- 1Assisted Reproduction Center, Northwest Women’s and Children’s Hospital, Xi’an, China
- 2Translational Medicine Center, Northwest Women’s and Children’s Hospital, Xi’an, China
Introduction: In frozen-thawed embryo transfer (FET) cycles, hormone replacement treatment (HRT) was associated with a higher risk of hypertensive disorders of pregnancy (HDP) compared with natural cycles (NC). Multiple pregnancy was a risk factor for HDP and several studies did not conduct subgroup analysis of singleton pregnancy and multiple pregnancy.
Objective: To investigate whether HRT regimen could be a risk factor for HDP in women undergoing FET cycles in singleton and twin pregnancies.
Methods: A retrospective cohort study at a tertiary hospital, including a total of 9120 women who underwent FET and achieved ongoing pregnancy; 7590 patients underwent HRT-FET and 1530 NC-FET. The main outcome was HDP. HDP were analyzed for singleton and twin pregnancies, respectively.
Results: In the singleton pregnancy, the risk of HDP in the HRT-FET group was significantly higher than that in the NC-FET group (6.21% vs. 4.09%; P=0.003). After adjusting for female age oocyte pick up, female age at FET and body mass index (BMI), HRT was found as a risk factor for HDP (adjusted odds ration [aOR]: 1.43; 95% confidence interval [CI]: 1.07 to 1.91; P=0.017). In the multiple pregnancy, the risk of HDP in the HRT-FET and NC-FET groups was similar.
Conclusion: HRT was associated with a higher risk of HDP in women who underwent FET and achieved singleton pregnancy.
Introduction
The application of frozen-thawed embryo transfer (FET) has dramatically increased worldwide over the past decade for its merits. The FET enables the storage of excess embryos, reduces the incidence of ovarian hyperstimulation syndrome (OHSS), provides time required for preimplantation genetic testing (PGT), and facilitates fertility preservation. However, several studies have suggested that compared with fresh embryo transfer, the FET is associated with the reduced incidence rates of preterm birth, low birthweight (LBW), small for gestational age (SGA), and perinatal mortality (1). However, the safety of FET is challenged by the increased incidence rates of large for gestational age (LGA) and hypertensive disorders of pregnancy (HDP) (2).
The commonly used endometrial preparation regimens for FET include natural cycle (NC) and hormone replacement treatment (HRT) cycle. Given that the HRT cycles rely on exogenous hormone supplement and lack of corpus luteum, this condition might be less ‘physiological’ than a natural ovulatory cycle. Recent studies reported that HRT-FET were associated with a higher risk of HDP compared with NC-FET. However, several studies did not conduct subgroup analysis of singleton pregnancy and multiple pregnancy. Moreover, multiple pregnancy was reported as a risk factor for HDP, indicating its importance. Besides, the effects of the endometrium preparation method on the outcomes of pregnancies conceived with FET have not been fully clarified.
The present study aimed to assess the effects of different FET regimens on the risk of HDP in women who underwent FET cycles, and the risk of HDP was analyzed in singleton pregnancy and multiple pregnancy. This retrospective cohort study was conducted to compare the risk of HDP between NC-FET and HRT-FET groups.
Materials and methods
Study design and patients
This was a retrospective cohort study, in which the in vitro fertilization (IVF) was performed from January 2018 to December 2020 in the Center for Assisted Reproductive Technology of Northwest Women’s and Children’s Hospital (Xi’an, China). The protocol of the study was approved by the institutional review board of the hospital. Data were extracted from electronic medical records. Patients who underwent FET and achieved ongoing pregnancy were enrolled. Ongoing pregnancy was defined as the presence of at least one fetal heart pulsation on ultrasound beyond 20 weeks. All patients were enrolled only once. Women with chronic hypertension before pregnancy were excluded. Written informed consent was obtained from the participants before treatment.
Controlled ovarian stimulation and vitrified cryopreservation
Ovarian stimulation protocols included gonadotropin-releasing hormone (GnRH) agonist protocol, GnRH antagonist protocol, and progestin-primed ovarian stimulation (PPOS) protocol. Recombinant human chorionic gonadotropin (OVIDREL; Merck Serono, Darmstadt, Germany) or GnRH-a (Decapeptyl; Ferring, Saint-Prex, Switzerland) were administered in patients when two leading follicles reached 18 mm in diameter. Oocyte retrieval was performed at 36 h after recombinant human chorionic gonadotropin or GnRH-a triggered by transvaginal ultrasound-guided aspiration. Insemination method was selected according to the sperm count after sperm preparation. A morphologic score of cleavage-stage embryo was given based on the number of blastomeres, the homogeneous degree of blastomeres, and the degree of cytoplasmic fragmentation, which has been extensively described in our previous study (3). If a couple has two or more high-quality cleavage-stage embryos on day 3 of embryo culture, the embryos were selected and cultured to blastocyst stage. Blastocyst evaluation was performed according to the Gardner’s grading system (4).
For patients who underwent GnRH agonist protocol and GnRH antagonist protocol, one to two fresh embryos were transferred into the uterus of women free of OHSS, hydrosalpinx, intrauterine adhesion and high progesterone level (> 1.5 ng/ml) on the day of triggering, and then, the spare embryos were cryopreserved for the next FET. Patients who underwent PPOS protocol had to freeze all their embryos. The vitrified cryopreservation was conducted according to standard protocols, as previously described (5).
Endometrial preparation before FET
The selection of FET regimen is performed based on patients’ conditions, including menstrual regularity, ovulation regularity, doctors’ preference, endometrial development, and the prevalence of endometriosis and adenomyosis. For instance, patients with regular menstrual cycles and ovulation mainly undergo NC-FET. Patients with ovulation disorders or impaired endometrium development often undergo HRT-FET, because these patients have trouble in preparing the endometrium with natural ovulation. Meanwhile, HRT-FET is also selected due to the convenience of scheduling the date of FET. Patients with endometriosis, adenomyosis or recurrent implantation failure mainly undergo combination of GnRH-a and HRT-FET.
In this study, patients in the NC-FET group underwent transvaginal ultrasound on days 8 to 10 of the menstrual cycle. Follicular growth was monitored through transvaginal ultrasound and measurement of serum luteinizing hormone (LH). When the leading follicle had reached a mean diameter of >17 mm and the serum LH level was 20 IU/L, the transvaginal ultrasound was performed every day until ovulation. The day of ovulation was confirmed by transvaginal ultrasound. Cleavage-stage embryo and blastocyst-stage embryo were thawed and transferred on 3 and 5 days after ovulation, respectively.
