- 1Department of Endocrinology and Metabolism, Hospital of Chengdu Office of People’s Government of Tibetan Autonomous Region (Hospital.C.T.), Chengdu, China
- 2Department of Orthopedics, Hospital of Chengdu Office of People’s Government of Tibetan Autonomous Region (Hospital.C.T.), Chengdu, China
Objective: A large body of literature has demonstrated the significant efficacy of antibiotic bone cement in treating infected diabetic foot wounds, but there is less corresponding evidence-based medical evidence. Therefore, this article provides a meta-analysis of the effectiveness of antibiotic bone cement in treating infected diabetic foot wounds to provide a reference basis for clinical treatment.
Methods: PubMed, Embase, Cochrane library, Scoup, China Knowledge Network (CNKI), Wanfang database, and the ClinicalTrials.gov were searched, and the search time was from the establishment of the database to October 2022, and two investigators independently. Two investigators independently screened eligible studies, evaluated the quality of the literature using the Cochrane Evaluation Manual, and performed statistical analysis of the data using RevMan 5.3 software.
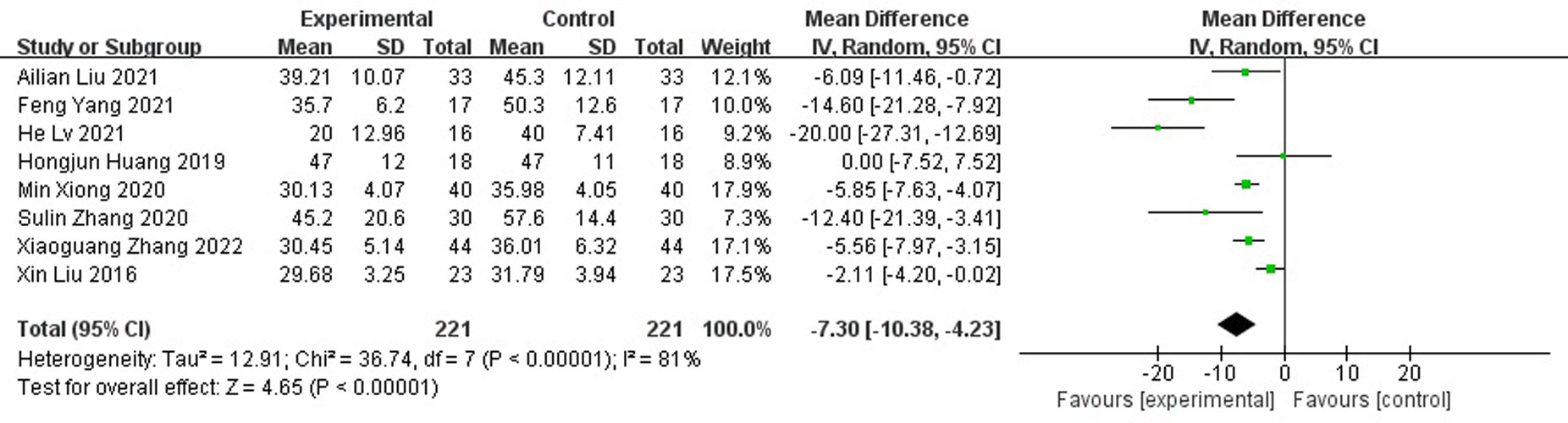
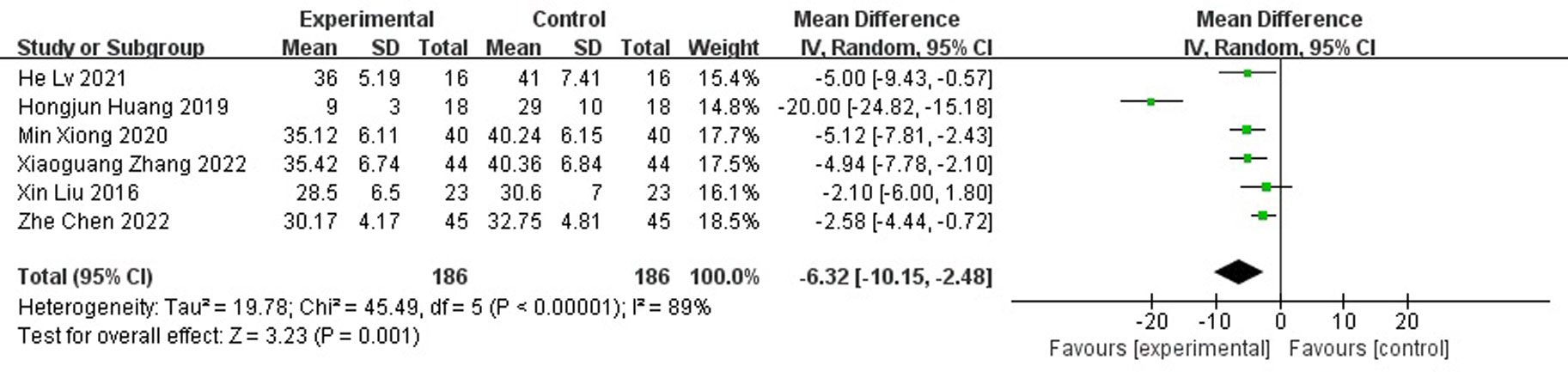
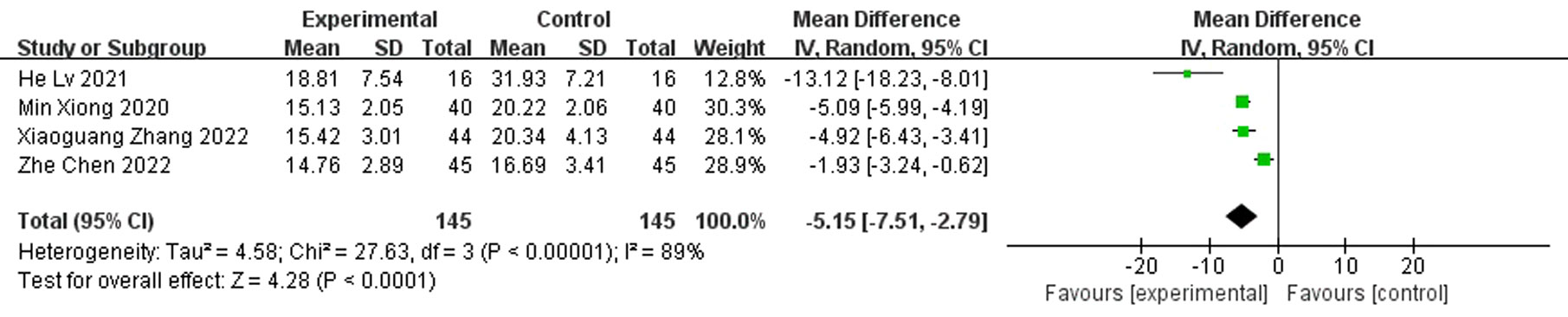
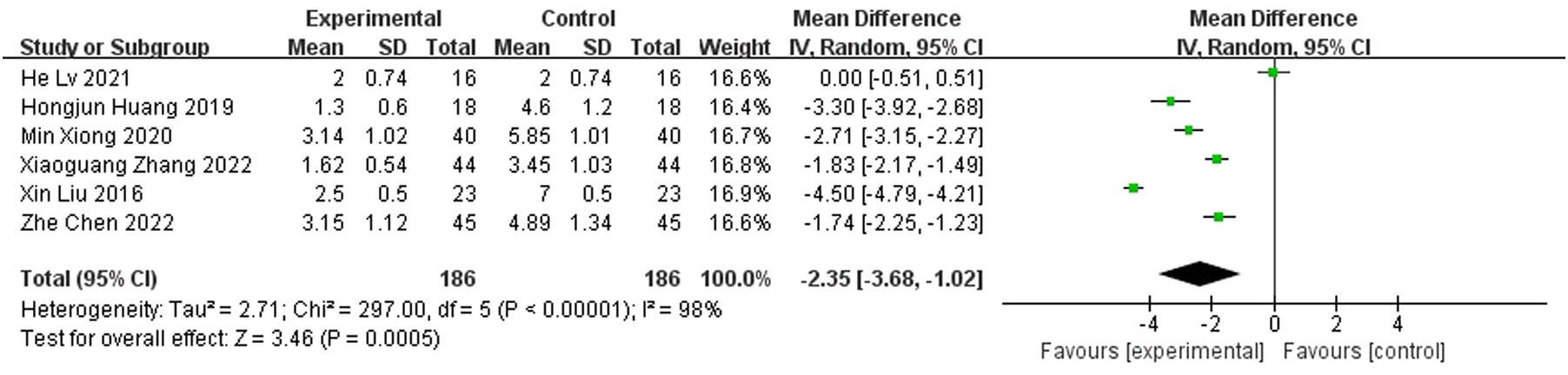
Results: A total of nine randomized controlled studies (n=532) were included and, compared with the control group, antibiotic bone cement treatment reduced the time to wound healing (MD=-7.30 95% CI [-10.38, -4.23]), length of hospital stay (MD=-6.32, 95% CI [-10.15, -2.48]), time to bacterial conversion of the wound (MD=-5.15, 95% CI [-7.15,-2.19]), and the number of procedures (MD=-2.35, 95% CI [-3.68, -1.02]).
Conclusion: Antibiotic bone cement has significant advantages over traditional treatment of diabetic foot wound infection and is worthy of clinical promotion and application.
Systematic review registration: PROSPERO identifier, CDR 362293.
Introduction
With the continuous improvement of socioeconomic and living standards, the global prevalence of diabetes is increasing rapidly and is expected to increase to 7.7% globally in 2030 (1). Diabetic foot infection (DFI) is one of the common complications of diabetic foot. The prevalence rate of diabetic foot is up to 25% (2, 3), of which the mortality rate is up to 12%. More than half of amputees are expected to die within 5 years. The mortality rate is higher than that of most cancers, posing a serious threat to patients’ health (4).
