- 1Division of Endocrinology and Metabolism, Department of Medicine, Taipei Veterans General Hospital, Taipei, Taiwan
- 2Faculty of Medicine, School of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan
- 3Division of Clinical Research, Department of Medical Research, Taipei Veterans General Hospital, Taipei, Taiwan
- 4Division of Medical Oncology, Center for Immuno-oncology, Department of Oncology, Taipei Veterans General Hospital, Taipei, Taiwan
- 5Institute of Biopharmaceutical Sciences, College of Pharmaceutical Sciences, National Yang Ming Chiao Tung University, Taipei, Taiwan
- 6Division of Endocrinology and Metabolism, Department of Medicine, Dalin Tzu Chi Hospital, Buddhist Tzu Chi Foundation, Chiayi, Taiwan
Background: The prognosis of adrenocortical carcinoma (ACC) is poor but highly variable. The present study aimed to characterize patients with ACC at a single center in Taiwan and to determine the prognostic predictors of overall and progression-free survival.
Methods: Medical records of patients, who were diagnosed with ACC at Taipei Veterans General Hospital between January 1992 and June 2021, were reviewed. Patient demographics, tumor characteristics, and subsequent treatment were analyzed with regard to overall survival and progression-free survival using Kaplan-Meier methods and a Cox regression model.
Results: Sixty-seven patients were included. Females (65.7%) were more susceptible to ACC, with a younger onset and active hormonal secretion. One-half of the patients exhibited distant metastases at the time of diagnosis. The European Network for the Study of Adrenal Tumours (ENSAT) stage (hazard ratio [HR] 3.60 [95% confidence interval (CI) 1.25–10.38]; p=0.018), large vessel invasion (HR 5.19 [95% CI 1.75–15.37]; p=0.003), and mitotane use (HR 0.27 [95% CI 0.11–0.70]; p=0.007) were significantly associated with overall survival (OS). There was no single factor independently associated with progression-free survival.
Conclusion: ENSAT stage had a substantial impact on overall survival though there was no difference in OS between patients with stage II and stage III ACC. Large vessel invasion portended poor prognosis and influenced OS significantly. Moreover, mitotane only improved clinical outcomes of patients with stage IV disease.
Introduction
Adrenocortical carcinoma (ACC) is a rare malignancy, with an incidence of 1–2 cases per million population (1). Prognosis is generally poor due to its aggressive nature and tendency to recur (2). Nevertheless, there is significant heterogeneity in individual outcomes. The median overall survival (OS) of ACC patients is 3.2 years, with stage-specific median survival ranging from 24.1 years for patients with stage I ACC to 0.9 years for those with stage IV ACC (3). However, some patients with metastatic disease survived more than 10 years after diagnosis (4). Therefore, the establishment of prognostic factors to predict patient survival is important to aid clinical decision-making.
A previous literature review revealed that tumor stage, surgical margin status, presence of distant metastases, and tumor grade were significantly associated with prognosis (5). Complete surgical resection with negative margins affords the greatest opportunity for cure (3). However, advanced stage and positive remote organ metastases are associated with poor outcomes (2). Several studies have also proposed advanced age as a negative prognostic factor (6–8), although others have not found such a correlation (9, 10). Hypercortisolism has been reported to be negatively correlated with OS. The mechanism was possibly related to immunocompromised status but was still poorly understood (11, 12).
The aforementioned studies were mostly restricted to European and American populations. Prognostic investigations of ACCs in the Chinese population are limited. Only Dong et al. reported a survival benefit in a cohort of patients who underwent surgical resection at Peking, China (13). As such, the aim of our study was to assess the clinical characteristics of patients with ACC treated at a single tertiary center in Taiwan, and to investigate the prognostic implications of each factor on OS and progression-free survival.
Methods
After the approval of research review board, the medical records of patients diagnosed with ACC at Taipei Veterans General Hospital between January 1992 and June 2021 were reviewed. Covariates included sex, age at diagnosis, presence of symptoms, tumor size, hormonal activity, tumor localization, surgical margins, European Network for the Study of Adrenal Tumours (ENSAT) stage, tumor grade, local invasion, metastatic status, large vessel invasion, and adjuvant therapy. Functional status of the tumor was determined through records of cortisol, androgen, and/or aldosterone hypersecretion before the operation. Surgical margins were assessed as follows: R0 resection was defined as the absence of residual disease; R1 resection was defined as a microscopically positive surgical margin; R2 resection was determined by macroscopic residual tumors; and RX resection indicated unknown status. Tumor grade was evaluated using the Ki-67 index, with Ki-67 ≤ 10% considered to be low-grade and Ki-67 > 10% considered to be high-grade (14).
The ENSAT classification system was adopted owing to its superiority in prognostic prediction compared with the International Union Against Cancer staging system (15). According to the ENSAT classification, stage I ACC was defined as a tumor measuring ≤ 5 cm in size without extra-adrenal invasion. Stage II ACC was a tumor > 5 cm but still confined to the adrenal gland. Stage III ACC was defined by the presence of local extension, vascular invasion, or lymphatic spread. Stage IV ACC was confirmed by positive distant metastases (16). OS was estimated from the date of diagnosis to the date of death. Progression-free survival was evaluated from the date of diagnosis to the date of documented progressive or recurrent disease.
Statistical analysis
All statistical analyses were performed using SPSS version 25 (IBM Corporation, Armonk, NY, USA) to evaluate OS and progression-free survival according to the Kaplan-Meier method. Univariate Cox regression analysis was used to determine the association between each factor and OS and progression-free survival. Factors that were statistically significant (p < 0.05) were included in the multivariate Cox regression analysis to confirm independent prognostic influence. Quantitative statistics are expressed as mean and standard deviation (SD).
