
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 08 March 2023
Sec. Bone Research
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1115834
This article is part of the Research Topic Advances in the endocrine role of the skeleton, volume II View all 10 articles
 Binglang Xiong1†
Binglang Xiong1† Zixing Bai1†
Zixing Bai1† Xuhan Cao1
Xuhan Cao1 Duorui Nie2
Duorui Nie2 Cheng Zhang3
Cheng Zhang3 Xudong Sun1
Xudong Sun1 Ziyan Guo1
Ziyan Guo1 Jianmin Wen1
Jianmin Wen1 Weidong Sun1*
Weidong Sun1*Introduction: Previous observational studies have reported that thyroid dysfunction is associated with hallux valgus (HV). However, the causal effect of thyroid dysfunction on hallux valgus is still unknown. To assess whether there is a causal relationship between thyroid dysfunction and hallux valgus, we performed a two-sample Mendelian randomization (MR) study.
Methods: The data of the two-sample Mendelian randomization study were obtained from public databases. In this study, hypothyroidism, hyperthyroidism, free thyroxine (FT4), and thyrotropin (TSH) were chosen as exposures. The single nucleotide polymorphisms (SNP) of hypothyroidism and hyperthyroidism were from the genome-wide association studies (GWAS) of the IEU database, including 337,159 subjects. Data for FT4 and TSH (72,167 subjects) were extracted from the ThyroidOmics Consortium. HV was used as the outcome. The SNPs associated with HV were selected from a GWAS of 202,617 individuals in the fignngen database. The inverse variance weighted (IVW) method was used as the primary analysis. Four complementary methods were applied, including MR-presso, MR-Egger, and weighted median. In addition, Cochran’s Q test, MR-presso, MR-Egger regression, and the leave-one-out test were used as sensitivity analysis, and the MR-pleiotropy test was performed to examine pleiotropy.
Results: According to the results of IVW, we found that there was a causal relationship between hypothyroidism and HV, and hypothyroidism increased the incidence of HV (OR = 2.838 (95% CI: 1.116–7.213); p = 0.028). There were no significant causal effects of hyperthyroidism, FT4, and TSH on HV (p > 0.05). Sensitivity analyses showed that the results were robust and reliable, and no horizontal pleiotropy was detected.
Conclusions: Our findings provided genetic support that hypothyroidism might increase the risk of HV. It will predict the occurrence of HV in patients with hypothyroidism and provide suggestions for early prevention and intervention.
Hallux valgus (HV) is one of the most common forefoot deformities (1), which is mainly characterized by the progressive aggravation of lateral hallux deviation and medial deviation of the first metatarsal, often leading to severe foot pain and walking dysfunction (2) and thus reducing the quality of life of patients (3). According to studies, women are more likely than men to develop HV, which affects 23% of individuals between the ages of 18 and 65 and 35.7% of adults over the age of 65 (2). However, the etiology of HV is currently unclear (4). Genetic factors, improper shoe habits, inflammatory joint disease, and neuromuscular disease can all contribute to the occurrence of this disease (5, 6). There are many treatments for HV, although etiology-specific therapies are still lacking. At present, there are hundreds of surgical procedures reported in the literature to correct HV deformity, but their postoperative complication rates range from 10% to 50% (7, 8). More than 25% to 33% of patients are dissatisfied with the outcomes of surgery (9), and the high expense of surgery also adds to the burden on the medical system (10). Therefore, it is of high clinical value and economic significance to actively explore the etiology of HV and find a treatment for the etiology.
Previous large-scale observational studies have found a significant correlation between hypothyroidism and HV (11), but no research has yet confirmed whether there is a causal relationship between the two disorders. Numerous earlier investigations have demonstrated a connection between thyroid dysfunction and various orthopedic diseases. For instance, Tagoe et al. (12) revealed that patients with higher antithyroid peroxidase antibody (TPOAb) were more likely to develop chondrocalcification. Cell research confirms that abnormal thyroid hormone signaling raises the risk of osteoporosis, osteoarthritis, and other degenerative orthopedic illnesses (13, 14). Other studies have demonstrated that thyroid hormones can affect the function of osteoblasts and osteoclasts (15). However, hallux valgus is a common orthopedic disease, and whether thyroid disease affects it has not been explored.
Based on previous studies, we hypothesize that there may be a causal relationship between thyroid dysfunction and the risk of HV, and in this study, two-sample Mendelian randomization (MR) analysis was used to verify this. MR is an epidemiological statistical method that uses genetic variation as an instrumental variable (IV) to infer causal relationships between exposures and outcomes (16). Because genetic variation follows Mendel’s second law and is randomly assigned when fertilized eggs are formed, MR can reduce the interference of confounding factors on the results compared with previous studies and achieve the same effect as randomized controlled trials (17). MR also overcomes reverse causation because genetic variation is not affected by disease status (18). In this study, we considered hypothyroidism, hyperthyroidism, free thyroxine (FT4), and thyrotropin (TSH) as exposure and HV as outcome. This is the first study on the causal relationship between thyroid dysfunction and HV, with the purpose of further studying the etiology of HV and providing new ideas for the clinical treatment of HV.
We aimed to investigate the causal relationship between thyroid dysfunction and the risk of HV. Because all of the data in this study were obtained from public databases, no consent was required from the participants. We reported our study according to the STROBE-MR statement (19). The key assumptions of the MR study can be seen in Figure 1.
