- 1Department of Paediatric Endocrinology, Royal Manchester Children’s Hospital, Manchester, United Kingdom
- 2Department of Computer Science, University of Manchester, Manchester, United Kingdom
- 3Section of Metabolic Diseases, Beatrix Children’s Hospital, University of Groningen, Groningen, Netherlands
- 4Department of Paediatrics, University of Cambridge, Cambridge, United Kingdom
- 5Faculty of Biology, Medicine and Health, University of Manchester, Manchester, United Kingdom
In 2023, childhood hypoglycaemia remains a major public health problem and significant risk factor for consequent adverse neurodevelopment. Irrespective of the underlying cause, key elements of clinical management include the detection, prediction and prevention of episodes of hypoglycaemia. These tasks are increasingly served by Continuous Glucose Monitoring (CGM) devices that measure subcutaneous glucose at near-continuous frequency. While the use of CGM in type 1 diabetes is well established, the evidence for widespread use in rare hypoglycaemia disorders is less than convincing. However, in the few years since our last review there have been multiple developments and increased user feedback, requiring a review of clinical application. Despite advances in device technology, point accuracy of CGM remains low for children with non-diabetes hypoglycaemia. Simple provision of CGM devices has not replicated the efficacy seen in those with diabetes and is yet to show benefit. Machine learning techniques for hypoglycaemia prevention have so far failed to demonstrate sufficient prediction accuracy for real world use even in those with diabetes. Furthermore, access to CGM globally is restricted by costs kept high by the commercially-driven speed of technical innovation. Nonetheless, the ability of CGM to digitally phenotype disease groups has led to a better understanding of natural history of disease, facilitated diagnoses and informed changes in clinical management. Large CGM datasets have prompted re-evaluation of hypoglycaemia incidence and facilitated improved trial design. Importantly, an individualised approach and focus on the behavioural determinants of hypoglycaemia has led to real world reduction in hypoglycaemia. In this state of the art review, we critically analyse the updated evidence for use of CGM in non-diabetic childhood hypoglycaemia disorders since 2020 and provide suggestions for qualified use.
1 Introduction
In 2023, non-diabetes hypoglycaemia remains a major global problem for children. Its effects are far reaching, with impacts on quality of life (1, 2), health economics (3), hypoglycaemia fear (4), reaching beyond the individual to the extended family (5, 6). Although recent studies (7), complimenting previous work (8, 9), have suggested a lesser effect of transient neonatal hypoglycaemia (10), there remains little doubt of the impact of severe childhood hypoglycaemia on neurodevelopmental delay, particularly in those children with severe and recurrent hypoglycaemia due to congenital hyperinsulinism (CHI) (9–11).
Essential to all hypoglycaemia management, irrespective of the cause, is the detection, prediction and prevention of episodes through glucose testing (12, 13). The first of these three tasks has been traditionally performed by fingerprick blood glucose testing (13), with prediction and prevention reliant on clinical skill and patient experience. However, over recent years, all three tasks are increasingly being performed by continuous glucose monitoring (CGM) in either its raw form or through its manipulation by modern computer algorithmics. For people living with diabetes, CGM and associated predictive algorithms are widely used and well established in the reduction of hypoglycaemia (14–17) and cost-effectiveness (18–20). However, for those with a non-diabetes hypoglycaemia disorder, the utility in diabetes has not been replicated and CGM has not been established in routine clinical practice.
The use of CGM in rare hypoglycaemia disorders is a rapidly evolving and expanding field. In this review we have followed on from a comprehensive review in 2020 (13), to provide an update on improvements in the technology and utility of CGM focusing mainly on CHI, glycogen storage diseases (GSD) and neonatal prematurity. We reflect on our predictions from 2020, synthesise current understanding and look to the future.
2 Accuracy
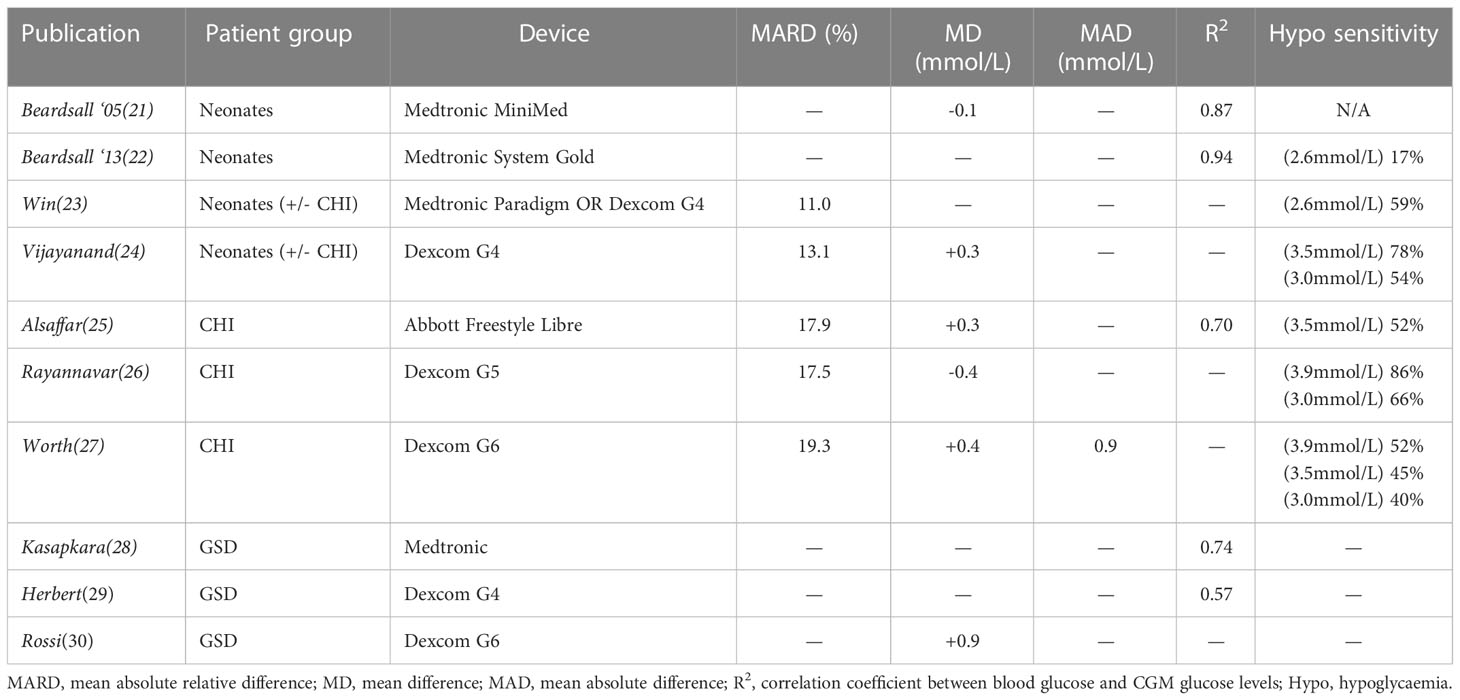
We have detailed the background to accuracy assessments in CGM elsewhere (13) but it is worth outlining the two differing approaches to accuracy assessment: 1) pairing CGM values with fingerprick glucometer values and measuring difference; 2) evaluating the ability of CGM devices to ‘detect’ hypo(or hyper)glycaemia within a time window and thus utilising to a fuller extent the semi-continuous nature of CGM. Measures of accuracy differ widely throughout the literature, but the former is more commonly used and tends to incorporate mean absolute relative difference (MARD), mean absolute difference and hypoglycaemia sensitivity/specificity. A summary of CGM accuracy studies by various groups using different CGM devices in non-diabetes hypoglycaemia is presented in Table 1.
