- 1The Sutherland Hospital, Endocrinology, Sydney, NSW, Australia
- 2UNSW Sydney, Faculty of Medicine, Sydney, NSW, Australia
- 3Garvan Institute of Medical Research, Healthy Ageing Theme, Sydney, NSW, Australia
- 4Launceston General Hospital, Endocrinology, Launceston, TAS, Australia
- 5Faculty of Medicine, The University of Sydney, Sydney, NSW, Australia
- 6St. George Hospital, Endocrinology, Sydney, NSW, Australia
Background: The prevalence of gestational diabetes mellitus (GDM) has been increasing in Australia and worldwide. The study aims were to examine, in comparison with dietary intervention, perinatal outcomes for women with gestational diabetes who were attending a single hospital clinic and to identify predictors for their pharmacological GDM treatment.
Methods: A prospective, observational study of women with GDM, treated with “Diet, N= 50”, “Metformin, N = 35”, “Metformin and Insulin, N = 46” or “Insulin, N = 20”.
Findings: The mean BMI for the whole cohort was 25.8 ± 4.7 kg/m2. The Metformin group, compared to the Diet group, had OR=3.1 (95% CI:1.13 to 8.25) for caesarean section birth (LSCS) compared to normal vaginal birth mode with no longer such a significant association after controlling for the number of their elective LSCS. The insulin treated group had the highest number of small for gestational age neonates (20%, p<0.05) with neonatal hypoglycaemia (25%, p< 0.05). Fasting glucose value on oral GTT (glucose tolerance test) was the strongest predictor for a pharmacological intervention requirement with OR = 2.77 (95CI%: 1.16 to 6.61), followed by timing of OGTT with OR=0.90 (95% CI: 0.83 to 0.97) and previous pregnancy loss with OR=0.28 (95% CI:0.10 to 0.74).
Interpretation: These data suggest that metformin may be a safe alternative treatment to insulin treatment in GDM. Raised fasting glucose on oral GTT was the strongest indicator that GDM women with BMI < 35 kg/m2 may require pharmacological therapy. Further studies are needed to identify the most effective and safe management of gestational diabetes within the public hospital setting.
Australian New Zealand Clinical Trial Registry ANZCTR Trial Id: ACTRN12620000397910.
1. Introduction
The prevalence of gestational diabetes mellitus (GDM) has been increasing in Australia and worldwide likely due to rising average maternal age and increasing obesity especially in young adults (1). In Australia, GDM is now becoming a common complication of pregnancy as it affects around 10% of pregnancies with up to 30% of pregnancies being affected by GDM in high-risk populations (2). In most cases, GDM occurs in pregnant women with impaired pancreatic function, which is insufficient to overcome the insulin resistance associated with the pregnant state.
In recent years, GDM has been diagnosed more frequently in Australia based on more stringent diagnostic criteria recommended by the International Association of Diabetes and Pregnancy Study Groups (IADPSG), which were endorsed by the Australasian Diabetes in Pregnancy Society (ADIPS) (2). The usual time point for GDM screening is recommended to be between 24-28 weeks of pregnancy (3). Earlier screening, recommended by these expert groups, in high risk women is desirable to enable lifestyle interventions focused on diet, physical activity, and weight control to be initiated during the first or early second trimesters of pregnancy (4).
The diagnosis of GDM carries important risks of adverse short and long-term clinical outcomes for women and their offspring. The main immediate consequences of GDM are increased risks of preeclampsia, large for gestational age (LGA) newborns, and caesarean birth, with their associated perinatal co-morbidities (5). GDM is associated with up to 10-times higher odds for the development of future maternal type 2 diabetes or prediabetes in comparison with individuals with a normoglycemic pregnancy (6). Women who are affected by GDM are not only at high risk of developing type 2 diabetes later in life, furthermore, having gestational diabetes is associated with a relative risk of 2.0 (95% CI, 1.6-2.5) for being affected by future cardiovascular disease (7).
Importantly, pharmacological treatment has been shown to improve perinatal outcomes of GDM with reductions in preeclampsia, macrosomia, shoulder dystocia and neonatal death (8). Insulin has been recommended as the first-line treatment agent for GDM in the U.S (9) while in the UK, the National Institute for Health and Care Excellence (NICE) together with Scottish and Canadian guidelines recommends that metformin, an insulin sensitizer, which reduces hepatic gluconeogenesis, and increases peripheral glucose uptake (10), may be considered as initial pharmacological glucose lowering treatment in GDM women (11). Although insulin therapy has been shown to reduce the risk of neonatal macrosomia and rate of serious perinatal outcomes such us shoulder dystocia or perinatal death (12), the benefits of insulin treatment in pregnancy often do not extend to preventing neonatal hypoglycaemia, frequently requiring intravenous glucose infusion and neonatal high-level nursery admission (13). Furthermore, gestational insulin therapy requires additional education, resources and training with the need for increased care for women throughout pregnancy and the act of injecting insulin can be stressful for some women.
At our institution, in our cohort of pregnant women with GDM, metformin has been endorsed as an alternative treatment to insulin therapy. We have therefore hypothesized that metformin use to treat GDM will result in similar pregnancy outcomes in comparison to pregnant women who are treated with insulin alone.
The present study examined perinatal outcomes for women with GDM who were treated with pharmacological interventions in comparison with dietary lifestyle changes alone while controlling for differences in baseline maternal characteristics. In particular, the primary aim was to examine the differences in composite maternal and in neonatal outcomes between four GDM treatment groups (“Diet”, “Metformin”, “Metformin and Insulin”, “Insulin”). In addition, specific maternal and neonatal outcomes were also reported and analysed separately. The second aim of the study was to identify early clinical maternal predictors for the use of pharmacological treatment in pregnancy affected by GDM.
2. Materials and Methods
2.1. Study design
We have conducted a prospective, observational, cohort study through a review of the medical records of women with GDM in singleton pregnancy who attended the multi-disciplinary Gestational Diabetes Clinic at Sutherland Hospital, Sydney, Australia, between years 2016 to 2018. The analyzed data were consecutively extracted from electronic and from hard copies of medical records.
Weight was measured to the nearest 0.1 kg on a digital scale (TANITA, Wedderburg) and height was measured to the nearest 0.1 cm with a scale-mounted stadiometer during the first antenatal visit. BMI (kg/m2) was calculated. Women with Type 1 and 2 pre-gestational diabetes as well as women with BMI exceeding 35 kg/m2 were excluded from the analysis, as their care was transferred to the tertiary referral centre. Furthermore, in our institution women who underwent previous lower segment caesarean sections (LSCS) were not being offered an option of vaginal birth after caesarean delivery (VBAC).
The Southern Eastern Sydney Local Health District Human Research Ethics Committee (Study Reference No. RESP/15/107) approved the study. The study was registered with Australian New Zealand Clinical Trial Registry ANZCTR Trial Id: ACTRN12620000397910. This cohort study, in accordance with the current rules of the local Research Ethics Committee, did not require the patient’s informed consent.
2.2. GDM diagnosis and treatment
GDM was diagnosed, as recommended for Australian women who are at 24–28 weeks gestation, using a 75-g oral glucose tolerance test (OGTT) following an overnight fast, applying the new diagnostic criteria, introduced in 2015, of a fasting plasma glucose ≥ 5.1 mmol/L, a 1-hour plasma glucose ≥ 10 mmol/L or a 2-h plasma glucose ≥ 8.5 mmol/L, as endorsed by ADIPS (14, 15). In our institution early screening (i.e., before 24 weeks gestation) is performed in high-risk patients including those with previous GDM, or other risk factors for GDM (pre-pregnancy BMI > 30 (kg/m2), previous birth of baby with birthweight above 4000 grams, family history of diabetes or those of a high-risk ethnicity)) (16).