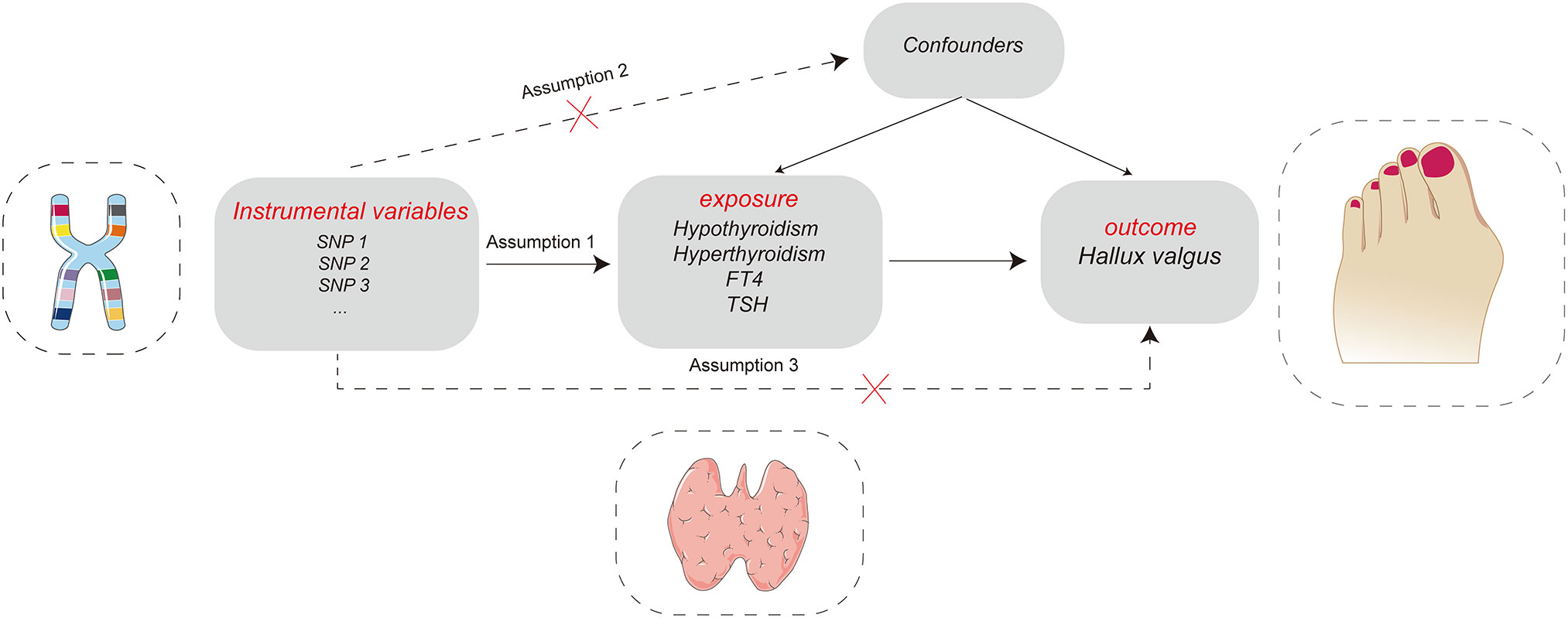
Figure 1 Key assumptions of the Mendelian randomization study: Assumption 1: instrumental variables should be robustly associated with exposure. Assumption 2: instrumental variables should not be associated with any confounders. Assumption 3: instrumental variables must not be associated with hallux valgus except through exposure.
In this study, hypothyroidism (increased TSH), hyperthyroidism (decreased TSH), FT4, and TSH were chosen as exposures. The single nucleotide polymorphisms (SNP) of hypothyroidism and hyperthyroidism were from the genome-wide association studies (GWAS) of the IEU database (https://gwas.mrcieu.ac.uk/), including 337,159 subjects and 10,894,596 SNPs. The study included 16,376 hypothyroidism samples and 320,783 control samples. There were also 2,547 hyperthyroidism cases and 334,612 ncases. The GWAS data of FT4 and TSH are all from The ThyroidOmics Consortium database (https://transfer.sysepi.medizin.uni-greifswald.de/thyroidomics/datasets/), containing 72,167 samples (Table 1).
HV was selected as the outcome, and its GWAS data were obtained from the FinnGen database (https://www.finngen.fi/en), which included 20,2617 samples (12,055 cases, 190,562 ncases) and 16,383,115 SNPs. All participants were of European ancestry.
We selected effective instrumental variables (IV) based on three assumptions (Figure 1). First, we set that each IV was significantly correlated with exposure (p< 5 × 10−8 means that the instrumental variable is strongly correlated with exposure). To remove the linkage disequilibrium between each SNP, we set the distance to 10,000 KB and the LD r2 to< 0.001 (20). To remove the possible horizontal pleiotropy of IV, use the Phenoscanner (http://www.phenoscanner.medschl.cam.ac.uk/) to search for phenotypes that may be affected by each SNP and remove the SNPs related to HV-associated phenotypes (21). SNPs for exposure and outcome should be harmonised, and palindromic and incompatible alleles should be removed (22). Finally, calculate the F value of each SNP (), and the SNPs with an F value<10 should be removed (23) (Figure 2).
All two-sample MR data analyses in this study were based on the TwoSampleMR package in the R software (version 4.5.0).