For patients in the HRT-FET group, endometrial preparation was initiated with oral estradiol valerate (Progynova; Bayer, Berlin, Germany) at a daily dose of 4 mg from day 5 of menstrual cycle. For patients with impaired endometrial development, a daily maximum dose of 6 mg oral estradiol valerate and 3 mg transdermal 17-β estradiol (Besins Healthcare, Paris, France) were given. The serum progesterone level was measured and the transvaginal ultrasound was performed 10-12 days after the usage of exogenous estrogen. When the endometrial thickness reached 7 mm or more and the serum progesterone level was <1.5 ng/mL, exogenous progesterone was added. The FET was scheduled for 5 days for cleavage-stage embryos and for 7 days for blastocyst-stage embryos.
Luteal support
Three methods of luteal support are implemented in our center. I. Vaginal progesterone gel (90 mg q.d; Crinone, Serono, Hertfordshire, UK); II. Vaginal progesterone soft capsules (0.2 g t.i.d; Utrogestan, Besins, France); III. Intramuscular progesterone (60 mg q.d; Xianju, Zhejiang, China). Patients from both groups could select one of these three luteal support methods and receive oral progesterone (10 mg t.i.d; Dydrogesterone, Abbott Biologicals B.V., Amsterdam, Netherlands) simultaneously. For patients who underwent HRT-FET, exogenous estrogen would be reduced after the confirmation of clinical pregnancy. The luteal support was maintained until week 10 of gestation.
Definition of outcomes
The primary outcome was the risk of HDP. It was attempted to define HDP as sustained (on at least two occasions 6 h apart) blood pressure ≥ 140/90 mmHg after 20 weeks, with or without proteinuria and other signs or symptoms of preeclampsia and without a history of hypertension. As secondary outcomes, we analyzed some other perinatal risks and neonatal risks. Other perinatal risks included gestational diabetes mellitus (GDM), placenta previa, premature rupture of membrane, anemia, miscarriage, and stillbirth. Miscarriage was defined as the spontaneous loss of clinical pregnancy before 28 weeks of gestational age. Stillbirth was defined as the absence of signs of life at or after 28 weeks of gestation. Neonatal risks included preterm birth (<37 weeks’ gestation), extremely preterm birth (<32 weeks’ gestation), low birth weight (<2500 g), very low birth weight (<1500 g), and macrosomia (birth weight ≥4000 g) (6).
Statistical analysis