All women diagnosed with GDM attended two separated education sessions containing dietary and lifestyle advice in pregnancy, which were run by a Diabetes Nurse Educator and Dietician. Women were advised to monitor their blood glucose levels (BGLs) 4 times daily using a blood glucose meter: in a fasting condition as well as at 2-hours post breakfast, lunch and dinner. Women were advised to follow a carbohydrate modified diet (30–45 grams of carbohydrate at main meals, 15–30 grams of carbohydrates at mid meals) and they were encouraged to consume low glycaemic index carbohydrates. In our institution, following Endocrinology advice, GDM women would commence on the pharmacological management when their BGLs, despite lifestyle and dietary modification, were exceeding fasting BGLs =< 5.0 and = <6.7 mmol/L 2-h postprandially. In line with NICE guidelines metformin use was discussed as first line of pharmaceutical therapy (11) together with an alternative choice of insulin treatment as dependent upon patient and physician preference. Insulin alone was a preferred treatment in high-risk women who due to their multiple risk factors underwent earlier OGTT. The insulin was commenced (insulin isophane and/or insulin aspart) based on the pattern of hyperglycaemia. Insulin doses were titrated to target fasting and postprandial BGLs by the treating Endocrinologist. The metformin group included GDM women who were prescribed metformin as the first line therapy. The initial metformin dose was 500 mg daily, which was up-titrated to 2000 mg per day (where tolerated) to aim for adequate glycaemic control. Treatment was intensified by the addition of insulin in women who did not achieve adequate glycaemic control with metformin alone.
Therefore, study patients were prospectively allocated to one of four treatment exposure groups (“Diet”, “Metformin”, “Metformin and Insulin”, “Insulin”).
2.2.1 Main primary outcome measure
The main composite study aim was to examine the differences in maternal and in neonatal outcomes between four GDM treatment groups. These perinatal outcomes were: maternal outcomes—mode and gestational age at delivery, timing of delivery and neonatal outcomes— neonatal birth weight, preterm birth, indicated by spontaneous birth before 37 weeks’ gestation; large-for-gestational-age (LGA; defined as birth weight > 90th centile for gestational age and gender), small-for-gestational-age (SGA; defined as birth weight (SGA; defined as birth weight < 10th centile for gestational age and gender) (17), presence of shoulder dystocia, neonatal respiratory distress, neonatal hypoglycaemia, jaundice, birth injury and neonatal death. The composite outcome was a binary variable defined as 1 if at least one maternal or neonatal outcome was present, or 0 in the absence of both maternal and neonatal outcomes.
2.2.2 Secondary outcomes measure
In order to identify the secondary study aim three pharmacological interventions were grouped together. For this aim, participants were classified in two groups: 1) any pharmacological intervention, including “Metformin”, “Metformin and Insulin”, and “Insulin” groups and 2) “Diet”.
2.2.3 Neonatal hypoglycaemia
In our institution the presence of formal BGL < 2.6 mmol/L in neonates who are less than 48 hours of age warrants immediate intervention. These neonates are admitted to the neonatal intensive care unit (NICU) for treatment. The definition of neonatal hypoglycaemia is based on the study, which demonstrated reversible disturbance in evoked potentials at BGL < 2.6mmol/l in a small cohort of asymptomatic term babies (18).
The aim of hypoglycaemia treatment is to return the neonatal BGL values to their safe range (> 3.9 mmol/L) through normal nutritional intake. For BGLs ranging from 1.5 to 2.5 mmol/L this occurs through the use of oral 40% Dextrose Gel, which is massaged into neonatal buccal mucosa, followed by refeed with either breast or formula. Severe symptomatic hypoglycaemia is corrected with an IV 10% dextrose bolus at 2 mL/kg and infusion at 60 – 80 ml/kg/day or IM glucagon. High risk neonates are monitored for at least the first 24 hours of life in NICU until the neonate’s BGLs remain at safe levels (≥ 2.6mmol/L) for at least 24 hours after the last episode of hypoglycaemia.
2.3. Statistical analysis
Baseline study data are presented as mean ( ± SD) for normally distributed variables and median (interquartile range) for non-normally distributed variables. Analysis of variance (ANOVA) and post-hoc pairwise Tukey honest significance difference test, or Kruskal–Wallis test and post-hoc pairwise Dunn’s test were used to examine the imbalance between the study groups for normally or non-normally distributed baseline data, respectively. Categorical variables are presented as number (%), and Fisher’s exact test was used for the between group comparisons.
The association between pharmacological intervention for GDM and adverse perinatal outcomes was determined using unadjusted and multivariable adjusted logistic regression analyses.
An exploratory analysis examined an association between mode of birth and treatment procedures after adjustment for the differences in baseline characteristics between study groups such as fasting glucose and BMI.
The secondary outcome of the study was to identify early clinical maternal predictors for the pharmacological treatment in GDM. The analyzed study data included maternal characteristic defined as age, ethnicity, body mass index (BMI), family history of diabetes, parity, pooled number of previous miscarriages and pregnancy terminations, previous history of GDM, history of thyroid disease and thyroid stimulating hormone (TSH) values, vitamin B12 levels, 25 (OH) D levels and the timing of OGTT with BGL values on OGTT and gestational age at diagnosis of GDM. The fasting glucose was analyzed as a continuous variable to avoid misclassification error of exposure variable.
Variables were firstly screened in univariate analysis, and those with a p-value < 0.25 were included in the multivariable model. The final model was selected using stepwise regression.
Statistical analyses were conducted using R software version 4.0.4 (2021–02–15) with P value of < 0.05, which was considered statistically significant.