Current clinical practice guidelines on the treatment of DFIs guide essentially the same pattern of negative pressure closed drainage therapy, debridement and dressing changes, hematologic reconstruction, wound dressing, and education of patients and families (5–7). However, DFI is currently difficult to treat and the wound takes a long time to heal, often leading to extended hospital stays and increased hospital costs, adding to the patient’s burden.
As a unique bone repair material, antibiotic bone cement can release high concentrations of antibiotics locally to lower the risk of systemic toxicity and accomplish the goal of preventing and treating infection. According to studies, the use of antimicrobial bone cement in the treatment of DFI wounds has also yielded positive therapeutic outcomes. However, there is still no relevant evidence-based medical evidence to confirm this, so our article systematically evaluates the clinical efficacy of antibiotic bone cement in the treatment of diabetic foot to provide a reference basis for the future clinical treatment of diabetic foot.
Methods
Search strategy and selection criteria
This systematic review and meta-analysis are reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (8) Statement and was registered in the International Prospective Register of Systematic Reviews (number CDR 362293).
The Embase, PubMed, Cochrane, Scoup, CNKI, CBM, and ClinicalTrials.gov were searched for relevant studies published between the database inception date and 11 October 2022. We applied no language restrictions. We used the following combined text and MeSH terms: “Diabetic Foot.” The complete search used for PubMed was: (“Diabetic Foot”[Mesh]) OR ((((Foot, Diabetic[Title/Abstract]) OR (Diabetic Feet[Title/Abstract])) OR (Feet, Diabetic[Title/Abstract])) OR (Foot Ulcer, Diabetic[Title/Abstract])). We considered all potentially eligible studies for review, irrespective of the primary outcome or language. We also conducted a manual search using the reference lists of key articles published in English (Supplementary materials).
Study selection and data extraction
Inclusion criteria
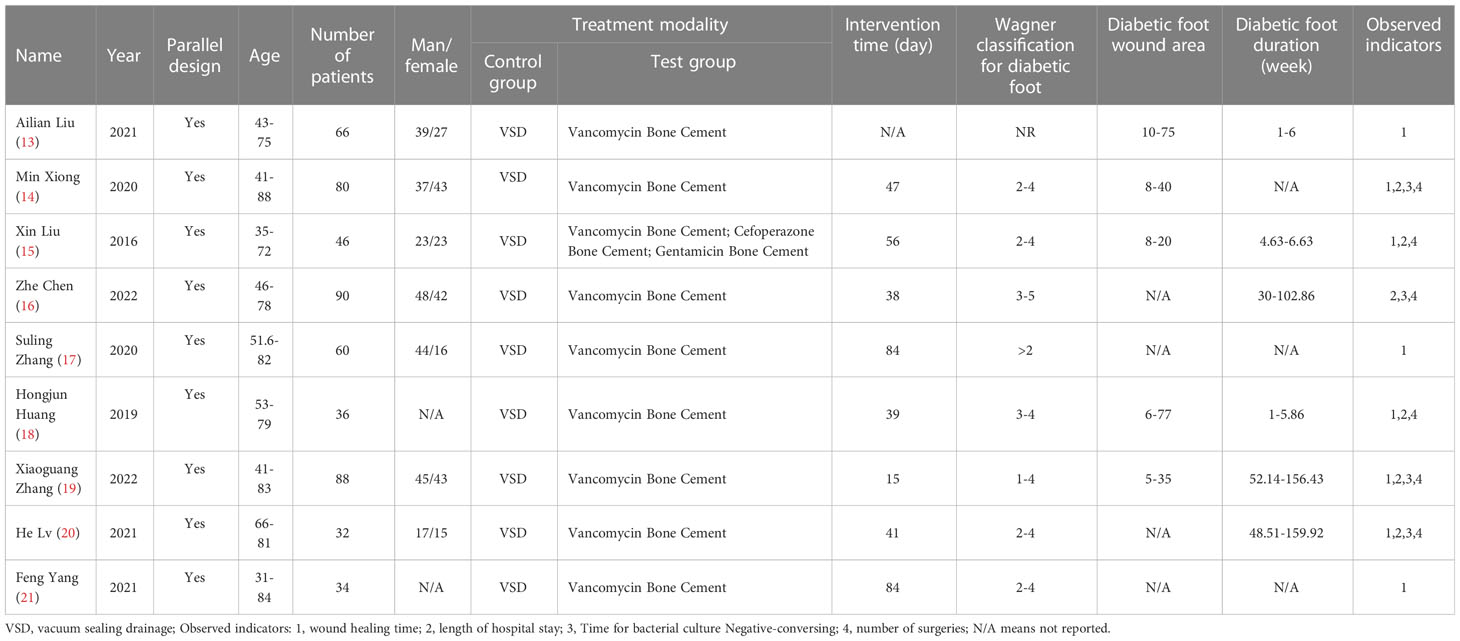
(1) meeting the management of diabetic foot: a clinical practice guideline by the Society for Vascular Surgery in collaboration with the American Podiatric Medical Association and the Society for Vascular Medicine (9); (2) meeting the criteria for the diagnosis and treatment of diabetic foot infection published by the American Diabetes Association (ADA) infection (10); (3) the study is a randomized clinical trial.
