
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 14 April 2023
Sec. Cancer Endocrinology
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1139222
This article is part of the Research Topic Gender Pressure in Cancer: Susceptibility, Progression and Treatment of the Neoplastic Disease View all 5 articles
Objective: The purpose of this study was to build nomograms for predicting the survival of individual advanced pleural mesothelioma (MPM) patients using the Surveillance, Epidemiology, and End Results (SEER) database.
Methods: The 1251 patients enrolled from the SEER database were randomized (in a 7:3 ratio) to a training cohort and an internal validation cohort. Eighty patients were enrolled from the Harbin Medical University Cancer Hospital as the external validation cohort. Nomograms were constructed from variables screened by univariate or multivariate Cox regression analyses and evaluated by consistency indices (C-index), calibration plots, and receiver operating characteristic (ROC) curves. Patients from the SEER database who received chemotherapy alone and chemoradiotherapy were statistically paired using propensity score matching of the two groups and performed subgroup analysis in the screened variables.
Results: The nomograms are well-structured and well-validated prognostic maps constructed from four variables: gender, histology, AJCC stage, and treatment. All individuals were allocated into high-risk versus low-risk groups based on the median risk score of the training cohort, with the high-risk group having worse OS and CSS in all three cohorts (P<0.05). The outcomes of the subgroup analysis indicated that the advanced MPM patients receiving chemotherapy with or without local radiotherapy do not affect OS or CSS.
Conclusion: The accurate nomograms to predict the survival of patients with advanced MPM were built and validated based on an analysis of the SEER database with an external validation cohort. The study suggests that the additional local radiotherapy to chemotherapy does not increase the survival benefit of patients.
Malignant pleural mesothelioma (MPM), with an international incidence of 1 in 1,000,000, is a rare, aggressive, and often lethal malignancy of pleural origin (1, 2). Survival rates for advanced MPM are very low, with newly published data showing that the median survival for patients with advanced, inoperable is only 12 months (3, 4). Findings from several recent studies have proposed superior efficacy of immunotherapy in patients with MPM, with ipilimumab plus nivolumab extending median survival by 4 months compared with chemotherapy in patients with advanced untreated MPM (5–7). Severe chest pain can occur when the tumor invades the chest wall, and radiotherapy is the preferred treatment for MPM to relieve pain (8).
Nomograms are graphical assessment systems that can quantify risk based on statistical prediction models (9, 10). Many studies have demonstrated that a nomogram can be used as an alternative to the AJCC TNM staging system to individually predict patient prognosis and help physicians to select the best treatment for individual clinicopathological conditions (11–13).
A prognostic model for MPM based on the AJCC I-IV stage has been constructed (14). However, treatment options and survival in advanced MPM differ significantly from those in the early stages. We aimed to build and validate The survival nomograms of patients with advanced inoperable MPM based on the Surveillance, Epidemiology, and End Results (SEER) database.
Data were obtained from SEER∗Stat (version 8.4.0.1). Patients included in this study must meet (1) the year of diagnosis was between 2005-2018, (2) site code: C34.0-C34.9, C38.4, and ICD- O- 3 histology/behavior codes: 9050-9055 (based on previous literature support) (15), (3) patients who are at stage IIIB and IV. Patients meeting any of the below conditions were excluded (1) patients with a second primary malignancy; (2) patients who survived <30 days or had an unknown cause of death; (3) patients who underwent surgery. All patients were restaged according to the AJCC eighth-edition staging principles. Overall survival (OS) is the time between the date of diagnosis and the date of death from any cause or the last follow-up visit. Tumor-specific survival (CSS) is described as the time between the date of diagnosis and the date of death due to tumor cause or last follow-up. OS and CSS are the primary and secondary endpoints of this study, respectively.
Patients from the SEER database who met the criteria were randomized (in a 7:3 ratio) to a training cohort and an internal validation cohort. The external validation cohort consisted of MPM patients who attended the Harbin Medical University Cancer Hospital from 2010-2018. We used the same screening criteria as those for patients from the SEER database and eventually recruited 80 patients. Follow-up was carried out via telephone communication with the patients, and the last follow-up date of November 23, 2022. This a retrospective study based on clinical data, informed patient consent was not required.
SPSS 26.0 (IBM Inc.) and R version 4.1.3 were used for statistical analysis. Patient characteristics were compared between cohorts using a chi-square test. The clinicopathological features significantly related to survival were screened by univariate and multivariate Cox regression analysis, and the variables were further screened using stepwise backward regression, where the model with the smallest Akaike Information Criterion (AIC) score is chosen as the ideal model.
Finally, nomogram prognostic models that predict the OS and CSS rate of advanced MPM patients at 0.5-, 1-, and 2- years were constructed using the screened variables. We then evaluated the performance in terms of the discriminatory power and accuracy of the models. The discriminatory ability was assessed using the consistency index (C-index) and the area under the receiver operating characteristics (ROC) curve (AUC); calibration curves measure how well the probabilities generated by the nomograms agree with the observed actual probability. A risk score was available for each individual from the nomograms, and the median risk score of the patients in the training cohort was used as the cut-off value to stratify all patients for risk. The Kaplan-Meier survival analysis was conducted to determine if there was a significant difference in OS versus CSS rates in the different risk groups. The flowchart of patient screening and study design is displayed in Figure 1.
Patients from the SEER database who received chemotherapy alone and chemoradiotherapy were statistically paired using propensity score matching of the two groups and performed subgroup analysis in the screened independent risk factors. Cox proportional risk models were used to analyze the relationship between treatment and prognosis in each subgroup. Finally, the results are shown in forest maps.
A total of 1251 individuals with MPM were identified in the SEER database and randomized into a training cohort (n = 879) and an internal validation cohort (n = 372), with no differences in clinicopathological and demographic characteristics between the two cohorts.
