- 1Transplant Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
- 2Gastroenterohepatology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
- 3Endocrinology and Metabolism Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
- 4Department of Tissue Engineering and Cell Therapy, School of Advanced Technologies in Medicine, Shiraz University of Medical Sciences, Shiraz, Iran
- 5Health Policy Research Center, Institute of Health, Shiraz University of Medical Sciences, Shiraz, Iran
Background: Modern societies face infertility as a global challenge. There are certain environmental conditions and disorders that damage testicular tissue and may cause male infertility. Melatonin, as a potential antioxidant, may protect testicular tissue. Therefore, we conducted this systematic review and meta-analysis to evaluate the effects of melatonin in animal models against physical, heat, and ischemic damage to the testicular tissue.
Methods: PubMed, Scopus, and Web of Science were systematically searched to identify animal trials evaluating the protective effect of melatonin therapy on rodent testicular tissue when it is exposed to physical, thermal, ischemic, or hypobaric oxygen stress. Random-effect modeling was used to estimate the standardized mean difference and 95% confidence intervals based on the pooled data. Additionally, the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE) tool was used to assess the risk of bias. The study protocol was prospectively registered in PROSPERO (CRD42022354599).
Results: A total of 41 studies were eligible for review out of 10039 records. Studies employed direct heat, cryptorchidism, varicocele, torsion-detorsion, testicular vascular occlusion, hypobaric hypoxia, ischemia-reperfusion, stress by excessive or restraint activity, spinal cord injury, and trauma to induce stress in the subjects. The histopathological characteristics of testicular tissue were generally improved in rodents by melatonin therapy. Based on the pooled data, sperm count, morphology, forward motility, viability, Johnsen’s biopsy score, testicular tissue glutathione peroxidase, and superoxide dismutase levels were higher in the melatonin treatment rodent arms. In contrast, the malondialdehyde level in testicular tissue was lower in the treatment rodent arms. The included studies suffered from a high risk of bias in most of the SYRCLE domains.
Conclusion: This study concludes that melatonin therapy was associated with improved testicular histopathological characteristics, reproductive hormonal panel, and tissue markers of oxidative stress in male rodents with physical, ischemic, and thermal testicular injuries. In this regard, melatonin deserves scientific investigations as a potential protective drug against rodent male infertility.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42022354599.
1 Introduction
The pathophysiology of male infertility is caused by a number of variables, including genetics and epigenetic changes, hormonal imbalances, environmental influences, and physical injuries like varicocele, cryptorchidism, and testicular torsion (1, 2). Some of these factors change the balance between the generation of free-radical species and the antioxidant defense system, which in turn disrupts functional male fecundity. Reactive oxygen species (ROS) are generally crucial for some common physiological processes like spermatogenesis, sperm capacitation, and the acrosome reaction; however, an increase in ROS production leads to “oxidative stress” (3, 4), which harms cells by inducing oxidative damages like lipid peroxidation, DNA damage, and protein misfolding resulting to abnormal semen parameters (5). Utilizing antioxidant supplements, such as melatonin, zinc, coenzyme Q10 (CoQ10), omega-3 fatty acids, vitamin E, and L-carnitine, as novel treatment strategies to address male infertility diseases has recently drawn increasing attention (6–8).
Melatonin, the sleep-wakefulness hormone, was initially discovered by Aaron Lerner in the pineal gland of a bovine in 1958 (9). Once believed that it only is secreted by the pineal gland, melatonin is now understood to be produced throughout the body in various tissues, including the cardiovascular, endocrine, immunological, male reproductive, skin, and gastrointestinal tract systems (10, 11). As an endogenous indole amine, melatonin plays a crucial role in many biological processes, like circadian rhythm, redox homeostasis, epigenetic regulation, body temperature regulation, fetal development, local and general immunity, and reproductive physiology (5, 12, 13). Melatonin has been extensively explored for its antioxidant properties (14–17). Melatonin can pass through the blood-testis barrier and is capable of entering testis cells (18). Melatonin has direct and indirect antioxidant properties. As an electron-rich molecule, it can negate free radicals, making stable products that are able to be excreted in urine. Melatonin’s antioxidant mechanism involves free-radical scavenger cascade indicating efficiency of secondary and tertiary metabolites. Furthermore, melatonin indirectly acts by stimulating antioxidant enzymes (19–21). In fact, it can modulate the mRNA levels and activity of some well-known antioxidants such as Glutathione Peroxidase (GPx), ascorbate, and superoxide dismutase (SOD) (15, 22). It plays a role in the production and secretion of testosterone by Leydig cells and increases the responsiveness of Sertoli cells to Follicle-Stimulating Hormone (FSH) during testis development and growth (23, 24). The detrimental disorders of unilateral testicular damages (such as undescended testis and torsion), which influence morphometric, spermatogenesis, and oxidative parameters, could be reversed by melatonin as an antioxidant (25, 26). Up to now, it’s widely recognized that melatonin has different effective roles in the male reproductive system, which exerts these effects directly (non-receptor mediated) or indirectly (receptor-mediated pathways) (27, 28). In humans, melatonin has two types of high-affinity G-protein-coupled receptors called melatonin type 1 and 2 receptors (MT1 and MT2) (29). In the male reproductive system, melatonin, through the effect of MT1, MT2, and retinoic acid receptor-related orphan receptor/retinoid Z receptor (ROR/RZR) implicates in proliferation (such as spermatogonial stem cells-SSCs), differentiation (such as spermatogonia to spermatids) and metabolic functions (23, 30, 31). On the other hand, melatonin acts directly as ROS and reactive nitrogen scavenger (RNS) according to its structure that had been suggested to be an electron donor (32, 33). Melatonin is believed to have an anti-apoptotic effect in the testes by decreasing mitochondrial-related apoptosis and ROS-related mitochondrial damage (34, 35).
Animal models have gained interest due to the limitations of human studies in reproductive research, such as ethical limitations in administering drugs and performing biopsies, and the long and ambiguous period of disease development and progression. As a result, we decided to systematically review the literature for rodent animal studies that used, evaluated, and reported melatonin and its protective effects on male genital systems against physical injuries.
2 Materials and method
This systematic review and meta-analysis was conducted following The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement (36), and the protocol is registered in the International Prospective Register of Systematic Reviews (PROSPERO: CRD42022354599).
2.1 Key question
This review aimed to investigate the protective effects of exogenous melatonin on the parameters of male reproductive function against physical, heat, or ischemic injuries compared to placebo in rodent subjects. We asked if melatonin therapy (whether before or after the induction of stress) prevents oxidative pathways in testicular cells.
2.2 Data sources and searches
Two reviewers (AS and SP) conducted a comprehensive search in PubMed, Scopus, and Web of Science for records from January 1, 1970, until September 9, 2022, for “melatonin”, “testicular function”, and their equivalents as keywords. The search strategies comprised a combination of Medical Subject Headings (MeSH) or their equivalent (where available), keywords, truncations, and boolean operators. A manual backward and forward citation search was also done for all the included studies. The detailed search strategy is provided in Supplementary Material 1.
2.3 Study selection and eligibility criteria
Firstly, duplicate records were removed electronically. Then, using the Rayyan online tool for managing systematic reviews (37), four reviewers (NDE, SP, NM, and AS) independently screened titles and abstracts, followed by the full-text screening of the identified records for eligibility criteria. Disagreements were resolved with discussion. Studies were included if they satisfied the following criteria: (1) controlled animal studies, (2) the population was rodents that were exposed to physical, electrical, ischemic, or thermal injuries as oxidative stress to the testicular tissue, (3) at least one intervention group received melatonin regimen, (4) at least one control group with similar stress, and (5) reported major hallmarks of testicular tissue (histopathologic, biochemical, and sperm analyses).
The exclusion criteria were: (1) in-vitro and ex-vivo studies, (2) non-rodent animals with other types of stresses (radiation, chemotherapy, toxins, and metabolic), (3) combination therapy of melatonin with other drugs, (4) treatment using derivatives of melatonin, (5) healthy controls without stress, and (6) reported irrelevant outcomes. Also, reviews, letters, and human trails were excluded from the review. Our search was not restricted by language.
2.4 Data extraction and risk of bias assessment
Two reviewers (ET and FSS) independently extracted the favorable data into Excel spreadsheets. Any disagreements were resolved by discussion and involvement of a third author (AS). Structured forms were used for data extraction of the following contents: (1) study characteristics (first author, publication year, and country), (2) population characteristics (species, age, sample size, and type of stress), (3) melatonin dose, duration, and route, (4) time of assessment of outcome, (5) tissue and plasma biochemical indices, and (6) histopathological characteristics. For missing data, we contacted the first or corresponding author and waited for a response for at least one month.
We assessed the risk of bias in the studies using the Systematic Review Centre for Laboratory animal Experimentation (SYRCLE) tool for animal intervention studies (38). Two authors (NDE and ET) independently reviewed each article and classified it as high, low, or unclear for each bias domain. Disagreements were resolved via consensus or by a third (AS) reviewer if necessary.
2.5 Data synthesis and statistical analysis
Data were analyzed using Stata MP Version 16 (StataCorp, College Station, TX, USA), and a p-value < 0.05 was considered statistically significant. Under a random-effects model (DerSimonian-Laird method), the pooled effect sizes were reported as standardized mean difference (Glass’s Δ) and 95% confidence interval (39). The statistical heterogeneity was examined using Cochran’s Q statistic, p-value, and I-squared. I-squared was employed to qualify heterogeneity as “perhaps not important”, “moderate heterogeneity”, “substantial heterogeneity”, and “considerable heterogeneity” if I-squared values were 0-40%, 30-60%, 50-90%, and 75-100%, respectively (40). Subgroup analyses were implemented only if three or more studies were available for each subgroup to identify possible sources of heterogeneity. In case of missing data, if crucial, studies were removed from the analysis. Also, funnel plots were used to detect visual asymmetry in publications when at least ten studies were available for the outcome (40).
3 Results
3.1 Search results
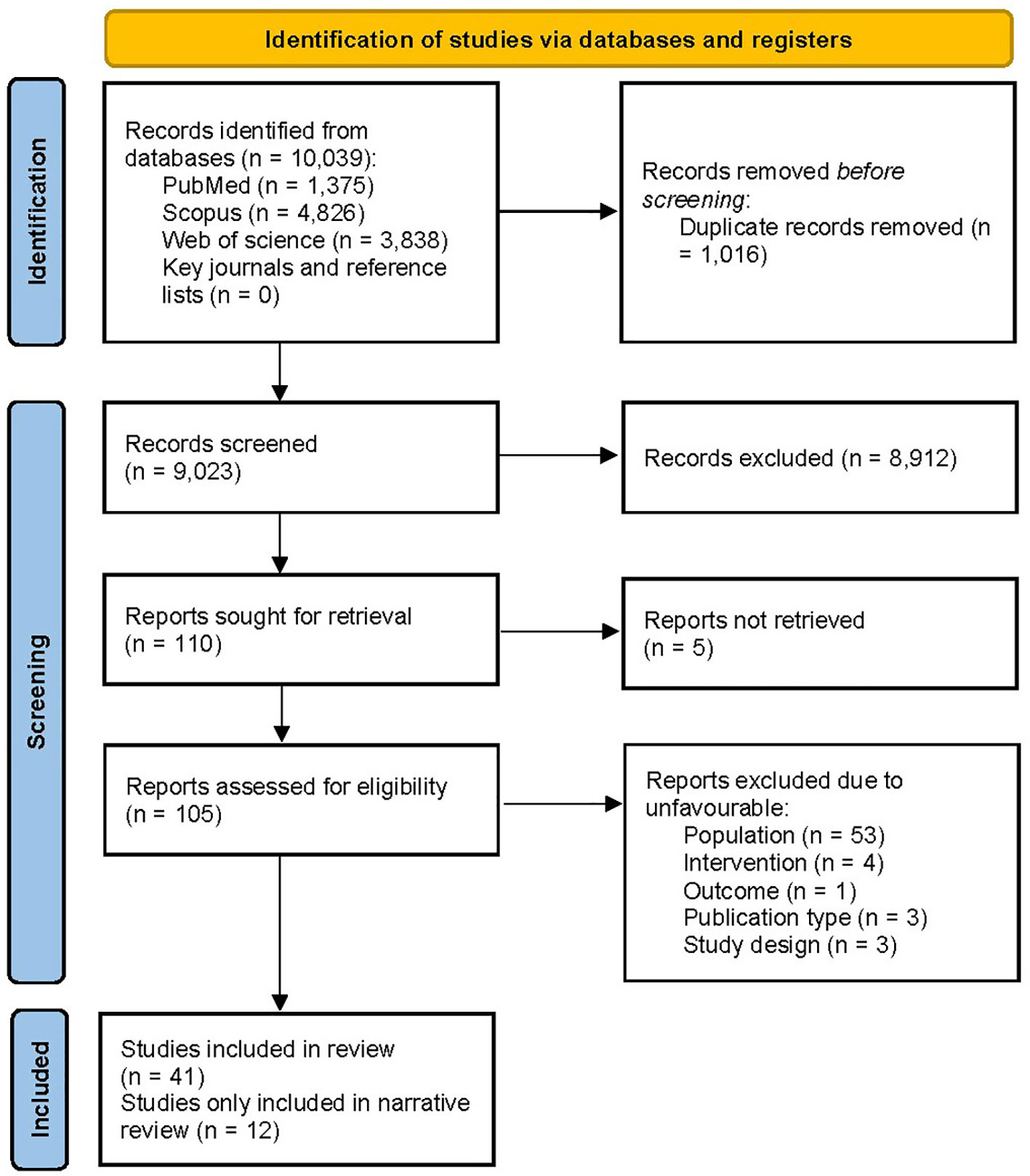
The PRISMA flow diagram of the literature search is presented in Figure 1. The systematic search yielded 10,039 records, while manual citation searching yielded no additional studies. The database searching included PubMed (n=1,375), Web of Science (n=3,838), and Scopus (n=4,826). 1,016 records were removed using automatic duplicate detection. Title and abstract screening was conducted on 9,023 records, and 110 studies were sought for retrieval. With the exclusion of 5 studies that we failed to retrieve (41–45), 105 articles were assessed for eligibility. A total of 64 articles were excluded due to ineligible population (n=53), design (n=3), intervention (n=4), outcome (n=1), and publication type (n=3). Finally, 41 articles were eligible for the study; 12 (46–57) were only included in narrative data synthesis. Two studies were on the same subject population; thus, for data synthesis, they were treated as one (47, 51). Despite our effort to contact the authors, 8 studies contained missing values; therefore, they were excluded from the analysis (47, 52–55, 57–59).
