- 1Department of Non-Communicable Chronic Disease Control, Jiangsu Provincial Center for Disease Control and Prevention, Nanjing, Jiangsu, China
- 2Department of Epidemiology, School of Public Health, Nanjing Medical University, Nanjing, China
- 3Department of Chronic Disease Prevention and Control, Suzhou City Center for Disease Control and Prevention, Suzhou, China
Objective: To investigate the associations of circulating liver function marker levels with the risk of chronic obstructive pulmonary disease (COPD).
Methods: We leveraged the data of 372,056 participants from the UK Biobank between 2006 and 2010. The assessed circulating liver function markers included alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), alkaline phosphatase (ALP), total bilirubin (TBIL), albumin (ALB), and total protein (TP). Incident COPD was identified through linkage to the National Health Service registries. Cox proportional hazards models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs).
Results: During a median follow-up period of 12.3 (interquartile range:11.4-13.2) years, we documented 10,001 newly diagnosed COPD cases. Lower levels of ALT, TBIL, ALB, and TP and higher levels of GGT and ALP were nonlinearly associated with elevated COPD risk. The HR (95% CI) for decile 10 vs. 1 was 0.92 (0.84-1.01) for ALT, 0.82 (0.75-0.89) for TBIL, 0.74 (0.67-0.81) for ALB, 0.96 (0.88-1.04) for TP, 1.45 (1.31-1.62) for GGT, and 1.31 (1.19-1.45) for ALP. Restricted cubic spline analyses suggested a U-shaped relationship between AST levels and COPD risk (P for nonlinearity <0.05).
Conclusion: We observed that all seven circulating liver function markers were nonlinearly associated with the risk of COPD, indicating the importance of liver function in COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) is a severe chronic disease characterized by persistent respiratory symptoms and airflow limitation (1). The pathological changes observed in COPD include chronic inflammation, and structural changes resulting from repeated injury and repair (2). Recent evidence suggests that increased innate immune response contributes directly to the risk of COPD (3–5). It has been shown that the liver is essential in immune regulation and actively participates in the innate immune response of systemic organs through the secretion of inflammatory cytokines and mediators (6). Some prospective studies and animal models have shown associations of circulating liver function markers with COPD exacerbations and mortality (6–10). These findings support the hypothesis of the ‘liver-lung axis’ that may affect COPD progression (6). However, direct evidence of the relationship between liver function and COPD risk remains limited.
Circulating liver function markers include alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), alkaline phosphatase (ALP), total bilirubin (TBIL), albumin (ALB), and total protein (TP). These biomarkers provide indicators of liver injury, metabolism, immunity, and nutrition. In the UK primary care study, higher bilirubin levels were associated with a reduced risk of respiratory disease (8); a prospective Korean study with a 13-year follow-up suggested that low ALT levels may be an underlying factor in COPD development (9). Additionally, a case-control study reported significant changes in the levels of ALT, AST, GGT, and ALP among COPD patients compared with healthy nonsmokers (11). However, previous studies were conducted only on single or partial liver function markers and were mostly case-control studies. There is still a lack of systematic analysis on the associations between liver function markers and COPD risk in the general population.
Therefore, we leveraged the data from the UK Biobank, a large prospective cohort study, to systematically examine the associations between circulating liver function markers (ALT, AST, GGT, ALP, TBIL, ALB, and TP) and COPD risk.
Methods
Study population
The UK Biobank is a population-based cohort that includes over a half million individuals aged between 40 and 69 years from 22 assessment centres across England, Scotland, and Wales. All participants completed touchscreen questionnaires on sociodemographics, personal medical history, and lifestyle factors, and physical measurements were conducted by trained nurses between 2006 and 2010. Blood samples were collected from all individuals at the time of recruitment and from approximately 18,000 individuals at repeat assessments in 2012-2013. The current study was conducted under UK Biobank application number 84525.
In our study, we excluded 3,516 participants with prevalent COPD, 8,349 participants with emphysema or chronic bronchitis at baseline, 41,779 participants with forced expiratory volume at 1 second/forced vital capacity (FEV1/FVC) <lower limit of normal (LLN) according to the Global Lung Function Initiative (GLI) 2012 criteria (12), 68,164 participants lacking data for any of the seven circulating liver function markers, and 12,460 participants who were identified as outliers according to the Studentized Deviate Many-Outlier procedure (13). In total, 372,056 participants were included in this study (Supplementary Figure 1).
Assessment of circulating liver function markers
Levels of circulating liver function markers, including ALT, AST, GGT, TBIL, ALP, TP, and ALB, were measured by biochemical assays utilizing the Beckman Coulter AU5800 Platform. ALT, AST, GGT, and ALP were analysed using the enzymatic method; TBIL, TP, and ALB were analyzed using the colorimetric method; high sensitivity C-reactive protein (CRP) was analysed using the immunoturbidimetric method. To reduce potential systematic error, the UK Biobank performed standardized procedures with strict quality assurance such that blood samples were collected, transported, processed, and stored in the same way. The protocol detailing the process and storage of blood samples has been extensively validated (14). Moreover, third-party internal quality control (IQC) materials were run for biochemical assays to provide an unbiased assessment of the analytical procedures. The average coefficients of variation (%) across high, medium, and low IQC levels of ALT, AST, GGT, ALP, TBIL, ALB, TP, and CRP were 1.2-2.9, 1.3-2.1, 1.4-2.8, 2.8-3.1,1.5-1.9, 2.1-2.2, 1.1-1.2, and 1.7-2.3, respectively. The details about circulating liver function marker assay performance have been described in the online UK Biobank Showcase (https://biobank.ndph.ox.ac.uk/showcase/ukb/docs/serum_biochemistry.pdf).
Ascertainment of COPD
Incident COPD cases and deaths were identified through linkage to the National Health Service registries and national death registries. COPD was defined as J40-44 according to the 10th revision of the International Classification of Diseases (ICD-10).
