
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol. , 17 May 2023
Sec. Clinical Diabetes
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1137406
This article is part of the Research Topic Pharmacological Approaches to the Prevention of Type 2 Diabetes View all 5 articles
 Weijuan Cui*
Weijuan Cui* Ling Zhao
Ling ZhaoObjective: Despite the fact that some evidence suggests that the administration of 17β-estradiol plus norethisterone acetate influences glucose and insulin metabolism in women, these findings are still contradictory. Thus, we aimed to examine the impact of the co-administration of 17β-estradiol and norethisterone acetate on glycated haemoglobin (HbA1c), fasting glucose, insulin and C-peptide concentrations in females by means of a systematic review and meta-analysis of randomized controlled trials (RCTs).
Methods: We searched four databases (PubMed/MEDLINE, Scopus, Embase, and Web of Science) using specific keywords and word combinations. The random-effects model (DerSimonian and Laird model) was employed to compute the weighted mean difference (WMD) and 95% confidence intervals (CIs) for the variations from baseline of HbA1c, fasting glucose, insulin, and C-peptide concentrations.
Results: In total, 14 RCTs were entered into the quantitative synthesis. The combined administration of 17β-estradiol and norethisterone acetate decreased HbA1c (WMD: -0.65%, 95% CI: -1.15 to -0.15; P=0.011), fasting glucose (WMD: -11.05 mg/dL, 95% CI: -16.6 to -5.5; P<0.001) and insulin (WMD: -1.35 mIU/L, 95% CI: -2.20 to -0.50; P=0.001) levels. C-peptide concentrations’ declined only in females diagnosed with overweight/obesity or diabetes.
Conclusion: Evidence to date points out that the administration of 17β-estradiol and norethisterone acetate has a positive impact on glucose metabolism in women by reducing fasting glucose, HbA1c, and insulin values. Future studies need to confirm the potential benefits of this drug combination in the prevention and/or management of cardiometabolic disorders.
There is a gradual decline in estrogen levels and in their cardioprotective effects in women as they approach the menopausal age (1). As females attain menopause, they are prone to develop several menopause-related symptoms or diseases, e.g., hot flashes, cardiovascular disorders, dementia, osteoporosis, sexual, and urogenital dysfunctions (2–4). To control or alleviate menopause-related symptomatology and to improve quality of life, women of postmenopausal age will require hormone replacement therapy (HRT) (2, 5). However, available evidence suggests that the administration of HRT can influence the metabolism of glucose and insulin (6–8).
An increase in insulin resistance and deterioration in glucose tolerance is known to occur with advancing age, although the effect of menopause remains controversial (9). Moreover, a lack of estrogen tends to promote an accumulation of abdominal fat, which is a major cardiovascular risk factor and one of the criteria for the diagnosis of metabolic syndrome (10). Estrogen may have beneficial effects on carbohydrate metabolism while the effects of progestogen may be deleterious; however, results may differ depending on the type of progestogen used (11). Due to conflicting results from previously conducted studies, the actions of 17β-estradiol plus norethisterone acetate on glucose homeostasis in women remain controversial (6–8, 12–22). Thus, we conducted a systematic review and meta-analysis of randomized controlled trials (RCTs) to investigate the effects of 17β-estradiol plus norethisterone acetate treatment on fasting glucose, fasting insulin, glycated haemoglobin (HbA1c) and C-peptide in women.
This meta-analysis was accomplished in agreement with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statements (23).
The PubMed/MEDLINE, Scopus, Embase, and Web of Science databases were systemically searched without language restrictions to identify papers indexed before June 14th, 2022. In addition, to identify other papers that could have been missed in the previous step, we checked the lists of references of any relevant review and/or eligible study. The MeSH (Medical subject heading) and non-MeSH search keywords used: (((“Glycated Hemoglobin A”[Mesh] OR “Blood Glucose”[Mesh] OR “Hyperglycemia”[Mesh] OR “Insulin Resistance”[Mesh] OR “Hyperinsulinism”[Mesh] OR “Hyperinsulinism”[Mesh] OR “Diabetes Mellitus”[Mesh] OR “Glycosylated Hemoglobin”[Title/Abstract] OR “HbA1”[Title/Abstract] OR “Hb A1c”[Title/Abstract] OR “Glycohemoglobin A”[Title/Abstract] OR “Glycated Hemoglobin”[Title/Abstract] OR “Blood Glucose”[Title/Abstract] OR “Blood Sugar”[Title/Abstract] OR Hyperglycemia[Title/Abstract] OR Glycemia[Title/Abstract] OR HOMA*[Title/Abstract] OR “homeostatic model for insulin resistance”[Title/Abstract] OR “fasting blood insulin”[Title/Abstract] OR FBI[Title/Abstract] OR FBG[Title/Abstract] OR FBS[Title/Abstract] OR “Insulin Resistance”[Title/Abstract] OR “Insulin Sensitivity”[Title/Abstract] OR “Hyperinsulinemia”[Title/Abstract] OR Hyperinsulinism[Title/Abstract] OR Insulin*[Title/Abstract] OR Hyperinsulin*[Title/Abstract] OR “Oral Glucose Tolerance Test”[Title/Abstract] OR OGTT[Title/Abstract] OR “Diabetes Mellitus”[Title/Abstract]))) AND (((estrogen [Mesh] OR estrogen [tiab] OR estrogen replacement therapy [Mesh] OR estrogen replacement therapy [tiab] OR hormone replacement therapy [Mesh] OR hormone replacement therapy [tiab] OR estradiol [Mesh] OR estradiol [tiab] OR progestin therapy [Mesh] OR progestin therapy [tiab] OR progestin [Mesh] OR progestin [tiab] OR *progesterone [Mesh] OR *progesterone [tiab] OR HRT [Mesh] OR HRT [tiab] OR tibolone [Mesh] OR tibolone [tiab] OR norethisterone [Mesh] OR norethisterone [tiab] OR norethindrone [Mesh] OR norethindrone [tiab] OR medrogestone [Mesh] OR medrogestone [tiab]))) AND (((((“Clinical Trials as Topic”[Mesh] OR “Cross-Over Studies”[Mesh] OR “Double-Blind Method”[Mesh] OR “Single-Blind Method”[Mesh] OR “Random Allocation”[Mesh] OR RCT[Title/Abstract] OR “Clinical Trial” [Publication Type] OR “Controlled Clinical Trials as Topic”[Mesh] OR “Intervention Studies”[Title/Abstract] OR “intervention”[Title/Abstract] OR Trial[Title/Abstract] OR “controlled trial”[Title/Abstract] OR “randomized”[Title/Abstract] OR “randomised”[Title/Abstract] OR “random”[Title/Abstract] OR “randomly”[Title/Abstract] OR “placebo”[Title/Abstract] OR “assignment”[Title/Abstract]))))).
To select the eligible RCTs, we used the PICO framework (P: women; I: 17β-estradiol plus norethisterone acetate in oral form; C: a group of women who did not receive 17β-estradiol plus norethisterone acetate and only received standard of care therapy or any other medication as control/placebo; O: mean and standard deviation (SD) for HbA1c, fasting glucose, insulin, and c-peptide. All papers other than original research publications (letter to the editor, correspondence, reviews), case series or case reports, studies on pregnant females and on participants below 18 years of age were excluded.