2.1 Neonates
Beardsall et al. first evaluated the accuracy of CGM devices in neonates in 2005 (21) and later in 2013 (22); they reported a correlation coefficient of 0.69-0.94 with safe results on an error grid (albeit one designed for those with diabetes). However, hypoglycaemia sensitivity was found to be only 17%. More recent results from the same group showed a relatively small MARD of 11% but a hypoglycaemia sensitivity of only 59% with the latest devices and technologies (23). These calculations were based on a lower threshold for hypoglycaemia (<2.6mmol/l) than is usually used outside the neonatal unit. Furthermore, as described above, sensitivity is based on point comparisons of accuracy which can underestimate the clinical value of sensor glucose trends in detecting hypoglycaemic events. Recent work in Australia by Vijayanand et al. (24) has confirmed the poor hypoglycaemia sensitivity seen in this group with results of 54% when using point comparisons.
2.2 Childhood hypoglycaemia disorders
CGM is not routinely used in patients with CHI and therefore data is relatively sparse (Table 1). In the first evaluation of CGM in CHI, Alsaffar et al. (25) reported a hypoglycaemia (3.5mmol/L) sensitivity of only 52% but did not report a MARD. While an evaluation of a more up to date device by Rayannavar et al. (26) showed a better hypoglycaemia sensitivity of 86%, this was calculated using a higher cut-off for hypoglycaemia (3.9mmol/L), as is standard practice in some countries. When hypoglycaemia <3.0mmol/L was investigated, a low sensitivity of 66% was demonstrated. As existing error grids (such as Parks and Clarke) are designed for evaluation of CGM accuracy for those with diabetes, they have not been used as standard in assessments in CHI. Recently Worth et al. (27) developed an expert-consensus error grid for use in CHI and used this to evaluate the accuracy of one of the most recent CGM sensors, the Dexcom G6. Results suggested the presence of significant clinical risk in the use of CGM for patients with CHI due to poor device accuracy on error grid analysis and hypoglycaemia sensitivity of only 45%. Analysis of the ability of the Dexcom G6 to detect glucometer-measured hypoglycaemia within a 30 minute window was marginally better but still unreliable at 51% (27).
Equally, CGM is also not used routinely in patients with GSD and assessments of CGM accuracy for this group have been largely incomplete (Table 1). These demonstrate correlation between CGM and glucometer values but the magnitude of error has not been reported. Papers (28, 29) report mean difference or correlation but due to the presence of both overestimation and underestimation, and no report of mean absolute difference, it is impossible to determine the average magnitude of errors. Rossi et al. (30) went on to evaluate CGM error by glucose value and also between those with GSD1a and healthy volunteers. They found that CGM overestimation was worse for those with GSD1a and at glucose values <3.9mmol/L, thereby increasing the risk of missed hypoglycaemia for the most vulnerable groups at the time of greatest need.
3 Efficacy of CGM to detect and prevent hypoglycaemia
We have previously summarised the efficacy of CGM for children with non-diabetes hypoglycaemia due to various conditions (13). Here we summarise recent developments in the field with regards to the conventional use of CGM to detect and prevent hypoglycaemia by simple provision to patients and clinicians. The non-conventional use of CGM is discussed later in Section 7.
3.1 Neonates
Previously summarised studies (13) have demonstrated the utility of CGM to reduce painful procedures, detect unsuspected hypoglycaemia and reduce hyperglycaemia. More recently, Fernández Martínez et al. (31) confirmed the ability of CGM to detect unsuspected and prolonged hypoglycaemia in very low birth weight (VLBW) neonates. Win et al. (23) have since demonstrated significant fluctuations in glucose in neonates; more pronounced in those with CHI. The same group recently published the results of an international, multi-centre RCT investigating the use of CGM in preterm neonates and clearly demonstrated a reduction in hypoglycaemia and hyperglycaemia for those in the CGM group (32) encouraging CGM as a potential tool for regular use in the neonatal intensive care unit.
3.2 Hypoglycaemia associated with rare endocrine conditions
At the time of our previous review in 2020, there was no evidence for CGM reducing hypoglycaemia for children with any endocrine conditions other than diabetes mellitus. In the absence of larger scale studies, we discussed (13) minimal evidence for use of CGM for both adults and children with adrenal insufficiency (AI) and the anecdotal reports of CGM use for those with CHI.
Further single-case, anecdotal reports of utility of CGM in CHI (33) and hypopituitarism (34) have since been published. Importantly however, Worth et al. have recently published non-randomised data on CHI patients with periods of blinded and unblinded CGM (35); suggesting that the simple provision of CGM (without expert or algorithmic interpretative support) does not reduce hypoglycaemia for those with CHI. The addition of interpretative algorithmic or clinical support is discussed in Section 7. However, at the time of writing, there are no comprehensive studies evaluating the efficacy of CGM to reduce hypoglycaemia for children with endocrine hypoglycaemia.
3.3 Hypoglycaemia associated with rare hereditary metabolic disorders
We have previously outlined (13) the utility of CGM to detect unsuspected hypoglycaemia and facilitate manipulation of diet and treatment for patients with GSD. Previous anecdotal reports highlighted the utility of retrospective CGM data analysis but advised against the provision of real-time CGM to patients for fear of inappropriate treatment alterations (36). Since our previous review, there have been further anecdotal reports of CGM utility in the detection of glycaemic variability and excursions for patients with metabolic causes of hypoglycaemia (37–39) but no systematic evaluations of the use of CGM to actually prevent or reduce hypoglycaemia.
4 Family perspectives
Our previous review discussed families with CHI and GSD reporting marginal benefit from the use of CGM as secondary outcomes of studies. Anecdotally, families found glucose trends helpful. Since 2020, the significant increase in the use of CGM in hypoglycaemia disorders has led to an increase in literature regarding families’ perceptions of this emerging technology, described below.
4.1 Patient charity reports
Patient charities fulfil a vital role of providing support to those with hypoglycaemia conditions but also provide an important window into the views and opinions of families. In a recent unpublished study (summarised in an opinion paper (40)), the UK Children’s Hyperinsulinism Charity (UK CHC) reported that families with CHI find CGM: offers a safety net, improves quality of life, and reduces worry. Patients reported (40) difficulty in access to CGM and a call was made for wider availability for families with CHI. While this survey is likely subject to significant positive sampling bias, it does offer an important insight into the opinions of some families with CHI.
The charity Congenital Hyperinsulinism International (CHI) recently revealed that 45.7% of respondents to a global registry use CGM but that access to devices is often a problem and trust in the data generated is often low (2). They also report that families generally find devices useful but that patients experience problems with poor accuracy (6). Again, this is likely open to sampling bias but offers an important user-perspective. Within GSD, CGM is a much higher research priority for healthcare professionals than it is for patients and carers who rank it as a lower priority (41).
4.2 Qualitative studies
While patient organisations have called for wider access to CGM, it is important to formally assess families’ experiences of CGM to actively seek out both positive and negative views. As recently highlighted by Peeks et al. (42), “glucose management as assessed with CGM should be balanced against psychosocial well-being and quality of life” which cannot be assumed to be higher with CGM than without.
In CHI patients, Auckburally et al. (43) undertook semi-structured interviews with families who had been provided with a CGM for 12 weeks as part of a research project. As there was no existing information on CHI families’ experiences of CGM, the authors performed a thematic analysis to identify themes important to patients and their families. Such detailed analysis revealed a rich and complex mixture of attitudes towards CGM. Families reported positive feelings about CGM’s function as an educational tool which could motivate behavioural changes to prevent hypoglycaemia. However, the problematic issues of poor accuracy and irritating alarms were raised by all participants.
