, Diabetes, and Bone Diseases, Department of Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, United States
Weight gain is a known adverse effect of ruxolitinib, a JAK1/2 inhibitor that is the mainstay of treatment for many patients with myelofibrosis. The mechanisms behind weight increase with ruxolitinib is incompletely understood, although decreased adipose tissue lipolysis and increased appetite due to blocking the effects of leptin in the hypothalamus have been proposed. In order to explore the metabolic changes in ruxolitinib-treated patients with myelofibrosis, we performed a pilot study to assess the feasibility of using a portable indirect calorimeter to quantify energy expenditure before and during ruxolitinib treatment and report the results of two patients. Waist circumference increased during ruxolitinib treatment in both patients. Energy expenditure initially increased followed by a decrease and then increase again, but to levels below baseline. These results suggest that weight gain secondary to ruxolitinib may be related to changes in whole body energy expenditure.
Introduction
Myelofibrosis (MF) is a hematologic malignancy clinically characterized by abnormal cytokine production, splenomegaly, and constitutional symptoms that include weight loss (1). Ruxolitinib (Jakafi, Incyte) is a JAK1/2 inhibitor and was the first Food and Drug Administration approved therapy for MF, based on the results of the COMFORT-I and -II studies that demonstrated efficacy in terms of spleen reduction and symptoms improvement (2, 3). Interestingly, ruxolitinib is associated with statistically and clinically significant weight gain. In the pivotal, phase 3 COMFORT-I and COMFORT-II trials, patients treated with ruxolitinib gained an average of 3.9 kg after 24 weeks (COMFORT-I) and 4.4 kg after 48 weeks (COMFORT-II) of ruxolitinib therapy (2–4). Weight gain occurs regardless of pretreatment body mass index (BMI), suggesting weight gain is not solely attributable to resolution of MF symptoms (e.g., early satiety, abdominal discomfort) in cachectic patients (5).
We have previously shown in a retrospective analysis of 179 patients with MPNs treated with ruxolitinib that 69% of patients experienced weight gain during treatment and the average gain for those patients was 12% of pretreatment body weight, with over 50% of patients gaining > 5% of their pretreatment body weight (5). There is uncertainty as to the pathogenesis of ruxolitinib-mediated weight gain. In a murine model, ruxolitinib reduced the normal JAK2/STAT3 phosphorylation in neuronal cells in mouse hypothalamus that occurs in response to feeding or to administration of exogenous leptin, which typically signals satiety, suggesting that increased appetite may be responsible (5). However, we have also demonstrated that ruxolitinib inhibits JAK/STAT signaling in the adipose tissue, which is an important regulator of adipose tissue lipolysis (6, 7).
To gain further insight into the metabolic changes that occur during ruxolitinib therapy, we performed a pilot and feasibility study of patients with MF using a portable indirect calorimeter to determine energy expenditure before and during treatment with ruxolitinib. Here, we describe two MF patients that highlight metabolic changes with this JAK1/2 inhibitor.
Methods
MF patients without prior JAK inhibitor exposure who were intending to start ruxolitinib as standard of care therapy were prospectively enrolled. Patients with chronic obstructive pulmonary disease, untreated endocrinopathy aside from diabetes, heart failure, or additional malignancies were excluded. Enrolled patients had resting energy expenditure measured by indirect calorimetry at screening and an additional baseline visit. Patients then had repeat measurements performed 2, 4, 8, 16, and 24 weeks after starting ruxolitinib. Weight, height, and waist circumference were recorded at each study visit, in addition to standard of care laboratory evaluation. A portable indirect calorimeter was kindly provided by PNOE (Palo Alto, California) (8). This device includes a wearable facemask, where the subject breathes through the Micro-Electro-Mechanical Systems based hot film anemometer flow sensor that operates on a breath-by-breath mode that continuously measures volume and determines expired gas concentrations simultaneously. Heart rate was measured using a heart rate monitor (Polar Electro, Lake Success, NY). Respiratory and cardiac measurements were transmitted via telemetry. Patients were required to fast for at least 8 hours prior to the testing, and not to exercise or use nicotine containing products for at least 4 hours prior to testing. They were seated at rest for at least 10 minutes before measurements were taken.