Two researchers accomplished the data extraction independently. In addition, the disagreements and discrepancies were resolved via consultation with the head author. The following data regarding the RCTs were extracted: (1) general information (i.e., publication year, title, authors, and country); (2) methodological information (i.e., treatment allocation, trial period duration, intervention description, and study design),; (3) participant-related data (i.e., sample size, group, age, sex, baseline characteristics and participant demographics); and (4) result-related data (i.e., mean and SD of HbA1c, fasting glucose, insulin, and c-peptide).
The quality of the RCTs was measured independently by two investigators using the Cochrane collaboration’s tool (24). This instrument evaluates the next parameters: incomplete outcome data, allocation concealment, sequence generation, blinding of participants and personnel, blinding of outcome assessment, selective outcome reporting, and other sources of bias.
The random-effects model (DerSimonian and Laird model) was used to compute the weighted mean difference (WMD) and 95% confidence intervals (CIs) for the variations from baseline of HbA1c, fasting glucose, insulin, and C-peptide values. We measured the heterogeneity by employing the I2 test. Values of <25%, 26–50%, and >50% indicated low, moderate and high degrees of heterogeneity, respectively.
Moreover, we evaluated the risk of publication bias by the Egger’s tests (quantitative) and funnel plot analysis (qualitative) (25). When significant publication bias was detected among the RCTs, the trim-and-fill test was applied to estimate the impact of unpublished papers (26). In order to ascertain the potential source of heterogeneity, stratified analyses were executed based on the following parameters: the length of the intervention, the participants’ body mass index (BMI) at baseline, the subjects’ health status, and the participants’ mean age. We employed a sensitivity analysis test to calculate the vigor of the overall results by exclusion of each comparison one-by-one and computing the final result. The statistical analysis of the current investigation was run using Stata software version 14 (StataCorp, TX, USA).
Figure 1 depicts the study selection method. Two researchers accomplished the study selection independently. In addition, the disagreements and discrepancies were resolved via consultation with the head author. A total of 6502 publications was found when the databases were searched. An overall of 2430 records were retrieved after the removal of duplicates. During the screening of titles and abstracts step, 4072 publications were excluded. In the next stage, of the 41 full-texts assessed, 16 papers were eliminated due to unavailability of the results, 4 articles were excluded because they lacked an RCT design, and 6 papers were excluded because they lacked a control group. Finally, 14 RCTs with 16 arms were included in the meta-analysis: 7 RCT arms on HbA1c, 15 RCT arms on fasting glucose, 12 RCT arms on insulin, and 6 RCT arms on c-peptide (13–22, 27–31).
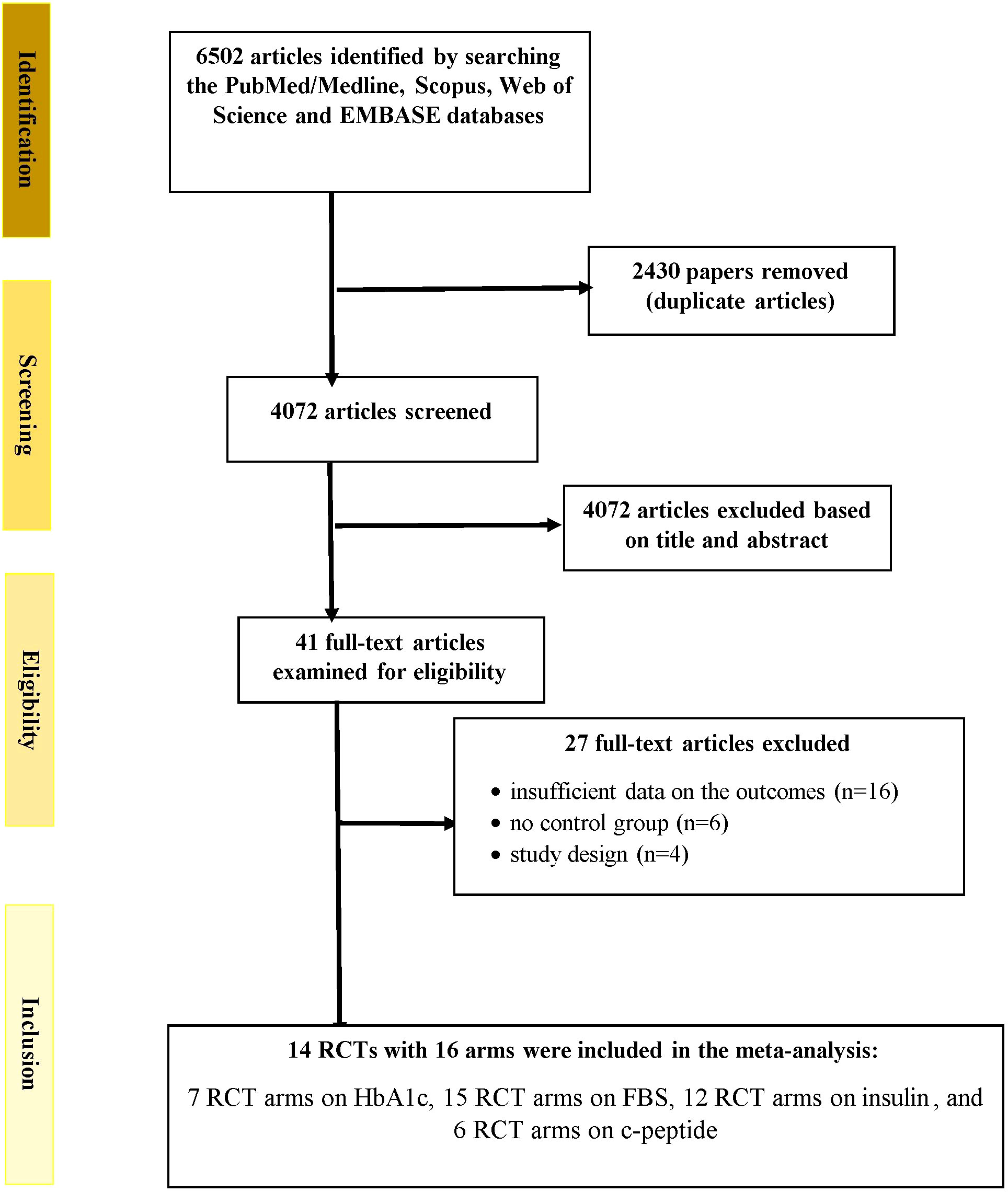
Figure 1 Flowchart depicting the study selection and inclusion processes for the current meta-analysis. RCTs, randomized controlled trials; HbA1c, glycated hemoglobin; FBS, fasting blood glucose.
Table 1 depicts the characteristics of the eligible publications. These RCTs were conducted in the United Kingdom, Brazil, Sweden, Turkey, Finland, Sweden, New Zealand, Italy, Germany, and Singapore. All of the 17β-estradiol plus norethisterone acetate doses were given orally, although the dosage varied between the eligible publications, ranging from 700 µg/day to 1 mg/day for norethisterone acetate and 1 mg/day to 2 mg/day for 17β-estradiol. All manuscripts were published between 1992 and 2013. The recruited subjects were healthy women or females diagnosed with type 2 diabetes mellitus (T2DM), overweight or obesity. The period of the intervention varied from 12 weeks to 2 years. The mean age of the participants varied from 25.4 years to 62.2 years. Supplementary Table 1 delineates the quality assessment of the included RCTs.
