- 1Department of Endocrinology and Metabolism, The Affiliated Hospital of Qingdao University, Qingdao, China
- 2Department of Nephrology, The Affiliated Hospital of Qingdao University, Qingdao, China
- 3Department of Cardiology, The Affiliated Hospital of Qingdao University, Qingdao, China
Chronic kidney diseases (CKD) and cardiovascular diseases (CVD) are the main complications in type 2 diabetic mellitus (T2DM), increasing the risk of cardiovascular and all-cause mortality. Current therapeutic strategies that delay the progression of CKD and the development of CVD include angiotensin-converting enzyme inhibitors (ACEI), angiotensin II receptor blockers (ARB), sodium-glucose co-transporter 2 inhibitors (SGLT-2i) and GLP-1 receptor agonists (GLP-1RA). In the progression of CKD and CVD, mineralocorticoid receptor (MR) overactivation leads to inflammation and fibrosis in the heart, kidney and vascular system, making mineralocorticoid receptor antagonists (MRAs) as a promising therapeutic option in T2DM with CKD and CVD. Finerenone is the third generation highly selective non-steroidal MRAs. It significantly reduces the risk of cardiovascular and renal complications. Finerenone also improves the cardiovascular-renal outcomes in T2DM patients with CKD and/or chronic heart failure (CHF). It is safer and more effective than the first- and second-generation MRAs due to its higher selectivity and specificity, resulting in a lower incidence of adverse effects including hyperkalemia, renal insufficiency and androgen-like effects. Finerenone shows potent effect on improving the outcomes of CHF, refractory hypertension, and diabetic nephropathy. Recently studies have shown that finerenone may have potential therapeutic effect on diabetic retinopathy, primary aldosteronism, atrial fibrillation, pulmonary hypertension and so on. In this review, we discuss the characteristics of finerenone, the new third-generation MRA, and compared with the first- and second-generation steroidal MRAs and other nonsteroidal MRAs. We also focus on its safety and efficacy of clinical application on CKD with T2DM patients. We hope to provide new insights for the clinical application and therapeutic prospect.
1 Introduction
Type 2 diabetes mellitus (T2DM) is a metabolic disorder characterized by hyperglycemia with insulin resistance. The management of T2DM requires multifactorial behavioral and pharmacological treatments to prevent or delay complications, and improves the quality of life. CKD and CVD are the common complications of T2DM (1). Among patients with T2DM, cardiovascular complications are the leading cause of morbidity and mortality, and kidney complications are highly prevalent in patients with T2DM (2). Available therapeutic strategies that delay the progression of CKD and the development of CVD include angiotensin-converting enzyme inhibitors (ACEI), angiotensin II receptor blockers (ARB), sodium-glucose co-transporter 2 inhibitors (SGLT-2i) and GLP-1 receptor agonists (GLP-1RA).
Finerenone is a structurally novel non-steroidal mineralocorticoid receptor antagonist (MRAs), which exhibits outstanding effect on cardio-renal protection (3). The mineralocorticoid receptors (MRs) are widely distributed in the heart, kidney, brain, lung, colon, skin, liver, skeletal muscle, saliva, sweat gland, and fat (4). MRs are mainly expressed in the cardiovascular system and kidney, and play vital role in ventricular remodeling and chronic heart failure (CHF) progression (5). Aldosterone, the MR, maintains the sodium/potassium homeostasis and the electrolyte balance of the body. In addition, an increasing number of studies have shown that inflammatory and fibrotic effect is mediated by excessive activation of MRs, leading to the adverse cardiac and renal outcomes. It could be an important therapeutic target for chronic kidney disease (CKD) induced by T2DM. Finerenone, a third-generation highly selective MRA, can directly and specifically block MR hyperactivation, and promote the anti-inflammatory and anti-fibrotic effects. In this way, finerenone exhibits cardiovascular and renal double-benefits, and is used in the treatment of T2DM-related CKD (diabetic kidney disease, DKD) to reduce the risk of persistent decline in glomerular filtration rate (eGFR) and the progression of end stage renal disease (ESRD). In general, finerenone could reduce the risk of cardiovascular and renal outcomes (3, 6).
A number of large-scaled clinical trials have proved that finerenone can significantly reduce both cardiorenal endpoints and the adverse reactions such as electrolyte disorders and sex hormone-like effects (7). In this review, we discuss the pharmacological characteristic, molecular mechanism, effectiveness and safety of finerenone in the treatment of T2DM with CKD/DKD and CVD, to provide clinical evidence and deep insight for therapeutic strategies.
2 The mechanism of MR activation on kidney and cardiovascular system
2.1 The physiological action of MR and MRAs
The physiological ligands of MR are mainly aldosterone and cortisol. MR is expressed in a variety of tissues and cells, including cardiomyocytes, vascular endothelial cells, vascular smooth muscle cells, renal tubular epithelial cells and macrophages (4). Aldosterone binds to MR in the distal renal tubular epithelial cells to form aldosterone-MR complex, which promotes the reabsorption of sodium and excretion of potassium and hydrogen ions, suggesting MR plays an important physiological role in the regulation of water and salt balance, blood pressure and circulating blood volume (8).
2.2 The pathological effect and mechanism of MR activation
MR is also involved in the inflammatory response, regulating the expression of cytokines and inflammatory mediators, the activation of the inflammatory pathways and infiltration of inflammatory cells (9). Excessive activation of MR promotes reactive oxygen species (ROS) production, mediates the inflammatory and fibrogenic processes, and ultimately leads to myocardial hypertrophy and ventricular remodeling (10), as well as the renal damage, glomerular hypertrophy, glomerulosclerosis, and vascular damage such as vascular endothelial dysfunction and vascular smooth muscle cells proliferation (5). MR overactivation act directly on vascular smooth muscle cells via the MR-VEGFR1 pathway, leading to cell proliferation and enhanced vascular fibrosis, thickness and stiffness. In addition, MR overactivation also promotes the differentiation of inflammatory cells such as macrophages, T lymph cells into a pro-inflammatory phenotype in mice model. It further promotes the chronic inflammation microenvironment, and damages target organs and accelerates the disease process (11). MR overactivation results in renal injury and mineralocorticoid sensitive hypertension directly through the MR-Rac1 pathway, and cause glomerular hyperfiltration. In animal study, MR gene knockdown in cardiovascular endothelial cells improves renal inflammation and fibrosis by reducing inflammatory macrophage differentiation and inhibiting the expression of inflammatory and fibrosis-related genes (12).
Therefore, blocking MR over-activation could be beneficial to improve target organ damage.
3 Cardio-renal protective mechanism of finerenone
3.1 Moderating mechanism of finerenone on cardiovascular protection
CVD is a common co-morbidity of T2DM. MR overactivation plays an important role in the cardiovascular progression of T2DM with CVD. The mechanism of MR overactivation involving in cardiovascular damage is as follows. (1) MR overactivation increases NADPH oxidase activity to induce a series of oxidative stress responses in adult rat models, leading to the inflammatory and fibrotic process, finally results in the cardiac lesions such as myocardial hypertrophy, ventricular remodeling, myocardial ischemia/infarction, and ultimately to the development and progression of cardiovascular disease and renal disease (13, 14). (2) In addition, high aldosterone levels cause water and sodium retention and sodium overload, and increase production of ROS, thus exerting inflammatory reaction, fibrotic progression and oxidative stress (15). Those factors act on the heart result in remodeling of the heart and arteries, triggering the risk of decreased left heart function, ventricular remodeling, and arrhythmias, all can deteriorate myocardial infarction and heart failure (HF) (12). (3) Furthermore, MR activation leads to vascular smooth muscle cell proliferation, increases vascular stiffness through vascular endothelial growth factor receptor 1 (VEGFR1), worsens vascular injury by decreasing nitric oxide, disturbs vascular endothelial dysfunction and vasoconstriction in rats (16). Therefore, targeted blockade of MR overactivation can ameliorate the inflammatory and fibrotic injury mediated by this pathway (17) (Figure 1). It is a key therapeutic target for patients with T2DM-related CVD.