Exclusion criteria
(1) Basic studies; (2) Conference reports and reviews; (3) Repeatedly published literature; (4) Literature with incomplete data; (5) Patients with non-diabetic foot infections; combined hematologic disorders other than anemia; and mental disorders were included in the literature.
Screening and selection of literature
Two independent investigators (DTT, HJ) reviewed study titles and abstracts, and studies that satisfied the inclusion criteria were retrieved for full-text assessment. Trials selected for detailed analysis and data extraction were analyzed by two investigators (DTT and HJ); disagreements were resolved by a third investigator (SZM).
We extracted the following data from each selected study: total number of participants, age, sex, trial duration, and treatment modality. Two independent reviewers (DTT, HJ) assessed the risk of bias according to the PRISMA recommendations.
Outcome indicators
The main outcome indicators were wound healing time, length of hospital stay, time for bacterial culture Negative-conversing, and number of surgeries.
Patient and public involvement
Patients and/or the public were not involved in the design, conduct, reporting, or dissemination plan of this study.
Quality evaluation of included studies
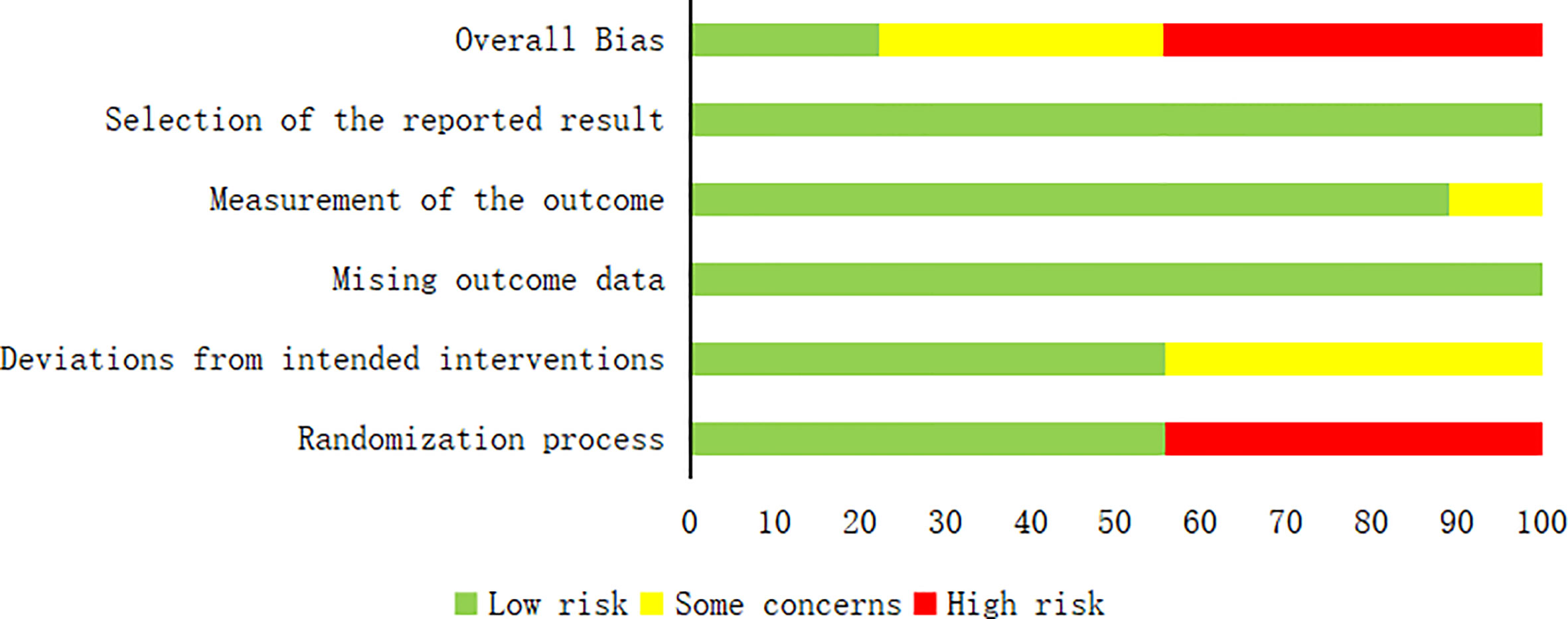
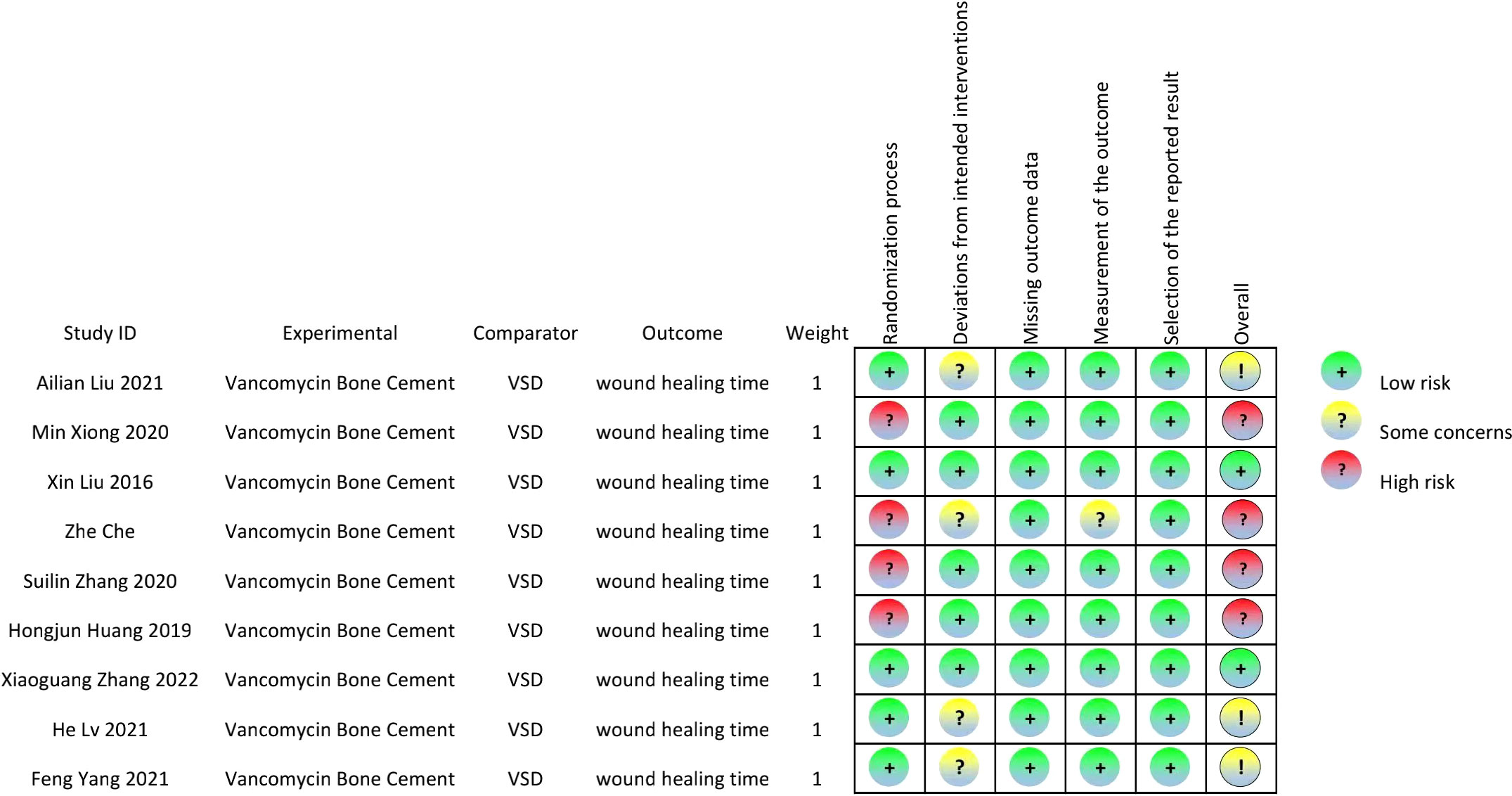
The quality of the included articles was assessed by the Cochrane RoB 2.0 tool (11, 12), which included the following: (1) risk of bias arising from the randomization process; (2) risk of bias due to deviations from the intended interventions (effect of assignment to