The Myeloproliferative Neoplasms Symptom Assessment Form Total Symptom Score (MPN-SAF-TSS) was calculated at each visit (9). The MPN-SAF-TSS includes ten symptoms: fatigue; early satiety; abdominal discomfort; inactivity; impaired concentration; night sweats; pruritis; bone pain; fever; unintentional weight loss over preceding 6 months. Each symptom is rated on a scale of 0-10, with 0 being absence of symptoms, and 10 being the worst imaginable symptoms. The MPN-SAF-TSS has a range from 0-100, with 100 being the most severe symptom severity.
All subjects signed a written informed consent prior to their participation in the study. The study was approved by the Program for the Protection of Human Subjects Institutional Review Board at the Icahn School of Medicine at Mount Sinai. All procedures were in accordance with the Declaration of Helsinki.
Results
Baseline characteristics
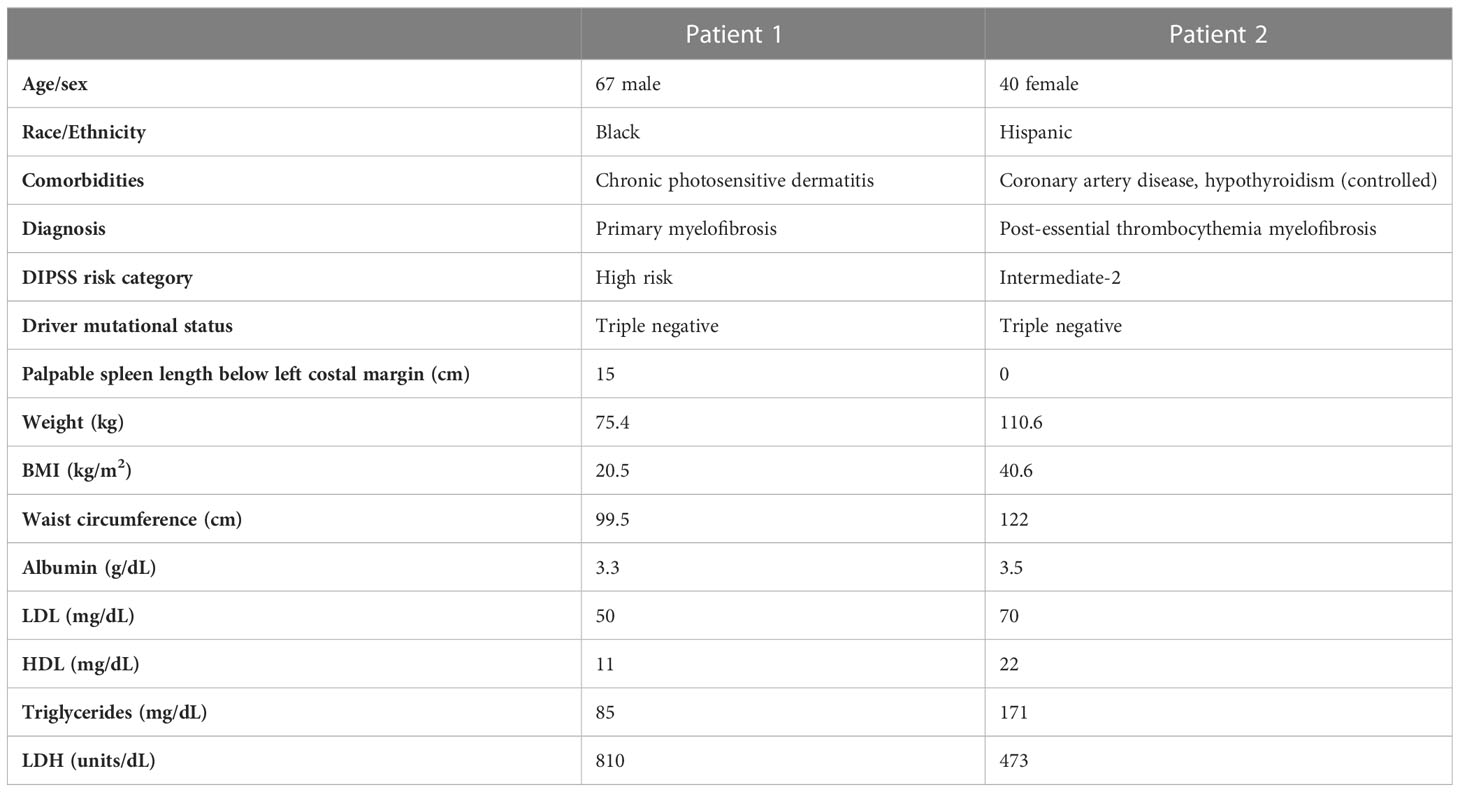
We report observations from two patients enrolled in this study. Additional patient enrollment was ceased because of the COVID-19 pandemic. Baseline metabolic characteristics are shown in Table 1. While Patient 1 was normal weight with a BMI of 20.5 kg/m2, Patient 2 had Class III obesity (BMI 40.6 kg/m2) and a waist circumference of 122 cm. Both patients were newly diagnosed and treatment naive. Patient 1 had baseline leukocytosis with a white blood cell (WBC) count of 94.1 x 109/L, hemoglobin of 8.9 g/dL and platelet count of 172 x 109/L while Patient 2 had a WBC of 9.8 g/dL, hemoglobin of 7.3 g/dL and a platelet count of 389 x 109/L. The Dynamic International Prognosis Scoring System (DIPSS) risk category was high and intermediate-2 for Patient 1 and Patient 2, respectively. Both patients were symptomatic with an MPN-SAF-TSS) of 31 and 49, respectively. Patient 1 had a baseline palpable spleen length of 15cm below the left costal margin while Patient 2 did not have palpable splenomegaly. Both patients were initiated ruxolitinib at 5mg twice daily, a dose selected considering baseline disease-related anemia.