In order to better understand the reasons for a high rate of dissatisfaction with CGM seen in CHI families, Ahmad et al. (44) performed semi-structured telephone interviews with those who had discontinued use. Primary reasons for discontinuation were pain, device inaccuracy, issues with technical setup and 90% of those surveyed thought that CGM device use would have been easier if their child had been a different age (either younger or older) (44). Comprehensive assessments of families’ experiences of CGM, with a focus on the reduction of selection bias, are essential in the journey to establish CGM as a therapeutic option for paediatric hypoglycaemia disorders. The authors are aware of two separate studies aiming to achieve this for families with CHI and the results are eagerly awaited.
5 Barriers to the use of CGM
In our 2020 review we highlighted the barriers to wider use of CGM in paediatric hypoglycaemia disorders and to date there are no improvements with regards to lag time, alarms or fingerprick testing. However, with regards to clinician inertia and cost, an update is worthwhile.
5.1 Clinician inertia and usability
Over the last three years, the authors have noticed a significant increase in the interest in CGM by clinicians working in paediatric hypoglycaemia disorders. There is now less suspicion of the technology and a higher acceptance of using CGM as a routine part of care. This is mirrored in the significant increase in publications relating to CGM in both hypoglycaemia disorders and neonatology. However, the interest and marketing strategy of device manufacturers remains firmly focused on diabetes mellitus, precluding wider adoption and development specific to hypoglycaemia.
5.2 Cost and widening access
As CGM technology develops, it is important that the availability of devices is considered, especially for those in low-income countries (LICs) and for patients with rare diseases. These groups are often marginalised and disadvantaged in the commercially-driven push for technological progression but efforts must be made to minimise access inequalities (45). As a technology, CGM could arguably have significant impact in LICs due to the added burden of hypoglycaemia from malaria, malnutrition, diarrhoea and sepsis (46). Additionally, for people living with diabetes, access to insulin is often intermittent in LICs (47), leading to hypoglycaemia and hyperglycaemia. CGM would also be highly valuable in the neonatal setting as capacity for regular glucose monitoring in neonatal units in LICs is often limited and neonatal mortality is high (48). Indeed, neonatal hypoglycaemia is often present in otherwise uncomplicated newborn infants, and recognition and treatment may have a significant impact on neonatal outcomes (49, 50).
Moreover, the long-term impacts associated with childhood hypoglycaemia, such as neurodisability, epilepsy and reduced cognitive function (9, 51) have a higher burden in LICs, being poorly understood by wider society and suboptimally managed due to meagre resources (52–54). So, while the costs of CGM may be high, its implementation may enable faster, accurate treatment modification, improving outcomes (38) and likely contributing to value based healthcare in both common, high volume disease (55) and rare, low volume disease such as GSD (56). However, it is important to recognise that technology developed for a high-income setting is not always appropriate for LICs where the environment is different; there can be extremes of temperatures, intermittent access to internet and electricity, high levels of dust and minimal access to engineers to repair devices (50, 57–59). A target product profile (TPP)-based approach has been developed to identify key specifications for product innovation in LICs. This approach has been particularly successful in development of neonatal devices, most notably in bubble CPAP, and a similar approach should be considered in the development of CGM devices (50, 60).
6 Updates on previously suggested developments
In our 2020 review we predicted that future developments would be focused on CGM device technology and predictive hypoglycaemia algorithms. Here we provide an update on the developments in these areas over the last three years before moving on to discuss alternative and novel areas for CGM use in Section 7.
6.1 CGM device technology
The direction for CGM device technology development continues towards miniaturisation, with a focus on reducing the invasive nature of some CGM devices. Dexcom® have since released the G7 device which is smaller, thinner and predicted to be more accurate. Abbott® have released the Freestyle Libre 3, also smaller and thinner and now offering real time readings with optional alerts. Eversense® now have an implantable sensor with a six month wear time and requiring only a single calibration per day.
There has also been significant interest in the last few years on optical sensors that detect photons to determine the glucose concentration via the interaction between glucose molecules and different wavelengths of light (61). Other sensor developments focus on the non-invasive measurement of sweat, urine, saliva, tears (62) and even thermal monitoring (63); however, these ideas have not yet translated to a commercially viable stage.
6.2 Predictive hypoglycaemia algorithms
Our 2020 review (13) outlined the background to the use of predictive algorithms for hypoglycaemia and the different forms that these can take; physiological, data-driven, and hybrid (64). While non-machine learning algorithms such as Model Predictive Control have been beneficial for adults (65) and neonates (66) using closed loop insulin delivery, these systems are of no use to the majority of patients with rare hypoglycaemia disorders whose hypoglycaemia is not caused by exogenous insulin. Work in the field of data-driven predictions continues to expand rapidly in diabetes and artificial intelligence and machine learning methods using large historical datasets continue to be used to derive theoretical prediction models (Figure 1). While, multiple groups have continued to publish increasingly accurate in-silico algorithms (67–70), these have been evaluated by systematic review (71) and meta-analysis (72) and found to have insufficient ability to detect and prevent hypoglycaemia. The authors conclude that improvement is required before application in clinical settings. As suggested, these algorithms have been evaluated in-silico only with no conclusive examples of Machine Learning-driven predictive algorithms reducing hypoglycaemia in the real world.
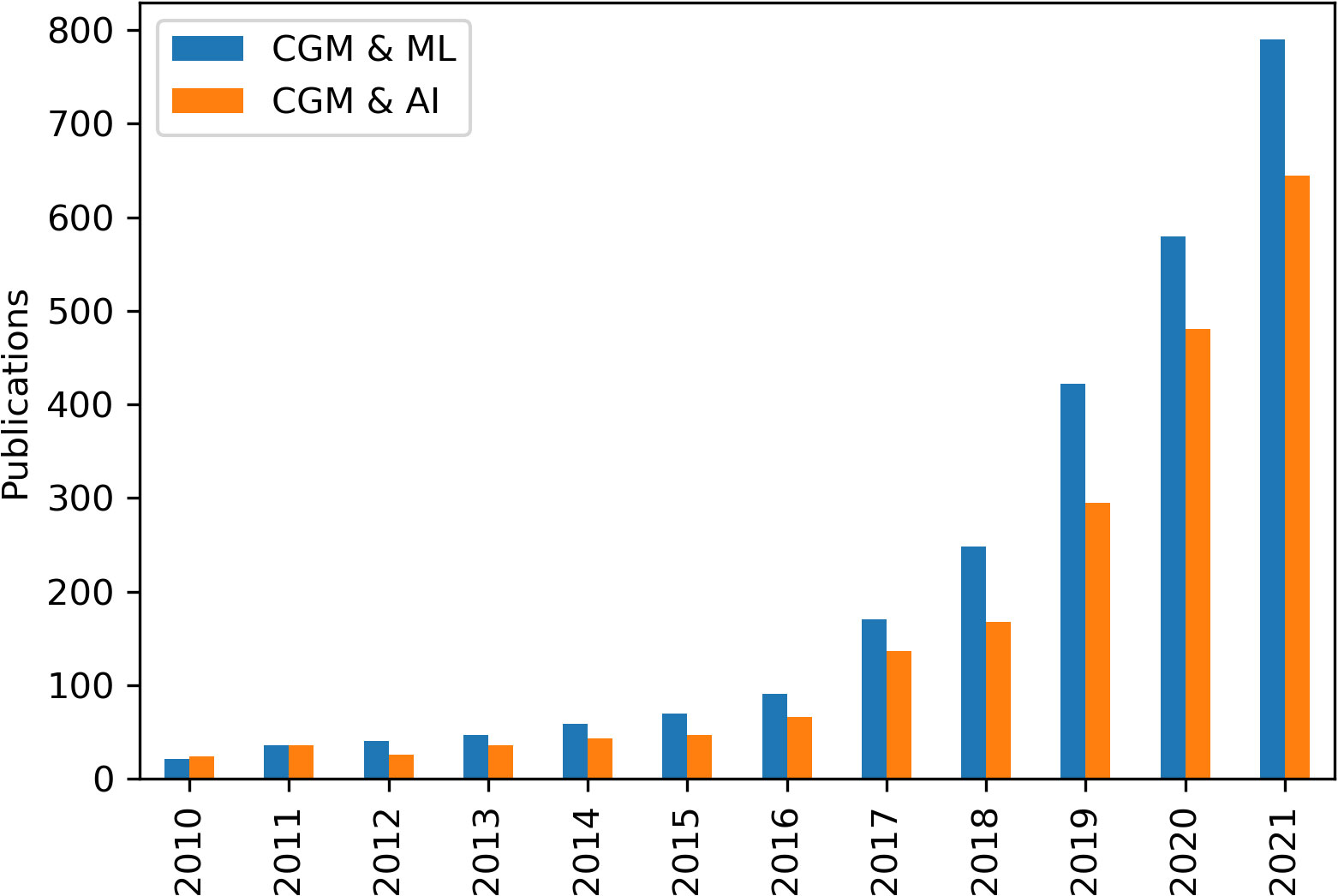
Figure 1 Publications by year with the search terms "continuous glucose monitoring" (CGM) and "machine learning" (ML) or “artificial intelligence” (AI), found on Google Scholar.