intervention); (3) missing outcome data; (4) risk of bias in the measurement of the outcome; and (5) risk of bias in the selection of the reported result. Each study was assessed as having a “low risk of bias,” “some concerns,” or “high risk of bias” according to the probability of bias.
Data synthesis
All data processing and statistical analyses were performed using Review Manager (RevMan) software version 5.3 (http://www.ims.cochrane.org/revman/). Heterogeneity between studies was evaluated by the Q statistic and the I (2) statistic. When I (2) < 50%, p > 0.05, indicating little or no heterogeneity, a fixed effects model was used; when I (2) > 50%, p < 0.05, indicating significant heterogeneity, a random effects model was used. Subgroup analysis was also conducted, with a sample size cut-off of n=50, to compare the combined effect results of small and large sample subgroups and to explore sources of heterogeneity. Publication bias was assessed using funnel plots, sensitivity analyses were conducted using the sequential omission of a single study from the total studies and evaluating the influence of each study on the pooled effect estimates, and subgroup analyses were conducted based on the general characteristics of the study population and sample size.
Results
Screening and selection of literature
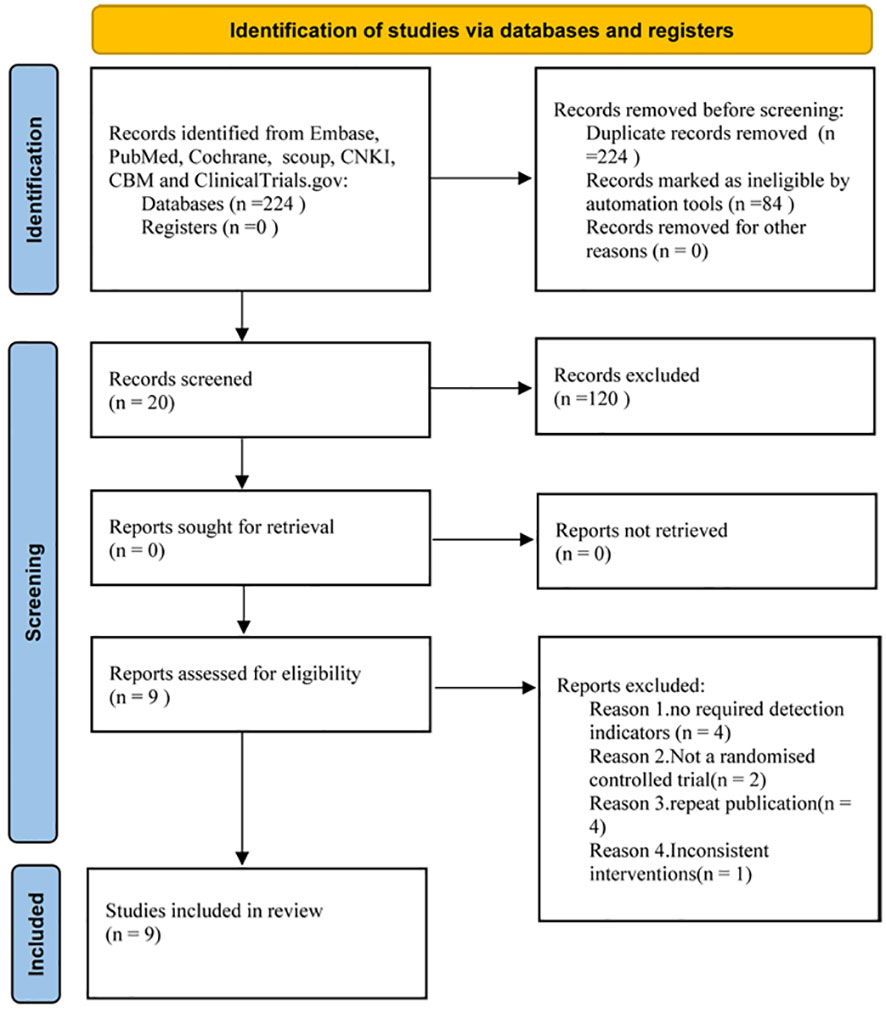
We identified 224 studies, downloaded the full texts to read, and excluded 11, of which, nine (with data for 532 participants) were included in our analysis (Figure 1), all of which were randomized controlled studies. The nine trials (13–21)were all published between 2016 and 2022.
