- 1Endometriosis Ultrasound and Advanced Endosurgery Unit, Sydney Medical School Nepean, University of Sydney, Nepean Hospital, Sydney, NSW, Australia
- 2University of New South Wales Microbiome Research Centre, St. George and Sutherland Clinical Campuses, School of Clinical Medicine, Faculty of Medicine and Health, University of New South Wales, Sydney, NSW, Australia
Endometriosis has been described by many different theories of pathogenesis over the years. It is now also appreciated to be a state of chronic inflammation, and the role of immune dysfunction in its development has been proven. There is increasing evidence to support the role of the microbiome in the formation and progression of endometriosis via inflammatory pathways. The dysbiosis seen in endometriosis is thought to be both causative and a consequence of the pathogenesis. Gut, peritoneal fluid and female reproductive tract microbiota has been studied to understand if there are any microbiome signatures specific to endometriosis. New research on how to manipulate the microbiome for better detection and treatment of endometriosis is emerging.
Introduction
Endometriosis is an inflammatory disease characterized by the presence of endometrium-like tissue outside of the endometrium and myometrium (1, 2). It has a variety of subtypes and clinical presentations, ranging from being asymptomatic to causing chronic pain and infertility (2, 3). Endometriosis affects a significant proportion of the world’s population – estimated to be present in up to 10% of females, and up to 50% of women with infertility (1). It also has significant healthcare costs, with the most recent study in 2022 appraising the direct cost of endometriosis to be US$1459 to US$20,239 per patient per year, and indirect cost to be between US$4,572 and US$14,079 (4). An Australian study in 2019 projected the total economic burden per year in the reproductive aged population (at 10% prevalence) to be Int$6.50 billion (5).
Recently, an increased understanding of the role of microbiota and immune dysbiosis in many diseases has also brought to light the possibility of their role in development of endometriosis. The knowledge on the human microbiome and its definition has been rapidly expanding due to new developments in sequencing methods and analytical techniques (6). With this growing field, terminology can lead to confusion. Microbiota is defined as the community of microorganisms living in or on the human body site – it includes bacteria, archaea (single celled organisms without nuclei), fungi, eukaryotes and viruses. Microbiome is defined as the collective genomes of these microbes (7, 8). Microbiota has an associated theatre of activity – structural elements, metabolites, signal molecules, and the surrounding environmental conditions – that supports local immune, metabolic and epithelial function (9). When there is dysbiosis – defined as an imbalance or impairment of the microbiota – this support breaks down (3, 9, 10). Microbes and their metabolites can translocate to different body sites and can trigger an immune response and inflammation that is involved in multitude of diseases such as metabolic disorders, many neurological disorders, arthritis, psoriasis, inflammatory bowel disease and cancer (10–12).
Pathogenesis of endometriosis
There have been many postulations of pathogenesis of endometriosis including retrograde menstruation, immune dysfunction, inflammation, hormone dysregulation, coelomic metaplasia, lymphatic or hematological metastasis, stem cell dysfunction and genetic and epigenetic factors (13–16). A combination of these theories is likely in play together to lead to this chronic disease.
Immunopathology of endometriosis
Peritoneal endometriosis has been described as a chronic inflammatory state (17, 18). It is thought that the inflammatory state and the immune dysfunction in the peritoneum are both the cause and result of endometriosis. Immune dysregulation also leads to poor immunosurveillance as an appropriate response cannot be mounted to the refluxed endometrial cells and debris. This allows ectopic endometrial cells to persist in the peritoneal cavity (10, 19).
Firstly, the peritoneal inflammation plays a role in the development of the disease as well as the symptomology of the disease – pain and subfertility (17). The theory of retrograde menstruation is the most widely accepted pathogenesis of endometriosis (15). However, this theory alone does not explain disease prevalence, as women without endometriosis also display retrograde menstruation. Once endometrial cells are in the peritoneal cavity, they are required to adhere and proliferate to lead to endometriosis (10, 19). The inflammation and altered immunity create the right environment for cellular adhesion and endometriosis development and disease progression (10, 17, 18).
Oxidative stress is thought to be a key contributor to this inflammatory process (18, 20, 21). Reactive oxygen species (ROS) are intermediaries produced by the normal oxygen metabolism and have been implicated in his process (17, 18, 21). As a protective mechanism, cells cultivate antioxidant systems to counteract the ROS. When there is an imbalance between the ROS and antioxidants, with an abundance of ROS and deficiency in antioxidants, oxidative stress occurs (18, 20). In endometriosis, this imbalance is postulated to arise from erythrocytes in the peritoneal cavity and their toxic by-products of heme and iron. Free heme and iron lead to formation of ROS (17, 18, 21). This oxidative stress not only leads to cellular damage but also can alter cellular function via affecting protein activity and gene expression. The transcription factor called nuclear factor kappa-B (NF-κB) induces expression of multiple genes encoding proinflammatory cytokines, growth factors, adhesion molecules and enzymes, and has been implicated in peritoneal endometriosis by aiding in endometrial cell adhesion, proliferation and neovascularization (10, 17, 21–23).