Categorical variables were presented as count and proportion; normally distributed continuous variables were expressed as the mean and standard deviation, and abnormally distributed continuous variables were presented as the median and interquartile range (IQR). The Chi-squared test or the Fisher’s exact test were utilized to compare the categorical variables. The Student’s t-test was used to compare the continuous variables. The effects of different FET regimens on the risk of HDP and other outcomes were estimated using the generalized linear model adjusted for female age at oocyte pick up (OPU), female age at FET and body mass index (BMI). To assess the influence of potential heterogeneity on the risk of HDP, the effects of different FET regimens were estimated in several subgroups. All data were analyzed using the SPSS 22.0 software (IBM Corp., Armonk, NY, USA). The level of significance was set at P < 0.05.
Results
Baseline characteristics of patients
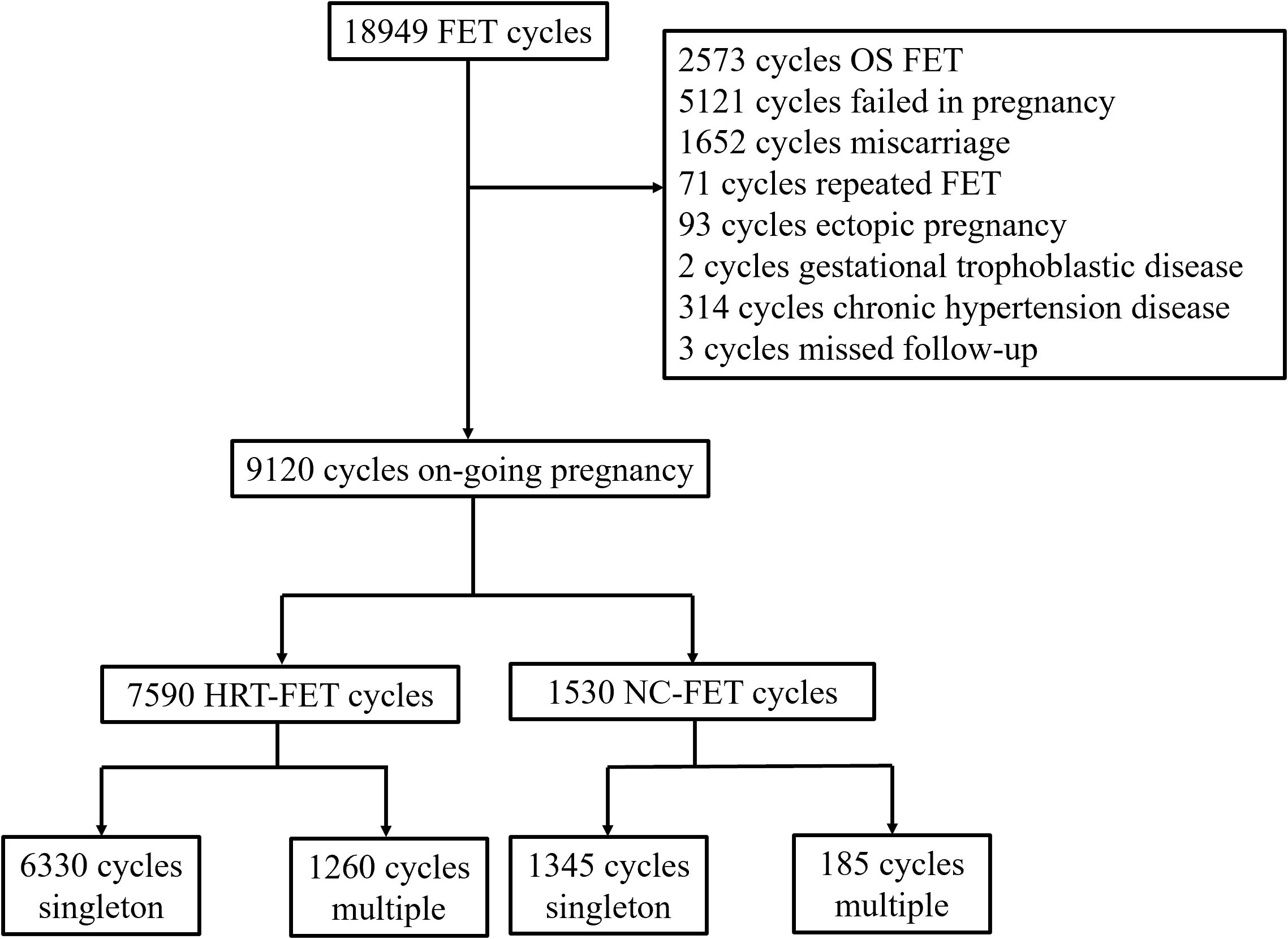
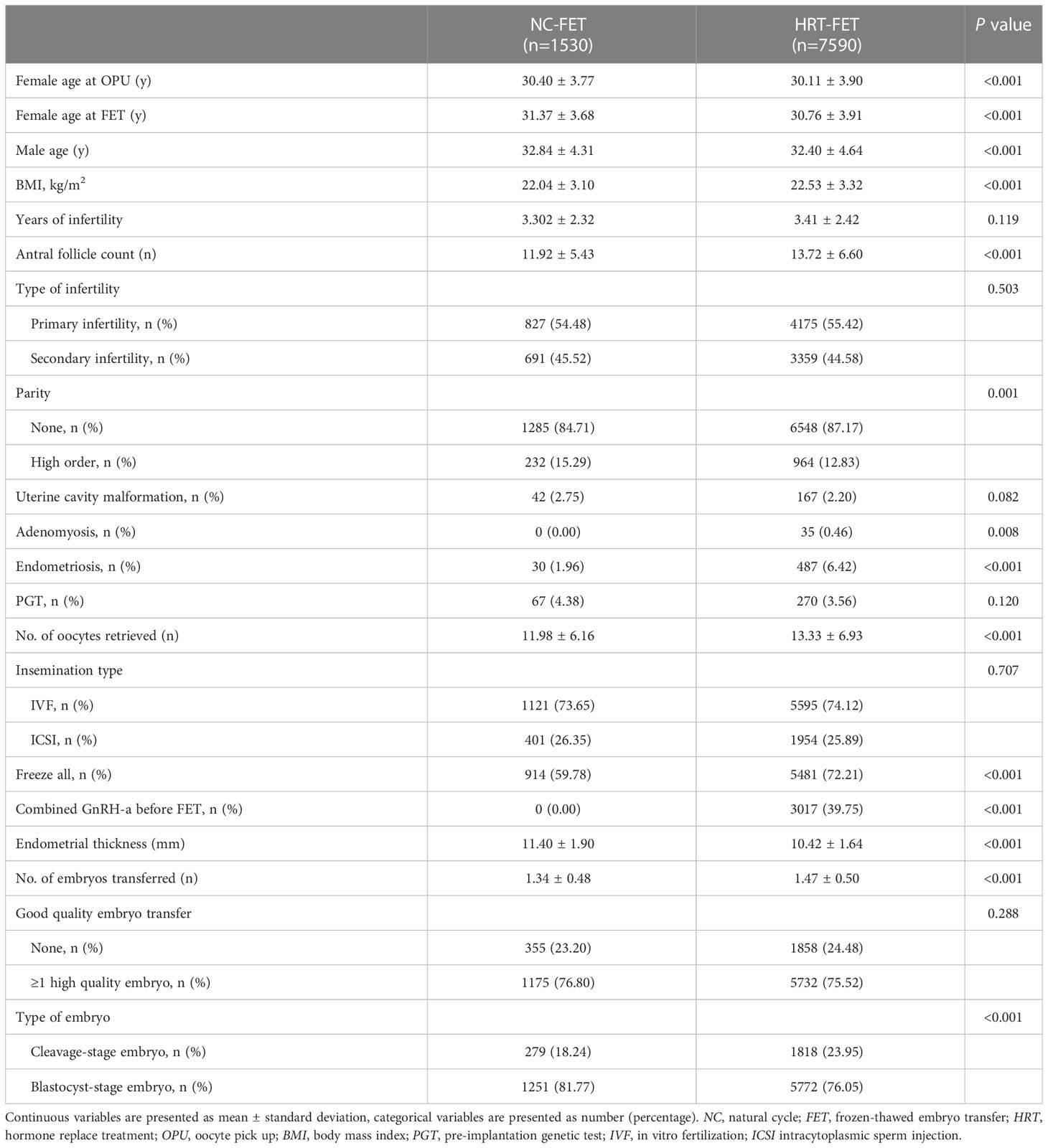
A total of 9120 patients who fulfilled the inclusion and exclusion criteria were included in this study (Figure 1). Of these, 7590 and 1530 patients were in HRT-FET and NC-FET groups, respectively. The baseline characteristics of patients are shown in Table 1. Women who underwent HRT-FET were significantly younger at OPU (30.11 ± 3.90 vs. 30.40 ± 3.77, P<0.001) and FET (30.76 ± 3.91 vs. 31.37 ± 3.68, P<0.001) compared with those in the NC-FET group. Husbands of women in the HRT-FET group were significantly younger than those of women in the NC-FET group. Besides, a significantly higher BMI, a greater antral follicle count (AFC), a higher number of nulliparous women, and higher incidence rates of adenomyosis and endometriosis were detected in the HRT-FET group compared with the NC-FET group. After ovarian stimulation, the number of oocytes retrieved in the HRT-FET group was significantly greater than that in the NC-FET group. More patients in the HRT-FET group froze all their embryos compared with the NC-FET group. The endometrial thickness in the HRT-FET group was significantly thinner than that in the NC-FET group. In terms of the number of embryos transferred, it was higher in the HRT-FET group compared with that in the NC-FET group. The proportion of D3 cleavage-stage embryo transfer was significantly higher in the HRT-FET group compared with that in the NC-FET group. There were no significant differences in infertility duration, infertility type, proportion of patients with uterine cavity malformation, proportion of patients undergoing PGT, insemination type, and proportion of patients transferred at least one high-quality embryo between the two groups.