Figure 2 presents the forest plot for HbA1c concentrations. A total of 7 RCT arms (sample size = 261 subjects; 17β-estradiol plus norethisterone acetate group = 137 subjects, placebo group = 124 subjects) evaluated the impact of 17β-estradiol plus norethisterone acetate administration on HbA1c levels in postmenopausal females. Women who were prescribed 17β-estradiol plus norethisterone acetate experienced significant reductions in HbA1c concentrations (WMD: -0.65%, 95% CI: -1.15 to -0.15; P=0.011) versus the placebo group. However, a significant heterogeneity was noticed among the trials (I2 = 97%, P<0.001). Moreover, a significant decrease in HbA1c values was observed in postmenopausal women aged ≥60 years (WMD: -0.50%, 95% CI: -0.60 to -0.45, P<0.001) versus <60 years (WMD: -0.73%, 95% CI: -1.80 to 0.33, P=0.17). In addition, a notable decline in HbA1c levels was demonstrated in postmenopausal women with a BMI of ≥30 kg/m2 (WMD: -1.05%, 95% CI: -2.05 to -0.05, P=0.03) versus <30 kg/m2 (WMD: -0.4%, 95% CI: -0.6 to -0.2, P<0.001). The subgroup analyses depicted significant reductions in HbA1c values in the RCTs with a duration of ≥6 months (WMD: -0.50%, 95% CI: -0.60 to -0.45, P<0.001) versus <6 months (WMD: -0.70%, 95% CI: -1.6 to 0.2, P=0.129). Moreover, a pronounced decrease in HbA1c concentrations was observed in postmenopausal women with T2DM (WMD: -0.85%, 95% CI: -1.50 to -0.2, P=0.01) versus healthy postmenopausal women (WMD: -0.25%, 95% CI: -0.40 to -0.08, P=0.004) (Supplementary Figure 1).
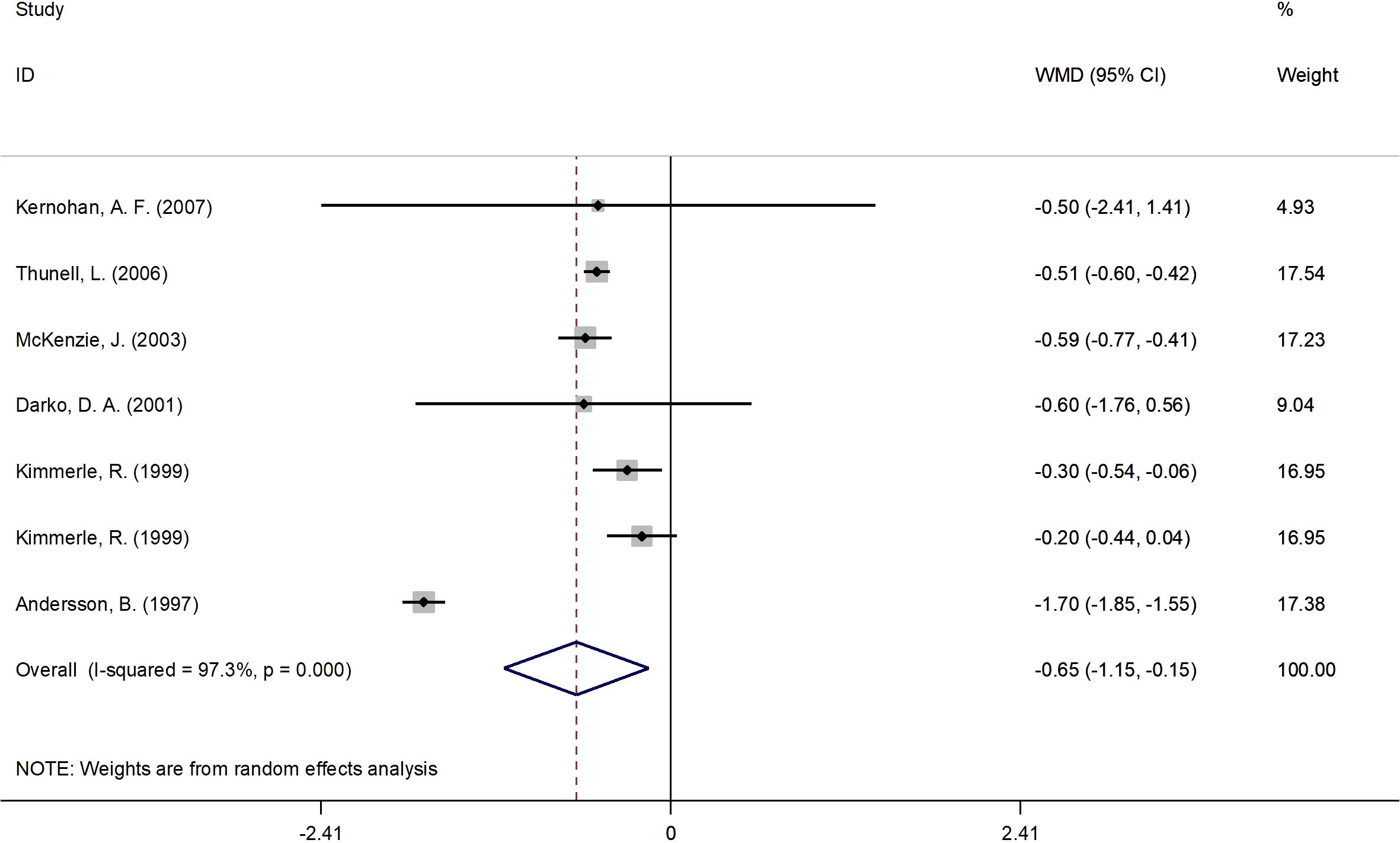
Figure 2 Forest plot of RCTs investigating the effects of 17β-estradiol plus norethisterone acetate on HbA1c. RCTs, randomized controlled trials; HbAlc, glycated hemoglobin; WMD, weighted mean difference; CL, confidence interval.
Figure 3 presents the forest plot for fasting glucose concentrations. A total of 15 RCT arms (sample size = 825 subjects; 17β-estradiol plus norethisterone acetate group = 423 subjects; placebo group = 402 subjects) assessed the effect of 17β-estradiol plus norethisterone acetate administration on fasting glucose levels in postmenopausal female. There was a notable decrease in fasting glucose concentrations in women who received 17β-estradiol plus norethisterone acetate (WMD: -11.05 mg/dL, 95% CI: -16.6 to -5.5; P<0.001) versus placebo. However, a significant heterogeneity was noticed among the trials (I2 = 98%, P<0.001). fasting glucose levels notably decreased in postmenopausal women aged <60 years (WMD: -8.45 mg/dL, 95% CI: -14.25 to -2.60, P<0.001) versus ≥60 years (WMD: -23.70 mg/dL, 95% CI: -48.5 to 1.05, P=0.06). In addition, a significant reduction in fasting glucose levels was detected in postmenopausal women with a BMI of ≥25 kg/m2 (WMD: -12.30 mg/dL, 95% CI: -19.25 to -5.40, P<0.001) versus <25 kg/m2 (WMD: -3.90 mg/dL, 95% CI: -17.65 to 9.90, P=0.58). The subgroup analyses also revealed a significant decrease in fasting glucose values in the RCTs with a duration of ≥6 months (WMD: -5.10 mg/dL, 95% CI: -9.55 to -0.70, P=0.02) versus <6 months (WMD: -25.60 mg/dL, 95% CI: -50.75 to -0.50, P=0.046). Moreover, a significant decrease in fasting glucose concentrations was observed in women with T2DM (WMD: -29.20 mg/dL, 95% CI: -53.50 to -4.90, P=0.01) versus healthy women (WMD: -3.50 mg/dL, 95% CI: -7.30 to 0.25, P=0.06) (Supplementary Figure 1).
