- 1Cardiometabolic Medicine Center, State Key Laboratory of Cardiovascular Disease, Beijing, China
- 2Cardiometabolic Medicine Center, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 3Department of Cardiology, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Background: Inflammatory processes crucially modulate the development, progression, and outcomes of coronary artery disease (CAD). Since hyperglycemia could alter inflammatory responses, this study aimed to investigate the effect of ANC, a novel and rapidly available inflammatory biomarker, on the prognosis of patients undergoing PCI with or without type 2 diabetes (T2D).
Methods: A total of 7,826 patients with CAD hospitalized for PCI at Fuwai Hospital were consecutively recruited. According to the median ANC value, patients were stratified as having high ANC (ANC-H) or low ANC (ANC-L) and were further classified into four groups by T2D. The primary endpoint was major adverse cardiovascular and cerebrovascular events (MACCEs), including all-cause mortality, myocardial infarction, stroke, and target vessel revascularization.
Results: During a median follow-up of 2.4 years, 509 (6.5%) MACCEs were documented. Diabetic patients with increased ANC were at significantly higher risk of MACCEs (aHR, 1.55; 95% CI, 1.21–1.99; P = 0.001) compared to those in the ANC-L/non-T2D group (P for interaction between T2D and ANC categories = 0.044). Meanwhile, multivariable regression analysis demonstrated the highest MACCE risk in diabetic patients with a higher level of ANC than others (P for trend <0.001).
Conclusion: This study suggests that stratification of patients with elevated ANC and T2D could provide prognostic information for CAD patients undergoing PCI.
Introduction
Inflammatory processes crucially modulate the development, progression, and outcomes of cardiovascular (CV) diseases (CAD), and accordingly, targeting inflammation is promising to reduce the atherosclerotic burden (1). Currently, several recent clinical trials have illustrated that there are clinical benefits for patients with CVD by targeting inflammatory pathways. The Canakinumab Anti-inflammatory Thrombosis Outcome Study (CANTOS) (2), followed by the Colchicine Cardiovascular Outcomes Trial (COLCOT) (3), and the second Low Dose Colchicine trial (LoDoCo-2) (4), collectively demonstrated the efficacy of anti-inflammatory treatments in the context of CV prevention. Notably, these contemporary studies not only increased interest in traditional inflammatory biomarkers, including interleukin (IL)-1, IL-6, and C-reactive protein (CRP), but also in finding a simple biomarker widely and rapidly available to clinical practice, such as the absolute neutrophil count (ANC) (5).
Neutrophils are the most abundant subtype of white blood cells in the human circulation and the main cell type during acute inflammatory responses. They belong to the first line of innate immunity and contribute to atherosclerosis in a stage-dependent manner (6). In the initial stage of atherosclerosis, neutrophils attract monocytes to plaques by secreting granule proteins and activate plaque endothelial cells through reactive oxygen species and proteases (7–9). During atherosclerotic progression, neutrophil-derived granule proteins promote the activation of plaque macrophages towards a proinflammatory phenotype (10). Further, myeloperoxidase is released by neutrophils, which increases foam cell formation (11). Also, neutrophil extracellular traps (NETs) activate plaque macrophages to secrete IL-1β and IL-18 through the nucleotide-binding domain and leucine-rich repeat protein 3 (NLRP3) inflammasome formation (10, 12). Regarding advanced atherosclerosis, neutrophils release NETs, which contain cytotoxic histone H4 and matrix metalloproteinases, leading to the breakdown of vascular smooth muscle cells and the extracellular matrix, eventually resulting in fibrous cap thinning, a characteristic of vulnerable plaques (6, 13). Previous studies demonstrated that ANC was positively associated with the rate of adverse clinical events (5, 14). Meanwhile, in the placebo group of the CANTOS trial, the ANC remained stable during the follow-up period (5), which also indicated that it could be a promising candidate biomarker to be accepted by the physicians.
Type 2 diabetes (T2D) is an established predictor for coronary artery disease (CAD), which has been previously suggested to be closely associated with a greater burden of atherosclerotic plaque and fuel the risk of adverse clinical outcomes. Cumulative translational evidence suggests a synergism between neutrophils and hyperglycemia (6, 13). However, the association of ANC with poor prognosis in CAD patients with different glycemic states after percutaneous coronary intervention (PCI) remains uncertain. Therefore, the aim of this study is to investigate the effect of ANC on the clinical outcomes of patients undergoing PCI with or without T2D.
Methods
Study design
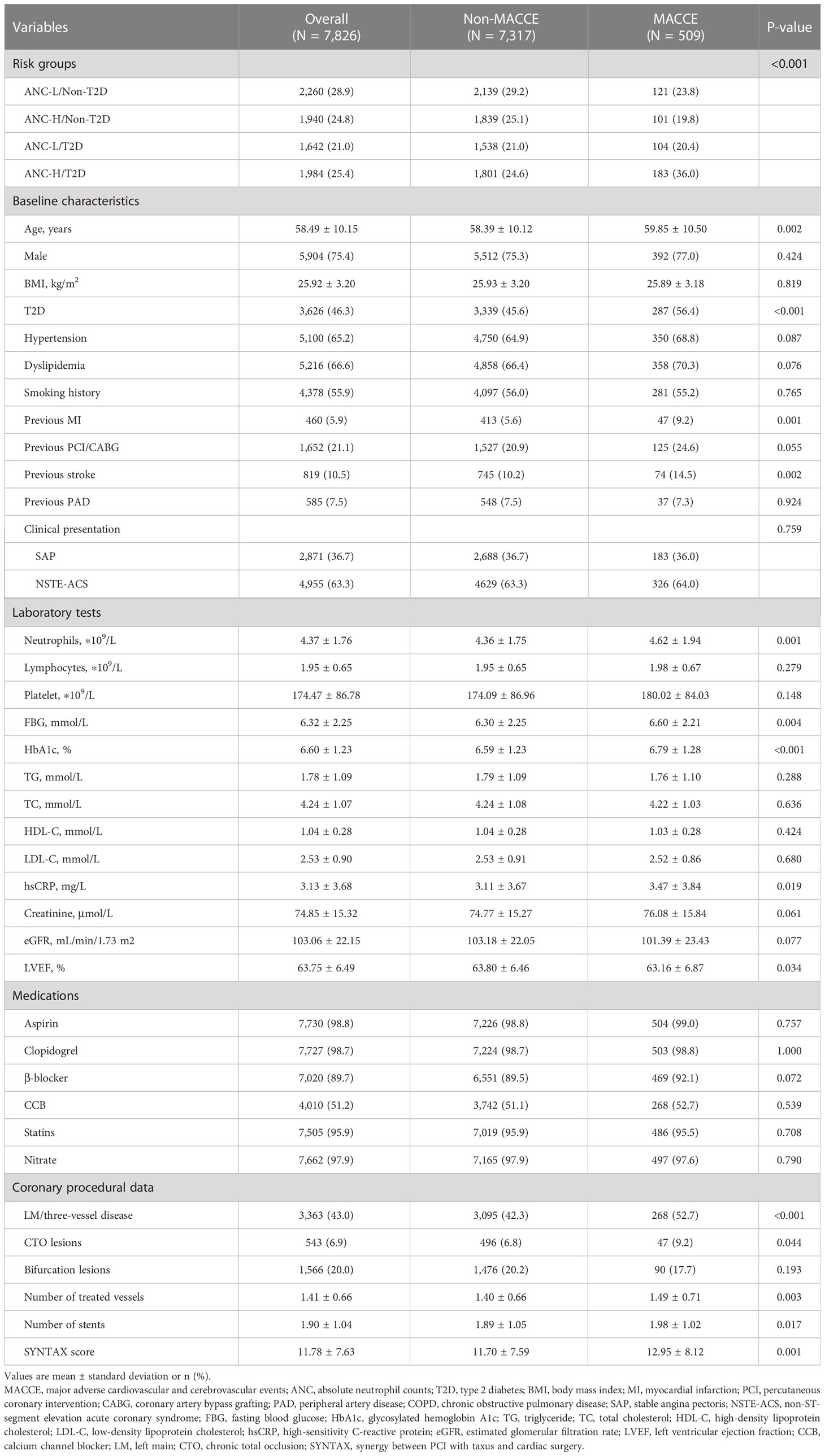
This study was a prospective, observational cohort study at Fuwai Hospital, Chinese Academy of Medical Sciences. Between January 2013 and December 2013, a total of 10,724 consecutive CAD patients hospitalized for PCI with drug-eluting stent (DES) implantation were screened. The exclusion criteria included (1): patients aged <18 years old; (2) patients with in-hospital death; (3) patients without ANC, fasting blood glucose (FBG), and glycosylated hemoglobin A1c (HbA1c) values; (4) patients with severe kidney or liver dysfunction; (5) patients with hematological disorders, active tumors, acute infections, or on immune-suppressant or steroid drugs; and (6) patients presenting with ST-segment-elevated myocardial infarction (STEMI) (Figure 1). A total of 7,826 patients were finally enrolled in this study and were categorized according to the presence of T2D and then by the median value of ANC (4.05 ∗ 109/L), as ANC-L/non-T2D (n = 2,260), ANC-H/non-T2D (n = 1,940), ANC-L/T2D (n = 1,642), and ANC-H/T2D (n = 1,984).