Different FET regimens and the risk of HDP
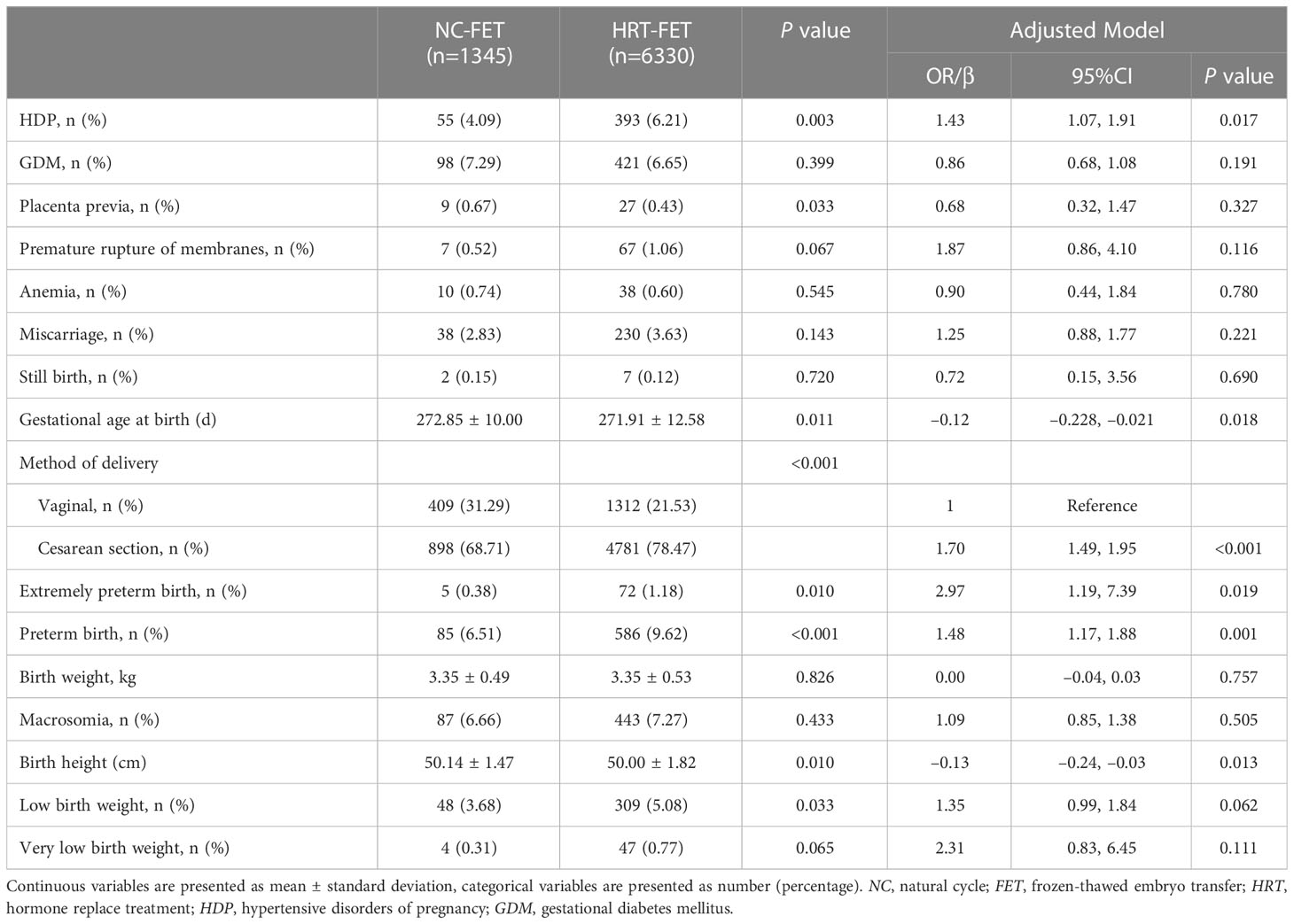
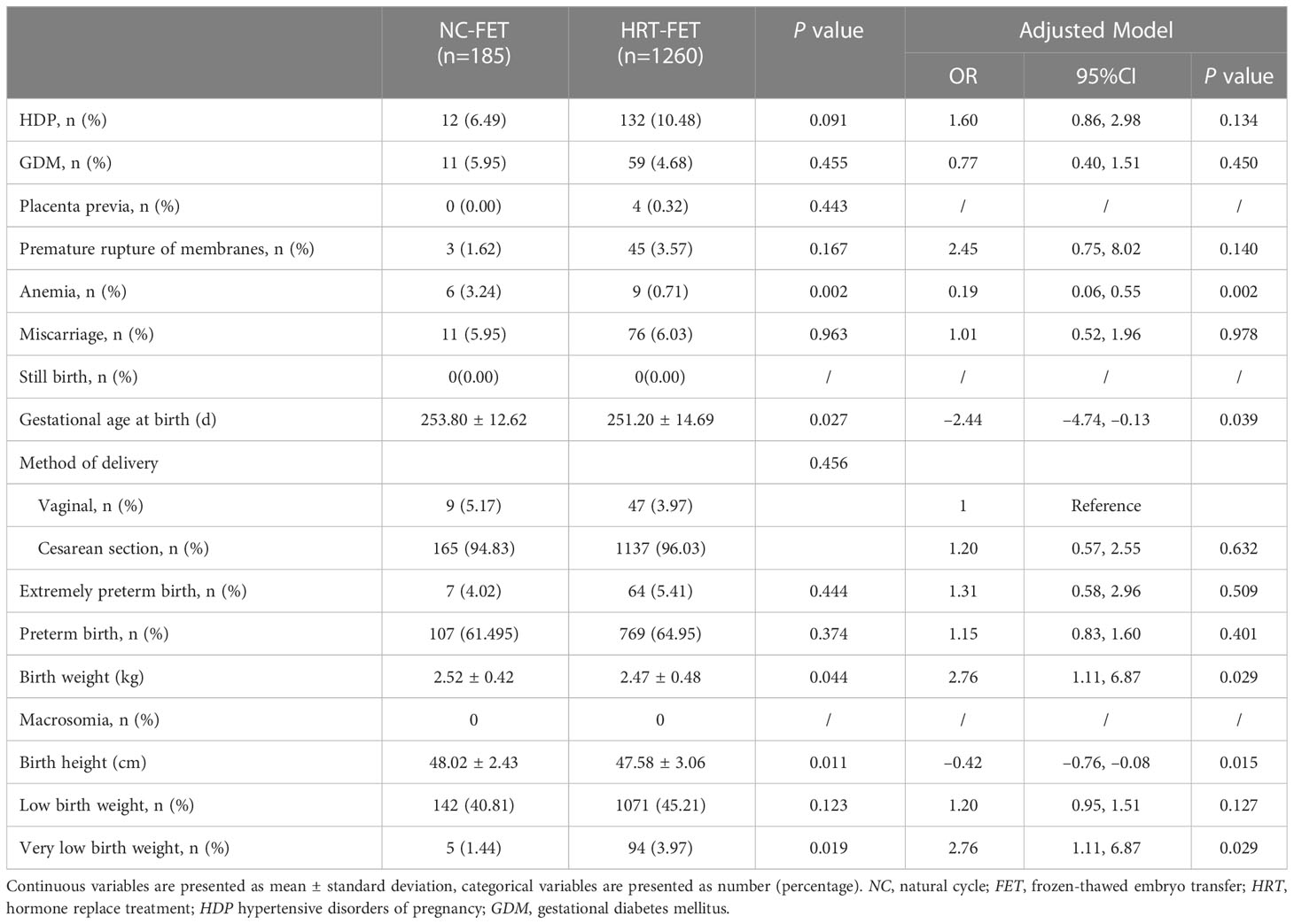
The maternal and neonatal outcomes were categorized by the type of FET regimen. The risk of HDP was regarded as the primary outcome. Perinatal outcomes were analyzed in singleton pregnancy (Table 2) and twin pregnancy (Table 3), respectively. In the singleton pregnancy, the risk of HDP in the HRT-FET group was significantly higher than that in the NC-FET group (6.21% vs. 4.09%, P=0.003). After adjusting for confounders, including female age at OPU, female age at FET and BMI, the HRT-FET group was associated with a higher risk of HDP compared with the NC-FET group in singleton pregnancy (adjusted odds ratio (aOR): 1.43; 95% confidence interval (CI): 1.07 to 1.91; P=0.017). In the twin pregnancy, the risk of HDP was similar between the HRT-FET and NC-FET groups (10.48% vs. 6.49%, P=0.091).
Different FET regimens and other perinatal outcomes
In the singleton pregnancy (Table 2), a higher cesarean section rate (78.47% vs. 68.71%, P<0.001) and a shorter gestational age at birth (271.91 ± 12.58 vs. 272.85 ± 10.00, P=0.011) were found in the HRT-FET group compared with the NC-FET group. This association remained essentially unchanged after adjusting for female age at oocyte retrieval, female age at FET and BMI (aOR: 1.70; 95% CI: 1.49 to 1.95; P<0.001) (aOR: −0.12; 95% CI: −0.23 to −0.02; P=0.018). A lower rate of placenta previa was detected in the NC-FET group compared with that in the HRT-FET group (0.63% vs. 0.67%), and this association was not significant after adjusting for covariates (aOR: 0.68; 95% CI: 0.32 to 1.47; P=0.327). Other perinatal outcomes, including the rates of GDM, premature rupture of membranes, anemia, miscarriage, and stillbirth were similar between the two groups in the singleton pregnancy (P≥0.05).
In the multiple pregnancy (Table 3), a lower rate of anemia (0.71% vs. 3.24%, P=0.002) and a shorter gestational age at birth (251.20 ± 14.69 vs. 253.80 ± 12.62, P=0.027) were identified in the HRT-FET group compared with those in the NC-FET group. This association remained essentially unchanged after adjusting for female age at oocyte retrieval, female age at FET and BMI (aOR: 0.19; 95% CI: 0.09 to 0.55; P=0.002) (aOR: −2.44; 95% CI: −4.74 to −0.13; P=0.039). Other perinatal outcomes, including the rates of GDM, placenta previa, premature rupture of membranes, miscarriage, stillbirth, and method of delivery were similar between the two groups in the multiple pregnancy (P≥0.05).