3.2 Study characteristics
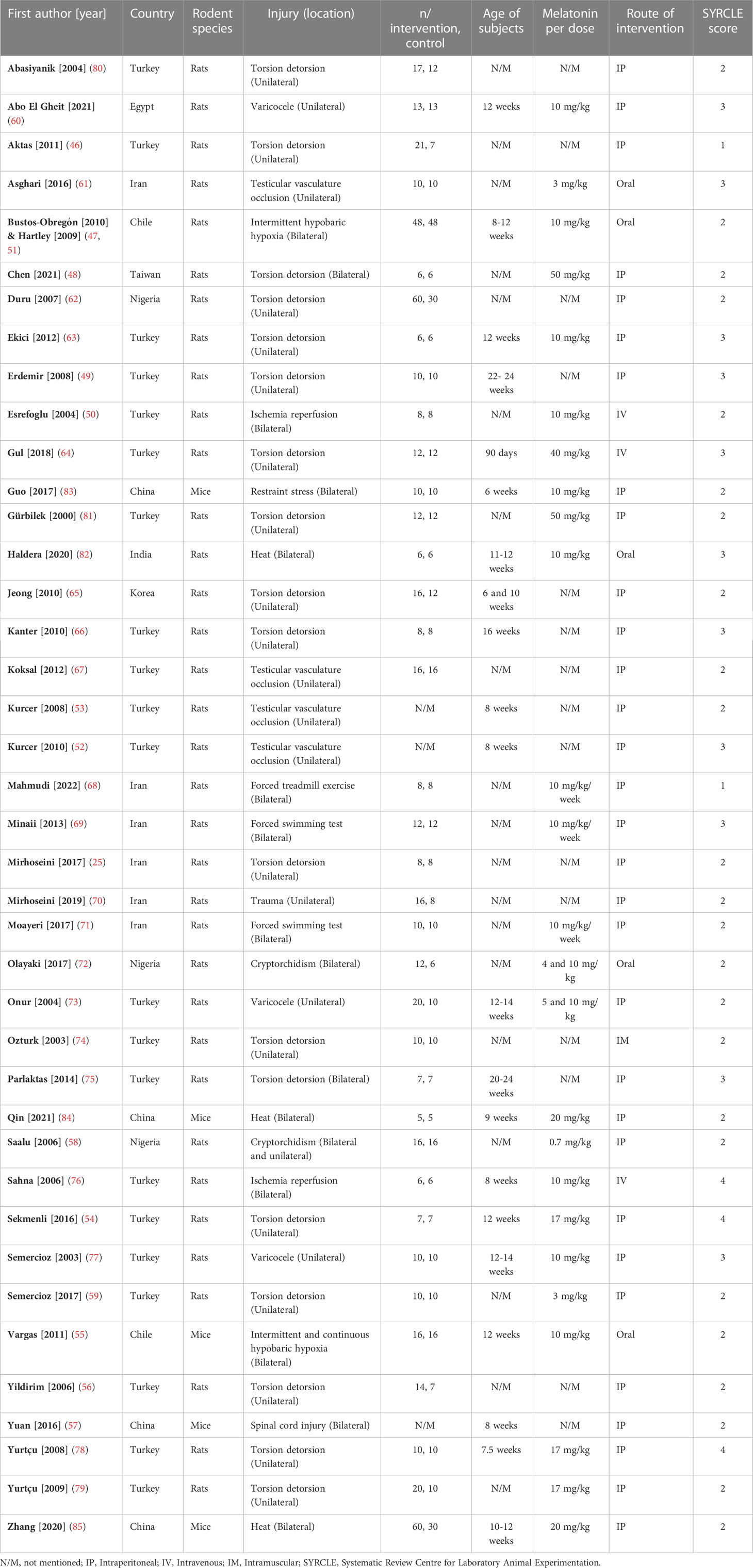
Included studies were published between 2000 and 2022 in English. Rats (n=36) (25, 46–54, 56, 58–82) and mice (n=5) (55, 57, 83–85) were the subjects in the included studies. Studies employed direct heat (n=3) (82, 84, 85), cryptorchidism (n=2) (58, 72), varicocele (n=3) (60, 73, 77), torsion-detorsion (n=18) (25, 46, 48, 49, 54, 56, 59, 62–66, 74, 75, 78–81), testicular vascular occlusion (n=4) (52, 53, 61, 67), hypobaric hypoxia (n=3) (47, 51, 55), ischemia-reperfusion (n=2) (50, 76), stress by excessive (n=3) (68, 69, 71) or restraint (n=1) (83) activity, spinal cord injury (n=1) (57), and trauma (n=1) (70). Studies induced the stress mechanisms bilaterally (n=16) (47, 48, 50, 51, 55, 57, 58, 68, 69, 71, 72, 75, 76, 82–85) and unilaterally (n=25) (25, 46, 49, 52–54, 56, 58–67, 70, 73, 74, 77–81). Mice aged between 6 - 12 weeks and rats aged between 6 – 24 weeks. Studies administered melatonin intraperitoneal (n=31) (25, 46, 48, 49, 52–54, 56–60, 62, 63, 65–71, 73, 75, 77–81, 83–85), oral (n=5) (47, 51, 55, 61, 72, 82), intravenous (n=3) (50, 64, 76), and intramuscular (n=1) (74). Characteristics of the included articles are summarized in Figure 2. The dose of melatonin therapy, descriptive histopathological findings, duration of stress induction, and outcomes assessment ranged significantly between the studies presented in Table 1 and Supplementary Materials 2.
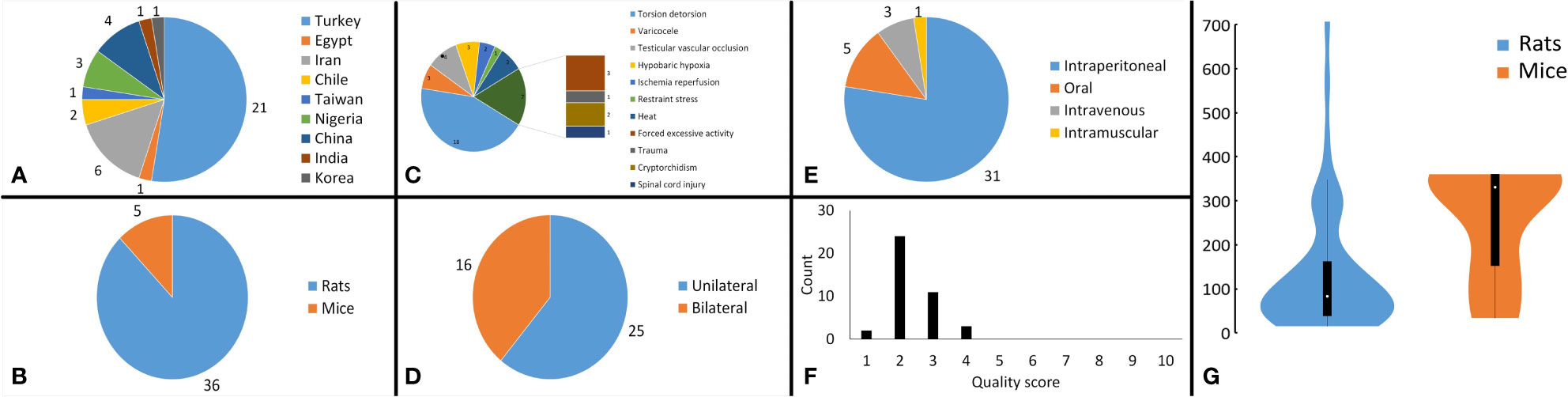
Figure 2 Analysis of study characteristics. Pie chart of (A) the countries that studies were published from, (B) the subject rodents across the studies, (C) the stress mechanism used in the studies, (D) the side that stresses were induced in the subjects, (E) the route of melatonin administration across the studies, (F) bar chart, illustrating the distribution of the methodological quality scores across the studies, and (G) violin plot demonstrating the distribution of overall cumulative dosages that were administered to the rodents (mg/kg).
3.3 Outcomes
18 outcomes were pooled from the included studies which were classified into five groups: (a) sperm parameters, (b) reproductive hormone profile, (c) markers of oxidative stress in testicular tissue, (d) body weight and testicular somatic indices, and (e) exploratory outcomes. Pooled outcomes included: total sperm count, forward progressive motility, normal sperm morphology, sperm viability, Johnson tubular biopsy score, serum testosterone and Inhibin-B levels, testicular tissue SOD, malondialdehyde (MDA), GPx, and catalase (CAT) activity, final body and testes weight, testis to body weight ratio, seminiferous tubular diameter, percentage of tubules with TUNEL-positive cells (TUNEL: Terminal deoxynucleotidyl transferase dUTP nick end labeling), and number of TUNEL-positive cells per tubule. The forest plots of the overall pooled effects sizes are presented in the Figure 3–7.