Design characteristics
A total of 532 patients with diabetic foot were included in nine randomized controlled trials (13–21), with 266 patients in the intervention group and 266 in the control group. The subjects included in the study were aged 31-88 years. All had diabetic foot with a Wagner classification of 1-5. In the intervention group, cefoperazone bone cement was used in 13 patients, gentamicin bone cement in six patients, and vancomycin bone cement in 27 patients in the study by Xin Liu et al.[13]. The remaining eight studies were treated with vancomycin bone cement and the control group was treated with VSD. The baseline characteristics of the study participants and design characteristics are presented in Table 1. The quality of the literature is assessed in Figures 2, 3.
Meta-analysis results
Wound healing time
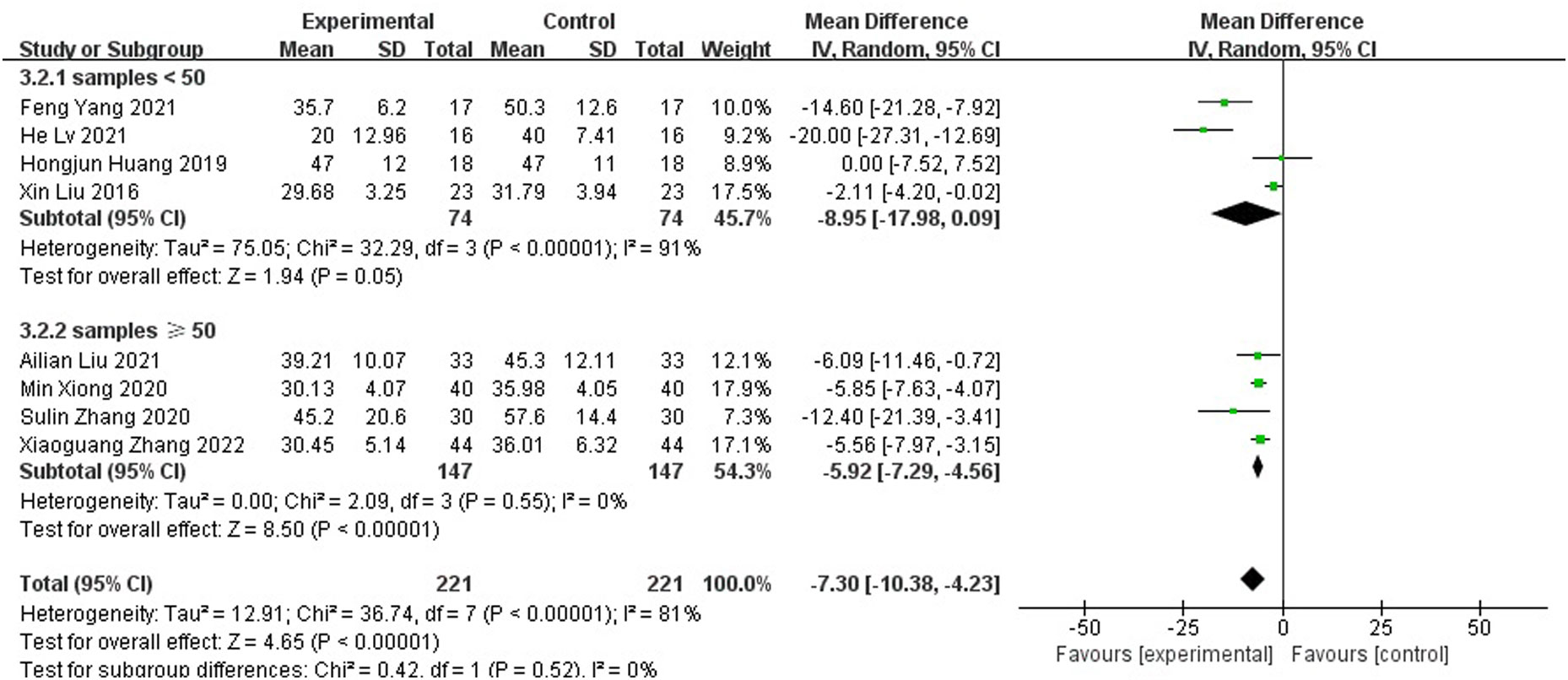
Compared with those of the control group, wound healing time was shortened (MD=-7.30 95% CI[-10.38, -4.23]), As the meta-analysis showed a high degree of heterogeneity with Tau2 = 12.91, I2 = 81%, p<0.001, a random effects model was used for the combined analysis (Figure 4). Subgroup analysis according to sample size showed significantly lower heterogeneity in both the subgroup with sample size < 50 (-8.95[-17.98,0.09], I2 = 91%, p<0.001) and the subgroup with sample size ≥ 50 (-5.92[-7.29,-4.56], I2 = 0%, p=0.52). The antibiotic bone cement treatment of diabetic foot was shorter than the control group in terms of wound healing time in all cases (Figure 5).
