- Department of Cardiology, The First Affiliated Hospital of Guangxi Medical University, Nanning, China
Background: Glycemic variability (GV) has been associated with vascular complications in patients with diabetes. However, the relationship between GV and risk of atrial fibrillation (AF) remains not fully determined. We therefore conducted a systematic review and meta-analysis to evaluate the above association.
Methods: Medline, Embase, Web of Science, Wanfang, and China National Knowledge Infrastructure were searched for longitudinal follow-up studies comparing the incidence of AF between patients with higher versus lower GV. A random-effects model incorporating the potential heterogeneity was used to pool the results.
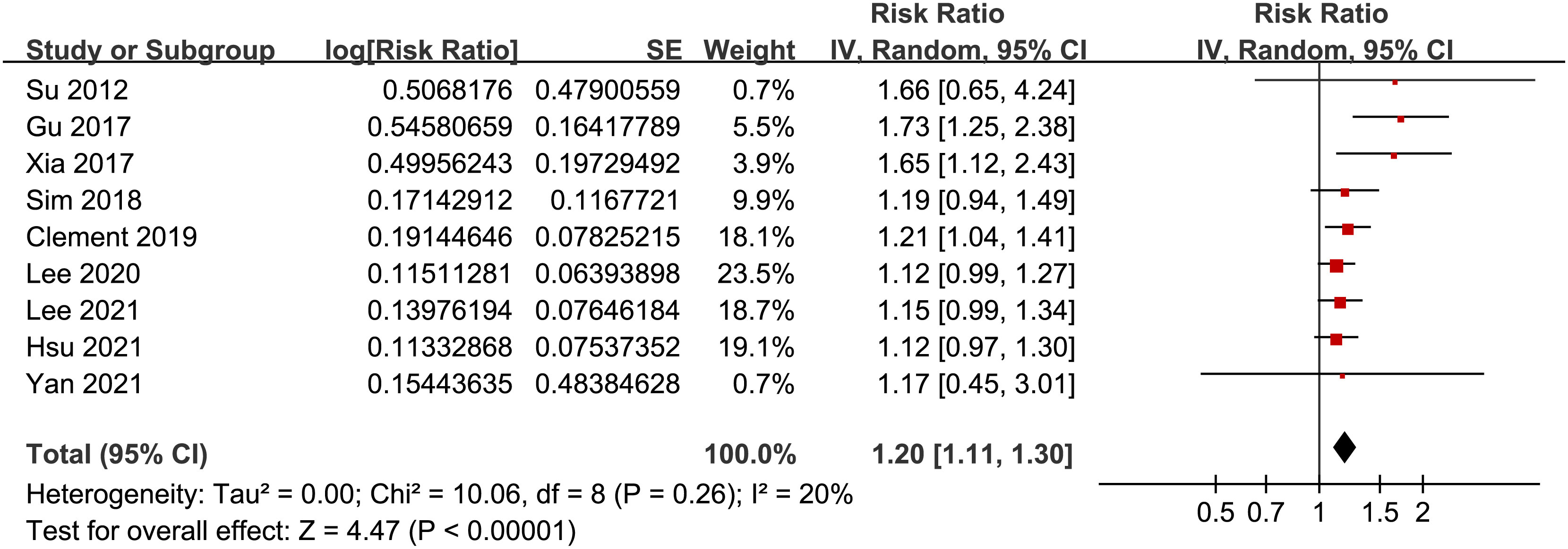
Results: Nine cohort studies with 6,877,661 participants were included, and 36,784 (0.53%) participants developed AF during follow-up. Pooled results showed that a high GV was associated with an increased risk of AF (risk ratio [RR]: 1.20, 95% confidence interval [CI]: 1.11 to 1.30, p < 0.001, I2 = 20%). Subgroup analyses suggested consistent association between GV and AF in prospective (RR: 1.29, 95% CI: 1.05 to 1.59, p = 0.01) and retrospective studies (RR: 1.18, 95% CI: 1.08 to 1.29, p = 0.002), in diabetic (RR: 1.24, 95% CI: 1.03 to 1.50, p = 0.03) and non-diabetic subjects (RR: 1.13, 95% CI: 1.00 to 1.28, p = 0.05), in studies with short-term (RR: 1.25, 95% CI: 1.11 to 1.40, p < 0.001) and long-term GV (RR: 1.18, 95% CI: 1.05 to 1.34, p = 0.006), and in studies with different quality scores (p for subgroup difference all > 0.05).
Conclusion: A high GV may predict an increased risk of AF in adult population.
Introduction
Atrial fibrillation (AF) is one of the most common arrhythmia, particularly in older population (1, 2). Although in recent decades, continuous efforts have been made to develop novel treatments for AF, substantial patients with AF still have poor clinical outcomes, such as a higher incidence of stroke and increased overall mortality (3, 4). Therefore, identification of risk factors for AF is important for the prevention of AF and related adverse events (5).
Accumulating evidence from clinical observations suggests that diabetes may be a risk factor of AF (6, 7). In diabetes patients, persistent hyperglycemia evidenced by elevated glycosylated hemoglobin (HbA1c) is widely accepted as being responsible for cardiovascular complications (8). Subsequent investigations suggest that besides persistent hyperglycemia, hypoglycemia may also be independently associated with the risk of cardiovascular events in patients with diabetes, including AF (9, 10). The concept of glycemic variability (GV) is therefore raised during the recent decades, which reflects the extent of glucose fluctuation within days (short-term GV) and months/years (long-term GV) (11). Independent of persistent hyperglycemia, high GV has been shown to be a predictor of adverse cardiovascular events in both the diabetic and the non-diabetic population (12). Indeed, a high GV has been related to a higher incidence of coronary artery disease (CAD) (13), stroke (14), and overall cardiovascular mortality (15). Besides, an increased acute GV has also recently been identified as a potential predictor of poor survival in patients with sepsis (16). However, previous studies investigating the relationship between GV and AF showed inconsistent results (17). Some studies showed that a high GV may be a risk factor of AF (18–23), while other studies did not support this association (24–26). Therefore, in this study, we conducted a systematic review and meta-analysis to systematically evaluate the association between GV and the incidence of AF.
Methods
The meta-analysis was performed in accordance with the MOOSE (Meta-analysis of Observational Studies in Epidemiology) (27) and Cochrane’s Handbook (28) guidelines.
Literature search
Studies were identified via systematic search of electronic databases including Medline, Embase, Web of Science, Wanfang, and China National Knowledge Infrastructure (CNKI) from inception to September 19, 2022. A combined search strategy was used, which included: (1) “glycemic” OR “glyceamic” OR “glucose” OR “hemoglobin A1c” OR “A1C”‘; (2) “variability” OR “variation” OR “fluctuation”; and (3) “atrial fibrillation” OR “AF”. The search was limited to clinical studies published in English. The reference lists of related original and review articles were also analyzed using a manual approach.