We used inverse variance weighting (IVW) as the primary method to evaluate the causal relationship between thyroid dysfunction and the risk of HV (24). IVW assumes that all selected IV are valid, so it has the highest statistical power and can provide the most accurate results (18). In addition, MR-Egger (25) and weighted median (26) were selected as supplementary methods. If all included SNPs match the effective IV assumption, then IVW can be considered the most reliable result (27). All results are expressed as OR values and 95% confidence intervals, with p< 0.05 representing statistical significance.
Cochrane’s Q was used as a heterogeneity test; p< 0.05 represents the existence of heterogeneity (28). MR-Egger regression was used to detect horizontal pleiotropy. The intercept value of MR-Egger regression represents the strength of horizontal pleiotropy, and a p-value of > 0.05 means that there is no horizontal pleiotropy (29). MR-PRESSO was used to detect SNPs that may lead to pleiotropic effects, remove outlier SNPs, and then perform MR analysis to compare whether the results have changed before and after correction (30). The leave-one-out test was used to detect the robustness of the results. This method gradually eliminated a single SNP and performed MR analysis on the remaining SNPs to detect whether the single SNP had a significant impact on the results.
After a series of quality evaluations, the number of SNPs selected as effective IV in hypothyroidism, hyperthyroidism, FT4, and TSH was 70, 4, 14, and 36, respectively. In the Supplementary Material, information on SNPs as IV was provided (Supplementary Tables S1–S4). The F values of all SNPs used as IV are greater than 10 (Supplementary Tables S1–S4), indicating that the included IV perform effectively.
IVW, MR-Egger, and weighted median methods were used to assess whether there is a causal relationship between hypothyroidism, hyperthyroidism, FT4, TSH, and the risk of HV. According to IVW results, we found that there is a positive causal relationship between hypothyroidism and HV; hypothyroidism can increase the risk of HV (OR = 2.838, 95% CI: 1.116–7.213); p = 0.028) (Table 2; Figure 3), and there is no statistical difference in MR-Egger and weighted median (Table 2; Figure 3). At the same time, these three methods showed no statistical significance in the assessment of the causal relationship between hyperthyroidism, FT4, TSH, and the risk of HV (Table 2; Figure 3).
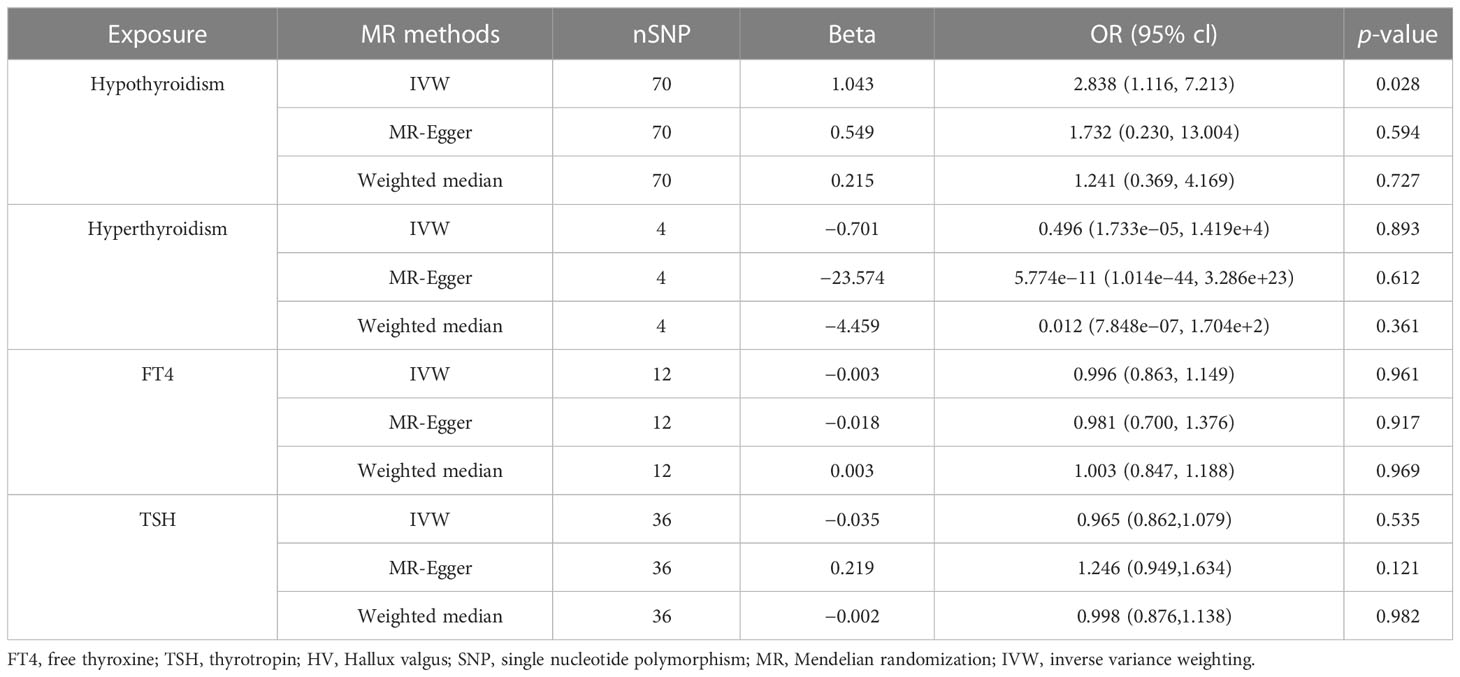
Table 2 MR estimates from different methods of assessing the causal effect of thyroid dysfunction on HV.