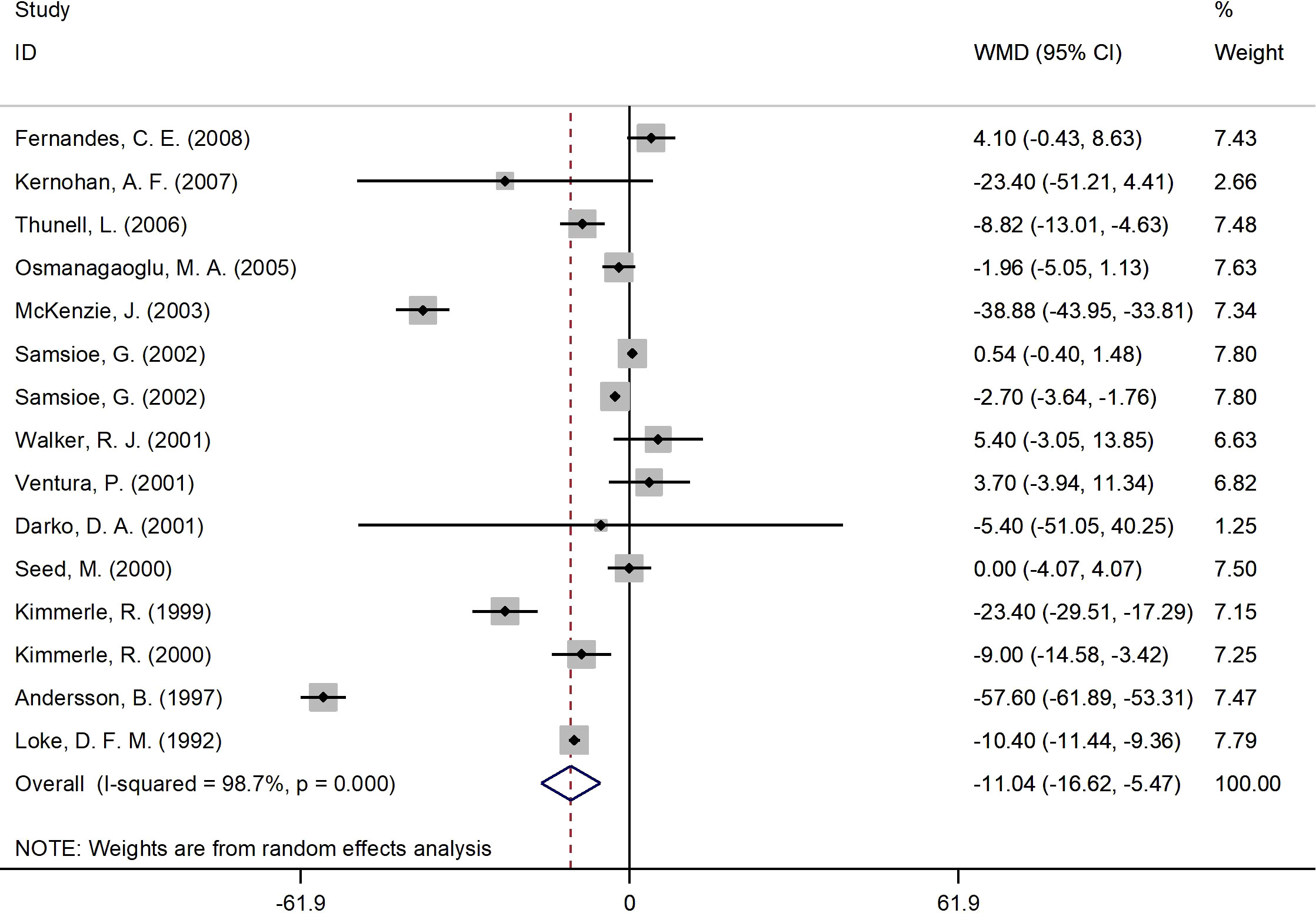
Figure 3 Forest plot of RCTs investigating the effects of 17β-estradiol plus norethisterone acetate administration on FBS. RCTs, randomized controlled trials. FBS, fasting blood glucose; WMD, weighted mean difference; CI, confidence interval.
Figure 4 presents the forest plot for insulin concentrations. A total of 12 RCT arms with (sample size = 810 subjects; 17β-estradiol plus norethisterone acetate group = 404 subjects; placebo = 406 subjects) investigated the action of 17β-estradiol plus norethisterone acetate administration on insulin levels in females. Following the intake of 17β-estradiol plus norethisterone acetate, insulin concentrations decreased (WMD: -1.35 mIU/L, 95% CI: -2.20 to -0.50; P=0.001), with a moderate heterogeneity among the trials (I2 = 38%, P=0.08). Moreover, a significant decline in insulin levels was noted in women aged ≥60 years (WMD: -1.60 mIU/L, 95% CI: -2.45 to -0.80, P<0.001) versus <60 years (WMD: -1.10 mIU/L, 95% CI: -2.25 to 0.06, P=0.06). In addition, a pronounced reduction in insulin concentrations was experienced by women with a BMI of ≥25 kg/m2 (WMD: -1.4 mIU/L, 95% CI: -2.20 to -0.50, P=0.002) versus <25 kg/m2 (WMD: 1.1 mIU/L, 95% CI: -6.20 to 8.30, P=0.77). The subgroup analyses discovered a significant decrease in insulin values in the RCTs with a duration of ≥6 months (WMD: -1.20 mIU/L, 95% CI: -2.05 to -0.40, P=0.005) versus <6 months (WMD: -1.40 mIU/L, 95% CI: -3.75 to 0.90, P=0.23). Moreover, a notable reduction in insulin concentrations was observed in women with T2DM (WMD: -2.20 mIU/L, 95% CI: -3.40 to -1.10, P<0.001) versus healthy women (WMD: -0.80 mIU/L, 95% CI: -1.80 to 0.20, P=0.12) (Supplementary Figure 1).