Study selection
The inclusion criteria for the studies were: (1) observational studies that are designed to follow up over time, such as cohort studies, post-hoc analyses of clinical trials, or nested case-control studies; (2) included adult population who were evaluated for GV at baseline; (3) incidence of AF during follow-up was observed and recorded; and (4) the incidence of AF between patients with higher versus lower GV was compared and reported as risk ratio (RR) and corresponding 95% confidence interval [CI], or these data could be calculated or estimated. Measuring methods and definition of GV were consistent with the criteria applied among the included studies. Reviews, editorials, preclinical studies, cross-sectional studies, studies that did not evaluate GV, studies that did not report the incidence of AF, or other studies that were irrelevant to the aim of the meta-analysis were excluded.
Data extracting and quality evaluation
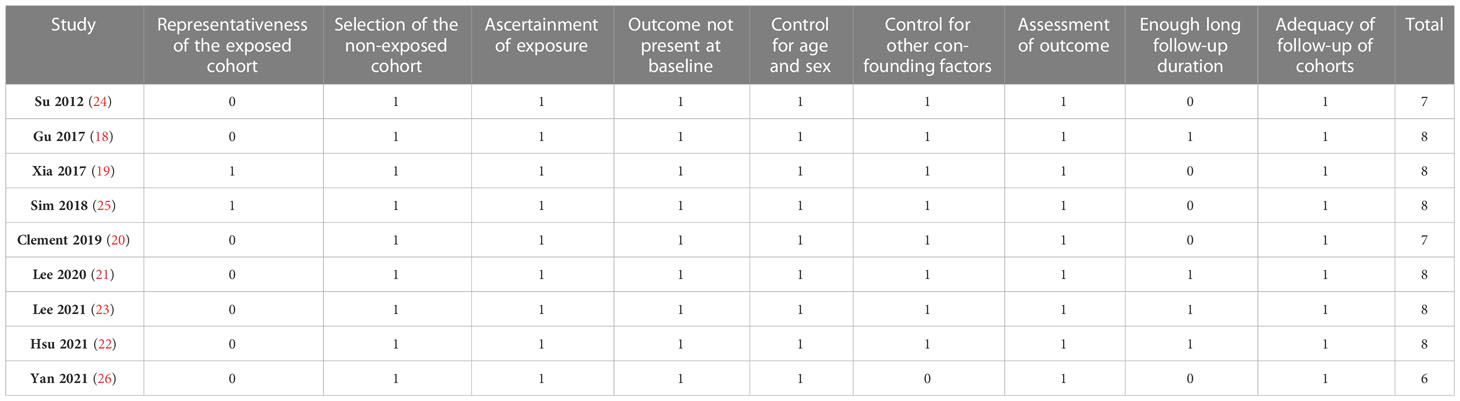
Literature search, data extraction, and quality assessment of the included studies were independently performed by two authors according to the predefined criteria. Discrepancies were resolved by consensus or discussion with the corresponding author. The extracted data included: (1) general information of the study (author, year, and country); (2) study design characteristics; (3) patient characteristics (diagnosis, age, sex, and diabetic status); (4) exposure characteristics (parameter for GV, cutoff for defining high GV, and number of subjects with high GV at baseline); (5) follow-up and outcome (follow-up durations, methods for validation the diagnosis of AF, and number of patients with AF incidence); and (6) potential confounding factors that were adjusted or matched when the association between GV and the incidence of AF was estimated. The quality of each study was evaluated using the Newcastle-Ottawa Scale (29) which ranges from 1 to 9 stars and judges each study regarding three aspects: selection of the study groups; the comparability of the groups; and the ascertainment of the outcome of interest.
Statistical analyses
The methods of the statistics are generally consistent with those used in the previous meta-analysis of cohort studies (30). We used RR and corresponding 95% CI as the general measure for association between GV at baseline and the incidence of AF during follow-up. Data of RR and the corresponding stand error (SE) were calculated from 95% CI or P values, and were logarithmically transformed to stabilize the variance and normalize the distribution (28). The Cochrane’s Q test was used to evaluate the heterogeneity among the included studies (31). Besides, the I2 statistic was also estimated (31). A significant heterogeneity was considered if I2 > 50%. We used a random-effect model to synthesize the RR data because this model is considered as a more generalized method which incorporates the potential heterogeneity among the included studies (32). Sensitivity analysis was used to evaluate the possible influence of each study on the pooled results (33). Predefined subgroup analyses were also performed to evaluate the influences of study characteristics on the association, such as study design, diabetic status of the participants, type of GV (short-term or long-term), and quality scores of the studies. The potential publication bias was assessed by funnel plots with the Egger’s regression asymmetry test (34). A P value < 0.05 indicates statistically significance. We used the RevMan (Version 5.1; Cochrane Collaboration, Oxford, UK) and Stata 12.0 software for the meta-analysis and statistical analysis.