3. Study results
3.1. Baseline characteristics of study patients
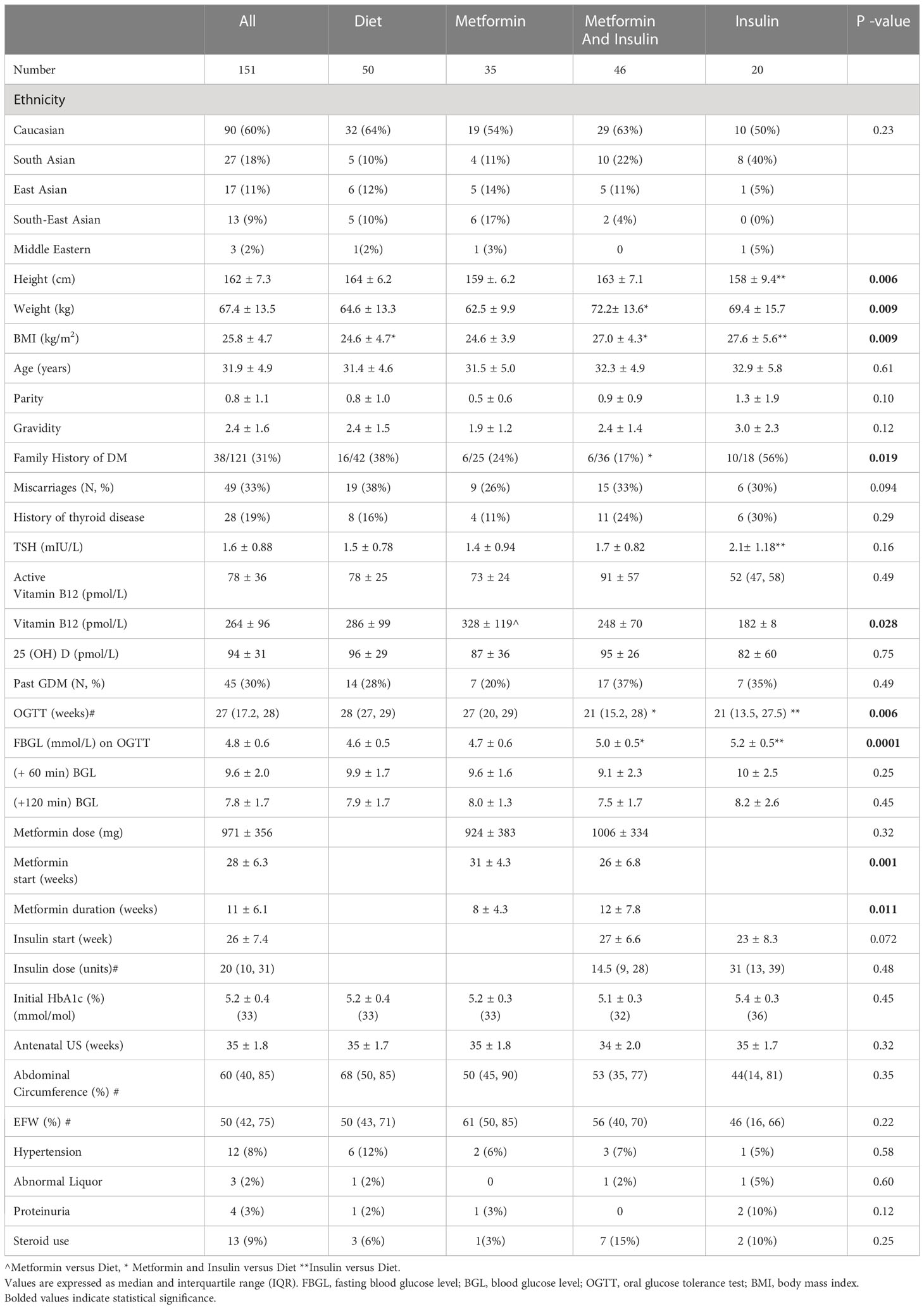
During the time period of September 2016 to April 2018, 151 women were identified as being treated with Diet (N = 50), Metformin (N = 35), taking Metformin and Insulin (N=46) or with Insulin alone (N=20) during singleton GDM pregnancy. The demographics of these groups are outlined in Table 1. There were differences in baseline characteristics between study groups in subjects’ height, weight, timing of their OGTT, value of fasting BGL on OGTT, family history of diabetes and total vitamin B12 (Table 1).
Compared to Diet, Metformin and Insulin and Insulin groups were heavier (p = 0.009). In addition, Metformin group, although not on vitamin B12 supplementation, had higher level of total vitamin B12 (p = 0.028), Metformin and Insulin group were more likely to have family history for diabetes (p = 0.019). There were no differences in age, the number of previous pregnancies and live births, number of previous pregnancies affected by GDM, initial HbA1c level, previous thyroid disease, TSH or 25 (OH) vitamin D levels.
3.2. Treatment of gestational diabetes
Metformin and Insulin and Insulin alone groups, as having identified risk factors for the GDM at their first antenatal visit, had earlier OGTTs, an average at 21 weeks, in comparison with the Diet treated group which had an average OGTT at 28 weeks (p = 0.006). There was no difference in the history GDM in previous pregnancies between study groups or in their HbA1c with an average initial HbA1c of 5.2% (± 0.34), (33 mmol/mol). Approximately a third of these pregnant women were diagnosed with GDM in their previous pregnancies and experienced previous spontaneous miscarriages or terminations of their pregnancies.
Women that were treated with insulin or with metformin and insulin had significantly higher fasting glucose on 75 g OGTT (5.19 mmol/L or 5.01 vs. 4.6 mmol/L, respectively, p= 0.0001), in comparison with women treated with diet and lifestyle modification alone without such difference for their 1- hourly and 2- hourly BSL. Caucasian women had a higher mean fasting BGL of 4.9 mmol/L (SD = 0.55), p = 0.015 on oral GTT in comparison with a mean fasting BGL of 4.7 mmol/L (SD = 0.56) in women of Asian ethnicity.
The timing of pharmacological intervention varied between groups. Women treated with insulin were initiated on their therapy earlier at 23 ± 8.3 weeks while women treated with metformin only on average commenced on metformin at 31 ± 4.3 weeks. The mean gestational age at which insulin was added to metformin was 27 ± 6.6 weeks (Table 1).
There was no difference in the total daily dose of insulin at delivery for women in the Metformin and Insulin and Insulin alone groups. There was no difference in the foetal abdominal circumference on the antenatal scans (34-36 weeks).
3.3. Perinatal (maternal and neonatal) outcomes
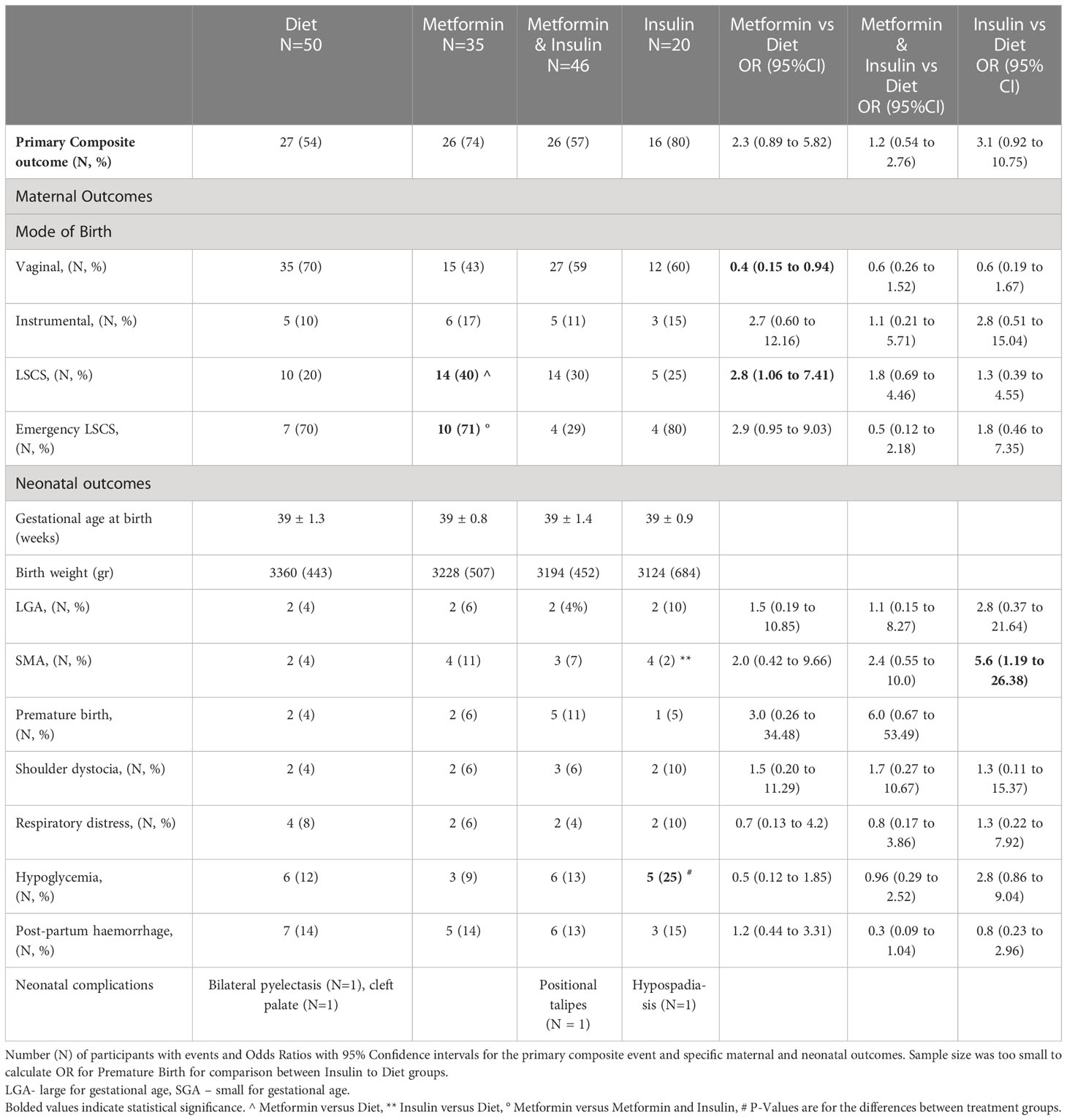
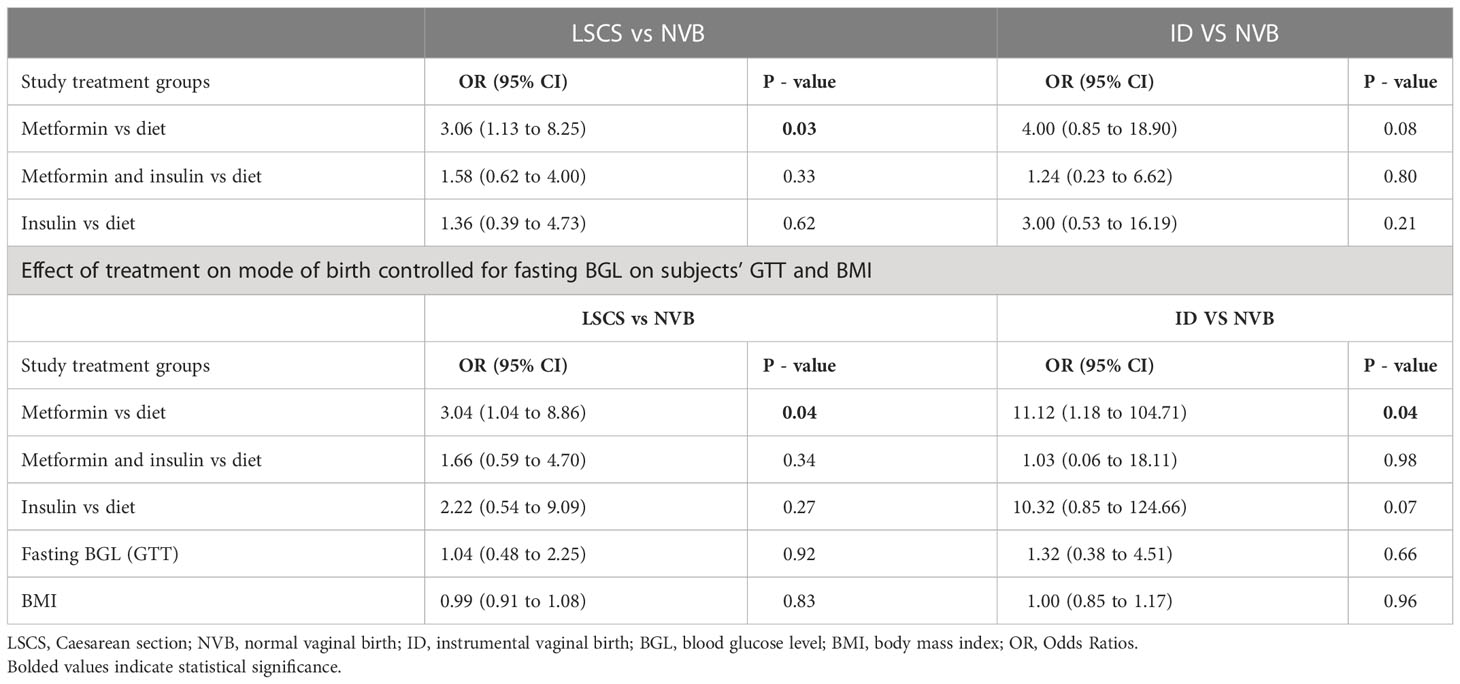