Results
Patient characteristics
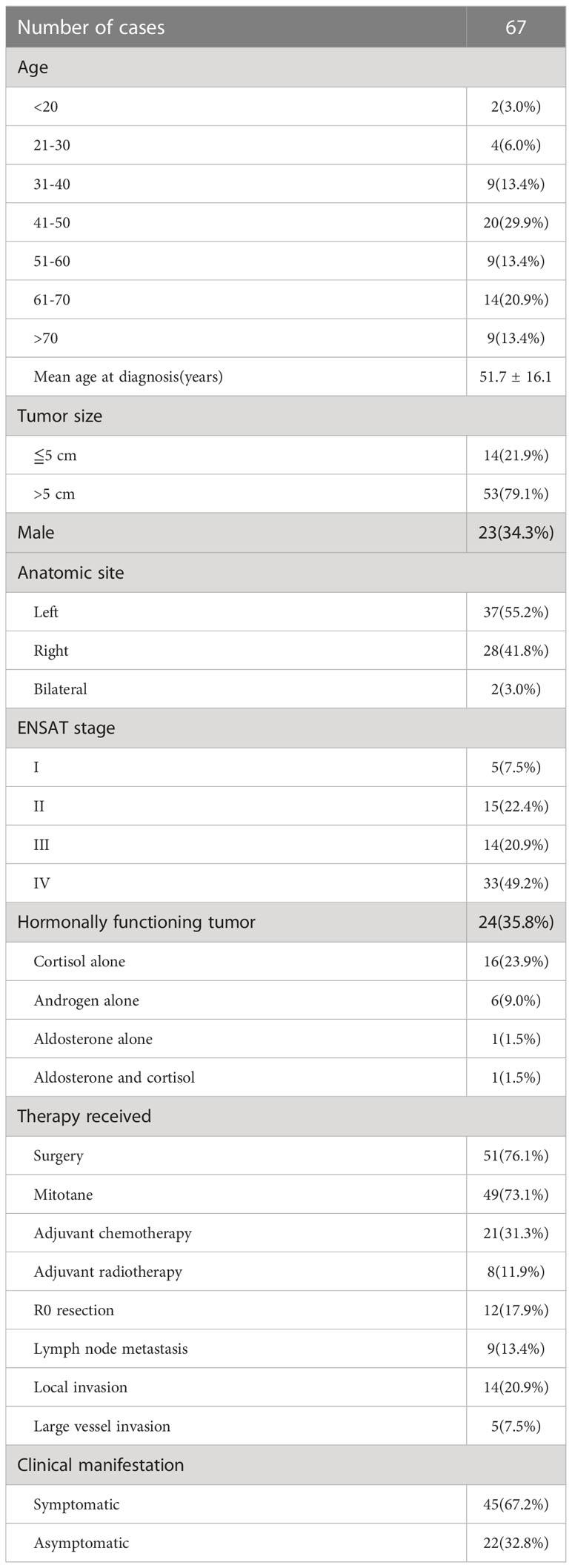
A total of 67 patients were included in the study. Demographic information and basic characteristics of the patients are summarized in Table 1. None of the patients had hereditary malignancy syndromes. The mean age at diagnosis was 51.7 ± 16.1 years (median age, 50 years). More than one-quarter of the patients were diagnosed in their 40s, and 44 (66.7%) patients were female. The mean age of the male patients was 58.3 years, while the mean age of the female patients was 48.3 years (p = 0.015). However, the mean age did not differ between the different ENSAT stages. Nearly one-half of the patients (n = 33/67 [49.2%]) had stage IV disease. Among these patients, twenty-five were diagnosed with late presentation, while the other 8 had missed diagnosis initially. Only 5 (7.5%) were diagnosed with stage I ACC. Fifteen (22.4%) patients had stage II ACC, and 14 (20.9%) were diagnosed with stage III ACC. Thirteen patients (19.4%) had incidentally found tumor without any clinical manifestation. Local invasion was observed in 14 (20.9%) patients. Lymphatic metastasis was observed in 9 (13.4%) patients. The most common locations of distant metastases were the liver and lungs. Nineteen patients had liver metastases and 12 had lung metastases. Five patients presented with large vessel thrombosis. Twenty-four (35.8%) patients had functional ACC. Among these patients, the age at diagnosis was significantly lower than that of those with non-functional cancer (mean, 46.8 versus 55.9 years; p = 0.020). Twenty-one (p = 0.001) patients were female. Seventeen patients exhibited hypercortisolism, 6 had elevated androgen levels, and 2 were screened positively with primary aldosteronism (PA) based on aldosterone-to-renin ratio. One patient exhibited elevated aldosterone and cortisol levels. Among patients with cortisol excess, sixteen presented with overt Cushing syndrome including Cushingoid appearance, weight gain, irregular menstrual cycles and recently worsening glycemic status. The other one had subclinical form of hypercortisolism. Those who had hyperandrogenism complained of amenorrhea and hirsutism.
Surgical results and post-surgical treatment
All the patients were involved in multidisciplinary team care including urologists, endocrinologists, oncologists, radiotherapists, pharmacists, nurses and nutrition specialists. Fifty-one (76.1%) patients underwent surgery, almost all of whom underwent surgical resection at Taipei Veterans General Hospital, except for 2, who underwent surgery at other hospitals. Pathologists at the Taipei Veterans General Hospital reviewed the pathological slides of these 2 patients. Most patients underwent open surgery (n = 39/51 [76.5%]). R0 resections were recorded in 12 patients, among whom 8 were diagnosed with local disease (stage I + II), and 4 had stage III ACC.
Mitotane was administered to 49 (73.1%) patients, with a median dose of 1.5 g/day. The average duration of mitotane therapy was 40.9 months. The side effects recorded in our cohort included nausea, vomiting, diarrhea, skin rash, dizziness, lethargy, anorexia, hypothyroidism, gynecomastia, impotence, gait disturbance, slow response, hand tremor and hypertension, most of which were relieved by symptomatic treatment and down-titration of mitotane. The incidence of mitotane-related adrenal insufficiency was 38.8% (19/49), with mean onset of 5.3 months. Steroid supplements were administered to these patients. Clinical examination of serum mitotane levels is not available in Taiwan. Twenty-one patients underwent adjuvant chemotherapy, eighteen of which received etoposide, doxorubicin and cisplatin (EDP) regimen. Two patients underwent therapy with cisplatin and etoposide due to poor cardiac function, while the other one received therapy with cisplatin, etoposide and bleomycin. Eight patients underwent post-surgical radiotherapy. Palliative care teams were available to provide relief of symptoms related to cancer or treatment.