Statistical analysis
Person-years of follow-up were calculated from the baseline assessment to the first COPD diagnosis, death, loss to follow-up, or the end of the study (30 September 2021 for England, 31 July 2021 for Scotland, and 28 February 2018 for Wales), whichever occurred first. Intraclass correlation coefficients (ICCs) were calculated to evaluate the consistency of repeatedly measured circulating liver function marker levels. We used the Cox proportional hazards model to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs), with age as the timescale. Model 1 was adjusted for sex, race, fasting status, assessment centre, and age at recruitment. Model 2 was further adjusted for education level, Townsend deprivation index, body mass index (BMI), physical activity, alcohol consumption frequency, smoking status, C-reactive protein, family history of respiratory disease, passive smoking, fine particulate matter (PM2.5), and occupations at risk of COPD. Model 3 further used ICCs to recalibrate the multivariate estimates.
Restricted cubic splines with 4 knots were used to fully assess the nonlinear associations between circulating liver function markers and COPD risk. The likelihood ratio test was used to compare the model with only the linear term of liver function marker levels to the model with both the linear term and cubic spline term. P <0.05 indicated the statistical significance of nonlinearity. Stratified analyses were conducted according to age at recruitment (<60, ≥60 years), sex (female, male), smoking status (never, previous, current), alcohol consumption (never or special occasions only, once a month to twice a week, three times a week to daily), BMI (<25, 25-30, ≥30 kg/m2), PM2.5 (<9.83, ≥9.83 μg/m3) (15), and occupations at risk of COPD (not at risk, at risk) (16). Multiplicative interactions were calculated using the likelihood ratio test to compare models with and without the cross-product terms.
In sensitivity analyses, we excluded participants who were diagnosed with COPD in the first two years of follow-up (n = 735), who had abnormally low or high levels of circulating liver function markers, who had hepatitis and other liver/hepatobiliary diseases (n = 8,680), who had asthma (n = 37,573), who had atherosclerotic diseases (n = 8,199), and who had diabetes at recruitment (n = 7,413). Moreover, we additionally adjusted cholesterol lowering medication including statins, etc. We also adjusted dietary and nutritional supplement intake, including frequency of poultry and livestock consumption, raw and cooked vegetable, fresh and dried fruit, coffee, and vitamin, mineral, and other dietary supplement intake.
All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC). Statistical tests were all two-sided, and P <0.05 was defined as statistically significant.
Results
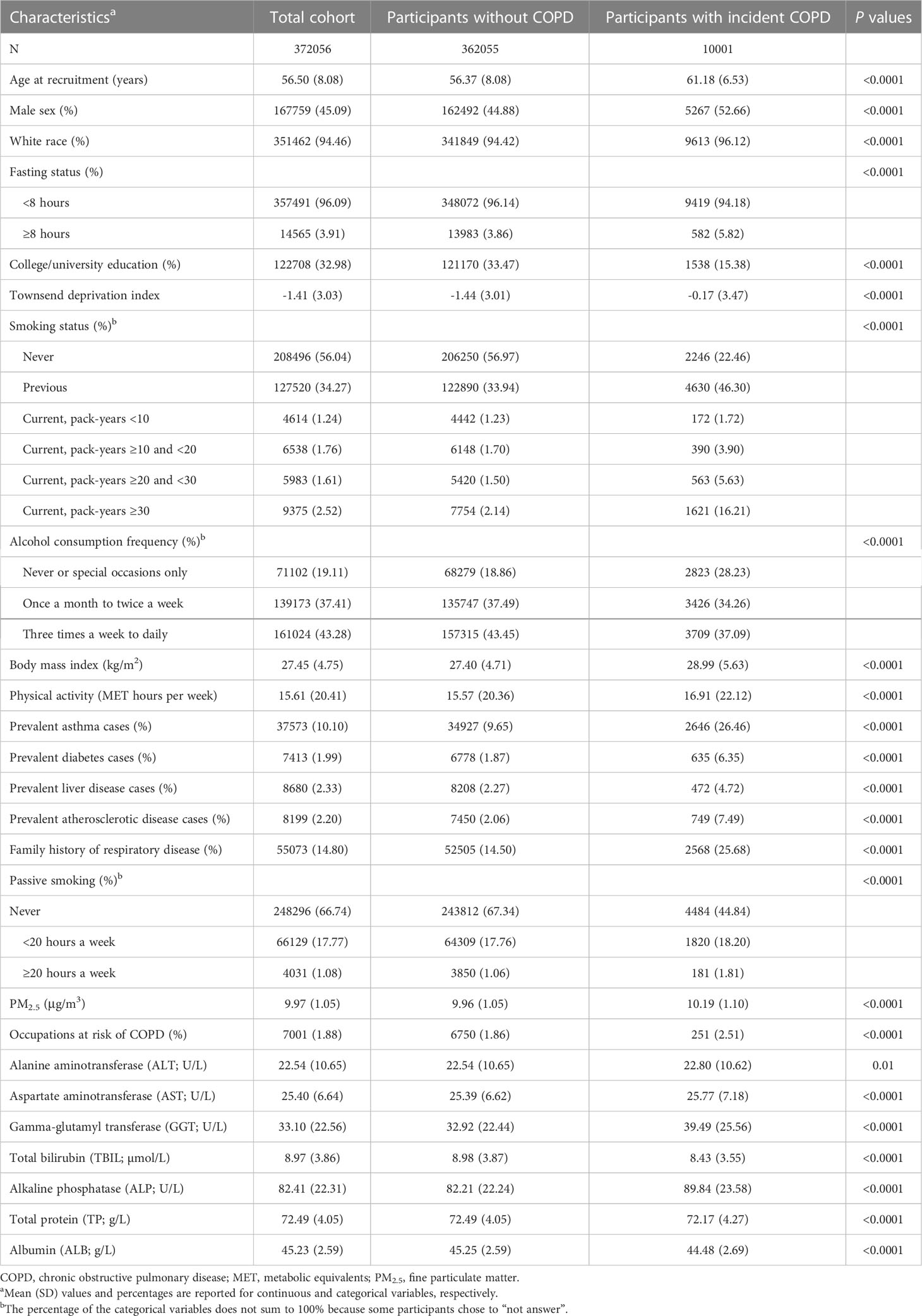
During a median follow-up period of 12.3 years (interquartile range:11.4-13.2), we documented 10,001 incident COPD cases among 372,056 participants. Individuals with COPD were older and more likely to be current smokers, had higher levels of physical activity, and BMI and had a higher prevalence of asthma (Table 1). All seven circulating liver function markers were generally within the normal range, with the lowest percentage of participants within a normal range being 84.58% for ALP (Supplementary Table 1). In Spearman correlation analysis, ALT and AST (rs = 0.69) and ALT and GGT (rs = 0.58) were strongly correlated (Supplementary Table 2). The ICCs value between the two measurements of these biomarkers were 0.50 (95% CI: 0.49-0.51) for ALT, 0.49 (95% CI: 0.47-0.50) for AST, 0.61 (95% CI: 0.59-0.62) for GGT, 0.72 (95% CI: 0.71-0.73) for ALP, 0.73 (95% CI: 0.73-0.74) for TBIL, 0.46 (95% CI: 0.44-0.47) for ALB, and 0.46 (95% CI: 0.44-0.47) for TP (Supplementary Table 3).
