- 1Department of Cardiovascular Medicine, The Second Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, China
- 2Jiangxi Provincial Cardiovascular Disease Clinical Medical Research Center, Nanchang, Jiangxi, China
- 3Jiangxi Sub-Center of National Clinical Research Center for Cardiovascular Diseases, Nanchang, China
- 4Department of Cardiovascular Medicine, Wuyuan People’s Hospital, Shangrao, Jiangxi, China
- 5Center for Prevention and Treatment of Cardiovascular Diseases, The Second Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, China
Objective: Exploring the relationship between (weight-adjusted waist index) WWI and arterial stiffness (AS) in the total and different BMI populations among patients with hypertension.
Methods: This study enrolled 5232 hypertensive subjects, a subset of the China H-type Hypertension Registry Study. WWI was calculated as WC (cm) divided by the square root of weight (kg). Brachial-ankle pulse wave velocity (baPWV) was measured to determine AS.
Results: The mean WWI was 10.97 (0.78)cm/√kg. In multiple logistic analyses showed that there were significant dose-dependent association between WWI with baPWV in a dose-dependent manner in total population (β 57.98, 95% CI 44.06-71.90), and in different BMI group: group 1 (BMI<18.5kg/m2) (β 94.30, 95% CI 39.36-149.23), group 2 (18.5-23.9kg/m2) (β 74.21, 95% CI 54.57-93.85), group 3 (≥24kg/m2) (β 26.11, 95% CI 5.22-47.01). In stratified analysis, stronger associations between WWI and baPWV were observed in patients with higher BP or lower BMI. Sensitivity analysis by excluding patients treated with lipid-lowering agents did not change the association between WWI and baPWV.
Conclusion: For hypertensive patients, we found that WWI was positively associated with baPWV in different BMI groups. WWI might be considered as an intervening factor in preventing and treatment of AS, besides BP management.
Introduction
Arterial stiffness (AS) is an independent predictor of cardiovascular diseases (CVD) and mortality (1, 2). Brachia-ankle pulse wave velocity (baPWV) measurement is commonly used to assess arterial wall stiffness in epidemiological studies, as it is accurate, simple and non-invasive. Recently, increasing numbers of studies have revealed that baPWV is positively related to future risk of CVD events (3), diabetes (4), and all-cause mortality (5).
As generally known, obesity is closely associated with AS and can independently predict the future risk of AS (6–8). Several studies have shown that weight loss achieved through lifestyle measures can improve AS (9, 10). Moreover, existing studies show that muscle indices are negatively related to the risk of AS (11). Body mass index (BMI) is the major measure of obesity and is widely used in many epidemiological studies because of its low cost, simplicity, and availability. Nevertheless, current researches are still controversial about the relationship between BMI and AS among different populations. In the past decade, some studies have found a positive association between BMI and AS (12–14), while others have found a negative association (15, 16), and others have found no association (17, 18). These discrepancies between the different studies may be attributed to different sample sizes, ethnic and regional variations. Moreover, BMI is not able to discriminate between muscle and fat. Weight-adjusted waist index (WWI) was first proposed in 2018 as a new anthropometric index for predicting the risk of CVD events and mortality (19). Moreover, a study from Korea shows that elevated WWI is closely related to high body fat and low muscle mass (20). Draw a question, whether WWI would better identify the risk of AS compared to BMI.
Hypertension is a significant risk factor for the development of AS (21). According to China Hypertension Survey (2012-2015), the prevalence of hypertension among Chinese adults is 23.2% (22). Obesity is a proven risk factor for hypertension (23). The co-existence of obesity and hypertension significantly increases the risk of developing AS. According to a cohort study of 10338 subjects, WWI is closed associated with future risk of hypertension (24). However, the ability of WWI for assessing the risk of AS among the hypertensive population is poorly understood. Therefore, this study aimed to explore the association between WWI and AS among hypertensive patients in the total population and different BMI populations. In addition, we further assess the mediating roles of blood pressure (BP) satisfaction on the association between WWI and AS.
Methods
Study participants
Our study participants came from China H-type Hypertension Registry Study that previously reported (25, 26) (Registration number: ChiCTR1800017274). In brief, this is a real-world, multicenter, observational registry study carried out in southern China from March 2018 to August 2018. Inclusion was carried out in participants with hypertension aged 18 years and older. Hypertension was defined as seated, resting BP≥140/90mmHg at the screening, self-report, or undergoing anti-hypertensive treatment. The exclusion criteria included neurological abnormalities, inability to follow up according to the study protocol, or plans to relocate shortly, and the patients, who were not suitable for inclusion or for long-term follow-up assessed by study physicians. All participants provided written informed consent. The protocol was approved by the Ethics Committee of Institute of Bio-medicine, Anhui Medical University (NO. CH1059) and the Second Affiliated Hospital of Nanchang University (NO. 2018019).
Our study was conducted on a subset of 5233 subjects with complete baPWV data from China H-type Hypertension Registry Study. After excluding participants with loss of WWI data (n =1), 5232 participants were included in the final analysis, as shown in Figure S1.
Assessment of covariates
According to a standard operating procedure, all subjects were interviewed by a trained study coordinator. The information related to age, sex (male or female), smoking, drinking, physical activity (mild, moderate or vigorous), and medical history (diabetes mellitus, hyperlipidemia, duration of hypertension and current medication usage) were abstracted from a standard questionnaire. The heights, weights, and waist circumference (WC) of all patients were collected by trained researchers according to the standard process. BMI was calculated as weight (kg) divided by the square of height (m2). BMI was defined as underweight (<18.5kg/m2), normal (18.5-23.9kg/m2), overweight or obesity (≥24kg/m2) according to the cut-off point for Chinese adults (27). WWI (cm/√kg) was calculated as WC (cm) divided by the square root of weight (kg). Resting blood pressure (BP) was measured by the automated electronic device (Omron; Dalian, China) in a standardized manner.
After a 10 h fasting period, blood samples were obtained from all subjects. The blood samples would be measured in Biaojia Biotechnology Laboratory, Shenzhen, China. Fasting plasma glucose (FPG), fasting lipids (total cholesterol, high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and triglycerides) and homocysteine(Hcy) were determined using automatic clinical analyzers (Beckman Coulter).
BaPWV measurements
The baPWV was assessed with Omron Colin BP-203RPE III device (Omron Health Care, Kyoto, Japan) following the recommended standard procedures. The specific details of baPWV measurement were described previously (26).