Survival outcomes
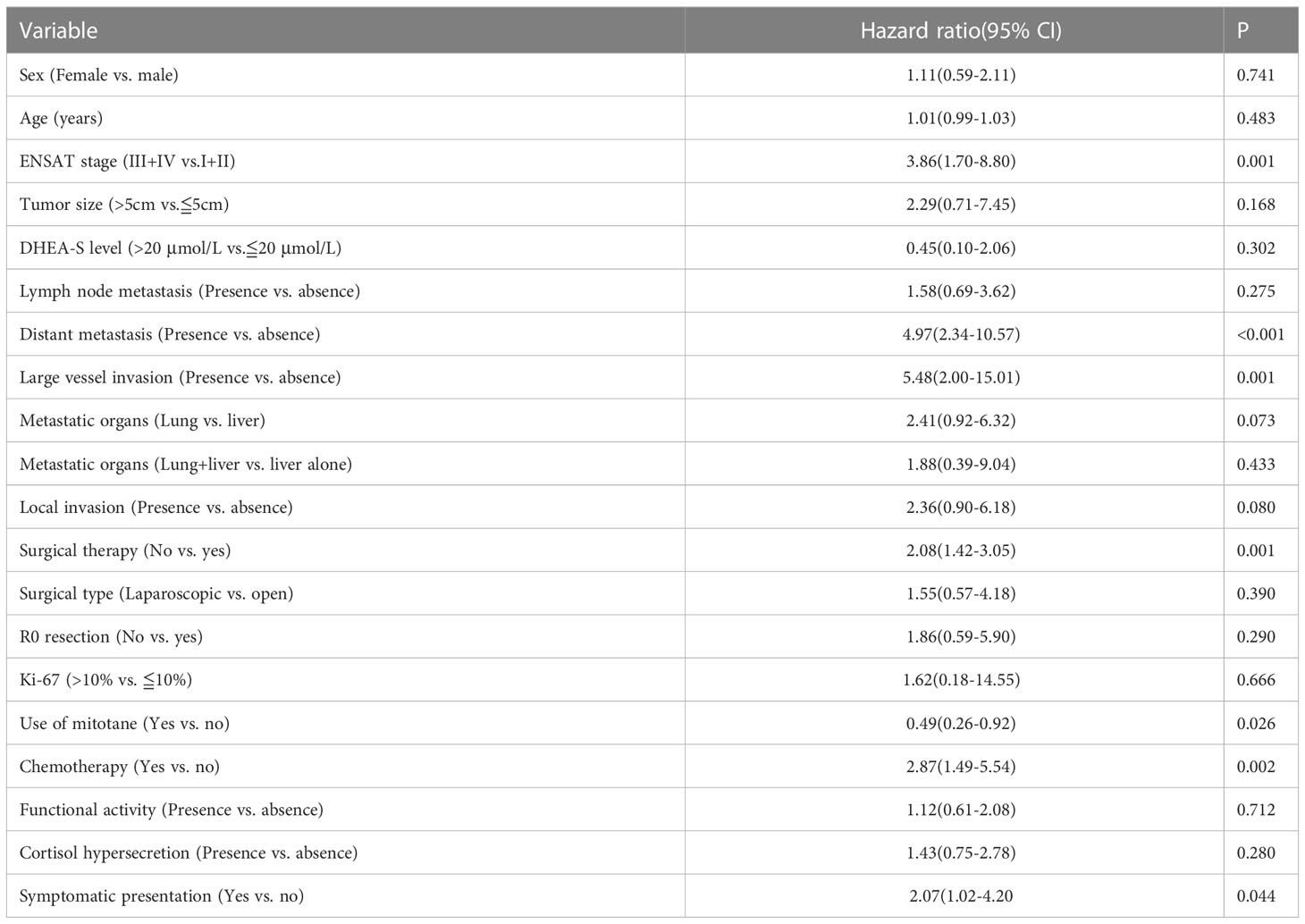
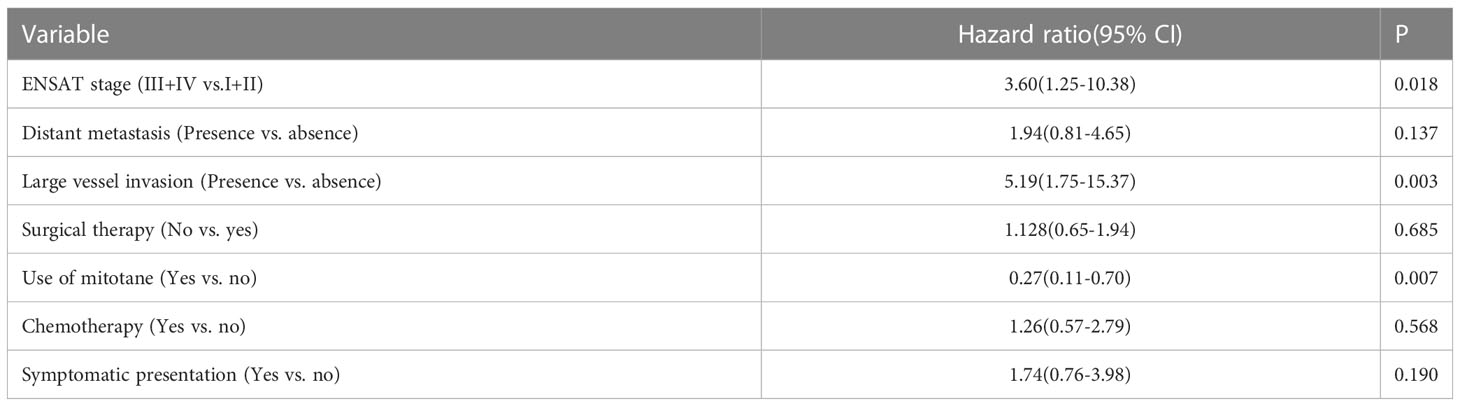
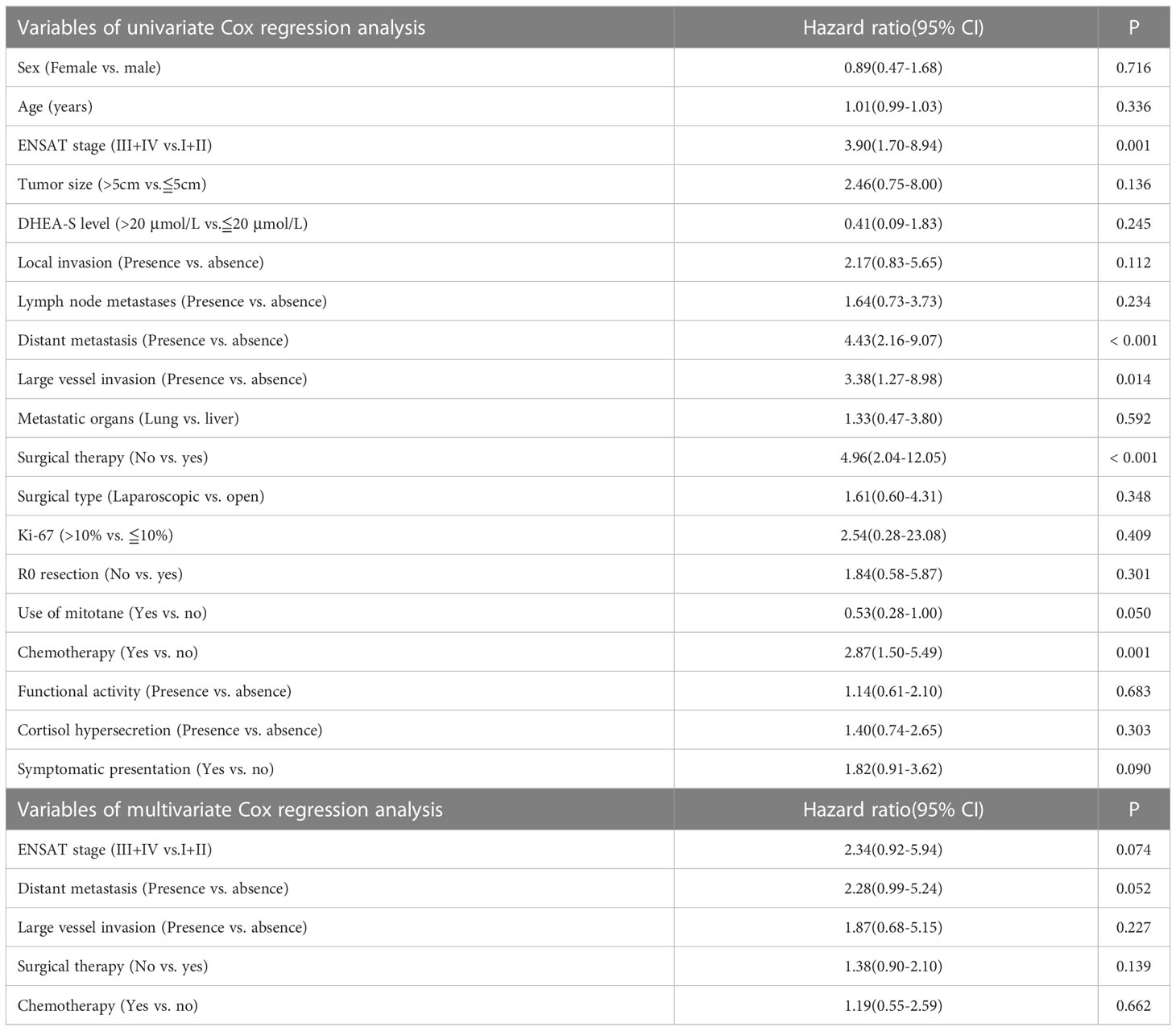
Univariate analysis revealed that ENSAT stage, distant metastases, large vessel invasion, symptomatic presentation, use of mitotane, chemotherapy, and surgical therapy were significantly associated with OS (Table 2 and Figure 1). Multivariate analysis (Table 3) revealed that ENSAT stage (hazard ratio [HR] 3.60 [95% confidence interval (CI) 1.25–10.38]), and great vessel invasion (HR 5.19 [95% CI 1.75-15.37]) were factors negatively and independently associated with OS. In contrast, administration of mitotane was associated with better outcomes (HR 0.27 [95% CI 0.11–0.70]). The mean OS was 160.8, 85.5, 81.8 and 20.3 months for stage I, II, III, and IV patients, respectively. The mean OS across all stages was 58.2 months. The 5-year OS percentage was 35.8%.