Different FET regimens and neonatal outcomes
In the singleton pregnancy (Table 2), the rates of extremely preterm birth (1.18% vs. 0.38%, P=0.010) and preterm birth (9.62% vs. 6.51%, P<0.001) were significantly higher in the HRT-FET group than those in the NC-FET group. This association remained essentially unchanged after adjusting for female age at oocyte retrieval, female age at FET and BMI (aOR: 2.97; 95% CI: 1.19 to 7.39; P=0019) (aOR: 1.48; 95% CI: 1.17 to 1.88; P=0.001). Meanwhile, the birth height in the HRT-FET group (47.58 ± 3.06 cm) was significantly shorter than that in the NC-FET group (48.02 ± 2.43 cm, P=0.011). This association remained essentially unchanged after adjustment (aOR: −0.013; 95% CI: −0.24 to −0.03; P=0.013). Low birth weight rate was significantly higher in the HRT-FET group compared with that in the NC-FET group (5.08% vs. 3.68%, P=0.033), and this association was not significant after adjusting for female age at OPU, female age at FET and BMI (aOR: 1.35; 95% CI: 0.99 to 1.84; P=0.062). Other neonatal outcomes, including birth weight, macrosomia rate, and very low birth weight rate were similar between the two groups in the singleton pregnancy (P≥0.05).
In the multiple pregnancy (Table 3), the birth weight in the HRT-FET group was significantly lower (2.47 ± 0.48 vs. 2.52 ± 0.42 kg, P=0.044), and birth height (47.58 ± 3.06 vs. 48.02 ± 2.42 cm, P=0.011) was significantly shorter compared with the NC-FET group. The rate of very low birth weight (3.97% vs. 1.44% P=0.019) was significantly higher in the HRT group. These associations remained essentially unchanged after adjustment (aOR: 2.76; 95% CI: 1.11 to 6.87; P=0.029). There was no macrosomia in both groups. Other neonatal outcomes, including extremely preterm birth rate, preterm birth rate, birth weight, and low birth weight rate were similar between the two groups in the multiple pregnancy (P≥0.05).