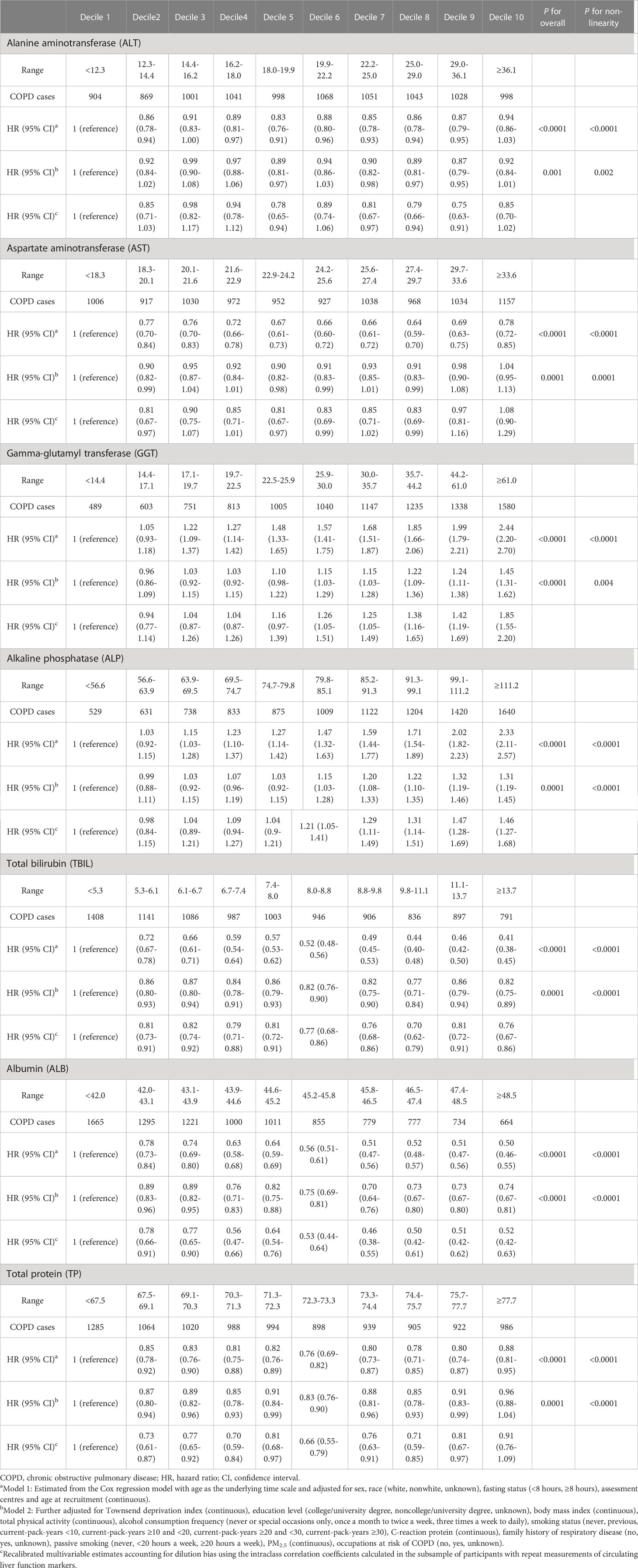
In the multivariate adjusted model, we found that lower levels of ALT, TBIL, ALB, TP, and higher levels of GGT and ALP were associated with an elevated risk of COPD. AST levels showed a U-shaped association with COPD risk (Table 2 and Figure 1). The corresponding HRs (95% CIs) for deciles 10 vs. 1 were 0.92 (0.84-1.01) for ALT, 0.82 (0.75-0.89) for TBIL, 0.74 (0.67-0.81) for ALB, 0.96 (0.88-1.04) for TP, 1.45 (1.31-1.62) for GGT, and 1.31 (1.19-1.45) for ALP. After correction for ICCs, the associations between circulating liver function markers and COPD risk became stronger (Table 2). Restricted cubic spline analyses showed a nonlinear relationship between these biomarkers and COPD risk (all P for nonlinearity <0.05) (Figure 1).