Once the endometriotic implants adhere to the peritoneum, they require the help of cytokines and growth factors such as vascular endothelial growth factor (VEGF), tumour necrosis factor-α (TNF-α) and interleukin-8 (IL-8) for angiogenesis and lesion proliferation (10, 19, 24). These are primarily expressed by macrophages in the peritoneal cavity via increased activation of NF-κB pathways. There have been studies that support this theory by demonstrating increased number of macrophages, monocytes, and inflammatory mediators such as complements and cytokines in the peritoneal fluid of women with endometriosis (25, 26).
The dysregulation of both the innate and adaptive immunity are involved in the immunopathology of endometriosis. It has been shown that ectopic endometrial deposits, compared to matched eutopic endometrium of the same patients as well as to the endometrial tissues of the control group, have elevated expression of molecular genes associated with immune system process activation (27). Genes encoding for proinflammatory cytokines and receptors, cell adhesion molecules, complement proteins and angiogenesis are increased, and genes involved in regulation of inflammation, NK and cytotoxic T-cell activity, and cellular apoptosis are aberrantly expressed in ectopic endometrial tissues (27). These findings support the immune dysregulation in endometriosis.
Role of genetics and epigenetics in pathogenesis of endometriosis
Higher rates of endometriosis are seen in the relatives of women affected with endometriosis (28, 29). Twin studies have also supported the genetic influences on endometriosis by demonstrating a concordance ratio of 2:1 between monozygotic and dizygotic twins and a genetic risk ratio of 2.34 for endometriosis for a sibling, as well as 47-51% of endometriosis variation to be attributable to additive genetic effects (30, 31). As our understanding of genes and their role in disease has exponentially grown, there have been many studies conducted to determine the genes involved in specific condition such as endometriosis. Genome-wide association studies (GWAS) have discovered up to 27 significant loci associated with endometriosis but the challenge of understanding the functional consequences of these loci remain (32–35). There are genes associated with steroidogenesis and sex hormone receptorial activity, leading to dysregulation of estrogen and progesterone receptor ligand signaling, genes involved in inflammation and immune response, neoangiogenesis and DNA reparation, and genes coding for metabolism regulation and cell growth postulated to be instrumental in establishment of endometriosis (33). Furthermore, genes have been shown to regulate their neighboring genes by epigenetic mechanisms. Epigenetics is defined as heritable changes in gene function that are not associated with DNA sequence changes but involves processes such as DNA methylation and histone modification (34, 36). Epigenetic mechanisms have been demonstrated to be involved in regulating immune processes such as cytokine expression, T-cell differentiation, antigen presentation and regulation of transcription factors, such as NF-κB, which has been implicated in immunopathogenesis of endometriosis (10, 36, 37). The genetic and epigenetic theory is also supported by the finding that there are gene expression and molecular differences found in the endometrium of women with endometriosis is compared to the endometrium of healthy controls, as well as between the eutopic and ectopic endometrium of women with endometriosis (36, 38–40).
Microbiome of endometriosis
Alterations in the microbiota of gut, peritoneal fluid and female reproductive tract in subjects with endometriosis compared to healthy controls have been demonstrated in increasing number of both human and animal studies (10, 41–51). It is not too clear whether these alterations are a result of endometriosis or whether they are the cause of endometriosis. However experimental animal models support a bidirectional relationship between endometriosis and microbiota changes (42, 50). In a particular study in mice who had surgically induced endometriosis, a reduction in the size of the endometriotic lesions was seen after treatment with antibiotics. After fecal microbiota transfer from endometriotic mice, regrowth of the lesions and associated inflammation was seen (42).
Gut microbiota
Gut microbiota has been the most studied body site in endometriosis microbiome research. The gut microbiome is dominated by bacteria, especially the members of the phyla Bacteroidetes and Firmicutes. In most healthy humans, percentage of each of these two dominant phyla can vary but the combined percentage tends to be approximately 95%. In a disease state, the gut microbiome can shift to represent large percentages of other bacterial phyla, such as Proteobacteria, Verrucomicrobia, Actinobacteria, or Fusobacteria (7, 52).
Interestingly, a systematic review conducted in 2019 on the microbial signatures of endometriosis found the following results (3). At the phylum level, Actinobacteria, Firmicutes, Proteobacteria and Verrucomicrobia were identified as being significantly higher in the gut of the endometriosis cohort, compared with controls. In contrast, Lactobacillaceae was found to be significantly decreased. They concluded that the levels of Proteobacteria, Enterobacteriaceae, Streptococcus and E. coli were elevated across various microbiome sites in endometriosis cohorts.