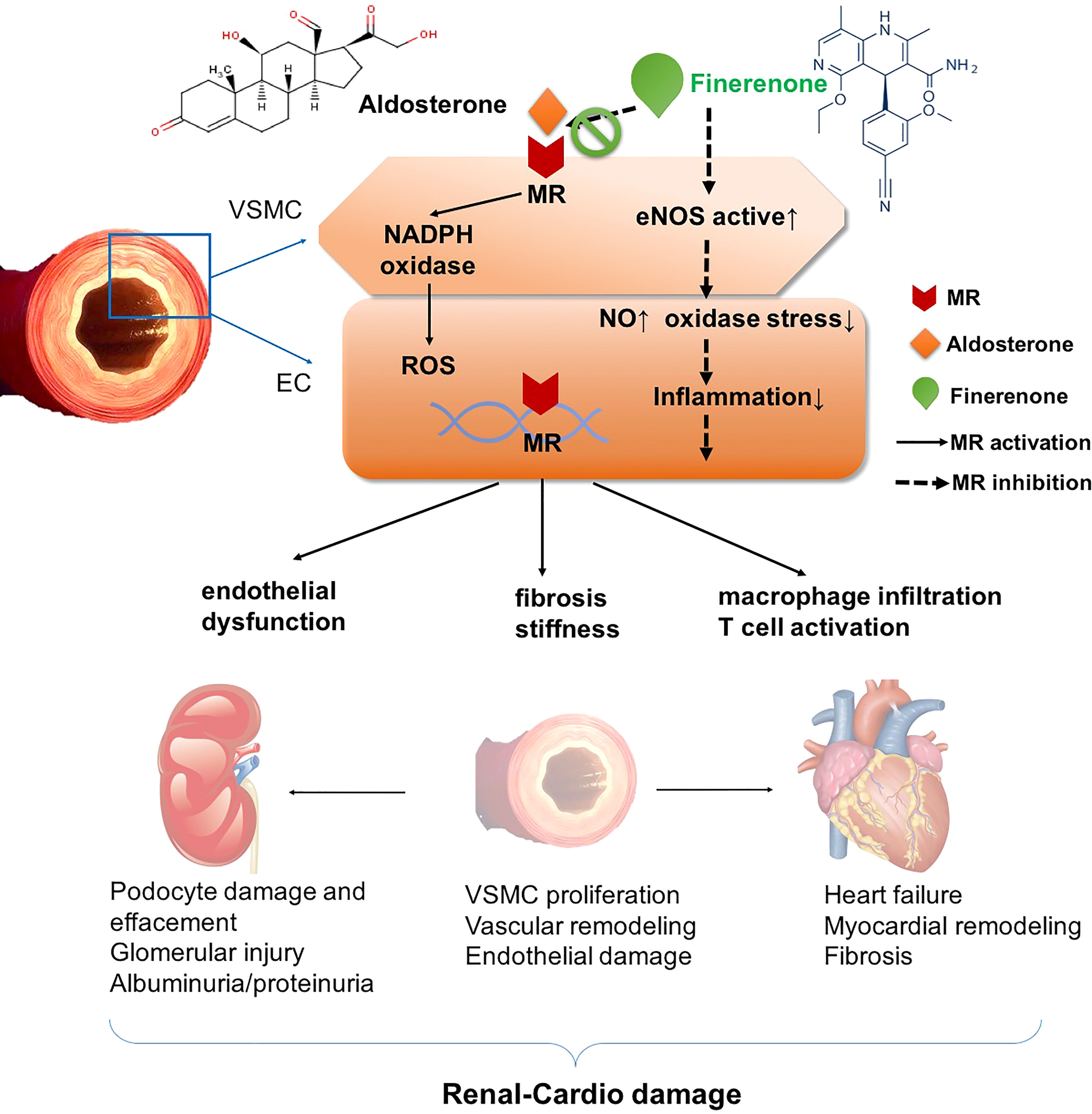
Figure 1 Mechanism of renal-cardio damage by mineralocorticoid receptor (MR) overactivation. MR activation plays important role in promoting NADPH oxidase, and enhancing ROS accumulation in VSMCs and ECs, and induces oxidase stress. In this way, MR agonist such as aldosterone results in endothelial dysfunction, macrophage infiltration and T cell activation, inflammatory progenitors including cytokines collection, and acts on VSMC leading to fibrosis and stiffness. MR activation affects kidney, aggravates podocyte damage and effacement, glomerular injury, and VSMC proliferation and endothelial damage, leading to vascular remodeling. On heart, MR activation exacerbates heart failure, myocardial remodeling and fibrosis. On contrast, MR antagonist finerenone blocks the binding of aldosterone and MR, then attenuates those pathophysiological progressions. In this way, finerenone shows renal-cardio protective effect. MR, mineralocorticoid receptor. EC, endothelial cells. VSMC, vascular smooth muscle cells. ROS, reactive oxygen species. NADPH, nicotinamide adenine dinucleotide phosphate.
MRAs promote co-factor SRC-1 recruitment to an MR-dependent promoter. The third-generation MRA finerenone has highly potent and selectivity for MR. Compared to spironolactone, finerenone binds to MR in a manner of unstable receptor-ligand complex, and leads to less recruit co-regulators (18). Finerenone delays the nuclear accumulation of MR-aldosterone complex, and blocks the recruitment of critical transcription cofactors. Thus, finerenone disturbs the steps downstream of MR pathway, and decreased expression of pro-inflammatory and pro-fibrotic factors. MRA acts on cardiomyocytes hypertrophy by affecting gene transcription (19). In animal study, knockdown of MR in T-cells attenuates cardiac hypertrophy. The administration of MRA in mice also blocks MR signaling, reduces oxidative stress in cardiomyocytes, inhibits inflammation and fibrosis, and reduces the extent of macrophage infiltration (20). MRAs attenuate proinflammatory molecule expression in the rat heart and subsequent vascular and myocardial damage. Thus, we can infer that finerenone treatment in rats with severe hypertension and the vascular inflammation phenotype in the heart is effective (21).
3.2 The mechanism of finerenone on kidney
On the kidney, MR overactivation leads to glomerular hypertrophy, sclerosis, and renal fibrosis with reduced renal blood flow, finally results in renal injury and renal dysfunction (22). Finerenone reduces the formation of damaged vascular neointima by reducing endothelial cell apoptosis and inhibiting smooth muscle cell proliferation. Finerenone can prevent adverse vascular remodeling while restoring vascular integrity, and it can also block the damage to the kidney from MR overactivation, delaying the progression of nephropathy and bringing renal benefit (23).
Finerenone reduces endothelial cell apoptosis, attenuates smooth muscle cells proliferation, and decreases leukocyte recruitment and inflammatory response after vascular injury, thereby promoting endothelial repair and preventing adverse vascular remodeling. In a mouse model of DM induced CKD, finerenone treatment shows a significant reduction of proteinuria (24). Kolkhof et al. reported that in a rat model, the expression of genes related with renal hypertrophy, proteinuria and renal inflammatory are down-regulated in finerenone-treated group compared to isodose eplerenone group (8). In addition, finerenone prevents from functional and structural heart and kidney damage in a dose-dependent manner, without affecting blood pressure. Finerenone reduced cardiac hypertrophy, plasma pro-BNP, and proteinuria more efficiently than eplerenone. In the mice model of non-diabetic nephropathy, finerenone reduces the levels of inflammatory factors, fibrogenic markers and deposition of perinephric macrophages, reduces proteinuria and tubulointerstitial fibrosis. This anti-fibrotic process is independent of blood pressure, and exhibit a dose-dependent reduction in fibroblast accumulation and collagen deposition (25). The MRA treatment also reduces glomerular pathological injury and improves renal function in glomerulonephritis mice models. In addition, finerenone treatment may also prevent ischemia-reperfusion-induced renal tubular injury (19).