Subgroup analysis
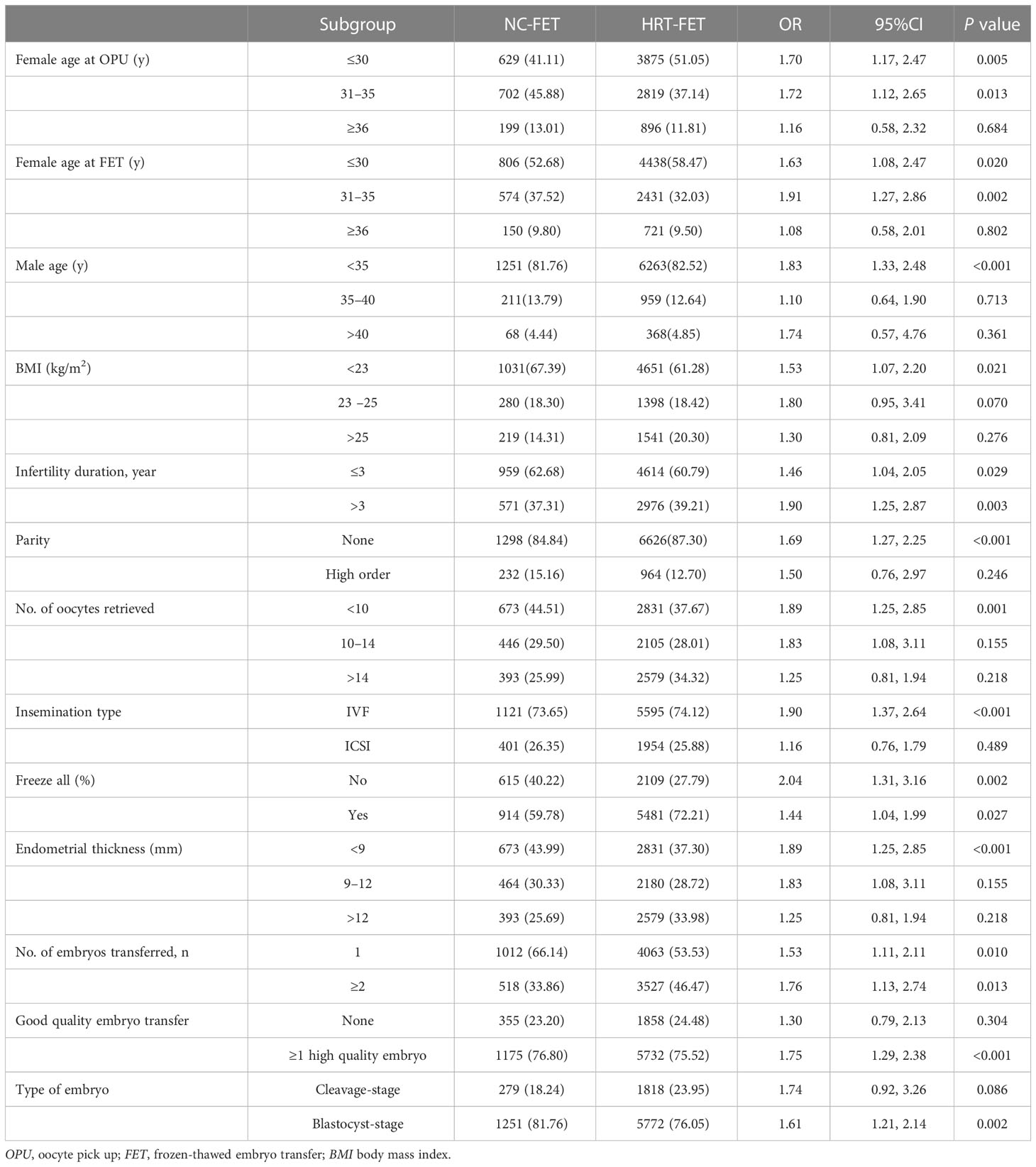
The effects of two different FET regimens on the risk of HDP were analyzed in different subgroups (Table 4; Supplementary Figure 1). It was revealed that HRT-FET was associated with a higher risk of HDP in all the subgroups of women with different infertility durations, whether they attempted to freeze all their embryos or not and the number of embryos transferred, in which these associations were statistically significant (P<0.05). For the other subgroups, HRT-FET was associated with a higher risk of HDP compared with NC-FET. Meanwhile, those associations were statistically significant for the subgroups of female age at OPU (≤ 30, between 31 and 35), female age at FET (≤ 30, between 31 and 35), male age (≤ 35), female BMI (< 23 kg/m2), nulliparity, number of oocytes retrieved (< 10), insemination method (IVF), transfer of ≥1 high-quality embryo, and transfer of blastocyst-stage embryo (P <0.05).
Discussion
Different FET regimens and the risk of HDP
Endometrial preparation protocols are commonly categorized into two categories: with and without corpus luteum protocols. HRT protocols combined with or without GnRH-a were classified into artificial preparation without corpus luteum, while with exogenous steroid. For patients with ovulation disorders, monitoring of ovarian follicular development is particularly troublesome. Preparing the endometrium with exogenous hormones is associated with some advantages, such as monitoring and scheduling of the timing of the procedure, making it more convenient and simpler. Therefore, HRT-FET cycle accounted for the majority of our FET cycles.
Hypertensive disorders were reported in 5.9% of assisted reproductive technology (ART) singleton and 12.6% of ART twin pregnancies (7). Multiple pregnancy was reported as a risk factor for HDP. Previous relevant studies have mainly compared the risk of HDP in NC and HRT groups without separate analysis of singleton pregnancy and multiple pregnancy. Therefore, we analyzed and reported the risk of HDP in singleton pregnancy and multiple pregnancy, respectively.
In our large retrospective cohort study of over 9120 FET cycles, the risk of HDP in the HRT-FET group was risen by 2.12% in the singleton pregnancy compared with that in the NC-FET group. In the multiple pregnancy, the risk of HDP in the HRT-FET group was escalated by 3.99% compared with that in the NC-FET group. This finding is similar to previously reported outcome (2, 8–10). Our results strengthened the pivotal etiopathogenic role of the corpus luteum. In HRT-FET cycles, the absence of corpus luteum was inevitably resulted in the lack of circulating vasoactive factors, such as relaxin (11), prorenin, and renin (12). These circulating vasoactive factors were required for maternal cardiovascular adaptation during the first trimester of pregnancy. Then, the lack of these circulating vasoactive factors may lead to the disruption of maternal circulatory adaptation. Von Versen-Höynck (11, 13) reported the decline of carotid-femoral pulse-wave velocity (cfPWV) and the increase of femoral pulse-wave transit time (fPWTT) during the first trimester in pregnant women who underwent HRT-FET compared with those who underwent NC-FET and fresh transfer.