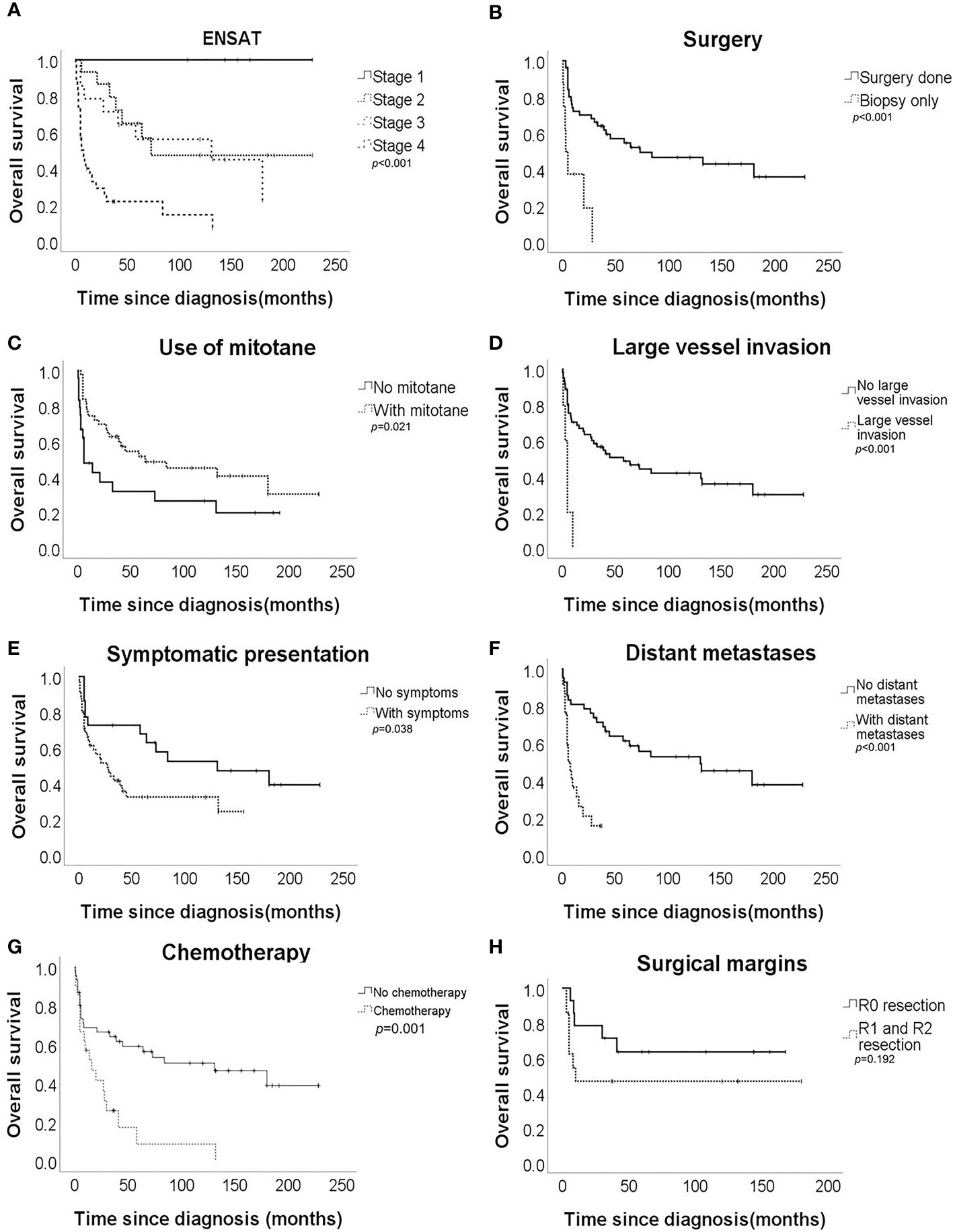
Figure 1 Overall survival of the adrenocortical carcinoma patients according to (A) European Network for the Study of Adrenal Tumours (ENSAT) stage, (B) surgical resection, (C) mitotane use, (D) large vessel invasion, (E) symptomatic presentation, (F) distant metastases, (G) chemotherapy, and (H) surgical margin.
In terms of progression-free survival, univariate analysis revealed that ENSAT stage, distant metastases, large vessel invasion, surgical therapy and chemotherapy had a statistically significant impact (Table 4). However, no single factor was associated with progression-free survival. The mean progression-free survival of the entire cohort was 39.0 months, while stage-specific progression-free survival was 160.8, 58.7, 52.6, and 5.8 months in patients with stage I, II, III, and IV disease, respectively. The 5-year progression-free survival was 25.4%.
Regarding survival among different ENSAT stages, there was still better OS for patients with stage III than for those with stage IV ACC (HR 0.30 [95% CI 0.13–0.70]). On the other hand, there was no difference in OS between those with stage II and stage III disease (HR 1.27 [95% CI 0.46–3.52]) or even between stage I+II and stage III ACC (HR 1.89 [95% CI 0.69–5.23]). Furthermore, there was no difference in progression-free survival between those with stage II and stage III ACC (HR 1.22 [95% CI 0.44–3.36]).
Regarding the effects of mitotane on clinical outcomes, the difference in OS was most apparent in patients with stage IV disease. The mean OS of patients with stage IV disease who underwent mitotane therapy (n = 18/33 [54.6%]) was 33.5 months, while those who did not receive mitotane (n = 15/33 [45.4%]) only survived 4.4 months on average (p = 0.010). Furthermore, mitotane therapy had a borderline beneficial effect on progression-free survival (HR 0.53 [95% CI 0.28–1.00]). Subgroup analysis revealed that the use of mitotane also resulted in better progression-free survival of patients with stage IV ACC (HR 0.39 [95% CI 0.17–0.91]). Moreover, the functional status of tumor did not affect the association between mitotane therapy and improved OS (HR 0.74 [95% CI 0.25-2.22]) and progression-free survival (HR 0.42 [95% CI 0.14-1.36]). However, mitotane administration to patients with stage I-III disease did not result in better survival.