Statistical analysis
Baseline characteristics are shown as mean ± standard deviation (SD) for continuous variables, and as n (%) for categorical variables. Descriptive analyses were conducted according to WWI quartiles using t-test or Chi-square tests to compare between-group differences. Dose-response association of WWI with baPWV was assessed using a generalized additive model (GAM) and a fitted smoothing curve (penalized spline method). We used a multivariate linear regression model (beta coefficient [β] and 95% confidence interval [CI]) to assess the relationship between WWI and baPWV in the total and different BMI populations by controlling the confounders in three models. Model 1: adjusted for age; Model 2: Model 1 plus sex, current smoking, current drinking, physical activity, BMI; Model 3: Model 2 plus systolic blood pressure (SBP), diastolic blood pressure (DBP), duration of hypertension, diabetes mellitus, hyperlipidemia, antihypertensive agents, antidiabetes agents, lipid-lowering agents, FPG, Hcy, triglyceride, HDL-C, and LDL-C. The general linear model was determined to compare the difference of baPWV in total and different BMI populations. Subgroup and stratified analyses were also done. A sensitivity analysis was also conducted. In sensitivity analyses, the same analyses were performed after excluding patients treated with lipid-lowering agents. In addition, To determine whether the association between WWI and baPWV was mediated by BP (SBP or DBP), a simple mediation analysis was completed (Figures S2A, B).
All data analyzed were using the statistical package R (http://www.r-project.org) and Stata software, version 14.0 (StataCorp). A 2-tailed P < 0.05 was considered to be statistically significant.
Results
Characteristics of the subjects
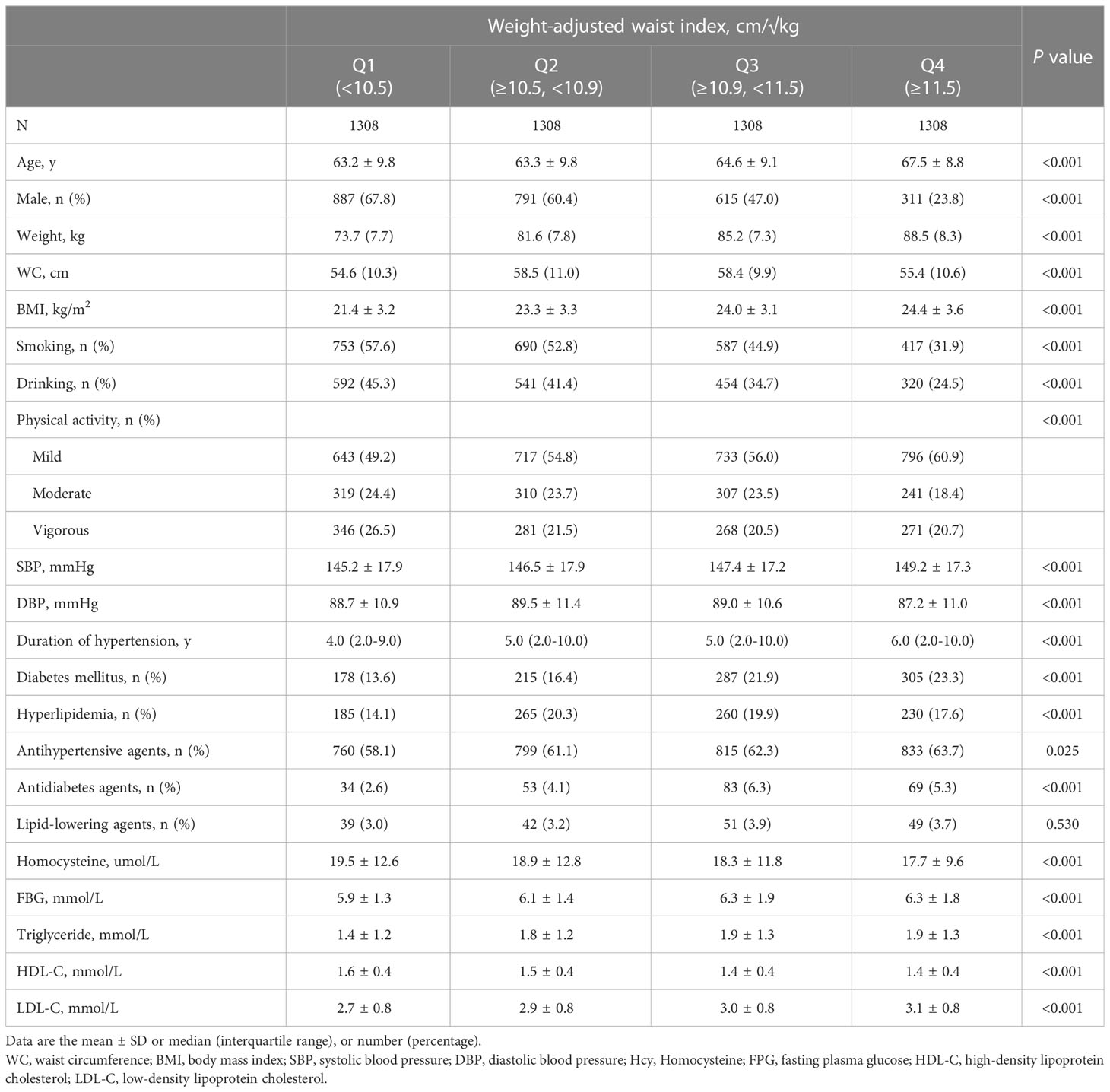
Overall, 5232 hypertensive subjects were enrolled in the final analysis (2603 men and 2629 women, aged 64.7 ± 9.5 years). The mean WWI was 10.97 (0.78) cm/√kg. Mean baPWV was 1858.4 (420.7) mm/s. Table 1 shows the characteristics of the patients according to the WWI quartiles. The participants with higher WWI had higher values of age, weight, WC, BMI, SBP, duration of hypertension, diabetes mellitus, antihypertensive agents, antidiabetes agents, FBG, and LDL-C. In addition, higher WWI level was negatively associated with lower values in male, smoking, drinking, physical activity, Hcy, and HDL-C.