Since this systematic review, a number of studies exploring the role of gut microbiome in endometriosis have been published (41, 48, 53). Svensson et al’s 2021 study did not find any significant differences in the abundance of bacterial classes between patient with or without isolated ovarian endometriosis, involvement of the gastrointestinal tract, gastrointestinal symptoms, or hormonal treatment (48). However, other studies have demonstrated that in the gut microbiota, more women in the endometriosis group had Shigella and Escherichia dominance (41).
Female reproductive tract microbiota
The female reproductive tract microbiota can be divided into the vagina, cervix, endometrium, fallopian tubes and ovaries. Majority of the studies in the microbiota of the female reproductive tract in endometriosis have focused on the cervix and the vagina. It has been found that the distribution of microbiota is similar in the cervical mucus of women with and without endometriosis regardless of the phases of the menstrual cycle, however the abundance of each changes (54). Lactobacilli is the predominant species in the vagina and the cervix. In addition to this, the abundance of Corynebacterium, Enterobacteriaceae, Flavobacterium, Pseudomonas, and Streptococcus are increased in the endometriosis group compared to the control group, with Enterobacteriaceae and Streptococcus being the more noteworthy candidates (54). A recent review has summarized that bacterial vaginosis-associated bacteria and Lactobacillus depletion in the cervicovaginal microbiome were associated with endometriosis and infertility in the majority of studies they analyzed (53). Another noteworthy finding is that reduced richness and diversity of cervical microbiome were detected in patients with more severe endometriosis symptoms including higher CA125 levels, more severe pain and infertility (55). This study suggested that cervical microbiome has an important role in regulating the pathogenesis of the associated complications of endometriosis and concluded that a more diverse cervical microbiome is associated with better clinical outcomes.
A small study of 14 participants with Stage III-IV endometriosis and 14 healthy controls revealed that the vaginal, cervical and gut microbiota composition among the endometriosis group were similar; but it showed that some potentially pathogenic species were increased in the cervical and stool microbiome in women with endometriosis compared to the control group (41). In the cervical microbiota, Gardnerella, Streptococcus, Escherichia, Shigella, and Ureoplasma were increased. Interestingly they observed a total absence of a particular genus, Atopobium in vaginal and cervical microbiota. Atopobium has been recently implicated as a gynecological pathogen potentially associated with endometrial cancer, and lower incidence of it was seen in women with benign gynecological pathologies (56). It is unclear that this association is causal or coincidental. It could be proposed that the absence of Atopobium can be related to occurrence benign gynecological pathologies, in which endometriosis a part of.
Less studies have been performed exploring the relationship with viruses, particularly human papilloma virus (HPV) with endometriosis (3). Majority of these studies have found HPV detection to be higher and therefore associated with endometriosis (57–59).
Peritoneal microbiota
Microbiota diversity of the peritoneal fluid was shown to be similar in women with an without endometriosis (51). However, the abundance of these microbiota differed (47, 51). Acidovorax, Devosia, Methylobacterium, Phascolarctobacterium, and Streptococcus were more abundant in the peritoneal fluid of endometriosis patients than the controls, while Brevundimonas and Stenotrophomonas were less abundant (51). Another study reported the abundance of Acinetobacter, Pseudomonas, Streptococcus, and Enhydrobacter to be significantly increased while the abundance of Propionibacterium, Actinomyces, and Rothia to be significantly decreased in the endometriosis group compared with those in the control group (47). A third study concluded Sphingobium, Pseudomonadaceae, Sphingomonas, Acinetobacter, Erysipelothrix, Clostridiales, Micrococcaceae, Vagococcus, Dysgonomonas, Pseudomonas viridiflava, Shewanella, Tissierellaceae were enriched in the peritoneal fluid of endometriosis patients compared to the control group (49). When the microbiota of deep endometriosis lesions were examined by Hernandes et al. in 2020, Alishewanella, Enterococcus and Pseudomonas were demonstrated to be more abundant (43). Acinetobacter and Pseudomonas prove to be persistently present in several of these studies (43, 47, 49). These findings not only support that microbiome composition is altered in the peritoneal environment in women with endometriosis but also point to the possibility of finding a peritoneal fluid microbial signature specific to endometriosis.
Microbiome’s role in pathogenesis of endometriosis
The effects of dysbiosis could be contributing to the pathogenesis of endometriosis via inflammation and immune modulation and there is new evidence suggesting a role of the microbiome in development of endometriosis (10, 41, 43, 49, 53, 60–62).