Length of hospital stay
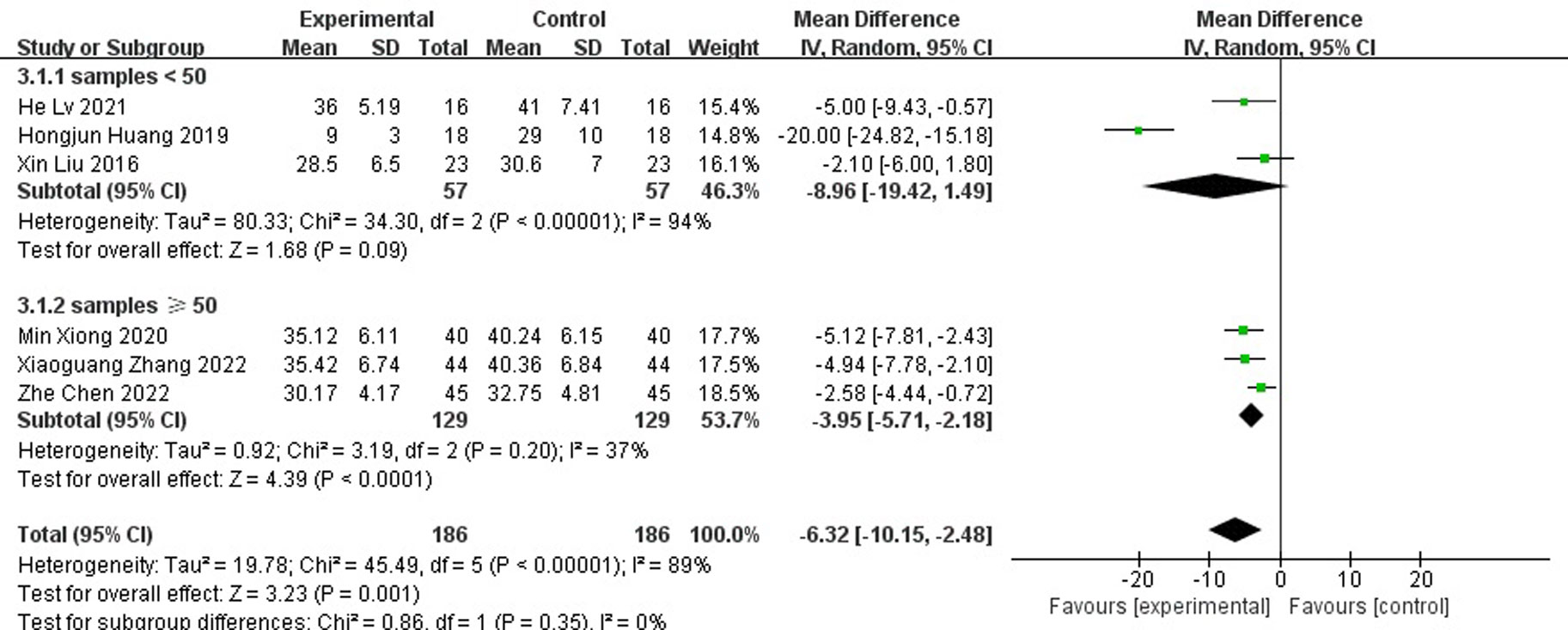
A total of six papers reported the length of hospital stay and the results showed that the length of hospital stay was shorter for antibiotic bone cement for diabetic foot than the control group (MD=-6.32, 95% CI [-10.15,-2.48]) and there was heterogeneity in the data (Tau2 = 19.78, I2 = 89%, P < 0.001) (Figure 6). Subgroup analysis according to the sample size showed significantly lower heterogeneity in both the subgroup with sample size <50 (-8.96[-19.42,1.49], I2 = 94%, p<0.001) and the subgroup with sample size ≥ 50 (-6.32[-10.15,-2.48], I2 = 37%, p=0.2). The length of stay was shorter for antibiotic bone cement for diabetic foot than the control group (Figure 7).
Time for bacterial culture negative-conversing
A total of four papers reported bacterial culture turnaround times, which showed shorter bacterial culture turnaround times in diabetic foot treated with antibiotic bone cement than in the control group (MD=-5.15,95% CI [-7.15,-2.19]) and there was heterogeneity in the data (Tau2 = 4.58, I2 = 89%, P < 0.001); therefore, a random effects model was used to calculate the combined effect size (Figure 8).
Number of surgeries
A total of six publications reported the number of procedures and showed that the number of procedures for diabetic foot treated with antibiotic bone cement was less than the control group (MD=-2.35,95% CI [-3.68, -1.02]) and there was heterogeneity in the data (Tau2 = 2.71, I2 = 89%, P < 0.001); therefore, a random effects model was used to calculate the combined effect size (Figure 9).
Discussion
Our results show that antibiotic bone cement treatment reduced wound healing time, length of hospital stay, time to negative bacterial culture of wound secretions, and the number of procedures compared with other diabetic foot treatments, and the results provide support for antibiotic bone cement as a treatment for diabetic foot infections.
Patients with DFI have low resistance and skin tissue regeneration capacity, resulting in slow wound healing, and these open wounds are susceptible to invasion by pathogenic bacteria, resulting in serious infections (22). Patients with DFI are treated with systemic antibiotics against infection, but the long-term application of antibiotics will produce adverse effects and bacterial resistance (23). Sun Shujuan et al. (24) investigated and analyzed the pathogenic bacteria of diabetic foot in Beijing and showed that multi-drug-resistant bacteria accounted for 16.9%. Due to the long trauma healing time, long hospital stay, and high medical costs of DFI patients, it not only imposes a heavy burden on clinical care but also causes increased anxiety and depression in patients, which affects treatment outcomes and quality of life (25, 26). In addition, routine debridement and surgical treatment for DFI may cause improper wound management, resulting in longer healing times, easy recurrence of ulcers, increased risk of metastatic ulcers, amputation, and death (27, 28).
As shown in Figures 4, 6, antibiotic bone cement for DFI reduced wound healing time and length of hospital stay compared to the controls, which is consistent with other results reported in the literature (13, 29). This may be due to the fact that when the bone cement covers the wound, it produces a 1-2 mm thick biofilm, which becomes an induced membrane and is capable of secreting relevant cytokines to promote wound healing, such as transforming growth factor-β1 (TGF-β1), vascular endothelial growth factor (VEGF), etc. The secreted cytokines also have angiogenic and potential osteogenic properties (30–32). Most patients with DFI have pathological manifestations of small blood vessels and capillary blockages at the ends of the limbs, and the formation of IM promotes fresh angiogenesis, which, in turn, improves blood flow to the extremities, facilitating wound healing and shortening the patient’s hospital stay (33–37).
Our meta-analysis research showed that antibiotic bone cement treatment shortened the bacterial turnaround time of the trauma and reduced the number of procedures compared with conventional debridement combined with negative pressure closure and drainage treatment. Due to poor blood flow to the foot and reduced peripheral perfusion in patients with DFI, intravenous systemic antibiotics reduce delivery to bone or soft tissues and limit their efficacy, thus not achieving effective bactericidal concentrations at the site of the lesion. Antibiotic bone cement has eluting properties, releasing a much higher concentration of antibiotics than systemically applied antibiotics, with an efficiency of 81%, effectively preventing the emergence of drug-resistant strains (38). The bone cement gradually creates a local sterile environment with a slow and continuous local release of antibiotics, which can act directly on the lesion area and, thus, kill the bacteria, shortening the time until bacterial transformation of the wound (39, 40). In addition, the drug released locally rarely enters the systemic circulatory system, thus reducing the side effects of antibiotics (41). The simultaneous application of antibiotic bone cement treatment is easy to perform, with short operative times, easy post-operative care and dressing changes, and lower overall treatment costs.
Vancomycin is a common additive to antibiotic bone cement, releasing topical vancomycin at a concentration of approximately 0.5-2.0 μg/mL to meet the minimum inhibitory concentration requirements (42). In assessing the breadth of response to the dynamic release of vancomycin, it was found that the plateau period for vancomycin release could be >10 days (43–45). Vancomycin has a broad spectrum of sensitive bacteria and kills the most common pathogenic microorganisms (46). In the intervention group, the wound was covered with vancomycin using bone cement as a carrier, and the infection was effectively controlled, facilitating wound healing.
Our study also has some shortcomings. First, the sample size of the included literature was small, with four papers having a sample size of fewer than 50 cases. Second, the different lengths of interventions and inconsistent outcome indicators in the included literature may cause some bias in the study results and increase the heterogeneity of the Meta-analysis results. Finally, the number of high-quality literature included is limited, and more multicenter, large sample randomized controlled clinical trials are needed.