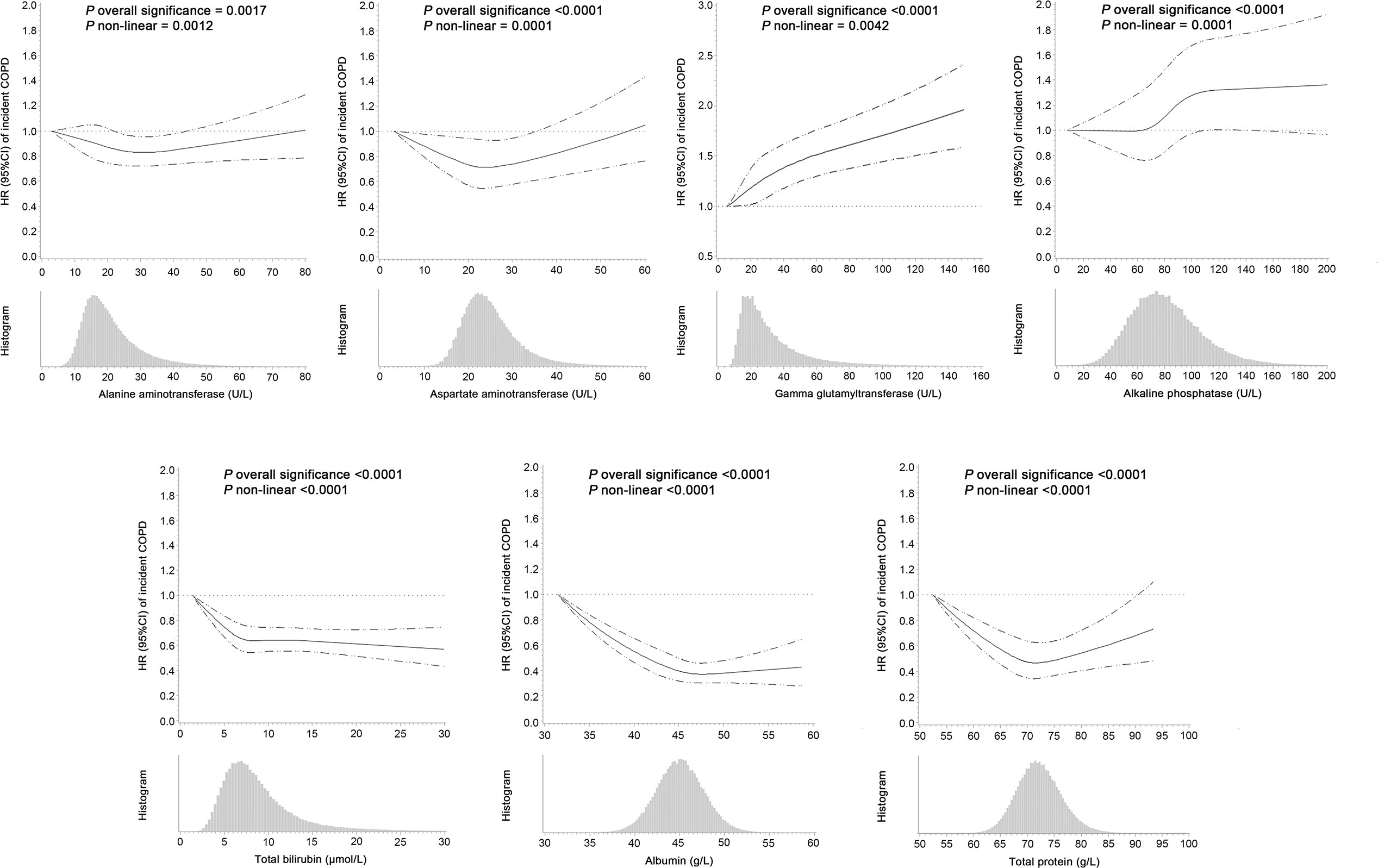
Figure 1 Hazard ratios (95% confidence intervals) of incident COPD associated with circulating levels of liver function markers and the frequency histograms of liver function markers in the UK Biobank. The associations were examined by multivariate Cox regression models based on restricted cubic splines. The solid line represents estimates of hazard ratios and dashed lines represent 95% confidence intervals.
Stratified analyses showed that the associations of ALP and ALB with COPD risk were stronger in male and younger participants. Interactions of ALT, AST, and GGT with smoking status were observed for COPD risk. Moreover, we found significant interactions of ALP, ALB, and TP with alcohol consumption and ALP, TBIL, and TP with BMI for the risk of COPD. The significant interactions of ALP with PM2.5 and occupations were also observed for COPD risk (all P for interaction <0.05) (Table 3).