Changes in metabolic parameters during treatment
The body weight of Patient 1 increased from 75.6kg to 83.9kg at 24 weeks, a gain of 8.3kg. Patient 2 experienced mild weight loss, with a change from110.8kg to 109.3kg at 24 weeks, a loss of 1.5kg. However, waist circumference increased in both patients: Patient 1 increased from 99.5cm at baseline to 104cm at 24 weeks, and Patient 2 increased from 122cm to 128cm at 24 weeks.
Indirect calorimetry readings displayed interesting dynamics in both patients. The resting energy expenditure for Patient 1 initially increased from a baseline of 3158 kcal/day to 3355 kcal/day at week 2 and then dropped to 2303 kcal/day at week 4 before rebounding to 2686 kcal/day at week 8 and then plateaued. Patient 2 had a baseline resting energy expenditure of 1870 kcal/day which increased to 2530 kcal/day on week 4 before decreasing to 1420 kcal/day on week 8 before ending at 1983 kcal/day at week 24.
Figures 1A, B show the energy expenditure over time plotted with changes in waist circumference. In both cases, there was an initial increase in resting energy expenditure followed by a decrease followed by an increase before returning to levels below baseline.

Figure 1 Changes in energy expenditure and waist circumferences over time. For patient 1 (A) and 2 (B), energy expenditure initially increased after initiation of ruxolitinib then decreased which was followed by an increase in waist circumference.
Changes in hemoglobin levels over time somewhat mirrored energy expenditure with initial decrease followed by an increase (Supplemental Figure 1).
Consistent with prior reports, albumin increased in both patients, with Patient 1 increasing from 3.3 g/dL to 3.9 g/dL at week 24 and increasing from 3.6 g/dL to 3.7 g/dL at week 24. Triglyceride levels also increased from a baseline of 85 mg/dL to 143 mg/dL and 171 mg/dL to 259 mg/dL for Patient 1 and 2, respectively. Patient reported outcome measure of early satiety during the treatment course steadily decreased from 8 out of 10 at baseline to 5 at week 24 in Patient 1, and from 8 to 7 in Patient 2.
