
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Endocrinol., 31 May 2023
Sec. Clinical Diabetes
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1131437
This article is part of the Research TopicClinical Practice of Monogenic DiabetesView all 7 articles
Background: Haploinsufficiency of A20 (HA20) is a monogenic autosomal-dominant genetic autoinflammatory disease caused by loss of function mutations in the TNFAIP3 gene. The predominant autoimmune phenotype associated with HA20 varies significantly, presenting with fever, recurrent oral and genital ulcers, skin rash, gastrointestinal and musculoskeletal symptoms, and other clinical manifestations, all of which indicate an early-onset of autoinflammatory disorder. Genetic linkage between TNFAIP3 and T1DM was reported in GWAS studies. However, only a few cases of HA20 combined with T1DM have been reported.
Case description: A 39-year-old man with a history of type 1 diabetes mellitus since 19 years was admitted to the Department of Endocrinology and Metabolism, First Affiliated Hospital of China Medical University. He also suffered from recurring and minor mouth ulcers since early childhood. His laboratory evaluation results revealed reduced islet function, normal lipid profile, HbA1c of 7%, elevated glutamate decarboxylase antibodies, elevated hepatic transaminases, and elevated thyroid-related antibodies with normal thyroid function. Notably, the patient was diagnosed in adolescence and never had ketoacidosis, the islets were functioning despite the long disease duration, his abnormal liver function could not be reasonably explained, and he had early onset Behcet’s-like disease symptom. Hence, although he was on routine follow-up for diabetes, we communicated with him and obtained consent for genetic testing. Whole-exome sequencing revealed a novel c.1467_1468delinsAT heterozygous mutation in the gene TNFAIP3, which is located in exon 7, resulting in a stop-gained type mutation p.Q490*. With good but mild fluctuating glycemic control, the patient received intensive insulin therapy with long-acting and short-acting insulin. The liver function was improved by using ursodeoxycholic acid 0.75 mg/d during the follow-up.
Conclusion: We report a novel pathogenic mutation in TNFAIP3 that results in HA20 in a patient with T1DM. In addition, we analyzed the clinical feathers of such patients and summarized the cases of five patients with HA20 co-presented with T1DM. When T1DM co-occurs with autoimmune diseases or other clinical manifestations, such as oral and/or genital ulcers and chronic liver damage, the possibility of an HA20 must be considered. Early and definitive diagnosis of HA20 in such patients may inhibit the progression of late-onset autoimmune diseases, including T1DM.
Monogenic conditions are known to cause autoimmune diabetes that is clinically indistinguishable from T1DM. To date, nine disease-causing genes have been reported, including AIRE, CTLA4, FOXP3, IL2RA, ITCH, LRBA, SIRT1, STAT1, and STAT3 (1–3). Compared with T1DM, autoimmune monogenic diabetes typically manifests earlier and is more likely to be associated with multiple autoimmune diseases, such as immune dysregulation, polyendocrinopathy, enteropathy, and X-linked (IPEX) syndrome, which is caused by mutations in the FOXP3 and AIRE genes (2).
The tumor necrosis factor alpha–induced protein 3 (TNFAIP3) gene encodes the ubiquitin-editing enzyme A20, which acts as a negative regulator of the nuclear factor-κB (NF-κB) pathway (4). Haploinsufficiency of A20 (HA20) is a monogenic autosomal-dominant genetic autoinflammatory disease, which was first described and named by Zhou et al. in 2016 (5). The predominant autoimmune phenotype associated with HA20 varies significantly in reported cases, presenting with fever, recurrent oral and genital ulcers, skin rash, gastrointestinal and musculoskeletal symptoms, and other clinical manifestations, which are all indicating an early-onset of autoinflammatory disorder (6). Genetic linkage between TNFAIP3 gene and T1DM was also reported in GWAS studies (7–9).
Although more than 100 cases of HA20 combined with multiple autoimmune disorders have been reported in the relevant literature, there are only a few reports of combined T1DM and their clinical feathers. Here we report the case of a patient with HA20 who initially presented with T1DM. The patient was diagnosed in adolescence and never had ketoacidosis, his islet function was still not completely failed after such a long time (fasting C-peptide: >200 pmol/L), his abnormal liver function could not be reasonably explained, and he had early onset Behcet’s-like disease symptom. Hence, although he was only on routine follow-up for diabetes, we communicated with him and obtained his consent for genetic testing. A new nonsense mutation in TNFAIP3 was identified and the patient was diagnosed with HA20. At present, there is no recommended treatment plan for HA20 combined with T1DM; therefore, we did not change his treatment plan and continued to use insulin to control blood glucose. Here we report the clinical characteristics of our patient and present the review cases of HA20 combined with T1DM based on the relevant literature.