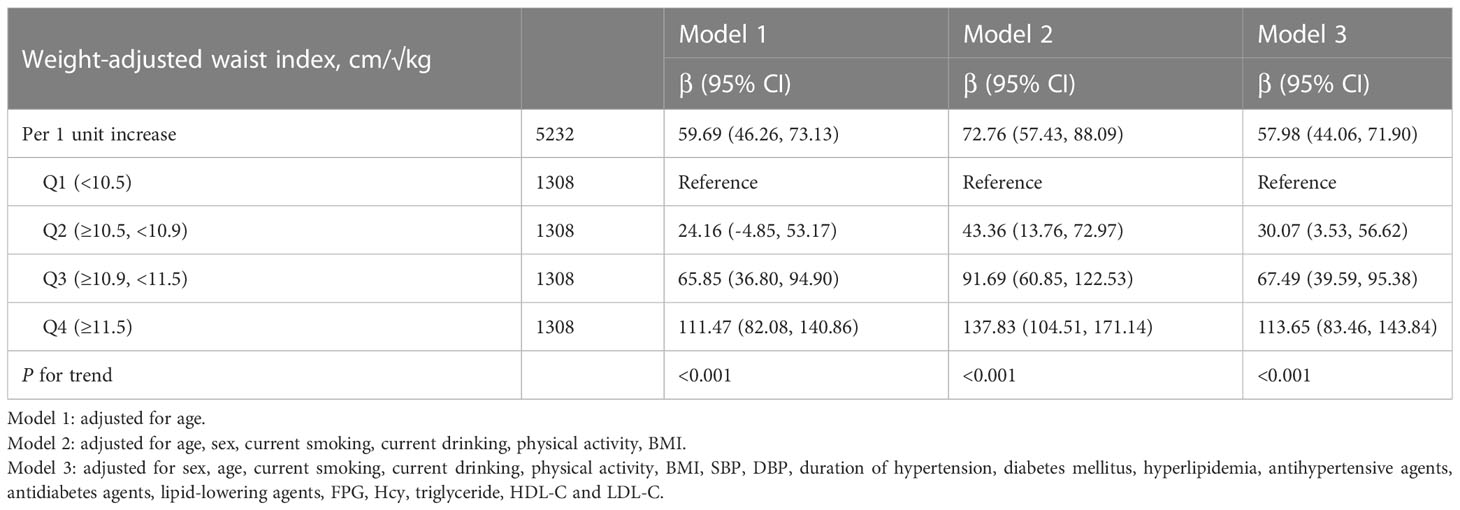
Association between WWI and baPWV in total population
As shown in Figure 1, there was a significantly positive association between WWI and baPWV. Moreover, the significant relationship between WWI with baPWV was observed in Table 2. After correction for different confounding factors, the positive associations between WWI and baPWV were found in three models (P for trend <0.001). For a 1-unit increase in WWI, the baPWV is changed in 57.98mm/s (95% CI 44.06-71.90) in the fully adjusted model. The subjects were stratified into four groups by the quartile value of WWI, comparing the Q1 (<10.5 cm/√kg), the adjusted β of Q2 (≥10.5, <10.9), Q3 (≥10.9, <11.5), and Q4 (≥11.5)were 30.07 (95% CI 3.53-56.62), 67.49 (95% CI 39.59-95.38), 113.65 (95% CI 83.46-143.84) in the model 3.
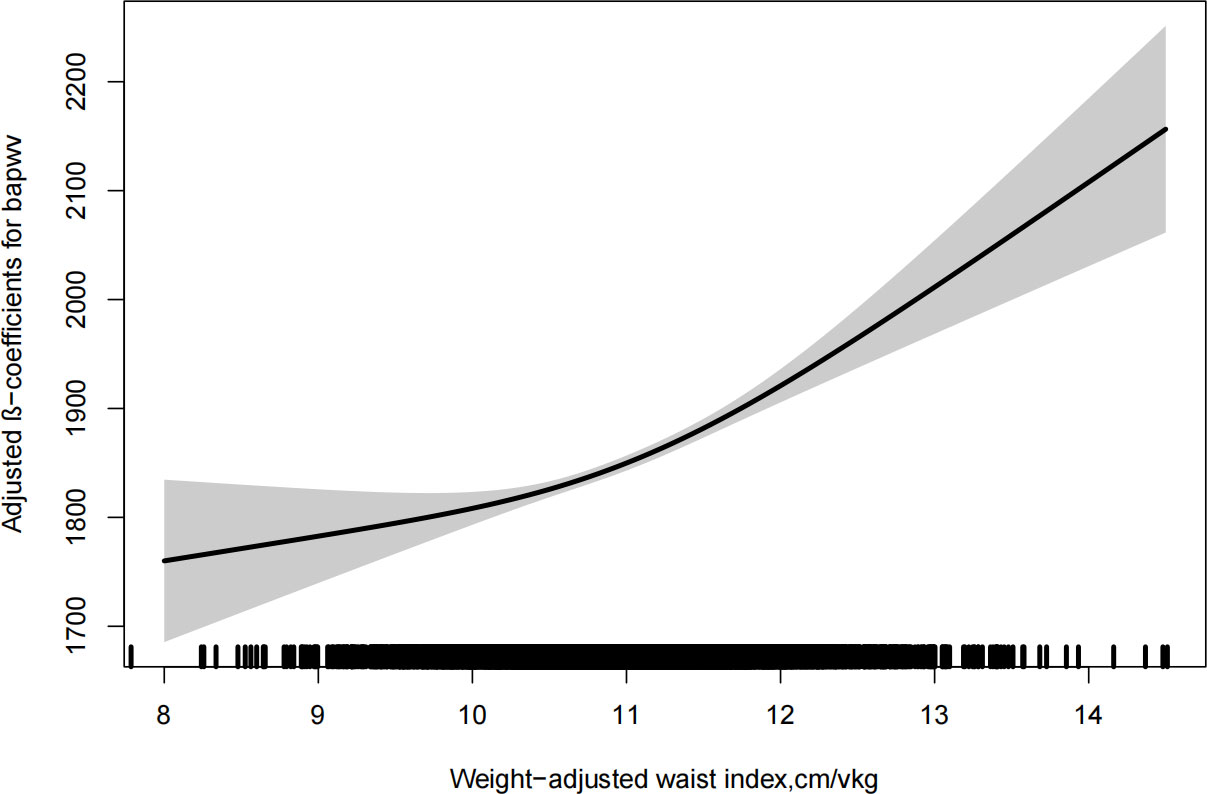
Figure 1 Dose-response relationship between weight-adjusted waist index and baPWV. Models were adjusted for age, sex, current smoking, current drinking, physical activity, BMI, SBP, DBP, duration of hypertension, diabetes mellitus, hyperlipidemia, antihypertensive agents, antidiabetes agents, lipidlowering agents, FPG, homocysteine, triglyceride, HDL-C and LDL-C.