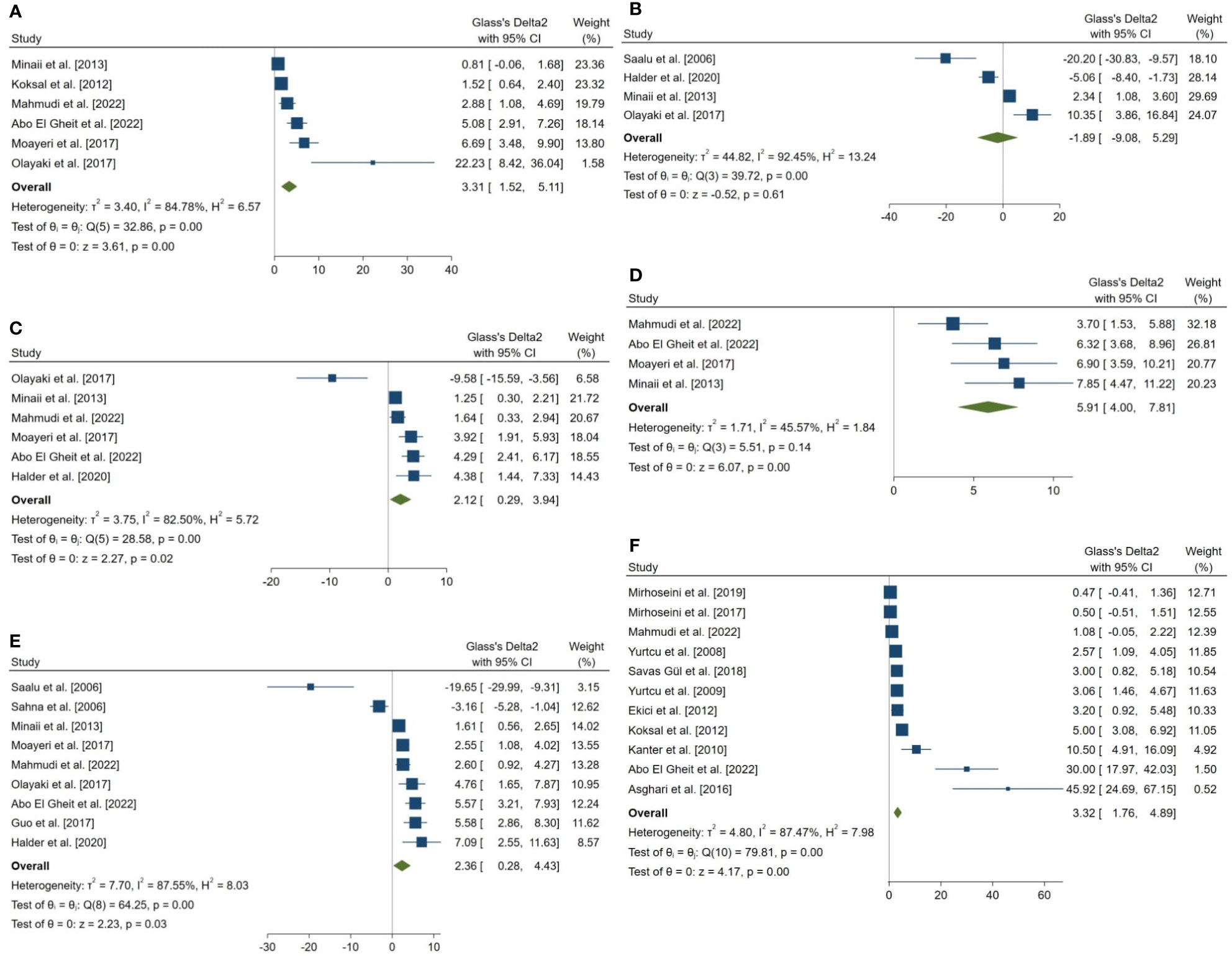
Figure 3 Forest plots for the overall pooled effects sizes of sperm parameters. (A) normal morphology (n=6), (B) total motility (n=4), (C) viability (n=6), (D) forward progressive motility (n=4), (E) total count (n=9), (F) Johnsen’s biopsy score (n=11).
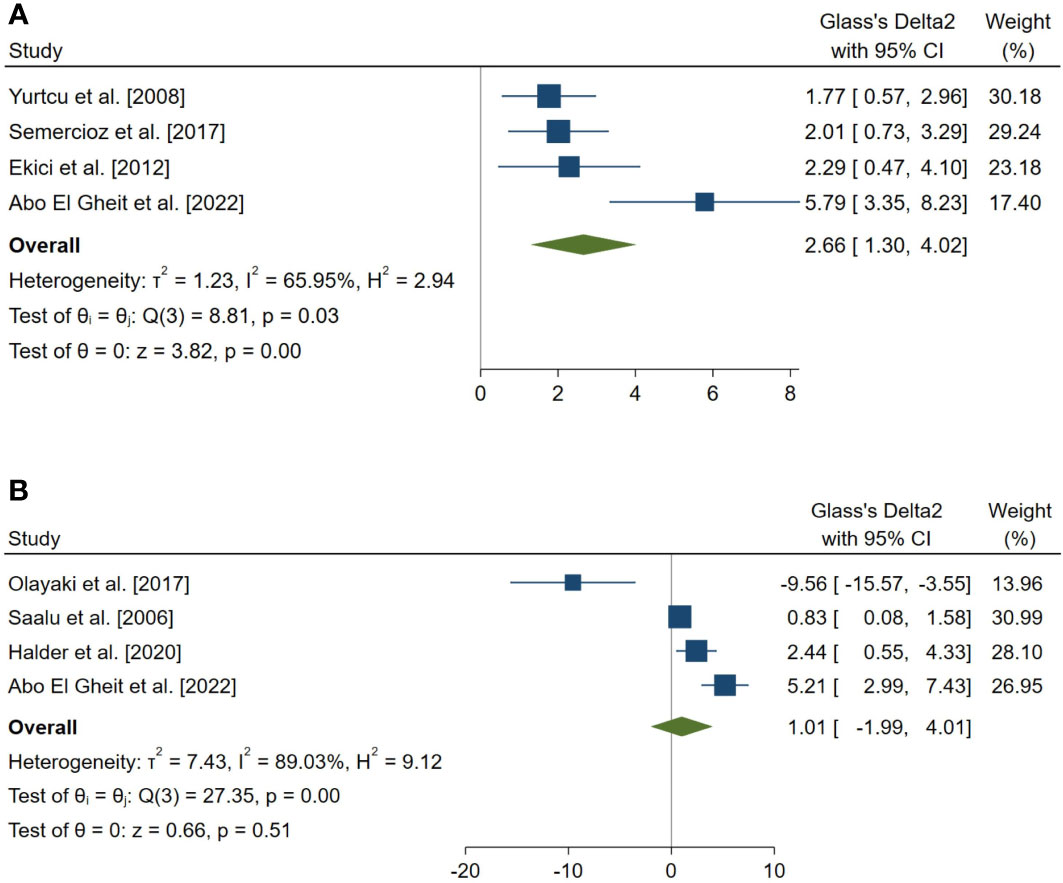
Figure 4 Forest plots for the overall pooled effects sizes of serum level of reproductive hormones. (A) Serum Inhibin-B (n=4), (B) testosterone (n=4).
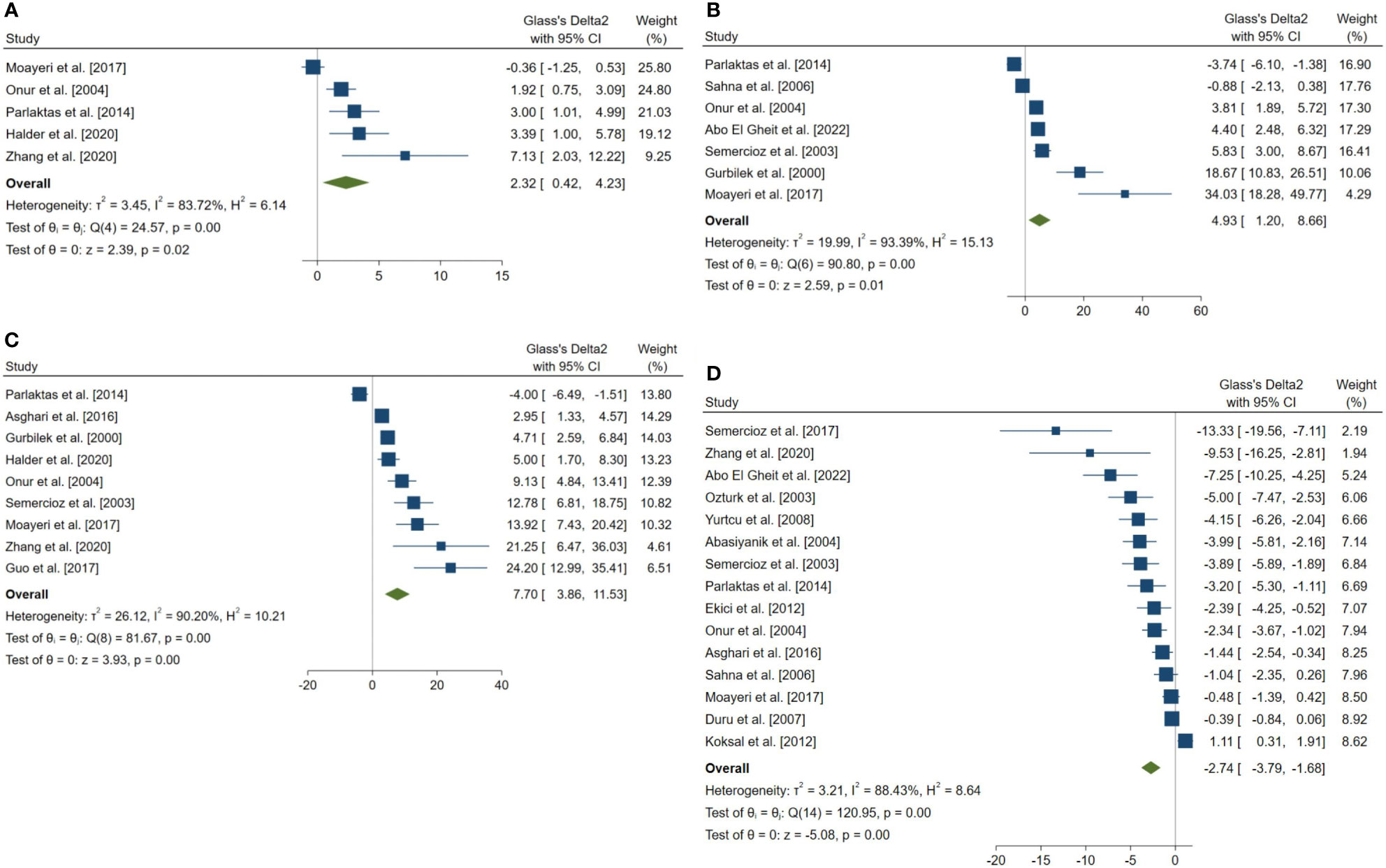
Figure 5 Forest plots for the overall pooled effects sizes of the testicular tissue oxidative stress markers. (A) CAT (n=5), (B) GPx (n=7), (C) SOD (n=9), (D) MDA (n=15). MDA, Malondialdehyde; GPx, Glutathione Peroxidase; CAT, Catalase; SOD, Superoxide Dismutase.
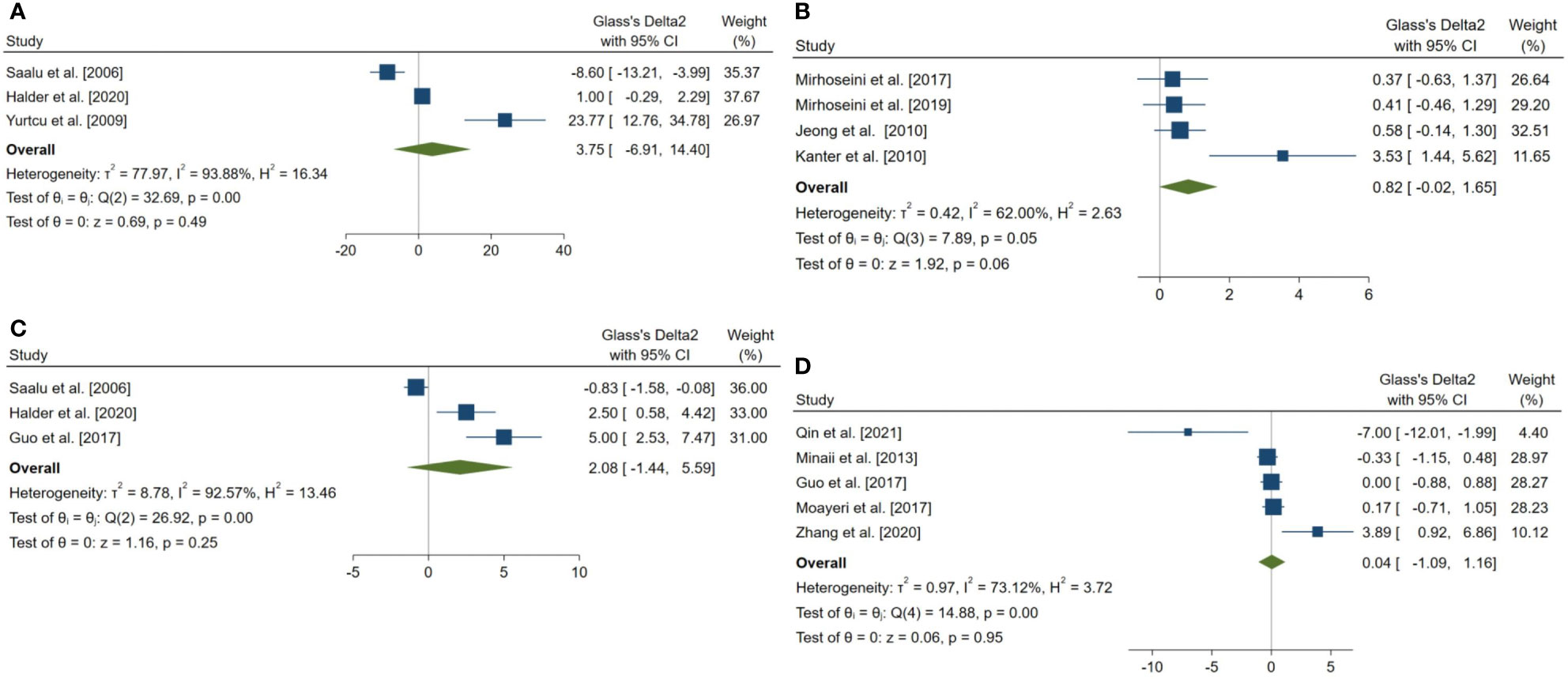
Figure 6 Forest plots for the overall pooled effects sizes of body weight and testicular somatic indices. (A) total testicular weight (n=3), (B) seminiferous tubular diameter (n=4), (C) final body weight (n=3), (D) testis to body relative weight (n=5).
