- 1Department of Molecular and Translational Oncology, Maria Sklodowska-Curie National Research Institute of Oncology, Warsaw, Poland
- 2Department of Experimental Immunotherapy, Maria Sklodowska-Curie National Research Institute of Oncology, Warsaw, Poland
- 3Department of Cancer Pathomorphology, Maria Sklodowska-Curie National Research Institute of Oncology, Warsaw, Poland
- 4Department of Neurosurgery, Military Institute of Medicine, Warsaw, Poland
- 5Department of Neurosurgery, Maria Sklodowska-Curie National Research Institute of Oncology, Warsaw, Poland
- 6Department of Internal Medicine, Endocrinology and Diabetes, Medical University of Warsaw, Warsaw, Poland
Objective: Pituitary neuroendocrine corticotroph tumors commonly cause Cushing’s disease (CD) that results from increased adrenocorticotropic hormone (ACTH) secretion by the pituitary tumor and consequent increase of cortisol levels in blood. However, in some patients, corticotroph tumors remain clinically non-functioning. Cortisol secretion is regulated by the hypothalamic–pituitary–adrenal axis and includes a negative feedback between cortisol and ACTH secretion. Glucocorticoids reduce ACTH level both by hypothalamic regulation and acting on corticotrophs via glucocorticoid (GR) and mineralocorticoid (MR) receptors. The aim of the study was to determine the role of GR and MR expression at mRNA and protein levels in both functioning and silent corticotroph tumors.
Methods: Ninety-five patients were enrolled, including 70 with CD and 25 with silent corticotroph tumors. Gene expression levels of NR3C1 and NR3C2 coding for GR and MR, respectively, were determined with qRT-PCR in the two tumor types. GR and MR protein abundance was assessed with immunohistochemistry.
Results: Both GR and MR were expressed in corticotroph tumors. Correlation between NR3C1 and NR3C2 expression levels was observed. NR3C1 expression was higher in silent than in functioning tumors. In CD patients NR3C1 and NR3C2 levels were negatively correlated with morning plasma ACTH levels and tumor size. Higher NR3C2 was confirmed in patients with remission after surgery and in densely granulated tumors. Expression of both genes and GR protein was higher in USP8-mutated tumors. Similar relationship between USP8 mutations and expression levels were observed in analysis of silent tumors that also revealed a negative correlation between GR and tumor size and higher NR3C1 expression in densely granulated tumors.
Conclusions: Although the associations between gene/protein expression and patients clinical features are not strong, they consistently show an evident trend in which higher receptor expression corresponds to more favorable clinical characteristics.
1 Introduction
Corticotroph pituitary neuroendocrine tumors (corticotroph PitNETs) are the subtype of tumors of pituitary gland (1). They develop as a result of neoplastic transformation of corticotropic cells of the anterior pituitary (1). Most of corticotroph PitNETs cause Cushing’s disease (CD) which is characterized by a set of symptoms caused by increased ACTH secretion and consequent serum cortisol excess. Approximately 20% of corticotroph tumors are clinically hormonally inactive (silent), i.e. do not cause rise in cortisol in patients’ circulation (2). The reason why a proportion of ACTH-secreting tumors remain clinically silent and do not cause Cushing’s disease remains unclear. Intriguingly, the molecular profiles of these two groups of corticotroph PitNETs are similar (3, 4).
Hypothalamic–pituitary–adrenal (HPA) axis is a neurohormonal system that provides a negative feedback between pituitary and adrenal secretion (5). Glucocorticoids inhibit ACTH secretion by reducing the level of hypothalamic CRH hormone as well as by direct inhibition of pituitary corticotroph cells activity (5). Partial resistance to glucocorticoids, a hallmark of Cushing’s disease, results from the impairment in HPA axis regulation (6).
Glucocorticoids signal through two steroid hormone receptors: glucocorticoid receptor (GR) and mineralocorticoid receptor (MR) (5, 7). Both receptors are intracellular proteins that upon ligand-dependent activation translocate to the nucleus, where they exert their role of transcription factors and regulate the expression of glucocorticoid-responsive genes. GR and MR have sequence homology and high similarity in DNA binding domains that are responsible for receptor interaction with glucocorticoid response elements (GREs) in DNA (5, 7). The receptors directly regulate the transcription of target genes and/or influence the expression indirectly by interacting with other transcription factors (5, 7, 8). In cells expressing both receptors, MR and GR bind GREs as homo- or heterodimers and the role of dimer composition is not fully understood (7).
The role of corticosteroid receptors in pituitary corticotroph tumors is relatively poorly understood (6, 9). In CD-causing tumors, loss of heterozygosity (LOH) in the NR3C1 (encoding GR) locus was observed (10). More recently, somatic point mutations in NR3C1 were found in 6.4% of patients with CD (9). NR3C1 expression may be also downregulated by hsa-miR-124-3p miRNA in pituitary corticotroph tumors (11). Considering the growing interest in the role of GR and nearly no information on the role of MR in corticotroph PitNETs, we aimed to investigate the expression levels of both receptors in a relatively large cohort of functioning and silent corticotroph tumors.
2 Materials and methods
2.1 Patients’ characteristics
The study included 95 patients with corticotroph tumors of which 70 suffered from CD and 25 present with silent corticotroph PitNETs (silent corticotroph adenomas, SCAs). CD patients had clear clinical signs and symptoms of hypercortisolism verified according to biochemical criteria: increased urinary free cortisol (UFC) in three 24h urine collections; disturbed cortisol circadian rhythm, increased serum cortisol levels accompanied by increased or not suppressed plasma ACTH levels at 8.00; no suppression of serum cortisol levels to <1.8 µg/dL during an overnight dexamethasone suppression test (1 mg at midnight). The pituitary etiology of disease was confirmed based on the measurement of serum cortisol levels or UFC suppression <50% with a high-dose dexamethasone suppression test (2 mg q.i.d. for 48 h) or a positive result of a corticotropin-releasing hormone stimulation test (100 mg i.v.) and magnetic resonance imaging of pituitary. Patients with SCA had no clinical or biochemical signs of hypercortisolism and showed normal levels of midnight cortisol and 24h UFC. ACTH levels were assessed using IRMA (ELSA-ACTH, CIS Bio International, Gif-sur-Yvette Cedex, France). Serum cortisol levels were determined by the Elecsys 2010 electrochemiluminescence immunoassay (Roche Diagnostics, Mannheim, Germany). UFC was assessed after extraction (liquid/liquid with dichloromethane) by electrochemiluminescence immunoassay (Elecsys 2010, Roche Diagnostics, Mannheim, Germany).
All CD-related and SCA tumor samples were ACTH-positive upon immunohistochemical staining (IHC) against pituitary hormones (ACTH, GH, PRL, TSH, FSH, LH, α-subunit) and revealed characteristic ultrastructural features of corticotroph tumors, as assessed with electron microscopy. Each SCA sample was T-PIT positive as determined in additional immunohistochemical staining. Evaluation of Ki-67 immunoreactivity score in tumor sample was performed for all the patients.
Status of hot-spot mutation in ubiquitin-specific peptidase 8 (USP8) and ubiquitin-specific peptidase 48 (USP48) genes was determined in entire patients cohort with a method described previously (12).