Decision Support Systems (DSS) are an extension of glucose predictive algorithms and facilitate decision making (e.g. food intake) based on various inputs (e.g. CGM data) and predicted outcomes (e.g. hypoglycaemia). Recent DSSs have shown in-silico (73) and possibly real world (74) reduction in hypoglycaemia through modification of insulin dosing for people living with diabetes. However, Tyler et al. (75) note in their systematic review that “it has not yet been shown that a DSS can improve time in range in human studies” and more work is required. Vitally, all DSSs focus on the use of exogenous insulin as either an input or output and are therefore of no use to those with a rare hypoglycaemia disorder such as CHI or GSD but may have potential in neonates on insulin therapy (66).
7 Novel directions and a possible future for CGM in hypoglycaemia
So far we have provided updates on areas covered in our previous review. In this section we move on to discuss novel areas and uses for CGM which have either emerged since 2020 or are now gaining prominence. Person-centred outcome measures have been defined for type 1 diabetes (76, 77) but are currently lacking for rare hypoglycaemia disorders. This causes difficulty in comparing studies and evaluating day to day impact for patients. Consensus, person-centred outcomes would greatly enhance routine healthcare and research for these groups, particularly with regards to emerging but as yet unproven technologies such as CGM.
7.1 CGM to elicit patterns and digital phenotypes
There is increasing recognition of phenotypes beyond those classically described by physical traits or cellular changes. Most recently established is the “digital phenotype” (78). The digital phenotype covers both aspects of behaviours related to technology such as social media use use as well as behaviours measured by technology such as heart rate monitors, accelerometers and CGM. These new measures facilitate a more comprehensive and individualised picture of patients’ health and contribute to “P4 medicine” (79); allowing for a predictive, preventative, personalised and participatory approach to management.
Worth et al. (80) took the first steps towards extending the digital phenotype of CHI with their analysis of retrospectively collected CGM data. Previously collected CGM data was used to identify periods of high hypoglycaemia risk in the early morning in patients with CHI; opening the door for targeted interventions on a group and individual level. Further work by this group (81) investigated patterns of hypoglycaemia at an individual level and found that each patient with CHI had clear and individual weekly patterns for repeated hypoglycaemia. Peeks et al. (42) performed a similar analysis in patients with hepatic GSD to provide the first insight into CGM profiles in this patient group and similarly concluded that analysis on a group level was of some use but improved when performed on an individual basis.
Further contributions to the digital phenotypes of hypoglycaemia disorders have been made by Rossi et al. (30) who provided CGM metrics for glycaemic variation and control in adult patients with GSD1a and compared this to healthy volunteers. Worth et al. (82) performed a similar analysis for patients with CHI on a larger scale (3.4 million data points) but without healthy controls to establish a national baseline of hypoglycaemia and confirm earlier reports (80) of daily hypoglycaemia patterns at a group level. Finally, Park et al. (83) recently reported preliminary data from the GRACE trial, establishing the extent of glucose variability in children with adrenal insufficiency compared to healthy controls.
7.2 CGM as a behaviour change tool
CGM is still in its infancy as a technology and new ways are being explored to derive positive impact for patients’ health. Traditional usage has focused on high frequency glucose data to allow patients to adjust insulin doses and to predict upcoming excursions from euglycaemia. As discussed above, CGM has been adopted by the computer science community with a focus on the development of glucose forecasting algorithms (64, 84) to improve the accuracy with which these excursions are predicted.
However, a new direction for CGM use is now being investigated, CGM as a behaviour change tool. In their review, Ehrhardt and Zaghal (85) conclude that “Rather than being used as a “reactionary device” for hypoglycaemia prevention and glycaemic management, CGM should be assessed for its use as a prevention tool. Its potential role as an adjunct to lifestyle changes [ … ] calls for further evaluation”. In a survey of 40 people living with diabetes (86), 90% commented that CGM contributed to a healthier lifestyle, with 87% modifying food choices and 47% increasing physical activity based on CGM. Recent publications have also suggested that CGM could act as a behaviour modification tool for those with obesity (87).
Combining pattern recognition with behaviour change has the potential to significantly improve self-management behaviours (88). Worth et al. used CGM to identify individual patterns in weekly hypoglycaemia risk of patients with CHI (81). The same group developed interpretative algorithms to facilitate patient understanding of patterns and provided suggestions for reflection designed to modify parental behaviours (35). The resulting change in fingerprick and self-management behaviours led to a reduction in real world hypoglycaemia of 25% (35, 81), demonstrating the potential power of using CGM as a tool to identify and modify the behavioural determinants of hypoglycaemia. Due to the focus on weekly patterns and behavioural determinants of hypoglycaemia, this approach is less subject to problems with poor point accuracy and patient dissatisfaction with alarms, suggesting a novel and sustainable path to CGM application.
7.3 CGM to diagnose and inform management
While children with rare hypoglycaemia disorders do not have exogenous insulin to adjust based on CGM readings, there are many other diagnostic and management decisions that can be made upon the basis of CGM outputs. Work evaluating the CGM profiles of healthy subjects (89, 90) provides more data with which researchers can compare results from disease cohorts and evaluate glycaemic control in context. Rossi et al. (30) have shown this with their own assessment of healthy subjects in comparison to those with GSDIa. Separately, Rossi et al. (91) propose the use of CGM in a hybrid approach to determine fasting tolerance in children with GSDs rather than the traditional “controlled fast” with multiple fingerprick tests. They go on to highlight the efficacy of CGM to determine incidence of nocturnal hypoglycaemia as well as the impact of diet and medications on glycaemic profiles. Peeks et al. (42) support this approach and have documented their use of CGM to monitor the impact of nocturnal dietary interventions, changes in starch loads, and treatment with empagliflozin for patients with hepatic GSDs. In the case of treatment with empagliflozin, the authors highlight the utility of CGM to detect the potential hypoglycaemia resulting from medication-induced glycosuria (42). Logel et al. (92) similarly used intermittent CGM to initiate and then titrate doses of diazoxide in a patient with Glut1 deficiency who had failed ketogenic diet; without the high granularity data of CGM it was felt that diazoxide would have been administered at incorrect doses, risking the loss of efficacy seen in other cases treated without CGM.
7.4 CGM as an outcome marker in clinical trials
In recent years CGM has become popular as an outcome in clinical trials to determine efficacy of interventions to reduce hypoglycaemia. The high granularity data generated by CGM reduces the chance of type II errors in clinical trials and allows investigators better insight into glycaemic changes secondary to therapeutics.