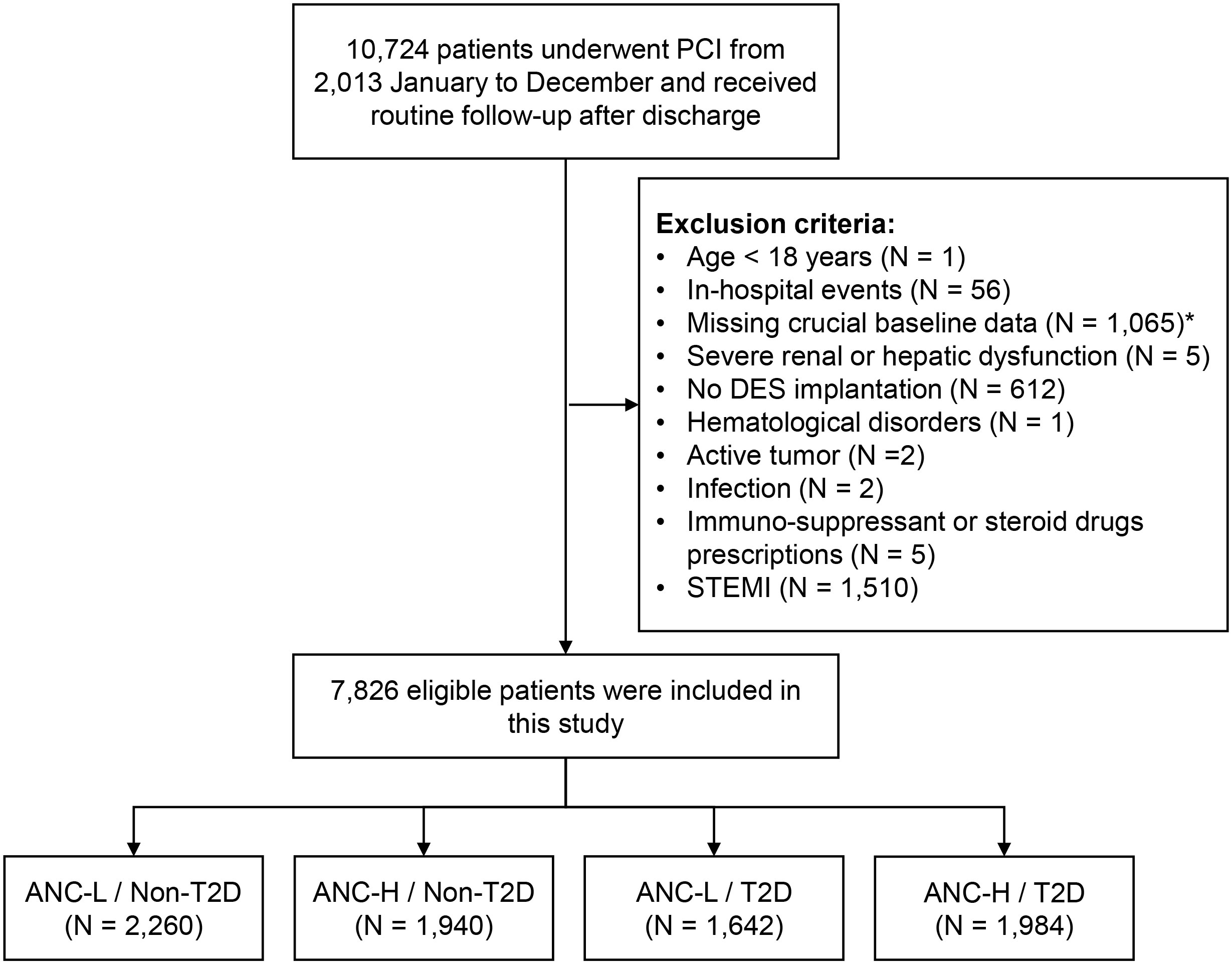
Figure 1 Study flowchart. *A total of 708 patients with missing ANC and 409 patients with missing FBG or HbA1c levels were excluded. PCI, percutaneous coronary intervention; DES, drug-eluting stent; STEMI, ST-segment-elevation myocardial infarction; ANC, absolute neutrophil counts; T2D, type 2 diabetes; FBG, fasting blood glucose; HbA1c, glycosylated hemoglobin A1c.
The study process complied with the Declaration of Helsinki and was approved by the Institutional Review Board of Fuwai Hospital. Written informed consent for long-term follow-up before PCI was obtained from all study patients.
Procedure and medications
PCI procedures were according to standard techniques, and the choice of devices and auxiliary examinations, including intravascular ultrasound and optical coherence tomography, was at the discretion of the interventionalists. Before PCI, unless already on dual antiplatelet therapy, patients were treated with a 300-mg loading dose of aspirin and a 180-mg loading dose of ticagrelor or a 300-mg loading dose of clopidogrel. The periprocedural administration of antiplatelet and antithrombotic medications was according to the guidelines in effect at the time. After the procedure, patients were prescribed aspirin (100 mg daily) indefinitely and ticagrelor (90 mg, twice daily) or clopidogrel (75 mg, daily) for at least 12 months. The data were entered into a dedicated database by independent research personnel (15).
Clinical assessment and angiographic information
Baseline characteristics based on demographic information, individual health habits, medical history, and drug prescriptions were acquired from each patient by well-trained cardiologists. T2D was defined as FBG ≥7.0 mmol/L (126 mg/dl), HbA1c ≥6.5%, 2-h blood glucose from an oral glucose tolerance test ≥11.1 mmol/L (200 mg/dl), or a previous definite diagnosis of T2D with hypoglycemic drugs or insulin treatment (16). Hypertension was defined as self-reported hypertension with antihypertensive drugs or newly recorded systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg for three or more consecutive times on different days. According to previous studies (15), dyslipidemia was diagnosed by fasting total cholesterol (TC) ≥5.2 mmol/L, low-density lipoprotein cholesterol (LDL-C) ≥3.4 mmol/L, high-density lipoprotein cholesterol (HDL-C) <1.0 mmol/L, or triglycerides (TG) ≥1.7 mmol/L, and/or receiving lipid-lowering medications. Kidney dysfunction was considered when the estimated glomerular filtration rate (eGFR) was less than 90 ml/min ∗ 1.73 m2 (17). Body mass index (BMI) was calculated by weight (in kg) divided by height (in meters)2.
Coronary angiograms were independently evaluated at the angiographic core laboratory of Fuwai Hospital for the process of coronary angiology and stenting procedures and recorded the number of coronary arteries with stenosis of 50% or more, special types of coronary stenotic lesions [such as left main (LM) disease, three-vessel disease, and chronic total occlusion (CTO) disease], the Synergy Between PCI with TAXUS and Cardiac Surgery (SYNTAX) score, and the number of treated vessels and implanted stents.