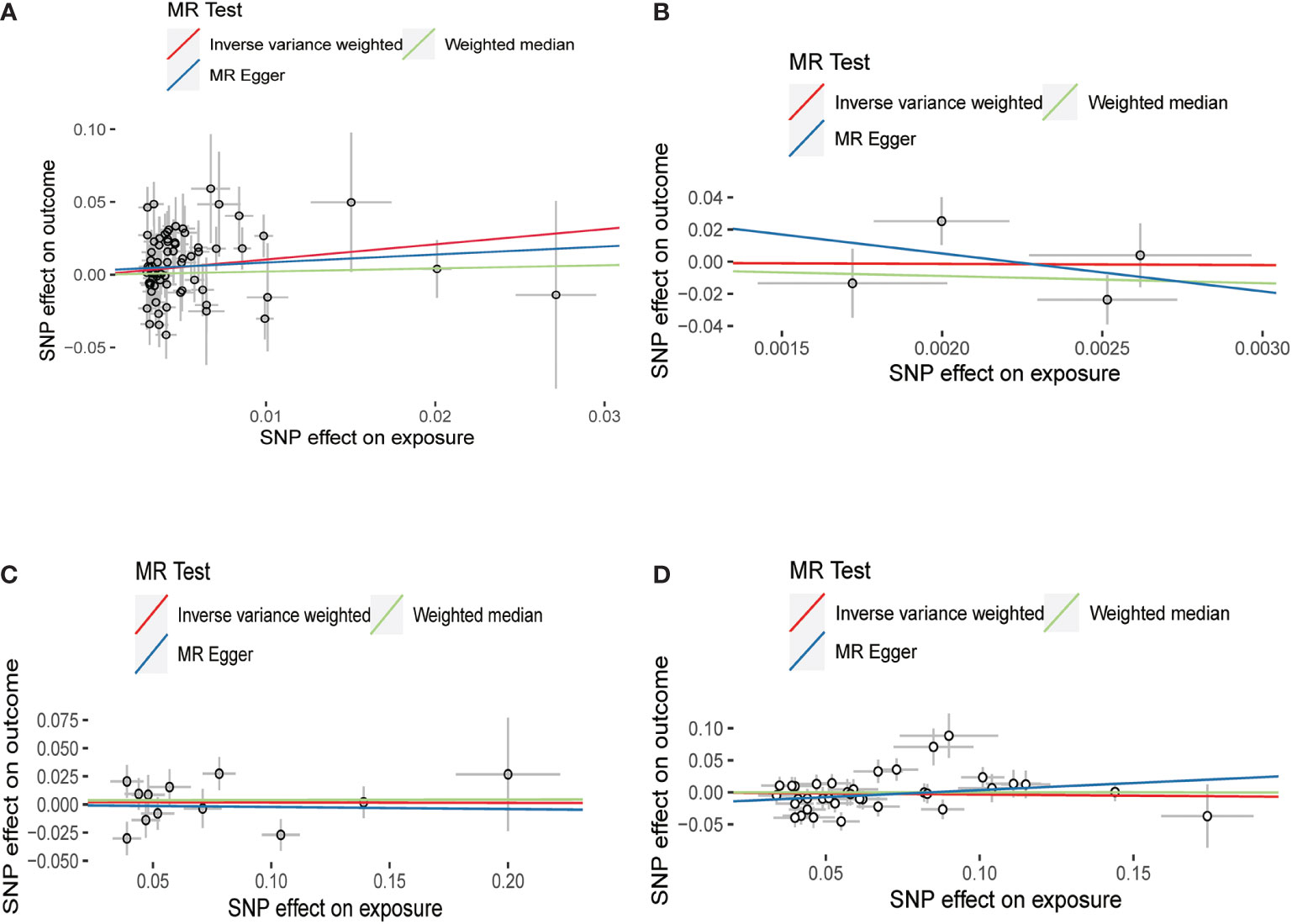
Figure 3 Scatter plots for Mendelian randomization (MR) analyses of the causal relationship between thyroid dysfunction and hallux valgus. (A) Hypothyroidism-HV. (B) Hyperthyroidism-HV. (C) FT4-HV. (D) TSH-HV.
All the results of this part are presented in Table 3. We found no heterogeneity when hyperthyroidism (p = 0.131) and FT4 (p = 0.132) were used as exposure. However, when hypothyroidism (p = 0.001) and TSH (p = 0.0004) were considered exposures, we discovered heterogeneity. The sources of these heterogeneities may be due to differences in the source of SNP data, experimental conditions, detection methods, and included populations. When heterogeneity was present, the random-effects model in IVW was chosen (30). MR-Egger regression showed that there was no horizontal pleiotropy for the SNPs of all exposures (Table 3). Neither MR-presso found outliers, and the results were consistent with IVW results (Table 3). the leave-one-out test further confirms that the results are stable, as shown in Figure 4. Funnel plots can be seen in Supplementary Figure S1, and the forest plots in MR analysis are shown in Supplementary Figure S2. Therefore, we considered the results of IVW to be reliable.