Maternal and neonatal outcomes for women taking metformin (with or without additional insulin) in comparison with those managed with diet and lifestyle modification are outlined in Table 2. There was no overall difference in maternal and neonatal composite study outcomes between groups (p = 0.13), (Table 2). There was no difference in ethnicity distribution between groups (Table 1). Furthermore, there was no interaction between study procedures and the ethnicity of women with GDM in primary composite study outcome (p = 0.38). In particular, there was no overall difference between study groups in the rate of normal vaginal birth (NVB) (p = 0.19), instrumental vaginal birth (p = 0.14) or LSCS (p = 0.36) or in the gestational age of the time of birth (p = 0.17). However, the comparison of each treatment group to the dietary intervention revealed that metformin treated group had 3.1 times the odds (95% CI: 1.13 to 8.25) for the birth by LSCS with the trend for the positive association between metformin treatment and instrumental vaginal birth (Table 3). Furthermore, once the effect of the treatment procedure on mode of birth was adjusted for the value of fasting glucose on subjects’ OGTT and their initial BMI this association became stronger with OR = 3.4 (95% CI: 1.04 to 8.86) for birth by LSCS for the metformin treated group and with OR = 11.12 (1.18 to 104.71) for the instrumental vaginal birth (Table 3). Once we excluded elective LSCS from the analysis, Metformin and Insulin group had lower rate of LSCS than Metformin group without difference to the Diet treated group (Table 2).
There were no differences between subjects treated with pharmacological intervention and dietary/lifestyle modifications in birth weight, numbers of shoulder dystocia, cases of respiratory distress, postpartum haemorrhage, rates of premature delivery or large-for-gestational-age neonates (Table 2). There were 19 neonates who required NICU admission: Diet with N = 7 (14%), Metformin with N=3 (9%), Metformin and Insulin with N = 7 (15%), Insulin with N = 2 (10%). The 8 newborns were separated from their mothers due to admission to NICU caused by: intrauterine growth retardation due to oligohydramnios (2 infants), significant hypoglycaemia (2 infants), respiratory distress requiring CPAP due to meconium aspiration (2 infants), foetal bradycardia (1 infant) and feeding problems due to undiagnosed cleft palate (1 infant).
One- fifth of insulin treated women delivered children who were small for their gestational age. The neonates in insulin alone treated group had the highest proportion (25%) of hypoglycaemic episodes however two of these children were affected by prematurity and by intrauterine growth retardation, respectively. The mothers of neonates with hypoglycaemia, prior to delivery, had high daily insulin requirements, which ranged between 58 units to 104 units.
3.4. Changes in vitamin B12 over time
The were 78 women who had measured total vitamin B12 level at their initial visit. The 8 of them (10%) were noted to have vitamin B12 level below the reference range (RR 150-700 pmol/L). When the cohort of these women was analyzed together, at their baseline and approximately 8 weeks later, there was a reduction in measured total vitamin B12 level from baseline vitamin B12 of 264 ± 96 pmol/L (RR 150-700pmol/L) to vitamin B12 of 242 ± 71 pmol/L (p = 0.019). Due to the limitation of small sample size, we were unable to compare differences between intervention groups.