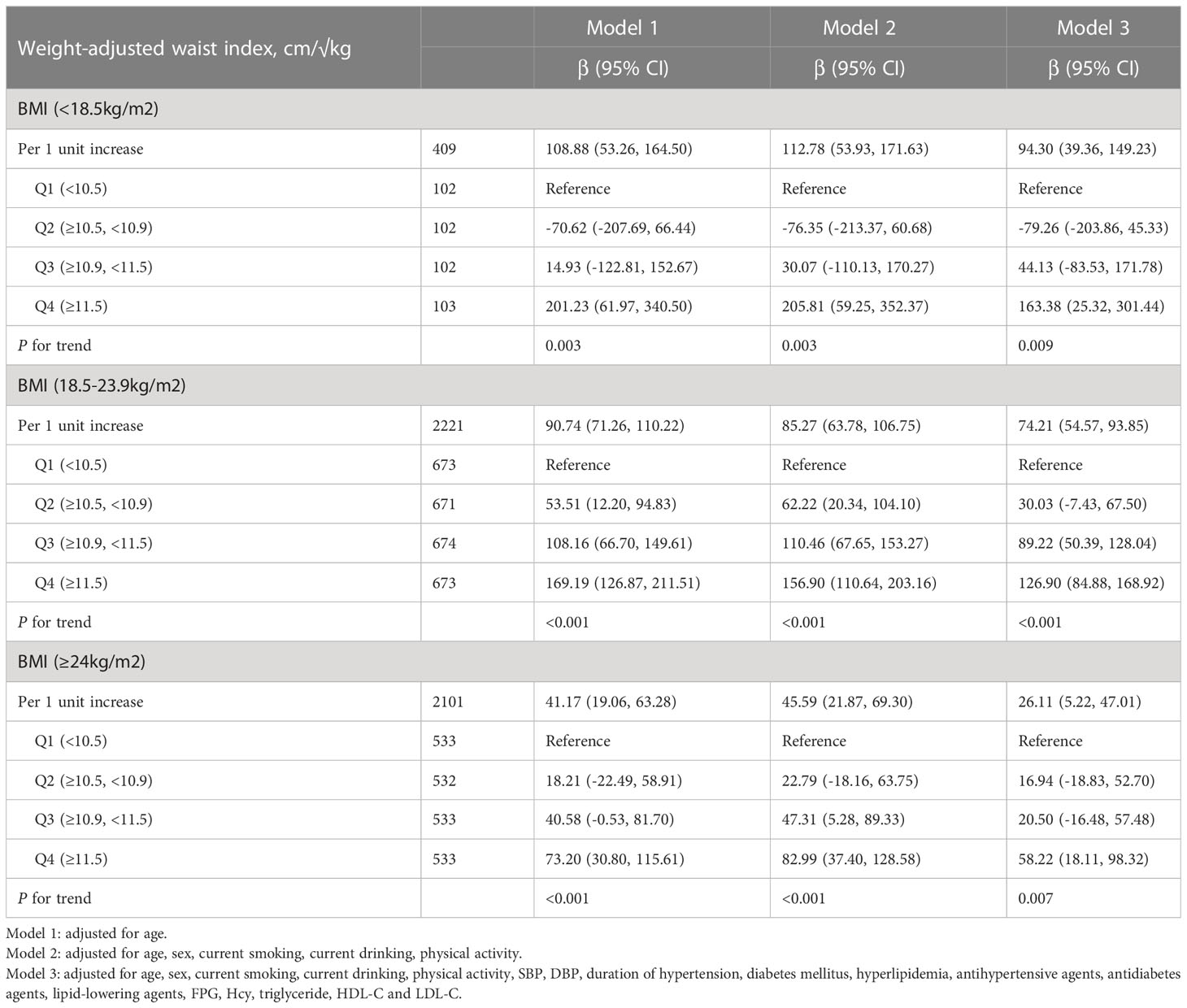
Association between WWI and baPWV in different BMI groups
As shown in Table 3, the positive associations between WWI and baPWV were maintained in different categories of BMI, even in the normal BMI group (18.5-23.9kg/m2). In the normal BMI group, per 1-unit increase in WWI, the baPWV increases by 74.21mm/s (95% CI 54.57-93.85) in model 3. Moreover, the baPWV values increased as the quartiles of WWI (P for trend<0.05). Consistently, the same associations between WWI and baPWV were observed among underweight (BMI <18.5) and overweight (BMI ≥24) subjects. Figure 2 shows the levels of baPWV by quartiles of WWI in total and different BMI populations. The levels of baPWV showed an increasing trend across quartiles of WWI in total and different BMI populations, but this trend was not evident in the underweight group.