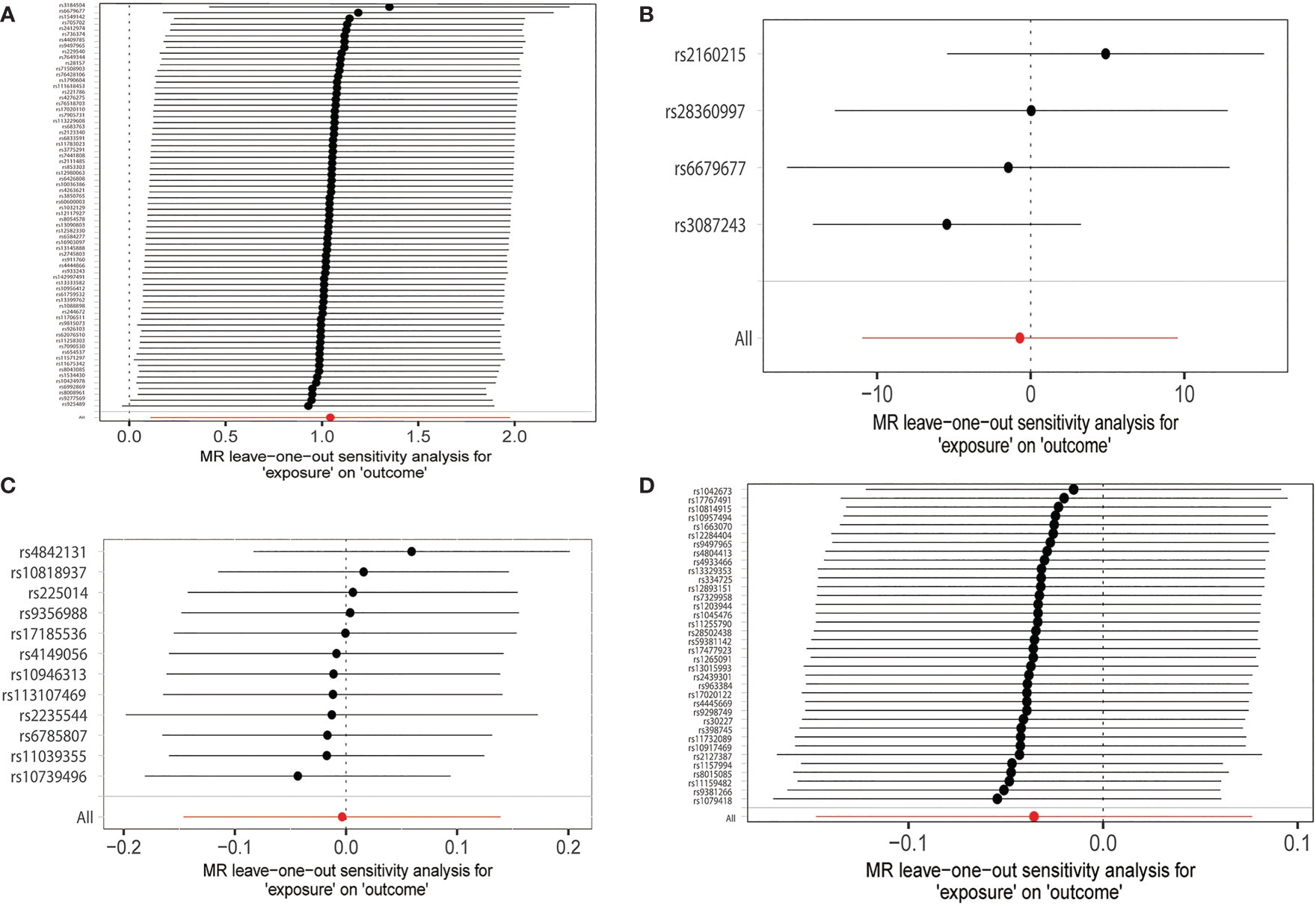
Figure 4 MR leave-one-out sensitivity analyses of the causal relationship between thyroid dysfunction and hallux valgus. (A) Hypothyroidism-HV. (B) Hyperthyroidism-HV. (C) FT4-HV. (D) TSH-HV.
In our two-sample MR study, we found a positive causal relationship between hypothyroidism and HV, with hypothyroidism associated with an increased risk of HV. However, there is no obvious causal relationship between hyperthyroidism, FT4, TSH, and HV. Our findings support the results of a previous observational study. Sterling et al. (11) investigated the prevalence of thyroid disease in 350 patients with forefoot deformity who visited the doctor for the first time and found that the prevalence of hypothyroidism was the highest among patients (19.1%) and that patients with hallux valgus were more likely to have thyroid disease (61.5%). Subsequently, the researchers analyzed a national public database of 905,924 patients with forefoot deformities and found that 321,656 (35.5%) patients were diagnosed with thyroid disease. Finally, it was concluded that forefoot deformities, especially HV, were significantly associated with thyroid dysfunction (11). A limitation of this study is that it did not examine the causal relationship between thyroid dysfunction and hallux valgus, which our findings provide support for. Our findings provide a new perspective on the etiology of hallux valgus, which is that hypothyroidism may contribute to the development of HV.