Bacterial contamination theory
A “bacterial contamination” theory for endometriosis progression of endometriosis has been postulated. Khan et al. (63) examined Escherichia coli (E. coli) concentrations in the menstrual blood of women with endometriosis in comparison to control groups. They found that there was increased number of E. coli colony formation in women with endometriosis, especially those with peritoneal endometriosis in addition to ovarian endometriomas. They suggested that E. coli contamination of the menstrual blood would be a constant source of bacterial endotoxin or a lipopolysaccharide in the peritoneal cavity. The primary inflammation caused by the lipopolysaccharides would lead to the secretion of secondary inflammatory mediators such as NF-κB in the peritoneal cavity via promoting Toll-like receptor 4 (TLR4) which are present on macrophages and other immune cells (60). This would start the cascade of endometriosis development as explained with the immunopathology of endometriosis.
The bacterial contamination theory has since been discussed (10, 60) and is supported by other research demonstrating increased levels of Proteobacteria, which is a phylum of bacteria that produces lipopolysaccharides, in endometriosis cohorts (41, 45, 50, 54, 63, 64). Furthermore, a large cohort study of over 140,000 women demonstrated that there is a three-fold increased risk of developing endometriosis in women with a history of pelvic inflammatory disease (PID) compared to the control cohort (62). A similar result has been exhibited by another study in which double the incidence of endometriosis was seen in women with a lower genital tract infection (61).
Estrobolomes in development of endometriosis
Another possible mechanism of how microbiome can influence endometriosis development and progression can be explained by the altered estrogen metabolism that is seen with dysbiosis (65). It is known that endometriosis is an estrogen driven condition (66, 67). Certain dysbiotic gut microbiota are known as ‘estrobolomes’ whose products can metabolize estrogen, increasing the circulating levels of estrogen in the body (3, 53, 68). These estrobolomes are known to secrete β-glucuronidase and β-glucosidases which deconjugate estrogen, which in turn increases the re-absorption of free estrogens in the gut (68, 69). It is theorized that this can lead to a hyperestrogenic state and contribute to progressing of endometriosis (53, 65). Multiple genera in the gut microbiome encode for β-glucuronidase, including Bacteroides, Bifidobacterium, Escherichia and Lactobacillus (3, 69). Interestingly, some studies have found higher levels of Bifidobacterium and Escherichia in endometriosis groups over control groups (50, 63). It is also known that there is dysbiosis in the Bacteroidetes/Firmicutes ratios, which are the two dominant phyla in the gut, in women with endometriosis (10, 50).
Genetic and epigenetic factors
Bacterial induced epigenetic deregulation of host cells has been well studied (70–72). Dysbiosis and female genital tract infections may induce genetic and epigenetic incidents, leading to increased oxidative stress and changes in the immune responses, which in turn could play a role in the formation of endometriosis (72). A study on Mycoplasma genitalium revealed that the gene expression of peritoneal fluid cells of women with endometriosis who are colonized with Mycoplasma genitalium were significantly downregulated which in turn would inhibit immune cells recruited to the site (73). Viruses are also known to be mutagenic and this has been proven in many malignancies caused by carcinogenic viruses such as HPV, human immunodeficiency virus (HIV), hepatitis B virus (HBV), hepatitis C virus (HCV) and Ebstein-Barr virus (EBV) (74–76). There have only been a few studies assessing the relationship between viruses, particularly HPV, and endometriosis, and future studies are needed in this area (57–59). Furthermore, it has been reported that inflammation itself can cause aberrant DNA methylation patterns leading to hypermethylation which in turn effects the production of certain transcription factors and receptors (such as HOXA10 and progesterone receptor B) that is seen in endometriosis (39, 77, 78).
Clinical implications
Performance of different body sites
Only a few studies have compared microbiota of different body sites to assess which site is the best performer in predicting endometriosis. One of the studies examined the gut, peritoneal fluid and cervical mucus. It demonstrated that the gut and peritoneal fluid have higher richness and diversity in microbiota compared to cervical mucus and concluded that the gut microbiota is the top performing predictor of endometriosis out of the three (44). Another study compared samples from lower third of vagina, posterior vaginal fornix, cervical mucus, endometrium and peritoneal fluid (49). Each site had a different microbiota distribution. Significant difference of the community diversity began showing in the cervical mucus of endometriosis patients and gradually increased upward the reproductive tract, suggesting the upper female reproductive tract is better indicator for the risk of endometriosis if used as a screening tool (49).