In general, MR activation promotes NADPH oxidase, and enhances ROS accumulation in VSMCs and ECs. In this way, MR agonist such as aldosterone results in endothelial dysfunction, macrophage infiltration and T cell activation, inflammatory cytokines accumulation, leading to fibrosis and stiffness. MR activation aggravates podocyte damage and effacement, glomerular injury and endothelial damage, leading to vascular remodeling. MRA finerenone blocks the binding of aldosterone and MR, then attenuates those pathophysiological progressions. In this way, finerenone shows renal-cardio protective effect (Figure 1).
4 Pharmacological characteristic and safety of finerenone
Finerenone is innovative in its molecular structure. Finerenone induces MR conformational changes mainly through its side chain, leading to the prominence of helix 12 of the c-terminal, activating functional domain of the MR receptor. It affects the recruitment of co-regulatory factors and alters MR stability, nuclear translocation and activation. Finerenone has a high selectivity and innovative molecular structure compared to the first- and second-generation of MRAs.
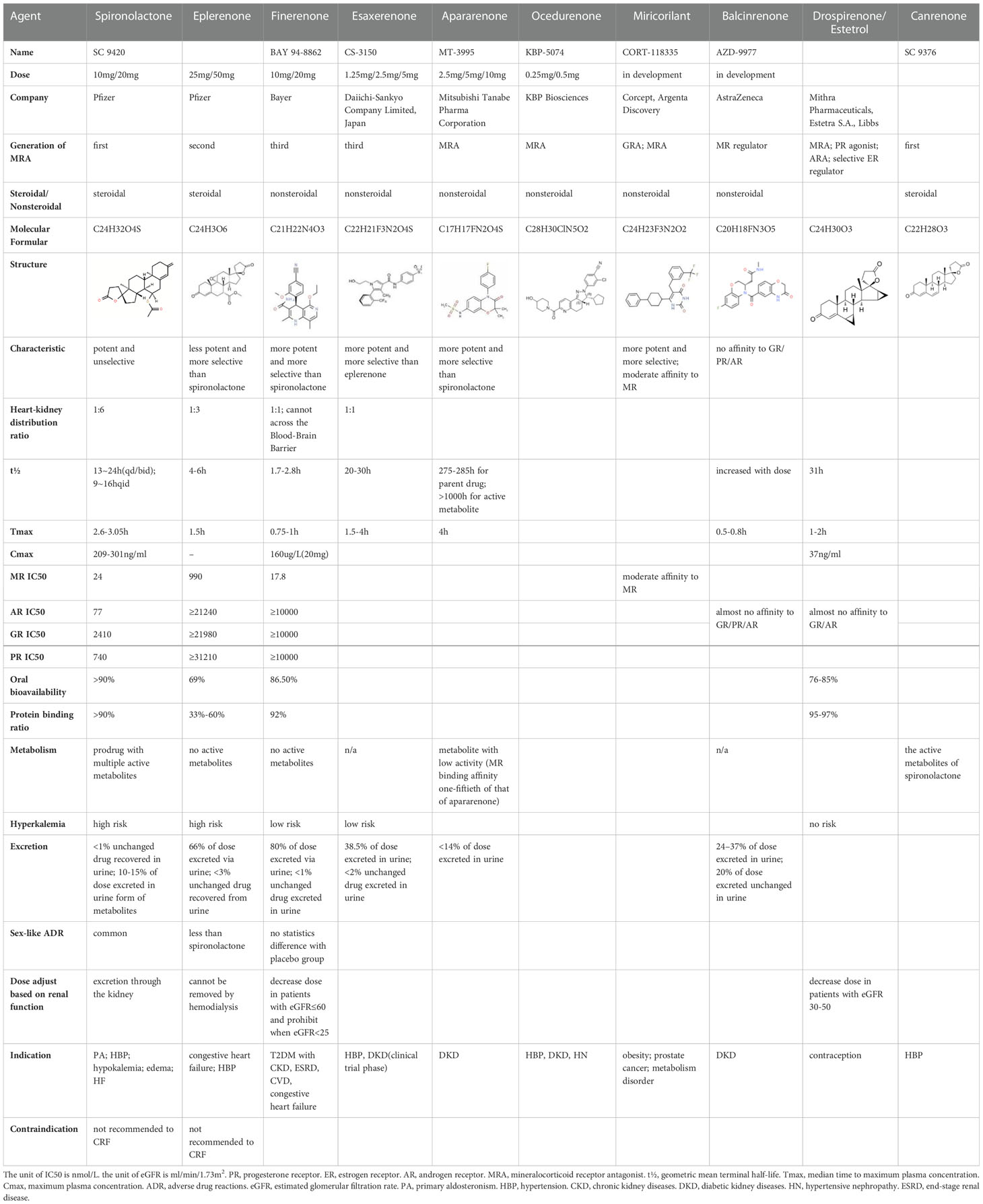
Finerenone binds to MR with greater affinity through a large number of van der Waals forces and hydrogen bonds, has a stronger antagonistic effect, completely blocks the transcription factor aggregation caused by aldosterone-MR receptor complex, and inhibits MR overactivation. Finerenone has a short elimination half-life of only two hours. Finerenone displays shorter Tmax of 0.5~0.75h than spironolactone and eplerenone of 1-2h. Spironolactone exhibits multiple active metabolites, such as canrenone, while elperenone and finerenone has no active metabolites. Finerenone has a weak affinity for androgen and progesterone receptors. Spironolactone displays non-specific binding to steroid receptors, thus, it shows anti-androgenic effect. Compared to the first-generation MRA spironolactone, eplerenone is 40-fold less potent than spironolactone. However, eplerenone and those non-steroidal MRAs (including finerenone, esaxerenone, ocedurenone, balcinrenone, ect.) generally display exhibits greater selectivity for MR over other steroid hormone receptors. Therefore, finerenone shows less risk of sex hormone related adverse effects (26).
Finerenone shows similar potency to spironolactone, but high affinity to MR. Compared to the previous steroidal MRAs spironolactone or eplerenone, the new-generation MRA finerenone shows its superiority (Table 1). The side effects including sex hormone associated adverse effects, risk of hyperkalemia and renal insufficiency are lower in finerenone (26). It is safer and more favorable for T2DM patients’ treatment. Finerenone also inhibits the expression of downstream pro-inflammatory and pro-fibrotic factors, providing effective anti-inflammatory and anti-fibrotic effects.
Finerenone, the new oral MRA is a kind of naphthyridine derivatives based on dihydropyridines (DHP) structure, inhibits MR activation precisely and potently, and show stronger anti-inflammatory and anti-fibrotic effects than the first- and second-generation of steroidal MRAs. Finerenone is balanced distributed in heart and kidney (8), therefore, it has cardio-renal double benefits. While spironolactone and eplerenone mainly distributes in the kidney (19). Otherwise, finerenone is not allowed to across the blood-brain barrier (BBB). Quantitative whole-body autoradiography with [14C]-labeled finerenone does not demonstrate in brain (27). Both finerenone and spironolactone act as affecting the transcriptional process. Spironolactone inhibits the binding of cortisol to the receptor while also acting as a partial agonist. However, finerenone acts as an inverse agonist after binding to the promoter, reducing the activation of a kind of transcriptional cofactors (SRC-1) and inhibiting the transcriptional process even in the absence of aldosterone (28). In phase II trials, the novel MRAs have comparable efficacy compared to the conventional MRAs, but exhibiting a significant safety profile in patients with HF and renal dysfunction (29).
In the safety analysis of FIDELITY study, the discontinuation of finerenone associated hyperkalemia is low, and is comparable to placebo (30). In the FIDELITY study, the incidence of treatment-emergent adverse events (TEAE) is similar in the finerenone and placebo groups, with no increase in sex hormone-related side effects compared to the placebo group. In addition, finerenone has no effect on glycated hemoglobin A1c (HbA1c). In terms of CKD stage, the safety profile of finerenone in T2DM patients with CKD stage 4 is consistent with that of CKD stages 1 to 3 (31). In the FIDELIO-DKD study, emergency adverse events, diarrhea, nausea, vomiting, and hypovolemia are analyzed, and those adverse events in the treatment group are similar to placebo group. The conventional steroidal MRAs have limited long-term usage due to the potential adverse effects such as hyperkalemia, but the advent of finerenone shows a promising direction for the treatment of T2DM with CKD and CVD (32).