Patients characteristics are presented in Table 1. The study was approved by the local Ethics Committee of Maria Sklodowska-Curie National Research Institute of Oncology, Warsaw, Poland (approval no. number 44/2018). Each patient provided informed consent for the use of tissue samples for scientific purposes.
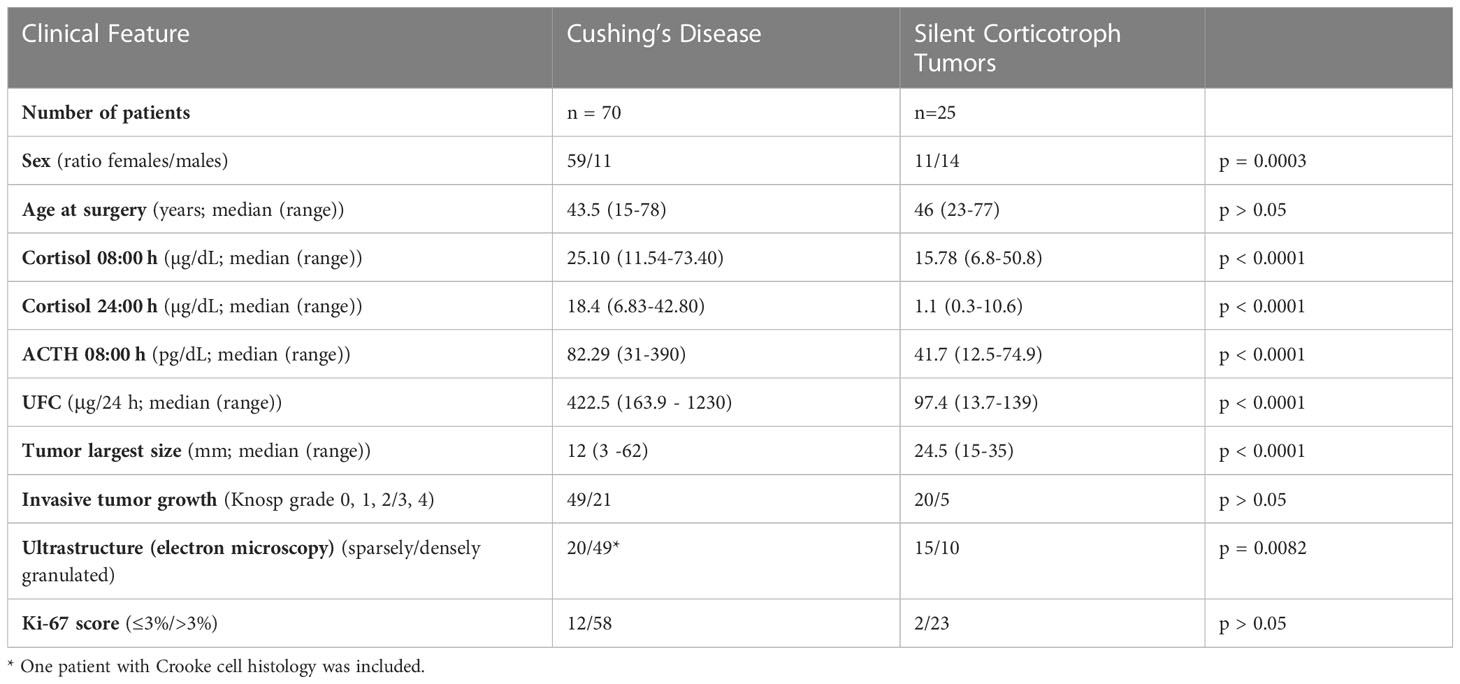
Table 1 Summary of clinical features of patients with Cushing’s disease and silent corticotroph tumors.
2.2 Quantitative real-time RT-PCR
Total RNA from formalin-fixed and paraffin-embedded (FFPE) tumor tissues was isolated with RecoverAll™ Total Nucleic Acid Isolation Kit for FFPE [Thermo Fisher Scientific, Waltham, Massachusetts, USA], measured using NanoDrop 2000 [Thermo Fisher Scientific, Waltham, Massachusetts, USA] and stored at -70 °C. One microgram of total RNA was subjected to reverse transcription using Transcriptor First Strand cDNA Synthesis Kit (Roche Diagnostics). Power SYBR Green PCR Master Mix (Thermo Fisher Scientific) was used for qRT-PCR reaction, according to manufacturer’s recommendations. The reactions were run in a volume of 5 μL, containing 2.25 pmol of each primer, using 384-well format in 7900HT Fast Real-Time PCR System (Applied Biosystems). Each sample was amplified in triplicates. Delta Ct method was used to calculate relative expression levels of NR3C1 and NR3C2, and GAPDH was used as a reference gene. The following oligonucleotides were used as PCR primers: NR3C1 forward 5’-TGCTCCTTCTGCGTTCACAA-3’, NR3C1 reverse 5’-CCATCAGTGAATATCAACTCTGGC-3’, NR3C2 forward 5’-GGGGATGAGGCTTCAGGATG-3’, NR3C2 reverse 5’-AGTTGTGTTGCCCTTCCACT-3’, GAPDH forward 5’-GAAGATGGTGATGGGATTTC-3’, GAPDH reverse 5’-GAGGTGAAGGTCGGAGTC-3’.
2.3 Immunohistochemistry
IHC was performed on 4-μm FFPE tissue sections using Envision Detection System (Dako, Glostrup, Denmark), according to manufacturer’s recommendations. Tissue sections were deparaffinized with xylene and subsequently rehydrated in a series of ethanol solutions of decreasing concentration. Target Retrieval Solution pH 9 (Dako) was used for heat-induced epitope retrieval in a 96°C water bath for 30 minutes. The samples were incubated for 1 h with the primary antibodies: anti-glucocorticoid receptor antibody (EPR19621, Abcam) and anti-mineralocorticoid receptor antibody (H10E4C9F, Abcam) in 1:100 and 1:200 dilutions, respectively. Color reaction product was developed with 3,3′-diaminobenzidine tetrahydrochloride (Dako) as a substrate. Hematoxylin counterstaining was applied. Analysis of nuclear immunohistochemical reactivity was performed by calculating H-score with a formula that combines information on both reaction intensity (scored from 0 to 3) and number of the cells with a given intensity (13). Scoring results were analyzed as continuous variables. Intensity of cytoplasmic reactivity was assessed in 4-grade scale (0-3), where 0 was considered as no expression, 1- low expression, 2 – moderate expression, 3 – high protein level and the results were analyzed as categorical variables.
2.4 Statistical analysis
Datasets of quantitative variables were tested for the normal distribution with Shapiro-Wilk test. Variables following normal distribution were analyzed with two-sided t-test whereas two-sided Mann–Whitney U-test was used when normal distribution was not determined. The Spearman correlation method was used for correlation analysis. Exact Fisher’s test was used for the analysis of proportions. Significance threshold of α = 0.05 was adopted. Data was analyzed using GraphPad Prism 6.07 (GraphPad Software, San Diego, California, USA).