Microbiome in predicting endometriosis stage
There is limited evidence available in the differences between microbiotas of different endometriosis stages and all the available evidence is based on studies with small numbers (44, 79). The studies that investigated this question used revised-ASRM (rASRM) stages (80). A study of 21 participants with endometriosis found no difference between gut microbiota of early (Stage I and II) and advanced stages (Stage III and IV) of endometriosis (44). A larger study with 59 participants (34 with endometriosis, 24 control) assessed gut and vaginal samples collected at two different time periods within the menstrual cycle (79). They reported that 9/35 (25.7%) had Stage I, 12/35 (34.2%) had Stage II, 3/35 (11.4%) had Stage III, and 10/35 (28.5%) had Stage IV endometriosis. They grouped Stage I and II together and Stage II and IV together for comparative analysis. The analysis did not show any significant differences in the microbiota between either two groups of stages of endometriosis or control groups. However, they concluded that vaginal microbiome was predictive of the stage of the disease based on an operational taxonomical unit (OTU) from the genus Anaerococcus. Both of these studies used 16S rRNA gene sequencing for analysis. Other studies that performed a sub-analysis on rASRM stages any briefly mentioned that there was no significant difference between the different stages (45, 73).
Evidence so far in altering microbiome to treat endometriosis
Evidence on the role of microbiome and dysbiosis in the development of endometriosis is rapidly mounting. Therapeutic manipulation of the microbiome in treatment and prevention of endometriosis is a very real possibility. Animal studies have already established this possibility (42). Figure 1 summaries the current interventions that have a potential in treatment of endometriosis. Chadchan et al’s study on mice (42) with surgically induced endometriosis found higher levels of Bacteroidetes and lower levels of Firmicutes in the gut microbiota composition compared to the control mice. Metronidazole was used on the mice with endometriosis to target Bacteroidetes and this demonstrated reduction in the endometriosis lesion size and cell proliferation. Furthermore, they proved a reduced inflammatory response in the treated mice by measuring lower levels of inflammatory cytokines and mediators in the peritoneal fluid and endometriotic lesions.
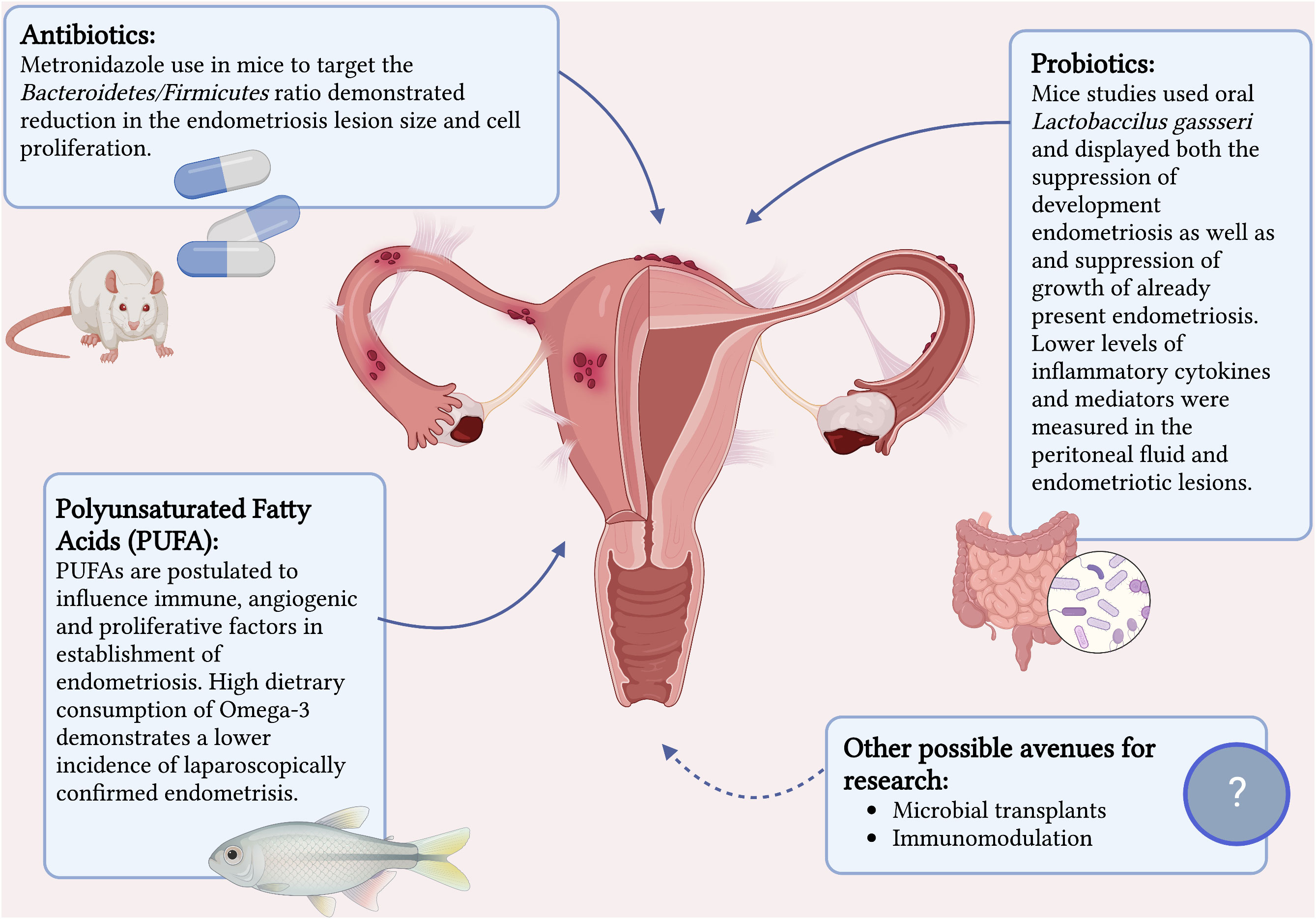
Figure 1 Current interventions that have a potential in treatment of endometriosis. Created with BioRender.com.