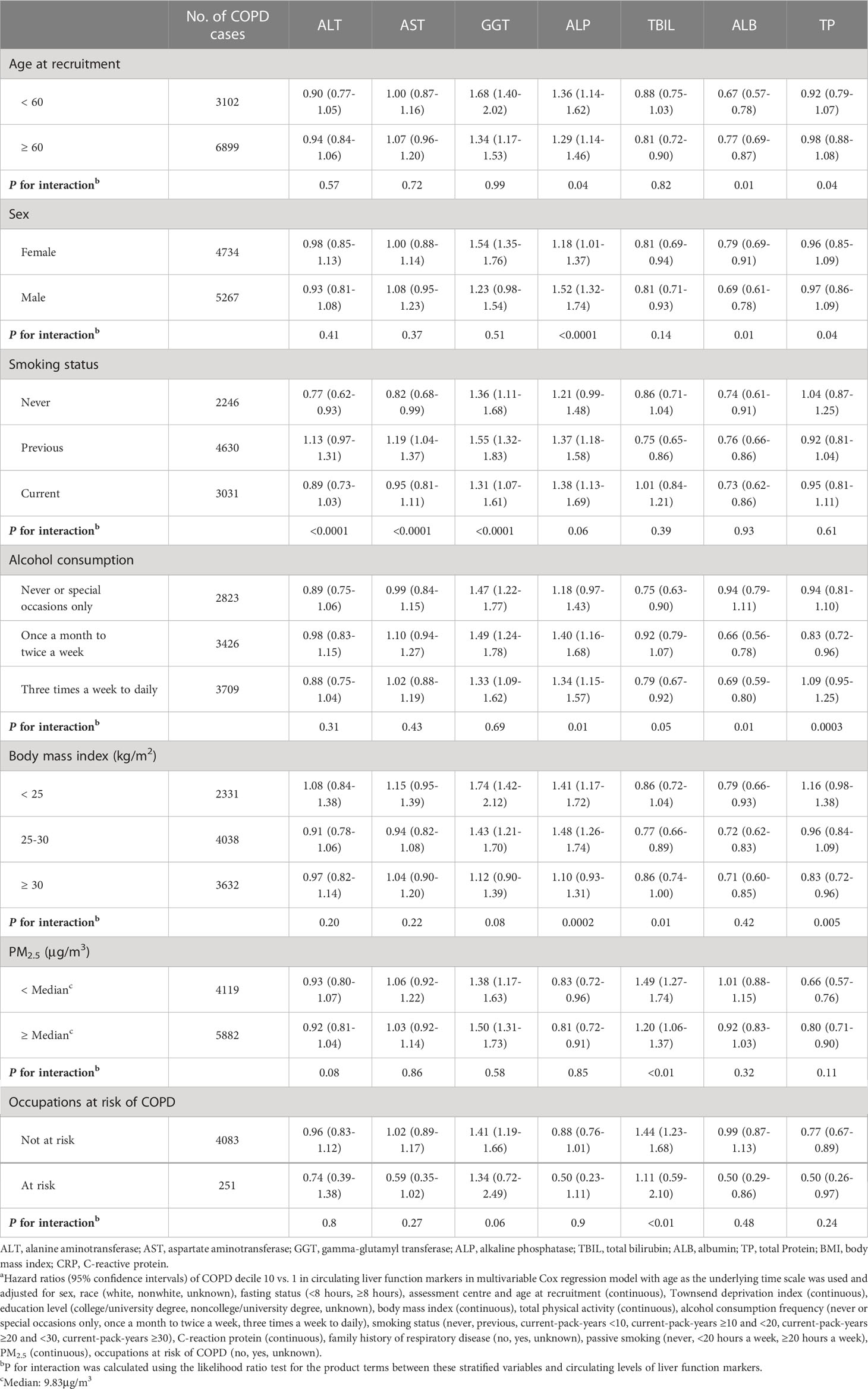
Table 3 Stratified analyses of the association between circulating levels of liver function markers and COPD riska.
In sensitivity analyses, the aforementioned associations were not materially altered after excluding incident COPD cases in the first two years of follow-up, participants with diabetes, participants with asthma, participants with atherosclerotic diseases, participants with liver/hepatobiliary disease at recruitment, or participants with abnormally low or high levels of liver function markers. Additionally, we adjusted for cholesterol lowering medication, dietary and nutritional supplement intake, and the associations remained robust (Supplementary Table 4).
Discussion
The results from this large cohort of approximately half a million individuals indicated that ALT, TBIL, ALB, and TP levels were inversely associated with COPD risk. In contrast, positive associations were found for GGT and ALP with the risk of COPD. A U-shaped association is apparent between AST and COPD risk. After several comprehensive sensitivity analyses, the observed associations did not essentially change. Additionally, we observed effect modification by several stratification factors in the associations of circulating liver function markers with COPD risk. To our knowledge, none of the previous studies systematically evaluated the relationship between circulating liver function markers and COPD risk.
Our analysis suggested that the levels of ALT were inversely associated with COPD risk. Previous studies also reported that low ALT levels play an important role in COPD development and exacerbations (9, 17). In clinical practice, circulating ALT levels commonly represent a specific liver dysfunction and injury marker. Recent studies indicated that low ALT activity has been linked to sarcopenia, frailty, and overall health (18, 19). The current knowledge on the characteristics of COPD is that in addition to airway obstruction, muscle, and weight loss are also prevalent (20). Therefore, levels of ALT may reflect the overall health status that contributes to the progression of COPD.
AST has a strong correlation with ALT and is generally known to be less specific to the liver than ALT because of its wide distribution. This study showed a U-shaped association of AST with COPD. However, few prior studies have reported associations between AST levels and COPD risk. Several meta-analyses demonstrated that levels of AST were significantly higher in patients with obstructive sleep apnoea and severe coronavirus disease 2019 (COVID-19) than in the general population (21, 22). The results from an animal study showed that liver steatosis promoted liver mitochondrial biogenesis in response to hypoxia (23). In addition, we found that the associations of ALT and AST levels with COPD risk were observed in never-smokers. A large cross-sectional study reported that smoking intensity was inversely associated with AST and ALT activities in individuals not drinking alcohol (24). The mechanism linking ALT, AST, and smoking is unclear. One hypothesis for this association is that hepatocellular vulnerability and the level of injury differ according to smoking status (24).
Regarding GGT, our finding of a positive association between circulating GGT levels and the risk of COPD is consistent with previous studies (25). Some case-control studies also indicated that serum GGT levels increased in individuals with stable COPD and were significantly higher in individuals with exacerbated COPD (26, 27). One possible explanation is that oxidative stress and inflammation have been demonstrated to be important amplifying mechanisms in COPD, and serum GGT is a sensitive biomarker of oxidative stress (28). Unexpectedly, we observed that the association of GGT with COPD risk was stronger among previous smokers in the stratified analysis. Nicotine might induce the elevation of GGT, and inflammation has a major role in the increase in GGT linked with smoking (24). Furthermore, airway inflammation causes structural changes that increase with COPD severity and last even after smoking cessation (1).