3 Results
3.1 GR and MR expression in silent and functioning corticotroph tumors
The expression levels of NR3C1 and NR3C2 were determined in 25 SCAs and 70 functioning corticotroph tumors with qRT-PCR. Protein GR and MR expression was assessed based on immunohistochemical staining in the same tumor samples as used for qRT-PCR. Predominant nuclear expression was observed for GR, and immunoreactivity was quantified by counting positive nuclei according to H-score formula. For MR, cytoplasmic immunoreactivity was observed with additional slight nuclear staining in few samples only. Due to difficulties with counting the individual MR-positive cells, a simplified score was applied which categorized MR expression in tissue samples as negative, weak, moderate and high. Five tumor samples were lacking MR expression, 35 had weak, 51 moderate and 1 high MR expression. Two CD patients that were included in qRT-PCR analysis were excluded from immunohistochemical assessment due to low quality of tissue samples. Intracellular localization of each protein was highly similar across the samples. Representative examples of results of immunohistochemical staining are presented in Figure 1A.
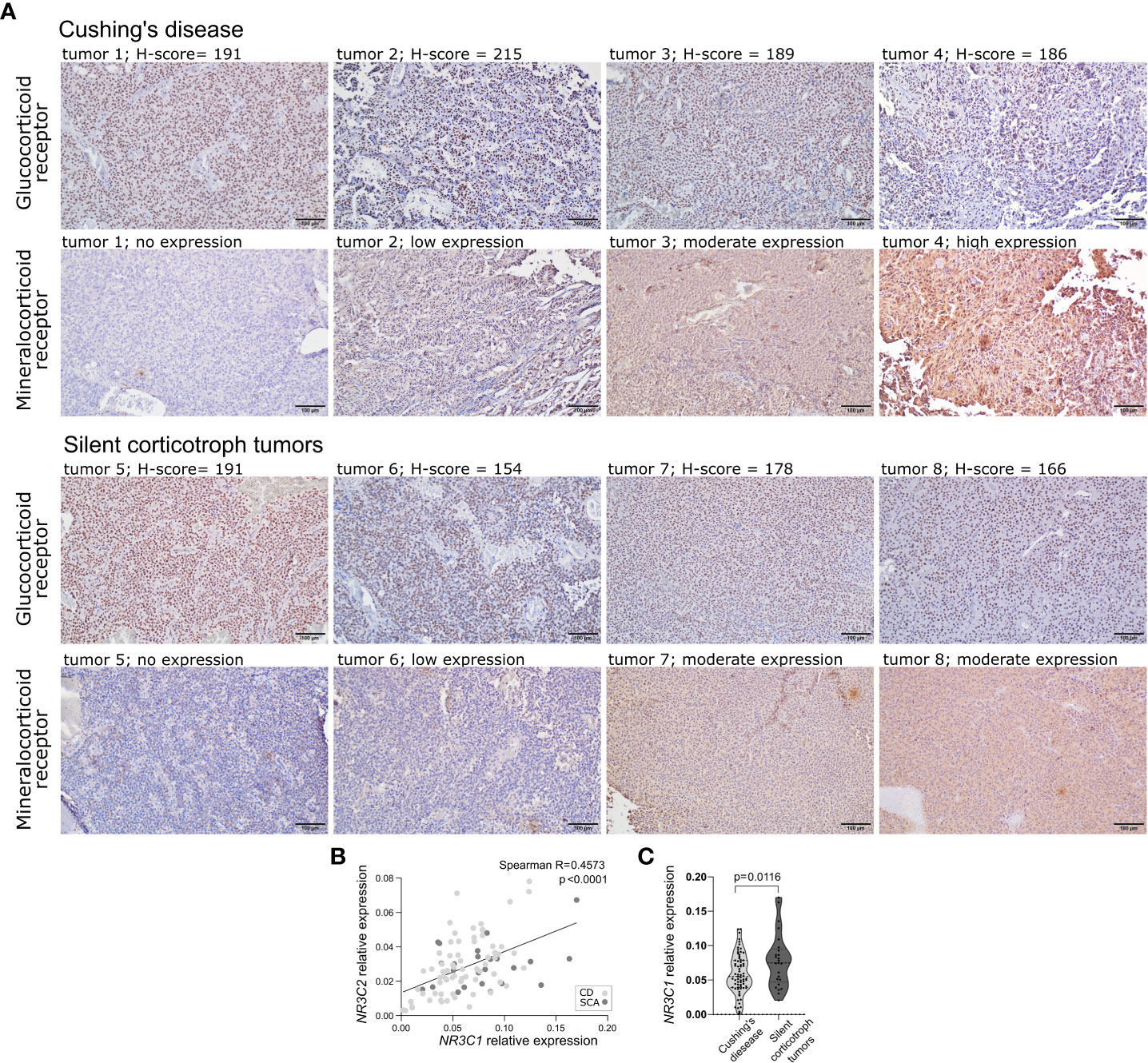
Figure 1 The expression of corticosteroid receptors in corticotroph tumors. (A) Representative examples of results of immunohistochemical staining against glucocorticoid receptor and mineralocorticoid receptor. (B) Difference in NR3C1 expression in silent corticotroph pituitary tumors and functioning corticotroph tumors causing Cushing’s disease (CD). (C) The correlation between the expression of NR3C1 and NR3C2 in corticotroph tumors.
We did not observe clear relationship between mRNA and protein levels. No correlation was observed between NR3C1 and GR immunoreactivity, quantified using H-score formula (Spearman R = 0.03801; p = 0.716). No difference in NR3C2 expression between samples with distinct MR immunoreactivity (no expression, weak, moderate, strong expression) was found (p = 0.9625, Kruskal-Wallis test). Clear correlation was found between NR3C1 and NR3C2 expression (Spearman R = 0.4573, p < 0.0001) (Figure 1B). However, no difference in GR immunoreactivity score between MR expression categories was observed indicating lack of relationship between the expression at the protein levels.
When comparing silent and functioning corticotroph tumors a significantly higher expression of NR3C1 was observed in SCAs than in tumors from CD patients (expression fold change (FC) = 1.36; p = 0.0166) (Figure 1C). No difference in NR3C2 expression, GR or MR immunoreactivity was found.
3.2 Relationship between the expression of corticosteroid receptors and clinical parameters in patients with functioning and silent corticotroph tumors
SCA and CD differ in terms of diagnosis and patients’ treatment, thus the roles of the expression of corticosteroid receptors in the two patients groups were assessed separately.
We analyzed the relationship between the expression of NR3C1, NR3C2, GR and MR with clinical and pathomorphological features: morning ACTH level, morning cortisol level, midnight cortisol level, 24h UFC, clinical remission in CD patients, tumor size, invasive growth status, USP8/USP48 mutation status, granulation pattern (sparsely granulated (SG) vs densely granulated (DG)) and Ki-67 score.
The analysis of CD patients data revealed that both NR3C1 and NR3C2 expression levels were negatively correlated with morning plasma ACTH levels (Spearman R = -0.3326; p = 0.0052 and R= -0.3717; p = 0.0017, respectively) and tumor size (Spearman R = -0.3345; p = 0.0053 and R = -0.4194; p = 0.0004, respectively) (Figures 2A, B). Patients with clinical remission after surgery had higher NR3C2 mRNA level (FC = 1.545; p = 0.013) (Figure 2C). Slightly higher NR3C2 expression level was also observed in DG tumors as compared to SG ones (FC = 1.2; p = 0.0295) (Figure 2D). We did not observe relationship between expression of the receptors and cortisol level, 24h UFC, invasive growth status and Ki-67 score. All the results are shown in details in Supplementary Table 1.