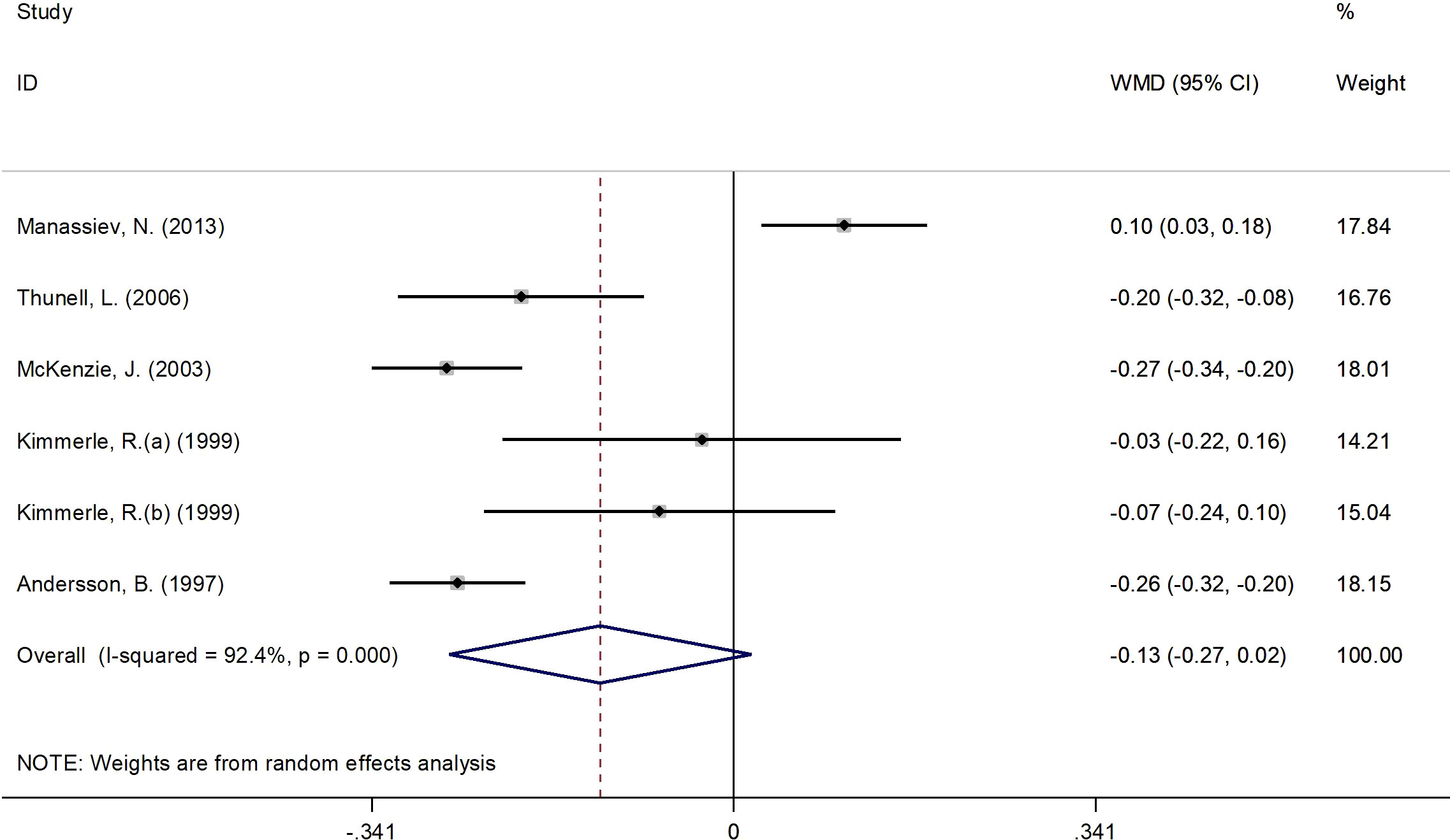
Figure 4 Forest plot of RCTs investigating the effects of 17β-estradiol plus norethisterone acetate on insulin levels. RCT, randomized controlled trials; WMD, weighted mean difference; Cl, confidence interval.
Figure 5 presents the forest plot for C-peptide concentrations. A total of 6 RCT arms (sample size = 268 subjects; 17β-estradiol plus norethisterone acetate group = 142 subjects; placebo = 126 subjects) evaluated the impact of 17β-estradiol plus norethisterone acetate administration on C-peptide levels in females. C-peptide concentrations did not change following the use of 17β-estradiol plus norethisterone acetate (WMD: -0.12 nmol/L, 95% CI: -0.30 to 0.02; P=0.08) versus placebo, with significant heterogeneity among the trials (I2 = 92%, P<0.001). However, a notable reduction in C-peptide levels was detected in women with a BMI of ≥25 kg/m2 (WMD: -0.20 nmol/L, 95% CI: -0.30 to -0.15, P<0.001) versus <25 kg/m2 (WMD: 0.10 nmol/L, 95% CI: 0.02 to 0.18, P=0.009). Moreover, a significant decrease in C-peptide values occurred in women with T2DM (WMD: -0.25 nmol/L, 95% CI: -0.30 to -0.20, P<0.001) versus healthy women (WMD: 0.02 nmol/L, 95% CI: -0.10 to 0.15, P=0.71) (Supplementary Figure 1).
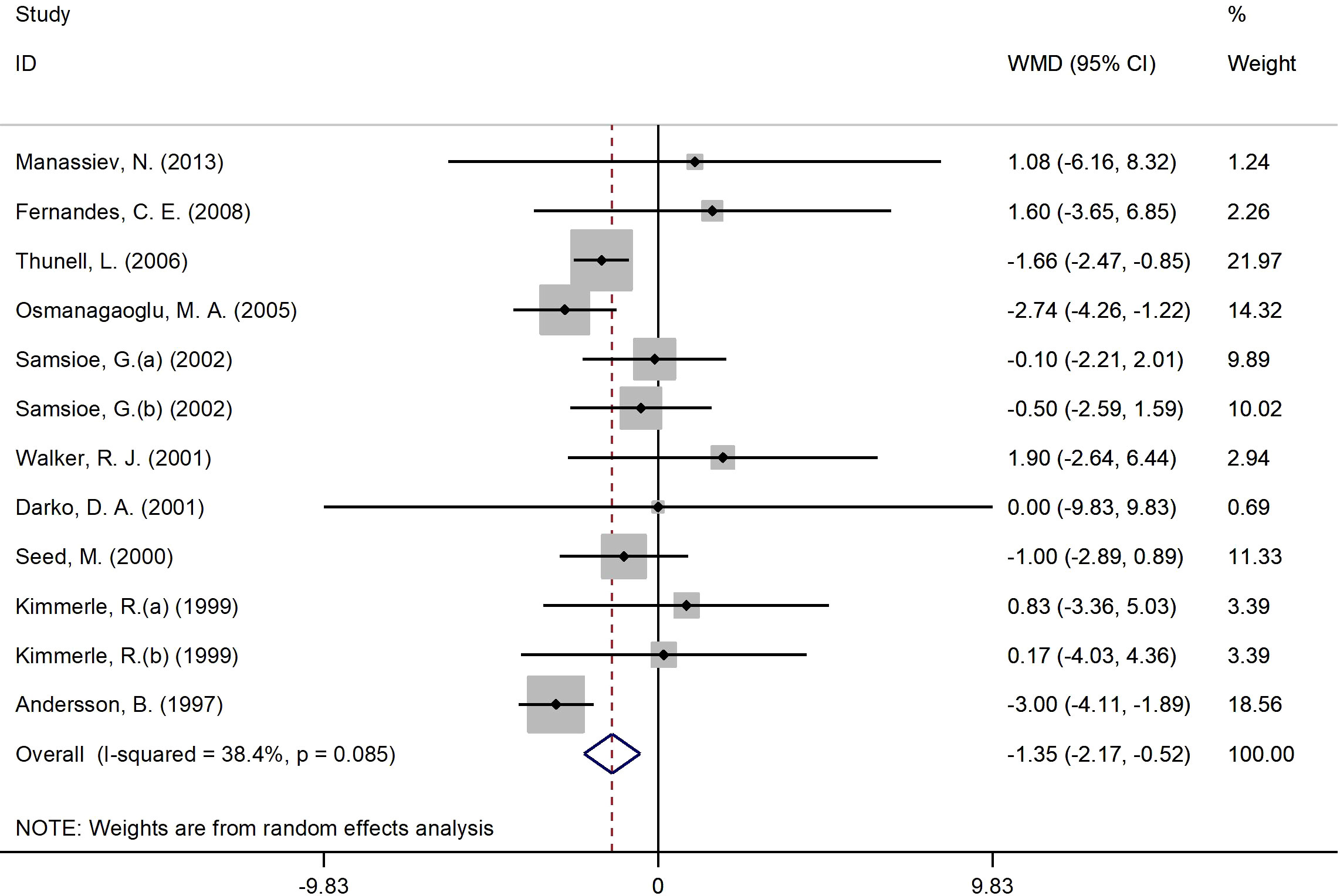
Figure 5 Forest plot of RCTs investigating the effects of 17β-estradiol plus eorethisterone acetate administration on C-peptide levels. RCTs, and contriled trials; WMD, weighted mean difference; Ct, confidence umurval.