Results
Literature search
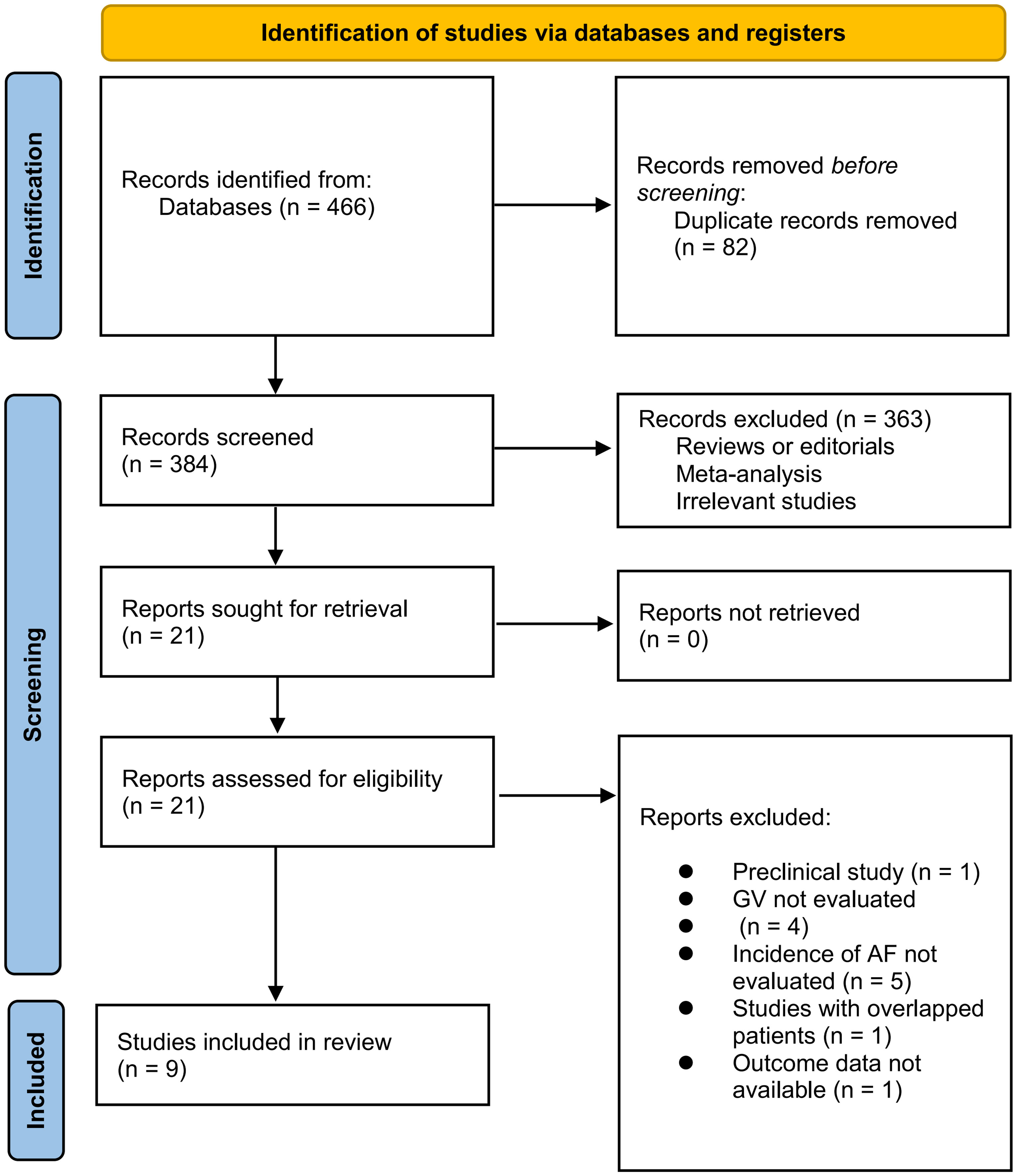
The process of database search is summarized in Figure 1. Briefly, 466 articles were found via initial literature search, and 384 articles were left after excluding the duplicated records. Subsequently, 363 articles were excluded by screening the titles and abstracts because they were not relevant to the objective of the meta-analysis. Accordingly, 21 articles underwent full-text review, and 12 of them were further excluded for the reasons listed in Figure 1. Finally, 9 studies were included in the meta-analysis (18–26).
Study characteristics and quality evaluation
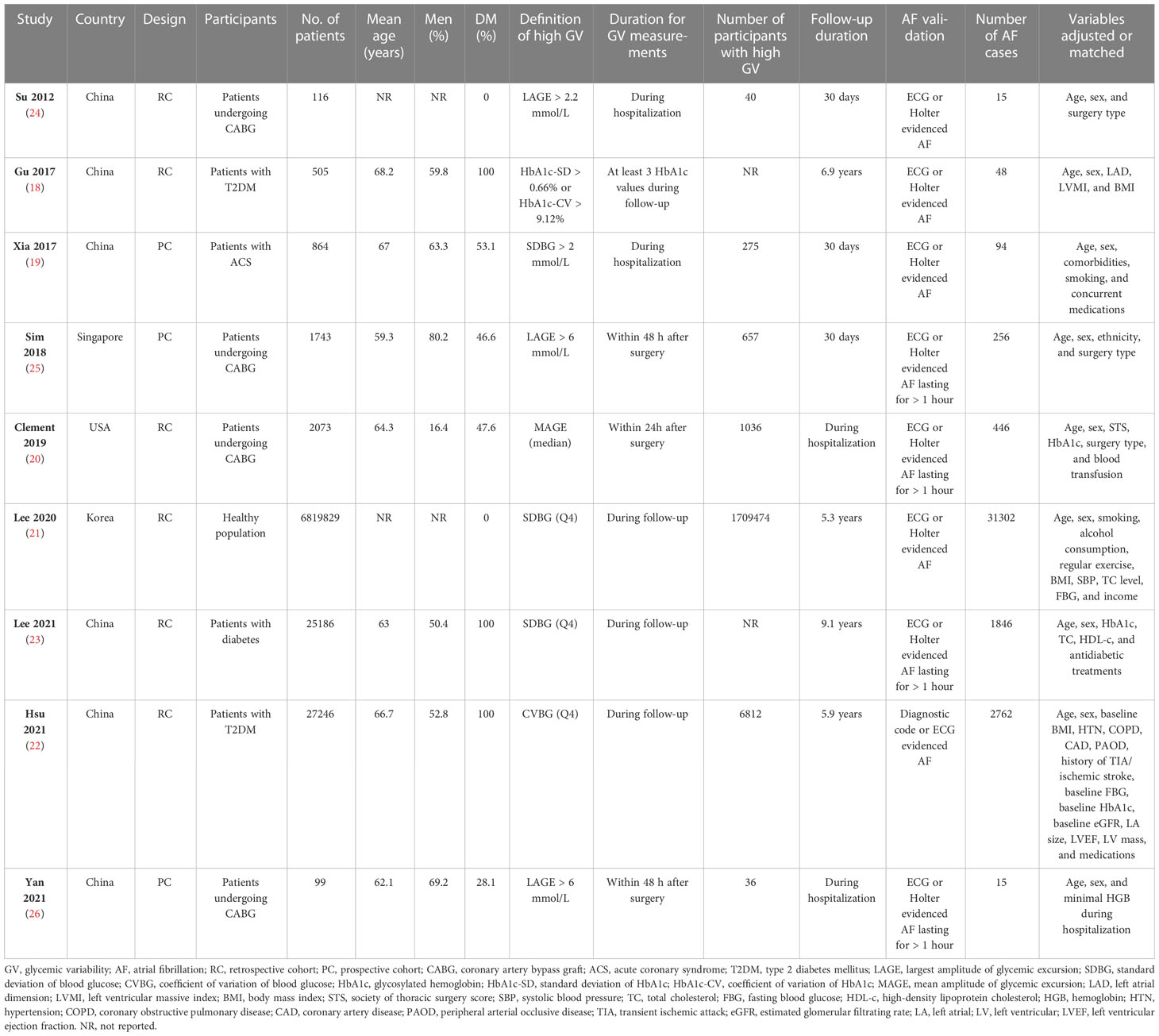
The characteristics of the included studies are summarized in Table 1. Overall, nine studies, including 3 prospective cohort studies (19, 25, 26) and 6 retrospective cohort studies (18, 20–24), were included in the meta-analysis. These studies were published between 2012 and 2021, and carried out in China (18, 19, 22–24, 26), Singapore (25), Korea (21), and the United States (20). Fours studies included patients with CAD who underwent coronary artery bypass graft (CABG) (20, 24–26), one study included patients with acute coronary syndrome (ACS) (19), three studies included subjects with diabetes (18, 22, 23), and the remaining one study included healthy population (21). The mean ages of the patients varied between 59.3 and 68.2 years, and the proportions of men ranged from 16.4% to 80.2%. Various GV parameters were applied in the included studies, such as the mean or largest amplitude of glycemic excursion (MAGE or LAGE) for as the indicators of short-term GV (19, 20, 24–26), and the standard deviation (SD) or coefficient of variation (CV) of HbA1c or blood glucose (BG) as the indicators of long-term GV (18, 21–23). The follow-up durations were within hospitalization or 30 days for studies with short-term GV, and from 5.3 to 9.1 years for studies with long-term GV. Patients with incidental AF were generally validated with electrocardiograph or Holter, and a total of 36,784 (0.53%) patients developed AF during follow-up. Possible confounding factors, such as age, sex, smoking, alcohol drinking, and comorbidities were adjusted to a varying degree among the included studies. The NOS scores of the included studies ranged from six to eight, indicating moderate to good study quality (Table 2).