5 Effect of finerenone on cardiovascular disease outcomes in T2DM
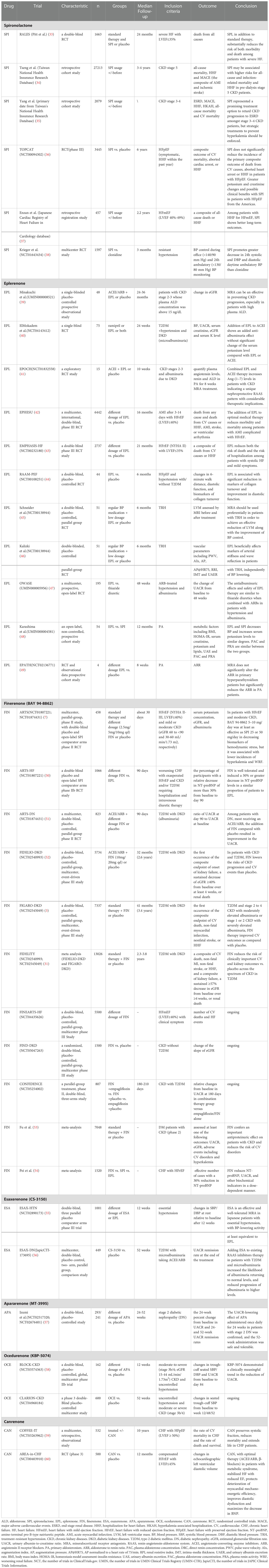
Clinical studies show the protective effect of finerenone on cardiovascular outcomes to provide evidence (Table 2). Both the Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and the Clinical Diagnosis of Diabetic Kidney Disease (FIGARO-DKD) and Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and Diabetic Kidney Disease (FIDELIO-DKD) studies are large-scaled multicenter phase III clinical studies, focusing on the effect of finerenone on the composite cardiovascular-renal outcomes as the primary endpoints in T2DM patients with CKD (3, 6, 26). The findings of FIGARO-DKD study ultimately show 13% reduction in cardiovascular composite endpoint events (including cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for HF) with finerenone in T2DM patients with CKD. In the patients with well-controlled blood pressure and glycemic levels, or using a combination of RAAS inhibitors, finerenone also shows the consistent results (6). There was no significant difference in safety from placebo. The results of the FIDELIO-DKD study shows that finerenone significantly reduce the risk of composite cardiovascular outcomes compared with placebo, with no significant difference in outcomes in patients with or without established ASCVD. Furthermore, the rate of treatment discontinuation due to hyperkalemia is low (26). The FIDELITY study is the meta-analysis study based on the FIDELIO-DKD study and the FIGARO-DKD study. The results of the study show that finerenone significantly reduce the risk of cardiovascular composite endpoint events in patients with T2DM with CKD by 14% (HR=0.86; 95% CI: 0.78-0.95; P=0.0018) compared to placebo independent of established ASCVD. The risk of HF associated hospitalization is significantly reduced by 18% (RR=0.82; 95% CI: 0.71-0.94), and the risk of all-cause mortality is reduced by 15% (RR=0.85; 95% CI: 0.74-0.99) (31, 61).
6 Effects of finerenone on renal outcomes in T2DM patients with CKD
MR overactivation is one of the key pathophysiological mechanism in patients with T2DM and CKD. The inflammatory and fibrotic mediated effects occur when MR is overactivated, leading to the progression of CKD (62). Finerenone reduces the urinary albumin-to-creatinine ratio (UACR) in T2DM with CKD patients (63). Proteinuria is also an independent predictor of CVD risk. Elevated proteinuria portends pre-existing endothelial damage (64). Overactivation of MR is an important mechanism leading to endothelial damage (Figure 1). Thus inhibition of MR overactivation is essential to suppress endothelial injury, reduce CV risk, and delay progression of CKD (65).
FIGARO-DKD and FIDELIO-DKD are two large-scaled phase III clinical studies (Table 2), involving T2DM with CKD patients, and the endpoints are cardiorenal outcomes. In the FIGARO-DKD study, finerenone significantly reduces renal composite endpoint events (occurrence of renal failure, sustained decline in eGFR ≥57% from baseline, or death from renal disease) by 23% (6). The results of the FIDELIO-DKD study show that finerenone significantly reduces the risk of renal composite endpoint events by 18% compared with placebo on the basis of standard treatment (HR=0.82; 95% CI[0.73-0.93]; P=0.0001) (52). Finerenone reduces albuminuria in short-term intervention involving T2DM patients with CKD. However, the long-term effects on renal and cardiovascular outcomes are unknown. Finerenone reduces the risk of major outcome events including renal failure, 40% reduction in eGFR or death due to renal diseases, while the adverse events are comparable to placebo group (52).
The results of the FIDELITY study, a pooled analysis of the FIDELIO-DKD and FIGARO-DKD studies, show that finerenone significantly reduce the risk of renal composite events by up to 23% (HR=0.77; 95% CI: 0.67-0.88; P=0.0002) and significantly reduced UACR by 32% compared to placebo. Further analysis reveals that finerenone decreases the incidence of all non-lethal renal outcomes, including end-stage renal disease (ESRD). Finerenone reduces cardiovascular risk in T2DM patients with CKD in all UACR and eGFR stages (51, 66). Finerenone significantly reduced the risk of renal composite events by 29% (RR=0.71; 95% CI: 0.57-0.88) in patients with established ASCVD and by 19% in patients without ASCVD history compared with placebo (RR=081; 95% CI: 0.68-0.97). The renal benefit of finerenone and the effect of reducing all-cause mortality are not affected by ASCVD history (67).
7 Finerenone in combination therapy with ACEI/ARB and SGLT-2i/GLP-1RA
Finerenone therapy improved cardiovascular and kidney outcomes in the FIDELITY pooled analysis (66). SGLT-2i and GLP-1RA can also improve cardio-renal endings independently, which play a significant role in inhibiting fibrillation, reducing urine protein, controlling inflammation, anti-oxidative stress and delaying atherosclerosis (68). However, the effect and mechanism on combination with ACEI/ARB and SGLT-2i/GLP-1RA, the established cardio-renal protective anti-hypertension or anti-diabetic agents, are unclear. Finerenone combines with either SGLT-2i/GLP-1 RA may enhance the effect of anti-inflammation, anti-oxidative stress, and endothelial protection. Whereas, clinical trials and deep mechanism research are needed to provide evidence. A meta-analysis based on the combination therapy of oral glycemic-lowering agents with finerenone included FIDELIO-DKD and FIGARO-DKD, with the primary outcomes of MACE events, illustrates that finerenone does not significantly increase cardiovascular benefit in T2DM patients with “add-on” the SGLT-2i or GLP-1RA, but confirms the significant efficacy of single-agent finerenone in cardio-renal improvement (69). It provides a basis for guiding clinical use. However, the evidence may be not conclusive due to the limited number of RCTs.
In the subgroup analysis from FIDELIO-DKD trial, finerenone reduces UACR by 31% in patients with or without GLP-1RA usage at baseline. It suggests that finerenone improve the kidney and CV outcomes independent of GLP-1RA use (66). It suggests the renal-protective effect of finerenone in patients already treated with GLP-1RA and demonstrates that GLP-1RA is also a UACR-reducing treatment since previous meta-analysis has shown that GLP-1RA is marginally reduced UACR (70, 71). Animal studies clarify that the combination of finerenone and SGLT-2i provides renal protection effect in a mouse model of hypertension-induced cardiorenal disease. The combination administration significantly reduces proteinuria levels in mice compared to single agents (72). However, several clinical studies, including FIDELIGO-DKD, have shown that finerenone alone reduces UACR independent of SGLT-2i, and similar in heart failure with reduced ejection fraction (HFrEF) patients, SGLT-2i alone significantly improves cardiovascular outcomes, even without finerenone (73).