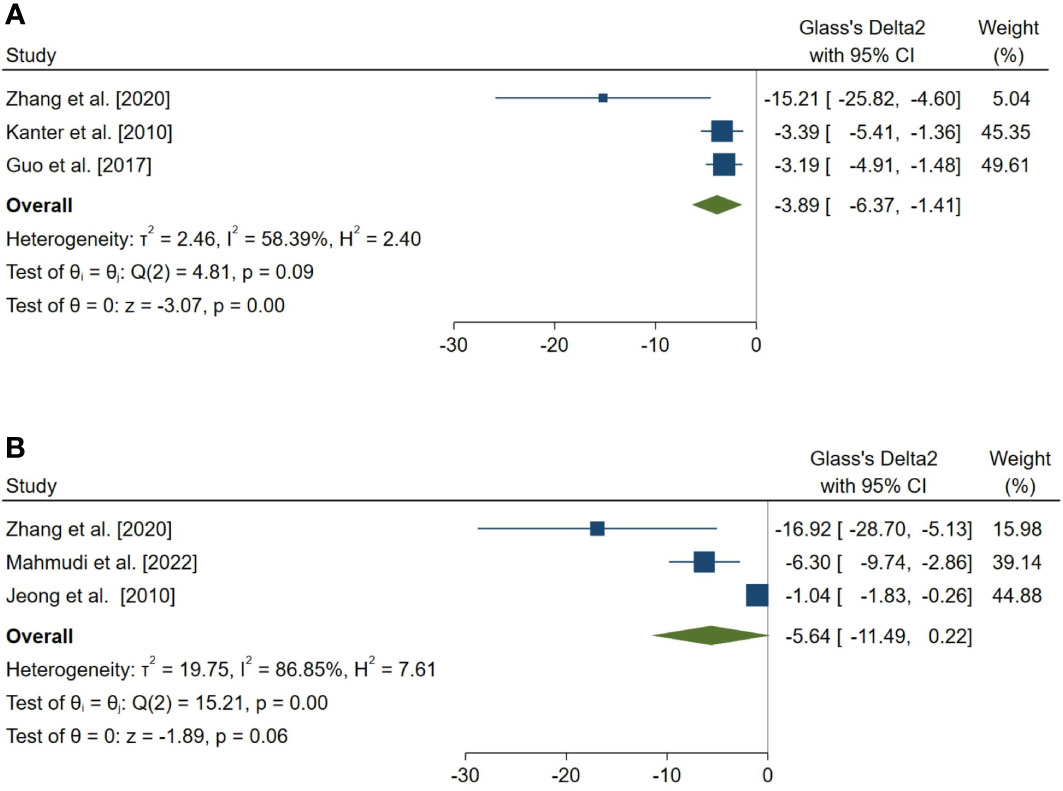
Figure 7 Forest plots for the overall pooled effects sizes of exploratory outcomes, including (A) the percentage of tubules with TUNEL-positive cells (n=3) and (B) the number of TUNEL-positive cells per tubule (n=3). TUNEL, Terminal deoxynucleotidyl transferase dUTP nick end labeling.
3.3.1 Sperm parameters
The combined SMDs for the effect of melatonin therapy on total sperm count (SMD = 2.358, 95% CI: 0.285 to 4.431, p-value = 0.026), forward progressive motility (SMD = 5.907, 95% CI: 4 to 7.814, p-value <0.001), normal sperm morphology (SMD = 3.312, 95% CI: 1.516 to 5.108, p-value <0.001), and sperm viability (SMD = 2.116, 95% CI: 0.291 to 3.941, p-value = 0.023) were statistically significant. On the other hand, total sperm motility was not significantly affected by melatonin therapy (SMD = -1.893, 95% CI: -9.076 to 5.29, p-value = 0.605). Between study heterogeneity was considerable for sperm viability (I2 = 82.5% and p-value for Q test <0.001), total sperm count (I2 = 87.55% and p-value for Q test <0.001), total sperm motility (I2 = 92.45% and p-value for Q test <0.001), and normal sperm morphology (I2 = 84.78% and p-value for Q test <0.001) and moderate for forward progressive sperm motility (I2 = 45.57% and p-value for Q test = 0.138).
Johnsen’s score was examined and reported in 14 studies, of which, 11 were included in the meta-analysis. The combined SMD for the effect of melatonin therapy on Johnsen’s mean testicular biopsy score was (SMD = 3.322, 95% CI: 1.759 to 4.885, p-value <0.001). Substantial between study heterogeneity was observed in the analysis.
3.3.2 Reproductive hormone profile
The overall pooled SMDs for the effect of melatonin therapy on rodents’ reproductive hormones were (SMD = 1.012, 95% CI: -1.991 to 4.015, p-value = 0.509) and (SMD = 2.659, 95% CI: 1.296 to 4.022, p-value <0.001) for serum testosterone and Inhibin-B levels, respectively. Substantial between study heterogeneity was observed in both analyses (I2 = 89.03% and p-value for Q test <0.001 and I2 = 65.95% and p-value for Q test = 0.032 for serum testosterone and Inhibin-B levels, respectively).
3.3.3 Markers of oxidative stress in testicular tissue
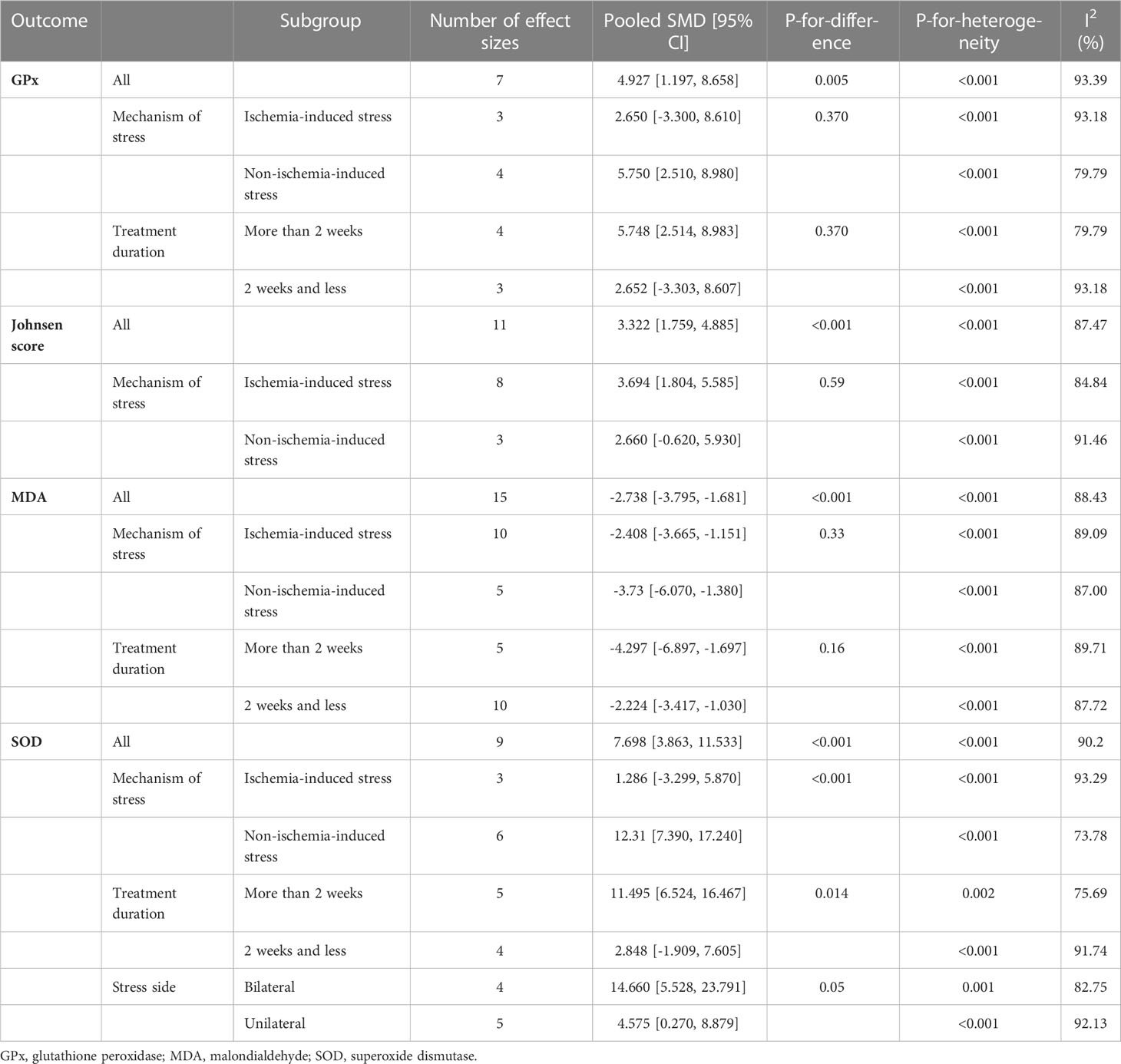
The pooled SMDs for the effect of melatonin therapy on testicular tissue antioxidant activity were for SOD (SMD = 7.698, 95% CI: 3.863 to 11.533, p-value <0.001), malondialdehyde (SMD = -2.738, 95% CI: -3.795 to -1.681, p-value <0.001), GPx (SMD = 4.927, 95% CI: 1.197 to 8.658, p-value = 0.005), and catalase (CAT, SMD = 2.323, 95% CI: 0.42 to 4.226, p-value = 0.017) were. However, considerable between-study heterogeneity (I2 = 90.2% and p-value for Q test <0.001 for SOD, I2 = 88.43% and p-value for Q test <0.001 for MDA, I2 = 93.39% and p-value for Q test <0.001 for GPx, and I2 = 83.72% and p-value for Q test <0.001 for CAT) was observed in testicular tissue antioxidant activities.
3.3.4 Body weight and testicular somatic indices
The pooled SMDs for the effect of melatonin therapy on body weight and testicular somatic indices were not statistically significant; for final body weight (SMD = 2.076, 95% CI: -1.438 to 5.59, p-value = 0.247), final total testis weight (SMD = 3.745, 95% CI: -6.905 to 14.396, p-value = 0.491), testis to body weight ratio (SMD = 0.036, 95% CI: -1.089 to 1.162, p-value = 0.95), and seminiferous tubular diameter (SMD = 0.818, 95% CI: -0.018 to 1.655, p-value = 0.055). The between-study heterogeneity was substantial to considerable for body weight and testicular somatic indices; for final body weight (I2 = 92.57% and p-value for Q test <0.001), final total testis weight (I2 = 93.88% and p-value for Q test <0.001), testis to body weight ratio (I2 = 73.12% and p-value for Q test = 0.005), and seminiferous tubular diameter (I2 = 62% and p-value for Q test 0.048).
3.4 Exploratory outcomes
In a complementary analysis, we assessed the effect of melatonin therapy on TUNEL assay of seminiferous tubular cells (54, 65, 66, 68, 83, 85). The pooled SMD for the effect of melatonin therapy on the percentage of tubules with TUNEL-positive cells (SMD = -3.886, 95% CI: -6.365 to -1.406, p-value = 0.002) was statistically significant. On the other hand, this measure was not statistically significant for the number of TUNEL-positive cells per tubule (SMD = -5.636, 95% CI: -11.495 to 0.222, p-value = 0.059).