CGM has recently been used as an outcome measure for: hypoglycaemia after paediatric cardiac surgery (93); treatment of CHI with Dasiglucagon (94); treatment of CHI with RZ358 (95); treatment of GSDIa with AAV8 gene transfer (96) and is planned for more upcoming therapeutic trials in rare hypoglycaemia disorders. An essential component of using CGM as an outcome measure is understanding the baseline data for each disease and population (42). This requires quantification of as many patients as possible (79); Rossi et al. (30) recently provided the first publication of CGM metrics for patients with GSD1a, as did Worth et al. (80, 82) for patients with CHI, essential datasets for those utilising baseline characteristics when designing future therapeutic trials using CGM for primary or secondary outcomes.
8 Conclusion
There has been considerable progress in the development of the relatively new technology of CGM. However, in childhood hypoglycaemia disorders many historical problems remain. CGM continues to be insufficiently accurate, somewhat burdensome for patients and their families, costly, and lacking in evidence for its ability to reduce hypoglycaemia when provided to families without support. However, there is scope for optimism. Devices continue to miniaturise, improve in accuracy and reduce patient burden. Research and clinical teams are working around suboptimal point accuracy and lack of patient educational resources to develop novel ways of utilising this technology. CGM is being used for diagnostics, monitoring changes in management, establishment of baseline characteristics, modifying behaviour, and ultimately to reduce hypoglycaemia when used retrospectively and combined with interpretative algorithms or clinical expertise. Use in neonatal medicine is becoming established, with good evidence for a reduction and early recognition in neonatal hypoglycaemia.
A lack of guidelines for the use of CGM in hypoglycaemia disorders has restricted progress but given rapid technological advances, it is predicted to play a larger role in all forms of childhood hypoglycaemia disorders. The challenge is to adapt CGM technology to clinical application with research designed to bring CGM innovations for patient benefit.
Author contributions
CW researched and wrote the first draft of the manuscript other than Section 5.2 which was written by LH. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kristensen K, Quitmann J, Witt S. Health-related quality of life of children and adolescents with congenital hyperinsulinism – a scoping review. Front Endocrinol (Lausanne) (2021) 12:784932. doi: 10.3389/fendo.2021.784932
2. Pasquini TLS, Mesfin M, Schmitt J, Raskin J. Global registries in congenital hyperinsulinism. Front Endocrinol (Lausanne) (2022) 13:876903. doi: 10.3389/fendo.2022.876903
3. Eljamel S, Griffiths A, Evans J, Banerjee I, Hussain K, Thompson R. The burden of congenital hyperinsulinism in the united kingdom: A cost of illness study. Orphanet J Rare Dis (2018) 13. doi: 10.1186/s13023-018-0867-6
4. Wild D, von Maltzahn R, Brohan E, Christensen T, Clauson P, Gonder-Frederick L. A critical review of the literature on fear of hypoglycemia in diabetes: Implications for diabetes management and patient education. Patient Educ Couns (2007) 68:10–5. doi: 10.1016/J.PEC.2007.05.003
5. Nordfeldt S, Jonsson D. Short-term effects of severe hypoglycaemia in children and adolescents with type 1 diabetes. A cost-of-illness study Acta Pædiatrica (2001) 90:137–42. doi: 10.1111/J.1651-2227.2001.TB00274.X
6. Raskin J, Pasquini TLS, Bose S, Tallis D, Schmitt J. Congenital hyperinsulinism international: A community focused on improving the lives of people living with congenital hyperinsulinism. Front Endocrinol (Lausanne) (2022) 13:886552. doi: 10.3389/fendo.2022.886552
7. Shah R, Dai DWT, Alsweiler JM, Brown GTL, Chase JG, Gamble GD, et al. Association of neonatal hypoglycemia with academic performance in mid-childhood. Jama (2022) 327:1158. doi: 10.1001/jama.2022.0992
8. McKinlay CJD, Alsweiler JM, Ansell JM, Anstice NS, Chase JG, Gamble GD, et al. Neonatal glycemia and neurodevelopmental outcomes at 2 years. N Engl J Med (2015) 373:1507–18. doi: 10.1056/NEJMoa1504909
9. McKinlay CJD, Alsweiler JM, Anstice NS, Burakevych N, Chakraborty A, Chase JG, et al. Association of neonatal glycemia with neurodevelopmental outcomes at 4.5 years. JAMA Pediatr (2017) 171:972–83. doi: 10.1001/jamapediatrics.2017.1579
10. Lucas A, Morley R, Cole TJ. Adverse neurodevelopmental outcome of moderate neonatal hypoglycaemia. (1988), British Medical Journal, Vol 297 1304–8. doi: 10.1136/bmj.297.6659.1304
11. Lord K, Radcliffe J, Gallagher PR, Adzick NS, Stanley CA, De León DD. High risk of diabetes and neurobehavioral deficits in individuals with surgically treated hyperinsulinism. J Clin Endocrinol Metab (2015) 100:4133–9. doi: 10.1210/jc.2015-2539
12. Worth C, Yau D, Salomon Estebanez M, O’Shea E, Cosgrove K, Dunne M, et al. Complexities in the medical management of hypoglycaemia due to congenital hyperinsulinism. Clin Endocrinol (Oxf) (2020) 92:387–95. doi: 10.1111/cen.14152
13. Worth C, Dunne M, Ghosh A, Harper S, Banerjee I. Continuous glucose monitoring for hypoglycaemia in children: Perspectives in 2020. Pediatr Diabetes (2020) 21:1–10. doi: 10.1111/pedi.13029
14. Šoupal J, Petruželkov´a L, Grunberger G, H´askov´a A, Flekač M, Matoulek M, et al. Glycemic outcomes in adults with T1D are impacted more by continuous glucose monitoring than by insulin delivery method: 3 years of follow-up from the COMISAIR study. Diabetes Care (2020) 43:37–43. doi: 10.2337/DC19-0888
15. Charleer S, De Block C, Van Huffel L, Broos B, Fieuws S, Nobels F, et al. Quality of life and glucose control after 1 year of nationwide reimbursement of intermittently scanned continuous glucose monitoring in adults living with type 1 diabetes (FUTURE): A prospective observational real-world cohort study. Diabetes Care (2020) 43:389–97. doi: 10.2337/DC19-1610
16. Wright EE, Kerr MSD, Reyes IJ, Nabutovsky Y, Miller E. Use of flash continuous glucose monitoring is associated with A1C reduction in people with type 2 diabetes treated with basal insulin or noninsulin therapy. Diabetes Spectr (2021) 34:184. doi: 10.2337/DS20-0069
17. Tauschmann M, Hermann JM, Freiberg C, Papsch M, Thon A, Heidtmann B, et al. Reduction in diabetic ketoacidosis and severe hypoglycemia in pediatric type 1 diabetes during the first year of continuous glucose monitoring: A multicenter analysis of 3,553 subjects from the DPV registry. Diabetes Care (2020) 43:e40–2. doi: 10.2337/DC19-1358
18. Wan W, Skandari MR, Minc A, Nathan AG, Winn A, Zarei P, et al. Cost-effectiveness of continuous glucose monitoring for adults with type 1 diabetes compared with self-monitoring of blood glucose: The DIAMOND randomized trial. Diabetes Care (2018) 41:1227–34. doi: 10.2337/DC17-1821