The CONFIDENCE study (A Study to Learn How Well the Treatment Combination of Finerenone and Empagliflozin Works and How Safe it is Compared to Each Treatment Alone in Adult Participants With Long-term Kidney Disease and Type 2 Diabetes, NCT05254002) is an ongoing randomized controlled study of the efficacy of finerenone in combination with SGLT-2i Empagliflozin in T2DM patients with CKD. Both finerenone and empagliflozin are guideline-recommended clinical agents for the treatment of DKD. The study was designed to investigate whether the two-agents combination is superior to monotherapy, focusing on the endpoints of UACR, eGFR change and incidence of hyperkalemia. Clinical evidence of the additional benefit of finerenone in combination with empagliflozin will be available at the end of the study (74). The analysis of the CONFIDENCE study, which is scheduled to end in May 2023, include both the combination group and the monotherapy group of empagliflozin and finerenone. Thus, the analysis of this study may provide stronger evidence to show whether the combination is superior to monotherapy for clinical use.
Another clinical study investigates the effect of finerenone on proteinuria in DKD patients, with the treatment combination with renin-angiotensin-aldosterone system (RAAS) inhibitors (ACEI/ARB) for 90 days. The results show a significant and dose-dependent improvement of UACR in all dose-groups of finerenone compared to the placebo group (51). A meta-analysis of combination therapy for DKD show that the combination of MRA with ACEI/ARB further reduce the urine albumin excretion rate (UAER) compared with ACEI/ARB monotherapy. eGFR is not statistically different between the two groups, but the serum creatinine level is significantly increased in the combination group. A subgroup analysis based on different MRAs yields that the relative risk of hyperkalemia with the ACEI/ARB combination with finerenone is lower than with eplerenone or spironolactone (75).
In summary, either finerenone alone or in combination with SGLT-2i or GLP-1RA may improve DKD outcomes and risk of cardiovascular events in T2DM patients, but the results of clinical studies for combination versus monotherapy varies. More clinical trials are needed to provide conclusive evidence. The deep-insight of molecular mechanism and the cross-talk links among finerenone and ACEI/ARB and SGLT-2i/GLP-1RA agents unclear. Thus, further basic studies are excepted.
8 Prospects for finerenone treatment
In clinical observation, finerenone show potential therapeutic effects in diabetic retinopathy (DR). A phase III clinical trial ReFineDR (NCT04477707)/DeFineDR (NCT04795726) on the effect of finerenone on slowing the progression of non-proliferative diabetic retinopathy (NPDR) is currently ongoing. A total of 244 patients with DR at baseline (134 in the finerenone group and 110 in the placebo group) are enrolled from the FIDELIO-DKD or FIGARO-DKD studies to investigate, with the primary outcome of the NPDR progression. At baseline, most patients had mild-to-moderate NPDR. After two-year observation, 3.7% and 6.4% of patients in the finerenone and placebo groups, respectively, show vision-threatening events, and fewer participants in the finerenone group require ocular intervention (76). The results of this trial are pending and the data are continuously being updated.
In the FIGARO-DKD study, HF or exacerbation of HF causing death as endpoints, finerenone reduces the risk of new-onset HF and improves exacerbation of HF in T2DM patients with CKD, regardless of the prior HF history (6). In the FIDELIO-DKD study, finerenone reduces the risk of new-onset atrial flutter or atrial fibrillation (AFF) in T2DM patients with CKD and T2DM, regardless of the AFF history at baseline (77).
Both pre-clinical and clinical studies support the correlation between increase adiposity and MR activation. In an cohort analysis, obesity was correlated with elevated aldosterone levels (78). In animal study, finerenone improves metabolic parameters, including the glucose-lipid metabolism and insulin resistance in high-fat-diet mice. Finerenone stimulates the brown adipose tissue function, and increases the expression of uncoupling protein-1 (UCP-1) through AMP-activated protein kinase (AMPK)-UCP-1 pathway (79). The effect of finerenone on anti-obesity and regulation of metabolic parameters needs more clinical evidence.
Primary hyperaldosteronism (PA) is a common cause of secondary increased hypertension, which also acceleration the progression of cardiovascular complication. Unilateral adrenal hyperplasia or adenoma is first-line treated by surgery, while bilateral adrenal hyperplasia or idiopathic hyperaldosteronism is treated by MRAs (80). Spironolactone and eplerenone are commonly recommended choice at present. Finerenone as a new MRAs, may have more prominent advantages in the treatment of PA. Further clinical studies on this agent will provide supportive evidence.
Obstructive sleep apnea hypopnea syndrome (OSAHS) is considered as an independent risk factor for hypertension, and the pathophysiological mechanisms include RAAS activation, oxidative stress, endothelial cell damage, and sympathetic nerve excitation. The elevated aldosterone levels can increase nocturnal fluid transfer, and aggravate OSAHS. There is an interaction between OSAHS and aldosterone, which aggravate the occurrence of hypertension in OSAHS patients. MRA can improve the control of hypertension and delay the development of OSAHS. Therefore, the application of aldosterone receptor antagonist can improve OSAHS related Hypertension. Finerenone as a novel MRA also may be a promising therapeutic strategy of OSAHS and OSA-related hypertension (81).
A rat experiment demonstrates that MR is overexpressed in experimental and human pulmonary arterial hypertension (PAH), with the monocrotaline and sugen/hypoxia rat models. In addition, hMR+ (human MR overexpressing) mice display increased right ventricular systolic pressure, right ventricular hypertrophy, and remodeling of pulmonary arterioles. Finerenone-feeding mice show reversed PAH in some extent and decreased inflammatory cell infiltration and vascular cell proliferation. This experiment confirmed that finerenone appears to a potential therapy for PAH (9).
9 Summary
Finerenone is marketed as the first third-generation highly selective non-steroidal MRA for improving cardiorenal prognosis in T2DM patients with CKD and CVD. Several large-scaled clinical trials show that, regardless of ASCVD history, finerenone reduces the risk of cardiovascular and renal adverse events in T2DM patients, delays the disease progression and improves cardiac and renal outcomes. Improving cardiorenal outcomes and delaying the progression of complications are the vital strategy for diabetic management. The integrated management of diabetic-cardio-renal contributes to long-term prognosis of diabetic patients, especially combined with CKD and CVD. Finerenone provides organ protection, also has a lower incidence of electrolyte disturbances such as hyperkalemia than those conventional MRAs due to its high selectivity and affinity to MR. There is potential effect on the treatment of primary aldosteronism (PA), diabetic retinopathy (DR), atrial fibrillation and pulmonary hypertension. It suggests that finerenone may be a potential therapeutic strategy for treatment of CKD and CVD.