The funnel plot of the effect sizes was essentially symmetrical and the Egger’s test confirm it (Supplementary Figure 2). These results remained essentially unchanged in the sensitivity analyses after we removed each RCT and combined the remaining ones (Supplementary Figure 3).
In this systematic review and meta-analysis, we examined the effect of the co-administration of 17β-estradiol and norethisterone acetate on markers of glucose and insulin metabolism, namely glycated haemoglobin (HbA1c), fasting glucose, insulin and C-peptide concentrations, in women. Based on data derived from 14 RCTs, our results suggest that the use of 17β-estradiol and norethisterone acetate can reduce fasting glucose, HbA1c, insulin and C-peptide levels in females. In the subgroup analyses, the age and BMI of the participants, the intervention length and the presence of type 2 diabetes mellitus (T2DM) seemed to mediate the impact of this drug combination on the examined markers of glucose and insulin metabolism.
Our findings clearly point out that the co-administration of 17β-estradiol and norethisterone acetate reduces HbA1c concentrations in females. The reduction was clinical significant (WMD: -0.65%, P=0.01) and was more notable when this drug combination was administered in elderly women (WMD: -0.62%, P<0.001 for females aged at least 60 years or more), in women diagnosed with obesity (WMD: -1.04%, P=0.03 for BMI ≥30 kg/m2) or T2DM (WMD: -0.84%, P=0.01), and when the length of the intervention was equal to or exceeded 6 months (WMD: -0.52%, P<0.001). This discovery is of particular importance as it has been revealed that HbA1c concentrations increase in women with advancing age. In a cohort study which enrolled nearly 170000 adults, Alghamdi et al. (2021) reported that HbA1c concentrations increase overall by 0.35% for each ten years increase in age, however, after the age of 50, the elevation in HbA1c is of 1.118% (32). Our results were also confirmed by other cohort studies. For example, Ferrara et al. demonstrated that the use of hormone replacement therapy (HRT) is linked with a reduction in HbA1c concentrations in women suffering from T2DM (P<0.001) (33). These conclusions were drawn based on a sub-analysis of the The Northern California Kaiser Permanente Diabetes Registry which recruited over 15000 females diagnosed with T2DM (33). Similarly, Kuh et al. depicted a positive association between HbA1c levels and age and communicated that women who were prescribed hormone replacement therapy (HRT) exhibited lower HbA1c, BMI and low-density lipoprotein cholesterol values (34). Likewise, in previous publications, we have pointed out that the co-administration of 17β-estradiol and norethisterone acetate reduces serum lipids’ concentrations, enabling thus a better metabolic control of T2DM in women (35, 36).
In terms of its impact on glycemia, the combined use of 17β-estradiol and norethisterone acetate was also successful in decreasing fasting glucose levels (WMD: -11.04 mg/dL, P<0.001). Similarly to the previously mentioned results, this type of hormone replacement therapy reduced fasting glucose when the duration of the administration was equal to or exceeded 6 months (WMD: -5.11 mg/dL, P=0.02) and when it was prescribed in females with concurrent T2DM (WMD: -29.20 mg/dL, P=0.01). However, fasting glucose values also declined in women who were at least overweight (WMD: -12.32 mg/dL, P<0.001 for BMI ≥25 kg/m2) or aged <60 years (WMD: -8.43 mg/dL, P<0.001). Taken as a whole, these findings reinforce the statement that the combination of 17β-estradiol and norethisterone acetate can be helpful in the management of T2DM in women as it improves glycemic control and is associated with a significant decrease in HbA1c and fasting glucose concentrations. This is particularly important in the prevention of T2DM-related complications, e.g., cardiovascular disease (CVD), as fasting glucose values have been linked to the 10-year risk of CVD in both pre- and postmenopausal women (37). What is more, the clinical guide for the management of T2DM during the menopause issued by the European Menopause and Andropause Society (EMAS) recommended a tailored-based approach in the prescription of HRT based on CVD risk: oral estrogens should be given to females with low risk of CVD, whereas transdermal estrogens should be administered in those with obesity or other risk factors for CVD. In addition to the use of estrogens, EMAS recommends supplementing the HRT regimen with a progestogen that does not influence carbohydrate metabolism, e.g., norethisterone (38). Moreover, the BMI seems to mediate the relationship between age and fasting glucose values in females, especially in those in whom menopause occurred later in life, e.g., after 54 years of age. Zhao et al. highlighted that the age at menopause was associated with the development of T2DM, overweight/obesity and fasting glucose concentrations (39). This information was confirmed by other investigations (40, 41). Even if these actions on glucose metabolism sound promising, we still need more RCT-based evidence to use HRT in the control or prevention of T2DM (42).
Insulin levels were also affected by the combined use of 17β-estradiol and norethisterone acetate. Our results suggest that, following the use of this drug combination, females exhibit a decline in insulin concentrations (WMD: -1.34 mIU/L, P=0.001). The decrease in insulin values was more notable in elderly women (WMD: -1.62 mIU/L, P=0.001 for age ≥60 years), women suffering from T2DM (WMD: -2.22 mIU/L, P<0.001) or overweight/obesity (WMD: -1.36 mIU/L, P=0.002) or when the length of the intervention exceeded or was equal to 6 months (WMD: -1.21 mIU/L, P=0.005). However, C-peptide concentrations only decreased in females diagnosed with overweight/obesity (WMD: -0.20 nmol/L, P<0.001 for BMI ≥25 kg/m2) or T2DM (WMD: -0.25 nmol/L, P<0.001). C-peptide concentrations are employed in clinical practice to evaluate the function of the beta cells of the pancreas, as this substance is generated in equimolar quantities to insulin as thus can reflect cardiometabolic health (43). These findings are in line with the results of previously published investigations which confirmed that HRT reduces fasting glucose, insulin and C-peptide values (44). In addition, Palla et al. pointed out that anthropometric indices indicative of an increased BMI, e.g., body perimeter and/or waist-to-hip ratio, are correlated with insulin and C-peptide levels, particularly in females with excessive body weight and other cardiometabolic ailments, e.g., metabolic syndrome (45). Thus, these women might be more sensitive to the action of 17β-estradiol and norethisterone acetate due to their excessive concentrations of insulin and C-peptide. It seems that 17β-estradiol can influence the levels of pro- and anti-inflammatory cytokines during the menopause (46). An assessment in a murine model reported that IL-10 concentrations increased, whereas IL-1β and TNF-α decreased, following the administration of this drug in aged rat hearts. Consequently, this compound was able to decrease insulin resistance and enhance insulin signalling which might explain the results of our meta-analysis (46).
Our study had several strengths and limitations. To our knowledge, this is the first meta-analysis to examine the impact of 17β-estradiol and norethisterone acetate on glucose metabolism in females. As the data was derived strictly from RCTs and the reported data is robust. In addition, we examined the inter-study heterogeneity and performed subgroup analyses based on several factors that could have influenced our findings, e.g., length of the intervention, age or BMI of the participants, presence of T2DM.