Chen et al. (14) reported a significantly higher (two times) risk of preeclampsia in HRT-FET cycles compared with that in fresh transfer cycles in women who were diagnosed with polycystic ovary syndrome. The possible reason is that the supra-physiologic number of corpora lutea in fresh transfer cycles could produce the circulating vasoactive factors and protect pregnancies against HDP.
Another possible mechanism underlining the increased risk of HDP after HRT-FET may be attributed to the prematurely elevated level of estradiol. A low estradiol level during implantation allows for extravillous trophoblasts into uterine spiral arteries with vascular remodeling, and elevation of estradiol level later prevents further remodeling (15). In HRT-FET cycles, estradiol level is elevated more prematurely than that in NC-FET cycles. Trophoblastic invasion of spiral arteries may be suppressed by the prematurely elevated estradiol level (16). A retrospective study reported a lower risk of HDP in letrozole-induced FET cycles than that in HRT-FET cycles (17). The administration of letrozole during modified NC-FET cycles might potentially lower estradiol rise and optimal extravillous trophoblasts into uterine spiral arteries with vascular remodeling.
For China the average rate for cesarean section was 54.9 percent in 2014 (18). Higher rates of cesarean section were reported among women who get pregnant after ART. Meanwhile, patients present multiple pregnancy are prone to have greater need for cesarean section. These may be the reasons for the high rate of cesarean section in this study.
Strengths and limitations
The major strength of this study is the large cohort size from a single-center, in which practice consistency can be assured. Controlled ovarian stimulation, IVF protocols, and laboratory conditions remained homogeneous. Additionally, maternal and neonatal outcomes of singleton and multiple pregnancies were analyzed separately, because multiple pregnancy was reported as a risk factor for HDP (19). Similar to multiple pregnancy, chronic hypertension, BMI>30 kg/m2, and female age were previously found as risk factors for HDP (20–22). We excluded women with chronic hypertension. Meanwhile, we adjusted female age at OPU, female age at FET and BMI in the assessment of the effects of different FET regimens on the risk of HDP. These analyses made our results more reliable.
Our study had several limitations. Firstly, this was a single-center retrospective study, in which inherent bias was inevitable. Regarding this deficiency, we screened patients with strict criteria.
Secondly, endometrial preparation was not randomly assigned in our study population, and imbalance in the number of enrolled patients in the two groups should be noted. However, ovulatory dysfunction was noted as the main indicator to pick HRT-FET rather than NC-FET. Ovulatory dysfunction was identified in approximately 15% of all infertile couples and accounted for up to 40% of female infertility (23). In our study, the number of patients in the HRT-FET group was 3.96 times greater than that in the NC-FET group. The main advantages of HRT-FET were its convenience, low-cost, and simplicity. Patients who underwent HRT-FET only need to visit the doctor for 2-3 times during their endometrial preparation period. Nevertheless, the imbalance in the number of study subjects could also be found in a previous study (24).
Thirdly, we failed in exact classification of patients into HDP categories after telephone follow-up. It is noteworthy that gestational hypertension and preeclampsia are involved in HDP (25). We followed up patients by telephone at one month after their expected date of delivery, thus, recall bias might be existed. Besides, some patients could not remember the exact diagnosis of HDP.
Conclusion
In conclusion, HRT was found to be associated with a higher risk of HDP in women who underwent FET and achieved singleton pregnancy. However, further large-scale, prospective, randomized controlled trials with a longer follow-up are required to verify the increased risk of HDP in women undergoing HRT-FET.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Northwest Women’s and Children’s Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
LF and NL: designed study, drafted the manuscript and reviewed the manuscript. XitL, XiaL, HC, DP, TW, WS and PQ: analyzed data. JS: Study conceptualization. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Shaanxi Provincial Department of science and technology (2022SF-564).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1133978/full#supplementary-material
Supplementary Figure 1 | Subgroup analysis of different FET regimens on HDP.
References
1. Trounson A, Mohr L. Human pregnancy following cryopreservation, thawing and transfer of an eight-cell embryo. Nature (1983) 305(5936):707–9. doi: 10.1038/305707a0
2. Saito K, Kuwahara A, Ishikawa T, Saito H, Morisaki N, Miyado M, et al. Endometrial preparation methods for frozen-thawed embryo transfer are associated with altered risks of hypertensive disorders of pregnancy, placenta accreta, and gestational diabetes mellitus. Hum Reprod (2019) 34(8):1567–75. doi: 10.1093/humrep/dez079
3. Li M, Wang H, Ma C, Shi J. Transferring two grades I cleavage-stage embryo might not be a good protocol. Gynecol Endocrinol (2017) 33(7):557–9. doi: 10.1080/09513590.2017.1302420
4. Gardner DK, Schoolcraft WB. Culture and transfer of human blastocysts. Curr Opin Obstet Gynecol (1999) 11(3):307–11. doi: 10.1097/00001703-199906000-00013
5. Shi W, Xue X, Zhang S, Zhao W, Liu S, Zhou H, et al. Perinatal and neonatal outcomes of 494 babies delivered from 972 vitrified embryo transfers. Fertil Steril (2012) 97(6):1338–42. doi: 10.1016/j.fertnstert.2012.02.051