19. Roze S, Isitt J, Smith-Palmer J, Javanbakht M, Lynch P. Long-term cost-effectiveness of dexcom G6 real-time continuous glucose monitoring versus self-monitoring of blood glucose in patients with type 1 diabetes in the U.K. Diabetes Care (2020) 43:2411–7. doi: 10.2337/DC19-2213
20. Fonda SJ, Graham C, Munakata J, Powers JM, Price D, Vigersky RA. The cost-effectiveness of real-time continuous glucose monitoring (RT-CGM) in type 2 diabetes. J Diabetes Sci Technol (2016) 10:898–904. doi: 10.1177/1932296816628547
21. Beardsall K, Ogilvy-Stuart AL, Ahluwalia J, Thompson M, Dunger DB. The continuous glucose monitoring sensor in neonatal intensive care. Arch Dis Child Fetal Neonatal Ed (2005) 90:307–10. doi: 10.1136/adc.2004.051979
22. Beardsall K, Vanhaesebrouck S, Ogilvy-Stuart AL, Vanhole C, VanWeissenbruch M, Midgley P, et al. Validation of the continuous glucose monitoring sensor in preterm infants. Arch Dis Child Fetal Neonatal Ed (2013) 98:136–40. doi: 10.1136/archdischild-2012-301661
23. Win M, Beckett R, Thomson L, Thankamony A, Beardsall K. Continuous glucose monitoring in the management of neonates with persistent hypoglycaemia and congenital hyperinsulinism. J Clin Endocrinol Metab (2020) 107:1–18. doi: 10.1210/clinem/dgab601
24. Vijayanand S, Stevenson PG, Grant M, Choong CS, Davis EA, Abraham MB. The utility of continuous glucose monitoring systems in the management of children with persistent hypoglycaemia. J Pediatr Endocrinol Metab (2021) 34:1567–72. doi: 10.1515/jpem-2021-0414
25. Alsaffar H, Turner L, Yung Z, Didi M, Senniappan S. Continuous flash glucose monitoring in children with congenital hyperinsulinism; first report on accuracy and patient experience. Int J Pediatr Endocrinol (2018) 2018. doi: 10.1186/s13633-018-0057-2
26. Rayannavar A, Elci OU, Mitteer L, De León DD. Continuous glucose monitoring systems: Are they useful for evaluating glycemic control in children with hyperinsulinism? Horm Res Paediatr (2020) 92:319–27. doi: 10.1159/000506230
27. Worth C, Dunne MJ, Salomon-Estebanez M, Harper S, Nutter PW, Dastamani A, et al. The hypoglycaemia error grid: a UK-wide consensus on CGM accuracy assessment in hyperinsulinism. Front Endocrinol (Lausanne) (2022). doi: 10.3389/fendo.2022.1016072
28. Kasapkara CS, Cinasal Demir G, Hasanoğlu A, Tümer L. Continuous glucose monitoring in children with glycogen storage disease type i. Eur J Clin Nutr (2014) 68:101–5. doi: 10.1038/ejcn.2013.186
29. Herbert M, Pendyal S, Rairikar M, Halaby C, Benjamin RW, Kishnani PS. Role of continuous glucose monitoring in the management of glycogen storage disorders. J Inherit Metab Dis (2018) 41:917–27. doi: 10.1007/s10545-018-0200-5
30. Rossi A, Venema A, Haarsma P, Feldbrugge L, Burghard R, Rodriguez-Buritica D, et al. A prospective study on continuous glucose monitoring in glycogen storage disease type ia: Toward glycemic targets. J Clin Endocrinol Metab (2022) 107:e3612–23. doi: 10.1210/clinem/dgac411
31. Fernández Martínez M del M, Llorente JLG, de Cabo JM, López MAV, Porcel M del CO, Rubio JDD, et al. Monitoring the frequency and duration of hypoglycemia in preterm infants and identifying associated factors. Fetal Pediatr Pathol (2021) 40:131–41. doi: 10.1080/15513815.2019.1692111
32. Beardsall K, Thomson L, Guy C, Iglesias-Platas I, van Weissenbruch MM, Bond S, et al. Real-time continuous glucose monitoring in preterm infants (REACT): an international, open-label, randomised controlled trial. Lancet Child Adolesc Heal (2021) 5:265–73. doi: 10.1016/S2352-4642(20)30367-9
33. Denkboy Ongen Y, Eren E, Saglam H. Maltodextrin may be a promising treatment modality after near-total pancreatectomy in infants younger than six months with persistent hyperinsulinism: A case report. J Clin Res Pediatr Endocrinol (2012) 0:0–0. doi: 10.4274/jcrpe.galenos.2021.2021.0121
34. Kiuchi K, Sato T, Nakano S, Ishii T, Hasegawa T. Continuous glucose monitoring in an infant with panhypopituitarism having hypoglycemia on growth hormone therapy. J Pediatr Endocrinol Metab (2022) 2975:1–4. doi: 10.1515/jpem-2022-0224
35. Worth C, Nutter PW, Salomon-Estebanez M, Auckburally S, Dunne MJ, Banerjee I, et al. The behaviour change behind a successful pilot of hypoglycaemia reduction with HYPO-CHEAT. Under Rev Digit Heal (2022).
36. White FJ, Jones SA. The use of continuous glucose monitoring in the practical management of glycogen storage disorders. J Inherit Metab Dis (2011) 34:631–42. doi: 10.1007/s10545-011-9335-3
37. Du CQ, Wei H, Zhang C, Zhang M, Hu MH, Hou L, et al. [The application of continuous glucose monitoring in the management of hepatic glycogen storage disease]. Chin J Pediatr (2021) 59:452–8. doi: 10.3760/cma.j.cn112140-20210321-00235
38. Kaiser N, Gautschi M, Bosanska L, Meienberg F, Baumgartner MR, Spinas GA, et al. Glycemic control and complications in glycogen storage disease type I: Results from the Swiss registry. Mol Genet Metab (2019) 126:355–61. doi: 10.1016/j.ymgme.2019.02.008
39. Esper BS, Carson M, Galvin-Parton P, Lane AH, Tafuri KS. Continuous glucose monitoring in the management of hypoglycemia in TANGO2. J Endocr Soc (2021) 5:A699–9. doi: 10.1210/jendso/bvab048.1423
40. Ng SM, Dearman S, Fisher M, Mushtaq T. Case for funding of continuous glucose monitoring systems for patients with recurrent hypoglycaemia. Arch Dis Child (2022), 1–2. doi: 10.1136/archdischild-2022-323872
41. Peeks F, Boonstra WF, De BL, Carøe C. Research priorities for liver glycogen storage disease : An international priority setting partnership with the. J Inherit Metab Dis (2020) 43:279–89. doi: 10.1002/jimd.12178
42. Peeks F, Hoogeveen IJ, Feldbrugge RL, Burghard R, de Boer F, Fokkert-Wilts MJ, et al. A retrospective in-depth analysis of continuous glucose monitoring datasets for patients with hepatic glycogen storage disease: Recommended outcome parameters for glucose management. J Inherit Metab Dis (2021) 44:1136–50. doi: 10.1002/jimd.12383
43. Auckburally S, Worth C, Salomon-Estebanez M, Nutter PW, Harper S, Nicholson J, et al. Families’ experiences of continuous glucose monitoring in the management of congenital hyperinsulinism: A thematic analysis. Front Endocrinol (Lausanne) (2022). doi: 10.3389/fendo.2022.894559
44. Ahmad S, Worth C, Auckburally S, Salomon-Estebanez M, O’Shea E, Worthington S, et al. Continuous glucose monitoring for hypoglycaemia: the unheard patient voice IN 60th annual meeting of the European society for paediatric endocrinology (ESPE)(2022). Available at: https://abstracts.eurospe.org/hrp/0095/hrp0095p1-295 (Accessed November 3, 2022).
45. UnitedNations. The sustainable development goals report 2022. United Nations Publ issued by Dep Econ Soc Aff (2022) 64.