Discussion
Results of our study provide insights into the prognostic predictors of ACC in a sample of ethnic Taiwanese population. The median overall survival of our cohort was similar to the literature which ranged from 15-44% (3). ENSAT stage had the greatest impact on OS. Significant differences in clinical outcomes were more pronounced in those with more advanced stages of disease. No difference in survival was observed between patients with stage II and III ACC. There have been inconsistent results regarding survival between stage II and stage III disease. Luton et al. confirmed that regional disease did not influence survival (6), whereas Icard et al. reported worse outcomes in patients with stage III ACC than in those with stage I+II ACC (17). Surgery remains the sole cure for ACC. Patients with stage I or II ACC have the best chance of complete tumor removal due to localized disease. However, tumors with invasion to adjacent tissues or lymph nodes can still be completely removed by experienced surgeons through meticulous resection (14). Open en bloc surgery was performed in most patients in our study. The open procedure is recommended by current practice guidelines owing to more extensive clearance of tumors and comprehensive sampling of lymph nodes (18). Lymphadenectomy performed during surgery has also been reported to improve survival (19). Moreover, centers with expertise in ACC have a more positive influence on clinical outcomes (20, 21). Care by multidisciplinary team evaluation was adopted in our cohort. Multidisciplinary care has been shown to improve overall and progression-free survival in patients with stage III-IV ACC (22). Similar survival benefits were also demonstrated in tumor stage I-III in a dutch study which revealed surgery performed outside the hospitals integrating care from multiple expertise was associated with increased risks of death (HR 1.96 [95% CI 1.01–3.81], p=0.047) (20). These facts may explain the good prognosis observed in patients with stage III ACC in our series. Additionally, patients with stage IV ACC are susceptible to disease progression. This may explain why patients with stage IV disease experience worse OS than those with stage III ACC. Nevertheless, the number of patients with local or regional ACC in our cohort was small. Given the various surgical methods, there was no difference in OS between patients who underwent open surgery and laparoscopic surgery in our study. A meta-analysis including 9 retrospective case-control studies revealed similar cancer-specific mortality rates between laparoscopic adrenalectomy, adopted mostly for patients with smaller tumors, and open adrenalectomy. However, increased peritoneal metastasis is more often reported in patients who underwent laparoscopic surgery (23). No prospective randomized trial has directly compared these two surgical methods.
Our cohort exhibited obvious survival benefits with mitotane administration among those with stage IV ACC. Mitotane is a derivative of dichlorodiphenyltrichloroethane (DDT) (14). Its active metabolites induce adrenal cell necrosis by blocking mitochondrial respiratory chain complexes I and IV, and disrupting the mitochondrial membrane (24). Mitotane also inhibits sterol-O-acyl transferase 1 (SOAT1), which contributes to the accumulation of free fatty acids and cholesterol. These redundant materials cause stress in the endoreticulum and activate the intrinsic apoptotic pathway (24). Initially, mitotane was administered to patients with advanced ACC, with a response rate ranging from 5–49% (25). These observations established the rationale for adjuvant mitotane therapy in patients at high risk for recurrent or metastatic disease. However, in these studies, mitotane was usually combined with chemotherapy. It is difficult to determine whether the therapeutic benefits result from mitotane alone. In a meta-analysis including 6 retrospective studies, mitotane therapy resulted in improved mortality but not in decreased recurrence (18). Treatment options based on predicted prognosis may have confounding effects in these studies, which can be hardly corrected. Although current guidelines recommend adjuvant mitotane therapy for patients with stage III-IV ACC or any stage with a Ki-67 index >10%, who do not have macroscopic residual disease after surgery (18), this recommendation is based on low-quality evidence and expert opinions. Moreover, the randomized ADIUVO trial reported no additional benefits of adjuvant mitotane in recurrence-free survival and OS among patients with stage I-III ACC, R0 surgery, and Ki-67 ≤10% compared with observation-alone group, which was similar to our finding that mitotane did not add survival benefits in stage I-III disease (26). Well-designed prospective randomized trials are still needed to confirm the efficacy of mitotane in more advanced ACC. On the other hand, higher concentration of mitotane above 14 mg/L had independently positive impact on survival if mitotane is administered (27). The time needed to reach this target range was also significantly associated with disease recurrence. Furthermore, longer period in target concentration during maintenance phase of mitotane therapy resulted in reduced recurrence (HR 0.93 [95% CI 0.88–0.98], p<0.01) (28). Last but not least, patients with advanced disease and use of mitotane often had multiple physical and mental illness. Early introduction of palliative care along with standard treatment improved quality of life, survival and psychological burden of both patients and caregivers (29).
Moreover, our study revealed a negative association between large-vessel invasion and OS, which is seldom discussed with regard to prognostic prediction in most studies. In our series, these patients usually had late-stage disease and positive surgical margins. The mean OS was only 4.8 months. Turbendian et al. reported a decrease in 3-year OS from 93% to 29% and in 3-year recurrence-free survival from 67% to 15% in patients with large-vessel invasion (30). An association between adrenal hormone hypersecretion and symptomatic clinical manifestations with large-vessel thrombosis was also found in that study. Although venous thrombosis can be removed using sophisticated techniques, Chiche et al. found that perioperative mortality in patients with inferior vena cava invasion was approximately 10–15%. The long-term survival of these patients was poor owing to the association with metastases and lag in correct diagnosis (31). However, the association between vascular invasion and survival may be confounded by advanced stage and multiple distant metastases.
Less patients presented with hormonal excess (35.8%) in our cohort compared with most series in which 45-70% of patients had active hormone production (2). The majority of patients (49.2%) in our study were diagnosed with stage IV disease, which was still true for patients diagnosed before and after 2010. However, with increased availability of advanced imaging techniques, more and more patients were diagnosed with stage II ACC recently (32). Regarding other prognostic factors, our cohort did not reveal significant association of hypercortisolism, advanced pathologic features or status of surgical margin with overall survival.
Our study had some limitations, among which included its single-center, retrospective design and inherent selection bias; moreover, the size of the cohort was relatively small. Referral bias may have also occurred because adrenocortical carcinoma is rare. The clinical presentations may have been altered by previous failed treatments at other hospitals. Furthermore, details including Eastern Cooperative Oncology Group (ECOG) performance status of the patients and mitotic index could not be traced due to missing records or incomplete documents. Assay for detection of mitotane level was also not available. The confirmation tests of PA were not performed at the diagnosis of ACC. All cases were only screened positively with PA based on aldosterone-to-renin ratio. However, the strengths of our study are that it analyzed prognostic predictors of patients with ACC in a sample of the ethnic Taiwanese population and that baseline characteristics were similar to those of the global population. A multicenter prospective cohort study, with complete records of patient characteristics and tumor grades, is needed to better stratify the prognostic factors of ACC in Taiwan.