3.5. Maternal predictors for the pharmacological treatment in pregnancy
3.5.1 Univariate analysis
In the univariate analysis the following variables were positively associated with the need for pharmacological GDM treatment: BMI with OR = 1.1 (95% CI; 1.00 to 1.18, p=0.038), timing of the OGTT in pregnancy with OR = 0.9 (95%CI; 0.86 to 0.98, p=0.0059), fasting BGL values on OGTT with OR = 3.1 (95% CI: 1.50 to 6.47, p=0.0024) and inversely with number of previous miscarriages with OR = 0.5 (95% CI: 0.23 to 1.07, p= 0.075).
There was no association between need for pharmacological GDM treatment and following variables: ethnicity (p = 0.10), age (p = 0.47), history of previous GDM (p = 0.83), TSH level (p = 0.32), previous thyroid disease (p = 0.61), 1-hourly BSL on OGTT (p = 0.20), 2-hourly BSL on OGTT (p = 0.20), initial vitamin B12 level (p = 0.82), initial 25 (OH) D level (p = 0.40) and with positive family history for DM (p = 0.25).
3.5.2 Logistic Regression (Multivariate Analysis).
In the multivariate analysis, the timing of OGTT and personal history of miscarriages or terminations of pregnancy were inversely and significantly associated with need for pharmacological treatment while fasting BGL was a strong and positive predictor of the need for escalating treatment intervention. Maternal BMI value was no longer significantly associated with the need for pharmacological therapy (Table 4).
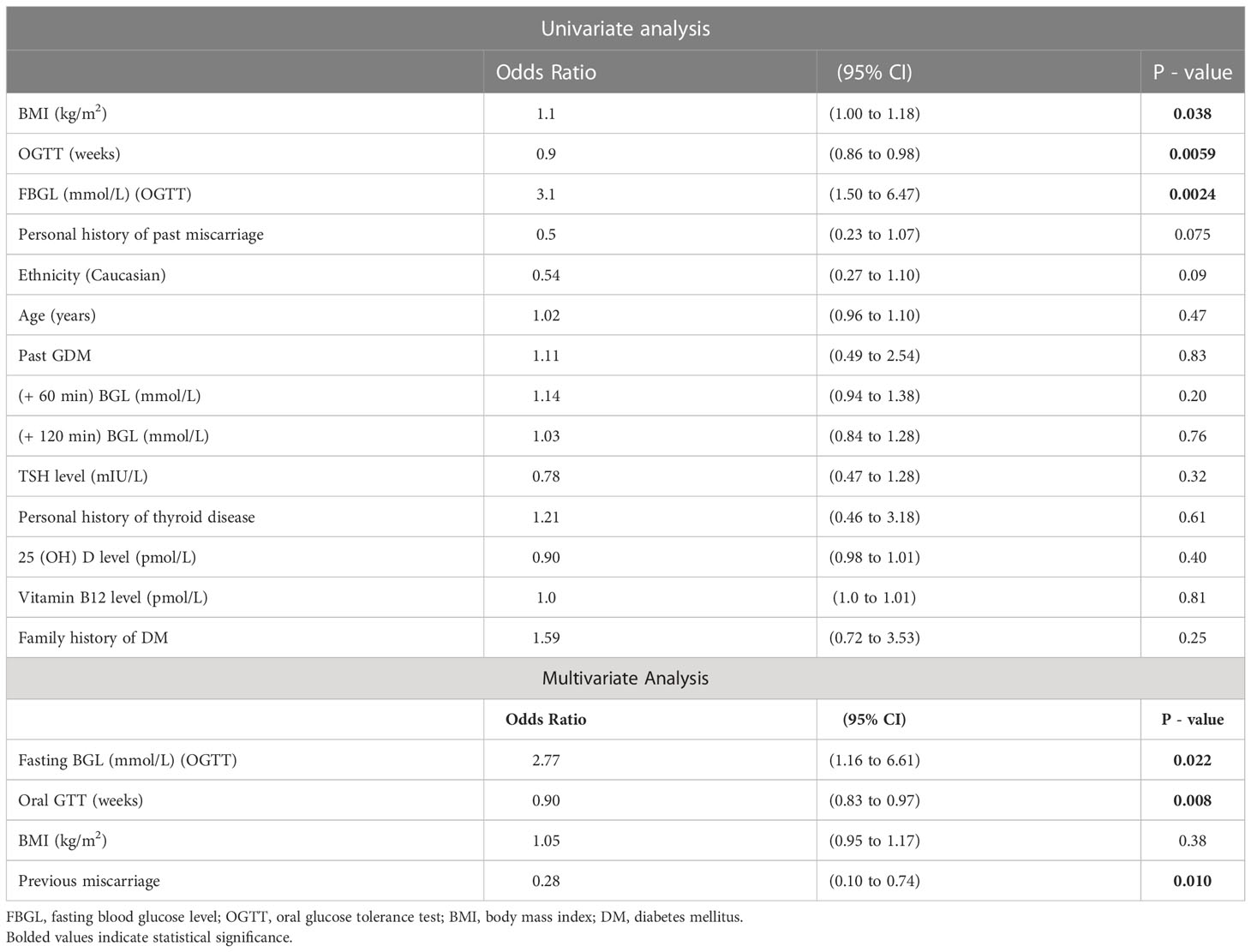
Table 4 Univariate and Multivariate analysis with predictors of pharmacological therapy in patients with gestational diabetes.
4. Discussion
The results from the present study support our hypothesis that metformin treatment (alone or combined with insulin) of women with GDM does not result in worse pregnancy outcomes as compared with those who were assigned to insulin. Despite no significant difference in main composite study aim between study groups and Diet group, insulin treatment alone, although prescribed for small number of GDM patients, was associated with a higher proportion of “small for gestational age” neonates and higher rates of neonatal hypoglycaemia in comparison with dietary or metformin treated groups. Such neonatal complications likely resulted from worse glycaemic control in this group of patients as indicated by their high daily maternal insulin requirements, which ranged between 58 units to 104 units.
Importantly, previous large retrospective cohort study reported that pregnancy outcomes are worse in GDM women with SGA neonates than in those without GDM with subsequent increased risks for respiratory distress syndrome, intrauterine foetal death, hypoglycaemia, jaundice and neonatal demise (19).Interestingly however, the metformin treated group in comparison with dietary intervention group had a higher risk for LSCS. Such association between mode of birth and metformin treatment is related not only to the treatment but also to the differences in our subjects basal characteristic. Previous studies have highlighted that, increased maternal BMI either in overweight or obese category without GDM, increased the risk of macrosomia and caesarean delivery when compared to normal weight women (20). In our metformin treated cohort we observed no evidence of foetal growth acceleration on third trimester ultrasound in majority of patients, likely reflective of satisfactory maternal glucose control (21). Indeed, once we excluded elective LSCS from the analysis there was no difference in number of emergency LSCS in metformin group in comparison with dietary intervention.