Several limitations must be also noted. The studies were conducted in multiple countries throughout the globe and thus the subjects had different demographics, clinical findings and most likely genetics. In addition, different brands of HRT drugs might have been administered based on the local availability. The studies conducted by Darko et al. (15) and Kernohan et al. (13) have wide confidence intervals due to their sample sizes, thus, their results do not provide a precise representation of the population mean. In addition, we combined just six studies that have selected C-peptide as an outcome variable and the findings of these investigations display significant differences.
Moreover, the inter-RCT heterogeneity was sometimes high and potential confounders apart from the ones explored in the subgroup analyses might have influenced the final results. The present study is the first systematic review and meta-analysis of randomized controlled trials (RCTs) which investigated the effects of 17β-estradiol plus norethisterone acetate treatment on fasting glucose, fasting insulin, glycated haemoglobin (HbA1c) and C-peptide concentrations in women based on articles published till June 14th, 2022. Future studies need to confirm the potential benefits of this drug combination in the prevention and/or management of cardiometabolic disorders.
The significant change in fasting glucose, HbA1c, insulin and C-peptide values by 17beta-estradiol plus norethisterone acetate reverses diabetes, obesity, and cardiometabolic disorders.
Evidence to date highlights that the co-administration of 17β-estradiol and norethisterone acetate in females reduces fasting glucose, HbA1c, insulin and, in some instances, C-peptide concentrations.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
WC and LZ carried out the concept, design and drafting of this study. WC and LZ reviewed literature, searched databases, screened articles, and extracted data. WC and LZ performed the acquisition, analysis, interpretation of data, and revision. All authors reviewed the manuscript for editorial and intellectual contents. All authors approved the final version of the manuscript.
No funding was received for this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1137406/full#supplementary-material
1. Naftolin F, Friedenthal J, Nachtigall R, Nachtigall L. Cardiovascular health and the menopausal woman: the role of estrogen and when to begin and end hormone treatment. F1000Research (2019) 8:F1000 Faculty Rev–1576. doi: 10.12688/f1000research.15548.1
2. Lee SR, Cho MK, Cho YJ, Chun S, Hong S-H, Hwang KR, et al. The 2020 menopausal hormone therapy guidelines. J menopausal Med (2020) 26(2):69. doi: 10.6118/jmm.20000
3. Santoro N, Epperson CN, Mathews SB. Menopausal symptoms and their management. Endocrinol Metab Clinics (2015) 44(3):497–515. doi: 10.1016/j.ecl.2015.05.001
4. Greenhalgh RM, Powell JT. Endovascular repair of abdominal aortic aneurysm. New Engl J Med (2008) 358(5):494–501. doi: 10.1056/NEJMct0707524
5. Group ECW. Hormones and cardiovascular health in women. Hum Reprod Update (2006) 12(5):483–97. doi: 10.1093/humupd/dml028
6. Zhang Y, Howard BV, Cowan LD, Yeh J, Schaefer CF, Wild RA, et al. The effect of estrogen use on levels of glucose and insulin and the risk of type 2 diabetes in american Indian postmenopausal women: the strong heart study. Diabetes Care (2002) 25(3):500–4. doi: 10.2337/diacare.25.3.500
7. Okada M, Nomura S, Ikoma Y, Yamamoto E, Ito T, Mitsui T, et al. Effects of postmenopausal hormone replacement therapy on HbA1c levels. Diabetes Care (2003) 26(4):1088–92. doi: 10.2337/diacare.26.4.1088
8. Triusu RJ, Cowie CC, Harris MI. Hormone replacement therapy and glucose metabolism. Obstetrics Gynecology (2000) 96(5):665–70. doi: 10.1016/S0029-7844(00)00980-7
9. Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab (2003) 88(6):2404–11. doi: 10.1210/jc.2003-030242
10. Schneider JG, Tompkins C, Blumenthal RS, Mora S. The metabolic syndrome in women. Cardiol Rev (2006) 14(6):286–91. doi: 10.1097/01.crd.0000233757.15181.67
11. Godsland IF, Crook D, Simpson R, Proudler T, Felton C, Lees B, et al. The effects of different formulations of oral contraceptive agents on lipid and carbohydrate metabolism. New Engl J Med (1990) 323(20):1375–81. doi: 10.1056/NEJM199011153232003
12. Espeland MA, Hogan PE, Fineberg SE, Howard G, Schrott H, Waclawiw MA, et al. Effect of postmenopausal hormone therapy on glucose and insulin concentrations. Diabetes Care (1998) 21(10):1589–95. doi: 10.2337/diacare.21.10.1589
13. Kernohan AFB, Sattar N, Hilditch T, Cleland SJ, Small M, Lumsden MA, et al. Effects of low-dose continuous combined hormone replacement therapy on glucose homeostasis and markers of cardiovascular risk in women with type 2 diabetes. Clin Endocrinol (2007) 66(1):27–34. doi: 10.1111/j.1365-2265.2006.02679.x
14. Thunell L, Andersson B, Glassell M, Mattsson LÅ. The effect of continuous combined HRT on glucose homeostasis and plasma lipids: a placebo-controlled study in postmenopausal women with type 2 diabetes. Maturitas (2006) 53(4):430–8. doi: 10.1016/j.maturitas.2005.07.008
15. Darko DA, Dornhorst A, Kennedy G, Mandeno RC, Seed M. Glycaemic control and plasma lipoproteins in menopausal women with type 2 diabetes treated with oral and transdermal combined hormone replacement therapy. Diabetes Res Clin Pract (2001) 54(3):157–64. doi: 10.1016/S0168-8227(01)00297-2
16. Fernandes CE, Pompei LM, Machado RB, Ferreira JAS, Melo NR, Peixoto S. Effects of estradiol and norethisterone on lipids, insulin resistance and carotid flow. Maturitas (2008) 59(3):249–58. doi: 10.1016/j.maturitas.2008.02.001
17. Osmanağaoğ lu MA, Osmanağ aoğ lu S, Osmanağ aoğ lu T, Okumuş B, Bozkaya H. Effect of different preparations of hormone therapy on lipid and glucose metabolism, coagulation factors, and bone mineral density in overweight and obese postmenopausal women. Fertility Sterility (2005) 84(2):384–93. doi: 10.1016/j.fertnstert.2005.01.131
18. McKenzie J, Jaap AJ, Gallacher S, Kelly A, Crawford L, Greer IA, et al. Metabolic, inflammatory and haemostatic effects of a low-dose continuous combined HRT in women with type 2 diabetes: potentially safer with respect to vascular risk? Clin Endocrinol (2003) 59(6):682–9. doi: 10.1046/j.1365-2265.2003.01906.x
19. Walker RJ, Lewis-Barned NJ, Sutherland WHF, Goulding A, Edwards EA, De Jong SA, et al. The effects of sequential combined oral 17β-estradiol norethisterone acetate on insulin sensitivity and body composition in healthy postmenopausal women: a randomized single blind placebo-controlled study. Menopause (2001) 8(1):27–32. doi: 10.1097/00042192-200101000-00006