Author contributions
RL and BD drafted the manuscript. LC, SL, and YW provided helpful suggestions. LX conceived the study. BD designed the study and take responsibility for this study. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by a grant from the National Natural Science Foundation of China (no. 81600691) and a China Postdoctoral Science Foundation-funded project (no. 2018M640615). The content of the article has not been influenced by the sponsors.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Davies MJ, Aroda VR, Collins BS, Gabbay RA, Green J, Maruthur NM, et al. Management of hyperglycaemia in type 2 diabetes, 2022. a consensus report by the American diabetes association (ADA) and the European association for the study of diabetes (EASD). Diabetologia (2022) 65(12):1925–66. doi: 10.1007/s00125-022-05787-2
2. Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. nature reviews. Endocrinology (2018) 14(2):88–98. doi: 10.1038/nrendo.2017.151
3. Pitt B, Filippatos G, Agarwal R, Anker SD, Bakris GL, Rossing P, et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. New Engl J Med (2021) 385(24):2252–63. doi: 10.1056/NEJMoa2110956
4. Jaisser F, Farman N. Emerging roles of the mineralocorticoid receptor in pathology: Toward new paradigms in clinical pharmacology. Pharmacol Rev (2016) 68(1):49–75. doi: 10.1124/pr.115.011106
5. Barrera-Chimal J, Bonnard B, Jaisser F. Roles of mineralocorticoid receptors in cardiovascular and cardiorenal diseases. Annu Rev Physiol (2022) 84:585–610. doi: 10.1146/annurev-physiol-060821-013950
6. Filippatos G, Anker SD, Agarwal R, Ruilope LM, Rossing P, Bakris GL, et al. Finerenone reduces risk of incident heart failure in patients with chronic kidney disease and type 2 diabetes: Analyses from the FIGARO-DKD trial. Circulation (2022) 145(6):437–47. doi: 10.1161/CIRCULATIONAHA.121.057983
7. Pitt B, Kober L, Ponikowski P, Gheorghiade M, Filippatos G, Krum H, et al. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double-blind trial. Eur Heart J (2013) 34(31):2453–63. doi: 10.1093/eurheartj/eht187
8. Kolkhof P, Delbeck M, Kretschmer A, Steinke W, Hartmann E, Bärfacker L, et al. Finerenone, a novel selective nonsteroidal mineralocorticoid receptor antagonist protects from rat cardiorenal injury. J Cardiovasc Pharmacol (2014) 64(1):69–78. doi: 10.1097/FJC.0000000000000091
9. Tu L, Thuillet R, Perrot J, Ottaviani M, Ponsardin E, Kolkhof P, et al. Mineralocorticoid receptor antagonism by finerenone attenuates established pulmonary hypertension in rats. Hypertension (Dallas Tex 1979) (2022) 79(10):2262–73. doi: 10.1161/HYPERTENSIONAHA.122.19207
10. van den Berg TNA, Rongen GA, Fröhlich GM, Deinum J, Hausenloy DJ, Riksen NP, et al. The cardioprotective effects of mineralocorticoid receptor antagonists. Pharmacol Ther (2014) 142(1):72–87. doi: 10.1016/j.pharmthera.2013.11.006
11. Ferreira NS, Tostes RC, Paradis P, Schiffrin EL. Aldosterone, inflammation, immune system, and hypertension. Am J Hypertension (2021) 34(1):15–27. doi: 10.1093/ajh/hpaa137
12. Bauersachs J, López-Andrés N. Mineralocorticoid receptor in cardiovascular diseases-clinical trials and mechanistic insights. Br J Pharmacol (2022) 179(13):3119–34. doi: 10.1111/bph.15708
13. Sinphitukkul K, Manotham K, Eiam-Ong S, Eiam-Ong S. Aldosterone nongenomically induces angiotensin II receptor dimerization in rat kidney: role of mineralocorticoid receptor and NADPH oxidase. Arch Med Sci AMS (2019) 15(6):1589–98. doi: 10.5114/aoms.2019.87135
14. Rude MK, Duhaney T-AS, Kuster GM, Judge S, Heo J, Colucci WS, et al. Aldosterone stimulates matrix metalloproteinases and reactive oxygen species in adult rat ventricular cardiomyocytes. Hypertension (Dallas Tex 1979) (2005) 46(3):555–61. doi: 10.1161/01.HYP.0000176236.55322.18
15. Connell JMC, Davies E. The new biology of aldosterone. J Endocrinol (2005) 186(1):1–20. doi: 10.1677/joe.1.06017
16. Callera GE, Montezano ACI, Yogi A, Tostes RC, He Y, Schiffrin EL, et al. C-src-dependent nongenomic signaling responses to aldosterone are increased in vascular myocytes from spontaneously hypertensive rats. Hypertension (Dallas Tex 1979) (2005) 46(4):1032–8. doi: 10.1161/01.HYP.0000176588.51027.35
17. Briet M, Schiffrin EL. Aldosterone: effects on the kidney and cardiovascular system. nature reviews. Nephrology (2010) 6(5):261–73. doi: 10.1038/nrneph.2010.30
18. Palanisamy S, Hernandez Funes M, Chang TI, Mahaffey KW. Cardiovascular and renal outcomes with finerenone, a selective mineralocorticoid receptor antagonist. Cardiol Ther (2022) 11(3):337–54. doi: 10.1007/s40119-022-00269-3
19. Agarwal R, Kolkhof P, Bakris G, Bauersachs J, Haller H, Wada T, et al. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur Heart J (2021) 42(2):152–61. doi: 10.1093/eurheartj/ehaa736
20. Grune J, Beyhoff N, Smeir E, Chudek R, Blumrich A, Ban Z, et al. Selective mineralocorticoid receptor cofactor modulation as molecular basis for finerenone's antifibrotic activity. Hypertension (Dallas Tex 1979) (2018) 71(4):599–608. doi: 10.1161/HYPERTENSIONAHA.117.10360
21. Rocha R, Rudolph AE, Frierdich GE, Nachowiak DA, Kekec BK, Blomme EAG, et al. Aldosterone induces a vascular inflammatory phenotype in the rat heart. Am J Physiol Heart Circulatory Physiol (2002) 283(5):H1802–10. doi: 10.1152/ajpheart.01096.2001
22. Barrera-Chimal J, Estrela R, Lechner SM, Giraud S, Moghrabi El S, Kaaki S, et al. The myeloid mineralocorticoid receptor controls inflammatory and fibrotic responses after renal injury via macrophage interleukin-4 receptor signaling. Kidney Int (2018) 93(6):1344–55. doi: 10.1016/j.kint.2017.12.016
23. Dutzmann J, Musmann R-J, Haertlé M, Daniel J-M, Sonnenschein K, Schäfer A, et al. The novel mineralocorticoid receptor antagonist finerenone attenuates neointima formation after vascular injury. PloS One (2017) 12(9):e0184888. doi: 10.1371/journal.pone.0184888
24. Hirohama D, Nishimoto M, Ayuzawa N, Kawarazaki W, Fujii W, Oba S, et al. Activation of Rac1-mineralocorticoid receptor pathway contributes to renal injury in salt-loaded mice. Hypertension (Dallas Tex 1979) (2021) 78(1):82–93. doi: 10.1161/HYPERTENSIONAHA.121.17263
25. Lattenist L, Lechner SM, Messaoudi S, Le Mercier A, El Moghrabi S, Prince S, et al. Nonsteroidal mineralocorticoid receptor antagonist finerenone protects against acute kidney injury-mediated chronic kidney disease: Role of oxidative stress. Hypertension (Dallas Tex 1979) (2017) 69(5):870–8. doi: 10.1161/HYPERTENSIONAHA.116.08526
26. Agarwal R, Joseph A, Anker SD, Filippatos G, Rossing P, Ruilope LM, et al. Hyperkalemia risk with finerenone: Results from the FIDELIO-DKD trial. J Am Soc Nephrol JASN (2022) 33(1):225–37. doi: 10.1681/ASN.2021070942
27. Kolkhof P, Bärfacker L. 30 YEARS OF THE MINERALOCORTICOID RECEPTOR: Mineralocorticoid receptor antagonists: 60 years of research and development. J Endocrinol (2017) 234(1):T125–40. doi: 10.1530/JOE-16-0600
28. Amazit L, Le Billan F, Kolkhof P, Lamribet K, Viengchareun S, Fay MR, et al. Finerenone impedes aldosterone-dependent nuclear import of the mineralocorticoid receptor and prevents genomic recruitment of steroid receptor coactivator-1. J Biol Chem (2015) 290(36):21876–89. doi: 10.1074/jbc.M115.657957