Probiotics is another promising treatment option that has proven itself with other benign gynecological conditions such as candida vulvovaginitis and bacterial vaginosis (81). There have been two mice studies that show some potential in its benefit in the treatment of endometriosis (82, 83). Both studies investigated the role of oral Lactobaccilus gassseri and displayed both the suppression of development endometriosis as well as and suppression of growth of already present endometriosis. The mechanism postulated was immunostimulatory activity via activation of NK cells and reduction in development of ectopic endometriotic lesions.
Although there are limited animal studies on antibiotics and probiotics on treatment of endometriosis (42, 82, 83), there have not been any studies to date to investigate the specific role of probiotics or prebiotics in helping resolve the dysbiosis associated with endometriosis in hopes to assist in its treatment. Urgent future research is needed to study the role of probiotic and antibiotic therapy further in human subjects. Conversely it is important to remember that excessive use of antibiotics can have the adverse effect of altering healthy commensal microbiota and contributing to antimicrobial resistance.
Lastly, the therapeutic anti-inflammatory effect of polyunsaturated fatty acids (PUFAs) such as omega-3 and omega-6 on multitude of diseases is well established (84, 85) and increasing evidence on beneficial effects of PUFAs on endometriosis is becoming available. Women with endogenously higher serum PUFAs levels have been shown to be 82% less likely to have endometriosis compared to women with low PUFAs levels (86) and a high dietary consumption of omega-3 demonstrates a lower incidence of laparoscopically confirmed endometriosis compared to individuals with a low dietary intake of omega-3 (87). A study on mice has confirmed this by exhibiting a 99-fold lower level of inflammatory cytokine IL-6 in mice with endogenously high levels of omega-3 PUFAs as well as a reduction of proliferation in endometriosis-like lesions when donor tissue was transferred to a PUFA rich host environment (88). They demonstrated that omega-3 PUFA levels influence immune, angiogenic, and proliferative factors implicated in the early establishment of endometriosis.
Future directions
The majority of research in microbiota and endometriosis has been focused on bacteria. The most common analytic method used in these studies has been the 16sRNA sequencing. 16sRNA sequencing uses a single gene in bacteria and is used to differentiate bacterial taxa and their relative abundance. However, it does not distinguish between the different strains of each genus of bacteria. Different strains of the genus of bacteria are genomically distinct and one strain can cause significant illness whilst another strain could be considered a probiotic (7). Shotgun metagenomics and metabolomics are newer analytical methods that have become more accessible in the recent years. They examine a wider range of microbiota and microbiome, although both come with analytical limitations due to the ongoing developments in the field. Shotgun metagenomics fragments all the DNA from a sample and sequences these fragments. It can infer a complete list of microbial strains including viruses and fungi. Metabolomics is the study of the nonprotein small molecules including products of metabolism (7, 89). Further research with these methods may yield different results, especially on different types of microbiota, such as fungi or viruses and new associations with endometriosis may be discovered.
Discussion
Microbiome testing has potential be used a non-invasive test to detect endometriosis. There is a significant delay in diagnosis of endometriosis, with the average time between onset of symptoms to diagnosis being 8 years and the range reported as 4-12 years in different studies (90–92). Previously, laparoscopic diagnosis was considered the gold standard but has the disadvantage of being invasive. As imaging techniques have improved over the years, transvaginal ultrasound and MRI for diagnosis of deep endometriosis have proven to have great accuracy and now accepted as first line diagnostic tools (1, 93). However, they are limited by the availability of skilled sonographers, sonologists and radiologists. In addition, the techniques to improve diagnosis of superficial endometriosis on ultrasound are still relatively new (94, 95). If available in the future, a simple microbiota test could complement the imaging modalities well in non-invasive diagnosis of endometriosis. Currently there is no evidence for microbiome signatures of different stages of endometriosis or predicting infertility. Further research is needed to be able to make this a possibility. Discovering the microbial signature of endometriosis would also create avenues for future research into developing methods to alter the microbiome via probiotics, microbial transplants, or immunomodulation to alter the disease.