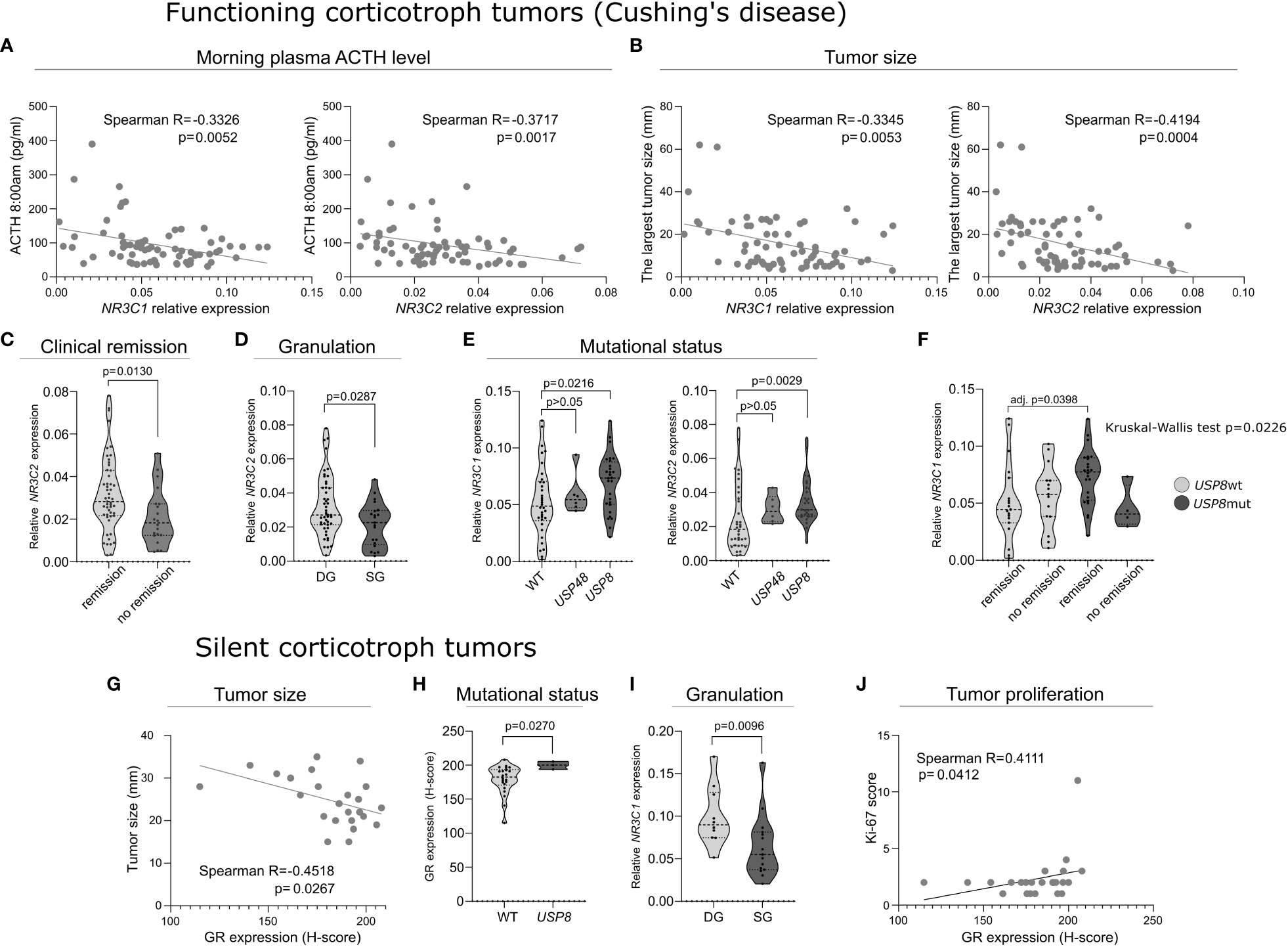
Figure 2 The expression of corticosteroid receptors’ genes and proteins in functioning and silent corticotroph tumors. (A) The correlation between morning plasma ACTH level and the expression of NR3C1 and NR3C2 expression in tumor tissue in Cushing’s disease patients. (B) The correlation between tumor size and the expression of NR3C1 and NR3C2 in Cushing’s disease patients. (C) Difference in NR3C2 expression in tumors of Cushing’s disease patients with clinical remission after surgery and those without remission. (D) Difference in NR3C2 expression between densely granulated (DG) and sparsely granulated (SG) functioning corticotroph pituitary tumors. (E) Difference in the expression of NR3C1 and NR3C2 in tumors with mutations in deubiquitinase-encoding genes (USP8 and USP48) and wild type functioning corticotroph pituitary tumors. (F) The expression of NR3C1 in patients with and without remission after surgery with respect to USP8 mutation status. (G) Correlation between the expression of glucocorticoid receptor (GR) and tumor size in silent corticotroph tumors. (H) GR expression in SCAs with and without USP8 mutation. (I) Difference in NR3C2 expression between densely granulated (DG) and sparsely granulated (SG) silent corticotroph pituitary tumors. (J) Correlation between GR expression and Ki-67 immunoreactivity score in silent tumors.
Mutations in genes encoding protein deubiquitinases USP8 and USP48 were determined in tumor tissue in the entire cohort of patients. USP8 mutations were found in 28/70 (40%) of patients with CD. The identified variants were: p.P720R (n = 13), p.S718SP (n = 5), pP720Q (n = 2), and in-frame deletion at position S719 (n = 8). USP48 missense mutations p.M415I were observed in 5 (7.14%) CD patients. USP48 and USP8 mutations were mutually exclusive. USP8 mutations were identified in 3 patients with silent tumors, that we already reported previously (4). They were p.S718SP (n=1) and in-frame deletion at position S719 (n = 2). No USP48 mutations were detected in SCA patients. CD patients with USP8 mutations had significantly higher expression of both NR3C1 and NR3C2 (FC = 1.508; p = 0.0216 and FC = 1.626; p = 0.0029, respectively) (Figure 2E).
Published data indicate that tumors with USP8 mutations and USP8-wild type tumors have distinct molecular profiles (3, 4). Since different expression of NR3C1 and NR3C2 was found in mutated and non-mutated tumors, we additionally ran analysis of relationship between the expression of each gene and clinical features in subgroups of tumors with USP8 mutation and wild type tumors (without USP8 and USP48 mutations). This showed negative correlation between NR3C2 expression and ACTH level, in analysis of both groups of mutated and wild type tumors (Spearman R = -0.406; p = 0.0356 and R = -0.4044; p = 0.0196, respectively). Additionally, analysis performed in the subgroup of patients with USP8 mutations showed higher NR3C1 expression in patients with clinical remission after surgery than in patients without remission. Similar result was not found in the group of patients with non-mutated tumors. Subsequent comparison of 4 groups of CD patients clustered according to USP8 mutation status and clinical remission status, with post hoc analysis showed that NR3C1 expression is significantly higher in USP8-mutated patients with clinical remission after surgery than in non-mutated patients with clinical remission (adj. p = 0.0398) (Figure 2F).