GV and the risk of AF
Pooled results of the nine cohort studies showed that overall, a high GV was associated with a higher risk of AF in adults population (RR: 1.20, 95% CI: 1.11 to 1.30, p < 0.001; Figure 2) with mild heterogeneity (p for Cochrane’s’ Q test = 0.26, I2 = 20%). Sensitivity analysis by excluding one study at a time did not significantly affect the results (data not shown). Subgroup analyses showed consistent association between a high GV and increased incidence of AF in prospective (RR: 1.29, 95% CI: 1.05 to 1.59, p = 0.01) and retrospective studies (RR: 1.18, 95% CI: 1.08 to 1.29, p = 0.002; p for subgroup difference = 0.44; Figure 3A), in diabetic (RR: 1.24, 95% CI: 1.03 to 1.50, p = 0.03) and non-diabetic subjects (RR: 1.13, 95% CI: 1.00 to 1.28, p = 0.05; p for subgroup difference = 0.43; Figure 3B), in studies with short-term (RR: 1.25, 95% CI: 1.11 to 1.40, p < 0.001) and long-term GV (RR: 1.18, 95% CI: 1.05 to 1.34, p = 0.006; p for subgroup difference = 0.56; Figure 4A), and in studies with quality scores of 6~7 (RR: 1.22, 95% CI: 1.05 to 1.42, p = 0.009) and in those of 8 (RR:1.21, 95% CI: 1.09 to 1.35, p < 0.001; p for subgroup difference = 0.94; Figure 4B).
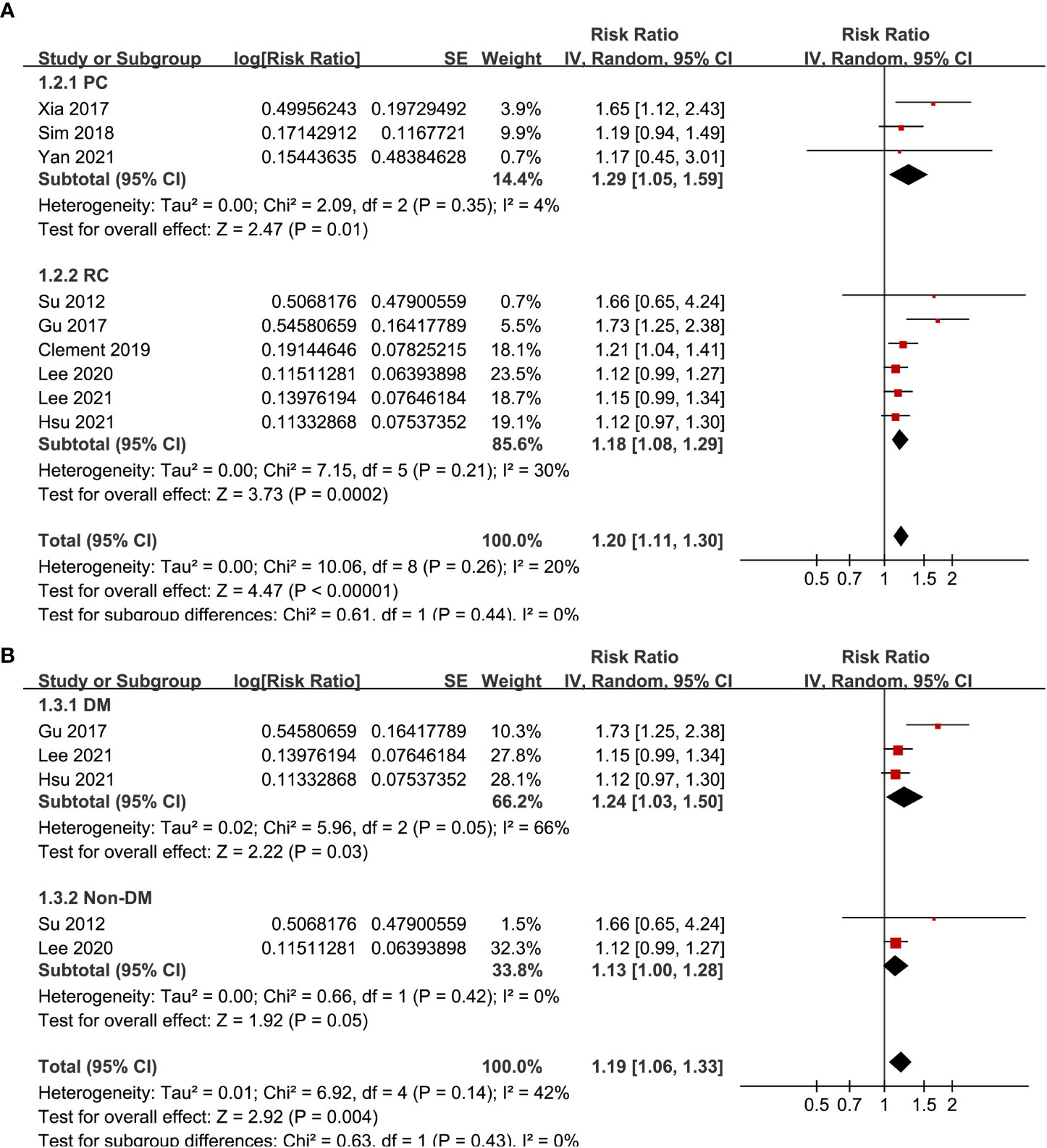
Figure 3 Subgroup analyses for the meta-analysis of the association between GV and the risk of AF. (A) subgroup analysis according to the study design; and (B) subgroup analysis according to the diabetic status of the subjects.