Conclusion
Advanced ENSAT stage and large vessel invasion were negatively associated with OS, whereas the use of mitotane improved clinical outcomes. However, no single factor had an independent influence on progression-free survival.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: Paper dataset. Requests to access these datasets should be directed to Liang-Yu Lin,dHJpc3RhbjA3NEBnbWFpbC5jb20=.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional review board, Taipei Veterans General Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
L-HP, and L-YL were the main conductors of this study and contributed to the study conception and design, implementation, statistical analysis, interpretation, the preparation and finalization of the manuscript. C-CY contributed to the study conception and design, implementation, and the preparation of the manuscript. C-JH and X-NN contributed to the study conception and design, and data collection. All authors contributed to the article and approved the submitted version.
Funding
This work was supported in part by research grants No. V108C-197, V109C-179, V110C-198, V111D62-002-MY3-1, V112C-183 and V111D62-002-MY3-2 to L-YL from Taipei Veterans General Hospital, Taipei, Taiwan and MOST 111-2314-B-075-040-MY2 to L-YL from National Science and Technology Council, Taiwan.
Acknowledgments
We are grateful to all the members for their contribution to the study. We also thank the Clinical Research Core Laboratory and Medical Sciences & Technology Building of Taipei Veterans General Hospital for providing us with an experimental space and facilities.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bilimoria KY, Shen WT, Elaraj D, Bentrem DJ, Winchester DJ, Kebebew E, et al. Adrenocortical carcinoma in the united states: treatment utilization and prognostic factors. Cancer (2008) 113:3130–6. doi: 10.1002/cncr.23886
2. Else T, Kim AC, Sabolch A, Raymond VM, Kandathil A, Caoili EM, et al. Adrenocortical carcinoma. Endocr Rev (2014) 35:282–326. doi: 10.1210/er.2013-1029
3. Ayala-Ramirez M, Jasim S, Feng L, Ejaz S, Deniz F, Busaidy N, et al. Adrenocortical carcinoma: Clinical outcomes and prognosis of 330 patients at a tertiary care center. Eur J Endocrinol (2013) 169:891–9. doi: 10.1530/EJE-13-0519
4. Hermsen IG, Gelderblom H, Kievit J, Romijn JA, Haak HR. Extremely long survival in six patients despite recurrent and metastatic adrenal carcinoma. Eur J Endocrinol (2008) 158:911–9. doi: 10.1530/EJE-07-0723
5. Clay MR, Pinto EM, Fishbein L, Else T, Kiseljak-Vassiliades K. Pathological and genetic stratification for management of adrenocortical carcinoma. J Clin Endocrinol Metab (2022) 107:1159–69. doi: 10.1210/clinem/dgab866
6. Luton JP, Cerdas S, Billaud L, Thomas G, Guilhaume B, Bertagna X, et al. Clinical features of adrenocortical carcinoma, prognostic factors, and the effect of mitotane therapy. N Engl J Med (1990) 322:1195–201. doi: 10.1056/NEJM199004263221705
7. Tella SH, Kommalapati A, Yaturu S, Kebebew E. Predictors of survival in adrenocortical carcinoma: An analysis from the national cancer database. J Clin Endocrinol Metab (2018) 103:3566–73. doi: 10.1210/jc.2018-00918
8. Li Y, Bian X, Ouyang J, Wei S, He M, Luo Z. Nomograms to predict overall survival and cancer-specific survival in patients with adrenocortical carcinoma. Cancer Manag Res (2018) 10:6949–59. doi: 10.2147/CMAR.S187169
9. Kim Y, Margonis GA, Prescott JD, Tran TB, Postlewait LM, Maithel SK, et al. Nomograms to predict recurrence-free and overall survival after curative resection of adrenocortical carcinoma. JAMA Surg (2016) 151:365–73. doi: 10.1001/jamasurg.2015.4516
10. Loncar Z, Djukic V, Zivaljevic V, Pekmezovic T, Diklic A, Tatic S, et al. Survival and prognostic factors for adrenocortical carcinoma: A single institution experience. BMC Urol (2015) 15:43. doi: 10.1186/s12894-015-0038-1
11. Abiven G, Coste J, Groussin L, Anract P, Tissier F, Legmann P, et al. Clinical and biological features in the prognosis of adrenocortical cancer: Poor outcome of cortisol-secreting tumors in a series of 202 consecutive patients. J Clin Endocrinol Metab (2006) 91:2650–5. doi: 10.1210/jc.2005-2730
12. Berruti A, Fassnacht M, Haak H, Else T, Baudin E, Sperone P, et al. Prognostic role of overt hypercortisolism in completely operated patients with adrenocortical cancer. Eur Urol (2014) 65:832–8. doi: 10.1016/j.eururo.2013.11.006
13. Dong D, Li H, Yan W, Ji Z, Mao Q. Surgical management and clinical prognosis of adrenocortical carcinoma. Urol Int (2012) 88:400–4. doi: 10.1159/000336134
14. Vaidya A, Nehs M, Kilbridge K. Treatment of adrenocortical carcinoma. Surg Pathol Clin (2019) 12:997–1006. doi: 10.1016/j.path.2019.08.010
15. Lughezzani G, Sun M, Perrotte P, Jeldres C, Alasker A, Isbarn H, et al. The European network for the study of adrenal tumors staging system is prognostically superior to the international union against cancer-staging system: A north American validation. Eur J Cancer (2010) 46:713–9. doi: 10.1016/j.ejca.2009.12.007