The analysis of silent corticotroph tumors showed a negative correlation between GR immunoreactivity score and tumor size (Spearman R = -0.4518; p = 0.0267) (Figure 2G), however, it was not observed for the relative NR3C1 and NR3C2 expression levels in this patients’ group. Lower immunoreactivity score was also found in USP8wt SCAs compared to in USP8-mutated tumors (H-score 200 vs 182.4, respectively; p = 0.0279) (Figure 2H). Higher relative expression of NR3C1 and NR3C2 in USP8-mutated tumors was also observed in the analyzed SCAs, however, it did not cross significance threshold (FC = 1.47; p = 0.0587 and FC = 1.80; p = 0.0587, respectively). Of note, in these analyses only 3 USP8-mutated samples were available. Densely granulated SCAs had higher NR3C1 expression level than sparsely granulated ones (FC = 1.63; p = 0.0116) (Figure 2I), but no difference in NR3C2 expression or corticosteroid receptors protein expression was found. We also found a significant correlation between GR expression and Ki-67 immunoreactivity score treated as continuous variable (Spearman R = 0.4111; p = 0.0412) (Figure 2J). However it has to be noted that our study group contains only 2 SCA patients with Ki-67>3% what limits the quality of the analysis.
In silent corticotroph tumors we did not observe relationship between NR3C1, NR3C2, GR or MR expression and clinical parameters (morning ACTH level, morning cortisol level, midnight cortisol level, 24h UFC, invasive growth status). All the results on silent corticotroph tumors are presented in details in Supplementary Table 2.
4 Discussion
Corticotroph PitNETs can be divided into functioning and silent tumors. They substantially differ in tumor clinical manifestation and results of biochemical tests. These differences are the basis for discriminating functioning corticotroph, CD-causing tumors and silent tumors. Functioning corticotroph PitNETs occur more frequently in women, while this sex-related prevalence is not observed in SCAs (14–16). Concordant difference in proportions of males and females in groups of CD and SCA patients was also observed in our patients cohort. Functioning corticotroph PitNETs are commonly small in size and are diagnosed due to excessive hormone secretion while SCAs are larger and commonly diagnosed following neurological symptoms caused by tumor volume. Accordingly, in our patients cohort SCAs are significantly larger than functioning tumors. In general, SCAs are considered as more aggressive tumors having higher rate of invasive growth and commonly they show sparse granulation pattern that is related to worse prognosis (17–19). In our study SG tumors were more prevalent in SCA patients while DG tumors were more frequent among CD patients that is also in line with previous reports (19). However, in our patient group we did not observe a difference in invasive growth status determined with Knosp’s grading or proliferation rate assessed based on Ki-67 staining. Majority of the tumors in each group were non-invasive ones with Ki-67 score below 3%. Low rate of invasive tumors may indicate that our group of SCA patients is not fully representative that should be taken into account with generalization of our results.
Glucocorticoids play a pivotal role in the regulation of hypothalamic–pituitary–adrenal (HPA) axis in a negative feedback mechanism. They inhibit ACTH secretion both indirectly via hypothalamus (by reducing CRH secretion) and directly acting on pituitary corticotroph cells (5). Glucocorticoids can be bound by two intracellular receptors GR and MR that regulate transcription of target genes (5, 7). It was shown in corticotroph cells that upon stimulation with glucocorticoids GR regulates POMC expression though various mechanisms including binding of GR to negative GREs (nGREs) in gene promoter (20), chromatin remodeling (21) or interaction with other nuclear receptor (NR4A1) involved in POMC regulation (22). Upon stimulation, GR may also act in nongenomic way by regulating membrane permeability for calcium influx (23). The function of GR and MR receptors in regulation of corticotroph cells activity suggests that they may play an important role in pituitary corticotroph tumors. According to our best knowledge, only few studies on limited number of patients were performed to address the role of GR expression and nearly no research on the role on MR expression in corticotroph PitNETs were published.
In our previous study comparing silent and functioning pituitary corticotroph tumors we found a difference in the expression level of genes encoding GR and MR (NR3C1 and NR3C2, respectively) (11). In the current investigation we included a notably higher number of patients to validate previous results. The obtained data confirmed a higher NR3C1 expression in silent tumors as compared to tumors causing CD, but we did not observe the difference in NR3C2 level. Our results show that, although statistically significant, the difference is slight. This observation supports our previous suggestion that higher NR3C1 expression has rather modulatory contribution to silent nature of SCAs than a strong causative role. The general model of GR activity indicates that its higher expression may directly decrease ACTH secretion (5) and contribute to non-secreting nature of SCAs. Importantly, secretory activity of corticotroph cells is regulated in a complex way. Previous studies comparing silent and functioning corticotroph tumors showed that they differ in several elements of this regulation including POMC expression, prohormone convertases level, dopamine or somatostatin receptor level and expression of specific ion channel (4, 24–28). We suppose that changes of few regulatory elements can occur simultaneously with additive effect on functioning of corticotroph tumors and GR/MR expression can be one of these changes. The possibly distinct expression of NR3C1 in functioning and silent corticotroph tumors was previously investigated in smaller series of patients but no significant difference was found in these studies (27, 28). This was probably due to lower patients’ numbers, i.e. n = 50 (36 CD, 14 SCA) and n = 20 (12 CD, 8 SCA), respectively and a small difference in gene expression levels. Previous analysis of GR protein expression in SCA and CD-causing tumors also did not reveal difference between the tumors of diverse functional status (29).
The change of functional status of corticotroph tumor can be observed in some patients. Cases of spontaneous conversion of silent adenoma into CD-causing corticotroph tumor as well as functioning to non-functioning tumor were reported (30, 31). Periodic changes of the secretory activity of the tumor are also considered as probable cause of cyclic Cushing's syndrome in patient with ACTH-related hypercortisolemia who experience temporal switch between hypercortisolemia and normocortisolemia status (32). The observed different NR3C1 expression between silent and functioning corticotroph tumors suggest that changes in GR expression may also contribute to temporary changes of tumor activity. Obviously, our speculation require further investigation. Recently, the role of GR in CD pathogenesis attracted the attention since new somatic point mutations in NR3C1 gene in functioning corticotroph tumors were identified (33). It has been estimated that these mutations occur in 6.2% of CD patients (34) although the functional role of most of identified somatic variants is unclear and was not investigated in vitro. Three NR3C1 mutations that were found in study by Miao et al. were shown to produce truncated GR protein or to reduce GR expression level (35).
The role of GR expression in patients with Cushing’s disease was not investigated extensively and the results of only a few studies were published. In general, these studies did not reveal any relationship of NR3C1 gene expression level and patients’ clinical features (27, 28, 36, 37) or GR protein expression with patients’ characteristics (29). One study indicated the relationship between low NR3C1 expression and the response to high dose dexamethasone suppression test, however, only six functioning corticotroph tumors were used in this evaluation (38). Of note, most of these previous studies included relatively low number of CD patients: n=17 (36), n=6 (38), n=12 (27), n=8 (29), n=36 (28) with only one study including more than 50 persons (37). Interestingly, although GR and MR are both involved in the response to glucocorticoids, to our knowledge, there is no data on NR3C2 or MR protein expression in CD-causing tumor available to date. Initially, the expression of MR was considered more tissue-specific as compared to GR, with highest levels in kidney and adipose tissue but its expression was identified in multiple human tissues (39). According to publicly available databases on tissue specific gene/protein expression in human MR is widely expressed in most tissue types/organs including normal pituitary gland (Geno-type-Tissue Expression project (https://gtexportal.org) or Protein atlas (https://www.proteinatlas.org), accessed on November, 2022). The expression of both MR and GR was documented in PVN neurons, adipocytes, osteoblasts, immune cells and kidney cells (40–44).