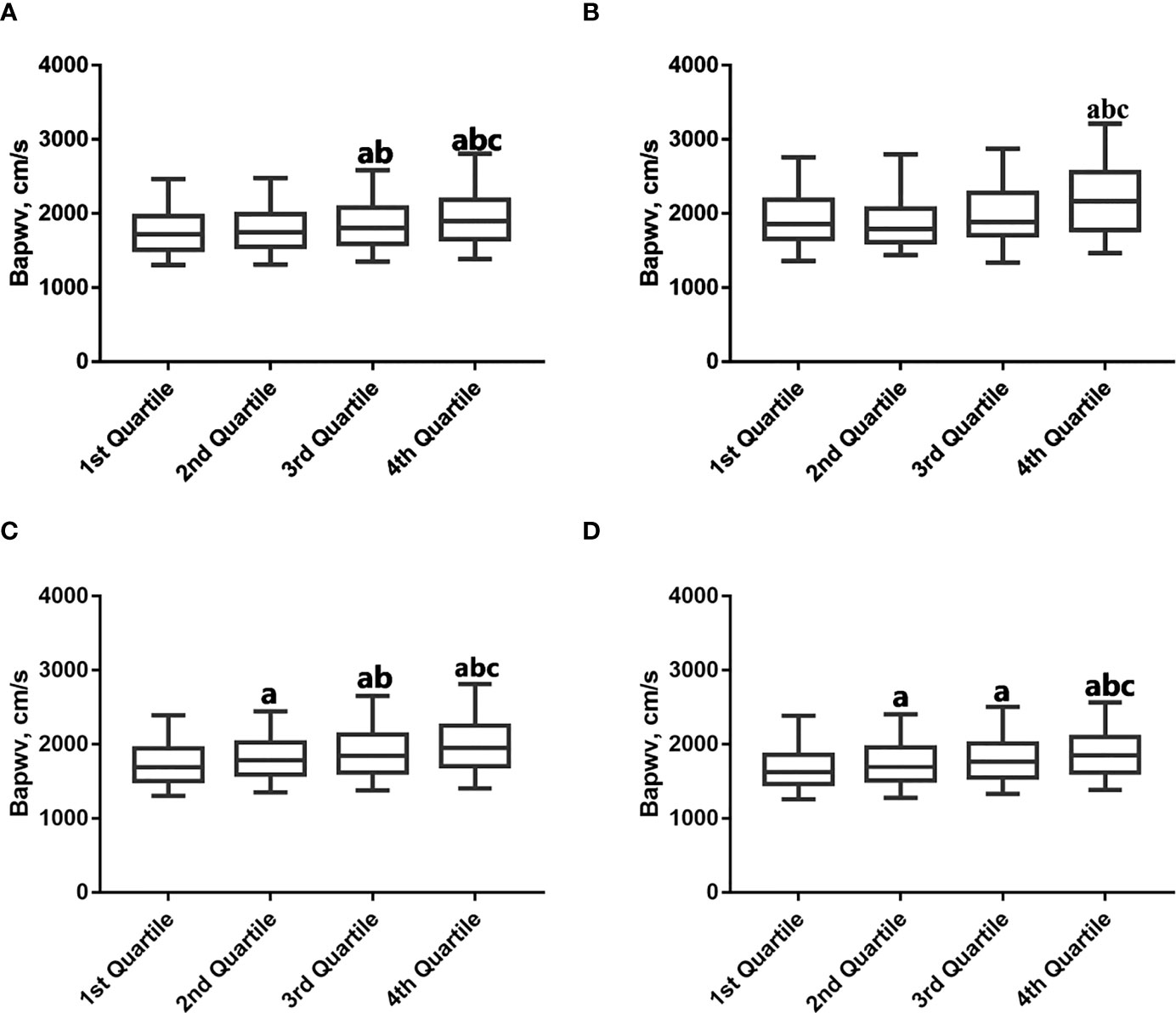
Figure 2 Mean baPWV by weight-adjusted waist index quartiles according to BMI category. (A) Total; (B) BMI (<18.5kg/m2); (C) BMI (18.5-23.9kg/m2); (D) BMI (≥24kg/m2). a p < 0.05 compared with the 1st Quartile. b p < 0.05 compared with the 2nd Quartile. c p < 0.05 compared with the 3rd Quartile.
Stratification analysis
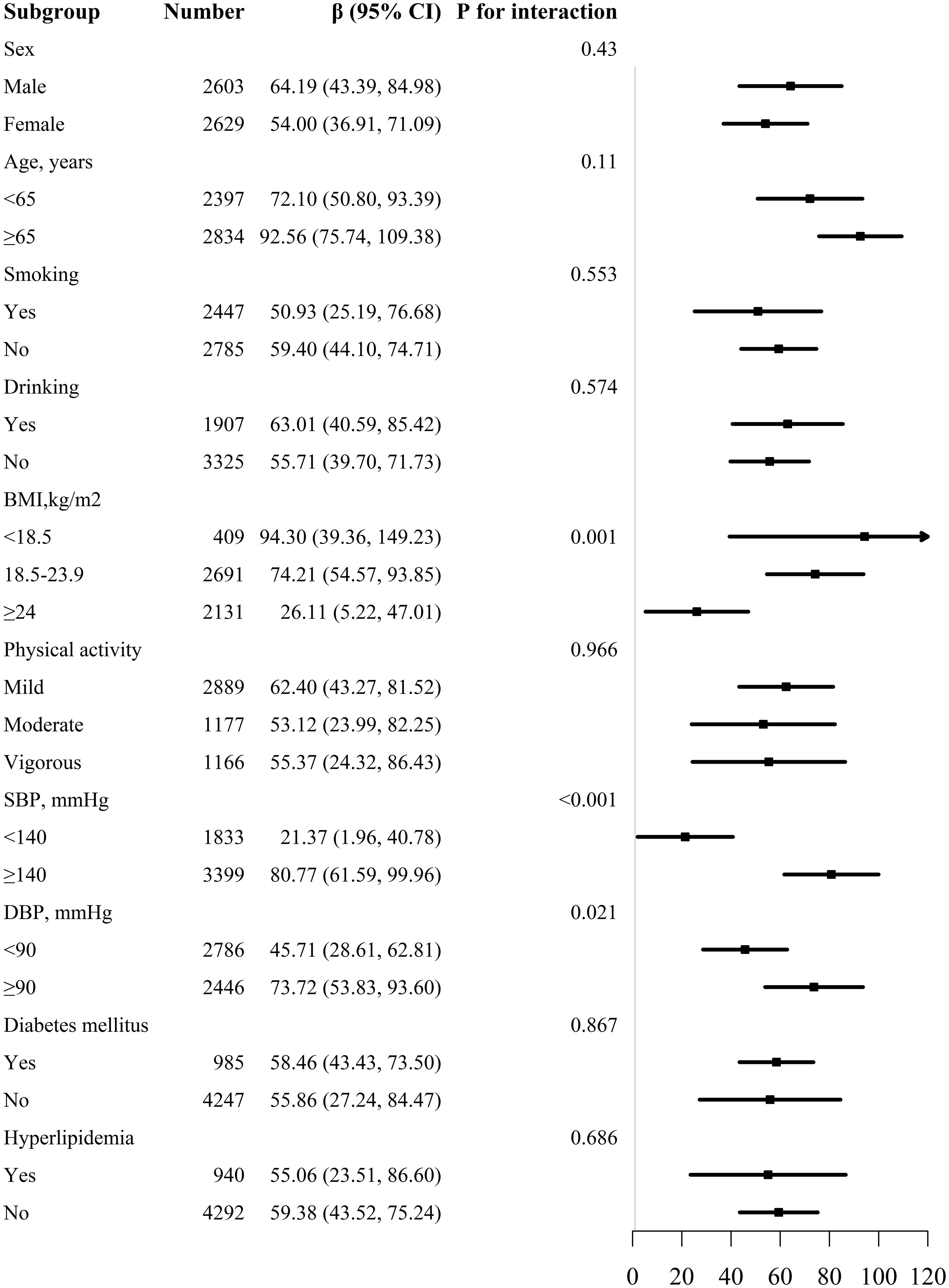
We conducted a list of stratified analyses by sex, age (<65 and ≥65 years), BMI (<18.5, 18.5-23.9 and ≥24kg/m2), smoking (yes/no), drinking (yes/no), physical activity (mild, moderate and vigorous), SBP (<140 and ≥140mmHg), DBP (<90 and ≥90mmHg), diabetes mellitus (yes/no), and hyperlipidemia (yes/no) as shown in Figure 3. We observed that the relationship between WWI and baPWV was consistent in all subgroups except for BMI (P for interaction =0.001), SBP (P for interaction <0.001), and DBP (P for interaction =0.021).