29. Barrera-Chimal J, Kolkhof P, Lima-Posada I, Joachim A, Rossignol P, Jaisser F. Differentiation between emerging non-steroidal and established steroidal mineralocorticoid receptor antagonists: head-to-head comparisons of pharmacological and clinical characteristics. Expert Opin On Investigational Drugs (2021) 30(11):1141–57. doi: 10.1080/13543784.2021.2002844
30. Goulooze SC, Snelder N, Seelmann A, Horvat-Broecker A, Brinker M, Joseph A, et al. Finerenone dose-Exposure-Serum potassium response analysis of FIDELIO-DKD phase III: The role of dosing, titration, and inclusion criteria. Clin Pharmacokinet (2022) 61(3):451–62. doi: 10.1007/s40262-021-01083-1
31. Agarwal R, Filippatos G, Pitt B, Anker SD, Rossing P, Joseph A, et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur Heart J (2022) 43(6):474–84. doi: 10.1093/eurheartj/ehab777
32. Lytvyn Y, Godoy LC, Scholtes RA, Raalte van DH, Cherney DZ. Mineralocorticoid antagonism and diabetic kidney disease. Curr Diabetes Rep (2019) 19(1):4. doi: 10.1007/s11892-019-1123-8
33. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. randomized aldactone evaluation study investigators. New Engl J Med (1999) 341(10):709–17. doi: 10.1056/NEJM199909023411001
34. Tseng W-C, Liu J-S, Hung S-C, Kuo K-L, Chen Y-H, Tarng D-C, et al. Effect of spironolactone on the risks of mortality and hospitalization for heart failure in pre-dialysis advanced chronic kidney disease: A nationwide population-based study. Int J Cardiol (2017) 238:72–8. doi: 10.1016/j.ijcard.2017.03.080
35. Yang C-T, Kor C-T, Hsieh Y-P. Long-term effects of spironolactone on kidney function and hyperkalemia-associated hospitalization in patients with chronic kidney disease. J Clin Med (2018) 7(11):459. doi: 10.3390/jcm7110459
36. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, et al. Spironolactone for heart failure with preserved ejection fraction. New Engl J Med (2014) 370(15):1383–92. doi: 10.1056/NEJMoa1313731
37. Enzan N, Matsushima S, Ide T, Kaku H, Higo T, Tsuchihashi-Makaya M, et al. Spironolactone use is associated with improved outcomes in heart failure with mid-range ejection fraction. ESC Heart Failure (2020) 7(1):339–47. doi: 10.1002/ehf2.12571
38. Krieger EM, Drager LF, Giorgi DMA, Pereira AC, Barreto-Filho JAS, Nogueira AR, et al. Spironolactone versus clonidine as a fourth-drug therapy for resistant hypertension: The ReHOT randomized study (Resistant hypertension optimal treatment). Hypertension (Dallas Tex 1979) (2018) 71(4):681–90. doi: 10.1161/HYPERTENSIONAHA.117.10662
39. Minakuchi H, Wakino S, Urai H, Kurokochi A, Hasegawa K, Kanda T, et al. The effect of aldosterone and aldosterone blockade on the progression of chronic kidney disease: a randomized placebo-controlled clinical trial. Sci Rep (2020) 10(1):16626. doi: 10.1038/s41598-020-73638-4
40. El Mokadem M, Abd El Hady Y, Aziz A. A prospective single-blind randomized trial of ramipril, eplerenone and their combination in type 2 diabetic nephropathy. Cardiorenal Med (2020) 10(6):392–401. doi: 10.1159/000508670
41. Kovarik JJ, Kaltenecker CC, Domenig O, Antlanger M, Poglitsch M, Kopecky C, et al. Effect of mineralocorticoid receptor antagonism and ACE inhibition on angiotensin profiles in diabetic kidney disease: An exploratory study. Diabetes Ther Research Treat Educ Diabetes Related Disord (2021) 12(9):2485–98. doi: 10.1007/s13300-021-01118-7
42. Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. New Engl J Med (2003) 348(14):1309–21. doi: 10.1056/NEJMoa030207
43. Zannad F, McMurray JJV, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. New Engl J Med (2011) 364(1):11–21. doi: 10.1056/NEJMoa1009492
44. Deswal A, Richardson P, Bozkurt B, Mann DL. Results of the randomized aldosterone antagonism in heart failure with preserved ejection fraction trial (RAAM-PEF). J Cardiac Failure (2011) 17(8):634–42. doi: 10.1016/j.cardfail.2011.04.007
45. Schneider A, Schwab J, Karg MV, Kalizki T, Reinold A, Schneider MP, et al. Low-dose eplerenone decreases left ventricular mass in treatment-resistant hypertension. J Hypertension (2017) 35(5):1086–92. doi: 10.1097/HJH.0000000000001264
46. Kalizki T, Schmidt BMW, Raff U, Reinold A, Schwarz TK, Schneider MP, et al. Low dose-eplerenone treatment decreases aortic stiffness in patients with resistant hypertension. J Clin Hypertension (Greenwich Conn.) (2017) 19(7):669–76. doi: 10.1111/jch.12986
47. Sawai T, Dohi K, Fujimoto N, Okubo S, Isaka N, Ichikawa T, et al. Antialbuminuric effect of eplerenone in comparison to thiazide diuretics in patients with hypertension. J Clin Hypertension (Greenwich Conn.) (2017) 19(10):990–8. doi: 10.1111/jch.13054
48. Karashima S, Yoneda T, Kometani M, Ohe M, Mori S, Sawamura T, et al. Comparison of eplerenone and spironolactone for the treatment of primary aldosteronism. Hypertension Res Off J Japanese Soc Hypertension (2016) 39(3):133–7. doi: 10.1038/hr.2015.129
49. Pilz S, Trummer C, Verheyen N, Schwetz V, Pandis M, Aberer F, et al. Mineralocorticoid receptor blockers and aldosterone to renin ratio: A randomized controlled trial and observational data. Hormone Metab Res = Hormon- Und Stoffwechselforschung = Hormones Et Metabolisme (2018) 50(5):375–82. doi: 10.1055/a-0604-3249
50. Filippatos G, Anker SD, Böhm M, Gheorghiade M, Køber L, Krum H, et al. A randomized controlled study of finerenone vs. eplerenone in patients with worsening chronic heart failure and diabetes mellitus and/or chronic kidney disease. Eur Heart J (2016) 37(27):2105–14. doi: 10.1093/eurheartj/ehw132
51. Bakris GL, Agarwal R, Chan JC, Cooper ME, Gansevoort RT, Haller H, et al. Effect of finerenone on albuminuria in patients with diabetic nephropathy: A randomized clinical trial. JAMA (2015) 314(9):884–94. doi: 10.1001/jama.2015.10081
52. Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. New Engl J Med (2020) 383(23):2219–29. doi: 10.1056/NEJMoa2025845
53. Fu Z, Geng X, Chi K, Song C, Wu D, Liu C, et al. Efficacy and safety of finerenone in patients with chronic kidney disease: a systematic review with meta-analysis and trial sequential analysis. Ann Palliative Med (2021) 10(7):7428–39. doi: 10.21037/apm-21-763
54. Pei H, Wang W, Zhao D, Wang L, Su G-H, Zhao Z. The use of a novel non-steroidal mineralocorticoid receptor antagonist finerenone for the treatment of chronic heart failure: A systematic review and meta-analysis. Medicine (2018) 97(16):e0254. doi: 10.1097/MD.0000000000010254
55. Ito S, Itoh H, Rakugi H, Okuda Y, Yoshimura M, Yamakawa S. Double-blind randomized phase 3 study comparing esaxerenone (CS-3150) and eplerenone in patients with essential hypertension (ESAX-HTN study). Hypertension (Dallas Tex 1979) (2020) 75(1):51–8. doi: 10.1161/HYPERTENSIONAHA.119.13569
56. Ito S, Kashihara N, Shikata K, Nangaku M, Wada T, Okuda Y, et al. Esaxerenone (CS-3150) in patients with type 2 diabetes and microalbuminuria (ESAX-DN): Phase 3 randomized controlled clinical trial. Clin J Am Soc Nephrol CJASN (2020) 15(12):1715–27. doi: 10.2215/CJN.06870520