ALP, a hydrolytic enzyme mainly distributed in the liver and bone, is used as an indicator for skeletal monitoring in clinical practice (29). Skeletal muscle dysfunction is a severe complication in patients with COPD. It exacerbates COPD by decreasing the function of the ventilatory muscle groups (30). Muscle integrity reflects overall nutritional status and is thus indirectly linked to COPD. In our analysis, higher ALP levels were related to an increased risk of COPD, and the associations were stronger in young individuals, male individuals, frequent alcohol drinkers, and overweight individuals. The sex interaction might be related to oestrogen, which can effectively inhibit osteoblast apoptosis and bone resorption (31). A European-based population study also supported our findings that alcohol intake was inversely associated with ALP levels, although no significant effect was observed for extremely low alcohol intake (32). Sustained alcohol intake could lead to mild biliary obstruction, thereby reducing the number of ALP-producing cells (32). Additionally, although ageing and low BMI have been acknowledged as independent risk factors contributing to COPD mortality (1), the relationship between overweight and COPD risk remains controversial. The COPDGene study reported that obesity was prevalent in COPD patients and was associated with worse COPD-related outcomes (33). One possible hypothesis for this finding is that weight gain may not be exactly representative of increased muscle mass (30).
The inverse relationship of TBIL with COPD risk was fairly consistent with several prospective studies (8, 34). However, a bidirectional Mendelian randomization analysis showed no causal relation between TBIL and COPD (35). Although higher TBIL levels within the normal range have been demonstrated to decrease the risk of cardiovascular disease through antioxidant and anti-inflammatory effects (36), the underlying mechanism for the protective effects of TBIL in COPD progression is unclear. The results from animal models indicated that the effects of TBIL inhibiting lipid peroxidation might improve the lung function of COPD patients (37). Oxidative stress may further influence chronic lung inflammation. Furthermore, the UK primary care study showed that significantly obese individuals had relatively lower TBIL levels (8). A cross-sectional study based on the Japanese population reported that TBIL levels decreased only in women with BMI ≥ 27.5 kg/m2 (38). These studies supported our findings on the interaction of TBIL with BMI for COPD risk.
Although there have been no previous studies on the association between ALB and COPD progression, a meta-analysis including 6 studies demonstrated that severe COVID-19 patients had a higher prevalence of COPD and were significantly associated with decreased ALB levels (21). In clinical practice, circulating ALB is commonly measured to assess protein nutritional adequacy. Some studies suggested that higher ALB levels had a protective effect on the myocardium and were independently associated with muscle strength (39, 40). ALB levels were higher in males than females among young adults and declined with age (41). Liver injury caused by alcohol consumption commonly results in a decreased ability to synthesize protein (32). Furthermore, ALB is a major component of TP. Our analysis suggested that the inverse associations of ALB and TP with COPD risk were fairly consistent, which may indicate that the associations were mainly attributed to the role of ALB.
Previous studies have demonstrated that the liver-lung axis plays a key role in regulating lung inflammation (6). Our data also indicated a link between multiple liver function markers and COPD. A better understanding of the liver-lung axis may provide valuable insights for clinical intervention in COPD patients. The typical feature of COPD progression is a progressive loss in health status and deteriorating symptoms, with acute exacerbations linked to a higher mortality risk (1). Although exacerbations are generally shorter in duration, they also have a negative impact on the organism. COPD patients with recurrent exacerbations are often in poorer health status, and patients hospitalized for COPD exacerbations also have a poor long-term prognosis. Considering the complexity of COPD comorbidity and prognosis, managing acute exacerbation of COPD is a significant event in clinical care.
The major strengths of our study include its prospective design and long-term follow-up. The large sample size allowed us to minimize residual confounding by comprehensively adjusting COPD risk factors. In addition, all biomarkers were measured using standardized methods in the same laboratory with strict third-party IQC, minimizing potential measurement error. Several limitations of this study should also be acknowledged. First, our analysis used a single measure of circulating liver function markers that may not reflect the levels during the entire follow-up. However, the relatively high ICCs calculated from repeated measurements of blood samples provide reproducibility of liver function markers. Second, as an observational study, the possibility of reverse causality cannot be ruled out. However, the sensitivity analysis showed that the results were stable after excluding baseline hepatitis disease or diabetes or the first two years of follow-up. Third, to minimize selection bias, approximately a quarter of the UK Biobank participants were excluded, including 10% for prevalent COPD at baseline and 15% for missing liver function marker data or outliers in measurement. Finally, participants in the UK Biobank are predominantly European Caucasians, which limits the generalizability of our findings to other ethnicities.
In this large prospective population-based study, all seven circulating liver function markers were associated with the risk of COPD. These findings support the importance of liver function in COPD and may spur future studies to elucidate the role of the liver-lung axis in COPD development.
Data availability statement
The data that support the findings of this study are available from the UK Biobank, but restrictions apply to the availability of these data, which were used under license for the current study and are not publicly available. Data are, however, available from the authors upon reasonable request and with the permission of the UK Biobank. Requests to access the datasets should be directed to https://www.ukbiobank.ac.uk/, via YWNjZXNzQHVrYmlvYmFuay5hYy51aw==.
Ethics statement
The UK Biobank study was approved by the North West Multi-centre Research Ethics Committee (06/MRE08/65). The patients/participants provided their written informed consent to participate in this study.
Author contributions
XF and XW have full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Conception and design of the study: JZ and XF. Statistical analysis: HG and XW. Drafting of the manuscript: WD and HG. Critical revision of the manuscript for important intellectual content: ZZ, HY, PL, LC, JS, YL, DH, RT, and MW. All authors contributed to the article and approved the submitted version.
Funding
Jiangsu Provincial Science and Technology Department Social Development Key Project (BE2019674); Suzhou Gusu Health Talent Program Training Project (GSWS2020098).
Acknowledgments
We are grateful to UK Biobank participants. This research has been conducted using the UK Biobank resource under application number 84525.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1121900/full#supplementary-material
References
1. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease. Report: Global initiative for chronic obstructive lung disease (2020). Available at: https://goldcopd.org/gold-reports/.
2. Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD Executive Sum Am J Respir Crit Care Med (2017) 195:557–82. doi: 10.1164/rccm.201701-0218PP
3. Gimeno D, Delclos GL, Ferrie JE, De Vogli R, Elovainio M, Marmot MG, et al. Association of CRP and IL-6 with lung function in a middle-aged population initially free from self-reported respiratory problems: the Whitehall II study. Eur J Epidemiol (2011) 26:135–44. doi: 10.1007/s10654-010-9526-5
4. Walter RE, Wilk JB, Larson MG, Vasan RS, Keaney JF Jr., Lipinska I, et al. Systemic inflammation and COPD: the framingham heart study. Chest (2008) 133:19–25. doi: 10.1378/chest.07-0058
5. Thomsen M, Dahl M, Lange P, Vestbo J, Nordestgaard BG. Inflammatory biomarkers and comorbidities in chronic obstructive pulmonary disease. Am J Respir Crit Care Med (2012) 186:982–8. doi: 10.1164/rccm.201206-1113OC
6. Hilliard KL, Allen E, Traber KE, Yamamoto K, Stauffer NM, Wasserman GA, et al. The lung-liver axis: A requirement for maximal innate immunity and hepatoprotection during pneumonia. Am J Respir Cell Mol Biol (2015) 53:378–90. doi: 10.1165/rcmb.2014-0195OC
7. Mroz RM, Lisowski P, Tycinska A, Bierla J, Trzeciak PZ, Minarowski L, et al. Anti-inflammatory effects of atorvastatin treatment in chronic obstructive pulmonary disease. a controlled pilot study. J Physiol Pharmacol (2015) 66:111–28.
8. Horsfall LJ, Rait G, Walters K, Swallow DM, Pereira SP, Nazareth I, et al. Serum bilirubin and risk of respiratory disease and death. Jama (2011) 305:691–7. doi: 10.1001/jama.2011.124
9. Choi YJ, Kwon DS, Kim T, Cho JH, Kim HJ, Byun MK, et al. Low alanine aminotransferase as a risk factor for chronic obstructive pulmonary disease in males. Sci Rep (2021) 11:14829. doi: 10.1038/s41598-021-94385-0
10. Young RP, Hopkins RJ, Marsland B. The gut-Liver-Lung axis. modulation of the innate immune response and its possible role in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol (2016) 54:161–9. doi: 10.1165/rcmb.2015-0250PS
11. Cepelak I, Dodig S, Romic D, Ruljancic N, Popovic-Grle S, Malic A. Enzyme catalytic activities in chronic obstructive pulmonary disease. Arch Med Res (2006) 37:624–9. doi: 10.1016/j.arcmed.2006.01.004
12. Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J (2012) 40:1324–43. doi: 10.1183/09031936.00080312
13. Rosner B. Percentage points for a generalized ESD many-outlier procedure. Technometrics (1983) 25:165–72. doi: 10.1080/00401706.1983.10487848
14. Elliott P, Peakman TC. The UK biobank sample handling and storage protocol for the collection, processing and archiving of human blood and urine. Int J Epidemiol (2008) 37:234–44. doi: 10.1093/ije/dym276
15. De Matteis S, Jarvis D, Hutchings S, Darnton A, Fishwick D, Sadhra S, et al. Occupations associated with COPD risk in the large population-based UK biobank cohort study. Occup Environ Med (2016) 73:378–84. doi: 10.1136/oemed-2015-103406
16. Doiron D, de Hoogh K, Probst-Hensch N, Fortier I, Cai Y, De Matteis S, et al. Air pollution, lung function and COPD: results from the population-based UK biobank study. Eur Respir J (2019) 54(1):1802140. doi: 10.1183/13993003.02140-2018
17. Lasman N, Shalom M, Turpashvili N, Goldhaber G, Lifshitz Y, Leibowitz E, et al. Baseline low ALT activity is associated with increased long-term mortality after COPD exacerbations. BMC Pulm Med (2020) 20:133. doi: 10.1186/s12890-020-1169-z
18. Irina G, Refaela C, Adi B, Avia D, Liron H, Chen A, et al. Low blood ALT activity and high FRAIL questionnaire scores correlate with increased mortality and with each other. a prospective study in the internal medicine department. J Clin Med (2018) 7(11):386. doi: 10.3390/jcm7110386
19. Portal D, Hofstetter L, Eshed I, Dan-Lantsman C, Sella T, Urban D, et al. L3 skeletal muscle index (L3SMI) is a surrogate marker of sarcopenia and frailty in non-small cell lung cancer patients. Cancer Manag Res (2019) 11:2579–88. doi: 10.2147/cmar.s195869
20. Wada H, Ikeda A, Maruyama K, Yamagishi K, Barnes PJ, Tanigawa T, et al. Low BMI and weight loss aggravate COPD mortality in men, findings from a large prospective cohort: the JACC study. Sci Rep (2021) 11:1531. doi: 10.1038/s41598-020-79860-4
21. Kovalic AJ, Huang G, Thuluvath PJ, Satapathy SK. Elevated liver biochemistries in hospitalized Chinese patients with severe COVID-19: Systematic review and meta-analysis. Hepatology (2021) 73:1521–30. doi: 10.1002/hep.31472
22. Sookoian S, Pirola CJ. Obstructive sleep apnea is associated with fatty liver and abnormal liver enzymes: a meta-analysis. Obes Surg (2013) 23:1815–25. doi: 10.1007/s11695-013-0981-4
23. Carabelli J, Burgueño AL, Rosselli MS, Gianotti TF, Lago NR, Pirola CJ, et al. High fat diet-induced liver steatosis promotes an increase in liver mitochondrial biogenesis in response to hypoxia. J Cell Mol Med (2011) 15:1329–38. doi: 10.1111/j.1582-4934.2010.01128.x