The 1251 patients from the SEER database were mostly elderly, with more than half of the patients concentrated between 65-85 years of age (67.7%), 974 (77.9%) patients were male, and epithelioid is the most common pathological type (35.6%) in patients with definitive histological diagnosis. AJCC staging showed that most patients were in T4 (64.3%) or N0 (48.4%), and the distribution of patients in stage IIIB and IV were more evenly distributed with 575 (46.0%) and 676 (54.0%) patients, respectively. 49% of patients received chemotherapy alone and 8.3% and 4.7% received chemoradiotherapy or radiotherapy alone, respectively. The external validation cohort from the Harbin Medical University Cancer Hospital had 80 patients, the majority of patients (90.1%) were less than 70 years old, all patients were married Asians, 45% received chemotherapy alone, and 20% received both systemic chemotherapy and radiotherapy to the primary lesion. The clinicopathological characteristics of all patients are outlined in Table 1.
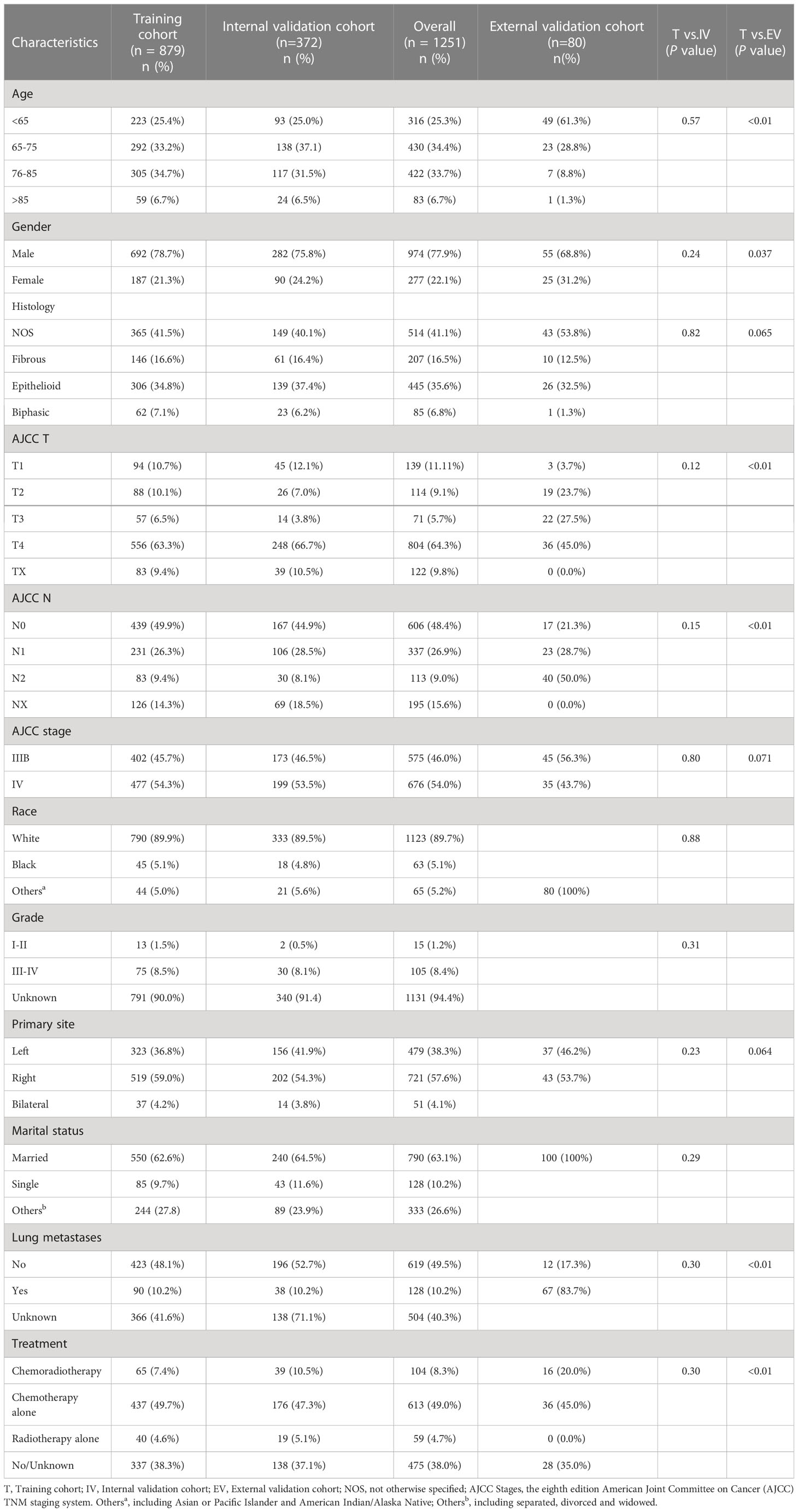
Table 1 Demographics and clinicopathologic characteristics of the training and external validation cohort.
Univariate Cox regression analysis of the training cohort showed age, gender, histology, AJCC stage, and treatment were significantly correlated with survival (P < 0.05). The above five variables were subjected to multivariate analysis, and the best model was determined using stepwise backward regression with minimum AIC values. Gender, histology, AJCC staging, and treatment were ultimately identified as independent prognostic factors for modeling the prognosis of advanced MPM. The outcomes of the Cox regression survival analysis based on OS and CSS are presented in Table 2 and S1, respectively.

Table 2 Selection of variables independently associated with OS by univariate and multivariate Cox proportional hazards analysis in the training cohort.
The nomograms for predicting late survival in MPM patients were established by the four variables screened above (Figure 2). By calculating the sum of the scores of the four variables through the nomograms, we could estimate the OS and CSS rate at 0.5-, 1-, and 2- years in patients with advanced MPM. C-index, time-dependent ROC curves, and calibration curves were used to validate the models’ performance. The C-index of the OS-based prediction models for the training, internal validation, and external validation groups were 0.656 (95% CI, 0.636-0.677), 0.668 (95% CI, 0.634-0.701) and 0.684 (95% CI, 0.605-0.763), respectively, as shown in Table S2. The CSS-based prediction models had a C-index of 0.654 (95% CI, 0.632-0.675), 0.657 (95% CI, 0.623-0.691), and 0.754 (95% CI, 0.670-0.838), respectively.