3.5 Publication bias
Two outcomes were eligible for analysis of publication bias. The funnel plots regarding the effects of melatonin therapy on testicular tissue MDA activity and Johnsen have been analyzed for publication bias. Both plots lack symmetry on visual inspection, suggesting a high risk of publication bias. Egger’s regression and Begg’s tests also showed consistent results: p-value <0.001 for both tests in both outcomes. The funnel plots are presented in Supplementary Material 4.
3.6 Subgroup analyses
Subgroup analyses were done on the side of induction of stress (unilateral vs. bilateral), mechanism of stress (ischemic vs. non-ischemic injuries), and duration of melatonin therapy (> 2 weeks vs. ≤ 2 weeks). Subgroup analyses revealed significant between-group differences for SOD based on the mechanism of stress and duration of melatonin therapy (p-value <0.001 and 0.01, respectively). All the meta-analyses are stratified in Table 2.
3.7 Risk of bias assessment
For each domain, studies scored 1 if they were assessed as low risk using SYRCLE tool. Studies scored between 1 and 4. All the studies were labeled as unclear risk on random sequence generation, allocation concealment, random housing, blinding, and random outcome assessment. For other sources of bias, all the studies were assessed as low risk. Studies were low risk based on baseline characteristics (n=16), incomplete outcome data (n=4), and selective outcome reporting (n= 35). All the details are presented in Figure 8 and Supplementary Material 5.
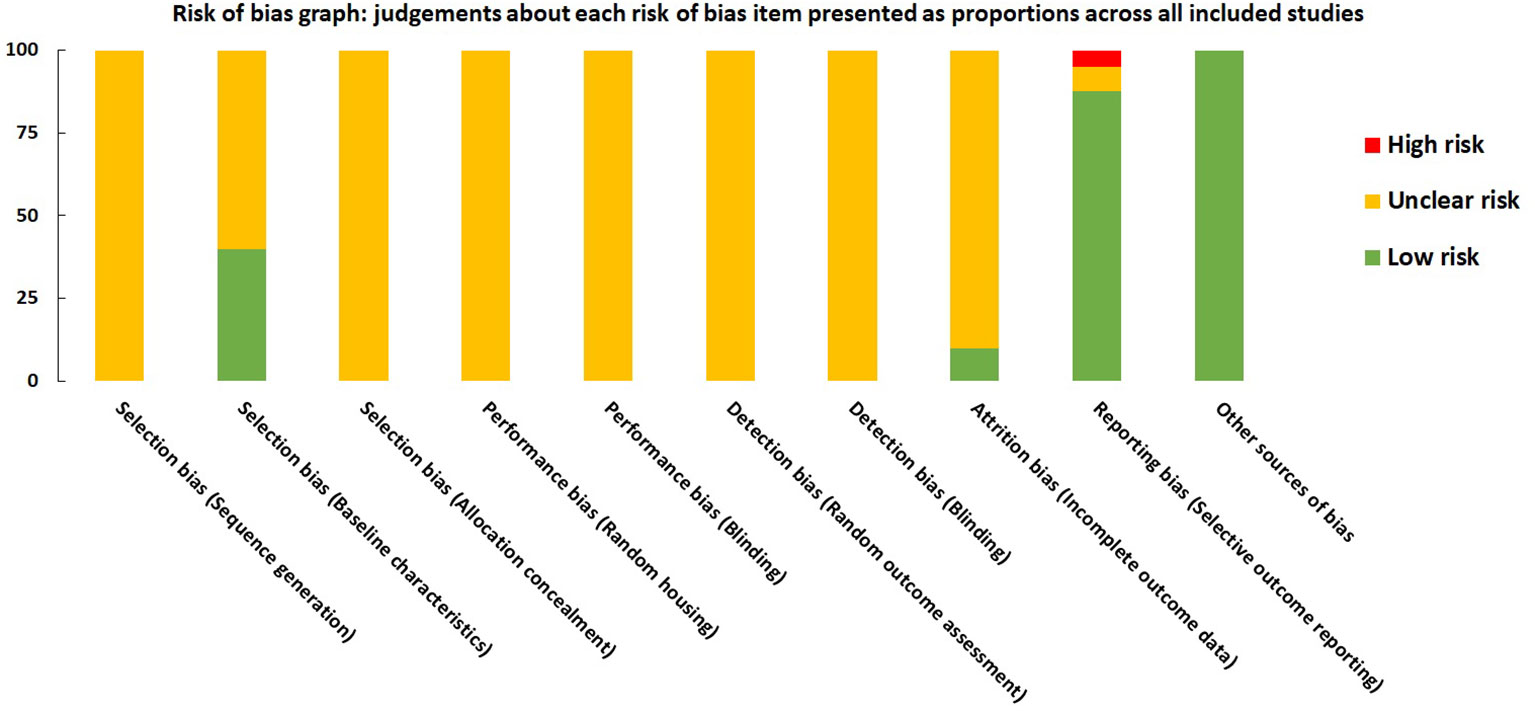
Figure 8 Risk of bias graph on judgements about each risk of bias item presented as proportions across all included studies (n=41).
4 Discussion
Our results suggest the beneficial effects of melatonin on male rodents’ infertility caused by physical testicular injuries. We hypothesized that melatonin might exert this favorable effect by influencing the reproductive system, inducing antioxidant defense, and suppressing apoptosis. Furthermore, subgroup analysis revealed a stronger impact of melatonin on testicular SOD in non-ischemia-induced injuries and studies with longer intervention duration compared to the comparison groups. Treatment duration and mechanism of stress were detected as possible sources of heterogeneity for testicular SOD and GPx analysis. In the following, we discussed the possible related mechanisms.
4.1 Effects on sperm and testis parameters
Male infertility is commonly caused by ejaculatory dysfunction, no or low sperm count, or abnormal morphology or motility of the sperms (86). In the present study, melatonin improved spermatogenesis, total sperm count, sperm viability and morphology, and forward progression sperm motility in animals with infertility induced by physical injuries. However, total testicular weight, testis to body weight ratio, sperm motility, and seminiferous tubule diameter were not affected by melatonin administration. Our results were partially in line with previous studies on other models of male infertility. Melatonin protects the reproductive system against the toxicity of chemotherapeutic agents (87). Zi et al. reported the favorable effects of melatonin on doxorubicin-induced impaired spermatogenesis and sperm quality, except for sperm motility (88). Melatonin also suppressed bleomycin, etoposide, and cisplatin-induced testicular damage by ameliorating histopathological alterations of testes, testicular weight, and sperm motility, viability, and morphology, but not sperm count and its progressive motility (89). These discrepancies may be due to the differences in the type of animals, experimental models of male infertility, and dose, duration, and route of melatonin administration. Melatonin may directly affect the male reproductive system by interacting with its receptors on the testes, epididymis, or spermatozoa (90, 91). Melatonin increased the expression of spermatogenesis-related genes and led bovine Sertoli cells to transit from G1 to S phase (92). In addition to the experimental studies, lower serum and seminal melatonin levels in patients with idiopathic oligoasthenoteratozoospermia compared to the fertile men and a significant positive correlation between serum melatonin and sperm motility were detected in a previous case-control study (93). Due to the conflicting evidence (55, 91), more studies should be performed to clarify the effects of melatonin on sperm and testis parameters.
4.2 Effects on reproductive hormone levels
Testosterone has fundamental roles in male reproductive system development and spermatogenesis (94). Our results revealed that melatonin does not affect plasma testosterone levels, similar to some reports on other animal models of male infertility (89, 95). Nonetheless, transgenic mammals with endogenously elevated melatonin levels revealed elevated testosterone production (96). Evidence also suggests that melatonin induces testosterone activation and inhibits its destruction (97). Although, the low number of included studies may conceal the possible influence of melatonin on testosterone; consequently, further studies are needed to address the issue.
The serum level of inhibin-B, a hormone produced primarily by the Sertoli cells of the testes, is a potential marker of testicular function and spermatogenesis (98, 99). Our findings demonstrated the improvement of inhibin-B levels following melatonin administration, which may be because of melatonin’s beneficial effects on preserving Sertoli cells against injuries. In this regard, melatonin upregulated inhibin-B expression in bovine Sertoli cells via its MT1 and MT2 receptors (92). In a randomized controlled trial by Lu et al., melatonin supplementation increased the peripheral blood inhibin B levels following varicocelectomy that could be attributed to its effects on spermatogenesis function (100).
4.3 Effects on oxidant/antioxidant balance
Testicular oxidative stress could be derived from intrinsic etiologies, including testicular torsion, varicocele, cryptorchidism, infection, inflammation, or aging (101). Some extrinsic factors, such as intense exercise, can also raise oxidative stress levels in the testes (69). Sperms are extremely sensitive to oxidative damage due to the high levels of polyunsaturated fatty acids in their membranes and the low content of enzymatic antioxidants. Oxidative stress is directly correlated with increased apoptosis in germ cells and mature spermatozoa by changing caspase activity and disrupting mitochondrial membrane (101). Therefore, multiple studies indicated the association between oxidative stress and abnormal sperm count, motility, viability, morphology, DNA integrity, and fertilization ability (5, 101, 102). According to our results, melatonin administration significantly increased the SOD, GPx, and CAT activities, essential enzymatic antioxidants, and reduced MDA levels, as a lipid peroxidation product, in the testicular tissue of animals. Antioxidant properties of melatonin are reported in previous literature. Melatonin could directly scavenge oxidants and indirectly increase enzymatic antioxidant levels (103). Morvaridzadeh et al. conducted a meta-analysis of randomized controlled trials on patients with a different health condition. They reported increased total antioxidant capacity, glutathione levels, SOD, GPx, and glutathione reductase, but not CAT activities, and reduced MDA levels following melatonin supplementation (104). Melatonin could upregulate the antioxidant nuclear factor erythroid 2-related factor 2 (Nrf2)/heme oxygenase-1 (HO-1) signaling pathway in the damaged testes (83). This pathway induces the transcription of antioxidant proteins and leads to ROS clearance (105). Melatonin may also exert its beneficial effects through upregulating micro-RNA-34a/silent information regulator 1 (SIRT1)/forkhead transcription factors-class O (type1) (FOXO1) epigenetic axis (60). This pathway stimulates antioxidants’ expression, inhibits pro-inflammatory pathways, decreases apoptosis, improves mitochondrial biogenesis, repairs cell damage, and prevents cells’ dysfunction and infertility (106). Therefore, the effects of melatonin on other oxidative stress-related conditions should be investigated.
4.4 Effects on apoptosis
Apoptosis, the programmed cell death, takes place during normal spermatogenesis. However, physical testicular injuries could elevate germ cell apoptosis and reduce seminiferous tubule diameter and sperm count (60, 68, 107, 108). We observed that melatonin decreases apoptotic germ cells and ameliorates the detrimental impact of physical injuries on the testes. Consistently, melatonin had protective effects against apoptotic cell damage caused by radiation (109, 110) or drugs (89) in the testes in other studies. Melatonin also inhibited endoplasmic reticulum stress-induced apoptosis in reproductive tissues, and therefore exerted protective effects on diabetes-related reproductive impairment (111). The anti-apoptotic effect of melatonin could be mediated via increasing Bcl-2 gene expression, an anti-apoptotic gene marker, and lowering mitochondrial membrane potentials and pro-apoptotic Bcl-2-associated X protein (BAX), P53, caspase 3, and nuclear factor-κB (NF-κB) expression (83, 89, 112, 113). Melatonin also activated the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) pathway in frozen-thawed human sperms (114). Activation of this pathway causes increased sperm motility, suppressed apoptotic cascade and caspase, and decreased membrane permeability and ROS production in spermatozoa (115). Furthermore, melatonin protected human spermatozoa from H2O2-induced DNA fragmentation and apoptosis via MT1 and extracellular signal–regulated kinase signaling (116). Some other studies also support the anti-apoptotic effects of melatonin on injuries to the female reproductive system (117, 118), heart (113), kidney (119), liver (120), and nervous tissue (121). Due to the limited evidence, we recommend that future studies assess the mechanisms related to the anti-apoptotic effects of melatonin in physical injuries to male testicular tissue.