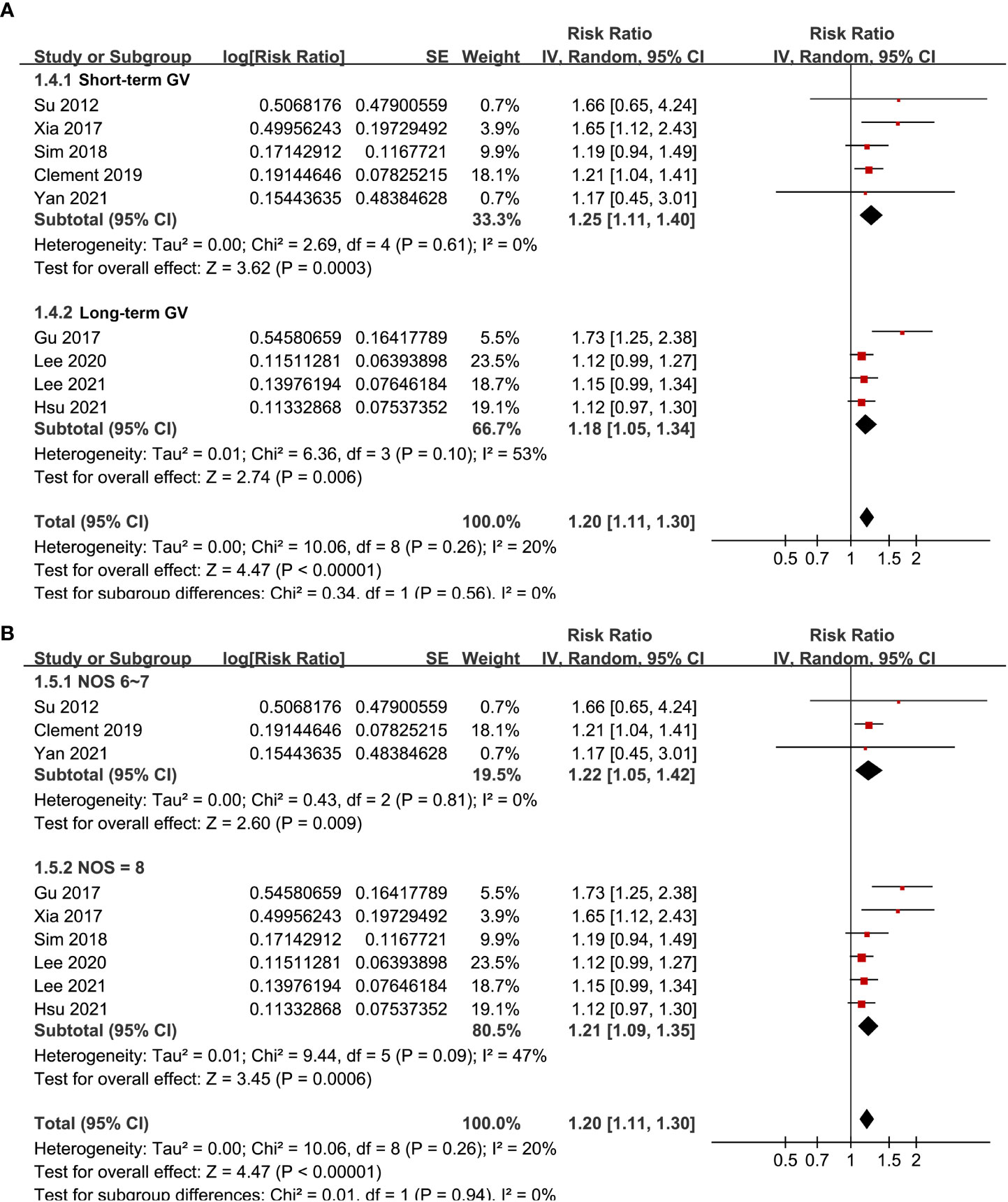
Figure 4 Subgroup analyses for the meta-analysis of the association between GV and the risk of AF. (A) subgroup analysis in studies with acute or long-term GV; and (B) subgroup analysis in studies with different quality scores.
Publication bias
The funnel plots for the meta-analysis of the association between GV and risk of AF are shown in Figure 5. The plots were symmetrical on visual inspection, suggesting low risk of publication bias. Egger’s regression test also suggested a low-risk publication underlying the meta-analysis (p = 0.23).