Laboratory measurements
After at least a 12-hour fast, blood samples were routinely collected from each patient on the day of admission. A complete blood count, including neutrophils, lymphocytes, and platelets, was performed using an automatic blood cell analyzer (XT-1800i; Sysmex Corporation). The HbA1c levels were estimated by the Tosoh Automated Glycohemoglobin Analyzer (HLC-723G8, Tokyo, Japan). Other laboratory parameters, including lipid profiles (TG, TC, HDL-C, and LDL-C), FBG, creatinine, and high-sensitivity CRP (hsCRP), were examined in the core laboratory at Fuwai Hospital, according to the standard procedures in effect at the time (15). Calculation of eGFR was performed using the Chinese-modified MDRD (Modification of Diet in Renal Disease) equation (18). Based on a modified Simpson’s rule, the left ventricular ejection fraction (LVEF) was estimated from two-dimensional echocardiography.
Follow-up and study endpoints
The clinical status was collected at 1, 6, and 12 months and yearly thereafter by an independent group of professional clinical research coordinators through telephone contacts or outpatient visits. The primary endpoint was major adverse cardiovascular and cerebrovascular events (MACCEs), defined as a composite of all-cause mortality, myocardial infarction (MI), stroke, and target vessel revascularization (TVR). The secondary end points included individual components of MACCEs. Consistent with our prior research (15), all-cause mortality was defined as mortality from any cause. MI was defined according to the Third Universal Definition of MI (19). Stroke was defined as neurological deficits, either ischemic or hemorrhagic, confirmed by a neurologist based on imaging findings. TVR was defined as a repeat PCI or coronary artery bypass graft (CABG) in the target vessel.
Statistical analyses
Continuous variables are expressed as the mean ± standard deviation and were compared by Student’s t-test, Mann–Whitney U test, or analysis of variance as appropriate. Categorical variables are presented as numbers with percentages and are tested by the χ2 test or Fisher’s exact test. In survival analysis, restricted cubic splines (RCSs) were created to assess the linearity assumptions of the association between ANC and MACCEs. The cumulative incidence of MACCEs among groups was estimated by Kaplan–Meier analysis and compared by the log-rank test. The proportional hazards assumption was assessed by Schoenfeld residuals. Univariable and multivariable Cox proportional hazard analyses were performed to estimate the association of ANC and T2D with the risk of adverse clinical events. Hazard ratios (HRs) and 95% confidence intervals (CIs) were presented. Variables, including age, male sex, BMI, hypertension, dyslipidemia, smoking history, previous MI, previous PCI/CABG, previous stroke, non-ST-segment-elevated acute coronary syndrome (NSTE-ACS), hsCRP, eGFR, LVEF, LM/three-vessel disease, CTO lesions, number of treated vessels, and SYNTAX score, were included in the multivariable Cox model for their statistical significance in univariable analysis or clinical importance in clinical trials. Additionally, compared with the predictive model containing clinical risk factors, the additional predictive performance of the new classification by ANC categories and T2D was determined by the receiver operating characteristic (ROC) analysis with area under the ROC curve (AUC), the category-free net reclassification index (NRI), and the integrated discrimination improvement (IDI). Furthermore, subgroup analyses were adopted to evaluate the risk of MACCEs according to six different subsets and illustrated as forest plots. A two-tailed P-value of <0.05 was considered statistically significant. Statistical analyses were performed using RStudio software (version 2021.09.0; http://www.rstudio.org/).
Results
Baseline characteristics
In general, a total of 7,826 CAD patients (58.49 ± 10.15 years, 75.4% male) who received PCI with DES implantation were consecutively enrolled in this study. During a median follow-up of 2.4 years, 69 (0.9%) all-cause mortality, 47 (0.6%) MIs, 111 (1.4%) strokes, 330 (4.2%) TVRs, and 509 (6.5%) MACCEs were observed.
Table 1 illustrates the baseline characteristics of the study population stratified by the occurrence of MACCEs. Individuals with any component of MACCEs tended to be older with a higher prevalence of T2D, previous MI, and previous stroke compared with those in the non-MACCE group. In addition, higher ANC, FBG, HbA1c, and hsCRP levels and lower LVEF levels were noted in patients who experienced MACCEs. Regarding angiographic information, LM/three-vessel disease and CTO lesions were more prevalent in patients who suffered MACCEs, with a higher SYNTAX score, number of treated vessels, and number of stents.
Baseline characteristics of individuals in four risk groups
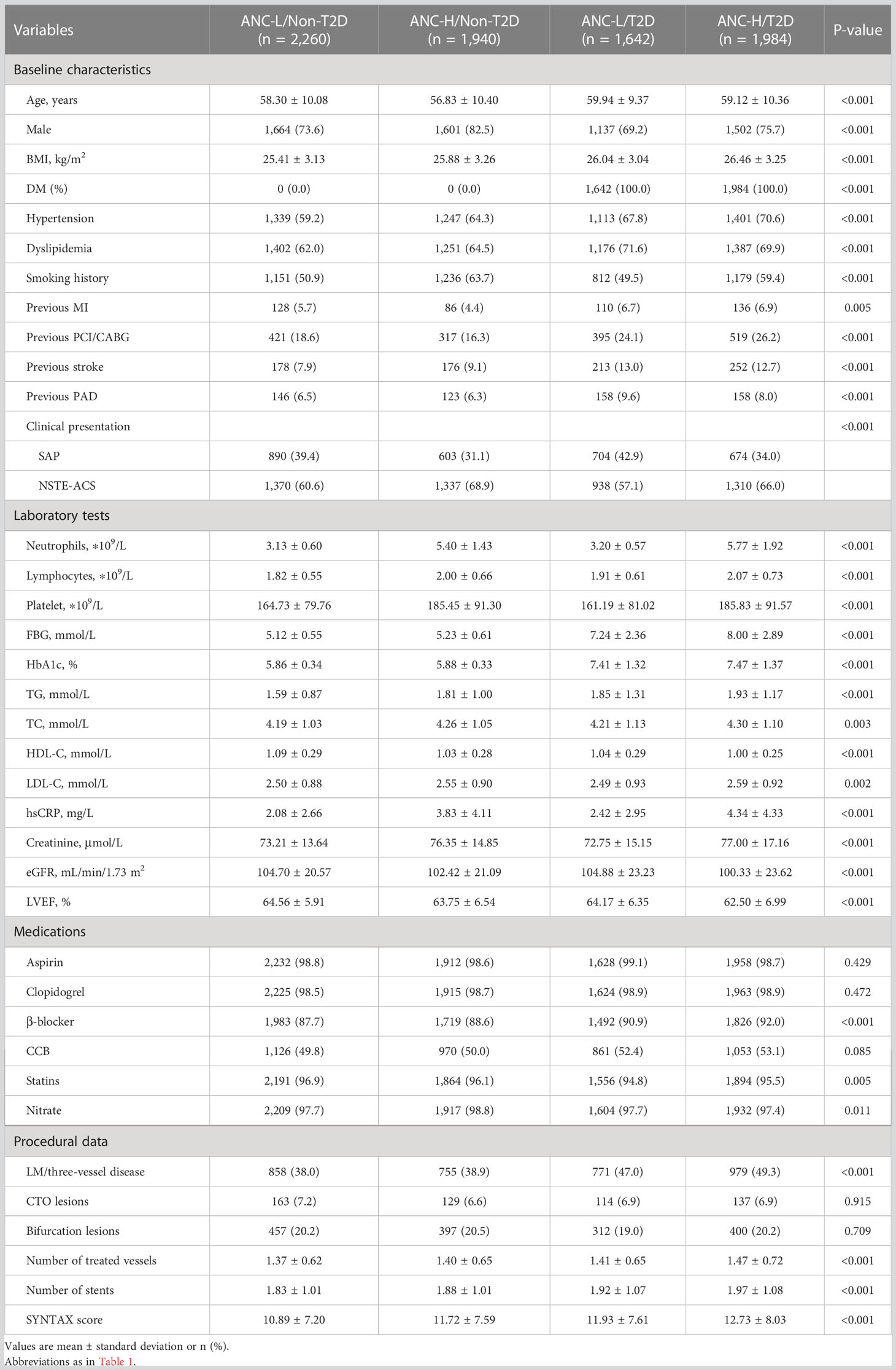
According to the ANC median value of 4.05 ∗ 109/L and T2D, patients were stratified into four risk groups. Subsequently, the baseline characteristics of the four risk groups are presented in Table 2.