57. Wada T, Inagaki M, Yoshinari T, Terata R, Totsuka N, Gotou M, et al. Apararenone in patients with diabetic nephropathy: results of a randomized, double-blind, placebo-controlled phase 2 dose-response study and open-label extension study. Clin Exp Nephrol (2021) 25(2):120–30. doi: 10.1007/s10157-020-01963-z
58. Bakris G, Pergola PE, Delgado B, Genov D, Doliashvili T, Vo N, et al. Effect of KBP-5074 on blood pressure in advanced chronic kidney disease: Results of the BLOCK-CKD study. Hypertension (Dallas Tex 1979) (2021) 78(1):74–81. doi: 10.1161/HYPERTENSIONAHA.121.17073
59. Derosa G, Maffioli P, Scelsi L, Bestetti A, Vanasia M, Cicero AFG, et al. Canrenone on cardiovascular mortality in congestive heart failure: CanrenOne eFFects on cardiovascular mortality in patiEnts with congEstIve hearT failure: The COFFEE-IT study. Pharmacol Res (2019) 141:46–52. doi: 10.1016/j.phrs.2018.11.037
60. Boccanelli A, Mureddu GF, Cacciatore G, Clemenza F, Lenarda Di A, Gavazzi A, et al. Anti-remodelling effect of canrenone in patients with mild chronic heart failure (AREA IN-CHF study): final results. Eur J Heart Failure (2009) 11(1):68–76. doi: 10.1093/eurjhf/hfn015
61. Bakris GL, Ruilope LM, Anker SD, Filippatos G, Pitt B, Rossing P, et al. A prespecified exploratory analysis from FIDELITY examined finerenone use and kidney outcomes in patients with chronic kidney disease and type 2 diabetes. Kidney Int (2022) 103(1):196–206. doi: 10.1016/j.kint.2022.08.040
62. Kolkhof P, Joseph A, Kintscher U. Nonsteroidal mineralocorticoid receptor antagonism for cardiovascular and renal disorders - new perspectives for combination therapy. Pharmacol Res (2021) 172:105859. doi: 10.1016/j.phrs.2021.105859
63. Bao W, Zhang M, Li N, Yao Z, Sun L. Efficacy and safety of finerenone in chronic kidney disease associated with type 2 diabetes: a systematic review and meta-analysis of randomized clinical trials. Eur J Clin Pharmacol (2022) 78(12):1877–87. doi: 10.1007/s00228-022-03408-w
64. Fukui A, Kaneko H, Okada A, Yano Y, Itoh H, Matsuoka S, et al. Semiquantitative assessed proteinuria and risk of heart failure: analysis of a nationwide epidemiological database. Nephrol Dialysis Transplant (2022) 37(9):1691–9. doi: 10.1093/ndt/gfab248
65. Liu LCY, Schutte E, Gansevoort RT, van der Meer P, Voors AA. Finerenone : third-generation mineralocorticoid receptor antagonist for the treatment of heart failure and diabetic kidney disease. Expert Opin On Investigational Drugs (2015) 24(8):1123–35. doi: 10.1517/13543784.2015.1059819
66. Rossing P, Agarwal R, Anker SD, Filippatos G, Pitt B, Ruilope LM, et al. Efficacy and safety of finerenone in patients with chronic kidney disease and type 2 diabetes by GLP-1RA treatment: A subgroup analysis from the FIDELIO-DKD trial. Diabetes Obes Metab (2022) 24(1):125–34. doi: 10.1111/dom.14558
67. Filippatos G, Anker SD, Pitt B, McGuire DK, Rossing P, Ruilope LM, et al. Finerenone efficacy in patients with chronic kidney disease, type 2 diabetes and atherosclerotic cardiovascular disease. Eur Heart J Cardiovasc Pharmacother (2022) 9(1):85–93. doi: 10.1093/ehjcvp/pvac054
68. Dong B, Lv R, Wang J, Che L, Wang Z, Hua Z, et al. The extraglycemic effect of SGLT-2is on mineral and bone metabolism and bone fracture. Front In Endocrinol (2022) 13:918350. doi: 10.3389/fendo.2022.918350
69. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ (Clinical Res ed.) (2022) 376:o109. doi: 10.1136/bmj.o109
70. Kristensen SL, Rørth R, Jhund PS, Docherty KF, Sattar N, Preiss D, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol (2019) 7(10):776–85. doi: 10.1016/S2213-8587(19)30249-9
71. Maruthur NM, Tseng E, Hutfless S, Wilson LM, Suarez-Cuervo C, Berger Z, et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: A systematic review and meta-analysis. Ann Internal Med (2016) 164(11):740–51. doi: 10.7326/M15-2650
72. Kolkhof P, Hartmann E, Freyberger A, Pavkovic M, Mathar I, Sandner P, et al. Effects of finerenone combined with empagliflozin in a model of hypertension-induced end-organ damage. Am J Nephrol (2021) 52(8):642–52. doi: 10.1159/000516213
73. Vaduganathan M, Docherty KF, Claggett BL, Jhund PS, Boer RA, Hernandez AF, et al. SGLT-2 inhibitors in patients with heart failure: a comprehensive meta-analysis of five randomised controlled trials. Lancet (London England) (2022) 400(10354):757–67. doi: 10.1016/S0140-6736(22)01429-5
74. Green JB, Mottl K, Bakris G, Heerspink HJL, Mann JFE, McGill JB, et al. Design of the COmbinatioN effect of FInerenone anD EmpaglifloziN in participants with chronic kidney disease and type 2 diabetes using an UACR endpoint study (CONFIDENCE). Nephrol Dialysis Transplant (2022) 0:1–10. doi: 10.1093/ndt/gfac198
75. Zuo C, Xu G. Efficacy and safety of mineralocorticoid receptor antagonists with ACEI/ARB treatment for diabetic nephropathy: A meta-analysis. Int J Clin Pract (2019) 00:e13413. doi: 10.1111/ijcp.13413
76. Rossing P, Garweg G, Anker SD, Osonoi T, Pitt B, Rosas SE, et al. Effect of finerenone on occurrence of vision-threatening complications in patients with non-proliferative diabetic retinopathy: pooled analysis of two studies using routine ophthalmological examinations from clinical trial participants (ReFineDR/DeFineDR). Diabetes Obes Metab (2022) 25(3):142–52. doi: 10.1111/dom.14915
77. Filippatos G, Bakris GL, Pitt B, Agarwal R, Rossing P, Ruilope LM, et al. Finerenone reduces new-onset atrial fibrillation in patients with chronic kidney disease and type 2 diabetes. J Am Coll Cardiol (2021) 78(2):142–52. doi: 10.1016/j.jacc.2021.04.079
78. Kathiresan S, Larson MG, Benjamin EJ, Corey D, Murabito JM, Fox CS, et al. Clinical and genetic correlates of serum aldosterone in the community: the framingham heart study. Am J Hypertension (2005) 18(5 Pt 1):657–65. doi: 10.1016/j.amjhyper.2004.12.005
79. Marzolla V, Feraco A, Gorini S, Mammi C, Marrese C, Mularoni V, et al. The novel non-steroidal MR antagonist finerenone improves metabolic parameters in high-fat diet-fed mice and activates brown adipose tissue via AMPK-ATGL pathway. FASEB J (2020) 34(9):12450–65. doi: 10.1096/fj.202000164R
80. Reincke M, Bancos I, Mulatero P, Scholl UI, Stowasser M, Williams TA. Diagnosis and treatment of primary aldosteronism. Lancet Diabetes Endocrinol (2021) 9(12):876–92. doi: 10.1016/S2213-8587(21)00210-2
Keywords: T2DM, finerenone, mineralocorticoid receptor antagonists, chronic kidney disease, cardiorenal protection
Citation: Lv R, Xu L, Che L, Liu S, Wang Y and Dong B (2023) Cardiovascular-renal protective effect and molecular mechanism of finerenone in type 2 diabetic mellitus. Front. Endocrinol. 14:1125693. doi: 10.3389/fendo.2023.1125693
Received: 16 December 2022; Accepted: 26 January 2023;
Published: 13 February 2023.
Edited by:
Ningning Hou, Affiliated Hospital of Weifang Medical University, ChinaReviewed by:
Lale Ertuglu, Vanderbilt University Medical Center, United StatesFernando P. Dominici, University of Buenos Aires, Argentina
Copyright © 2023 Lv, Xu, Che, Liu, Wang and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bingzi Dong, ZG9uZ2Jpbmd6aUBob3RtYWlsLmNvbQ==; Lili Xu, cWRmeXhsbEBxZHUuZWR1LmNu
 Ruolin Lv
Ruolin Lv Lili Xu
Lili Xu Lin Che
Lin Che Song Liu3
Song Liu3 Yangang Wang
Yangang Wang Bingzi Dong
Bingzi Dong