6. Brown CC, Moore JE, Felix HC, Tilford JM, Bird TM, Lowery CL, et al. Association of state medicaid expansion status with low birth weight and preterm birth. JAMA (2019) 321(16):1598–609. doi: 10.1001/jama.2019.3678
7. Opdahl S, Henningsen AA, Tiitinen A, Bergh C, Pinborg A, Romundstad PR, et al. Risk of hypertensive disorders in pregnancies following assisted reproductive technology: a cohort study from the CoNARTaS group. Hum Reprod (2015) 30(7):1724–31. doi: 10.1093/humrep/dev090
8. Wang B, Zhang J, Zhu Q, Wang Y. Effects of different cycle regimens for frozen embryo transfer on perinatal outcomes of singletons. Hum Reprod (2020) 35(7):1612–22. doi: 10.1093/humrep/deaa093
9. Asserhøj LL, Spangmose AL, Aaris Henningsen AK, Pinborg A. Adverse obstetric and perinatal outcomes in 1,136 singleton pregnancies conceived after programmed frozen embryo transfer (FET) compared with natural cycle FET. Fertil Steril (2021) 115(4):947–56. doi: 10.1016/j.fertnstert.2020.10.039
10. Zaat TR, Brink AJ, de Bruin JP, Mol F. Increased obstetric and neonatal risks in artificial cycles for frozen embryo transfers? Reprod BioMed Online (2021) 42(5):919–29. doi: 10.1016/j.rbmo.2021.01.015
11. von Versen-Ho¨ynck F, Schaub AM, Chi Y-Y, Chiu K-H, Liu J, Lingis M, et al. Increased preeclampsia risk and reduced aortic compliance with in vitro fertilization cycles in the absence of a corpus luteum. Hypertension (2019) 73(3):640–9. doi: 10.1161/HYPERTENSIONAHA.118.12043
12. Wiegel RE, Jan Danser AH, Steegers-Theunissen RPM, Laven JSE, Willemsen SP, Baker VL, et al. Determinants of maternal renin-angiotensin-aldosterone-system activation in early pregnancy: insights from 2 cohorts. J Clin Endocrinol Metab (2020) 105(11):3505–17. doi: 10.1210/clinem/dgaa582
13. von Versen-Höynck F, Narasimhan P, Selamet Tierney ES, Martinez N, Conrad KP, Baker VL, et al. Absent or excessive corpus luteum number is associated with altered maternal vascular health in early pregnancy. Hypertention (2019) 73(3):680–90. doi: 10.1161/HYPERTENSIONAHA.118.12046
14. Chen ZJ, Shi Y, Sun Y, Zhang B, Liang X, Cao Y, et al. Fresh versus frozen embryos for infertility in the polycystic ovary syndrome. New Engl J Med (2016) 375(6):523–33. doi: 10.1056/NEJMoa1513873
15. Bonagura TW, Pepe GJ, Enders AC, Albrecht ED. Suppression of extravillous trophoblast vascular endothelial growth factor expression and uterine spiral artery invasion by estrogen during early baboon pregnancy. Endocrinology (2008) 149(10):5078–87. doi: 10.1210/en.2008-0116
16. Bonagura TW, Babischkin JS, Aberdeen GW, Albrecht ED. Prematurely elevating estradiol in early baboon pregnancy suppresses uterine artery remodeling and expression of extravillous placental vascular endothelial growth factor and a1b1 and a5b1 integrins. Endocrinology (2012) 153(6):2897–906. doi: 10.1210/en.2012-1141
17. Zhang J, Wei MJ, Bian XJ, Wu L, Zhang S, Mao XY, et al. Letrozole-induced frozen embryo transfer cycles are associated with a lower risk of hypertensive disorders of pregnancy among women with polycystic ovary syndrome. Am J Obstet Gynecol (2021) 225(1):e1–9. doi: 10.1016/j.ajog.2021.01.024
18. Liu Y, Li G, Chen Y, Wang X, Ruan Y, Zou L, et al. A descriptive analysis of the indications for caesarean section in mainland China. BMC Pregnancy Childbirth (2014) 14:410. doi: 10.1186/s12884-014-0410-2
19. Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, et al. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens (2018) 13:291–310. doi: 10.1016/j.preghy.2018.05.004
20. Bartsch E, Medcalf KE, Park AL, Ray JG. Clinical risk factors for pre-eclampsia determined in early pregnancy: systematic review and meta-analysis of large cohort studies. BMJ (2016) 353:i1753. doi: 10.1136/bmj.i1753
21. Kawakita T, Downs SK, Franco S, Ghofranian A, Thomas A. Interpregnancy body mass index change and risk of hypertensive disorders in pregnancy. J Matern-Fetal Neo M (2022) 35(17):3223–8. doi: 10.1080/14767058.2020.1817371
22. Choi H, Lim JY, Lim NK, Ryu HM, Kwak DW, Chung JH, et al. Impact of pre-pregnancy body mass index and gestational weight gain on the risk of maternal and infant pregnancy complications in Korean women. Int J Obes (2022) 46(1):59–67. doi: 10.1038/s41366-021-00946-8
23. Mosher WD, Pratt WF. Fecundity and infertility in the united states: incidence and trends. Fertil Steril (1991) 56(2):192–3. doi: 10.1016/S0015-0282(16)54469-0
24. Kaponis A, Chatzopoulos G, Paschopoulos M, Georgiou I, Paraskevaidis V, Zikopoulos K, et al. Ultralong administration of gonadotropin-releasing hormone agonists before in vitro fertilization improves fertilization rate but not clinical pregnancy rate in women with mild endometriosis: a prospective, randomized, controlled trial. Fertil Steril (2020) 113(4):828–35. doi: 10.1016/j.fertnstert.2019.12.018
Keywords: frozen-thawed embryo transfer, endometrial preparation protocol, hormone replacement treatment, hypertensive disorders of pregnancy, obstetric outcomes
Citation: Fan L, Li N, Liu X, Li X, Cai H, Pan D, Wang T, Shi W, Qu P and Shi J (2023) Hormone replacement treatment regimen is associated with a higher risk of hypertensive disorders of pregnancy in women undergoing frozen-thawed embryo transfer. Front. Endocrinol. 14:1133978. doi: 10.3389/fendo.2023.1133978
Received: 29 December 2022; Accepted: 13 February 2023;
Published: 24 February 2023.
Edited by:
Gamal Serour, Al-Azhar University, EgyptReviewed by:
Yanying Lin, Fujian Women and Children Hospital, ChinaTian Yao, Xi’an No.4 Hospital, China
Copyright © 2023 Fan, Li, Liu, Li, Cai, Pan, Wang, Shi, Qu and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Na Li, RHJsaW5hX0FSVEAxNjMuY29t
 Lijuan Fan
Lijuan Fan Na Li
Na Li Xitong Liu
Xitong Liu Xiaofang Li
Xiaofang Li He Cai1
He Cai1 Pengfei Qu
Pengfei Qu Juanzi Shi
Juanzi Shi