Diabetic patients with a higher level of ANC were older and more likely to be female, with a greater burden of concomitant diseases, including hypertension, dyslipidemia, smoking history, previous MI, previous stroke, and previous peripheral artery disease (PAD), and clinical presentations as NSTE-ACS compared with those in other groups. Additionally, a higher prevalence of prior PCI/CABG and LM/three-vessel disease was observed in the ANC-H/T2D group. Moreover, there was also higher BMI, FBG, HbA1c, TG, TC, LDL-C, hsCRP, creatinine, number of treated vessels, number of stents, and SYNTAX score, and lower HDL-C, eGFR, and LVEF in the ANC-H/T2D group.
Association of ANC and type 2 diabetes with clinical outcomes
During a median follow-up of 2.4 years, the incidence of MACCE in the ANC-L/Non-T2D, ANC-H/Non-T2D, ANC-L/T2D, and ANC-H/T2D groups was 5.4% (121/2260), 5.2% (101/1940), 6.3% (104/1642), and 9.2% (183/1984), respectively.
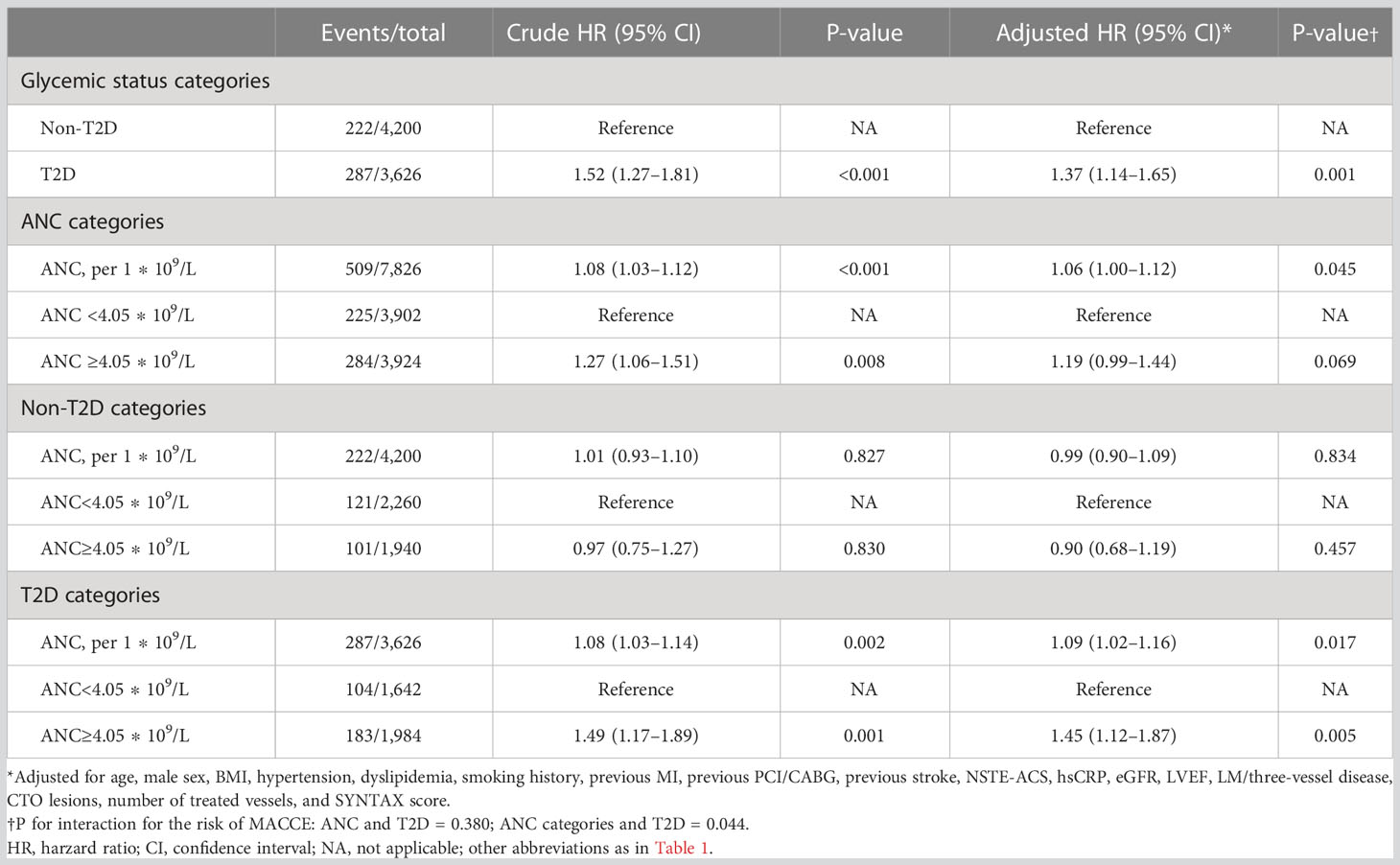
As depicted in Figure 2, the Kaplan–Meier curves revealed that T2D and a higher level of ANC conferred a higher risk of MACCEs compared with the other groups (Log rank P <0.001 and P = 0.008, respectively). In addition, there was a greater rate of MACCEs in patients with higher levels of ANC among diabetic patients (log rank P = 0.001), whereas no significant difference in MACCE risk was observed among non-diabetic patients (log rank P = 0.830). Table 3 displays the results of the Cox proportional analysis evaluating the association of ANC and glycemic status with clinical outcomes. A significant interaction was observed between T2D and ANC categories after adjusting for age, male sex, BMI, hypertension, dyslipidemia, smoking history, previous MI, previous PCI/CABG, previous stroke, NSTE-ACS, hsCRP, eGFR, LVEF, LM/three-vessel disease, CTO lesions, number of treated vessels, and SYNTAX score (P = 0.044 for interaction). In patients without T2D, no significant association between ANC and MACCE risk was detected when evaluated either by ANC [adjusted HR (aHR), 0.99; 95% CI, 0.90–1.09] or a categorical threshold of 4.05 ∗ 109/L (aHR, 0.90; 95% CI, 0.68–1.19). On the other hand, in diabetic patients, elevation of ANC, either by a 1 ∗ 109/L increase (aHR, 1.09; 95% CI, 1.02–1.16; P = 0.017) or a categorical threshold of 4.05 ∗ 109/L (aHR, 1.45; 95% CI, 1.12–1.87; P = 0.005), was significantly associated with a higher risk of MACCEs.