46. Bila R, Varo R, Madrid L, Sitoe A, Bassat Q. Continuous glucose monitoring in resource-constrained settings for hypoglycaemia detection: Looking at the problem from the other side of the coin. Biosensors (2018) 8:2–7. doi: 10.3390/bios8020043
47. Martínez C, Romero C, Villalobos NS. What are pharma companies doing to expand access to insulin – and how can efforts be scaled up? (2022). Available at: https://accesstomedicinefoundation.org/medialibrary/633ed0a126443_atmf-diabetes_access-to-insulin.pdf.
48. Newborn mortality, in: World heal organ (2022). Available at: https://www.who.int/news-room/fact-sheets/detail/levels-and-trends-in-child-mortality-report-2021 (Accessed November 16, 2022).
49. Anderson S, Shakya KN, Shrestha LN, De AM. Hypoglycaemia: A common problem among uncomplicated newborn infants in Nepal. J Trop Pediatr (1993) 39:273–7. doi: 10.1093/tropej/39.5.273
50. Maynard KR, Causey L, Kawaza K, Dube Q, Lufesi N, Oden ZM, et al. New technologies for essential newborn care in under-resourced areas: What is needed and how to deliver it. Paediatr Int Child Health (2015) 35:192–205. doi: 10.1179/2046905515Y.0000000034
51. Burns CM, Rutherford MA, Boardman JP, Cowan FM. Patterns of cerebral injury and neurodevelopmental outcomes after symptomatic neonatal hypoglycemia. Pediatrics (2008) 122:65–74. doi: 10.1542/peds.2007-2822
52. Kariuki SM, Thomas PT, Newton CR. Epilepsy stigma in children in low-income and middle-income countries. Lancet Child Adolesc Heal (2021) 5:314–6. doi: 10.1016/S2352-4642(21)00090-0
53. Dua T, De Boer HM, Prilipko LL, Saxena S. Epilepsy care in the world: Results of an ILAE/IBE/WHO global campaign against epilepsy survey. Epilepsia (2006) 47:1225–31. doi: 10.1111/j.1528-1167.2006.00595.x
54. Smythe T, Adelson JD, Polack S. Systematic review of interventions for reducing stigma experienced by children with disabilities and their families in low- and middle-income countries: state of the evidence. Trop Med Int Heal (2020) 25:508–24. doi: 10.1111/tmi.13388
55. Deerberg-Wittram J. Diabeter value-based healthcare delivery in diabetes diabeter: Value-based health care delivery in diabetes 1. (2016). NA - Medtronic online publication
56. Venema A, Peeks F, Rossi A, Jager EA, Derks TGJ. Towards values-based healthcare for inherited metabolic disorders: An overview of current practices for persons with liver glycogen storage disease and fatty acid oxidation disorders. J Inherit Metab Dis (2022) 45:1018–27. doi: 10.1002/jimd.12555
57. Schopman S, Kalchthaler K, Malthern R, Mehta K, Butler P. Ruggedising biomedical devices for field-testing in resource-constrained environments: Context, issues and solutions. J Humanit Eng (2013) 2:17–26. doi: 10.36479/jhe.v2i1.17
58. Howitt P, Darzi A, Yang GZ, Ashrafian H, Atun R, Barlow J, et al. Technologies for global health. Lancet (2012) 380:507–35. doi: 10.1016/S0140-6736(12)61127-1
59. Free MJ. Achieving appropriate design and widespread use of health care technologies in the developing world. overcoming obstacles that impede the adaptation and diffusion of priority technologies for primary health care. Int J Gynecol Obstet (2004) 85:3–13. doi: 10.1016/j.ijgo.2004.01.009
60. Kasali BA, Gururaj A, Batra M. Newborn care technology investments for LMIC settings: a CPAP approach. BMJ Innov (2021) 7:519–22. doi: 10.1136/bmjinnov-2020-000598
61. Ahmed I, Jiang N, Shao X, Elsherif M, Alam F, Salih A, et al. Recent advances in optical sensors for continuous glucose monitoring. Sensors Diagnostics (2022). doi: 10.1039/d1sd00030f
62. Zafar H, Channa A, Jeoti V, Stojanović GM. Comprehensive review on wearable sweat-glucose sensors for continuous glucose monitoring. Sensors (2022) 22:1–35. doi: 10.3390/s22020638
63. Kumar Das S, Nayak KK, Krishnaswamy PR, Kumar V, Bhat N. Review–electrochemistry and other emerging technologies for continuous glucose monitoring devices. ECS Sensors Plus (2022) 1:031601. doi: 10.1149/2754-2726/ac7abb
64. Oviedo S, Vehí J, Calm R, Armengol J. A review of personalized blood glucose prediction strategies for T1DM patients. Int J numer method BioMed Eng (2017) 33. doi: 10.1002/cnm.2833
65. Leelarathna L, English SW, Thabit H, Caldwell K, Allen JM, Kumareswaran K, et al. Feasibility of fully automated closed-loop glucose control using continuous subcutaneous glucose measurements in critical illness: A randomized controlled trial. Crit Care (2013) 17. doi: 10.1186/cc12838
66. Beardsall K, Thomson L, Elleri D, Dunger DB, Hovorka R. Feasibility of automated insulin delivery guided by continuous glucose monitoring in preterm infants. Arch Dis Child Fetal Neonatal Ed (2020) 105:F279–84. doi: 10.1136/archdischild-2019-316871
67. Bertachi A, Viñals C, Biagi L, Contreras I, Vehí J, Conget I, et al. Prediction of nocturnal hypoglycemia in adults with type 1 diabetes under multiple daily injections using continuous glucose monitoring and physical activity monitor. Sensors 2020 Vol 20 Page 1705 (2020) 20:1705. doi: 10.3390/S20061705
68. Amar Y, Shilo S, Oron T, Amar E, Phillip M, Segal E. Clinically accurate prediction of glucose levels in patients with type 1 diabetes(2020). Available at: https://home.liebertpub.com/dia.