A 39-year-old male patient with hyperglycemia for 20 years was admitted to the Department of Endocrinology and Metabolism, First Affiliated Hospital of China Medical University. He was diagnosed with type 1 diabetes mellitus at the age of 19 years and has been on insulin therapy since then. He also had recurring and minor oral and genital ulcers since early childhood. His parents are healthy and nonconsanguineous. He is a married man with no children. The physical examination revealed the following characteristics: height, 170.0 cm; weight, 65.0 kg; body mass index, 22.5 kg/m2; and vision and hearing were normal.
As shown in Table 1, laboratory examination results revealed reduced islet function, a normal lipid profile, an HbA1c of 7%, increased glutamate decarboxylase (GAD) antibodies, elevated liver transaminases, and increased thyroid-related antibodies with normal thyroid function. Electromyography, cardiac and vascular color Doppler ultrasound, and abdominal color Doppler ultrasound were normal.
Genetic testing was performed using whole-exome high-throughput sequencing technology, and the data were analyzed using the Verita Trekker® Variant Site Detection System and Enliven® Variant Site Annotation Interpretation System developed by Berry Genetics. As a result, a c.1467_1468delinsAT heterozygous mutation in the gene TNFAIP3, located in exon 7, was discovered, resulting in a stop-gained type mutation, p.Q490* (Figure 1). The patient’s parents and sister were found to be devoid of this variant.
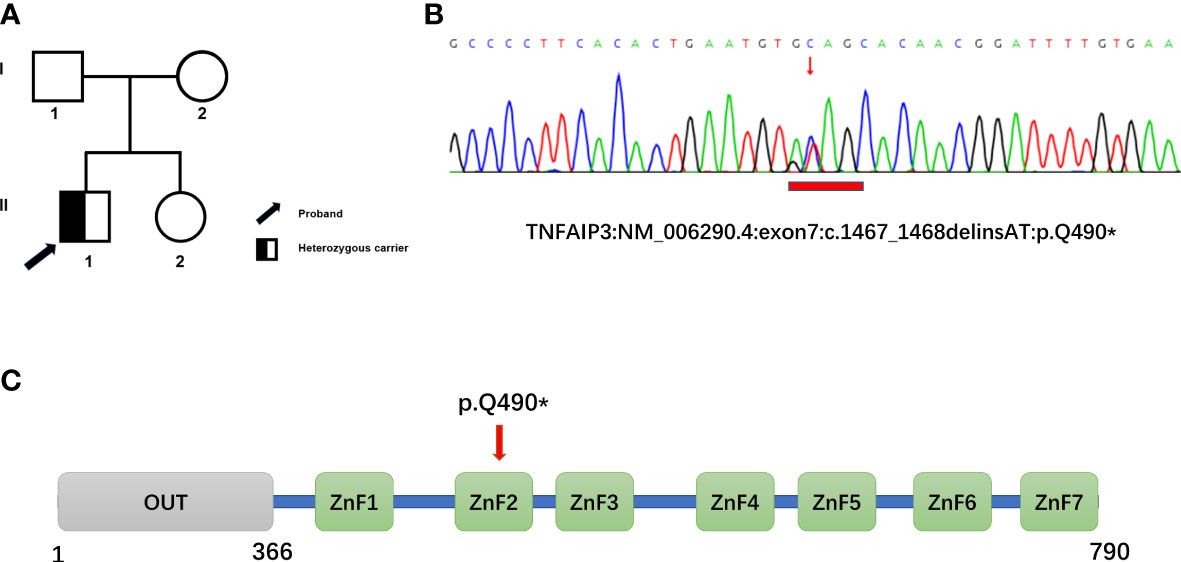
Figure 1 Family pedigree and genetic analyses. (A) Pedigree of the family with heterozygous mutation in TNFAIP3. (B) Whole-exome sequencing revealed a novel heterozygous mutation, c.1467_1468delinsAT (p.Glu490*), in TNFAIP3 in the patient, which was confirmed by Sanger Sequencing. (C) Schematic diagram of A20 protein and the location of the new identified mutation. OUT, ovarian tumor domain; ZnF 1-7, zinc finger domains.
With good but mild fluctuating glycemic control, the patient received intensive insulin therapy with long-acting and short-acting insulin. The liver function was improved by using ursodeoxycholic acid 0.75 mg/d during the follow-up.