A large number of experimental studies have confirmed that bones are sensitive to thyroid hormones, and the biological role of thyroid hormones in bone tissue and cartilage has also attracted more and more attention (13). However, the specific biological mechanism behind the positive causal relationship between hypothyroidism and HV remains unclear. Previous literature holds that the thyroid hormone is an important regulator of bone metabolism and is crucial for bone remodeling; any deficiency or excess may lead to bone metabolism disorder (31). Hyperthyroidism and TSH levels lower than normal can accelerate bone metabolism and lead to accelerated bone loss (32, 33). In contrast, histomorphological data from hypothyroid patients showed decreased bone turnover and increased bone calcification (34). The study by Robinson et al. (35) found that there may be a specific cell population in the soft tissue surrounding HV, and cell experiments proved that when stimulated by fibroblast growth factor (FGF), it would lead to increased osteogenesis and the formation of HV. From this, we can speculate that patients with hypothyroidism may suffer from HV due to regionally impaired bone metabolism. Sclerostin, an osteocyte-derived protein encoded by the SOST gene, acts as an inhibitor of bone formation by stimulating the apoptosis of osteoblasts (36). Studies have shown that the content of sclerostin in the blood circulation of patients with hyperthyroidism is significantly higher than that of patients with hypothyroidism, and the level of sclerostin is positively correlated with FT4 and negatively correlated with TSH (37). This provides further evidence that hypothyroid patients are more likely to exhibit osteogenic dominance.
Some studies have studied the distribution of thyroid hormone receptors in human bone tissue. They found that at sites of endochondral ossification in osteophytes, TRalpha1, TRalpha2 variants, TRbeta1, and TRbeta2 mRNA were widely distributed in undifferentiated, proliferating, mature, and hypertrophic chondrocytes. Most osteoblasts (> 90%) express TRalpha-1 mRNA at regions of bone remodeling (38). A part of the concurrent clinical symptoms of HV is the formation of osteophytes, and studies have shown that there is a significant correlation between the cartilage degeneration of the first metatarsophalangeal joint and the severity of hallux valgus (39). Therefore, we can speculate that hypothyroidism may mediate cartilage degeneration leading to hallux valgus.
Other studies have found that the synovium of patients with rheumatoid arthritis (RA) and osteoarthritis (OA) contains a network of thyroid hormones. Thyroid hormones are strongly biodegraded in the synovium, and synoviocytes play an important role in the activation and degradation of thyroid hormones (40). HV is frequently present alongside synovitis, which is strongly connected with pain in this condition (41, 42). This leads us to hypothesize that a series of pathological changes caused by the action of thyroid hormone on the synovium may also contribute to the development of hallux valgus. However, the above speculations on the mechanism are all based on existing research results. No studies have investigated the mechanism of thyroid hormones on HV, which may be the subject of our upcoming study.
Most of the previous knowledge about the etiology of HV is that abnormalities in anatomy or biomechanics lead to the occurrence of the disease (43). No studies have investigated endocrine disorders as an etiology of HV. The surgical approaches for treating HV are varied since there are numerous pathologic variables, and the choice of surgical method is often based on the preference of the surgeon (5). However, the treatment satisfaction of the operation is poor (9). No consensus has been reached about the gold standard of HV treatment, despite the fact that there are several studies comparing the effectiveness of various surgical techniques (44, 45). However, there are still a large number of patients waiting for treatment every year. A 1994 study estimated that there are approximately 209,000 HV operations in the USA each year (46). Therefore, it is particularly important to actively explore other possible causes and establish a standardized treatment system. Our research suggests that hypothyroidism may be one of the causes of HV, and people with hypothyroidism are 2.838 times more likely to develop HV than normal people. Based on the results of this study, it may provide new ideas for the treatment of HV and provide a theoretical basis for the development of drug therapy for HV in the future. Early treatment of hypothyroid patients may reduce the incidence of hallux valgus and improve the quality of life of patients. Thyroid function screening and timely intervention in the HV patient population may slow the progression of HV and avoid the eventual development of surgery. In the USA, per HV surgery costs about $18,332 (10). Numerous surgical expenditures can be avoided if the amount of operations is decreased. Therefore, our findings have important implications for public health.
There are some advantages to our study. First of all, compared with observational studies, we use MR studies for causal inference and select genetic variation as IV, which can avoid the interference of other confounding factors and reverse causality. Second, all samples were drawn from European populations, and our results are less susceptible to differences in demographics. Most importantly, we demonstrate for the first time a causal relationship between hypothyroidism and HV, which may provide new strategies for the treatment of HV.
There are also some deficiencies in this study. First, thyroid hormones include multiple subtypes, such as T3 and T4, but due to research limitations, we cannot obtain the SNP data of the remaining subtypes. We were not able to explore other thyroid hormone subtypes as exposures for causal effects on HV. Second, FT4 and TSH are often used as indicators for the diagnosis of hypothyroidism, but they have not shown a causal relationship with hallux valgus. This may be caused by a relatively insufficient sample size of FT4 and TSH. Third, the participants we included were all from European populations, so it is not clear whether our findings are applicable to other populations. In addition, some of the methods we used in this study did not produce the same results as IVW, but all of the SNPs included in our study met the assumption of effective IV, and the MR-presso results were consistent with IVW, so our results are still reliable.
We used the two-sample MR to study the causal relationship between thyroid function and HV and proved that there is a positive causal relationship between hypothyroidism and HV. Hypothyroidism could increase the risk of HV. It will predict the occurrence of HV in patients with hypothyroidism and provide suggestions for early prevention and intervention. However, the results of this study need to be further confirmed by basic experiments.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
BX: research design and paper writing. ZB, XC, and DN: collection and organization of data. CZ, XS, and ZG: analysis and visualization of results. JW and WS: research quality assessment. All authors contributed to the article and approved the submitted version.