To our knowledge no randomised trials (RCTs) compared effects of metformin directly to dietary/lifestyle intervention in pregnancy, although several previous studies compared metformin and insulin interventions alongside dietary interventions for both trial arms. The analysis of 16 RCTs or follow-up of a RCTs revealed that metformin in comparison with insulin treatment did not increase the risk of caesarean section (RR = 0.97; 95% CI, 0.80 to 1.19) (22). Furthermore, the meta-analysis consisting of 11 trials reported that women randomised to metformin had lower risk for adverse maternal and neonatal outcomes including lower risk for the instrumental delivery compared to those randomised to insulin (23). The above data points to metformin being a useful alternative to insulin therapy with a high degree of patient acceptability.
Importantly, in comparison with insulin, metformin can significantly decrease maternal weight gain, and therefore metformin is now the preferred treatment option for an increasing number of women with a BMI in obese category (24). The efficacy of metformin treatment in GDM is not without limitation, as congruent with present study, approximately 14% to 46% of pregnant women fail to achieve adequate glycaemic control with metformin alone (25).
The lack of longer-term safety studies and that metformin can cross the placenta raise potential concerns associated with metformin therapy in pregnancy. The safety and optimal metformin doses in pregnancy have not been yet defined, however most studies use metformin doses ranging from 500 mg to 2500 mg a day (22). In the present study the metformin treatment was commenced in later stages of pregnancy, on average at 28 ± 6.3 weeks of pregnancy, therefore without effect on early embryonic growth.
To date, no increased risk for non-genetic congenital anomalies has been identified following foetal exposure to metformin during the first trimester of pregnancy. A randomized, placebo-controlled trial of PCOS women who were either randomised to metformin (500 mg twice daily increasing to 1000 mg twice daily) or placebo from the first trimester gestational age between 5 and 12 weeks found no difference in the primary composite study outcome of preeclampsia, GDM and preterm delivery (26). Furthermore, no adverse safety signal was detected in randomised, placebo controlled trial of pregnant women with type 2 diabetes in pregnancy who were randomised either to metformin or placebo at 16.5 weeks of pregnancy. This study found no significant difference in congenital anomaly with 7/227 (3%) affected infants in metformin treated group in in comparison with 13/227 (6%) infants in dietary intervention group (p value of 0.16, RR 0.52 (0.22 to 1.28)) (27). Reassuringly, in previous studies, exposure in utero in children of GDM women to metformin ( ± insulin) or insulin alone led to similar total and abdominal body fat percent and metabolic measures at children at 7–9 years of life (28). Conversely, in a recent study, metformin exposure in the first trimester of pre-gestational diabetes was associated with an increased risk of birth defects and pregnancy loss; however, these adverse pregnancy outcomes were attributed to underlying disease rather than to metformin therapy (29). On-going long term follow-up studies of children born to mothers affected by GDM will help answer this current uncertainty.
In the present study, only 33% of GDM patients achieved satisfactory glycaemic control through the dietary therapy while the majority of pregnant GDM women (77%) required pharmacological treatment. The ability to predict a-priori which GDM group of patients will fail their dietary intervention would help to plan steps more effectively in their GDM management.
Past studies have identified the following predictors of the requirement to introduce pharmacological treatment in GDM. These predictors included early GDM diagnosis (e.g., at <25 weeks gestation), a family history of diabetes, non-European ethnicity, an older age, elevated fasting blood glucose level, HbA1c at GDM diagnosis, and an elevated pre- pre-pregnancy BMI (20, 30). In our unique group of pregnant non-obese women, we have identified fewer predictors of the need for pharmacological GDM treatment. Those predictors included maternal characteristics such as baseline maternal BMI, value of fasting BGL on their OGTT, the number of previous miscarriages and early GDM diagnosis (e.g., at <24 weeks gestation). Although on average Caucasian women had a higher fasting glucose on their GTT than women of Asian ethnicity, their ethnicity was not a significant predictor of the need for escalating GDM therapy beyond diet alone. Maternal BMI, once controlled for the fasting BGL level on OGTT and timing of the OGTT and number of previous miscarriages, was no longer a significant indicator of the need for pharmacological GDM therapy.
In previous research, elevated pre-pregnancy maternal BMI predicted failure of dietary therapy (20) with higher maternal BMI being significantly associated with the need for medical treatment (31–33). Although obesity is associated with increasing insulin resistance and pancreatic β-cell dysfunction, it remains unclear whether weight control during pregnancy, as recommended by the Institute of Medicine, would reduce the risk of GDM or the need for insulin therapy (34). Considering that our study patients had an average BMI close to the normal range at 25.8 ± 4.7 kg/m2, we may hypothesise that having an initial BMI in the obese category would have been more closely associated with the need for pharmacological GDM intervention.
In the present research, the value of fasting glucose level on the OGTT was the strongest indicator that women with GDM may not respond to the dietary intervention alone. This study finding is important considering that some at risk women are unable to complete OGTT in pregnancy. Interestingly we have also found the increased risk for LSCS and instrumental vaginal birth with raised fasting BGL in metformin treated group. Interestingly, once the effect of metformin treatment on mode of birth was controlled for fasting glucose on subjects’ OGTT and subjects initial BMI, the risk for LSCS or instrumental vaginal birth increased significantly in metformin treated GDM women. The previous retrospective cohort study of 14,741 pregnant women found that fasting hyperglycaemia was associated with increased risk for caesarean birth (OR: 1.33, 95% CI: 1.15-1.55,P < 0.001) (35). Such strong positive association between fasting hyperglycaemia on OGTT and adverse perinatal outcomes, including caesarean birth, was noted in previous systematic review and meta-analysis of GDM women (36).
Multiple studies have highlighted the link between fasting hyperglycaemia in the first and 2nd trimester of GDM pregnancies with increased occurrence of adverse pregnancy outcomes including the need for surgical birth (4, 37, 38). In previous study only fasting plasma glucose value on the oral glucose tolerance test in pregnancy was significantly associated with pregnancy adverse outcomes, irrespectively of pharmacological intervention (39). Therefore, effective treatment of fasting BGL in women affected by GDM may potentially improve maternal and neonatal health outcomes with a great potential for the early detection of women at risk of having more adverse perinatal outcomes, irrespective of their other risk factors, such as obesity and maternal age.
Interestingly, we observed that having at least one previous miscarriage or pregnancy termination may influence the need for pharmacological treatment for GDM women. Several murine studies have reported that progesterone, which is essential to sustain pregnancy, promotes insulin resistance by multiple mechanisms during pregnancy (40–42). Interestingly, previous case control study of 1567 Korean women demonstrated that threatened miscarriage is associated with decreased risk of GDM and the severity of glucose intolerance (43). Conversely, a retrospective cohort study found that having a spontaneous miscarriage was linked to a higher risk for having subsequent gestational diabetes (44). Further research is recommended to confirm these relationships and to evaluate the pathophysiologic mechanisms that interplay between these common obstetric complications.
Several studies have shown that vitamin B12 status during pregnancy is important to the health of mother and her offspring (45). In the present study, limited by the small sample size, approximately 10% of women had total vitamin B12 level in the insufficiency range and their vitamin B12 levels declined significantly during pregnancy. As metformin treatment may reduce ileal absorption of dietary vitamin B12 (46), GDM women treated with metformin would likely benefit from monitoring of their vitamin B12 status.