HA20 is an autosomal-dominant genetic disease in which the NF-kB signaling pathway is less inhibited because of insufficient A20 production. The most common manifestation of HA20 is a Behcet’s-like disease symptom, which causes increased expression of proinflammatory cytokines mediated by the nuclear factor-κB pathway owing to A20-incuded weakened negative regulation of the nuclear factor-κB pathway. Further, the main manifestations of HA20 include oral and/or genital ulcers, fever, skin and musculoskeletal involvements, and gastrointestinal symptoms. No standard treatment has been established for the disease, and the treatment method is generally based on the dominant clinical phenotypes (6, 8, 9). Nearly half of the patients were reported to respond well to colchicine, with or without immunosuppressive or biological agents (6). However, even when the pathogenic variants are the same, patients’ clinical presentations and responses to treatment are highly heterogeneous (6).
TNFAIP3/A20 has been identified as the most upregulated anti-apoptotic gene in pancreatic islet beta cells (10) and prevents islet graft rejection in animal transplantation models (11). By increasing the threshold of inflammatory signaling, therapeutic administration of A20 promotes immune tolerance and survival of transplanted islets (11). In the absence of adequate A20 expression, soluble secretory factors released by cells may interfere with the expression of genes required for normal β-cell function (12).
We searched the literature and found 117 cases (in 60 families) of genetically confirmed HA20 (only cases with clinical information were counted), with 62 pathogenic variants in TNFAIP3. However, the number of cases of coexisting HA20 and T1DM remain small, with five cases (5/118, 4.2%) being added to our case (13–15). They almost always have oral and/or genital ulcers (4/5), autoimmune thyroid disease (AITD) (3/5), and other autoimmune diseases; moreover, they are particularly susceptible to hepatic cytolysis (3/5), which is generally uncommon in T1DM and APS2 (Table 2). Notably, these five patients did not present with other clinical manifestations of HA20, including early onset IBD, muscle-skeletal disorders with arthralgia and arthritis, and recurrent fever.
The majority of the pathogenic variants are loss-of-function variants that cause protein truncation (54/62, 87.1%), with point mutations accounting for only 12.9% (8/62). The variants in HA20 patients with combined T1DM were nonsense or frameshift variants in the carboxy-terminal zinc finger (ZnF) coding regions or amino-terminal ovarian tumor (OUT) coding regions (Table 2).
The persistence of C-peptide secretion widely varies among patients after a long duration of type 1 diabetes (16). In the present case, even after 20 years, islet function has not completely failed (fasting C-peptide: 319.7 pmol/L). Unfortunately, data regarding changes in islet function after the onset of the disease were missing, and the reports of the remaining four patients in the literature did not present their islet functions; therefore, we could not summarize the characteristics of the process of islet function changes in HA20 combined with T1DM. However, if HA20 is speculated at the onset of the disease when the islet function is still good, early treatment using immunosuppressive or biological agents may limit or reverse the loss of beta cell function in T1DM with HA20. The clinical symptoms of our patients with HA20 were not serious and insulin administration provided adequate blood sugar control; therefore, we did not further try to use immunosuppressive or biological agents.
In this case, liver function revealed abnormally elevated transaminases, excluding other liver diseases. Recent research reported that patients with HA20 develop chronic liver involvement due to hepatic fibrosis, hepatocyte injury, and/or inflammatory T lymphocyte infiltrates with moderate NF-κB and/or NLRP3 staining (15). This indicates that HA20 may be present in patients who have multiple concurrent autoimmune diseases, Behcet’s-like symptoms, and unexplained liver function abnormalities.
In conclusion, we report a novel pathogenic mutation in TNFAIP3 that results in HA20 in a T1DM patient. We analyzed its clinical feathers and summarized five patients with HA20 in combination with T1DM. If T1DM is combined with other autoimmune diseases or clinical manifestations other than AITD, such as oral and/or genital ulcers and chronic liver injury, be aware of the possibility of HA20. Early diagnosis and timely treatment of HA20 may delay the progression of autoimmune diseases, including T1DM.
The original contributions presented in the study are publicly available. This data can be found here: https://figshare.com/articles/online_resource/0F2X000186_vcf/22873334.
The ethics committee approved the study of First Affiliated Hospital of China Medical University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Study design: XW. Data collection: CC and XF. Manuscript drafting: CC and XF. Data interpreting: XW. Revision of the manuscript: XW. Approval of final version of the manuscript: CC, XF, and XW. All authors contributed to the article and approved the submitted version.
The study received funding from Sinocare Diabetes Foundation (2020SD05).