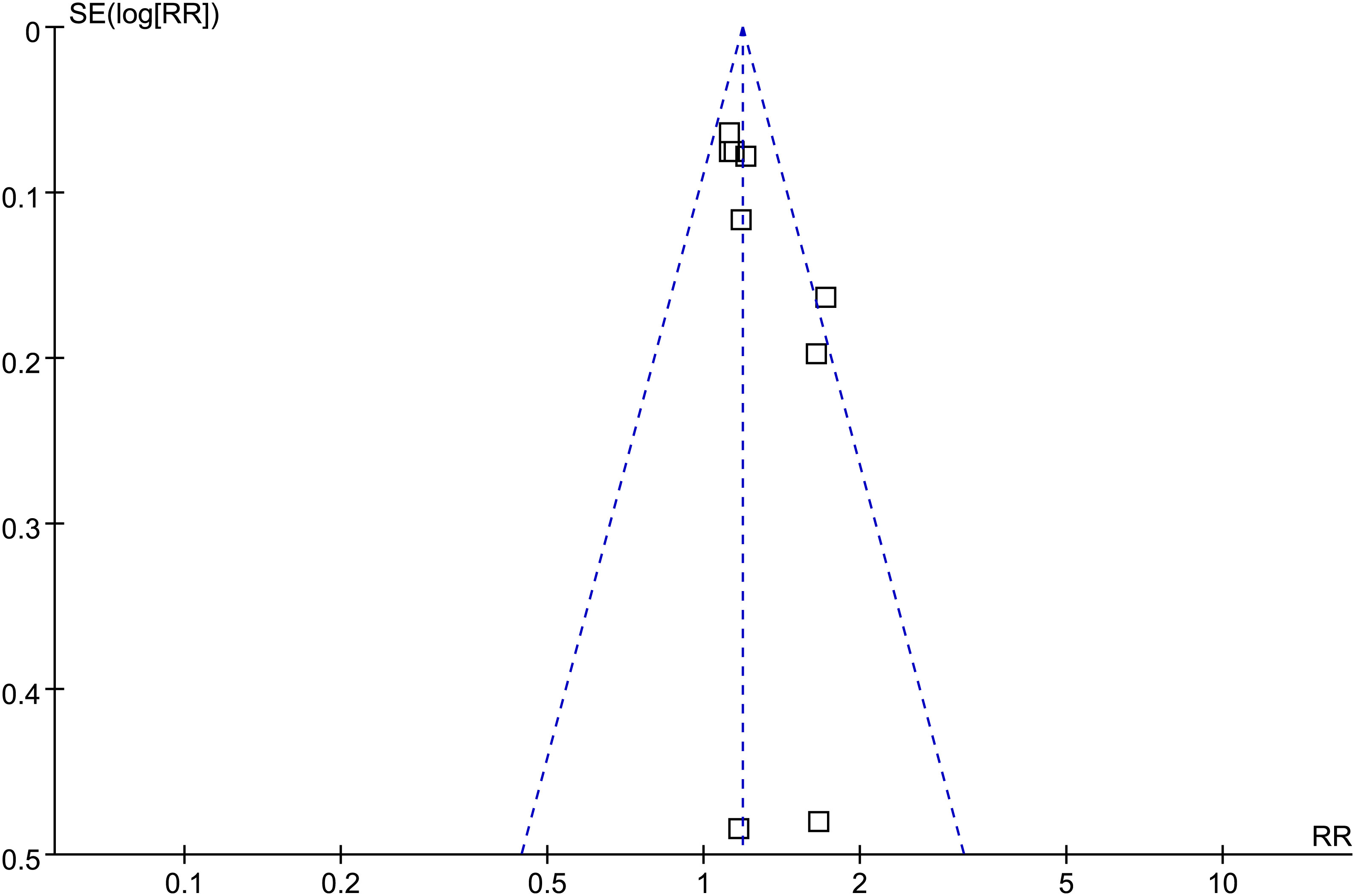
Figure 5 Funnel plots for the meta-analysis of the association between GV and the risk of AF. □ indicates each study included in the meta-analysis.
Discussion
In this systematic review and meta-analysis, by pooling the results of nine cohort studies, we found that subjects with a high GV at baseline was associated with a higher risk of AF as compared to those with a low GV. The stability of the finding was confirmed by similar results of sensitivity analyses by excluding one study at a time. Moreover, subsequent predefined subgroup analyses showed that the association between a high GV and the increased risk of AF was consistent in prospective and retrospective cohort studies, in diabetic and non-diabetic subjects, in studies with short-term and long-term GV, and in studies with different quality scores. Taken together, these results indicate that a high GV at baseline may be a risk factor of AF in adult population. Although large-scale prospective studies are needed to validate these findings, results of the meta-analysis suggest that measuring GV may be useful to predict the risk of AF.
To the best of our knowledge, this study may be the first systematic review and meta-analysis which investigated the potential relationship between GV and the incidence of AF. We extensively searched for relevant studies in five electronic databases, and nine up-to-date cohort studies were retrieved, with about half of them published within the recent 3 years. Besides, only cohort studies were included, which could therefore provide a longitudinal association between GV and AF. Moreover, multivariate analyses were used to estimate the association between GV and AF among all the included studies, and potential confounding factors such as age, sex, and comorbidities etc. were adjusted, which therefore could indicate an independent relationship between GV and AF. Finally, consistent results were obtained in multiple sensitivity and subgroup analyses, which further confirmed the robustness of the finding. Taken together, this meta-analysis confirmed that a high GV may be a risk factor of AF in adult population, which expanded the previous observations that a high GV is associated with an increased risk of adverse cardiovascular events, such as CAD, stroke, and cardiovascular deaths.
The mechanisms underlying the association between GV and AF remain to be fully understood. An early experimental study in streptozotocin-induced diabetic rats confirmed that glucose fluctuation in uncontrolled diabetes was associated with a high inducibility of AF (35). Meanwhile, an enhanced expression of markers of cardiac fibrosis (collagen type 1, collagen type 3, and α-smooth muscle actin) was also observed in rats with high glucose fluctuation, together with the unregulated markers of reactive oxygen species (ROS) and apoptosis (35). These findings may suggest that glucose fluctuation may accelerate the pathogenesis of AF via inducing oxidative stress, cardiomyocyte apoptosis, and atrial fibrosis. In addition, a previous clinical study in patients with newly diagnosed and well-controlled type 2 diabetes showed that a high GV may be associated with reduced cardiac autonomic modulation (36). Interestingly, changes of cardiac autonomic innervation or outflow have been shown to be involved in the pathogenesis of atrial arrhythmias, including AF (37), suggesting GV may induce AF via affecting the autonomic function. Finally, emerging evidence from preclinical studies suggested that a high glucose fluctuation may adversely affect the structural and electrical remodeling of the atrium, which may also be the underlying mechanisms for the association between GV and AF (38, 39). Studies are warranted in the future for determination of the molecular signaling pathways involved.
Our study has several limitations. First, the number of the included studies was limited, and some of them were of retrospective design. Therefore, results of the meta-analysis should be validated in large-scale prospective studies. Second, the parameters and definitions of patients with high GV varied among the included studies, which may be a main source of heterogeneity of the meta-analysis. However, as mentioned previously, no consensus has been reached regarding the optimal parameter and corresponding cutoff value for defining subjects with high GV. Additionally, AF is usually classified according to its temporal pattern as paroxysmal, persistent, or permanent (40). Studies are needed to determine if the association between GV and AF is consistent according to the different types of AF. Moreover, subgroup analysis was performed according to the diabetic status of the patients. In prediabetic patients, especially those with impaired glucose tolerance, postprandial glucose fluctuation has been closely related to cardiovascular complications (41). Accordingly, it could be hypothesized that GV in patients with prediabetes may be related to a higher risk of AF. However, to the best of our knowledge, no study has particularly investigated the association between GV and AF in this important population. Studies are warranted to investigate the potential relationship between GV and risk of AF in people with prediabetes. Additionally, although the results regarding the association between GV and AF in this meta-analysis were on the basis of studies with multivariate analyses, there may be residual confounding factors which may affect the association. For example, different antidiabetic strategies may affect the extent of GV differently (42). Accordingly, difference of antidiabetic drugs used in the included patients may affect the association between GV and the risk of AF. Among the strategies with low risk of hypoglycemia, the blood glucose fluctuation of patients is relatively small (43). Most of the new hypoglycemic drugs have low risk of hypoglycemia, and the GV is also small. Insulin-based treatment has a relatively high risk of hypoglycemia and high GV (43). Therefore, the effect of hypoglycemic strategies and drugs on GV and atrial fibrillation is important. On the other hand, insulin-based treatment has a relatively high risk of hypoglycemia and high GV (44). Moreover, the novel hypoglycemic agents such as sodium-glucose cotransporter (SGLT)-2 inhibitors can reduce heart load and improve the prognosis of heart failure, which may also reduce the risk of AF (45). However, for the three studies including diabetic population, none of them investigated the influences of hypoglycemic strategies on the potential relationship between GV and AF. Studies are needed in the future to determine if the potential differences in antidiabetic strategies may modify the relationship between GV and AF. Finally, a causative relationship between a high GV and an increased risk of AF could not be derived because this meta-analysis was based on observational studies. Clinical studies may be considered to determine if reduce the extent of GV could decrease or prevent the incidence of AF.