In summary, antibiotic bone cement is effective in treating DFI wounds, saving medical resources and costs, and it is worth promoting its use in clinical practice. In the future, more multicenter and large sample size randomized controlled clinical trials should be conducted to increase the in-depth discussion on the induction of film formation by trauma bone cement in different periods of the diabetic foot and the most suitable types of trauma for antibiotic bone cement, to comprehensively evaluate the effectiveness of antibiotic bone cement in the treatment of diabetic foot and provide a more rigorous and objective reference basis for the treatment of diabetic foot in the clinic in the future.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
TD and QH contributed equally to the design of the study. TD, QH, and ZS collected the data and performed the qualitative analysis. QH performed the quantitative analysis. TD, QH, and ZS drafted the manuscript. TD, QH, and ZS participated in the discussion and interpretation of the data. All authors approved this final version for publication. ZS acted as guarantor, assumed full responsibility for the completion of the article, had access to all data, and controlled the decision to publish.
Funding
This study was supported by Hospital-level research projects of the Hospital of the People’s Government of Tibet Autonomous Region in Chengdu(2020-YJ-11).
Acknowledgments
We thank QH for his help in literature screening and data collection, and ZS for judging the data collection when it was controversial and for his guidance in writing the article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pérez-Panero AJ, Ruiz-Muñoz M, Cuesta-Vargas AI, Gónzalez-Sánchez M. Prevention, assessment, diagnosis and management of diabetic foot based on clinical practice guidelines: a systematic review. Med (2019) 98(35). doi: 10.1097/MD.0000000000016877
2. Lim JZ, Ng NS, Thomas C. Prevention and treatment of diabetic foot ulcers. J Royal Soc of Med (2017) 110(3):104–9. doi: 10.1177/0141076816688346
3. Lepäntalo M, Apelqvist J, Setacci C, Ricco JB, de Donato G, Becker F, et al. Diabetic foot. Euro J Vasc and Endovasc Surg (2011) 42(S2):S60–74. doi: 10.1016/S1078-5884(11)60012-9
4. Armstrong DG, Swerdlow MA, Armstrong AA, Conte MS, Padula WV, Bus SA. Five year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J Foot Ankle Res (2020) 13:16. doi: 10.1186/s13047-020-00383-2
5. Schaper NC, van Netten JJ, Apelqvist J, Bus SA, Hinchliffe RJ, Lipsky BA. Practical guidelines on the prevention and management of diabetic foot disease (IWGDF 2019 update). Diabetes Metab Res Rev (2020) 36(Suppl 1):e3266. doi: 10.1002/dmrr.3266
6. Lim JZ, Ng NS, Thomas C. Prev Treat Diabetic foot ulcer. J R Soc Med (2017) 110(3):104109. doi: 10.1177/0141076816688346
7. Jones NJ, Harding K. 2015 international working group on the diabetic foot guidance on the prevention and management of foot problems in diabetes. Int Wound J (2015) 12(4):373374. doi: 10.1111/iwj.12475
8. Hingorani A, LaMuraglia GM, Henke P, Meissner MH, Loretz L, Zinszer KM, et al. The management of diabetic foot:a clinical practice guideline by the society for vascular surgery in collaboration with the American podiatric medical association and the society for vascular medicine. J Vasc Surg (2016) 63(2 Suppl):S3–S21. doi: 10.1016/j.jvs.2015.10.003
9. Boulton AJM, Armstrong DG, Hardman MJ, Malone M, Embil JM, Attinger CE, et al. Diagnosis and management of diabetic foot infections. Arlington(VA):American Diabetes Assoc (2020). doi: 10.2337/db2020-01
10. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (2021) 29(372):n71. doi: 10.1136/bmj.n71
11. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (2019) 28(366):l4898. doi: 10.1136/bmj.l4898
12. Higgins JPT, Sterne JAC, Savović J, et al. Revised cochrane risk of bias tool for randomized trials (RoB 2.0) (2016). Available at: https://www.riskofbias.info/welcome/rob-2-0-tool/archive-rob-2-0-2016/.
13. Liu AL, Liu YS, Cao TY. Analysis of the application of antibiotic bone cement in the repair of diabetic foot ulcer wounds. New World Diabetes (2021) .24(02):157–9. doi: 10.16658/j.cnki.1672-4062.2021.02.157
14. Xiong M. Application of VSD technique combined with antibiotic bone cement in infected diabetic foot wounds. New World Diabetes (2020) 23(24):176–8. doi: 10.16658/j.cnki.1672-4062.2020.24.176
15. Xin L, Jiulong L, Jun Z, Liangliang Q, Xunyuan J, Mingchao LI, et al. Vacuum sealing drainage treatment combined with antibiotic-impregnated bone cement for treatment of soft tissue defects and infection. Med Sci monitor: Int Med J Exp Clin Res (2016) 9(22):1959–65. doi: 10.12659/MSM.896108
16. Chen Z, He ZY. Analysis of the efficacy of antibiotic bone cement combined with negative pressure closed suction in the treatment of diabetic foot. Zhejiang Trauma Surg (2022) 27(02):249–51. doi: 10.3969/j.issn.1009-7147.2022.02.022
17. Zhang S, Luo YJ, Peng QH. Effect of antibiotic bone cement filling combined with closed negative pressure drainage in the treatment of diabetic foot with infected wounds. Chin J Bone Joint Injury (2020) 35(08):877–9. doi: 10.7531/j.issn.1672-9935.2020.08.035
18. Huang HJ, Niu XH, Yang GL, Wang LY, Shi FCC, Xu SJ, et al. Clinical effects of antibiotic bone cement applied to diabetic foot ulcer wounds. Chin J Burns (2019) 11(06):464–6. doi: 10.3760/cma.j.issn.1009-2587.2019.06.013
19. Zhang XG, Li JM, Huang HJ, DaoXuan LI, Wang W, PW D, et al. Effect of debridement combined with antibacterial drug bone cement coverage for diabetic foot ulcer wounds. Shenzhen J Integr Med (2022) 32(02):126–8. doi: 10.3760/cma.j.issn.1009-2587.2019.06.013
20. Lv H, Zhu H-B, Ma Y-P, Zhang Y-T, Hu C-T, Ying Y-F. A case-control study of vancomycin-loaded bone cement for Wagner grade II-IV diabetic foot. China Orthopaedic Injury (2021) 34(10):947–52. doi: 10.12200/j.issn.1003-0034.2021.10.012
21. Yang F, Li J, Hao LH, Ren PY, Liu S. Efficacy analysis of VSD sequential antibiotic bone cement interposition for Wagner grade 2 to 4 diabetic foot. Electronic J Foot Ankle Surg (2021) 8(04):56–60. doi: 10.3969/j.issn.2095-7793.2021.04.015
22. Lew DP, Waldvogel FA. Osteomyelitis. Lancet (2004) 364(9431):369–79. doi: 10.1016/S0140-6736(04)16727-5
23. Wang A-H, Xue J, Xu Z-R. Progress and prospects of clinical treatment of diabetic foot. Chin J Diabetes (2022) 14(07):643–9.