Our study is not free of limitations, due to pragmatic method of data collection as part of subjects’ routine clinical care, rather than at fixed short time intervals. Therefore detailed trajectories of weight gain and glycaemic control during pregnancies were not analysed in this study. However an absence of foetal growth acceleration on third trimester ultrasound in the majority of patients was likely reflective of their satisfactory maternal glucose control (21). Exclusion from the analysis of severely obese women with BMI> 35 kg/m2 might have reduced the risk of their pregnancy complications and a rate of instrumental or caesarean birth. Due to our small number of adverse perinatal events, and in order to improve the ability to detect differences in the primary study endpoints as well as to increase study statistical power, we have designed the main composite study outcome of combined maternal and neonatal events. Additionally, we have reported detailed information on clinically important events of which the composite outcome is based on, with their measures of association (Table 2). Additional advantage of this cohort study is consistent and uniform nutritional counselling as well as consistency of care being provided by the same physician.
Our clinical cohort study was relatively small. It is possible that with a bigger cohort, the association between pharmacological interventions and adverse perinatal outcomes may reach statistical significance. However, for the second study aim, when we grouped all pharmacological interventions together, we were able to identify significant predictors for the need of pharmacological treatment in GDM.
In summary, our study addressed the paucity of existing data comparing the effect of metformin intervention in pregnancy with dietary/lifestyle intervention in women with BMI below 35 kg/m2 and gestational diabetes. The present study has highlighted that metformin treatment of GDM may not be associated with different pregnancy outcomes compared to the GDM managed by diet except for the increased risk for the LSCS. These study findings, however, were no longer significant once the analysis was controlled for the number of elective caesarean sections.
We have also observed that elevated fasting blood glucose on OGTT, in metformin treated GDM women, is a stronger predictor of their need for either instrumental delivery or caesarean section. Moreover, due to combined demographic, obstetric and medical data we have identified the local characteristics of women with GDM, which would help to predict their need for pharmacological therapy. This predictive model will improve streamlining of our patients’ care and improve utilization of local hospital resources. Further studies are still needed to identify the most effective and safe management of gestational diabetes within the public hospital setting.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by The Southern Eastern Sydney Local Health District Human Research Ethics Committee (Study Reference No. RESP/15/107). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
Study design: MMB, AP, DB, AO'S. Study conduct: MMB. Data collection: MMB, AP. Data analysis: MMB, AP, DB, AKP. Data interpretation: MMB, AP, DB, AKP, AZ, AO'S. Drafting manuscript: MMB. Revising manuscript content: MMB, AP, DB, AKP, AZ, AO’S. Approving final version of manuscript: all authors. MB is the guarantor of this work. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to thank Dr Fariba Daniel and Dr Ruby Chang for their help with data entry for this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Shah NS, Wang MC, Freaney PM, Perak AM, Carnethon MR, Kandula NR, et al. Trends in gestational diabetes at first live birth by race and ethnicity in the US, 2011-2019. JAMA (2021) 326(7):660–9. doi: 10.1001/jama.2021.7217
2. Nankervis A, McIntyre HD, Moses RG, Ross GP, Callaway LK. Testing for gestational diabetes mellitus in Australia. Diabetes Care (2013) 36(5):e64. doi: 10.2337/dc12-2345
3. American Diabetes A. 2. classification and diagnosis of diabetes: Standards of medical care in diabetes-2018. . Diabetes Care (2018) 41(Suppl 1):S13–27.
4. Wang C, Wei Y, Zhang X, Zhang Y, Xu Q, Sun Y, et al. A randomized clinical trial of exercise during pregnancy to prevent gestational diabetes mellitus and improve pregnancy outcome in overweight and obese pregnant women. Am J Obstet Gynecol (2017) 216(4):340–51. doi: 10.1016/j.ajog.2017.01.037
5. Group HSCR, Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med (2008) 358(19):1991–2002.
6. Vounzoulaki E, Khunti K, Abner SC, Tan BK, Davies MJ, Gillies CL. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ (2020) 369:m1361.
7. Kramer CK, Campbell S, Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia (2019) 62(6):905–14. doi: 10.1007/s00125-019-4840-2
8. Hartling L, Dryden DM, Guthrie A, Muise M, Vandermeer B, Donovan L. Benefits and harms of treating gestational diabetes mellitus: a systematic review and meta-analysis for the U.S. preventive services task force and the national institutes of health office of medical applications of research. Ann Intern Med (2013) 159(2):123–9.
9. American Diabetes A. 13. management of diabetes in pregnancy: Standards of medical care in diabetes-2018. Diabetes Care (2018) 41(Suppl 1):S137–S43.
10. Scarpello JH, Howlett HC. Metformin therapy and clinical uses. Diabetes Vasc Dis Res (2008) 5(3):157–67. doi: 10.3132/dvdr.2008.027
11. Diabetes in pregnancy: management from preconception to the postnatal period. London: National Institute for Health and Care Excellence: Guidelines (2020).
12. Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med (2005) 352(24):2477–86. doi: 10.1056/NEJMoa042973
13. Bogdanet D, Egan A, Reddin C, Kirwan B, Carmody L, Dunne F. ATLANTIC DIP: Despite insulin therapy in women with IADPSG diagnosed GDM, desired pregnancy outcomes are still not achieved. what are we missing? Diabetes Res Clin Pract (2018) 136:116–23. doi: 10.1016/j.diabres.2017.12.003
14. Tsakiridis I, Giouleka S, Mamopoulos A, Kourtis A, Athanasiadis A, Filopoulou D, et al. Diagnosis and management of gestational diabetes mellitus: An overview of national and international guidelines. Obstet Gynecol Surv (2021) 76(6):367–81. doi: 10.1097/OGX.0000000000000899
15. Blumer I, Hadar E, Hadden DR, Jovanovic L, Mestman JH, Murad MH, et al. Diabetes and pregnancy: an endocrine society clinical practice guideline. J Clin Endocrinol Metab (2013) 98(11):4227–49. doi: 10.1210/jc.2013-2465
16. Practice bulletin no. 137: Gestational diabetes mellitus. Obstet Gynecol (2013) 122(2 Pt 1):406–16.
17. Villar J, Cheikh Ismail L, Victora CG, Ohuma EO, Bertino E, Altman DG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the newborn cross-sectional study of the INTERGROWTH-21st project. Lancet (2014) 384(9946):857–68. doi: 10.1016/S0140-6736(14)60932-6
18. Koh TH, Aynsley-Green A, Tarbit M, Eyre JA. Neural dysfunction during hypoglycaemia. Arch Dis Child (1988) 63(11):1353–8. doi: 10.1136/adc.63.11.1353
19. Esakoff TF, Guillet A, Caughey AB. Does small for gestational age worsen outcomes in gestational diabetics? J Matern Fetal Neonatal Med (2017) 30(8):890–3. doi: 10.1080/14767058.2016.1193142
20. Barnes RA, Wong T, Ross GP, Jalaludin BB, Wong VW, Smart CE, et al. A novel validated model for the prediction of insulin therapy initiation and adverse perinatal outcomes in women with gestational diabetes mellitus. Diabetologia (2016) 59(11):2331–8. doi: 10.1007/s00125-016-4047-8
21. Group HSCR. Hyperglycemia and adverse pregnancy outcome (HAPO) study: associations with neonatal anthropometrics. Diabetes (2009) 58(2):453–9.