This research was supported by the Beijing Municipal Science and Technology Project (Z191100006619024) and the Beijing Municipal Natural Science Foundation of China (7172244).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1115834/full#supplementary-material
1. Ota T, Nagura T, Kokubo T, Kitashiro M, Ogihara N, Takeshima K, et al. Etiological factors in hallux valgus, a three-dimensional analysis of the first metatarsal. J Foot Ankle Res (2017) 10:43. doi: 10.1186/s13047-017-0226-1
2. Nix S, Smith M, Vicenzino B. Prevalence of hallux valgus in the general population: a systematic review and meta-analysis. J foot ankle Res (2010) 3:21. doi: 10.1186/1757-1146-3-21
3. Menz HB, Fotoohabadi MR, Wee E, Spink MJ. Validity of self-assessment of hallux valgus using the Manchester scale. BMC musculoskelet Disord (2010) 11:215. doi: 10.1186/1471-2474-11-215
4. Easley ME, Trnka HJ. Current concepts review: hallux valgus part 1: pathomechanics, clinical assessment, and nonoperative management. Foot ankle Int (2007) 28(5):654–9. doi: 10.3113/FAI.2007.0654
5. Smyth NA, Aiyer AA. Introduction: Why are there so many different surgeries for hallux valgus? Foot ankle Clinics (2018) 23(2):171–82. doi: 10.1016/j.fcl.2018.01.001
6. Piqué-Vidal C, Maled-García I, Arabi-Moreno J, Vila J. Radiographic angles in hallux valgus: differences between measurements made manually and with a computerized program. Foot ankle Int (2006) 27(3):175–80. doi: 10.1177/107110070602700304
7. Monteagudo M, Martínez-de-Albornoz P. Management of complications after hallux valgus reconstruction. Foot ankle clinics (2020) 25(1):151–67. doi: 10.1016/j.fcl.2019.10.011
8. Raikin SM, Miller AG, Daniel J. Recurrence of hallux valgus: a review. Foot ankle clinics (2014) 19(2):259–74. doi: 10.1016/j.fcl.2014.02.008
9. Ferrari J, Higgins JP, Williams RL. Interventions for treating hallux valgus (abductovalgus) and bunions. Cochrane Database syst Rev (2000) 2):CD000964. doi: 10.1002/14651858.CD000964.pub2
10. Willey JC, Reuter LS, Belatti DA, Phisitkul P, Amendola N. Availability of consumer prices for bunion surgery. Foot ankle Int (2014) 35(12):1309–15. doi: 10.1177/1071100714549045
11. Tran SK, Carr JB, Hall MJ, Park JS, Cooper MT. Incidence of thyroid disease in patients with forefoot deformity. Foot ankle Surg (2020) 26(4):445–8. doi: 10.1016/j.fas.2019.05.014
12. Tagoe CE, Wang W, Wang S, Barbour KE. Association of anti-thyroid antibodies with radiographic knee osteoarthritis and chondrocalcinosis: a NHANES III study. Ther Adv musculoskelet dis (2021) 13:1759720x211035199. doi: 10.1177/1759720X211035199
13. Bassett JH, Williams GR. Role of thyroid hormones in skeletal development and bone maintenance. Endocr Rev (2016) 37(2):135–87. doi: 10.1210/er.2015-1106
14. Nehls V. [Osteoarthropathies and myopathies associated with disorders of the thyroid endocrine system]. Deutsche medizinische Wochenschrift (2018) 143(16):1174–80. doi: 10.1055/s-0043-121381
15. Williams GR. Thyroid hormone actions in cartilage and bone. Eur Thyroid J (2013) 2(1):3–13. doi: 10.1159/000345548
16. Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet (2014) 23(R1):R89–98. doi: 10.1093/hmg/ddu328
17. Davies NM, Holmes MV, Davey Smith G. Reading mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ (2018) 362:k601. doi: 10.1136/bmj.k601
18. Burgess S, Davey Smith G, Davies NM, Dudbridge F, Gill D, Glymour MM, et al. Guidelines for performing mendelian randomization investigations. Wellcome Open Res (2019) 4:186. doi: 10.12688/wellcomeopenres.15555.2
19. Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomization: The STROBE-MR statement. Jama (2021) 326(16):1614–21. doi: 10.1001/jama.2021.18236
20. Shen Y, Li F, Cao L, Wang Y, Xiao J, Zhou X, et al. Hip osteoarthritis and the risk of lacunar stroke: A two-sample mendelian randomization study. Genes (2022) 13(9):1584 doi: 10.3390/genes13091584
21. Staley JR, Blackshaw J, Kamat MA, Ellis S, Surendran P, Sun BB, et al. PhenoScanner: a database of human genotype-phenotype associations. Bioinformatics (2016) 32(20):3207–9. doi: 10.1093/bioinformatics/btw373
22. Emdin CA, Khera AV, Kathiresan S. Mendelian randomization. Jama (2017) 318(19):1925–6. doi: 10.1001/jama.2017.17219
23. Burgess S, Thompson SG. Avoiding bias from weak instruments in mendelian randomization studies. Int J Epidemiol (2011) 40(3):755–64. doi: 10.1093/ije/dyr036
24. Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med (2008) 27(8):1133–63. doi: 10.1002/sim.3034
25. Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan NA, Thompson JR. Assessing the suitability of summary data for two-sample mendelian randomization analyses using MR-egger regression: the role of the I2 statistic. Int J Epidemiol (2016) 45(6):1961–74. doi: 10.1093/ije/dyw220
26. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol (2016) 40(4):304–14. doi: 10.1002/gepi.21965
27. Hartwig FP, Davies NM, Hemani G, Davey Smith G. Two-sample mendelian randomization: avoiding the downsides of a powerful, widely applicable but potentially fallible technique. Int J Epidemiol (2016) 45(6):1717–26. doi: 10.1093/ije/dyx028
28. Greco MF, Minelli C, Sheehan NA, Thompson JR. Detecting pleiotropy in mendelian randomisation studies with summary data and a continuous outcome. Stat Med (2015) 34(21):2926–40. doi: 10.1002/sim.6522
29. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int J Epidemiol (2015) 44(2):512–25. doi: 10.1093/ije/dyv080
30. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nat Genet (2018) 50(5):693–8. doi: 10.1038/s41588-018-0099-7
31. Tuchendler D, Bolanowski M. The influence of thyroid dysfunction on bone metabolism. Thyroid Res (2014) 7(1):12. doi: 10.1186/s13044-014-0012-0
32. Eriksen EF, Mosekilde L, Melsen F. Trabecular bone remodeling and bone balance in hyperthyroidism. Bone (1985) 6(6):421–8. doi: 10.1016/8756-3282(85)90218-2
33. Bjerkreim BA, Hammerstad SS, Eriksen EF. Bone turnover in relation to thyroid-stimulating hormone in hypothyroid patients on thyroid hormone substitution therapy. J Thyroid Res (2022) 2022:8950546. doi: 10.1155/2022/8950546
34. Tsourdi E, Colditz J, Lademann F, Rijntjes E, Köhrle J, Niehrs C, et al. The role of dickkopf-1 in thyroid hormone-induced changes of bone remodeling in Male mice. Endocrinology (2019) 160(3):664–74. doi: 10.1210/en.2018-00998
35. Robinson D, Hasharoni A, Halperin N, Yayon A, Nevo Z. Mesenchymal cells and growth factors in bunions. Foot ankle Int (1999) 20(11):727–32. doi: 10.1177/107110079902001109
36. Sharifi M, Ereifej L, Lewiecki EM. Sclerostin and skeletal health. Rev endocr Metab Disord (2015) 16(2):149–56. doi: 10.1007/s11154-015-9311-6
37. Mihaljević O, Živančević-Simonović S, Lučić-Tomić A, Živković I, Minić R, Mijatović-Teodorović L, et al. The association of circulating sclerostin level with markers of bone metabolism in patients with thyroid dysfunction. J Med Biochem (2020) 39(4):436–43. doi: 10.5937/jomb0-24943
38. Abu EO, Horner A, Teti A, Chatterjee VK, Compston JE. The localization of thyroid hormone receptor mRNAs in human bone. Thyroid (2000) 10(4):287–93. doi: 10.1089/thy.2000.10.287
39. Bock P, Kristen KH, Kröner A, Engel A. Hallux valgus and cartilage degeneration in the first metatarsophalangeal joint. J Bone Joint Surg Br (2004) 86(5):669–73. doi: 10.1302/0301-620x.86b5.14766
40. Pörings AS, Lowin T, Dufner B, Grifka J, Straub RH. A thyroid hormone network exists in synovial fibroblasts of rheumatoid arthritis and osteoarthritis patients. Sci Rep (2019) 9(1):13235. doi: 10.1038/s41598-019-49743-4
41. Siclari A, Piras M. Hallux metatarsophalangeal arthroscopy: indications and techniques. Foot ankle clinics (2015) 20(1):109–22. doi: 10.1016/j.fcl.2014.10.012
42. Lui TH. First metatarsophalangeal joint arthroscopy in patients with hallux valgus. Arthroscopy (2008) 24(10):1122–9. doi: 10.1016/j.arthro.2008.05.006
43. Zirngibl B, Grifka J, Baier C, Götz J. [Hallux valgus : Etiology, diagnosis, and therapeutic principles]. Der Orthop (2017) 46(3):283–96. doi: 10.1007/s00132-017-3397-3
44. Alimy AR, Polzer H, Ocokoljic A, Ray R, Lewis TL, Rolvien T, et al. Does minimally invasive surgery provide better clinical or radiographic outcomes than open surgery in the treatment of hallux valgus deformity? a systematic review and meta-analysis. Clin orthop related Res (2022) 4. doi: 10.1097/CORR.0000000000002471
45. Fukushi JI, Tanaka H, Nishiyama T, Hirao M, Kubota M, Kakihana M, et al. Comparison of outcomes of different osteotomy sites for hallux valgus: A systematic review and meta-analysis. J orthop Surg (2022) 30(2):10225536221110473. doi: 10.1177/10225536221110473
Keywords: thyroid, hypothyroidism, hallux valgus, causality, Mendelian randomization analysis
Citation: Xiong B, Bai Z, Cao X, Nie D, Zhang C, Sun X, Guo Z, Wen J and Sun W (2023) Causal relationship between thyroid dysfunction and hallux valgus: A two-sample Mendelian randomization study. Front. Endocrinol. 14:1115834. doi: 10.3389/fendo.2023.1115834
Received: 04 December 2022; Accepted: 21 February 2023;
Published: 08 March 2023.
Edited by:
Michela Rossi, Bambino Gesù Children’s Hospital (IRCCS), ItalyReviewed by:
Shihua Gao, Guangzhou University of Chinese Medicine, ChinaCopyright © 2023 Xiong, Bai, Cao, Nie, Zhang, Sun, Guo, Wen and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weidong Sun, c3Vud2VpZG9uZzgyMzlAYWxpeXVuLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.