In conclusion, results of the meta-analysis indicate that a high GV may be a risk factor of AF in adults. Although the results should be validated in prospective studies and the mechanisms should be further determined, results of the meta-analysis suggest that measuring GV may be useful to predict the risk of AF in adult population.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
WL and GZ designed the study. WL and YW performed database search, literature review, study quality evaluation, data collection, and statistical analyses. WL, YW, and GZ interpreted the results. WL drafted the manuscript. YW and GZ critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhang J, Johnsen SP, Guo Y, Lip GYH. Epidemiology of atrial fibrillation: Geographic/Ecological risk factors, age, sex, genetics. Card Electrophysiol Clin (2021) 13(1):1–23. doi: 10.1016/j.ccep.2020.10.010
2. Kornej J, Borschel CS, Benjamin EJ, Schnabel RB. Epidemiology of atrial fibrillation in the 21st century: Novel methods and new insights. Circ Res (2020) 127(1):4–20. doi: 10.1161/CIRCRESAHA.120.316340
3. Wolfes J, Ellermann C, Frommeyer G, Eckardt L. Evidence-based treatment of atrial fibrillation around the globe: comparison of the latest ESC, AHA/ACC/HRS, and CCS guidelines on the management of atrial fibrillation. Rev Cardiovasc Med (2022) 23(2):56. doi: 10.31083/j.rcm2302056
4. Garside T, Bedford JP, Vollam S, Gerry S, Rajappan K, Watkinson PJ. Increased long-term mortality following new-onset atrial fibrillation in the intensive care unit: A systematic review and meta-analysis. J Crit Care (2022) 72:154161. doi: 10.1016/j.jcrc.2022.154161
5. Sagris M, Vardas EP, Theofilis P, Antonopoulos AS, Oikonomou E, Tousoulis D. Atrial fibrillation: Pathogenesis, predisposing factors, and genetics. Int J Mol Sci (2021) 23(1). doi: 10.3390/ijms23010006
6. Wang A, Green JB, Halperin JL, Piccini JPSR. Atrial fibrillation and diabetes mellitus: JACC review topic of the week. J Am Coll Cardiol (2019) 74(8):1107–15. doi: 10.1016/j.jacc.2019.07.020
7. Alijla F, Buttia C, Reichlin T, Razvi S, Minder B, Wilhelm M, et al. Association of diabetes with atrial fibrillation types: a systematic review and meta-analysis. Cardiovasc Diabetol (2021) 20(1):230. doi: 10.1186/s12933-021-01423-2
8. Giugliano D, Maiorino MI, Bellastella G, Chiodini P, Esposito K. Glycemic control, preexisting cardiovascular disease, and risk of major cardiovascular events in patients with type 2 diabetes mellitus: Systematic review with meta-analysis of cardiovascular outcome trials and intensive glucose control trials. J Am Heart Assoc (2019) 8(12):e012356. doi: 10.1161/JAHA.119.012356
9. Ko SH, Park YM, Yun JS, Cha SA, Choi EK, Han K, et al. Severe hypoglycemia is a risk factor for atrial fibrillation in type 2 diabetes mellitus: Nationwide population-based cohort study. J Diabetes Complications (2018) 32(2):157–63. doi: 10.1016/j.jdiacomp.2017.09.009
10. Andersen A, Bagger JI, Baldassarre MPA, Christensen MB, Abelin KU, Faber J, et al. Acute hypoglycemia and risk of cardiac arrhythmias in insulin-treated type 2 diabetes and controls. Eur J Endocrinol (2021) 185(2):343–53. doi: 10.1530/EJE-21-0232
11. Monnier LO, Owens D, Colette C, Bonnet F. Glycaemic variabilities: Key questions in pursuit of clarity. Diabetes Metab (2021) 47(6):101283. doi: 10.1016/j.diabet.2021.101283
12. Alfieri V, Myasoedova VA, Vinci MC, Rondinelli M, Songia P, Massaiu I, et al. The role of glycemic variability in cardiovascular disorders. Int J Mol Sci (2021) 22(16). doi: 10.3390/ijms22168393
13. Xia J, Yin C. Glucose variability and coronary artery disease. Heart Lung Circ (2019) 28(4):553–9. doi: 10.1016/j.hlc.2018.10.019
14. Ren X, Wang Z, Guo C. Long-term glycemic variability and risk of stroke in patients with diabetes: a meta-analysis. Diabetol Metab Syndr (2022) 14(1):6. doi: 10.1186/s13098-021-00770-0
15. Chen J, Yi Q, Wang Y, Wang J, Yu H, Zhang J, et al. Long-term glycemic variability and risk of adverse health outcomes in patients with diabetes: A systematic review and meta-analysis of cohort studies. Diabetes Res Clin Pract (2022) 192:110085. doi: 10.1016/j.diabres.2022.110085
16. Li X, Zhang D, Chen Y, Ye W, Wu S, Lou L, et al. Acute glycemic variability and risk of mortality in patients with sepsis: a meta-analysis. Diabetol Metab Syndr (2022) 14(1):59. doi: 10.1186/s13098-022-00819-8
17. Joung B. Risk factor management for atrial fibrillation. Korean Circ J (2019) 49(9):794–807. doi: 10.4070/kcj.2019.0212
18. Gu J, Fan YQ, Zhang JF, Wang CQ. Impact of long-term glycemic variability on development of atrial fibrillation in type 2 diabetic patients. Anatol J Cardiol (2017) 18(6):410–6. doi: 10.14744/AnatolJCardiol.2017.7938
19. Xia J, Xu J, Li B, Liu Z, Hao H, Yin C, et al. Association between glycemic variability and major adverse cardiovascular and cerebrovascular events (MACCE) in patients with acute coronary syndrome during 30-day follow-up. Clin Chim Acta (2017) 466:162–6. doi: 10.1016/j.cca.2017.01.022
20. Clement KC, Alejo D, DiNatale J, Whitman GJR, Matthew TL, Clement SC, et al. Increased glucose variability is associated with atrial fibrillation after coronary artery bypass. J Card Surg (2019) 34(7):549–54. doi: 10.1111/jocs.14071
21. Lee SR, Choi EK, Han KD, Lee SH, Oh S. Effect of the variability of blood pressure, glucose level, total cholesterol level, and body mass index on the risk of atrial fibrillation in a healthy population. Heart Rhythm (2020) 17(1):12–9. doi: 10.1016/j.hrthm.2019.07.006
22. Hsu JC, Yang YY, Chuang SL, Yu CC, Lin LY. Higher long-term visit-to-visit glycemic variability predicts new-onset atrial fibrillation in patients with diabetes mellitus. Cardiovasc Diabetol (2021) 20(1):148. doi: 10.1186/s12933-021-01341-3
23. Lee S, Zhou J, Wong WT, Liu T, Wu WKK, Wong ICK, et al. Glycemic and lipid variability for predicting complications and mortality in diabetes mellitus using machine learning. BMC Endocr Disord (2021) 21(1):94. doi: 10.1186/s12902-021-00751-4
24. Su JL, Li YB, Lu YH, Yang ZH, Liu B, Huang BT, et al. Intraoperative glycemia fluctuation is highly related to postoperative hyperglycemia and short-term clinical outcomes in patients after coronary artery bypass graft with cardiopulmonary bypass. J Clin Anesthesiol (2012) 28(7):640–2.