We further divided the population into four groups based on SBP and DBP (group 1: SBP<140mmHg and DBP<90mmHg, group 2: SBP≥140mmHg or DBP≥90mmHg, group 3: SBP≥160mmHg or DBP≥100mmHg, and group 4: SBP≥180mmHg or DBP≥110mmHg), and to explore the association of WWI and baPWV (Table S1). Per 1-unit increase in WWI, the baPWV increase by 23.39mm/s (95% CI 2.36-44.42), 72.80mm/s (95% CI 53.84-91.75), 79.03mm/s (95% CI 50.21-107.85), 189.74mm/s (95% CI 96.37-283.11) respectively. A more accentuated increase was observed in the higher BP group.
WWI (cm/√kg) was calculated as WC (cm) divided by the square root of weight (kg). As shown in Table S2, significant differences in weight and WC between BMI subgroups. Therefore, there was an interaction between WWI and BMI.
Sensitivity analyses
As shown in Table S3A, the sensitivity analysis was conducted by the exclusion of subjects treated with lipid-lowering agents, and the result was stable. Moreover, we performed a sensitivity analysis restricting the patients treated with lipid-lowering agents, the results remained significant and consistent (Table S3B).
Mediation effect of BP
The unstandardized regression coefficients for the effect of WWI on baPWV without and with SBP and DBP as mediators are shown in Table 4. As in Table 4, the results indicated that SBP and DBP mediated 28.3% and 8.6% of the relationship of increasing WWI and baPWV, respectively.
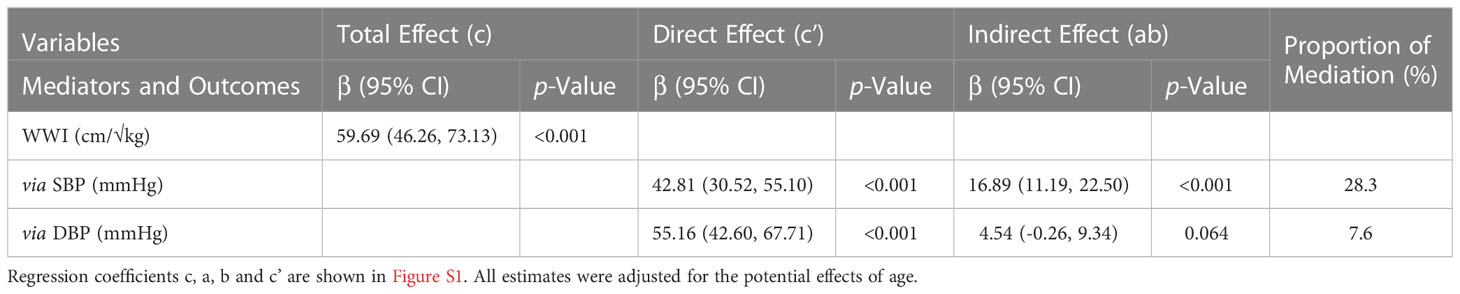
Table 4 Direct and indirect effects of WWI on markers of Bapwv with blood pressure as mediators in hypertensive patients.
Discussion
This cross-sectional study evaluated the association between WWI and baPWV in a group of middle-aged and older hypertensive patients. The main findings encompassed the following. We observed that WWI was positively associated with baPWV among patients with hypertension, even though these patients had normal BMI. The association was more significant in patients with higher BP or lower BMI levels. Moreover, this association was stable after excluding the patients treated with lipid-lowering agents. In this relationship, SBP mediated around 28.3% of the total effects. These findings suggest that WWI could be considered as an intervening factor in preventing and treatment of AS, besides BP management.
Numbers of epidemiological studies have demonstrated that obesity was significantly associated with high arterial stiffness (7, 8). A previous meta-analysis of 20 studies showed that modest weight loss was associated with reduced PWV (9). Obesity is usually defined by BMI. Many clinical studies indicated that BMI is positively associated with increased PWV (12, 13). However, several studies suggested that BMI is negatively associated with high arterial stiffness (15, 16). Moreover, Liao et al. conducted a longitudinal study of 1553 subjects found that there was no relationship between the BMI and AS after correction for SBP and DBP (18). There were several reasons for these apparent discrepancies including different study cohorts, ethnic and regional disparity. Furthermore, BMI does not differentiate between lean mass and body fat mass. WWI is an anthropometric index that can be calculated easily. A cross-sectional study suggested that WWI could reflect both body fat and muscle mass (20). In addition, several clinical studies showed that WWI is closely related to an increased risk of diabetes (28), hypertension (24), metabolic syndrome (29), CVD (19), and mortality (30). However, to our knowledge, this is the first study to explore the association between WWI and baPWV in a large population. In this study, it seems that WWI is positively related to baPWV in total and different BMI populations. Moreover, this relationship between 2 was independent of other variables in the multivariate linear regression and mediation analysis.
Notably, the association between WWI and baPWV appeared to be more pronounced in patients with SBP ≥140mmHg or DBP ≥90mmHg. As is well known, hypertension is a major risk for AS (21). Therefore, it is reasonable to postulate that higher BP status may amplify the adverse impact of WWI on baPWV. Our results support this assumption. In stratified analysis by BP, a larger association between WWI and baPWV is observed in patients with higher BP status (Table S1).
Several underlying mechanisms might explain the association between WWI and baPWV. WWI was positively associated with fat mass and negatively associated with lean body mass (20). Numbers experimental and human studies suggested that adipocyte hyperplasia and hypertrophy might induce adipokine dysregulation (31). And adipokine dysregulation may lead to vascular inflammation, endothelial dysfunction, and vascular remodeling, resulting in AS (32, 33). Moreover, lower lean mass induces less glucose intake into the muscle and physical activity increases lean mass and improves endothelial function, oxidative stress, insulin resistance, and inflammation, and subsequently improves AS (9). In addition, WWI was an independent risk factor for diabetes (28) and metabolic syndrome (29), all of these diseases can worsen arteriosclerosis.