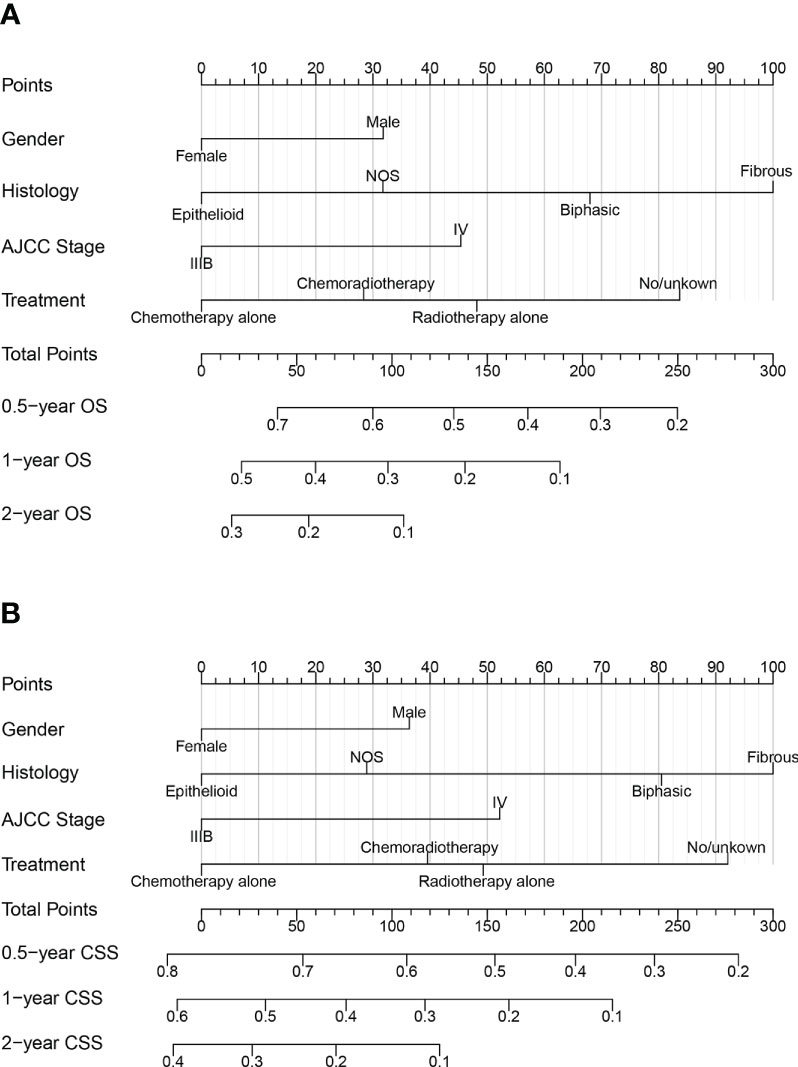
Figure 2 Nomograms for predicting 0.5, 1, and 2year (A) OS and (B) CSS of patients with advanced MPM.
Figures 3A–C shows the AUC values of a nomogram predicting 0.5-, 1-, and 2-year OS in three cohorts [training cohort:0.5 year OS 0.724 (95% CI, 0.690-0.758); 1 year OS 0.686 (95% CI, 0.647-0.725); 2 years OS 0.696 (95% CI, 0.641-0.750); internal validation cohort:0.5 year OS 0.731 (95% CI, 0.679-0.784); 1 year OS 0.711 (95% CI, 0.650 -0.771); 2-year OS 0.733 (95% CI, 0.645-0.820); external validation cohort:0.5-year OS 0.767 (95% CI, 0.628-0.905); 1-year OS 0.751 (95% CI, 0.635-0.867); 2-year OS 0.757 (95% CI, 0.596-0.917), respectively]. Figures 3D–F demonstrate the AUC values of a nomogram predicting 0.5-, 1-, and 2-year CSS in the three cohorts, respectively.
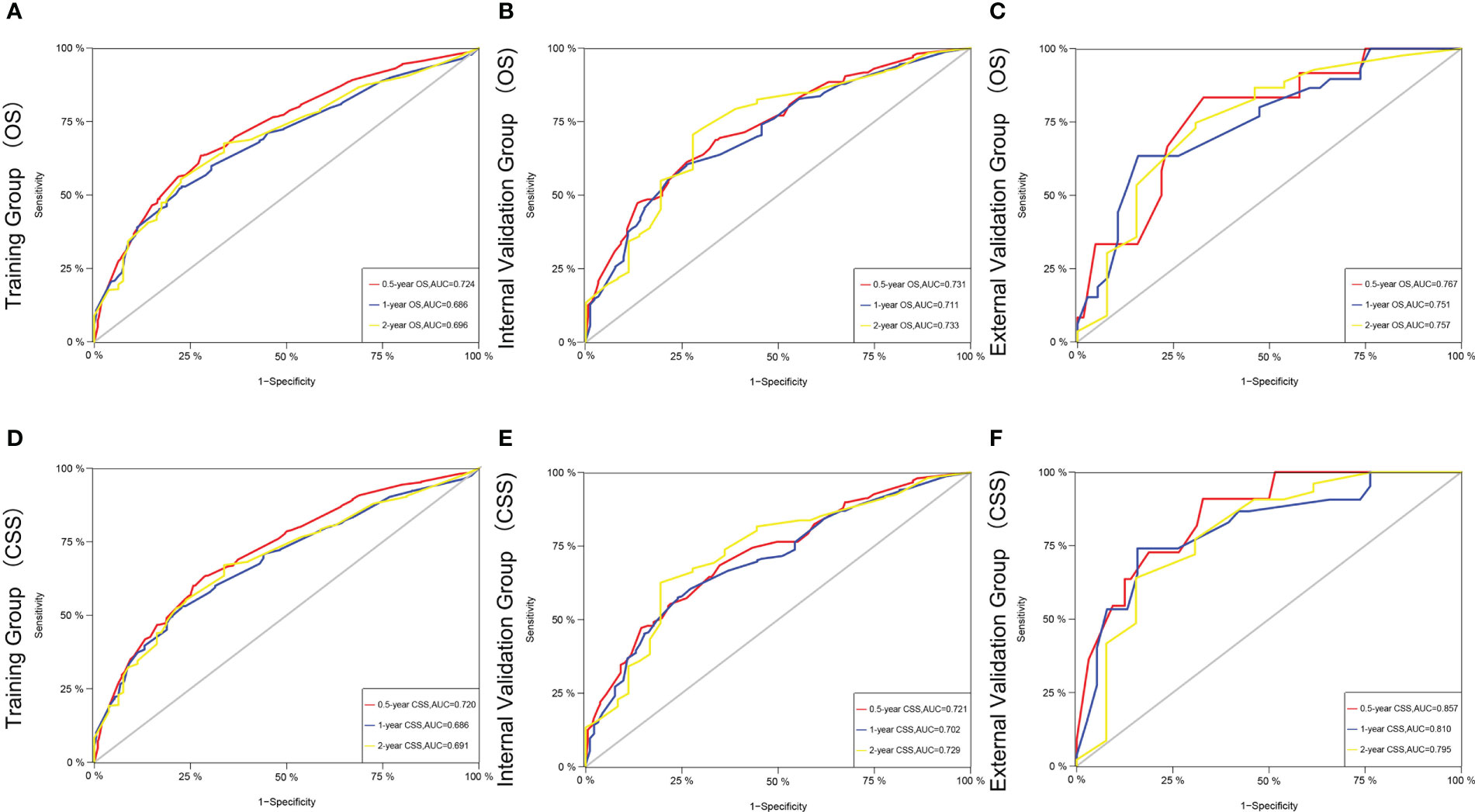
Figure 3 The time-dependent ROC curves of the nomogram predicting OS at (A) 0.5-year and 1-year and 2-year in the training cohort, and at (B) 0.5-year and 1-year and 2-year in the internal validation cohort, (C) 0.5-year and 1-year and 2-year in the