In our study we found some interesting relationships between NR3C1 and NR3C2 expression and clinical/histological features in CD patients. The expression levels of both receptors were negatively correlated with plasma ACTH level and tumor size. The expression of NR3C2 was also higher in patients with post-surgery clinical remission than in patients who did not experience biochemical remission after surgery and in patients with DG tumors as compared to SG tumors. DG histology is considered as related to favorable clinical outcome (19). Expression levels of both genes were also higher in tumors with USP8 mutations, which are the most common driver mutations in corticotroph PitNETs. These mutations are generally related to smaller tumor size and less aggressive growth (28, 34) USP8-mutatated and wild type tumors are also characterized by different biological features (34). The expression of NR3C1 turned out to be the highest in USP8-mutated tumors from patients with clinical remission after surgery indicating that CD patients with this mutation and high NR3C1 level have the best chance for clinical remission.
The relationships between the expression levels of GR/MR-encoding genes and clinical parameters revealed in our study were not strong but statistically significant. This allows to cautiously generalize the results of this study. They clearly show a trend in which higher receptor expression corresponds to more favorable clinical profile in patients, including lower plasma ACTH level, smaller tumor size, clinical remission after surgery and favorable features of sparsely granulated, USP8-mutated tumors.
Due to a weak relationship with patients characteristics it is difficult to indicate any clinical usefulness of testing NR3C1 or NR3C2 expression but our data support the clinically relevant roles of the receptors expression levels in functioning corticotroph pituitary tumors.
Probably the clinically relevant results were not observed in previous studies on NR3C1 expression in CD due to lower number of patient and the fact that the observed relationship is not strong. We suppose that the small difference in gene expression affects the general lack of the finding of the significant associations in our GR and MR protein level analysis. While qRT-PCR results are numerical data by definition, immunohistochemistry is a qualitative method and based on subjective microscopic observations, thus, it is generally less accurate. In our setting, GR immunostaining evaluation produced a relatively small range of immunoreactivity scores and similarly for MR we observed mainly low or moderate immunoreactivity. This is probably also the reason why we did not observe a correlation between qRT-PCR results and protein quantification in our study, and any difference in GR expression when comparing SCAs and functioning tumors.
Targeting GR with receptor antagonist is one of the main pharmacological options in Cushing’s syndrome (CS) aimed to reduce cortisol-related complications (9). The efficacy of two GR modulators mifepristone and relacorilant were already tested and subjected to phase III clinical trials (9). In case of ACTH-related hypercortisolemia this therapeutical approach may provide both therapeutical benefit and adverse effect due to direct effect on corticotroph tumor. Blocking the receptors in tumor may decline the inhibitory effect of GR on ACTH secretion i.e. direct negative adrenal-pituitary feedback. In fact, the use of mifepristone in CD was shown to result in more than 2-fold increase of ACTH secretion in patients (45). More promising preliminary results were obtained in the evaluation of relacorilant (46), but phase III clinical trials are still in progress. Our observation of the negative correlation between the tumor expression of corticosteroid receptor genes and plasma ACTH level suggests that lower receptors levels contribute to less effective negative regulation and possibly could affect lower adverse effect of GR antagonist on direct negative adrenal-pituitary feedback. We suppose that evaluation of expression status of GR/MR receptors (at both gene and protein level) may provide some predictive value in CD patients treated with GR modulators. Perhaps, patients with low expression could benefit more from the treatment. This would be important because our data indicate that these patients are of worse prognosis. This issue requires further specific research. The roles of GR and MR are considered cell-type specific. These receptors may play distinct, even opposite functions such as in immune cells where MR mediates proinflammatory effects, while GR mediates immune suppression (47). Both receptors have pivotal roles in the regulation of HPA axis and reducing activity of pituitary corticotrophs. MR is considered to be ligand-activated throughout the entire circadian cycle, including periods of low circulating glucocorticoid concentrations (inactive phase), when GR receptor is inactive. In turn, GR receptor is activated at high glucocorticoid circadian phase (active phase) or during a stress response (5). These different roles probably result from notably higher affinity of MR to glucocorticosteroids and longer substrate binding time as compared to GR (5). However, more recent data indicate more dynamic activity of MR and circadian differences in its occupancy at particular target DNA loci, as well as cooperative action of both receptors forming MR : GR heterodimers (48, 49). Most of the published data on MR and GR role in regulation of HPA-axis concern their role in hypothalamic neurons while the mechanism of their interaction in corticotroph cells was not determined. Importantly, our observations of co-expression of NR3C1 and NR3C2 in corticotroph PitNETs and negative correlation between the expression of each gene and morning plasma ACTH level suggest that in pituitary corticotroph cells the receptors may synergistically regulate secretory activities of hypothalamic neurons. This hypothesis needs verification in functional research regarding the role of MR in pituitary corticotrophs and cooperation between GR and MR in this type of cells.
5 Conclusions
GR and MR are both expressed in corticotroph tumors and there is a correlation between mRNA levels of genes encoding these receptors. NR3C1 expression level is higher in silent than in functioning corticotroph tumors. In CD patients there is an evident trend towards more favorable clinical characteristics in tumors with higher receptors’ expression.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Maria Sklodowska-Curie National Research Institute of Oncology, Warsaw, Poland. The patients/participants provided their written informed consent to participate in this study.