The strength of our study is that we assess the association between WWI and baPWV in different BMI groups. Moreover, the present study included a large hypertension cohort, subgroup analysis, and mediation analysis. However, this study contains several limitations. First, this study was a cross-sectional study. Hence, the association cannot prove causality. Second, although multivariate correction, it was difficult to exclude any potential confounding effect. Third, the conclusion of the current study applies to the Chinese hypertensive population that may not be directly extrapolated.
Conclusion
For hypertensive patients, we found that WWI was positively associated with baPWV in different BMI groups. Further longitudinal studies are required to corroborate our findings. Moreover, WWI might be considered as an intervening factor in preventing and treatment of AS, besides BP management.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. Requests to access these datasets should be directed to XC,eGlhb3NodW1lbmZhbjEyNkAxNjMuY29t.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Institute of Bio-medicine, Anhui Medical University and the Second Affiliated Hospital of Nanchang University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
YX, XH, HB and XC conceived and designed the study. YX, XH, WZ and were involved in the acquisition of data. YX and CY analyzed the data. YX wrote this paper. HB and XC contributed to the revision of manuscript for important intellectual content. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Cultivation of backup projects for National Science and Technology Awards (20223AEI91007), Jiangxi Science and Technology Innovation Base Plan - Jiangxi Clinical Medical Research Center (20223BCG74012), Science and Technology Innovation Base Construction Project (20221ZDG02010), Jiangxi Science and Technology Innovation Platform Project (20165BCD41005), Jiangxi Provincial Natural Science Foundation (20212ACB206019, 20224BAB206090), Key R&D Projects, Jiangxi (20203BBGL73173), Jiangxi Provincial Health Commission Science and Technology Project (202130440, 202210495, 202310528), Jiangxi Provincial Drug Administration Science and Technology Project (2022JS41), Fund project of the Second Affiliated Hospital of Nanchang University (2016YNQN12034, 2019YNLZ12010, 2021efyA01, 2021YNFY2024).
Acknowledgments
The investigators are grateful to the dedicated participants and all research staff of the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1134065/full#supplementary-material
Supplementary Figure 1 | Study flow diagram.
Supplementary Figure 2 | Represent a single mediator model used to test the association between WWI and Bapwv, SBP and DBP as mediators. (A) Path c, represents the simple total effect of WWI on baPWV, without adjusting mediators; (B) Path c, represents the direct (Path c’) and indirect effect (product of path a and b, ab) of WWI on Bapwv after controlling for the effect of the mediator.
References
1. Mitchell GF, Hwang S-J, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, et al. Arterial stiffness and cardiovascular events. CIRCULATION (2010) 121:505–11. doi: 10.1161/CIRCULATIONAHA.109.886655
2. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol (2010) 55:1318–27. doi: 10.1016/j.jacc.2009.10.061
3. Ohkuma T, Ninomiya T, Tomiyama H, Kario K, Hoshide S, Kita Y, et al. Brachial-ankle pulse wave velocity and the risk prediction of cardiovascular disease: An individual participant data meta-analysis. HYPERTENSION (2017) 69:1045–52. doi: 10.1161/HYPERTENSIONAHA.117.09097
4. Zheng M, Zhang X, Chen S, Song Y, Zhao Q, Gao X, et al. Arterial stiffness preceding diabetes: A longitudinal study. Circ Res (2020) 127:1491–8. doi: 10.1161/CIRCRESAHA.120.317950
5. Sheng CS, Li Y, Li LH, Huang QF, Zeng WF, Kang YY, et al. Brachial-ankle pulse wave velocity as a predictor of mortality in elderly Chinese. HYPERTENSION (2014) 64:1124–30. doi: 10.1161/HYPERTENSIONAHA.114.04063
6. Para I, Albu A, Porojan MD. Adipokines and arterial stiffness in obesity. Medicina (2021) 57:653. doi: 10.3390/medicina57070653
7. Wildman RP, Mackey RH, Bostom A, Thompson T, Sutton-Tyrrell K. Measures of obesity are associated with vascular stiffness in young and older adults. HYPERTENSION (2003) 42:468–73. doi: 10.1161/01.HYP.0000090360.78539.CD
8. Safar ME, Czernichow S, Blacher J. Obesity, arterial stiffness, and cardiovascular risk. J Am Soc Nephrol (2006) 17:S109–11. doi: 10.1681/ASN.2005121321
9. Petersen KS, Blanch N, JB K, Clifton PM. Effect of weight loss on pulse wave velocity. Arteriosclerosis Thrombosis Vasc Biol (2015) 35:243–52. doi: 10.1161/ATVBAHA.114.304798
10. Hughes TM, Althouse AD, Niemczyk NA, Hawkins MS, Kuipers AL, Sutton-Tyrrell K. Effects of weight loss and insulin reduction on arterial stiffness in the SAVE trial. Cardiovasc Diabetol (2012) 11:114. doi: 10.1186/1475-2840-11-114
11. Aminuddin A, Noor Hashim MF, Mohd Zaberi NAS, Zheng Wei L, Ching Chu B, Jamaludin NA, et al. The association between arterial stiffness and muscle indices among healthy subjects and subjects with cardiovascular risk factors: An evidence-based review. Front Physiol (2021) 12:742338. doi: 10.3389/fphys.2021.742338
12. Li G, Yao T, Wu X-W, Cao Z, Tu Y-C, Ma Y, et al. Novel and traditional anthropometric indices for identifying arterial stiffness in overweight and obese adults. Clin Nutr (2020) 39:893–900. doi: 10.1016/j.clnu.2019.03.029
13. Zhang J, Fang L, Qiu L, Huang L, Zhu W, Yu Y. Comparison of the ability to identify arterial stiffness between two new anthropometric indices and classical obesity indices in Chinese adults. ATHEROSCLEROSIS (2017) 263:263–71. doi: 10.1016/j.atherosclerosis.2017.06.031