external training cohort. The time-dependent ROC curves of the nomogram predicting CSS at (D) 0.5-year and 1-year and 2-year in the training cohort, and at (E) 0.5-year and 1-year and 2-yearin the internal validation cohort, (F) 0.5-year and 1-year and 2-year and 5-year in the external training cohort.
The C-index and AUC values indicate that the prognostic models have an excellent discriminatory ability for the survival rate of advanced MPM patients. Figures 4, 5 show the calibration curves of the prediction model between the actual OS or CSS rates at 0.5-, 1-, and 2- years and the predicted probabilities in the three cohorts, respectively, showing a good consistency between the survival rates generated by the nomograms and the observed survival rates in the actual population.
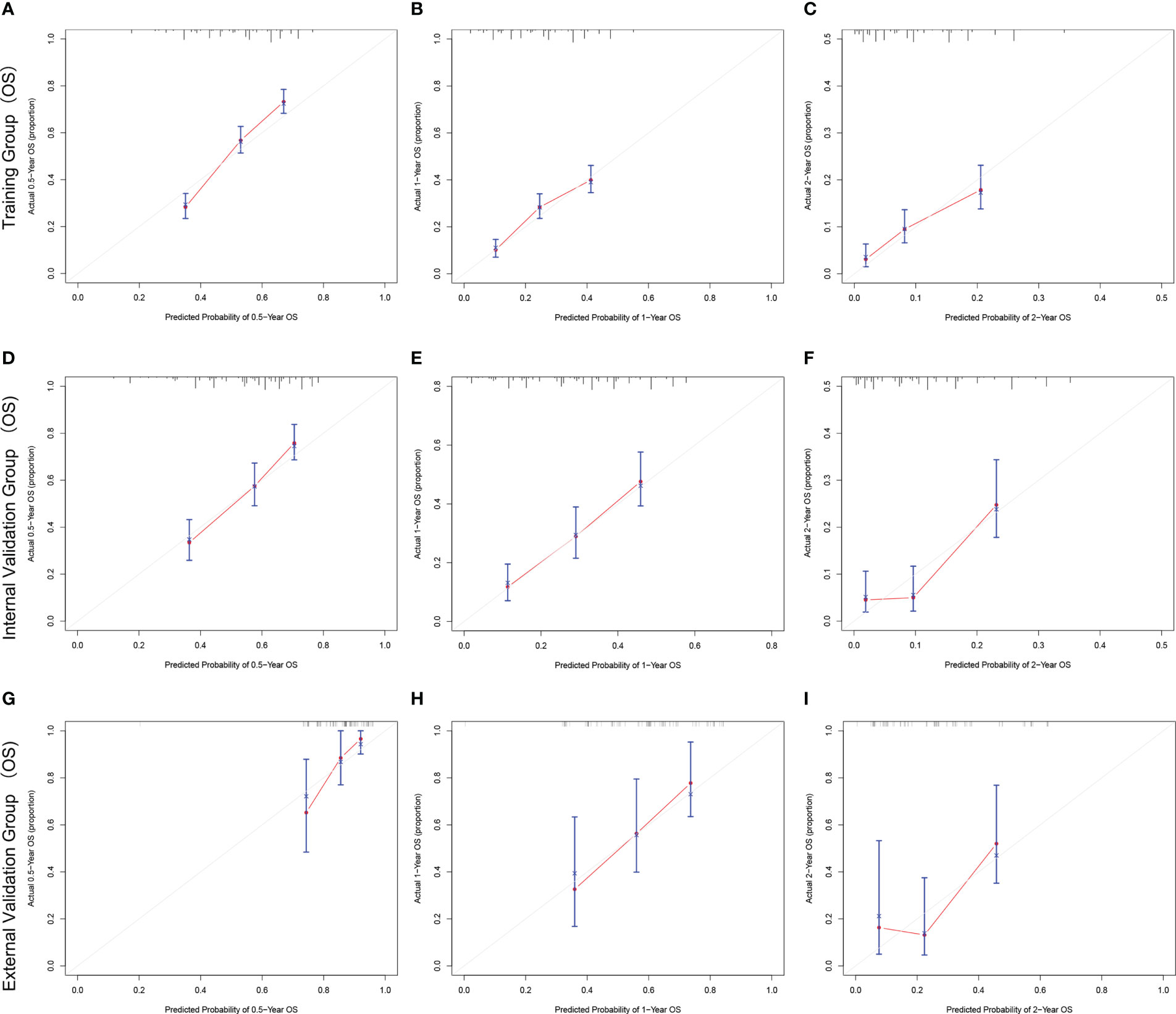
Figure 4 The calibration curves for predicting OS at (A) 0.5-year and (B) 1-year and (C) 2-year in the training cohort, and at (D) 0.5-year (E) 1-year and (F) 2-year in the internal validation cohort, and at (G) 0.5-year (H) 1-year and (I) 2-year in the external validation cohort.