25. Sim MA, Liu W, Chew STH, Ti LK. Wider perioperative glycemic fluctuations increase risk of postoperative atrial fibrillation and ICU length of stay. PloS One (2018) 13(6):e0198533. doi: 10.1371/journal.pone.0198533
26. Yan ZY, Li ZY, Mo GX, Liang HD, Cao DQ, Tang J, et al. Effect of perioperative glycemic fluctuations on incidence of postoperative atrial fibrillation and duration of intensive care unit in patients underwent coronary artery bypass grafting. South Chin J Cardiovasc Dis (2021) 27(1):57–61.
27. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA (2000) 283(15):2008–12. doi: 10.1001/jama.283.15.2008
28. Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane handbook for systematic reviews of interventions version 6.2. In: The cochrane collaboration (2021) (London UK: Wiley Press). Available at: www.training.cochrane.org/handbook.
29. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses (2010). Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
30. Du W, Guo K, Jin H, Sun L, Ruan S, Song Q. Association between metabolic syndrome and risk of renal cell cancer: A meta-analysis. Front Oncol (2022) 12:928619. doi: 10.3389/fonc.2022.928619
31. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med (2002) 21(11):1539–58. doi: 10.1002/sim.1186
32. Higgins J, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0. In: The cochrane collaboration (2011) (London UK: Wiley Press). Available at: www.cochranehandbook.org.
33. Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol (2008) 37(5):1148–57. doi: 10.1093/ije/dyn065
34. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
35. Saito S, Teshima Y, Fukui A, Kondo H, Nishio S, Nakagawa M, et al. Glucose fluctuations increase the incidence of atrial fibrillation in diabetic rats. Cardiovasc Res (2014) 104(1):5–14. doi: 10.1093/cvr/cvu176
36. Fleischer J, Lebech Cichosz S, Hoeyem P, Laugesen E, Loegstrup Poulsen P, Sandahl Christiansen J, et al. Glycemic variability is associated with reduced cardiac autonomic modulation in women with type 2 diabetes. Diabetes Care (2015) 38(4):682–8. doi: 10.2337/dc14-0654
37. Chen PS, Chen LS, Fishbein MC, Lin SF, Nattel S. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ Res (2014) 114(9):1500–15. doi: 10.1161/CIRCRESAHA.114.303772
38. Al Kury LT, Chacar S, Alefishat E, Khraibi AA, Nader M. Structural and electrical remodeling of the sinoatrial node in diabetes: New dimensions and perspectives. Front Endocrinol (Lausanne) (2022) 13:946313. doi: 10.3389/fendo.2022.946313
39. Vrachatis DA, Papathanasiou KA, Kossyvakis C, Giotaki SG, Raisakis K, Iliodromitis KE, et al. Atrial fibrillation risk in patients suffering from type I diabetes mellitus. a review of clinical and experimental evidence. Diabetes Res Clin Pract (2021) 174:108724. doi: 10.1016/j.diabres.2021.108724
40. Cheung CC, Nattel S, Macle L, Andrade JG. Management of atrial fibrillation in 2021: An updated comparison of the current CCS/CHRS, ESC, and AHA/ACC/HRS guidelines. Can J Cardiol (2021) 37(10):1607–18. doi: 10.1016/j.cjca.2021.06.011
41. Monnier L, Colette C, Owens D. Glucose variability and diabetes complications: Risk factor or biomarker? can we disentangle the "Gordian knot"? Diabetes Metab (2021) 47(3):101225. doi: 10.1016/j.diabet.2021.101225
42. Frontoni S, Di Bartolo P, Avogaro A, Bosi E, Paolisso G, Ceriello A. Glucose variability: An emerging target for the treatment of diabetes mellitus. Diabetes Res Clin Pract (2013) 102(2):86–95. doi: 10.1016/j.diabres.2013.09.007
43. Oh S, Purja S, Shin H, Kim M, Kim E. Hypoglycemic agents and glycemic variability in individuals with type 2 diabetes: A systematic review and network meta-analysis. Diabetes Vasc Dis Res (2022) 19(3):14791641221106866. doi: 10.1177/14791641221106866
44. Guerci B, Sauvanet JP. Subcutaneous insulin: pharmacokinetic variability and glycemic variability. Diabetes Metab (2005) 31(4 Pt 2):4S7–4S24. doi: 10.1016/S1262-3636(05)88263-1
Keywords: glycemic variability, atrial fibrillation, risk factor, meta-analysis, incidence
Citation: Li W, Wang Y and Zhong G (2023) Glycemic variability and the risk of atrial fibrillation: a meta-analysis. Front. Endocrinol. 14:1126581. doi: 10.3389/fendo.2023.1126581
Received: 18 December 2022; Accepted: 22 February 2023;
Published: 18 May 2023.
Edited by:
Lu Cai, University of Louisville, United StatesReviewed by:
Xinxing Feng, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaZhen-Ye Zhang, Wuxi People’s Hospital, China
Copyright © 2023 Li, Wang and Zhong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guoqiang Zhong, Z3VvcWlhbmd6aG9uZzIzMjFAMjFjbi5jb20=
 Wei Li
Wei Li Guoqiang Zhong
Guoqiang Zhong