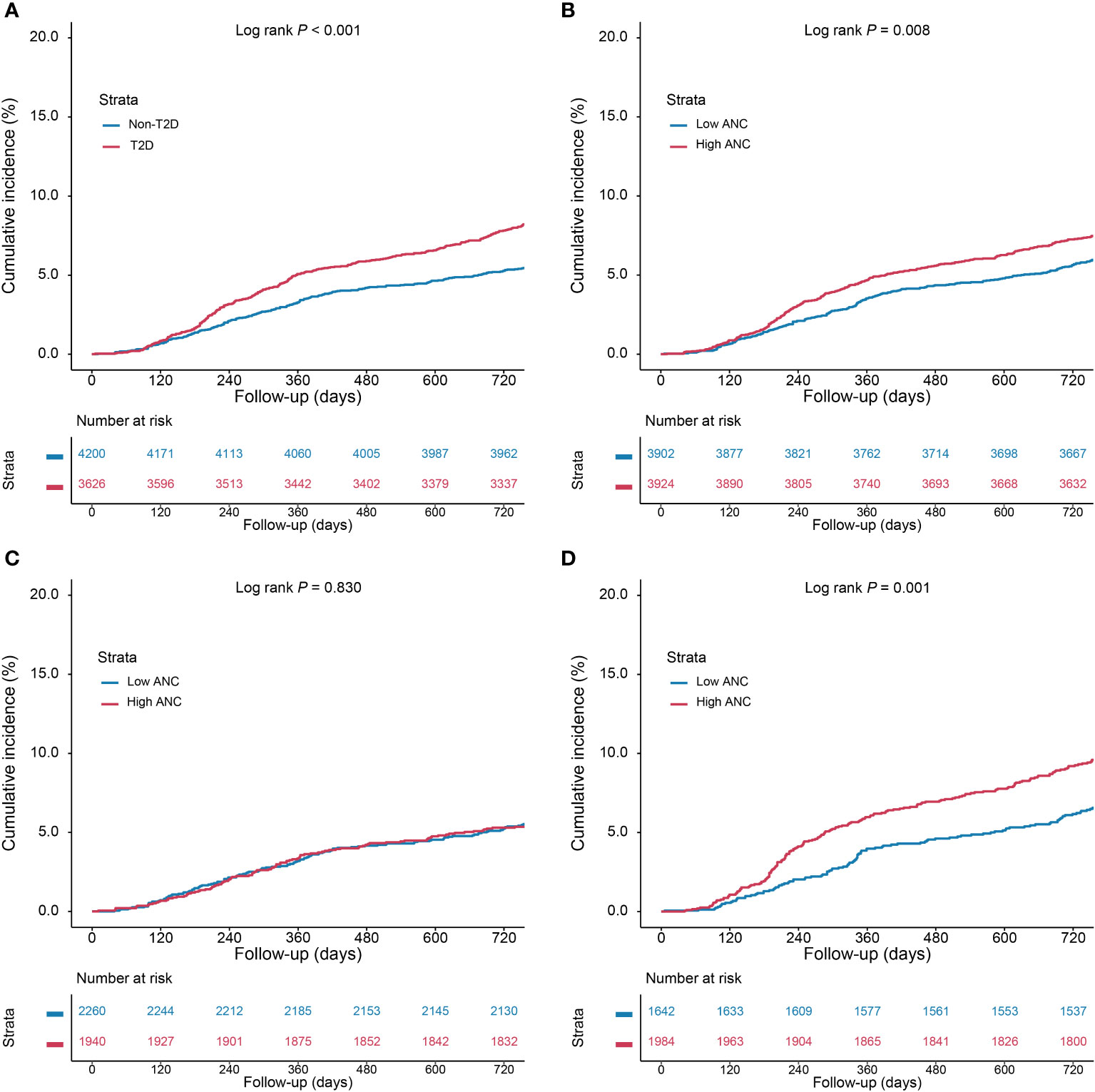
Figure 2 Kaplan–Meier (KM) curves for cumulative incidence of MACCEs. (A) KM curves according to type 2 diabetes, (B) KM curves according to ANC categories, (C) KM curves according to ANC categories in non-diabetic patients, and (D) KM curves according to ANC categories in diabetic patients. Abbreviations as in Figure 1.
Risk of adverse events in different risk groups classified by type 2 diabetes and ANC categories
As shown in Figure 3, the cumulative incidence of the MACCE rate was highest in the ANC-H/T2D group. HRs were estimated for each group using the ANC-L/non-T2D group as a reference (Additional File 1: Tables S1, S2). In fully adjusted analyses, patients in the ANC-H/T2D group were at significantly higher risk of MACCEs (aHR, 1.55; 95% CI, 1.21–1.99; P = 0.001) and TVR (aHR, 1.55; 95% CI, 1.15–2.10; P = 0.004). In addition, multivariable Cox proportional analysis also revealed the highest risk of MACCEs in diabetic patients with a higher level of ANC than others (P <0.001 for trend). RCS analysis exhibited a linear association between ANC and the MACCE risk regardless of the univariable and multivariable model (Additional file 1: Figure S1; P >0.05 for a non-linear association for both).
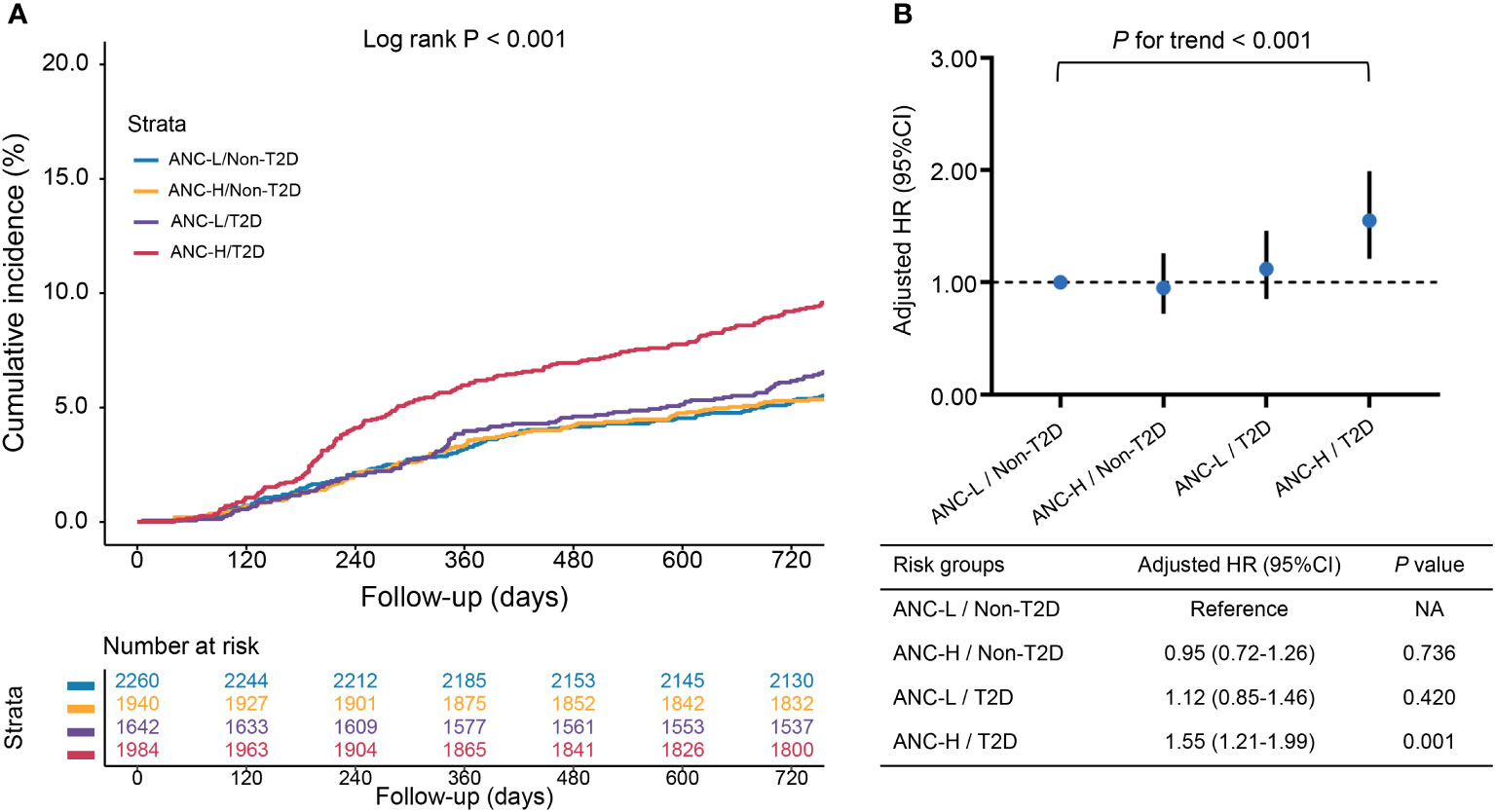
Figure 3 The associations of risk groups with the risk of MACCEs. (A) Kaplan–Meier curves for cumulative incidence of MACCEs according to four risk groups; (B) Hazard ratios (HRs) [95% confidence intervals (CIs)] for MACCEs according to four risk groups. Model adjusted for age, male sex, BMI, hypertension, dyslipidemia, smoking history, previous MI, previous PCI/CABG, previous stroke, NSTE-ACS, hsCRP, eGFR, LVEF, LM/three-vessel disease, CTO lesions, number of treated vessels, and SYNTAX score. Abbreviations as in Figure 1.
In the original model, the AUC was 0.587 (95% CI, 0.562–0.612) with clinical risk factors, including age, male sex, hypertension, dyslipidemia, smoking history, previous stroke, previous MI, NSTE-ACS, LVEF, eGFR, and the SYNTAX score. When we added the combination of ANC categories and T2D to the original model, there was a significant but slight improvement in AUC to 0.607 (95% CI, 0.582–0.632) (ΔAUC, 0.020; P = 0.027) (Additional File 1: Figure S2).