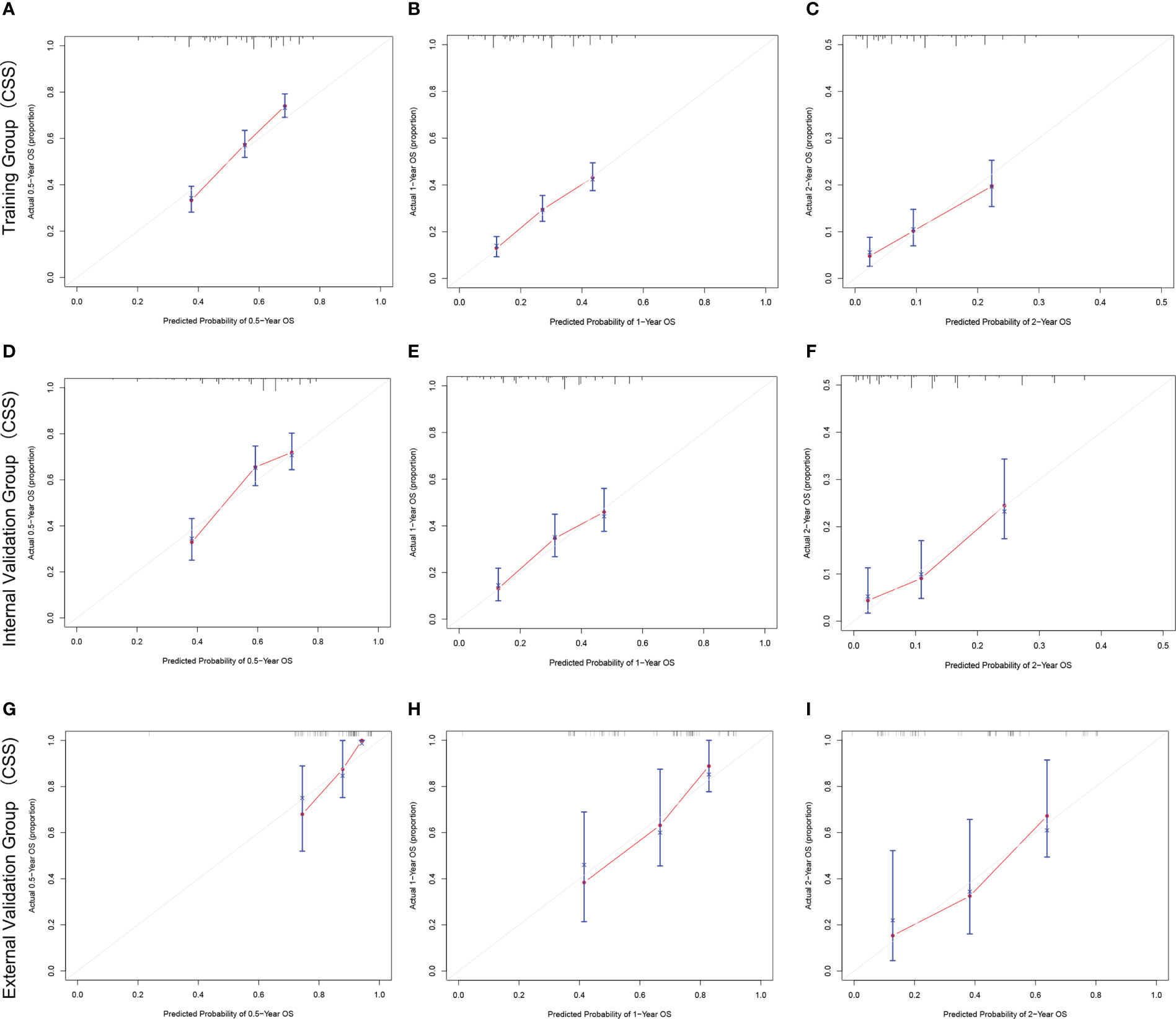
Figure 5 The calibration curves for predicting CSS at (A) 0.5-year and (B) 1-year and (C) 2-year in the training cohort, and at (D) 0.5-year (E) 1-year and (F) 2-year in the internal validation cohort, and at (G) 0.5-year (H) 1-year and (I) 2-year in the external validation cohort.
A risk score was calculated for all patients by nomograms, and the median risk score in the training cohort (OS: 116, CSS: 128) was used as the cut-off value to allocate patients into a high-risk group (OS: risk score ≥116, CSS: risk score ≥128) and a low-risk group (OS: risk score <116, CSS: risk score <128). Kaplan - Meier survival analysis revealed significant differences in OS or CSS across different risk groups (Figure 6), suggesting that nomograms can help us to accurately stratify risk in patients with advanced MPM.