69. Elhadd T, Mall R, Bashir M, Palotti J, Fernandez-Luque L, Farooq F, et al. Artificial intelligence (AI) based machine learning models predict glucose variability and hypoglycaemia risk in patients with type 2 diabetes on a multiple drug regimen who fast during ramadan (The PROFAST – IT Ramadan study). Diabetes Res Clin Pract (2020) 169:108388. doi: 10.1016/J.DIABRES.2020.108388
70. Dave D, DeSalvo DJ, Haridas B, McKay S, Shenoy A, Koh CJ, et al. Feature-based machine learning model for real-time hypoglycemia prediction. J Diabetes Sci Technol (2020) 15(4), 842–855. doi: 10.1177/1932296820922622
71. Mujahid O, Contreras I, Vehi J. Machine learning techniques for hypoglycemia prediction: Trends and challenges. Sensors (Switzerland) (2021) 21:1–21. doi: 10.3390/s21020546
72. Kodama S, Fujihara K, Shiozaki H, Horikawa C, Yamada MH, Sato T, et al. Ability of current machine learning algorithms to predict and detect hypoglycemia in patients with diabetes mellitus: meta-analysis. JMIR Diabetes (2021) 6. doi: 10.2196/22458
73. Tyler NS, Mosquera-Lopez CM, Wilson LM, Dodier RH, Branigan DL, Gabo VB, et al. An artificial intelligence decision support system for the management of type 1 diabetes. Nat Metab (2020) 2:612–9. doi: 10.1038/s42255-020-0212-y
74. Liu C, Avari P, Leal Y, Wos M, Sivasithamparam K, Georgiou P, et al. A modular safety system for an insulin dose recommender: A feasibility study. J Diabetes Sci Technol (2020) 14:87–96. doi: 10.1177/1932296819851135
75. Tyler NS, Jacobs PG. Artificial intelligence in decision support systems for type 1 diabetes. Sensors (Switzerland) (2020) 20:1–26. doi: 10.3390/s20113214
76. Nano J, Carinci F, Okunade O, Whittaker S, Walbaum M, Barnard-Kelly K, et al. A standard set of person-centred outcomes for diabetes mellitus: results of an international and unified approach. Diabetes Med (2020) 37:2009–18. doi: 10.1111/dme.14286
77. Agiostratidou G, Anhalt H, Ball D, Blonde L, Gourgari E, Harriman KN, et al. Standardizing clinically meaningful outcome measures beyond HbA1c for type 1 diabetes: A consensus report of the American association of clinical endocrinologists, the American association of diabetes educators, the American diabetes association, the endo. Diabetes Care (2017) 40:1622–30. doi: 10.2337/dc17-1624
78. Jain SH, Powers BW, Hawkins JB, Brownstein JS. The digital phenotype. Nat Biotechnol (2015) 33:462–3. doi: 10.1038/nbt.3223
79. Flores M, Glusman G, Brogaard K, Price ND, Hood L. P4 medicine: How systems medicine will transform the healthcare sector and society. Per Med (2013) 10:565–76. doi: 10.2217/pme.13.57
80. Worth C, Harper S, Salomon-Estebanez M, O’Shea E, Nutter PW, Dunne MJ. Indraneel Banerjee,MBBS m. clustering of hypoglycemia events in patients with hyperinsulinism: Extension of the digital phenotype through retrospective data analysis. J Med Internet Res (2021) 23:1–14. doi: 10.2196/26957
81. Worth C, Nutter PW, Dunne MJ, Salomon-Estebanez M, Banerjee I, Harper S. HYPO-CHEAT’s aggregated weekly visualisations of risk reduce real world hypoglycaemia. Digit Heal (2022) 8:1–22. doi: 10.1177/20552076221129712
82. Worth C, Tropeano Y, Gokul PR, Cosgrove KE, Salomon-Estebanez M, Senniappan S, et al. Insight into hypoglycemia frequency in congenital hyperinsulinism: evaluation of a large UK CGM dataset. BMJ Open Diabetes Res Care (2022) 10:e002849. doi: 10.1136/BMJDRC-2022-002849
83. Park J, Hawcutt D, Shantsila H, Lip G, Blair J. Glucose regulation in children with primary adrenal insufficiency: preliminary data(2021). Available at: https://abstracts.eurospe.org/hrp/0094/hrp0094p1-10 (Accessed November 7, 2022).
84. Woldaregay AZ, Årsand E, Walderhaug S, Albers D, Mamykina L, Botsis T, et al. Data-driven modeling and prediction of blood glucose dynamics: Machine learning applications in type 1 diabetes. Artif Intell Med (2019) 98:109–34. doi: 10.1016/j.artmed.2019.07.007
85. Ehrhardt N, Al Zaghal E. Behavior modification in prediabetes and diabetes: Potential use of real-time continuous glucose monitoring. J Diabetes Sci Technol (2019) 13:271–5. doi: 10.1177/1932296818790994
86. Ehrhardt N, Al Zaghal E. Continuous glucose monitoring as a behavior modification tool. Clin Diabetes (2020) 38:126–31. doi: 10.2337/cd19-0037
87. Schembre SM, Liao Y, Jospe MR. Continuous glucose monitors as wearable lifestyle behavior change tools in obesity and diabetes. Obes Diabetes (2020), 591–603. doi: 10.1007/978-3-030-53370-0_43
88. Lee SK, Shin DH, Kim YH, Lee KS. Effect of diabetes education through pattern management on self-care and self-efficacy in patients with type 2 diabetes. Int J Environ Res Public Health (2019) 16. doi: 10.3390/ijerph16183323
89. Shah VN, Dubose SN, Li Z, Beck RW, Peters AL, Weinstock RS, et al. Continuous glucose monitoring profiles in healthy nondiabetic participants: A multicenter prospective study. J Clin Endocrinol Metab (2019) 104:4356–64. doi: 10.1210/jc.2018-02763
90. Dubose SN, Kanapka LG, Bradfield B, Sooy M, Beck RW, Steck AK. Continuous glucose monitoring profiles in healthy, nondiabetic young children. J Endocr Soc (2022) 6:1–7. doi: 10.1210/jendso/bvac060
91. Rossi A, Rutten MGS, van Dijk TH, Bakker BM, Reijngoud DJ, Oosterveer MH, et al. Dynamic methods for childhood hypoglycemia phenotyping: A narrative review. Front Endocrinol (Lausanne) (2022) 13:858832. doi: 10.3389/fendo.2022.858832
92. Logel SN, Connor EL, Hsu DA, Fenske RJ, Paloian NJ, De Vivo DC. Exploring diazoxide and continuous glucose monitoring as treatment for Glut1 deficiency syndrome. Ann Clin Transl Neurol (2021) 8:2205–9. doi: 10.1002/ACN3.51462
93. Sadhwani A, Asaro LA, Goldberg CS, Ware J, Butcher J, Gaies M, et al. Impact of tight glycemic control and hypoglycemia after pediatric cardiac surgery on neurodevelopmental outcomes at three years of age: Findings from a randomized clinical trial. BMC Pediatr (2022) 22:1–10. doi: 10.1186/s12887-022-03556-z
94. Banerjee I, De Leon DD, Kendall DM, Birch S, Bøge E, Ivkovic J, et al. Dasiglucagon treatment over 21 days in infants with congenital hyperinsulinism results in glycaemic stability and reduces requirement for intravenous glucose IN 60th annual meeting of the European society for paediatric endocrinology (ESPE). Horm Res Paediatr (2022) 95(2). doi: 10.1159/000525606
95. Demirbilek H, Melikyan M, Galcheva S, Dastamani A, Thornton P, De Leon D, et al. Results from a global, multi-center, phase 2b study (RIZE) in congenital hyperinsulinism: Characterization of a high unmet treatment need and glycemic response to RZ358 IN 60th annual meeting of the European society for paediatric endocrinology (ESPE). Horm Res Paediatr (2022) 95(2). doi: 10.1159/000525606
96. NIH. ClinicalTrials: A study of adeno-associated virus serotype 8-mediated gene transfer of glucose-6-Phosphatase in patients with glycogen storage disease type ia (GSDIa) (2021). Available at: https://clinicaltrials.gov/ct2/show/NCT05139316 (Accessed December 2, 2022).
Keywords: hypoglycaemia, continuous glucose monitoring, children, hyperinsulinism, glycogen storage disease, prematurity
Citation: Worth C, Hoskyns L, Salomon-Estebanez M, Nutter PW, Harper S, Derks TGJ, Beardsall K and Banerjee I (2023) Continuous glucose monitoring for children with hypoglycaemia: Evidence in 2023. Front. Endocrinol. 14:1116864. doi: 10.3389/fendo.2023.1116864
Received: 05 December 2022; Accepted: 02 January 2023;
Published: 23 January 2023.
Edited by:
Huseyin Demirbilek, Hacettepe University, TürkiyeReviewed by:
Maria Melikyan, Yerevan State Medical University, ArmeniaNino Kheladze, Evex Hospitals, Georgia
Copyright © 2023 Worth, Hoskyns, Salomon-Estebanez, Nutter, Harper, Derks, Beardsall and Banerjee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chris Worth, Y2hyaXMud29ydGhAbWZ0Lm5ocy51aw==
 Chris Worth
Chris Worth Lucy Hoskyns1
Lucy Hoskyns1 Maria Salomon-Estebanez
Maria Salomon-Estebanez Terry G.J Derks
Terry G.J Derks Indraneel Banerjee
Indraneel Banerjee