Discussion
Our results suggest that weight gain due to ruxolitinib may not only be mediated by changes in appetite due to impaired JAK/STAT signaling in the hypothalamus, but may also related to changes in whole body energy expenditure.
In this pilot study, we used a portable indirect calorimeter, which allowed us to bring the device to the patients, facilitating point of care testing in the oncology rather than requiring separate visits to a metabolic testing location. Having the ability to perform indirect calorimetry in the oncology center could allow us to perform larger future studies to develop a greater understanding of the links between systemic metabolism and cancer.
Leptin receptors are found not only in the hypothalamus, but are expressed in other parts of the brain, and also in other tissues including the liver, spleen, kidneys, and adipose tissue. Leptin signaling in the brain and in adipose tissue has been found to increase energy expenditure in pre-clinical models (10). Interestingly, in pre-clinical models with intact leptin signaling in the hypothalamus, but adipose tissue leptin receptor deficiency, mice gained weight despite normal food intake, and developed insulin resistance and hypertriglyceridemia (11). Growth hormone binding to the growth hormone receptor also activates the JAK/STAT pathway. Interestingly, both chronic growth hormone excess and deficiency have been associated with increased energy expenditure (12). People with growth hormone receptor deficiency have higher percent body fat; however, in contrast to the leptin deficient models, do not develop insulin resistance or hypertriglyceridemia. Therefore, the effects of ruxolitinib on energy expenditure and triglycerides may be mediated through the leptin receptor. Interestingly, none of the previous studies have found biologically significant hyperglycemia with ruxolitinib despite the weight gain. This lack of hyperglycemia could be related to inhibition of the antagonistic effects of growth hormone on insulin signaling.
Ruxolitinib treatment has also been shown to increase muscle mass in MF patients (13). It is possible that this increase in muscle mass may be responsible for return of energy expenditure to near baseline as skeletal muscle is a significant driver of resting energy expenditure (14). Unfortunately, abdominal imaging was not obtained in our study and thus we are unable to explore this possibility.
Our study has several limitations that limit generalizability, including the fact that both patients were negative for MPN driver mutations, a rare subset of MF patients. In addition, the limited sample size and observational nature of our study did not allow us to evaluate the impact of ruxolitinib dosing on metabolic parameters. Overall, this pilot study shows the potential value of portable indirect calorimeters for measuring energy expenditure in individuals with malignancies, and suggests that the changes in energy expenditure may contribute in part to the weight gain associated with ruxolitinib.
Author contributions
DT, JM and EG designed the study, enrolled the patients, performed the indirect calorimetry and wrote the manuscript. MD performed regulatory functions and assisted with enrollment and design of the study. All authors contributed to the article and approved the submitted version.
Funding
E.G received research support from the National Institutes of Health/National Cancer Institute R37CA266853. The rest of the authors have no funding to disclose.
Acknowledgments
The authors would like to acknowledge PNOĒ for graciously providing the portable indirect calorimetry. We would also like to thank Panos Papadiamantis and Apostolos Atsalakis for their technical support.