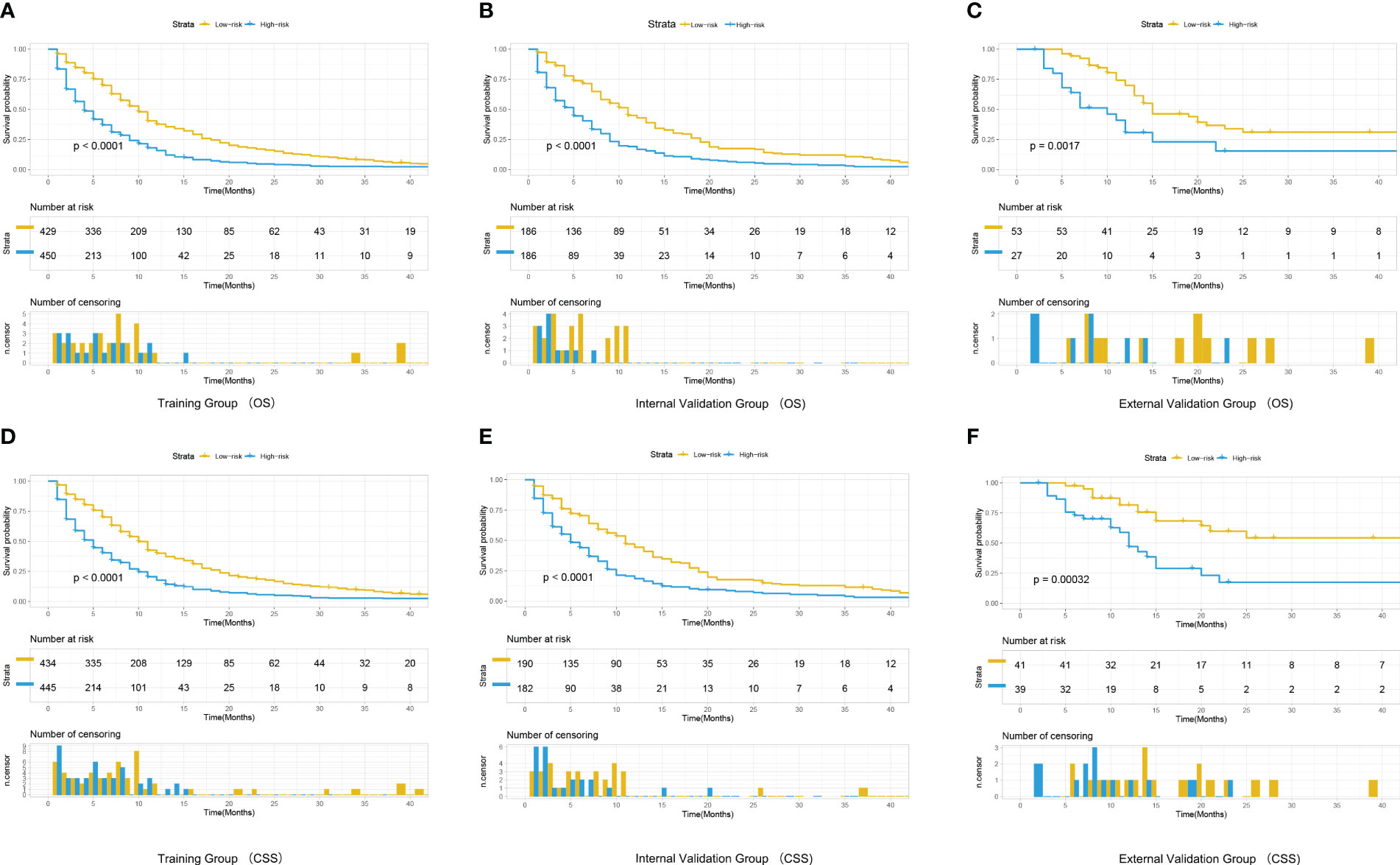
Figure 6 Kaplan-Meier curves for correlation with OS for the low and high- risk groups in the training cohort (A), internal validation cohort (B) and external validation cohort (C) Kaplan-Meier curves for correlation with CSS for the low and high-risk groups in the training cohort (D), internal validation cohort (E) and external validation cohort (F).
Radiotherapy plays a secondary role in patients with MPM compared to the importance of systemic therapy. In addition to systemic treatment, local palliative radiotherapy can relieve pain and improve patients’ symptoms. In our study, SPSS was used for propensity score matching analysis. Pairs of patients receiving chemoradiotherapy or chemotherapy-only were derived using1:1 greedy nearest neighbor matching within PS score of 0.1. This strategy resulted in 104 matched pairs in each group. resulting in 208 patients included in the subgroup analysis. It is evident from the results that the chemotherapy-only group tended to have better OS and CSS in all subgroups, however, all results failed to reach statistically significant (Figure 7), indicating that the addition of local radiotherapy to chemotherapy did not provide a survival benefit to patients with advanced MPM.
MPM is a low-incidence rate malignancy, and in previous studies, scholars have had difficulty collecting sufficient case data from a single center. The SEER database is the definitive cancer statistics database in the United States, containing a large sample of U.S. patients from different years, and is convenient and uniquely valuable for studying rare tumors (16–18).
The 1251 patients with advanced MPM from the SEER database in this work were randomized to the training and internal validation cohorts. Univariate analysis indicates that age, gender, histology, AJCC stage, and treatment regimen were significantly related to survival. These five variables were included in a multifactorial Cox regression analysis and further screened for variables using a backward stepwise regression approach. The age variable was excluded from the multifactorial Cox regression, probably because the 2-year survival rate of advanced MPM patients is extremely low and survival status is less affected by age. Nomograms were created based on the other four prognostic variables, then, the C-index, AUC values, and calibration curves validated the discriminatory power and accuracy of the models.
Previous studies have shown a significant difference in the gender distribution of MPM onset and survival rate (19), mainly because the onset of MPM is closely related to asbestos exposure, and those working in asbestos-related industries are mostly male (20). In the present study, the majority of individuals in the three cohorts were male (training cohort: n=692, 78.7%; internal validation cohort: n=282, 75.8%; external validation cohort: n=55, 68.8%) and had worse 2-year survival rates than female (training cohort: 8.9% vs. 16.1%; internal validation cohort. 9.6% vs. 18.6%; external validation cohort: 17.9% vs. 52.7%), further validating gender as an important predictor for survival in MPM patients. Histology significantly affects the survival of patients with advanced MPM, consistent with previous findings that the type of epithelioid was the most common and had a significantly better prognosis than fibrous (14, 21).
Patients with advanced MPM have a single treatment modality, with platinum/pemetrexed chemotherapy being the preferred regimen (3, 22), In recent years, the value of immunotherapy in advanced MPM is also being gradually demonstrated (23). The treatments in this study included: chemotherapy alone, chemoradiotherapy, and radiotherapy alone. Multifactorial analysis revealed that the selection of treatment options was significantly connected to the clinical outcome of MPM patients (P < 0.05). Among them, patients who received chemotherapy alone had the best 2-year survival rate (14.1%).
Mesothelioma has long been recognized to be highly radioresistant. Whether radiotherapy is beneficial in MPM patients who have not received extrapleural pneumonectomy has been highly controversial.