16. Fassnacht M, Johanssen S, Quinkler M, Bucsky P, Willenberg HS, Beuschlein F, et al. Limited prognostic value of the 2004 international union against cancer staging classification for adrenocortical carcinoma: Proposal for a revised TNM classification. Cancer (2009) 115:243–50. doi: 10.1002/cncr.24030
17. Icard P, Goudet P, Charpenay C, Andreassian B, Carnaille B, Chapuis Y, et al. Adrenocortical carcinomas: Surgical trends and results of a 253-patient series from the French association of endocrine surgeons study group. World J Surg (2001) 25:891–7. doi: 10.1007/s00268-001-0047-y
18. Fassnacht M, Dekkers OM, Else T, Baudin E, Berruti A, de Krijger R, et al. European Society of endocrinology clinical practice guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European network for the study of adrenal tumors. Eur J Endocrinol (2018) 179:G1–g46. doi: 10.1530/EJE-18-0608
19. Gerry JM, Tran TB, Postlewait LM, Maithel SK, Prescott JD, Wang TS, et al. Lymphadenectomy for adrenocortical carcinoma: Is there a therapeutic benefit? Ann Surg Oncol (2016) 23:708–13. doi: 10.1245/s10434-016-5536-1
20. Kerkhofs TM, Verhoeven RH, Bonjer HJ, van Dijkum EJ, Vriens MR, De Vries J, et al. Surgery for adrenocortical carcinoma in the Netherlands: Analysis of the national cancer registry data. Eur J Endocrinol (2013) 169:83–9. doi: 10.1530/EJE-13-0142
21. Hermsen IG, Kerkhofs TM, den Butter G, Kievit J, van Eijck CH, Nieveen van Dijkum EJ, et al. Surgery in adrenocortical carcinoma: Importance of national cooperation and centralized surgery. Surgery (2012) 152:50–6. doi: 10.1016/j.surg.2012.02.005
22. Tizianel I, Caccese M, Torresan F, Lombardi G, Evangelista L, Crimì F, et al. The overall survival and progression-free survival in patients with advanced adrenocortical cancer is increased after the multidisciplinary team evaluation. Cancers (Basel) (2022) 14(16):3904. doi: 10.3390/cancers14163904
23. Autorino R, Bove P, De Sio M, Miano R, Micali S, Cindolo L, et al. Open versus laparoscopic adrenalectomy for adrenocortical carcinoma: A meta-analysis of surgical and oncological outcomes. Ann Surg Oncol (2016) 23:1195–202. doi: 10.1245/s10434-015-4900-x
24. Corso CR, Acco A, Bach C, Bonatto SJR, de Figueiredo BC, de Souza LM. Pharmacological profile and effects of mitotane in adrenocortical carcinoma. Br J Clin Pharmacol (2021) 87:2698–710. doi: 10.1111/bcp.14721
25. Postlewait LM, Ethun CG, Tran TB, Prescott JD, Pawlik TM, Wang TS, et al. Outcomes of adjuvant mitotane after resection of adrenocortical carcinoma: A 13-institution study by the US adrenocortical carcinoma group. J Am Coll Surg (2016) 222:480–90. doi: 10.1016/j.jamcollsurg.2015.12.013
26. Terzolo M, Fassnacht M, Perotti P, Libe R, Lacroix A, Kastelan D, et al. Results of the ADIUVO study, the first randomized trial on adjuvant mitotane in adrenocortical carcinoma patients. J Endocrine Soc (2021) 5:A166–7. doi: 10.1210/jendso/bvab048.336
27. Haak HR, Hermans J, van de Velde CJ, Lentjes EGWM, Goslings BM, Fleuren G-J, et al. Optimal treatment of adrenocortical carcinoma with mitotane: Results in a consecutive series of 96 patients. Br J Cancer (1994) 69:947–51. doi: 10.1038/bjc.1994.183
28. Puglisi S, Calabrese A, Basile V, Ceccato F, Scaroni C, Simeoli C, et al. Mitotane concentrations influence the risk of recurrence in adrenocortical carcinoma patients on adjuvant treatment. J Clin Med (2019) 8:1850. doi: 10.3390/jcm8111850
29. Ruggiero E, Tizianel I, Caccese M, Lombardi G, Pambuku A, Zagonel V, et al. Advanced adrenocortical carcinoma: From symptoms control to palliative care. Cancers (Basel) (2022) 14:5901. doi: 10.3390/cancers14235901
30. Turbendian HK, Strong VE, Hsu M, Ghossein RA, Fahey TJ 3rd. Adrenocortical carcinoma: The influence of large vessel extension. Surgery (2010) 148:1057–64. doi: 10.1016/j.surg.2010.09.024
31. Chiche L, Dousset B, Kieffer E, Chapuis Y. Adrenocortical carcinoma extending into the inferior vena cava: Presentation of a 15-patient series and review of the literature. Surgery (2006) 139:15–27. doi: 10.1016/j.surg.2005.05.014
Keywords: adrenocortical carcinoma, mitotane, overall survival (OS), progression-free survival (PFS), adrenal carcinomas
Citation: Pan L-H, Yen C-C, Huang C-J, Ng X-N and Lin L-Y (2023) Prognostic predictors of adrenocortical carcinoma: A single-center thirty-year experience. Front. Endocrinol. 14:1134643. doi: 10.3389/fendo.2023.1134643
Received: 30 December 2022; Accepted: 27 February 2023;
Published: 10 March 2023.
Edited by:
Amir Hekmat Hamrahian, Johns Hopkins Medicine, United StatesReviewed by:
Filippo Ceccato, University of Padua, ItalySina Jasim, Washington University in St. Louis, United States
Copyright © 2023 Pan, Yen, Huang, Ng and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liang-Yu Lin, bGlubHlAdmdodHBlLmdvdi50dw==; dHJpc3RhbjA3NEBnbWFpbC5jb20=
 Li-Hsin Pan
Li-Hsin Pan Chueh-Chuan Yen
Chueh-Chuan Yen Chun-Jui Huang
Chun-Jui Huang Xin-Ning Ng
Xin-Ning Ng Liang-Yu Lin
Liang-Yu Lin