Subgroup analysis
In subgroup analyses, the relationship between four risk groups and MACCE risk was consistent across different subgroups (age, sex, BMI, hypertension, kidney dysfunction, and clinical presentation), with comparable interactions (all P >0.05 for interactions) (Figure 4, Additional File 1: Table S3).
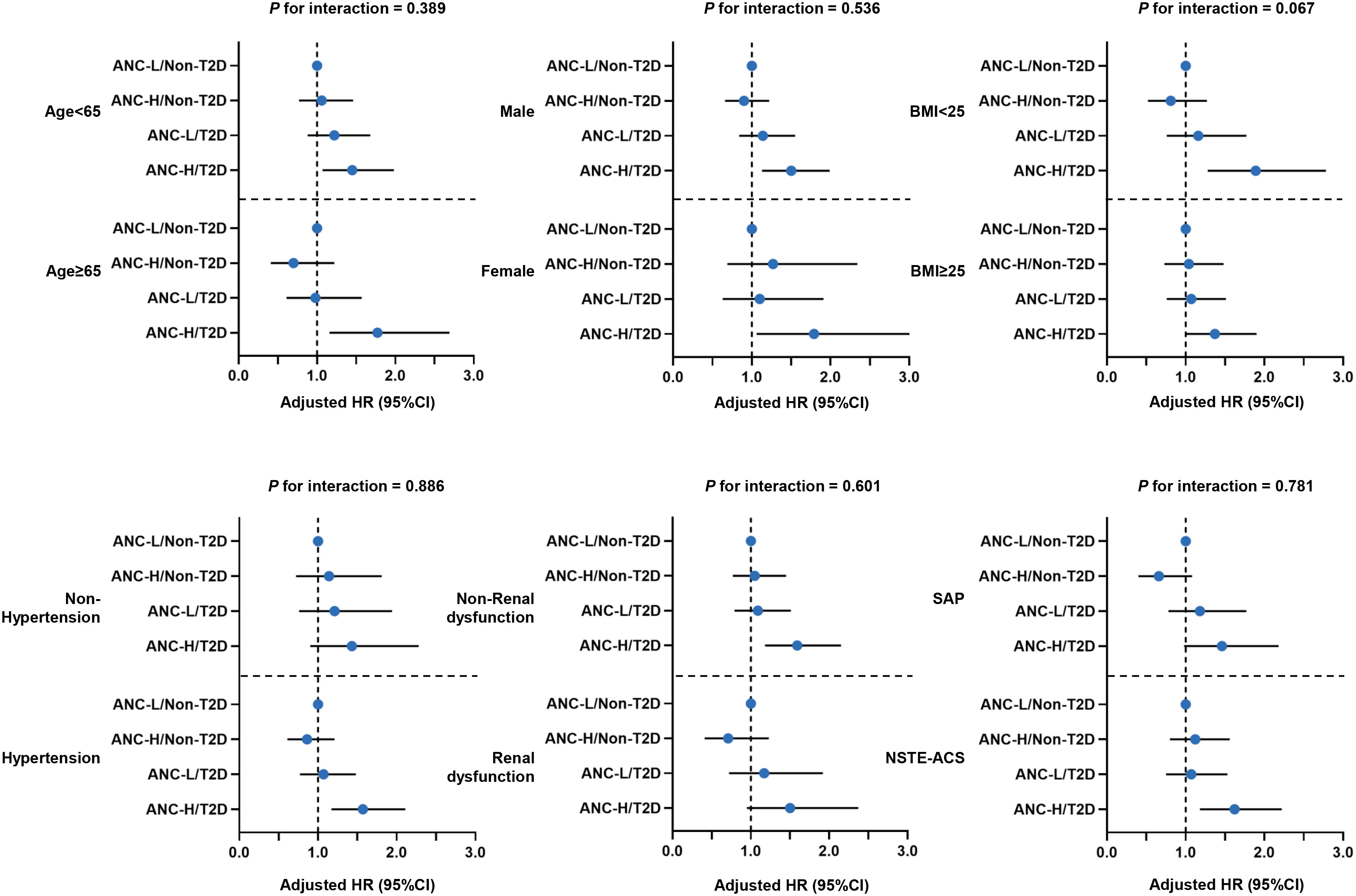
Figure 4 Forest plot of MACCE risk according to various subgroups. Model adjusted for age, male sex, BMI, hypertension, dyslipidemia, smoking history, previous MI, previous PCI/CABG, previous stroke, NSTE-ACS, hsCRP, eGFR, LVEF, LM/three-vessel disease, CTO lesions, number of treated vessels, and SYNTAX score. Abbreviations as in Figure 1.
Discussion
In this real-world, large-scale, prospective, observational cohort study, the prognosis of diabetic and non-diabetic CAD patients undergoing PCI with different levels of ANC was investigated. The major findings of this study are as follows (1): T2D modified the effect of ANC on clinical outcomes in CAD patients who underwent PCI after adjustment for confounding factors. A higher level of ANC was independently associated with MACCEs in T2D. While in non-diabetic patients, no significant association between ANC and MACCE risk was found; (2) elevated ANC with concomitant T2D conferred an increased MACCE risk in CAD patients undergoing PCI; (3) diabetic patients with elevated ANC tended to have unfavorable cardiovascular risk profiles and increased lesion complexity, assessed by the SYNTAX score; and (4) the association of ANC categories and T2D with adverse clinical outcomes was consistent according to different subgroups. Our findings suggested that more precise and accurate risk assessment and appropriate clinical management should be performed in CAD patients with concomitant T2D and elevated ANC.
Current evidence has indicated that inflammation might be an essential and modifiable process in the development of CAD (20). Previous studies reported a range of inflammatory biomarkers that were associated with an increased risk of cardiovascular events (21–23). Among them, white blood cells (WBCs) were one of the simplest and most measured blood-based inflammatory biomarkers. Since Friedman et al. (24) first demonstrated the WBC count could be a predictor of myocardial infarction in 1974, it has been suggested to be positively associated with higher rates of incident CV events and mortality (25, 26). Subsequently, several researchers evaluated the ability of different subpopulations of WBC to estimate the risk of CV events and mortality, which yielded controversial conclusions (26, 27). Wheeler et al. (27) conducted a meta-analysis to investigate the relationship between differential leucocyte count and incidence of CAD in 28,528 patients with or without a history of CAD from five large cohorts with a mean follow-up ranging from 3 to 18 years. Comparing the top tertiles vs. bottom tertiles of different leucocyte counts, they found the association of CAD with ANC was stronger than that of granulocytes, lymphocytes, and monocytes, with a risk ratio of 1.33, 1.32, 1.11, and 1.10, respectively. Besides, Welsh et al. (26) also found a similar conclusion from 478,259 UK biobank participants with a 7-year follow-up. After adjusting for a range of classical risk factors, they found only ANC was most consistently associated with the risk of fatal and nonfatal CVD in both men and women. Furthermore, Shah et al. (28) found strong associations between ANC and heart failure, unheralded coronary death, abdominal aortic aneurysm, nonfatal MI, and ischemic stroke. Overall, these studies indicated ANC had a strong positive association with the risk of all-cause or CV mortality in the general population.
Currently, several studies have explored the impact of neutrophils on CVD risk from biological mechanisms and clinically modifiable risk factors. From a biological standpoint, during the past decades, studies have demonstrated the important role of neutrophils in the process of atherosclerosis and CV inflammation by several mechanisms, as follows (1): In coronary lesions, neutrophils could be observed near plaques, especially rupture-prone ones (29). (2) Neutrophils could enhance the adhesion of monocytes, which might transform into foam cells and form the lipid core of vulnerable plaques (30). Meanwhile, neutrophils also contribute to endothelial cell dysfunction and oxidative stress via releasing myeloperoxidase, lipoxygenases, and NETs (30, 31). NETs can also promote atherothrombosis and present tissue factors at sites of infarction (31). (3) Neutrophils could promote fibrous cap rupture by releasing proteases that degrade matrix elements (29). (4) An early massive neutrophil influx into the ischemia-injured myocardium can cause collateral damage and impair myocardial healing (30). On the other hand, from clinical sights, acute inflammation, chronic inflammation background and genetics of patients might help to explain the relationship between ANC and CVD. Of note, low-grade and chronic inflammation might be the initial but modifiable factor in the causal pathways of CVD, including air pollution, obesity, a lack of physical exercise, and periodontal disease (32, 33). Here, we focus on T2D. Previous studies indicated that low-grade inflammation is a key component in the pathophysiology of T2D (34). Meanwhile, increased diabetic risk (35) and insulin resistance (36) have been described in patients with chronic inflammatory diseases such as rheumatoid arthritis and psoriasis. One previous cohort study assessed the role of neutrophils in the pathogenesis of T2D (37). However, no evidence indicated a relationship between neutrophils, T2D, and the risk of CVD. In our study, we found that for T2D patients, a higher level of ANC was independently associated with 2-year MACCEs. However, for patients without T2D, no statistically significant association was observed between ANC and 2-year MACCEs.