Author contributions
CU wrote the manuscript. JM, FE-A and GC outlined the content of the review, reviewed, edited and approved the final draft. GC supervised the project. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Becker CM, Bokor A, Heikinheimo O, Horne A, Jansen F, Kiesel L, et al. ESHRE guideline: endometriosis. Hum Reprod Open (2022) 2022(2):hoac009. doi: 10.1093/hropen/hoac009
2. International working group of Aagl EE, Wes TC, Johnson NP, Petrozza J, Abrao MS, Einarsson JI, et al. An international terminology for endometriosis, 2021. J Minim Invasive Gynecol (2021) 28(11):1849–59. doi: 10.1093/hropen/hoab029
3. Leonardi M, Hicks C, El-Assaad F, El-Omar E, Condous G. Endometriosis and the microbiome: A systematic review. BJOG (2020) 127(2):239–49. doi: 10.1111/1471-0528.15916
4. Darba J, Marsa A. Economic implications of endometriosis: A review. Pharmacoeconomics (2022) 40(12):1143–58. doi: 10.1007/s40273-022-01211-0
5. Armour M, Lawson K, Wood A, Smith CA, Abbott J. The cost of illness and economic burden of endometriosis and chronic pelvic pain in Australia: A national online survey. PloS One (2019) 14(10):e0223316. doi: 10.1371/journal.pone.0223316
6. Ursell LK, Metcalf JL, Parfrey LW, Knight R. Defining the human microbiome. Nutr Rev (2012) 70 Suppl 1:S38–44. doi: 10.1111/j.1753-4887.2012.00493.x
7. Allaband C, McDonald D, Vazquez-Baeza Y, Minich JJ, Tripathi A, Brenner DA, et al. Microbiome 101: Studying, analyzing, and interpreting gut microbiome data for clinicians. Clin Gastroenterol Hepatol (2019) 17(2):218–30. doi: 10.1016/j.cgh.2018.09.017
8. Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature (2007) 449(7164):804–10. doi: 10.1038/nature06244
9. Berg G, Rybakova D, Fischer D, Cernava T, Verges MC, Charles T, et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome (2020) 8(1):103. doi: 10.1186/s40168-020-00875-0
10. Jiang I, Yong PJ, Allaire C, Bedaiwy MA. Intricate connections between the microbiota and endometriosis. Int J Mol Sci (2021) 22(11):5644. doi: 10.3390/ijms22115644
11. Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat Rev Genet (2012) 13(4):260–70. doi: 10.1038/nrg3182
12. Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, Gasbarrini A, et al. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms (2019) 7(1):14. doi: 10.3390/microorganisms7010014
13. Vercellini P, Vigano P, Somigliana E, Fedele L. Endometriosis: Pathogenesis and treatment. Nat Rev Endocrinol (2014) 10(5):261–75. doi: 10.1038/nrendo.2013.255
14. Riccio L, Santulli P, Marcellin L, Abrao MS, Batteux F, Chapron C. Immunology of endometriosis. Best Pract Res Clin Obstet Gynaecol (2018) 50:39–49. doi: 10.1016/j.bpobgyn.2018.01.010
15. Sampson JA. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am J Pathol (1927) 3(2):93–110 43.
16. Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril (2012) 98(3):511–9. doi: 10.1016/j.fertnstert.2012.06.029
17. Lousse JC, Van Langendonckt A, Defrere S, Ramos RG, Colette S, Donnez J. Peritoneal endometriosis is an inflammatory disease. Front Biosci (Elite Ed) (2012) 4(1):23–40. doi: 10.2741/e358
18. Van Langendonckt A, Casanas-Roux F, Donnez J. Oxidative stress and peritoneal endometriosis. Fertil Steril (2002) 77(5):861–70. doi: 10.1016/S0015-0282(02)02959-X
19. Symons LK, Miller JE, Kay VR, Marks RM, Liblik K, Koti M, et al. The immunopathophysiology of endometriosis. Trends Mol Med (2018) 24(9):748–62. doi: 10.1016/j.molmed.2018.07.004
20. Clower L, Fleshman T, Geldenhuys WJ, Santanam N. Targeting oxidative stress involved in endometriosis and its pain. Biomolecules (2022) 12(8):1055. doi: 10.3390/biom12081055
21. Donnez J, Binda MM, Donnez O, Dolmans MM. Oxidative stress in the pelvic cavity and its role in the pathogenesis of endometriosis. Fertil Steril (2016) 106(5):1011–7. doi: 10.1016/j.fertnstert.2016.07.1075