14. Brunner EJ, Shipley MJ, Ahmadi-Abhari S, Tabak AG, McEniery CM, Wilkinson IB, et al. Adiposity, obesity, and arterial aging: longitudinal study of aortic stiffness in the Whitehall II cohort. HYPERTENSION (2015) 66:294–300. doi: 10.1161/HYPERTENSIONAHA.115.05494
15. Hu F, Yu R, Han F, Li J, Zhou W, Wang T, et al. Does body mass index or waist-hip ratio correlate with arterial stiffness based on brachial-ankle pulse wave velocity in Chinese rural adults with hypertension? BMC Cardiovasc DISOR (2021) 21:573. doi: 10.1186/s12872-021-02390-y
16. Huang J, Chen Z, Yuan J, Zhang C, Chen H, Wu W, et al. Association between body mass index (BMI) and brachial-ankle pulse wave velocity (baPWV) in males with hypertension: A community-based cross-section study in north China. Med Sci Monit (2019) 25:5241–57. doi: 10.12659/MSM.914881
17. Kim HL, Ahn DW, Kim SH, Lee DS, Yoon SH, Zo JH, et al. Association between body fat parameters and arterial stiffness. Sci Rep (2021) 11:20536. doi: 10.1038/s41598-021-00175-z
18. Liao Y, Chu C, Wang Y, Zheng W-L, Ma Q, Hu J-W, et al. Sex differences in impact of long-term burden and trends of body mass index and blood pressure from childhood to adulthood on arterial stiffness in adults: A 30-year cohort study. ATHEROSCLEROSIS (2020) 313:118–25. doi: 10.1016/j.atherosclerosis.2020.10.003
19. Park Y, Kim NH, Kwon TY, Kim SG. A novel adiposity index as an integrated predictor of cardiometabolic disease morbidity and mortality. Sci REP-UK (2018) 8:16753. doi: 10.1038/s41598-018-35073-4
20. Kim NH, Park Y, Kim NH, Kim SG. Weight-adjusted waist index reflects fat and muscle mass in the opposite direction in older adults. AGE Ageing (2021) 50:780–6. doi: 10.1093/ageing/afaa208
21. Safar ME, Asmar R, Benetos A, Blacher J, Boutouyrie P, Lacolley P, et al. Interaction between hypertension and arterial stiffness. HYPERTENSION (2018) 72:796–805. doi: 10.1161/HYPERTENSIONAHA.118.11212
22. Wang Z, Chen Z, Zhang L, Wang X, Hao G, Zhang Z, et al. Status of hypertension in China: Results from the China hypertension survey, 2012-2015. CIRCULATION (2018) 137:2344–56. doi: 10.1161/CIRCULATIONAHA.117.032380
23. Williams B, Mancia G, Spiering W, Agabiti RE, Azizi M, Burnier M, et al. ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J (2018) 39:3021–104. doi: 10.1093/eurheartj/ehy339
24. Li Q, Qie R, Qin P, Zhang D, Guo C, Zhou Q, et al. Association of weight-adjusted-waist index with incident hypertension: The rural Chinese cohort study. Nutrition metabolism Cardiovasc Dis (2020) 30:1732–41. doi: 10.1016/j.numecd.2020.05.033
25. Xiong Y, Yu Y, Cheng J, Zhou W, Bao H, Cheng X. Association of sleep duration, midday napping with atrial fibrillation in patients with hypertension. Clin Epidemiol (2022) 14:385–93. doi: 10.2147/CLEP.S351045
26. Li M, Zhan A, Huang X, Hu L, Zhou W, Wang T, et al. Positive association between triglyceride glucose index and arterial stiffness in hypertensive patients: the China h-type hypertension registry study. Cardiovasc Diabetol (2020) 19:139. doi: 10.1186/s12933-020-01124-2
27. Chen C, Lu FC. The guidelines for prevention and control of overweight and obesity in Chinese adults. BioMed Environ Sci (2004) 17 Suppl:1–36.
28. Liu Y, Liu X, Zhang S, Zhu Q, Fu X, Chen H, et al. Association of anthropometric indices with the development of diabetes among hypertensive patients in China: A cohort study. Front Endocrinol (2021) 12:736077. doi: 10.3389/fendo.2021.736077
29. Wu L, Zhu W, Qiao Q, Huang L, Li Y, Chen L. Novel and traditional anthropometric indices for identifying metabolic syndrome in non-overweight/obese adults. Nutr Metab (2021) 18:3. doi: 10.1186/s12986-020-00536-x
30. Ding C, Shi Y, Li J, Li M, Hu L, Rao J, et al. Association of weight-adjusted-waist index with all-cause and cardiovascular mortality in China: A prospective cohort study. Nutr Metab Cardiovasc Dis (2022) 32:1210–7. doi: 10.1016/j.numecd.2022.01.033
31. Rodriguez A, Ezquerro S, Mendez-Gimenez L, Becerril S, Fruhbeck G. Revisiting the adipocyte: a model for integration of cytokine signaling in the regulation of energy metabolism. Am J Physiol Endocrinol Metab (2015) 309:E691–714. doi: 10.1152/ajpendo.00297.2015
32. Recio-Rodriguez JI, Gomez-Marcos MA, Patino-Alonso MC, Agudo-Conde C, Rodriguez-Sanchez E, Garcia-Ortiz L. Abdominal obesity vs general obesity for identifying arterial stiffness, subclinical atherosclerosis and wave reflection in healthy, diabetics and hypertensive. BMC Cardiovasc Disord (2012) 12:3. doi: 10.1186/1471-2261-12-3
Keywords: weight-adjusted waist index, arterial stiffness, BMI, hypertension, obesity
Citation: Xiong Y, Shi W, Huang X, Yu C, Zhou W, Bao H and Cheng X (2023) Association between weight-adjusted waist index and arterial stiffness in hypertensive patients: The China H-type hypertension registry study. Front. Endocrinol. 14:1134065. doi: 10.3389/fendo.2023.1134065
Received: 29 December 2022; Accepted: 28 February 2023;
Published: 17 March 2023.
Edited by:
Changhee Jung, University of Ulsan, Republic of KoreaReviewed by:
Magdalena Olszanecka-Glinianowicz, Medical University of Silesia, PolandVannina Gonzalez Marrachelli, University of Valencia, Spain
Copyright © 2023 Xiong, Shi, Huang, Yu, Zhou, Bao and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huihui Bao, aHVpaHVpX2Jhbzc3QDEyNi5jb20=; Xiaoshu Cheng, eGlhb3NodW1lbmZhbjEyNkAxNjMuY29t
 Yurong Xiong
Yurong Xiong Weidong Shi4
Weidong Shi4 Xiao Huang
Xiao Huang Chao Yu
Chao Yu Wei Zhou
Wei Zhou Huihui Bao
Huihui Bao Xiaoshu Cheng
Xiaoshu Cheng