4.5 Strengths and limitations
To the best of our knowledge, this is the first systematic review and meta-analysis on the protective effects of melatonin against physical injuries to rodents’ testicular tissue. Animal models are indispensable tools for assessing new agents’ effectiveness and side effects for disease management. However, they do not completely imitate human models. Therefore, the interpretation of our findings should be conducted with caution. High statistical heterogeneity, publication bias, and low quality of the eligible studies are other limitations of our meta-analysis. Between-study methodological heterogeneity was also found in our study due to the differences between animals’ characteristics, the dose of melatonin, the intervention schedule, and model of infertility induction. Furthermore, the low number of the included studies prohibited us from doing subgroup analysis for some variables to detect other sources of heterogeneity. Almost all the available studies that have utilized animal models to evaluate the effects of melatonin therapy against male infertility were on rodent subjects. None of the included studies have evaluated and reported possible adverse effects of melatonin therapy. Finally, there are other outcomes that could have been helpful in explaining the mechanisms behind the effects of exogenous melatonin on male rodents’ reproductive system such as dihydrotestosterone, corticosterone, testicular and general immunity which were not investigated by the included studies.
5 Conclusion and future direction
In conclusion, melatonin protects against male infertility caused by physical injuries through direct effects on rodent male reproductive system cells, inducing antioxidant defense, and inhibiting apoptosis. More well-designed animal studies should be performed to clarify other mechanisms underlying these effects. To avoid methodological variations, future studies should be more harmonized regarding the mechanism of injury and design of treatment to develop consensus on definitions and methods in this field of research. Also, we recommend employing non-rodent subjects to assess generalizability of the results of this review. Discussions should be made about concerns on melatonin therapy with doses that are needed for anti-infertility effects. Future studies should consider detecting possible adverse effects and other outcomes such as dihydrotestosterone, corticosterone in their protocols.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
NA and NDE conceptualized the study. AS, NDE, SS-Z, and ARS designed the study. NDE, AS, and SS-Z searched databases. NDE, NM, and SP screened the records. NDE, ET, FSS, and AS extracted the data. NDE and AS performed quality assessment. AS, SS-Z, and ARS performed meta-analysis. AS, SS-Z, SD, NH, and ZM provided the draft of the manuscript. AS visualized the data. NA and ARS supervised the work. All authors contributed to the article and approved the final version. AS and NDE have contributed equally to this work and share first authorship. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1123999/full#supplementary-material
References
1. Babakhanzadeh E, Nazari M, Ghasemifar S, Khodadadian A. Some of the factors involved in male infertility: a prospective review. Int J Gen Med (2020) 13:29. doi: 10.2147/IJGM.S241099
2. Sahoo DK, Roy A. Compromised rat testicular antioxidant defence system by hypothyroidism before puberty. Int J Endocrinol (2012) 2012:637825. doi: 10.1155/2012/637825
3. Aitken RJ, Baker MA, Nixon B. Are sperm capacitation and apoptosis the opposite ends of a continuum driven by oxidative stress? Asian J Androl (2015) 17(4):633. doi: 10.4103/1008-682X.153850
4. Shim E, Lee JW, Park H, Zuccarello GC, Kim GH. Hydrogen peroxide signalling mediates fertilization and post-fertilization development in the red alga bostrychia moritziana. J Exper Botany (2022) 73(3):727–41. doi: 10.1093/jxb/erab453
5. Agarwal A, Virk G, Ong C, Du Plessis SS. Effect of oxidative stress on male reproduction. wjomsh (2014) 32(1):1–17. doi: 10.5534/wjmh.2014.32.1.1
6. Torres-Arce E, Vizmanos B, Babio N, Marquez-Sandoval F, Salas-Huetos AJB. Dietary antioxidants in the treatment of male infertility: Counteracting oxidative stress. Biology (2021) 10(3):241. doi: 10.3390/biology10030241
7. Agarwal A, Selvam MKP, Baskaran S, Finelli R, Leisegang K, Barbăroşie C, et al. Highly cited articles in the field of male infertility and antioxidants: A scientometric analysis. World J Mens World (2021) 39(4):760. doi: 10.5534/wjmh.200181
8. Sahoo DK, Roy A, Chainy GB. Protective effects of vitamin e and curcumin on l-thyroxine-induced rat testicular oxidative stress. J Am Chem Soc (2008) 176(2-3):121–8. doi: 10.1016/j.cbi.2008.07.009
9. Lerner AB, Case JD, Takahashi Y, Lee TH, Mori W. Isolation of melatonin, the pineal gland factor that lightens melanocytes1. J Am Chem Soc (1958) 80(10):2587–. doi: 10.1021/ja01543a060
10. Slominski RM, Reiter RJ, Schlabritz-Loutsevitch N, Ostrom RS, Slominski AT. Melatonin membrane receptors in peripheral tissues: distribution and functions. Endocrinol c. (2012) 351(2):152–66. doi: 10.1016/j.mce.2012.01.004
11. Singh M, Jadhav HR. Melatonin: functions and ligands. Drug Discov Today (2014) 19(9):1410–8. doi: 10.1016/j.drudis.2014.04.014
12. Bhattacharya K, Sengupta P, Dutta S. Role of melatonin in male reproduction. Asian Pacific J Reprod (2019) 8(5):211. doi: 10.4103/2305-0500.268142
13. Ahmad R, Haldar C. Effect of intra-testicular melatonin injection on testicular functions, local and general immunity of a tropical rodent funambulus pennanti. Endocrine (2010) 37(3):479–88. doi: 10.1007/s12020-010-9331-7
14. Hacışevki A, Baba B. An overview of melatonin as an antioxidant molecule: a biochemical approach. clin approaches p (2018) 5:59–85. doi: 10.5772/intechopen.79421
15. Rodriguez C, Mayo JC, Sainz RM, Antolín I, Herrera F, Martín V, et al. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res (2004) 36(1):1–9. doi: 10.1046/j.1600-079X.2003.00092.x
16. Chainy GBN, Sahoo DK. Hormones and oxidative stress: an overview. Free Radic Res (2020) 54(1):1–26. doi: 10.1080/10715762.2019.1702656
17. Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre-Jimenez M, Qin L. Melatonin as an antioxidant: under promises but over delivers. J Pineal Res (2016) 61(3):253–78. doi: 10.1111/jpi.12360
18. Sun T, Song L, Ma J, Yu H, Zhou S, Wang S, et al. Melatonin and its protective role against male reproductive toxicity induced by heavy metals, environmental pollutants, and chemotherapy: A review. BIOCELL (2020) 44(4):479. doi: 10.32604/biocell.2020.011675
19. Sahoo DK, Chainy GBN. Hormone-linked redox status and its modulation by antioxidants. Vitamins and Hormones. Academic Press (2023). doi: 10.1016/bs.vh.2022.10.007
20. Ma X, Idle JR, Krausz KW, Gonzalez FJ. Metabolism of melatonin by human cytochromes p450. Drug Metab Dispos (2005) 33(4):489–94. doi: 10.1124/dmd.104.002410
21. Rodriguez C, Mayo JC, Sainz RM, Antolín I, Herrera F, Martín V, et al. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res (2004) 36(1):1–9. doi: 10.1046/j.1600-079X.2003.00092.x
22. Milczarek R, Hallmann A, Sokołowska E, Kaletha K, Klimek J. Melatonin enhances antioxidant action of α-tocopherol and ascorbate against NADPH-and iron-dependent lipid peroxidation in human placental mitochondria. J Pineal Res (2010) 49(2):149–55. doi: 10.1111/j.1600-079X.2010.00779.x
23. Deng S-L, Wang Z-P, Jin C, Kang X-L, Batool A, Zhang Y, et al. Melatonin promotes sheep leydig cell testosterone secretion in a co-culture with sertoli cells. Theriogenology (2018) 106:170–7. doi: 10.1016/j.theriogenology.2017.10.025
24. Yu K, Deng S-L, Sun T-C, Li Y-Y, Liu Y-X. Melatonin regulates the synthesis of steroid hormones on male reproduction: a review. Molecules (2018) 23(2):447. doi: 10.3390/molecules23020447
25. Mirhoseini M, Talebpour Amiri F, Karimpour Malekshah AA, Rezanejad Gatabi Z, Ghaffari E. Protective effects of melatonin on testis histology following acute torsion-detorsion in rats. Int J Reprod Biomed (2017) 15(3):141–6. doi: 10.29252/ijrm.15.3.141
26. Mirhoseini M, Gatabi ZR, Saeedi M, Morteza-Semnani K, Amiri FT, Kelidari HR, et al. Protective effects of melatonin solid lipid nanoparticles on testis histology after testicular trauma in rats. Res Pharma Sci (2019) 14(3):201. doi: 10.4103/1735-5362.258486
27. Rocha C, Rato L, Martins A, Alves M, Oliveira P. Melatonin and male reproductive health: relevance of darkness and antioxidant properties. Current Mol Med (2015) 15(4):299–311. doi: 10.2174/1566524015666150505155530
28. Sun T-C, Li H-Y, Li X-Y, Yu K, Deng S-L, Tian LJC. Protective effects of melatonin on male fertility preservation and reproductive system. Cryobiology (2020) 95:1–8. doi: 10.1016/j.cryobiol.2020.01.018
29. Liu J, Clough SJ, Hutchinson AJ, Adamah-Biassi EB, Popovska-Gorevski M, Dubocovich ML, et al. MT1 and MT2 melatonin receptors: a therapeutic perspective. Ann Rev Pharmacol Toxicol (2016) 56:361. doi: 10.1146/annurev-pharmtox-010814-124742
30. Navid S, Abbasi M, Hoshino Y. The effects of melatonin on colonization of neonate spermatogonial mouse stem cells in a three-dimensional soft agar culture system. Stem Cell Res Ther (2017) 8(1):1–10. doi: 10.1186/s13287-017-0687-y
31. Navid S, Rastegar T, Baazm M, Alizadeh R, Talebi A, Gholami K, et al. In vitro effects of melatonin on colonization of neonate mouse spermatogonial stem cells. Stem Cell Res Ther (2017) 63(6):370–81. doi: 10.1186/s13287-017-0687-y
32. Zhang G, Yang W, Jiang F, Zou P, Zeng Y, Ling X, et al. PERK regulates Nrf2/ARE antioxidant pathway against dibutyl phthalate-induced mitochondrial damage and apoptosis dependent of reactive oxygen species in mouse spermatocyte-derived cells. Toxicol Let (2019) 308:24–33. doi: 10.1016/j.toxlet.2019.03.007
33. Jockers R, Delagrange P, Dubocovich ML, Markus RP, Renault N, Tosini G, et al. Update on melatonin receptors: IUPHAR review 20. Bri J Pharmacol (2016) 173(18):2702–25. doi: 10.1111/bph.13536
34. Reiter RJ, Rosales-Corral S, Tan DX, Jou MJ, Galano A, Xu BJC, et al. Melatonin as a mitochondria-targeted antioxidant: one of evolution’s best ideas. Cell Mol Life Sci (2017) 74(21):3863–81. doi: 10.1007/s00018-017-2609-7
35. Reiter RJ, Tan DX, Rosales-Corral S, Galano A, Zhou XJ, Xu BJM. Mitochondria: central organelles for melatonin′ s antioxidant and anti-aging actions. Molecules (2018) 23(2):509. doi: 10.3390/molecules23020509
36. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (2021) 372:n71. doi: 10.1136/bmj.n71
37. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev (2016) 5(1):210. doi: 10.1186/s13643-016-0384-4
38. Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE’s risk of bias tool for animal studies. BMC Med Res methodol (2014) 14:43. doi: 10.1186/1471-2288-14-43
39. DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin Trials. (1986) 7(3):177–88. doi: 10.1016/0197-2456(86)90046-2
40. Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA eds. Cochrane handbook for systematic reviews of interventions. 2nd ed. Chichester (UK: John Wiley & Sons (2019).
41. Liu ZM, Zhu WP, Huang DP, Bai PM. [Effects of melatonin on oxidative stress and apoptosis-related gene signaling pathways following testicular torsion in rats]. Zhonghua Nan Ke Xue. (2019) 25(5):309–14.
42. Yurtçu M, Abasiyanik A, Avunduk M, Karagözoğlu E, Abasiyanik F. The effects of one dose and seven days’ managements of melatonin and steroid to prevent ischemia-reperfusion injury in testicular torsion. Turkiye Klinikleri J Med Sci (2005) 25:496–500.
43. Yurtçu M, Abasiyanik A, Gökçe R, Avinduk MC, Özdamar MY. Investigation of the effects of melatonin and steroid (st) in preventing of testicular atrophy after surgical management performed with fowler-stephens procedure of intraabdominal testis in late period. Cocuk Cerrahisi Dergisi (2008) 22:33–8.