24. Sun S, Zhao S, Yang F, Zhao H. Screening and risk factor analysis of diabetic high-risk foot in wanshulu community of Beijing. J Chronic Dis (2022) 23(07):1082–5. doi: 10.16440/J.CNKI.1674-8166.2022.07.34
25. Schaper NC, Netten JJ, Apelqvist J, Bus SA, Hinchliffe RJ, Lipsky BA. Practical guidelines on the prevention and management of the diabetic foot (IWGDF 2019 update). Diabetes Metab Res Rev (2020) 36. doi: 10.1002/dmrr.3266
26. Singh R, Kishore L, Kaur N. Diabetic peripheral neuropathy: current perspective and future directions. Pharmacol Res (2014) 80:21–35. doi: 10.1016/j.phrs.2013.12.005
27. Mouzopoulos G, Kanakaris NK, Kontakis G, Obakponovwe O, Townsend R, Giannoudis PV. Management of bone infections in adults: the surgeon’s and microbiologist’s perspectives. Injury (2011) 42(Suppl 5):S18–23. doi: 10.1016/S0020-1383(11)70128-0
28. Kelm J, Anagnostakos K, Regitz T, Schmitt E, Schneider G, Ahlhelm F. MRSA-infections-treatment with intraoperatively produced gentamycin -vancomycin PMMA beads. Chirurg (2004) 75(10):988–95. doi: 10.1007/s00104-004-0847-3
29. Zhong YX, Li L, Wang DL. Study on the application of dilation combined with antibiotic bone cement in the treatment of severely infected diabetic foot ulcers. Chin J Injury Repair (electronic version) (2022) 17(1):60–4. doi: 10.3877/cma.j.issn.1673-9450.2022.01.010
30. Fischer C, Doll J, Tanner M, Bruckner T, Zimmermann G, Helbig L, et al. Quantification of TGF-β1,PDGF and IGF-1 cytokine expression after fracture treatment vs.non union therapy via masquelet. Injury (2016) 47(2):342–9. doi: 10.1016/j.injury.2015.11.007
31. Giotikas D, Tarazi N, Spalding L, Nabergoj M, Krkovic M. Results of the induced membrane technique in the management of traumatic bone loss in the lower limb:a cohort stud. J Orthop Trauma (2019) 33(3):131–6. doi: 10.1097/BOT.0000000000001384
32. Gruber HE, Ode G, Hoelscher G, Ingram J, Bethea S, Bosse MJ. Osteogenic,stem cell and molecular characterisation of the human induced membrane from extremity bone defects. Bone Joint Res (2016) 5(4):106–15. doi: 10.1302/2046-3758.54.2000483
33. Liu C, You JX, Chen YX, Zhu WF, Wang Y, Lv PP, et al. Effect of induced membrane formation followed by polymethylmethacrylate implantation on diabetic foot ulcer healing when revascularization is not feasible. J Diabetes Res (2019), 2429136. doi: 10.1155/2019/2429136
34. Christou C, Oliver RA, Yu Y, Walsh WR. The masquelet technique for membrane induction and the healing of ovine critical sized segmental defects. PloS One (2014) 9(12):e114122. doi: 10.1371/journal.pone.0114122
35. Aho OM, Lehenkari P, Ristiniemi J, Lehtonen S, Risteli J, Leskelä HV. The mechanism of action of induced membranes in bone repair. J Bone Joint Surg Am (2013) 95(7):597604. doi: 10.2106/JBJS.L.00310
36. Masquelet AC. Induced membrane technique: pearls and pitfalls. J Orthop Trauma (2017) 31 Suppl 5:S36S38. doi: 10.1097/BOT.0000000000000979
37. Oh Y, Yoshii T, Okawa A. Ankle arthrodesis using a modified masquelet induced membrane technique for open ankle fracture with a substantial osteochondral defect: a case report of novel surgical technique. Injury (2019) 50(11):21282135. doi: 10.1016/j.injury.2019.09.020
38. Xiannan S, Dapeng Z, Xinwei LIU. Experimental study on elution characteristics of antibiotic composite bone cement. J Local Surg (2021) 30(2):99–102. doi: 10.11659/jjssx.08E020071
39. Qiu X, Chen Y, Qi X, Yin Z, Zhang Y, Wang Z. Efficacy analysis of induced membrane technique for the treatment of infected bone defects. Chin J Restorative Reconstructive Surg (2017) 31(9):1064–8. doi: 10.7507/1002-1892.201704002
40. Wang X, Wang Z, Fu J, Huang K, Xie Z. Induced membrane technique for the treatment of chronic hematogenous tibiaosteomyelitis. BMC Musculoskelet Disord (2017) 18(1):33. doi: 10.1186/s12891-017-1395-6
41. Mendel V, Simanowski HJ, Scholz H. Synergy of HBO2 and a local antibiotic carrier for experimental osteomyelitis due to staphylococcus aureus in rats. Undersea Hyperb Med (2004) 31:407–16.
42. Patel N, Lubanski P, Ferro S, Bonafede M, Harrington S, Evans A, et al. Correlation between vancomycin MIC values and those of other agents against gram-positive bacteria among patients with bloodstream infections caused by methicillin-resistant staphylococcus aureus. Antimicrob Agents Chemother (2009) 53:5141–4. doi: 10.1128/AAC.00307-09
43. Lee SH, Tai CL, Chen SY, Chang CH, Chang YH, Hsieh PH. Elution and mechanical strength of vancomycin-loaded bone cement: in vitro study of the influence of brand combination. PloS One (2016) 11:e0166545. doi: 10.1371/journal.pone.0166545
44. Bishop AR, Kim S, Squire MW, Rose WE, Ploeg HL. Vancomycin elution, activity and impact on mechanical properties when added to orthopedic bone cement. J Mech Behav BioMed Mater (2018) 87:80–6. doi: 10.1016/j.jmbbm.2018.06.033
45. Zalavras CG, Patzakis MJ, Holtom P. Local antibiotic therapy in the treatment of open fractures and osteomyelitis. Clin Orthop Relat Res (2004) 427:86–93. doi: 10.1097/01.blo.0000143571.18892.8d
Keywords: bone cement, diabetic foot, infection, Antibiotics, meta-analysis, clinical efficacy
Citation: Dong T, Huang Q and Sun Z (2023) Antibiotic-laden bone cement for diabetic foot infected wounds: A systematic review and meta-analysis. Front. Endocrinol. 14:1134318. doi: 10.3389/fendo.2023.1134318
Received: 30 December 2022; Accepted: 21 February 2023;
Published: 16 March 2023.
Edited by:
Pranav Kumar Prabhakar, Lovely Professional University, IndiaReviewed by:
Ram Bajpai, Keele University, United KingdomMarco Meloni, University of Rome Tor Vergata, Italy
Copyright © 2023 Dong, Huang and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zengmei Sun, c3VuemVuZ21laTIwMjJAMTYzLmNvbQ==
 Tingting Dong
Tingting Dong Qi Huang2
Qi Huang2