24. Breitling LP, Arndt V, Drath C, Brenner H. Liver enzymes: interaction analysis of smoking with alcohol consumption or BMI, comparing AST and ALT to γ-GT. PloS One (2011) 6:e27951. doi: 10.1371/journal.pone.0027951
25. Kim HW, Lee SH, Lee DH. Relationship of serum gamma-glutamyltransferase levels with pulmonary function and chronic obstructive pulmonary disease. Lung (2014) 192:719–27. doi: 10.1007/s00408-014-9616-3
26. Ermis H, Celik MR, Gulbas G, Tavli D, Aytemur ZA. Relationship between serum γ-glutamyltransferase levels and acute exacerbation of chronic obstructive pulmonary disease. Pol Arch Med Wewn (2013) 123:85–90. doi: 10.20452/pamw.1617
27. Sun D, Liu H, Ouyang Y, Liu X, Xu Y. Serum levels of gamma-glutamyltransferase during stable and acute exacerbations of chronic obstructive pulmonary disease. Med Sci Monit (2020) 26:e927771. doi: 10.12659/msm.927771
28. Domej W, Oettl K, Renner W. Oxidative stress and free radicals in COPD–implications and relevance for treatment. Int J Chron Obstruct Pulmon Dis (2014) 9:1207–24. doi: 10.2147/copd.s51226
29. Sciacqua A, Tripepi G, Perticone M, Cassano V, Fiorentino TV, Pititto GN, et al. Alkaline phosphatase affects renal function in never-treated hypertensive patients: effect modification by age. Sci Rep (2020) 10:9847. doi: 10.1038/s41598-020-66911-z
30. Jaitovich A, Barreiro E. Skeletal muscle dysfunction in chronic obstructive pulmonary disease. what we know and can do for our patients. Am J Respir Crit Care Med (2018) 198:175–86. doi: 10.1164/rccm.201710-2140CI
31. Robinson LJ, Yaroslavskiy BB, Griswold RD, Zadorozny EV, Guo L, Tourkova IL, et al. Estrogen inhibits RANKL-stimulated osteoclastic differentiation of human monocytes through estrogen and RANKL-regulated interaction of estrogen receptor-alpha with BCAR1 and Traf6. Exp Cell Res (2009) 315:1287–301. doi: 10.1016/j.yexcr.2009.01.014
32. Tolstrup JS, Grønbaek M, Tybjaerg-Hansen A, Nordestgaard BG. Alcohol intake, alcohol dehydrogenase genotypes, and liver damage and disease in the Danish general population. Am J Gastroenterol (2009) 104:2182–8. doi: 10.1038/ajg.2009.370
33. Lambert AA, Putcha N, Drummond MB, Boriek AM, Hanania NA, Kim V, et al. Obesity is associated with increased morbidity in moderate to severe COPD. Chest (2017) 151:68–77. doi: 10.1016/j.chest.2016.08.1432
34. Brown KE, Sin DD, Voelker H, Connett JE, Niewoehner DE, Kunisaki KM. Serum bilirubin and the risk of chronic obstructive pulmonary disease exacerbations. Respir Res (2017) 18:179. doi: 10.1186/s12931-017-0664-0
35. Dai C, Wang Z, Yang H, Xiao S, Xu J, Deng Z, et al. Association between serum total bilirubin and COPD: Results from a cross-sectional study and a bidirectional mendelian randomization analysis. Clin Epidemiol (2022) 14:289–98. doi: 10.2147/clep.s353389
36. Lin JP, Vitek L, Schwertner HA. Serum bilirubin and genes controlling bilirubin concentrations as biomarkers for cardiovascular disease. Clin Chem (2010) 56:1535–43. doi: 10.1373/clinchem.2010.151043
37. Sedlak TW, Saleh M, Higginson DS, Paul BD, Juluri KR, Snyder SH. Bilirubin and glutathione have complementary antioxidant and cytoprotective roles. Proc Natl Acad Sci U.S.A. (2009) 106:5171–6. doi: 10.1073/pnas.0813132106
38. Tanaka M, Budhathoki S, Hirata A, Morita M, Kono S, Adachi M, et al. Behavioral and clinical correlates of serum bilirubin concentrations in Japanese men and women. BMC Endocr Disord (2013) 13:39. doi: 10.1186/1472-6823-13-39
39. Schalk BW, Deeg DJ, Penninx BW, Bouter LM, Visser M. Serum albumin and muscle strength: a longitudinal study in older men and women. J Am Geriatr Soc (2005) 53:1331–8. doi: 10.1111/j.1532-5415.2005.53417.x
40. Grimm G, Haslacher H, Kampitsch T, Endler G, Marsik C, Schickbauer T, et al. Sex differences in the association between albumin and all-cause and vascular mortality. Eur J Clin Invest (2009) 39:860–5. doi: 10.1111/j.1365-2362.2009.02189.x
Keywords: chronic obstructive pulmonary disease, liver function, serum biomarker, prospective cohort study, liver-lung axis
Citation: Du W, Guan H, Wan X, Zhu Z, Yu H, Luo P, Chen L, Su J, Lu Y, Hang D, Tao R, Wu M, Zhou J and Fan X (2023) Circulating liver function markers and the risk of COPD in the UK Biobank. Front. Endocrinol. 14:1121900. doi: 10.3389/fendo.2023.1121900
Received: 12 December 2022; Accepted: 08 March 2023;
Published: 22 March 2023.
Edited by:
Zhu Cuiling, Tongji University, ChinaReviewed by:
Yumin Zhang, Southeast University, ChinaStanislav Kotlyarov, Ryazan State Medical University named after academician I.P. Pavlov, Russia
Copyright © 2023 Du, Guan, Wan, Zhu, Yu, Luo, Chen, Su, Lu, Hang, Tao, Wu, Zhou and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xikang Fan, eGlrYW5nZmFuMTk5MUBqc2NkYy5jbg==; Jinyi Zhou, emhvdWppbnlpNzRAc2luYS5jb20=
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work
 Wencong Du
Wencong Du Haoyu Guan
Haoyu Guan Xinglin Wan
Xinglin Wan Zheng Zhu1
Zheng Zhu1 Pengfei Luo
Pengfei Luo Jian Su
Jian Su Dong Hang
Dong Hang Ming Wu
Ming Wu Jinyi Zhou
Jinyi Zhou Xikang Fan
Xikang Fan