Conflict of interest
DT receives research funding paid to his institution from CTI Biopharma, Astellas Pharma and Gilead and consulting fees from AbbVie, Sierra Oncology, GSK and Cogent. JM receives research funding paid to his institution from Incyte, CTI Bio, Novartis, PharmaEssentia, Merck, Kartos, Geron, BMS, Roche, and consulting fees from Incyte, CTI Bio, Novartis, Roche, BMS, Celgene, Geron, PharmaEssentia, Morphosys, Sierra Oncology, GSK, Galecto, Karyopharm, Kartos, and Imago.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1141029/full#supplementary-material
References
1. Mascarenhas J, Gleitz HFE, Chifotides HT, Harrison CN, Verstovsek S, Vannucchi AM, et al. Biological drivers of clinical phenotype in myelofibrosis. Leukemia (2022). doi: 10.1038/s41375-022-01767-y
2. Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, Dipersio JF, et al. Placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med (2012) 366:799–807. doi: 10.1056/NEJMoa1110557
3. Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med (2012) 366:787–98. doi: 10.1056/NEJMoa1110556
4. Mesa RA, Verstovsek S, Gupta V, Mascarenhas JO, Atallah E, Burn T, et al. Effects of ruxolitinib treatment on metabolic and nutritional parameters in patients with myelofibrosis from COMFORT-I. Clin Lymphoma Myeloma Leuk (2015) 15:214–221.e211. doi: 10.1016/j.clml.2014.12.008
5. Molle N, Krichevsky S, Kermani P, Silver RT, Ritchie E, Scandura JM. Ruxolitinib can cause weight gain by blocking leptin signaling in the brain via JAK2/STAT3. Blood (2020) 135:1062–6. doi: 10.1182/blood.2019003050
6. Sapre M, Tremblay D, Wilck E, James A, Leiter A, Coltoff A, et al. Metabolic effects of JAK1/2 inhibition in patients with myeloproliferative neoplasms. Sci Rep (2019) 9:16609. doi: 10.1038/s41598-019-53056-x
7. Xu D, Yin C, Wang S, Xiao Y. JAK-STAT in lipid metabolism of adipocytes. JAKSTAT (2013) 2:e27203. doi: 10.4161/jkst.27203
8. Tsekouras YE, Tambalis KD, Sarras SE, Antoniou AK, Kokkinos P, Sidossis LS. Validity and reliability of the new portable metabolic analyzer PNOE. Front Sports Act Living (2019) 1:24. doi: 10.3389/fspor.2019.00024
9. Emanuel RM, Dueck AC, Geyer HL, Kiladjian JJ, Slot S, Zweegman S, et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol (2012) 30:4098–103. doi: 10.1200/JCO.2012.42.3863
10. Harris RB. Direct and indirect effects of leptin on adipocyte metabolism. Biochim Biophys Acta (2014) 1842:414–23. doi: 10.1016/j.bbadis.2013.05.009
11. Huan JN, Li J, Han Y, Chen K, Wu N, Zhao AZ. Adipocyte-selective reduction of the leptin receptors induced by antisense RNA leads to increased adiposity, dyslipidemia, and insulin resistance. J Biol Chem (2003) 278:45638–50. doi: 10.1074/jbc.M304165200
12. Salomon F, Cuneo RC, Hesp R, Morris JF, Poston L, Sonksen PH. Basal metabolic rate in adults with growth hormone deficiency and in patients with acromegaly: relationship with lean body mass, plasma insulin level and leucocyte sodium pump activity. Clin Sci (Lond) (1992) 83:325–30. doi: 10.1042/cs0830325
13. Lucijanic M, Galusic D, Soric E, Sedinic M, Cubela M, Huzjan Korunic R, et al. Ruxolitinib treatment improves muscle mass in patients with myelofibrosis. Ann Hematol (2021) 100:1105–6. doi: 10.1007/s00277-020-04243-8
Keywords: ruxolitinib, energy expenditure, weight gain, intolerance, leptin
Citation: Tremblay D, Dougherty M, Mascarenhas J and Gallagher EJ (2023) Energy expenditure in myelofibrosis patients treated with a JAK1/2 inhibitor. Front. Endocrinol. 14:1141029. doi: 10.3389/fendo.2023.1141029
Received: 09 January 2023; Accepted: 19 June 2023;
Published: 29 June 2023.
Edited by:
Chen Wu, First People’s Hospital of Changzhou, ChinaReviewed by:
Marko Lucijanic, Clinical Hospital Dubrava, CroatiaCopyright © 2023 Tremblay, Dougherty, Mascarenhas and Gallagher. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Douglas Tremblay, ZG91Z2xhcy50cmVtYmxheUBtc3NtLmVkdQ==
 Douglas Tremblay
Douglas Tremblay Mikaela Dougherty1
Mikaela Dougherty1 John Mascarenhas
John Mascarenhas Emily Jane Gallagher
Emily Jane Gallagher