The authors thank the patient and his family members who agreed to participate in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Johnson MB, Cerosaletti K, Flanagan SE, Buckner JH. Genetic mechanisms highlight shared pathways for the pathogenesis of polygenic type 1 diabetes and monogenic autoimmune diabetes. Curr Diabetes Rep (2019) 19(5):20. doi: 10.1007/s11892-019-1141-6
2. Johnson MB, Hattersley AT, Flanagan SE. Monogenic autoimmune diseases of the endocrine system. Lancet Diabetes Endocrinol (2016) 4(10):862–72. doi: 10.1016/S2213-8587(16)30095-X
3. Biason-Lauber A, Böni-Schnetzler M, Hubbard BP, Bouzakri K, Brunner A, Cavelti-Weder C, et al. Identification of a SIRT1 mutation in a family with type 1 diabetes. Cell Metab (2013) 17(3):448–55. doi: 10.1016/j.cmet.2013.02.001
4. Wertz IE, Newton K, Seshasayee D, Kusam S, Lam C, Zhang J, et al. Phosphorylation and linear ubiquitin direct A20 inhibition of inflammation. Nature (2015) 528(7582):370–5. doi: 10.1038/nature16165
5. Zhou Q, Wang H, Schwartz DM, Stoffels M, Park YH, Zhang Y, et al. Loss-of-function mutations in TNFAIP3 leading to A20 haploinsufficiency cause an early-onset autoinflammatory disease. Nat Genet (2016) 48(1):67–73. doi: 10.1038/ng.3459
6. Yu MP, Xu XS, Zhou Q, Deuitch N, Lu MP. Haploinsufficiency of A20 (HA20): updates on the genetics, phenotype, pathogenesis and treatment. World J Pediatr (2020) 16(6):575–84. doi: 10.1007/s12519-019-00288-6
7. Fung EY, Smyth DJ, Howson JM, Cooper JD, Walker NM, Stevens H, et al. Analysis of 17 autoimmune disease-associated variants in type 1 diabetes identifies 6q23/TNFAIP3 as a susceptibility locus. Genes Immun (2009) 10(2):188–91. doi: 10.1038/gene.2008.99
8. Westra HJ, Martínez-Bonet M, Onengut-Gumuscu S, Lee A, Luo Y, Teslovich N, et al. Fine-mapping and functional studies highlight potential causal variants for rheumatoid arthritis and type 1 diabetes. Nat Genet (2018) 50(10):1366–74. doi: 10.1038/s41588-018-0216-7
9. Chen Y, Ye Z, Chen L, Qin T, Seidler U, Tian D, et al. Association of clinical phenotypes in haploinsufficiency A20 (HA20) with disrupted domains of A20. Front Immunol (2020) 11:574992. doi: 10.3389/fimmu.2020.574992
10. Liuwantara D, Elliot M, Smith MW, Yam AO, Walters SN, Marino E, et al. Nuclear factor-kappaB regulates beta-cell death: a critical role for A20 in beta-cell protection. Diabetes (2006) 55(9):2491–501. doi: 10.2337/db06-0142
11. Zammit NW, Walters SN, Seeberger KL, O’Connell PJ, Korbutt GS, Grey ST. A20 as an immune tolerance factor can determine islet transplant outcomes. JCI Insight (2019) 4(21):e131028. doi: 10.1172/jci.insight.131028
12. Ratajczak W, Atkinson SD, Kelly C. A20 controls expression of beta-cell regulatory genes and transcription factors. J Mol Endocrinol (2021) 67(4):189–201. doi: 10.1530/JME-21-0076
13. Duncan CJA, Dinnigan E, Theobald R, Grainger A, Skelton AJ, Hussain R, et al. Early-onset autoimmune disease due to a heterozygous loss-of-function mutation in TNFAIP3 (A20). Ann Rheum Dis (2018) 77(5):783–6. doi: 10.1136/annrheumdis-2016-210944
14. Rossi MN, Federici S, Uva A, Passarelli C, Celani C, Caiello I, et al. Identification of a novel mutation in TNFAIP3 in a family with poly-autoimmunity. Front Immunol (2022) 13:804401. doi: 10.3389/fimmu.2022.804401
15. Deshayes S, Bazille C, El Khouri E, Kone-Paut I, Giurgea I, Georgin-Lavialle S, et al. Chronic hepatic involvement in the clinical spectrum of A20 haploinsufficiency. Liver Int (2021) 41(8):1894–900. doi: 10.1111/liv.14935
Keywords: TNFAIP3, type 1 diabetes mellitus, HA20, mutation, case report
Citation: Cao C, Fu X and Wang X (2023) Case Report: A novel mutation in TNFAIP3 in a patient with type 1 diabetes mellitus and haploinsufficiency of A20. Front. Endocrinol. 14:1131437. doi: 10.3389/fendo.2023.1131437
Received: 25 December 2022; Accepted: 27 April 2023;
Published: 31 May 2023.
Edited by:
Fabrizio Barbetti, University of Rome Tor Vergata, ItalyReviewed by:
Anette S. B. Wolff, Haukeland University Hospital, NorwayCopyright © 2023 Cao, Fu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoli Wang, d2xpdHRsZXBlYXJAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.