Author contributions
PK, MB and MM contributed to conception and design. PK, NR, BM and MP contributed to investigation and acquiring laboratory results. GZ, JK, LD, PW and MM contributed to investigation and acquiring of resources (tissue samples and data for the analysis). PK, MM, MB contributed to visualization. PK, MB contributed to data analysis. PK, MB wrote the first draft of the manuscript. PW, MM contributed to writing—review and editing the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by National Science Centre, Poland, grant number 2021/05/X/NZ5/01874. Publication fee was covered by the subvention for the maintenance and development of research potential of the Maria Sklodowska-Curie National Research Institute of Oncology.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1124646/full#supplementary-material
References
1. Inomoto C, Tahara S, Oyama K, Kimura M, Matsuno A, Teramoto A, et al. Molecular, functional, and histopathological classification of the pituitary neuroendocrine neoplasms. Brain Tumor Pathol (2021) 38:183–188. doi: 10.1007/s10014-021-00410-5
2. Ben-Shlomo A, Cooper O. Silent corticotroph adenomas. Pituitary (2018) 21:1–11. doi: 10.1007/s11102-018-0864-8
3. Neou M, Villa C, Armignacco R, Jouinot A, Raffin-Sanson ML, Septier A, et al. Pangenomic classification of pituitary neuroendocrine tumors. Cancer Cell (2020) 37:123–134.e5. doi: 10.1016/j.ccell.2019.11.002
4. Bujko M, Kober P, Boresowicz J, Rusetska N, Paziewska A, Dabrowska M, et al. USP8 mutations in corticotroph adenomas determine a distinct gene expression profile irrespective of functional tumour status. Eur J Endocrinol (2019) 181:615–27. doi: 10.1530/EJE-19-0194
5. Lightman SL, Birnie MT, Conway-Campbell BL. Dynamics of ACTH and cortisol secretion and implications for disease. Endocr Rev (2021) 41:bnaa002. doi: 10.1210/ENDREV/BNAA002
6. Ciato D, Albani A. Molecular mechanisms of glucocorticoid resistance in corticotropinomas: New developments and drug targets. Front Endocrinol (Lausanne) (2020) 11:21. doi: 10.3389/fendo.2020.00021
7. Daskalakis NP, Meijer OC, de Kloet ER. Mineralocorticoid receptor and glucocorticoid receptor work alone and together in cell-type-specific manner: Implications for resilience prediction and targeted therapy. Neurobiol Stress (2022) 18:100455. doi: 10.1016/j.ynstr.2022.100455
8. Koning ASCAM, Buurstede JC, van Weert LTCM, Meijer OC. Glucocorticoid and mineralocorticoid receptors in the brain: A transcriptional perspective. J Endocr Soc (2019) 3:1917–30. doi: 10.1210/js.2019-00158
9. Regazzo D, Mondin A, Scaroni C, Occhi G, Barbot M. The role of glucocorticoid receptor in the pathophysiology of pituitary corticotroph adenomas. Int J Mol Sci (2022) 23:6469. doi: 10.3390/ijms23126469
10. Huizenga NATM, de Lange P, Koper JW, Clayton RN, Farrell WE, van der Lely AJ, et al. Human adrenocorticotropin-secreting pituitary adenomas show frequent loss of heterozygosity at the glucocorticoid receptor gene locus. J Clin Endocrinol Metab (1998) 83:917–21. doi: 10.1210/jc.83.3.917
11. Mossakowska BJ, Kober P, Rusetska N, Boresowicz J, Maksymowicz M, Pękul M, et al. Difference in miRNA expression in functioning and silent corticotroph pituitary adenomas indicates the role of miRNA in the regulation of corticosteroid receptors. Int J Mol Sci (2022) 23:2867. doi: 10.3390/ijms23052867
12. Bujko M, Kober P, Boresowicz J, Rusetska N, Zeber-Lubecka N, Paziewska A, et al. Differential microRNA expression in USP8-mutated and wild-type corticotroph pituitary tumors reflect the difference in protein ubiquitination processes. J Clin Med (2021) 10:1–15. doi: 10.3390/jcm10030375
13. McCarty KS, Szabo E, Flowers JL, Cox EB, Leight GS, Miller L, et al. Use of a monoclonal anti-estrogen receptor antibody in the immunohistochemical evaluation of human tumors. Cancer Res (1986) 46:4244–9.
14. Valassi E, Santos A, Yaneva M, Tóth M, Strasburger CJ, Chanson P, et al. The European registry on cushing’s syndrome: 2-year experience. baseline demographic and clinical characteristics. Eur J Endocrinol (2011) 165:383–92. doi: 10.1530/EJE-11-0272
15. Langlois F, Shao D, Lim T, Yedinak CG, Cetas I, Mccartney S, et al. Predictors of silent corticotroph adenoma recurrence; a large retrospective single center study and systematic literature review. Pituitary (1234) 21:32–40. doi: 10.1007/s11102-017-0844-4
16. Jahangiri A, Wagner JR, Pekmezci M, Hiniker A, Chang EF, Kunwar S, et al. A comprehensive long-term retrospective analysis of silent corticotrophic adenomas vs hormone-negative adenomas. Neurosurgery (2013) 738–17. doi: 10.1227/01.neu.0000429858.96652.1e
17. Kontogeorgos G, Thodou E, Osamura Y, Lloyd RV. High-risk pituitary adenomas and strategies for predicting response to treatment. Hormones (2022) 1:3. doi: 10.1007/s42000-021-00333-y
18. Osamura RY, Lopes MBS, Grossman A, Kontogeorge G, Trouillas J. In: WHO classification of tumours of endocrine organs. 4th. Lloyd RV, Osamura RY, Rosari J, editors. Lyon: IARC Press (2017) p. 12–3.
19. Rak B, Maksymowicz M, Pękul M, Zieliński G. Clinical, biological, radiological pathological and immediate post-operative remission of sparsely and densely granulated corticotroph pituitary tumors: A retrospective study of a cohort of 277 patients with cushing’s disease. Front Endocrinol (Lausanne) (2021) 12:672178. doi: 10.3389/fendo.2021.672178
20. Drouin J, Trifiro MA, Plante RK, Nemer M, Eriksson P, Wrange O. Glucocorticoid receptor binding to a specific DNA sequence is required for hormone-dependent repression of pro-opiomelanocortin gene transcription. Mol Cell Biol (1989) 9:5305–14. doi: 10.1128/mcb.9.12.5305
21. Bilodeau S, Vallette-Kasic S, Gauthier Y, Figarella-Branger D, Brue T, Berthelet F, et al. Role of Brg1 and HDAC2 in GR trans-repression of the pituitary POMC gene and misexpression in cushing disease. Genes Dev (2006) 20:2871–86. doi: 10.1101/gad.1444606
22. Martens C, Bilodeau S, Maira M, Gauthier Y, Drouin J. Protein-protein interactions and transcriptional antagonism between the subfamily of NGFI-B/Nur77 orphan nuclear receptors and glucocorticoid receptor. Mol Endocrinol (2005) 19:885–97. doi: 10.1210/me.2004-0333
23. Deng Q, Riquelme D, Trinh L, Low MJ, Tomic M, Stojilkovic S, et al. Rapid glucocorticoid feedback inhibition of ACTH secretion involves ligand-dependent membrane association of glucocorticoid receptors. Endocrinol (United States) (2015) 156:3215–27. doi: 10.1210/EN.2015-1265
24. Tateno T, Kato M, Tani Y, Oyama K, Yamada S, Hirata Y. Differential expression of somatostatin and dopamine receptor subtype genes in adrenocorticotropin (ACTH)- secreting pituitary tumors and silent corticotroph adenomas. Endocrine J Endocrine J (2009) 56:579–84. doi: 10.1507/endocrj.K08E-186
25. Righi A, Faustini-Fustini M, Morandi L, Monti V, Asioli S, Mazzatenta D, et al. The changing faces of corticotroph cell adenomas: the role of prohormone convertase 1/3. Endocrine (2017) 56:286–97. doi: 10.1007/s12020-016-1028-0