The interaction between inflammation, T2D, and CVD might be explained by the co-signaling of IL-1β and IL-6 in diabetes and atherosclerosis. Firstly, IL-1β was one of the key components of inflammatory activation by producing the inflammasome, which could regulate the activation of caspase-1 and mediate NLRP3. For T2D, one previous study had indicated IL-1β could impair insulin secretion, contributing to the development of glucose intolerance and T2D (38). Meanwhile, IL-1β could also promote insulin resistance via the NLRP3 inflammasome (39) and impair adipocyte insulin signaling via the activation of caspase-1 (40). For atherosclerosis, the formation of foam cells was initiated by the release of IL-1β and NLRP3 inflammasomes, which were activated by cholesterol and saturated fatty acids (41). Meanwhile, IL-β could regulate the chemotaxis and adhesion of monocytes (42). Secondly, current evidence indicates that IL-6 is a central stimulus for the acute phase response, for example by increasing the production of CRP (43). For atherosclerosis, the results from an animal model shows that IL-6 signaling contributed to the initiation of atherosclerosis and plaque destabilization in mice (44). For T2D, current studies have indicated that IL-6 has a negative effect on metabolism. It was supported by elevated circulating IL-6 in patients with obesity and the onset of diabetes (45). Meanwhile, one previous study indicated that IL-6 could induce hepatic insulin resistance (46).
It was the first and largest cohort study to investigate the interaction between neutrophils, T2D, and poor prognosis in CAD patients. However, there were still some limitations. First, even though the present study adjusted for a considerable number of potential confounding factors, it was hard to fully adjust all confounding factors (such as diet, air pollution, and physical exercise) due to the limitations of data collection and the nature of the observational study design. Second, data regarding dynamic changes in ANC and glycemic status during follow-up are unavailable. Thirdly, the ethnic composition of this study was single, only Chinese patients were included (47, 48). It was still unclear whether the conclusion was suitable for other populations. Fourth, in this study, we performed multivariable Cox analysis to adjust for potential confounding factors. However, a sensitivity analysis (propensity score matching or a nested case–control design) was not adopted to strengthen our results. Future, well-designed prospective studies are warranted to confirm our findings.
Conclusions
Elevated ANC confers increased MACCE risk with T2D. Patients with concomitantly elevated ANC and T2D have greater MACCE risk; stratification of patients with elevated ANC and T2D could provide valuable information for risk stratification of CAD patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The study process complied with the Declaration of Helsinki and was approved by the Institutional Review Board of Fuwai hospital. Written informed consent for long term follow-up before PCI was obtained from all study patients.
Author contributions
KD and QD conceived and designed the study. JH, ZL, CS, and RZ acquired, analyzed, and interpreted the data. JH, ZL, and SY contributed to drafting the article. JH, ZL, HW, and XB revised it critically for important intellectual content. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was supported by the CAMS Innovation Fund for Medical Sciences (CIFMS) [grant number, 2021-I2M-1-008], and the Beijing Municipal Science and Technology Project [grant number, Z211100002921009].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1129633/full#supplementary-material
References
1. Lawler PR, Bhatt DL, Godoy LC, Lüscher TF, Bonow RO, Verma S, et al. Targeting cardiovascular inflammation: next steps in clinical translation. Eur Heart J (2021) 42(1):113–31. doi: 10.1093/eurheartj/ehaa099
2. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med (2017) 377(12):1119–31. doi: 10.1056/NEJMoa1707914
3. Tardif J-C, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med (2019) 381(26):2497–505. doi: 10.1056/NEJMoa1912388
4. Nidorf SM, Fiolet ATL, Mosterd A, Eikelboom JW, Schut A, Opstal TSJ, et al. Colchicine in patients with chronic coronary disease. N Engl J Med (2020) 383(19):1838–47. doi: 10.1056/NEJMoa2021372
5. Adamstein NH, MacFadyen JG, Rose LM, Glynn RJ, Dey AK, Libby P, et al. The neutrophil-lymphocyte ratio and incident atherosclerotic events: analyses from five contemporary randomized trials. Eur Heart J (2021) 42(9):896–903. doi: 10.1093/eurheartj/ehaa1034
6. Silvestre-Roig C, Braster Q, Ortega-Gomez A, Soehnlein O. Neutrophils as regulators of cardiovascular inflammation. Nat Rev Cardiol (2020) 17(6):327–40. doi: 10.1038/s41569-019-0326-7
7. Sager HB, Koenig W. Immune cell-based cardiovascular risk assessment: spotlight on the neutrophil-lymphocyte ratio. Eur Heart J (2021) 42(9):904–6. doi: 10.1093/eurheartj/ehaa1104
8. Soehnlein O, Lindbom L, Weber C. Mechanisms underlying neutrophil-mediated monocyte recruitment. Blood (2009) 114(21):4613–23. doi: 10.1182/blood-2009-06-221630
9. Soehnlein O, Kai-Larsen Y, Frithiof R, Sorensen OE, Kenne E, Scharffetter-Kochanek K, et al. Neutrophil primary granule proteins HBP and HNP1-3 boost bacterial phagocytosis by human and murine macrophages. J Clin Invest. (2008) 118(10):3491–502. doi: 10.1172/JCI35740
10. Warnatsch A, Ioannou M, Wang Q, Papayannopoulos V. Inflammation. neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science (2015) 349(6245):316–20. doi: 10.1126/science.aaa8064
11. Delporte C, Boudjeltia KZ, Noyon C, Furtmüller PG, Nuyens V, Slomianny M-C, et al. Impact of myeloperoxidase-LDL interactions on enzyme activity and subsequent posttranslational oxidative modifications of apoB-100. J Lipid Res (2014) 55(4):747–57. doi: 10.1194/jlr.M047449
12. Soehnlein O, Ortega-Gómez A, Döring Y, Weber C. Neutrophil-macrophage interplay in atherosclerosis: protease-mediated cytokine processing versus NET release. Thromb Haemost. (2015) 114(4):866–7. doi: 10.1160/TH15-08-0623
13. Burn GL, Foti A, Marsman G, Patel DF, Zychlinsky A. The neutrophil. Immunity (2021) 54(7):1377–91. doi: 10.1016/j.immuni.2021.06.006
14. Horne BD, Anderson JL, John JM, Weaver A, Bair TL, Jensen KR, et al. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol (2005) 45(10):1638–43. doi: 10.1016/j.jacc.2005.02.054
15. He J, Bian X, Song C, Zhang R, Yuan S, Yin D, et al. High neutrophil to lymphocyte ratio with type 2 diabetes mellitus predicts poor prognosis in patients undergoing percutaneous coronary intervention: a large-scale cohort study. Cardiovasc Diabetol (2022) 21(1):156. doi: 10.1186/s12933-022-01583-9
16. Association AD. 2. classification and diagnosis of diabetes: Standards of medical care in diabetes-2022. Diabetes Care (2022) 45(Suppl 1):S17–38. doi: 10.2337/dc22-S002
17. Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med (2013) 158(11):825–30. doi: 10.7326/0003-4819-158-11-201306040-00007