44. Duru FIO, Oshiozokhai Y, Noronha CC, Okanlawon A. Alterations in morphometry and malondialdehyde levels in adult sprague-dawley rat testes in three obstructive vasectomy models: Effect of melatonin. Asian J Pharm Clin Res (2011) 4:27–30.
45. Duru FI, Olabiyi O, Noronha CC, Akinwande AI, Okanlawon AO. Brief ischaemia reduces testicular lipid peroxidation following subsequent ischaemia: an evidence for ischaemic preconditioning. Nig Q J Hosp Med (2008) 18(3):149–52. doi: 10.4314/nqjhm.v18i3.45016
46. Aktaş A, Tuncer M, Yıldırım A, Nergiz Y, Akkus M. Protective effects of melatonin on testicular torsion and detorsion damage in sprague-dawley rats. Int J Morphol (2011) 29:7–15. doi: 10.4067/S0717-95022011000100001
47. Bustos-Obregon E, Sánchez R, Ramos B, Torres-Diaz L. Rat spermatogenesis damage in intermittent hypobaric hypoxia and the protective role of melatonin. II: Testicular parameters. Int J Morphol (2010) 28:537–47. doi: 10.4067/S0717-95022010000200034
48. Chen YT, Chuang FC, Yang CC, Chiang JY, Sung PH, Chu YC, et al. Combined melatonin-adipose derived mesenchymal stem cells therapy effectively protected the testis from testicular torsion-induced ischemia-reperfusion injury. Stem Cell Res Ther (2021) 12(1):370. doi: 10.1186/s13287-021-02439-x
49. Erdemir F, Parlaktaş BS, Özyurt H, Boztepe Ö, Atiş Ö, Şahin Ş. Antioxidant effect of melatonin in systemic circulation of rats after unilateral testicular torsion. Turkish J Med Sci (2008) 38:1–6.
50. Eşrefoğlu M, Gül M, Parlakpinar H, Acet A. Effects of melatonin and caffeic acid phenethyl ester on testicular injury induced by myocardial ischemia/reperfusion in rats. Fundam Clin Pharmacol (2005) 19(3):365–72. doi: 10.1111/j.1472-8206.2005.00331.x
51. Hartley R, Castro-Sánchez R, Ramos-Gonzalez B, Bustos-Obregón E. Rat spermatogenesis damage in intermittent hypobaric hypoxia and the protective role of melatonin: I cauda epididymal spermatozoa. Int J Morphol (2009) 27:1275–84. doi: 10.4067/S0717-95022009000400049
52. Kurcer Z, Hekimoglu A, Aral F, Baba F, Sahna E. Effect of melatonin on epididymal sperm quality after testicular ischemia/reperfusion in rats. Fertil Steril (2010) 93(5):1545–9. doi: 10.1016/j.fertnstert.2009.01.146
53. Kurcer Z, Oguz E, Ozbilge H, Baba F, Aksoy N, Celik N. Effect of melatonin on testicular ischemia/reperfusion injury in rats: is this effect related to the proinflammatory cytokines? Fertil Steril (2008) 89(5 Suppl):1468–73. doi: 10.1016/j.fertnstert.2007.04.065
54. Sekmenli T, Gunduz M, Öztürk B, Karabağlı P, Ciftci I, Tekin G, et al. The effects of melatonin and colchicine on ischemia-reperfusion injury in experimental rat testicular torsion model. J Pediatr Surg (2017) 52(4):582–6. doi: 10.1016/j.jpedsurg.2016.11.035
55. Vargas A, Bustos-Obregón E, Hartley R. Effects of hypoxia on epididymal sperm parameters and protective role of ibuprofen and melatonin. Biol Res (2011) 44(2):161–7. doi: 10.4067/S0716-97602011000200008
56. Yildirim A, Akkus M, Nergiz Y, Baran OP. The effect of melatonin on ductus epididymis. unilateral testicular torsion in rats. Saudi Med J (2007) 28(2):288–9.
57. Yuan XC, Wang P, Li HW, Wu QB, Zhang XY, Li BW, et al. Effects of melatonin on spinal cord injury-induced oxidative damage in mice testis. Andrologia (2017) 49(7). doi: 10.1111/and.12692
58. Saalu LC, Togun VA, Oyewopo AO, Raji Y. Artificial cryptorchidism and the moderating effect of melatonin (N-acetyl. 5 methoxy tryptamin) in sprague-dawley rats. J Appl Sci (2006) 6:2889–94. doi: 10.3923/jas.2006.2889.2894
59. Semercioz A, Baltaci AK, Mogulkoc R, Avunduk MC. Effect of zinc and melatonin on oxidative stress and serum inhibin-b levels in a rat testicular torsion-detorsion model. Biochem Genet (2017) 55(5-6):395–409. doi: 10.1007/s10528-017-9826-5
60. Abo El Gheit RE, Soliman NA, Nagla SA, El-Sayed RM, Badawi GA, Emam MN, et al. Melatonin epigenetic potential on testicular functions and fertility profile in varicocele rat model is mediated by silent information regulator 1. Br J Pharmacol (2022) 179(13):3363–81. doi: 10.1111/bph.15804
61. Asghari A, Akbari G, Meghdadi A, Mortazavi P. Effects of melatonin and metformin co-administration on testicular ischemia/reperfusion injury in rats. J Pediatr Urol (2016) 12(6):410.e1–.e7. doi: 10.1016/j.jpurol.2016.06.017
62. Duru FI, Noronha CC, Akinwande AI, Okanlawon AO. Effects of torsion, detorsion and melatonin on testicular malondialdehyde level. West Afr J Med (2007) 26(4):312–5. doi: 10.4314/wajm.v26i4.28333
63. Ekici S, Doğan Ekici AI, Öztürk G, Benli Aksungar F, Sinanoğlu O, Turan G, et al. Comparison of melatonin and ozone in the prevention of reperfusion injury following unilateral testicular torsion in rats. Urology (2012) 80(4):899–906. doi: 10.1016/j.urology.2012.06.049
64. Gul SS, Gurgul S, Uysal M, Erdemir F. The protective effects of pulsed magnetic field and melatonin on testis torsion and detorsion induced rats indicated by scintigraphy, positron emission Tomography/Computed tomography and histopathological methods. Urol J (2018) 15(6):387–96. doi: 10.22037/uj.v0i0.4404
65. Jeong SJ, Choi WS, Chung JS, Baek M, Hong SK, Choi H. Preventive effects of cyclosporine a combined with prednisolone and melatonin on contralateral testicular damage after ipsilateral torsion-detorsion in pubertal and adult rats. J Urol (2010) 184(2):790–6. doi: 10.1016/j.juro.2010.03.109
66. Kanter M. Protective effects of melatonin on testicular torsion/detorsion-induced ischemia-reperfusion injury in rats. Exp Mol Pathol (2010) 89(3):314–20. doi: 10.1016/j.yexmp.2010.07.006
67. Koksal M, Oğuz E, Baba F, Eren MA, Ciftci H, Demir ME, et al. Effects of melatonin on testis histology, oxidative stress and spermatogenesis after experimental testis ischemia-reperfusion in rats. Eur Rev Med Pharmacol Sci (2012) 16(5):582–8.
68. Mahmudi SAA, Ghasemi Hamidabadi H, Moayeri A, Nazm Bojnordi M, Zahiri M, Madani Z, et al. Melatonin ameliorates testes against forced treadmill exercise training on spermatogenesis in rats. Folia Med (Plovdiv). (2022) 64(1):75–83. doi: 10.3897/folmed.64.e57544
69. Minaii B, Moayeri A, Shokri S, Habibi Roudkenar M, Golmohammadi T, Malek F, et al. Melatonin improve the sperm quality in forced swimming test induced oxidative stress in nandrolone treated wistar rats. Acta Med Iran. (2014) 52(7):496–504.
70. Mirhoseini M, Rezanejad Gatabi Z, Saeedi M, Morteza-Semnani K, Talebpour Amiri F, Kelidari HR, et al. Protective effects of melatonin solid lipid nanoparticles on testis histology after testicular trauma in rats. Res Pharm Sci (2019) 14(3):201–8. doi: 10.4103/1735-5362.258486
71. Moayeri A, Mokhtari T, Hedayatpour A, Abbaszadeh HA, Mohammadpour S, Ramezanikhah H, et al. Impact of melatonin supplementation in the rat spermatogenesis subjected to forced swimming exercise. Andrologia (2018) 50(3). doi: 10.1111/and.12907
72. Olayaki LA, Alagbonsi IA, Abdulkadir HO, Idowu FO. Low dose of melatonin ameliorates cryptorchidism-induced spermatotoxicity in rats. J Anatom Soc India (2017) 66(1):67–71. doi: 10.1016/j.jasi.2017.05.010
73. Onur R, Semerciöz A, Orhan I, Yekeler H. The effects of melatonin and the antioxidant defence system on apoptosis regulator proteins (Bax and bcl-2) in experimentally induced varicocele. Urol Res (2004) 32(3):204–8. doi: 10.1007/s00240-004-0403-0
74. Ozturk A, Baltaci AK, Mogulkoc R, Ozturk B. The effect of prophylactic melatonin administration on reperfusion damage in experimental testis ischemia-reperfusion. Neuro Endocrinol Lett (2003) 24(3-4):170–2.
75. Parlaktas BS, Atilgan D, Ozyurt H, Gencten Y, Akbas A, Erdemir F, et al. The biochemical effects of ischemia-reperfusion injury in the ipsilateral and contralateral testes of rats and the protective role of melatonin. Asian J Androl (2014) 16(2):314–8. doi: 10.4103/1008-682X.122202
76. Sahna E, Türk G, Atessahin A, Yilmaz S, Olmez E. Remote organ injury induced by myocardial ischemia and reperfusion on reproductive organs, and protective effect of melatonin in male rats. Fertil Steril (2007) 88(1):188–92. doi: 10.1016/j.fertnstert.2006.11.068
77. Semercioz A, Onur R, Ogras S, Orhan I. Effects of melatonin on testicular tissue nitric oxide level and antioxidant enzyme activities in experimentally induced left varicocele. Neuro Endocrinol Lett (2003) 24(1-2):86–90.
78. Yurtçu M, Abasiyanik A, Avunduk MC, Muhtaroğlu S. Effects of melatonin on spermatogenesis and testicular ischemia-reperfusion injury after unilateral testicular torsion-detorsion. J Pediatr Surg (2008) 43(10):1873–8. doi: 10.1016/j.jpedsurg.2008.01.065
79. Yurtçu M, Abasiyanik A, Biçer S, Avunduk MC. Efficacy of antioxidant treatment in the prevention of testicular atrophy in experimental testicular torsion. J Pediatr Surg (2009) 44(9):1754–8. doi: 10.1016/j.jpedsurg.2008.11.043
80. Abasiyanik A, Dağdönderen L. Beneficial effects of melatonin compared with allopurinol in experimental testicular torsion. J Pediatr Surg (2004) 39(8):1238–41. doi: 10.1016/j.jpedsurg.2004.04.018
81. Gurbilek M, Vatansev H, Gültekin F, Dilsiz A, Vatansev C, Aköz M. Prevention of testicular damage by free radical scavengers after acute experimental torsion. Biomed Res (2000) 11:315–9.