26. Nagaya T, Seo H, Kuwayama A, Sakurai T, Tsukamoto N, Nakane T, et al. Pro-opiomelanocortin gene expression in silent corticotroph-cell adenoma and cushing’s disease. J Neurosurg (1990) 72:262–7. doi: 10.3171/jns.1990.72.2.0262
27. Tateno T, Izumiyama H, Doi M, Yoshimoto T, Shichiri M, Inoshita N, et al. Differential gene expression in ACTH -secreting and non-functioning pituitary tumors. Eur J Endocrinol (2007) 157:717–24. doi: 10.1530/EJE-07-0428
28. Raverot G, Wierinckx A, Jouanneau E, Auger C, Borson-Chazot F, Lachuer J, et al. Clinical, hormonal and molecular characterization of pituitary ACTH adenomas without (silent corticotroph adenomas) and with cushing’s disease. Eur J Endocrinol (2010) 163:35–43. doi: 10.1530/EJE-10-0076
29. Ebisawa T, Tojo K, Tajima N, Kamio M, Oki Y, Ono K, et al. Immunohistochemical analysis of 11-β-hydroxysteroid dehydrogenase type 2 and glucocorticoid receptor in subclinical cushing’s disease due to pituitary macroadenoma. Endocr Pathol (2008) 19:252–60. doi: 10.1007/s12022-008-9052-0
30. Zheng G, Lu L, Zhu H, You H, Feng M, Liu X, et al. Clinical, laboratory, and treatment profiles of silent corticotroph adenomas that have transformed to the functional type: A case series with a literature review. Front Endocrinol (Lausanne) (2020) 11:558593. doi: 10.3389/fendo.2020.558593
31. Rotman LE, Vaughan TB, Hackney JR, Riley KO. Long-term survival after transformation of an adrenocorticotropic hormone–secreting pituitary macroadenoma to a silent corticotroph pituitary carcinoma. World Neurosurg (2019) 122:417–23. doi: 10.1016/j.wneu.2018.11.011
32. Świątkowska-Stodulska R, Berlińska A, Stefańska K, Kłosowski P, Sworczak K. Cyclic cushing’s syndrome – a diagnostic challenge. Front Endocrinol (Lausanne) (2021) 12:658429. doi: 10.3389/fendo.2021.658429
33. Theodoropoulou M. Glucocorticoid receptors are making a comeback in corticotroph tumorigenesis. Endocrinol (United States) (2022) 163:bqab257. doi: 10.1210/endocr/bqab257
34. Sbiera S, Kunz M, Weigand I, Deutschbein T, Dandekar T, Fassnacht M. The new genetic landscape of cushing’s disease: Deubiquitinases in the spotlight. Cancers (Basel) (2019) 11:1–14. doi: 10.3390/cancers11111761
35. Miao H, Liu Y, Lu L, Gong F, Wang L, Duan L, et al. Effect of 3 NR3C1 mutations in the pathogenesis of pituitary ACTH adenoma. Endocrinol (United States) (2021) 162:bqab167. doi: 10.1210/endocr/bqab167
36. Dahia PLM, Honegger J, Reincke M, Jacobs RA, Mirtella A, Fahlbusch R, et al. Expression of glucocorticoid receptor gene isoforms in corticotropin- secreting tumors. J Clin Endocrinol Metab (1997) 82:1088–93. doi: 10.1210/jc.82.4.1088
37. Cassarino MF, Sesta A, Pagliardini L, Losa M, Lasio G, Cavagnini F, et al. Proopiomelanocortin, glucocorticoid, and CRH receptor expression in human ACTH-secreting pituitary adenomas. Endocrine (2017) 55:853–60. doi: 10.1007/s12020-016-0990-x
38. Mu YM, Takayanagi R, Imasaki K, Ohe K, Ikuyama S, Yanase T, et al. Low level of glucocorticoid receptor messenger ribonucleic acid in pituitary adenomas manifesting cushing’s disease with resistance to a high dose-dexamethasone suppression test. Clin Endocrinol (Oxf) (1998) 49:301–6. doi: 10.1046/j.1365-2265.1998.00520.x
39. Viengchareun S, le Menuet D, Martinerie L, Munier M, Pascual-Le Tallec L, Lombès M. The mineralocorticoid receptor: insights into its molecular and (patho)physiological biology. Nucl Recept Signal (2007) 5:e012. doi: 10.1621/nrs.05012
40. Han F, Ozawa H, Matsuda KI, Nishi M, Kawata M. Colocalization of mineralocorticoid receptor and glucocorticoid receptor in the hippocampus and hypothalamus. Neurosci Res (2005) 51:371–81. doi: 10.1016/j.neures.2004.12.013
41. Beavan S, Horner A, Bord S, Ireland D, Compston J. Colocalization of glucocorticoid and mineralocorticoid receptors in human bone. J Bone Mineral Res (2001) 16:1496–504. doi: 10.1359/jbmr.2001.16.8.1496
42. Marzolla V, Armani A, Zennaro MC, Cinti F, Mammi C, Fabbri A, et al. The role of the mineralocorticoid receptor in adipocyte biology and fat metabolism. Mol Cell Endocrinol (2012) 350:281–8. doi: 10.1016/j.mce.2011.09.011
43. Farman N, Oblin ME, Lombes M, Delahaye F, Westphal HM, Bonvalet JP, et al. Immunolocalization of gluco- and mineralocorticoid receptors in rabbit kidney. Am J Physiol Cell Physiol (1991) 260:C226–33. doi: 10.1152/ajpcell.1991.260.2.c226
44. Armanini D, Endres S, Kuhnle U, Weber PC. Parallel determination of mineralocorticoid and glucocorticoid receptors in T-and b-lymphocytes of human spleen. Acta Endocrinol (Copenh) (1988) 118:479–82. doi: 10.1530/acta.0.1180479
45. Fleseriu M, Findling JW, Koch CA, Schlaffer SM, Buchfelder M, Gross C. Changes in plasma ACTH levels and corticotroph tumor size in patients with cushing’s disease during long-term treatment with the glucocorticoid receptor antagonist mifepristone. J Clin Endocrinol Metab (2014) 99:3718–27. doi: 10.1210/jc.2014-1843
46. Terzolo M, Iacuaniello D, Pia A, Adriano P, Moraitis A, Pivonello R. SUN-463 tumor shrinkage with preoperative relacorilant therapy in two patients with cushing disease due to pituitary macroadenomas. J Endocr Soc (2019) 3(Supp 1):SUN–463. doi: 10.1210/js.2019-sun-463
47. Chantong B, Kratschmar DV, Nashev LG, Balazs Z, Odermatt A. Mineralocorticoid and glucocorticoid receptors differentially regulate NF-kappaB activity and pro-inflammatory cytokine production in murine BV-2 microglial cells. J Neuroinflamm (2012) 9:260. doi: 10.1186/1742-2094-9-260
48. Mifsud KR, Reul JMHM. Mineralocorticoid and glucocorticoid receptor-mediated control of genomic responses to stress in the brain. Stress (2018) 21:389–402. doi: 10.1080/10253890.2018.1456526
Keywords: pituitary neuroendocrine tumor (PitNET), Cushing’s disease (CD), silent corticotroph adenoma, NR3C1, NR3C2, hypothalamic - pituitary - adrenal axis, glucocorticoid receptor, mineralocorticoid receptor
Citation: Kober P, Rusetska N, Mossakowska BJ, Maksymowicz M, Pękul M, Zieliński G, Styk A, Kunicki J, Działach Ł, Witek P and Bujko M (2023) The expression of glucocorticoid and mineralocorticoid receptors in pituitary tumors causing Cushing’s disease and silent corticotroph tumors. Front. Endocrinol. 14:1124646. doi: 10.3389/fendo.2023.1124646
Received: 15 December 2022; Accepted: 13 March 2023;
Published: 29 March 2023.
Edited by:
Corin Badiu, Carol Davila University of Medicine and Pharmacy, RomaniaReviewed by:
Erika Peverelli, University of Milan, ItalyMarek Bolanowski, Wroclaw Medical University, Poland
Copyright © 2023 Kober, Rusetska, Mossakowska, Maksymowicz, Pękul, Zieliński, Styk, Kunicki, Działach, Witek and Bujko. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mateusz Bujko, bWF0ZXVzei5idWprb0BwaWItbmlvLnBs
 Paulina Kober1
Paulina Kober1 Grzegorz Zieliński
Grzegorz Zieliński Łukasz Działach
Łukasz Działach Przemysław Witek
Przemysław Witek Mateusz Bujko
Mateusz Bujko