22. Butalia S, Gutierrez L, Lodha A, Aitken E, Zakariasen A, Donovan L. Short- and long-term outcomes of metformin compared with insulin alone in pregnancy: A systematic review and meta-analysis. Diabetes Med (2017) 34(1):27–36. doi: 10.1111/dme.13150
23. Farrar D, Simmonds M, Bryant M, Sheldon TA, Tuffnell D, Golder S, et al. Treatments for gestational diabetes: a systematic review and meta-analysis. BMJ Open (2017) 7(6):e015557. doi: 10.1136/bmjopen-2016-015557
24. D'Ambrosio V, Brunelli R, Vena F, Di Mascio D, Marchetti C, Boccherini C, et al. Metformin reduces maternal weight gain in obese pregnant women: A systematic review and meta-analysis of two randomized controlled trials. Diabetes Metab Res Rev (2019) 35(6):e3164. doi: 10.1002/dmrr.3164
25. Rowan JA, Hague WM, Gao W, Battin MR, Moore MP, Mi GTI. Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med (2008) 358(19):2003–15. doi: 10.1056/NEJMoa0707193
26. Vanky E, Stridsklev S, Heimstad R, Romundstad P, Skogoy K, Kleggetveit O, et al. Metformin versus placebo from first trimester to delivery in polycystic ovary syndrome: a randomized, controlled multicenter study. J Clin Endocrinol Metab (2010) 95(12):E448–55. doi: 10.1210/jc.2010-0853
27. Feig DS, Donovan LE, Zinman B, Sanchez JJ, Asztalos E, Ryan EA, et al. Metformin in women with type 2 diabetes in pregnancy (MiTy): a multicentre, international, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol (2020) 8(10):834–44. doi: 10.1016/S2213-8587(20)30310-7
28. Rowan JA, Rush EC, Plank LD, Lu J, Obolonkin V, Coat S, et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition and metabolic outcomes at 7-9 years of age. BMJ Open Diabetes Res Care (2018) 6(1):e000456.
29. Panchaud A, Rousson V, Vial T, Bernard N, Baud D, Amar E, et al. Pregnancy outcomes in women on metformin for diabetes or other indications among those seeking teratology information services. Br J Clin Pharmacol (2018) 84(3):568–78. doi: 10.1111/bcp.13481
30. Watanabe M, Katayama A, Kagawa H, Ogawa D, Wada J. Risk factors for the requirement of antenatal insulin treatment in gestational diabetes mellitus. J Diabetes Res (2016) 2016:9648798. doi: 10.1155/2016/9648798
31. Meshel S, Schejter E, Harel T, Maslovitz S, Germez N, Elimelech B, et al. Can we predict the need for pharmacological treatment according to demographic and clinical characteristics in gestational diabetes? J Matern Fetal Neonatal Med (2016) 29(13):2062–6. doi: 10.3109/14767058.2015.1077225
32. Wong VW, Jalaludin B. Gestational diabetes mellitus: who requires insulin therapy? Aust N Z J Obstet Gynaecol (2011) 51(5):432–6. doi: 10.1111/j.1479-828X.2011.01329.x
33. Ali A, Shastry S, Nithiyananthan R, Ali A, Ganapathy R. Gestational diabetes-predictors of response to treatment and obstetric outcome. Eur J Obstet Gynecol Reprod Biol (2018) 220:57–60. doi: 10.1016/j.ejogrb.2017.11.014
34. Al-Goblan AS, Al-Alfi MA, Khan MZ. Mechanism linking diabetes mellitus and obesity. Diabetes Metab Syndr Obes (2014) 7:587–91. doi: 10.2147/DMSO.S67400
35. Feng H, Zhu WW, Yang HX, Wei YM, Wang C, Su RN, et al. Relationship between oral glucose tolerance test characteristics and adverse pregnancy outcomes among women with gestational diabetes mellitus. Chin Med J (Engl) (2017) 130(9):1012–8. doi: 10.4103/0366-6999.204928
36. Farrar D, Simmonds M, Bryant M, Sheldon TA, Tuffnell D, Golder S, et al. Hyperglycaemia and risk of adverse perinatal outcomes: systematic review and meta-analysis. BMJ (2016) 354:i4694.
37. Seabra G, Saunders C, de Carvalho Padilha P, Zajdenverg L, da Silva LB, de Souza Santos MM. Association between maternal glucose levels during pregnancy and gestational diabetes mellitus: an analytical cross-sectional study. Diabetol Metab Syndr (2015) 7:17. doi: 10.1186/s13098-015-0013-8
38. Riskin-Mashiah S, Younes G, Damti A, Auslender R. First-trimester fasting hyperglycemia and adverse pregnancy outcomes. Diabetes Care (2009) 32(9):1639–43. doi: 10.2337/dc09-0688
39. Ryan EA, Savu A, Yeung RO, Moore LE, Bowker SL, Kaul P. Elevated fasting vs post-load glucose levels and pregnancy outcomes in gestational diabetes: a population-based study. Diabetes Med (2020) 37(1):114–22. doi: 10.1111/dme.14173
40. Ryan EA, Enns L. Role of gestational hormones in the induction of insulin resistance. J Clin Endocrinol Metab (1988) 67(2):341–7. doi: 10.1210/jcem-67-2-341
41. Wada T, Hori S, Sugiyama M, Fujisawa E, Nakano T, Tsuneki H, et al. Progesterone inhibits glucose uptake by affecting diverse steps of insulin signaling in 3T3-L1 adipocytes. Am J Physiol Endocrinol Metab (2010) 298(4):E881–8. doi: 10.1152/ajpendo.00649.2009
42. Picard F, Wanatabe M, Schoonjans K, Lydon J, O'Malley BW, Auwerx J. Progesterone receptor knockout mice have an improved glucose homeostasis secondary to beta -cell proliferation. Proc Natl Acad Sci U S A. (2002) 99(24):15644–8. doi: 10.1073/pnas.202612199
43. Lee HJ, Norwitz E, Lee B. Relationship between threatened miscarriage and gestational diabetes mellitus. BMC Pregnancy Childbirth (2018) 18(1):318. doi: 10.1186/s12884-018-1955-2
44. Zhao Y, Zhao Y, Fan K, Jin L. Association of history of spontaneous or induced abortion with subsequent risk of gestational diabetes. JAMA Netw Open (2022) 5(3):e220944. doi: 10.1001/jamanetworkopen.2022.0944
45. Rogne T, Tielemans MJ, Chong MF, Yajnik CS, Krishnaveni GV, Poston L, et al. Associations of maternal vitamin B12 concentration in pregnancy with the risks of preterm birth and low birth weight: A systematic review and meta-analysis of individual participant data. Am J Epidemiol (2017) 185(3):212–23. doi: 10.1093/aje/kww212
Keywords: gestational diabetes mellitus, dietary intervention, perinatal outcomes, metformin, treatment predictors
Citation: Brzozowska MM, Puvanendran A, Bliuc D, Zuschmann A, Piotrowicz AK and O’Sullivan A (2023) Predictors for pharmacological therapy and perinatal outcomes with metformin treatment in women with gestational diabetes. Front. Endocrinol. 14:1119134. doi: 10.3389/fendo.2023.1119134
Received: 08 December 2022; Accepted: 18 January 2023;
Published: 30 January 2023.
Edited by:
Giulio Frontino, San Raffaele Hospital (IRCCS), ItalyReviewed by:
Antonella Poloniato, San Raffaele Hospital (IRCCS), ItalySally Abell, Tasmanian Health Service (THS), Australia
Copyright © 2023 Brzozowska, Puvanendran, Bliuc, Zuschmann, Piotrowicz and O’Sullivan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Malgorzata M. Brzozowska, TWFsZ29yemF0YS5Ccnpvem93c2thQGhlYWx0aC5uc3cuZ292LmF1; bWJyem96b3dza2FAaG90bWFpbC5jb20=
 Malgorzata M. Brzozowska
Malgorzata M. Brzozowska Anita Puvanendran1
Anita Puvanendran1