82. Soma H, Mrinmoy S, Sananda D, Prasanta G, Sujay KB, Debasish B, et al. Melatonin ameliorates heat stress induced dysregulation of testicular function in wistar rat by restoring tissue health, hormone and antioxidant status and modulating heat shock protein expression. Int J Life Sci Pharma Res (2020) 10(5):31–43. doi: 10.22376/ijpbs/lpr.2020.10.5.L31-43
83. Guo Y, Sun J, Li T, Zhang Q, Bu S, Wang Q, et al. Melatonin ameliorates restraint stress-induced oxidative stress and apoptosis in testicular cells via NF-κB/iNOS and Nrf2/HO-1 signaling pathway. Sci Rep (2017) 7(1):9599. doi: 10.1038/s41598-017-09943-2
84. Qin DZ, Cai H, He C, Yang DH, Sun J, He WL, et al. Melatonin relieves heat-induced spermatocyte apoptosis in mouse testes by inhibition of ATF6 and PERK signaling pathways. Zool Res (2021) 42(4):514–24. doi: 10.24272/j.issn.2095-8137.2021.041
85. Zhang P, Zheng Y, Lv Y, Li F, Su L, Qin Y, et al. Melatonin protects the mouse testis against heat-induced damage. Mol Hum Reprod (2020) 26(2):65–79. doi: 10.1093/molehr/gaaa002
86. Reiter RJ, Tan D-X, Galano A. Melatonin reduces lipid peroxidation and membrane viscosity. Front Media SA (2014) 5:377. doi: 10.3389/fphys.2014.00377
87. Haghi-Aminjan H, Asghari MH, Farhood B, Rahimifard M, Hashemi Goradel N, Abdollahi M. The role of melatonin on chemotherapy-induced reproductive toxicity. J Pharm Pharmacol (2018) 70(3):291–306. doi: 10.1111/jphp.12855
88. Zi T, Liu Y, Zhang Y, Wang Z, Wang Z, Zhan S, et al. Protective effect of melatonin on alleviating early oxidative stress induced by DOX in mice spermatogenesis and sperm quality maintaining. Reprod Biol Endocrinol (2022) 20(1):1–11. doi: 10.1186/s12958-022-00977-4
89. Moradi M, Goodarzi N, Faramarzi A, Cheraghi H, Hashemian AH, Jalili C. Melatonin protects rats testes against bleomycin, etoposide, and cisplatin-induced toxicity via mitigating nitro-oxidative stress and apoptosis. Biomed Pharmacother (2021) 138:111481. doi: 10.1016/j.biopha.2021.111481
90. Izzo G, Francesco A, Ferrara D, Campitiello MR, Serino I, Minucci S, et al. Expression of melatonin (MT1, MT2) and melatonin-related receptors in the adult rat testes and during development. Zygote (2010) 18(3):257–64. doi: 10.1017/S0967199409990293
91. Gwayi N, Bernard R. The effects of melatonin on sperm motility in vitro in wistar rats. Andrologia (2002) 34(6):391–6. doi: 10.1046/j.1439-0272.2002.00522.x
92. Yang W-C, Tang K-Q, Fu C-Z, Riaz H, Zhang Q, Zan L-S. Melatonin regulates the development and function of bovine sertoli cells via its receptors MT1 and MT2. Anim Reprod Sci (2014) 147(1-2):10–6. doi: 10.1016/j.anireprosci.2014.03.017
93. Hassan MH, El−Taieb MA, Fares NN, Fayed HM, Toghan R, Ibrahim HM. Men with idiopathic oligoasthenoteratozoospermia exhibit lower serum and seminal plasma melatonin levels: Comparative effect of night−light exposure with fertile males. Exp Ther Med (2020) 20(1):235–42. doi: 10.3892/etm.2020.8678
94. Wang R-S, Yeh S, Tzeng C-R, Chang C. Androgen receptor roles in spermatogenesis and fertility: lessons from testicular cell-specific androgen receptor knockout mice. Endocr Rev (2009) 30(2):119–32. doi: 10.1210/er.2008-0025
95. Bahrami N, Goudarzi M, Hosseinzadeh A, Sabbagh S, Reiter RJ, Mehrzadi S. Evaluating the protective effects of melatonin on di (2-ethylhexyl) phthalate-induced testicular injury in adult mice. Biomed Pharmacother (2018) 108:515–23. doi: 10.1016/j.biopha.2018.09.044
96. Yang M, Guan S, Tao J, Zhu K, Lv D, Wang J, et al. Melatonin promotes male reproductive performance and increases testosterone synthesis in mammalian leydig cells. Biol Reprod (2021) 104(6):1322–36. doi: 10.1093/biolre/ioab046
97. Cipolla-Neto J, Amaral FG, Soares JM Jr., Gallo CC, Furtado A, Cavaco JE, et al. The crosstalk between melatonin and sex steroid hormones. Neuroendocrinology (2022) 112(2):115–29. doi: 10.1159/000516148
98. Kumanov P, Nandipati K, Tomova A, Agarwal A. Inhibin b is a better marker of spermatogenesis than other hormones in the evaluation of male factor infertility. Fertil steril (2006) 86(2):332–8. doi: 10.1016/j.fertnstert.2006.01.022
99. Jankowska K, Suszczewicz N, Rabijewski M, Dudek P, Zgliczyński W, Maksym RB. Inhibin-b and FSH are good indicators of spermatogenesis but not the best indicators of fertility. Life (2022) 12(4):511. doi: 10.3390/life12040511
100. Lu XL, Liu JJ, Li JT, Yang QA, Zhang JM. Melatonin therapy adds extra benefit to varicecelectomy in terms of sperm parameters, hormonal profile and total antioxidant capacity: A placebo-controlled, double-blind trial. Andrologia (2018) 50(6):e13033. doi: 10.1111/and.13033
101. Ko EY, Sabanegh ES Jr, Agarwal A. Male Infertility testing: reactive oxygen species and antioxidant capacity. Fertil steril (2014) 102(6):1518–27. doi: 10.1016/j.fertnstert.2014.10.020
102. Agarwal A, Sharma RK, Sharma R, Assidi M, Abuzenadah AM, Alshahrani S, et al. Characterizing semen parameters and their association with reactive oxygen species in infertile men. Reprod Biol Endocrinol (2014) 12(1):1–9. doi: 10.1186/1477-7827-12-33
103. Reiter RJ, Manchester LC, Tan D-X. Neurotoxins: free radical mechanisms and melatonin protection. Curr neuropharmacol (2010) 8(3):194–210. doi: 10.2174/157015910792246236
104. Morvaridzadeh M, Sadeghi E, Agah S, Nachvak SM, Fazelian S, Moradi F, et al. Effect of melatonin supplementation on oxidative stress parameters: a systematic review and meta-analysis. Pharmacol Res (2020) 161:105210. doi: 10.1016/j.phrs.2020.105210
105. Wardyn JD, Ponsford AH, Sanderson CM. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem Soc Trans (2015) 43(4):621–6. doi: 10.1042/BST20150014
106. Alam F, Syed H, Amjad S, Baig M, Khan TA, Rehman R. Interplay between oxidative stress, SIRT1, reproductive and metabolic functions. Curr Res Physiol (2021) 4:119–24. doi: 10.1016/j.crphys.2021.03.002
107. Fan X, Liu Y, Yue M, Yue W, Ren G, Zhang J, et al. Effect of cryptorchidism on the histomorphometry, proliferation, apoptosis, and autophagy in boar testes. Animals (2021) 11(5):1379. doi: 10.3390/ani11051379
108. Barqawi A, Caruso A, Meacham RB. Experimental varicocele induces testicular germ cell apoptosis in the rat. J urol (2004) 171(1):501–3. doi: 10.1097/01.ju.0000088775.69010.61
109. Take G, Erdogan D, Helvacioglu F, Göktas G, Ozbey G, Uluoglu C, et al. Effect of melatonin and time of administration on irradiation-induced damage to rat testes. Braz J Med Biol Res (2009) 42:621–8. doi: 10.1590/S0100-879X2009000700006
110. Khan S, Adhikari JS, Rizvi MA, Chaudhury NK. Radioprotective potential of melatonin against 60Co γ-ray-induced testicular injury in male C57BL/6 mice. J Biomed Sci (2015) 22(1):1–15. doi: 10.1186/s12929-015-0156-9
111. Armandeh M, Bameri B, Haghi-Aminjan H, Foroumadi R, Ataei M, Hassani S, et al. A systematic review on the role of melatonin and its mechanisms on diabetes-related reproductive impairment in non-clinical studies. Front Endocrinol (2022) 13. doi: 10.3389/fendo.2022.1022989
112. Nazeri T, Hedayatpour A, Kazemzadeh S, Safari M, Safi S, Khanehzad M. Antioxidant effect of melatonin on proliferation, apoptosis, and oxidative stress variables in frozen-thawed neonatal mice spermatogonial stem cells. Biopreserv Biobanking (2022) 20(4):374–83. doi: 10.1089/bio.2021.0128
113. Chen S, Li Y, Fu S, Li Y, Wang C, Sun P, et al. Melatonin alleviates arginine vasopressin-induced cardiomyocyte apoptosis via increasing Mst1-Nrf2 pathway activity to reduce oxidative stress. Biochem Pharmacol (2022) 206:115265. doi: 10.1016/j.bcp.2022.115265
114. Najafi A, Adutwum E, Yari A, Salehi E, Mikaeili S, Dashtestani F, et al. Melatonin affects membrane integrity, intracellular reactive oxygen species, caspase3 activity and AKT phosphorylation in frozen thawed human sperm. Cell Tissue Res (2018) 372(1):149–59. doi: 10.1007/s00441-017-2743-4
115. Koppers AJ, Mitchell LA, Wang P, Lin M, Aitken RJ. Phosphoinositide 3-kinase signalling pathway involvement in a truncated apoptotic cascade associated with motility loss and oxidative DNA damage in human spermatozoa. Biochem J (2011) 436(3):687–98. doi: 10.1042/BJ20110114
116. Espino J, Ortiz Á, Bejarano I, Lozano GM, Monllor F, García JF, et al. Melatonin protects human spermatozoa from apoptosis via melatonin receptor–and extracellular signal–regulated kinase-mediated pathways. Fertil steril (2011) 95(7):2290–6. doi: 10.1016/j.fertnstert.2011.03.063
117. Xue R, Li S, Wei Z, Zhang Z, Cao Y. Melatonin attenuates di-(2-ethylhexyl) phthalate-induced apoptosis of human granulosa cells by inhibiting mitochondrial fission. Reprod Toxicol (2022) 113:18–29. doi: 10.1016/j.reprotox.2022.08.004
118. Feng J, Ma W-W, Li H-X, Pei X-Y, Deng S-L, Jia H, et al. Melatonin prevents cyclophosphamide-induced primordial follicle loss by inhibiting ovarian granulosa cell apoptosis and maintaining AMH expression. Front Endocrinol (2022) 13. doi: 10.3389/fendo.2022.895095
119. Sun J, Pan J, Liu Q, Cheng J, Tang Q, Ji Y, et al. Melatonin attenuates mitochondrial damage in aristolochic acid-induced acute kidney injury. Biomol Ther (2022) 31(1):97–107. doi: 10.4062/biomolther.2022.054
120. Sokolović D, Lazarević M, Milić D, Stanojković Z, Mitić K, Sokolović DT. Melatonin arrests excessive inflammatory response and apoptosis in lipopolysaccharide-damaged rat liver: A deeper insight into its mechanism of action. Tissue Cell (2022) 79:101904. doi: 10.1016/j.tice.2022.101904
121. Zhang Y, Cook A, Kim J, Baranov SV, Jiang J, Smith K, et al. Melatonin inhibits the caspase-1/cytochrome c/caspase-3 cell death pathway, inhibits MT1 receptor loss and delays disease progression in a mouse model of amyotrophic lateral sclerosis. Neurobiol dis (2013) 55:26–35. doi: 10.1016/j.nbd.2013.03.008
Keywords: infertility, melatonin, rodents, oxidative stress, ischemia, reperfusion, heat
Citation: Dehdari Ebrahimi N, Shojaei-Zarghani S, Taherifard E, Dastghaib S, Parsa S, Mohammadi N, Sabet Sarvestani F, Moayedfard Z, Hosseini N, Safarpour H, Sadeghi A, Azarpira N and Safarpour AR (2023) Protective effects of melatonin against physical injuries to testicular tissue: A systematic review and meta-analysis of animal models. Front. Endocrinol. 14:1123999. doi: 10.3389/fendo.2023.1123999
Received: 14 December 2022; Accepted: 17 January 2023;
Published: 31 January 2023.
Edited by:
Dipak Kumar Sahoo, Iowa State University, United StatesReviewed by:
Chandana Haldar, Department of Zoology, Banaras Hindu University, IndiaChristopher Zdyrski, Iowa State University, United States
Copyright © 2023 Dehdari Ebrahimi, Shojaei-Zarghani, Taherifard, Dastghaib, Parsa, Mohammadi, Sabet Sarvestani, Moayedfard, Hosseini, Safarpour, Sadeghi, Azarpira and Safarpour. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Negar Azarpira, bmVnYXJhemFycGlyYUBnbWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
 Niloofar Dehdari Ebrahimi
Niloofar Dehdari Ebrahimi Sara Shojaei-Zarghani
Sara Shojaei-Zarghani Ehsan Taherifard
Ehsan Taherifard Sanaz Dastghaib3
Sanaz Dastghaib3 Shima Parsa
Shima Parsa Fatemeh Sabet Sarvestani
Fatemeh Sabet Sarvestani Alireza Sadeghi
Alireza Sadeghi Negar Azarpira
Negar Azarpira Ali Reza Safarpour
Ali Reza Safarpour