Several studies have confirmed that the radiosensitivity of mesothelioma cells is not as low as believed and is even higher than that of non-small cell lung cancer cells (24). Strukowska L et al. found durable local control with palliative radiotherapy targeting primary sites or chest wall metastases at a single dose of ≥4Gy (25).
Ghirardelli et al. performed a retrospective analysis of clinical information on 37 patients with inoperable MPM who progressed locally after first-line chemotherapy, showed that focal radiotherapy (FRT) delayed further systemic therapy in patients (median time of 6 months) and achieved a 1-year regional disease control of 76% in all patients, even better than most reported systemic therapies (26). However, others have suggested that a larger volume of lung irradiated in patients with preserved whole lungs may produce severe pulmonary toxicity and that higher-grade radiation pneumonia is a risk factor in patient mortality (27, 28). Given the persistent lack of high-value clinical evidence on whether local radiotherapy has a survival benefit for advanced MPM, we conducted a subgroup analysis of patients in the well-matched chemotherapy alone versus chemoradiotherapy groups. It was found that there was no remarkable difference in the prognosis with or without radiotherapy in patients who received systemic chemotherapy in advanced stages. Suggesting that radiotherapy may serve as a palliative treatment for local pain relief, but the improvement in symptoms may not translate into a benefit in OS or CSS. However, the patients in the SEER database are mostly from earlier years with outdated radiotherapy techniques. Current sophisticated radiotherapy equipment and precise target volume delineation may improve the benefit of radiotherapy. In addition, the rapid development of proton radiotherapy in recent years may hold great promise for the treatment of superficial malignancies like pleural mesothelioma.
In our study, the median survival of the external validation cohort was better than that of patients from the SEER database (7 months vs. 14 months). Possible reasons for this are (1) the differences in the distribution of clinicopathological factors such as race, marital status, and treatment status among patients in the external validation group compared with those from the SEER database, (2) A relatively large proportion of patients in the external validation group were female compared to the training group, and (3) the smaller number of cases in the external validation group due to the low incidence of MPM and the difficulty of collecting sufficient cases in a single center, which may have led to biased results.
However, the prediction model based on the training cohort was still well validated in the external validation cohort, with remarkable differences in survival among patients in the different risk groups (Figure 6). This is the first prognostic model focused on advanced inoperable MPM that differs from previous studies based only on the SEER database, the external validation group in this work demonstrates that the predictive model still has good applicability to patients in real-world Asia. As a retrospective analysis based on a public database, this study has several limitations. First, the SEER database lacks much important clinical information, including specific regimens and timing of treatment for chemotherapy as well as radiotherapy, which are important for the prognosis of MPM patients; second, many variables in the SEER database contain a large number of unknowns (No/Unknow), which may confound the statistical results of the study; third, immunotherapy has been shown to improve the prognosis of advanced MPM patients’ prognosis, however, the SEER database lacks information related to immunotherapy; finally, considering the small sample size in external validation, we only performed a subgroup analysis of patients from the SEER database and lacked validation from real-world data. In summary, real-world multicenter studies are needed to further validate our findings.
In summary, we successfully constructed and validated two nomograms to predict the survival of advanced MPM patients, providing a more accurate basis for treatment decisions in such patients. Systemic chemotherapy is predominant in patients with advanced MPM, and the addition of radiotherapy failed to improve the probability of survival in advanced MPM.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This is a retrospective study based on clinical data and informed patient consent was not required. The study was conducted with the review and approval of the Board of Directors of the Harbin Medical University Cancer Hospital.
XZ and LC conceived the original ideas of this manuscript and executed supervision throughout the process. YZ and YM performed the data collection, analysis. TZ and QZ prepared tables, figures and manuscript. CW reviewed and supervised the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1139222/full#supplementary-material
1. Zervos MD, Bizekis C, Pass HI. Malignant mesothelioma 2008. Curr Opin Pulm Med (2008) 14(4):303–9. doi: 10.1097/MCP.0b013e328302851d
2. Carbone M, Adusumilli PS, Alexander HR Jr, Baas P, Bardelli F, Bononi AT, et al. Mesothelioma: Scientific clues for prevention, diagnosis, and therapy. CA Cancer J Clin (2019) 69(5):402–29. doi: 10.3322/caac.21572
3. Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol (2003) 21(14):2636–44. doi: 10.1200/JCO.2003.11.136
4. Ai J, Stevenson JP. Current issues in malignant pleural mesothelioma evaluation and management. Oncologist (2014) 19(9):975–84. doi: 10.1634/theoncologist.2014-0122
5. Saunders J, Ashton M, Hall C, Laird B, MacLeod N. Pain management in patients with malignant mesothelioma: challenges and solutions. Lung Cancer (Auckl) (2019) 10:37–46. doi: 10.2147/LCTT.S192558
6. Baas P, Scherpereel A, Nowak AK, Fujimoto N, Peters S, Tsao AS, et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet (2021) 397(10272):375–86. doi: 10.1016/S0140-6736(20)32714-8