18. Ma Y-C, Zuo L, Chen J-H, Luo Q, Yu X-Q, Li Y, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. (2006) 17(10):2937–44. doi: 10.1681/ASN.2006040368
19. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third universal definition of myocardial infarction. Eur Heart J (2012) 33(20):2551–67. doi: 10.1093/eurheartj/ehs184
20. Back M, Hansson GK. Anti-inflammatory therapies for atherosclerosis. Nat Rev Cardiol (2015) 12(4):199–211. doi: 10.1038/nrcardio.2015.5
21. Welsh P, Murray HM, Ford I, Trompet S, de Craen AJ, Jukema JW, et al. Circulating interleukin-10 and risk of cardiovascular events: a prospective study in the elderly at risk. Arterioscler Thromb Vasc Biol (2011) 31(10):2338–44. doi: 10.1161/ATVBAHA.111.231795
22. Danesh J, Kaptoge S, Mann AG, Sarwar N, Wood A, Angleman SB, et al. Long-term interleukin-6 levels and subsequent risk of coronary heart disease: two new prospective studies and a systematic review. PloS Med (2008) 5(4):e78. doi: 10.1371/journal.pmed.0050078
23. Emerging Risk Factors C, Kaptoge S, Di Angelantonio E, Pennells L, Wood AM, White IR, et al. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med (2012) 367(14):1310–20. doi: 10.1056/NEJMoa1107477
24. Friedman GD, Klatsky AL, Siegelaub AB. The leukocyte count as a predictor of myocardial infarction. N Engl J Med (1974) 290(23):1275–8. doi: 10.1056/NEJM197406062902302
25. Kabat GC, Kim MY, Manson JE, Lessin L, Lin J, Wassertheil-Smoller S, et al. White blood cell count and total and cause-specific mortality in the women's health initiative. Am J Epidemiol. (2017) 186(1):63–72. doi: 10.1093/aje/kww226
26. Welsh C, Welsh P, Mark PB, Celis-Morales CA, Lewsey J, Gray SR, et al. Association of total and differential leukocyte counts with cardiovascular disease and mortality in the UK biobank. Arterioscler Thromb Vasc Biol (2018) 38(6):1415–23. doi: 10.1161/ATVBAHA.118.310945
27. Wheeler JG, Mussolino ME, Gillum RF, Danesh J. Associations between differential leucocyte count and incident coronary heart disease: 1764 incident cases from seven prospective studies of 30,374 individuals. Eur Heart J (2004) 25(15):1287–92. doi: 10.1016/j.ehj.2004.05.002
28. Shah AD, Denaxas S, Nicholas O, Hingorani AD, Hemingway H. Neutrophil counts and initial presentation of 12 cardiovascular diseases: A CALIBER cohort study. J Am Coll Cardiol (2017) 69(9):1160–9. doi: 10.1016/j.jacc.2016.12.022
29. Soehnlein O. Multiple roles for neutrophils in atherosclerosis. Circ Res (2012) 110(6):875–88. doi: 10.1161/CIRCRESAHA.111.257535
30. Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science (2013) 339(6116):161–6. doi: 10.1126/science.1230719
31. Doring Y, Soehnlein O, Weber C. Neutrophil extracellular traps in atherosclerosis and atherothrombosis. Circ Res (2017) 120(4):736–43. doi: 10.1161/CIRCRESAHA.116.309692
32. Newby DE, Mannucci PM, Tell GS, Baccarelli AA, Brook RD, Donaldson K, et al. Expert position paper on air pollution and cardiovascular disease. Eur Heart J (2015) 36(2):83–93b. doi: 10.1093/eurheartj/ehu458
33. Nakamura K, Fuster JJ, Walsh K. Adipokines: a link between obesity and cardiovascular disease. J Cardiol (2014) 63(4):250–9. doi: 10.1016/j.jjcc.2013.11.006
34. Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol (2011) 11(2):98–107. doi: 10.1038/nri2925
35. Solomon DH, Love TJ, Canning C, Schneeweiss S. Risk of diabetes among patients with rheumatoid arthritis, psoriatic arthritis and psoriasis. Ann Rheum Dis (2010) 69(12):2114–7. doi: 10.1136/ard.2009.125476
36. Dessein PH, Joffe BI, Stanwix A, Botha AS, Moomal Z. The acute phase response does not fully predict the presence of insulin resistance and dyslipidemia in inflammatory arthritis. J Rheumatol (2002) 29(3):462–6.
37. Guo X, Zhang S, Zhang Q, Liu L, Wu H, Du H, et al. Neutrophil:lymphocyte ratio is positively related to type 2 diabetes in a large-scale adult population: a tianjin chronic low-grade systemic inflammation and health cohort study. Eur J Endocrinol (2015) 173(2):217–25. doi: 10.1530/EJE-15-0176
38. Eguchi K, Manabe I, Oishi-Tanaka Y, Ohsugi M, Kono N, Ogata F, et al. Saturated fatty acid and TLR signaling link beta cell dysfunction and islet inflammation. Cell Metab (2012) 15(4):518–33. doi: 10.1016/j.cmet.2012.01.023
39. Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med (2011) 17(2):179–88. doi: 10.1038/nm.2279
40. Stienstra R, Joosten LA, Koenen T, van Tits B, van Diepen JA, van den Berg SA, et al. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab (2010) 12(6):593–605. doi: 10.1016/j.cmet.2010.11.011
41. Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature (2010) 464(7293):1357–61. doi: 10.1038/nature08938
42. Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol (2009) 27:519–50. doi: 10.1146/annurev.immunol.021908.132612
43. Ridker PM. Anticytokine agents: Targeting interleukin signaling pathways for the treatment of atherothrombosis. Circ Res (2019) 124(3):437–50. doi: 10.1161/CIRCRESAHA.118.313129
44. Zhang K, Huang XZ, Li XN, Feng M, Li L, Cai XJ, et al. Interleukin 6 destabilizes atherosclerotic plaques by downregulating prolyl-4-hydroxylase alpha1 via a mitogen-activated protein kinase and c-jun pathway. Arch Biochem Biophys (2012) 528(2):127–33. doi: 10.1016/j.abb.2012.09.007
45. Spranger J, Kroke A, Mohlig M, Hoffmann K, Bergmann MM, Ristow M, et al. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European prospective investigation into cancer and nutrition (EPIC)-potsdam study. Diabetes (2003) 52(3):812–7. doi: 10.2337/diabetes.52.3.812
46. Klover PJ, Zimmers TA, Koniaris LG, Mooney RA. Chronic exposure to interleukin-6 causes hepatic insulin resistance in mice. Diabetes (2003) 52(11):2784–9. doi: 10.2337/diabetes.52.11.2784
47. He J, Zhang D, Zhang R, Wang H, Wu S, Feng L, et al. Validation of the V-RESOLVE (Visual estimation for risk prEdiction of side branch OccLusion in coronary bifurcation interVEntion) score system in unprotected left main bifurcation. Catheter Cardiovasc Interv. (2022) 99 Suppl 1:1465–72. doi: 10.1002/ccd.30111
Keywords: coronary artery disease, neutrophils, percutaneous coronary intervention, prognosis, type 2 diabetes
Citation: He J, Lin Z, Song C, Zhang R, Wang H, Yuan S, Bian X, Dong Q and Dou K (2023) High absolute neutrophil count with type 2 diabetes is associated with adverse outcome in patients with coronary artery disease: A large-scale cohort study. Front. Endocrinol. 14:1129633. doi: 10.3389/fendo.2023.1129633
Received: 22 December 2022; Accepted: 21 March 2023;
Published: 11 April 2023.
Edited by:
Gaetano Santulli, Albert Einstein College of Medicine, United StatesReviewed by:
Lucia Natarelli, LMU Munich University Hospital, GermanyCarmen Spaccarotella, Università Federico II Napoli, Italy
Copyright © 2023 He, Lin, Song, Zhang, Wang, Yuan, Bian, Dong and Dou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kefei Dou, ZHJkb3VrZWZlaUAxMjYuY29t; Qiuting Dong, Ymx1ZTEwMDVkcXRAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Jining He
Jining He Zhangyu Lin1,2†
Zhangyu Lin1,2† Rui Zhang
Rui Zhang Haoyu Wang
Haoyu Wang Sheng Yuan
Sheng Yuan Xiaohui Bian
Xiaohui Bian Qiuting Dong
Qiuting Dong Kefei Dou
Kefei Dou