20. Ventura P, Cagnacci A, Malmusi S, Panini R, Baldassari F, Arangino S, et al. Continuous combined hormone replacement therapy with oral 17beta-estradiol and norethisterone acetate improves homocysteine metabolism in postmenopausal women. Menopause (2001) 8(4):252–8. doi: 10.1097/00042192-200107000-00006
21. Kimmerle R, Heinemann L, Heise T, Bender R, Weyer C, Hirschberger S, et al. Influence of continuous combined estradiol-norethisterone acetate preparations on insulin sensitivity in postmenopausal nondiabetic women. Menopause (1999) 6(1):36–42. doi: 10.1097/00042192-199906010-00008
22. Andersson B, Mattsson LÅ, Hahn L, Mårin P, Lapidus L, Holm G, et al. Estrogen replacement therapy decreases hyperandrogenicity and improves glucose homeostasis and plasma lipids in postmenopausal women with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab (1997) 82(2):638–43. doi: 10.1210/jc.82.2.638
23. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Reprint–preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Phys Ther (2009) 89(9):873–80. doi: 10.1093/ptj/89.9.873
24. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. Bmj (2011) . 343:d5928. doi: 10.1136/bmj.d5928
25. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
26. Duval S. The trim and fill method. Publ bias meta-analysis: Prevention Assess adjustments (2005) p:127–44. doi: 10.1002/0470870168.ch8
27. Manassiev N, Godsland IF, Proudler AJ, Whitehead MI, Stevenson JC. Effects of tibolone or continuous combined oestradiol/norethisterone acetate on glucose and insulin metabolism. Clin Endocrinol (2013) 78(2):297–302. doi: 10.1111/j.1365-2265.2012.04491.x
28. Li C, Samsioe G, Borgfeldt C, Bendahl PO, Wilawan K, Åberg A. Low-dose hormone therapy and carbohydrate metabolism. Fertility Sterility (2003) 79(3):550–5. doi: 10.1016/S0015-0282(02)04762-3
29. Seed M, Sands RH, McLaren M, Kirk G, Darko D. The effect of hormone replacement therapy and route of administration on selected cardiovascular risk factors in post-menopausal women. Family Pract (2000) 17(6):497–507. doi: 10.1093/fampra/17.6.497
30. Loke DFM, Ng CSA, Holck S, Hall PE, Ratnam SS. Lipid and biochemical changes after low-dose oral contraception. Contraception (1992) 46(3):227–41. doi: 10.1016/0010-7824(92)90004-D
31. Samsioe G, Li C, Borgfeldt C, Wilawan K, Åberg A, Larsen S. Changes in lipid and lipoprotein profile in postmenopausal women receiving low-dose combinations of 17β-estradiol and norethisterone acetate. Menopause (2002) 9(5):335–42. doi: 10.1097/00042192-200209000-00006
32. Alghamdi AS, Alqadi A, Jenkins RO, Haris PI. The influence of gender and menopausal status on Hba1c variation in a big data study of a Saudi population. Curr Diabetes Rev (2021) 17(3):365–72. doi: 10.2174/1573399816999200729143238
33. Ferrara A, Karter AJ, Ackerson LM, Liu JY, Selby JV. Hormone replacement therapy is associated with better glycemic control in women with type 2 diabetes: the northern California kaiser permanente diabetes registry. Diabetes Care (2001) 24(7):1144–50. doi: 10.2337/diacare.24.7.1144
34. Kuh D, Langenberg C, Hardy R, Kok H, Cooper R, Butterworth S, et al. Cardiovascular risk at age 53 years in relation to the menopause transition and use of hormone replacement therapy: a prospective British birth cohort study. Bjog (2005) 112(4):476–85. doi: 10.1111/j.1471-0528.2005.00416.x
35. Tao W, Cai X, Al Masri MK, Găman MA, Prabahar K, Baradwan S, et al. The effect of transdermal 17β-estradiol combined with norethisterone acetate treatment on the lipid profile in postmenopausal women: a meta-analysis and systematic review of randomized controlled trials. Steroids (2022) 185:109061. doi: 10.1016/j.steroids.2022.109061
36. Abu-Zaid A, Gaman MA, Jamilian P, Ilesanmi-Oyelere BL, Jamilian P, Baradwan S, et al. The effect of 17β-estradiol plus norethisterone acetate treatment on the lipid profile in women: a dose-response meta-analysis of randomized controlled trials. Exp Gerontol (2022) 165:111855. doi: 10.1016/j.exger.2022.111855
37. Zhou H, Zhang C, Ni J, Han X. Prevalence of cardiovascular risk factors in non-menopausal and postmenopausal inpatients with type 2 diabetes mellitus in China. BMC Endocr Disord (2019) 19(1):98. doi: 10.1186/s12902-019-0427-7
38. Slopien R, Wender-Ozegowska E, Rogowicz-Frontczak A, Meczekalski B, Zozulinska-Ziolkiewicz D, Jaremek JD, et al. Menopause and diabetes: EMAS clinical guide. Maturitas (2018) 117:6–10. doi: 10.1016/j.maturitas.2018.08.009
39. Zhao Y, Wang S, Yang Y, Cao W, Chen K, Wang K. Mediation effect of body mass index on the association between age at menopause and type 2 diabetes mellitus in postmenopausal Chinese women. Menopause (2022) 29(5):590–8. doi: 10.1097/GME.0000000000001946
40. Ren Y, Zhang M, Liu Y, Sun X, Wang B, Zhao Y, et al. Association of menopause and type 2 diabetes mellitus. Menopause (2019) 26(3):325–30. doi: 10.1097/GME.0000000000001200
41. Clayton GL, Soares AG, Kilpi F, Fraser A, Welsh P, Sattar N, et al. Cardiovascular health in the menopause transition: a longitudinal study of up to 3892 women with up to four repeated measures of risk factors. BMC Med (2022) 20(1):299. doi: 10.1186/s12916-022-02454-6
42. Lambrinoudaki I, Paschou SA, Armeni E, Goulis DG. The interplay between diabetes mellitus and menopause: clinical implications. Nat Rev Endocrinol (2022). doi: 10.1038/s41574-022-00708-0
43. Leighton E, Sainsbury CAR, Jones GC. A practical review of c-peptide testing in diabetes. Diabetes Ther (2017) 8(3):475–87. doi: 10.1007/s13300-017-0265-4
44. Godsland IF, Manassiev NA, Felton CV, Proudler AJ, Crook D, Whitehead MI, et al. Effects of low and high dose oestradiol and dydrogesterone therapy on insulin and lipoprotein metabolism in healthy postmenopausal women. Clin Endocrinol (Oxf) (2004) 60(5):541–9. doi: 10.1111/j.1365-2265.2004.02017.x
45. Palla G, Ramírez-Morán C, Montt-Guevara MM, Salazar-Pousada D, Shortrede J, Simoncini T, et al. Perimenopause, body fat, metabolism and menopausal symptoms in relation to serum markers of adiposity, inflammation and digestive metabolism. J Endocrinol Invest (2020) 43(6):809–20. doi: 10.1007/s40618-019-01168-6
Keywords: glucose markers, insulin, insulin resistance, HbA1c, 17β-estradiol, norethisterone acetate
Citation: Cui W and Zhao L (2023) The influence of 17β-estradiol plus norethisterone acetate treatment on markers of glucose and insulin metabolism in women: a systematic review and meta-analysis of randomized controlled trials. Front. Endocrinol. 14:1137406. doi: 10.3389/fendo.2023.1137406
Received: 04 January 2023; Accepted: 03 May 2023;
Published: 17 May 2023.
Edited by:
Elaine Chow, The Chinese University of Hong Kong, ChinaReviewed by:
Eija K. Laakkonen, University of Jyväskylä, FinlandCopyright © 2023 Cui and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weijuan Cui, MjAyMnpoYW96aGFvQHNpbmEuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.