7. Bongiovanni A, Frassoldati A, Calabro L. Immunotherapy in malignant pleural mesothelioma: a long story ended in success. J Cancer Metastasis Treat (2022) 8:44. doi: 10.20517/2394-4722.2022.78
8. de Perrot M, Wu L, Wu M, Cho BCJ. Radiotherapy for the treatment of malignant pleural mesothelioma. Lancet Oncol (2017) 18(9):e532–42. doi: 10.1016/S1470-2045(17)30459-X
9. Makkouk A, Sundaram V, Chester C, Chang S, Colevas AD, Sunwoo JB, et al. Characterizing CD137 upregulation on NK cells in patients receiving monoclonal antibody therapy. Ann Oncol (2017) 28(2):415–20. doi: 10.1093/annonc/mdw570
10. Huang Y, Yu X, Li W, Li Y, Yang J, Hu Z, et al. Development and validation of a nomogram for predicting late-onset sepsis in preterm infants on the basis of thyroid function and other risk factors: Mixed retrospective and prospective cohort study. J Adv Res (2020) 24:43–51. doi: 10.1016/j.jare.2020.02.005
11. Li J, Liu Y, Yan Z, Wan X, Xia Y, Wang K, et al. A nomogram predicting pulmonary metastasis of hepatocellular carcinoma following partial hepatectomy. Br J Cancer (2014) 110(5):1110–7. doi: 10.1038/bjc.2014.19
12. Nör F, Warner KA, Zhang Z, Acasigua GA, Pearson AT, Kerk SA, et al. Therapeutic inhibition of the MDM2-p53 interaction prevents recurrence of adenoid cystic carcinomas. Clin Cancer Res (2017) 23(4):1036–48. doi: 10.1158/1078-0432.CCR-16-1235
13. Yang N, Xu L, Wang Q, Chen F, Zhou Y. Construction and validation of a prognostic nomogram for anal squamous cell carcinoma. Cancer Med (2022) 11(2):392–405. doi: 10.1002/cam4.4458
14. Chen S, Yu W, Shao S, Xiao J, Bai H, Pu Y, et al. Establishment of predictive nomogram and web-based survival risk calculator for malignant pleural mesothelioma: A SEER database analysis. Front Oncol (2022) 12:1027149. doi: 10.3389/fonc.2022.1027149
15. Thompson AB, Quinn TJ, Siddiqui ZA, Almahariq MF, Grills IS, Stevens CW. Addition of radiotherapy to surgery and chemotherapy improves survival in localized malignant pleural mesothelioma: A surveillance, epidemiology, and end results (SEER) study. Lung Cancer (2020) 146:120–6. doi: 10.1016/j.lungcan.2020.05.032
16. Alfaar AS, Hassan WM, Bakry MS, Qaddoumi I. Neonates with cancer and causes of death; lessons from 615 cases in the SEER databases. Cancer Med (2017) 6(7):1817–26. doi: 10.1002/cam4.1122
17. Jia Z, Yan Y, Wang J, Yang H, Zhan H, Chen Q, et al. Development and validation of prognostic nomogram in ependymoma: A retrospective analysis of the SEER database. Cancer Med (2021) 10(17):6140–8. doi: 10.1002/cam4.4151
18. Ni X, Ma X, Qiu J, Zhou S, Cheng W, Luo C. Development and validation of a novel nomogram to predict cancer-specific survival in patients with uterine cervical adenocarcinoma. Ann Transl Med (2021) 9(4):293. doi: 10.21037/atm-20-6201
19. Brusselmans L, Arnouts L, Millevert C, Vandersnickt J, van Meerbeeck JP, Lamote K. Breath analysis as a diagnostic and screening tool for malignant pleural mesothelioma: a systematic review. Transl Lung Cancer Res (2018) 7(5):520–36. doi: 10.21037/tlcr.2018.04.09
20. Shaikh F, Zauderer MG, von Reibnitz D, Wu AJ, Yorke ED, Foster A, et al. Improved outcomes with modern lung-sparing trimodality therapy in patients with malignant pleural mesothelioma. J Thorac Oncol (2017) 12(6):993–1000. doi: 10.1016/j.jtho.2017.02.026
21. Brcic L, Kern I. Clinical significance of histologic subtyping of malignant pleural mesothelioma. Transl Lung Cancer Res (2020) 9(3):924–33. doi: 10.21037/tlcr.2020.03.38
22. Ceresoli GL, Zucali PA, Favaretto AG, Grossi F, Bidoli P, Del Conte G, et al. Phase II study of pemetrexed plus carboplatin in malignant pleural mesothelioma. J Clin Oncol (2006) 24(9):1443–8. doi: 10.1200/JCO.2005.04.3190
23. Parikh K, Hendriks LEL, Bironzo P, Remon J. Immune checkpoint inhibitors a new player in the therapeutic game of mesothelioma: New reality with new challenges. Cancer Treat Rev (2021) 99:102250. doi: 10.1016/j.ctrv.2021.102250
24. Carmichael J, Degraff WG, Gamson J, Russo D, Gazdar AF, Levitt ML, et al. Radiation sensitivity of human lung cancer cell lines. Eur J Cancer Clin Oncol (1989) 25(3):527–34. doi: 10.1016/0277-5379(89)90266-6
25. de Graaf-Strukowska L, van der Zee J, van Putten W, Senan S. Factors influencing the outcome of radiotherapy in malignant mesothelioma of the pleura–a single-institution experience with 189 patients. Int J Radiat Oncol Biol Phys (1999) 43(3):511–6. doi: 10.1016/S0360-3016(98)00409-X
26. Ghirardelli P, Franceschini D, D'Aveni A, Dominici L, Ravasio A, Marzo M, et al. Salvage radiotherapy for oligo-progressive malignant pleural mesothelioma. Lung Cancer (2021) 152:1–6. doi: 10.1016/j.lungcan.2020.11.022
27. Maasilta P, Kivisaari L, Holsti LR, Tammilehto L, Mattson K. Radiographic chest assessment of lung injury following hemithorax irradiation for pleural mesothelioma. Eur Respir J (1991) 4(1):76–83. doi: 10.1183/09031936.93.04010076
28. Liao Z, Lee JJ, Komaki R, Gomez DR, O'Reilly MS, Fossella FV, et al. Bayesian Adaptive randomization trial of passive scattering proton therapy and intensity-modulated photon radiotherapy for locally advanced non-Small-Cell lung cancer. J Clin Oncol (2018) 36(18):1813–22. doi: 10.1200/JCO.2017.74.0720
Keywords: advanced malignant pleural mesothelioma, Nomograms, Radiotherapy, SEER database, prognosis
Citation: Zhang X, Chang L, Zhu Y, Mao Y, Zhang T, Zhang Q and Wang C (2023) Establishment and validation of nomograms to predict survival probability of advanced malignant pleural mesothelioma based on the SEER database and a Chinese medical institution. Front. Endocrinol. 14:1139222. doi: 10.3389/fendo.2023.1139222
Received: 06 January 2023; Accepted: 29 March 2023;
Published: 14 April 2023.
Edited by:
Teresa Gagliano, University of Udine, ItalyReviewed by:
Alberto Bongiovanni, IRCCS Istituto Romagnolo per lo Studio dei Tumori (IRST) “Dino Amadori”, ItalyCopyright © 2023 Zhang, Chang, Zhu, Mao, Zhang, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunbo Wang, MTM5MzYwMDk1NTBAMTM5LmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.