22. Gonzalez-Ramos R, Donnez J, Defrere S, Leclercq I, Squifflet J, Lousse JC, et al. Nuclear factor-kappa b is constitutively activated in peritoneal endometriosis. Mol Hum Reprod (2007) 13(7):503–9. doi: 10.1093/molehr/gam033
23. Gonzalez-Ramos R, Van Langendonckt A, Defrere S, Lousse JC, Mettlen M, Guillet A, et al. Agents blocking the nuclear factor-kappaB pathway are effective inhibitors of endometriosis in an in vivo experimental model. Gynecol Obstet Invest (2008) 65(3):174–86. doi: 10.1159/000111148
24. Iwabe T, Harada T, Tsudo T, Nagano Y, Yoshida S, Tanikawa M, et al. Tumor necrosis factor-alpha promotes proliferation of endometriotic stromal cells by inducing interleukin-8 gene and protein expression. J Clin Endocrinol Metab (2000) 85(2):824–9. doi: 10.1210/jcem.85.2.6335
25. Deaton JL, Honore GM, Huffman CS, Bauguess P. Early transvaginal ultrasound following an accurately dated pregnancy: The importance of finding a yolk sac or fetal heart motion. Hum Reprod (1997) 12(12):2820–3. doi: 10.1093/humrep/12.12.2820
26. Gazvani R, Templeton A. Peritoneal environment, cytokines and angiogenesis in the pathophysiology of endometriosis. Reproduction (2002) 123(2):217–26. doi: 10.1530/rep.0.1230217
27. Ahn SH, Khalaj K, Young SL, Lessey BA, Koti M, Tayade C. Immune-inflammation gene signatures in endometriosis patients. Fertil Steril (2016) 106(6):1420–31.e7. doi: 10.1016/j.fertnstert.2016.07.005
28. Simpson JL, Elias S, Malinak LR, Buttram VC Jr. Heritable aspects of endometriosis. I. Genet Stud Am J Obstet Gynecol (1980) 137(3):327–31. doi: 10.1016/0002-9378(80)90917-5
29. Moen MH, Magnus P. The familial risk of endometriosis. Acta Obstet Gynecol Scand (1993) 72(7):560–4. doi: 10.3109/00016349309058164
30. Treloar SA, O'Connor DT, O'Connor VM, Martin NG. Genetic influences on endometriosis in an Australian twin sample. Fertil Steril (1999) 71(4):701–10. doi: 10.1016/S0015-0282(98)00540-8
31. Saha R, Pettersson HJ, Svedberg P, Olovsson M, Bergqvist A, Marions L, et al. Heritability of endometriosis. Fertil Steril (2015) 104(4):947–52. doi: 10.1016/j.fertnstert.2015.06.035
32. Rahmioglu N, Nyholt DR, Morris AP, Missmer SA, Montgomery GW, Zondervan KT. Genetic variants underlying risk of endometriosis: Insights from meta-analysis of eight genome-wide association and replication datasets. Hum Reprod Update (2014) 20(5):702–16. doi: 10.1093/humupd/dmu015
33. Deiana D, Gessa S, Anardu M, Daniilidis A, Nappi L, D'Alterio MN, et al. Genetics of endometriosis: A comprehensive review. Gynecol Endocrinol (2019) 35(7):553–8. doi: 10.1080/09513590.2019.1588244
34. Borghese B, Zondervan KT, Abrao MS, Chapron C, Vaiman D. Recent insights on the genetics and epigenetics of endometriosis. Clin Genet (2017) 91(2):254–64. doi: 10.1111/cge.12897
35. Giacomini E, Minetto S, Li Piani L, Pagliardini L, Somigliana E, Vigano P. Genetics and inflammation in endometriosis: Improving knowledge for development of new pharmacological strategies. Int J Mol Sci (2021) 22(16):9033. doi: 10.3390/ijms22169033
36. Guo SW. Epigenetics of endometriosis. Mol Hum Reprod (2009) 15(10):587–607. doi: 10.1093/molehr/gap064
37. Fitzpatrick DR, Wilson CB. Methylation and demethylation in the regulation of genes, cells, and responses in the immune system. Clin Immunol (2003) 109(1):37–45. doi: 10.1016/S1521-6616(03)00205-5
38. Koninckx PR, Ussia A, Adamyan L, Wattiez A, Gomel V, Martin DC. Pathogenesis of endometriosis: The genetic/epigenetic theory. Fertil Steril (2019) 111(2):327–40. doi: 10.1016/j.fertnstert.2018.10.013
39. Wu Y, Halverson G, Basir Z, Strawn E, Yan P, Guo SW. Aberrant methylation at HOXA10 may be responsible for its aberrant expression in the endometrium of patients with endometriosis. Am J Obstet Gynecol (2005) 193(2):371–80. doi: 10.1016/j.ajog.2005.01.034
40. Meola J, Rosa e Silva JC, Dentillo DB, da Silva WA Jr., Veiga-Castelli LC, Bernardes LA, et al. Differentially expressed genes in eutopic and ectopic endometrium of women with endometriosis. Fertil Steril (2010) 93(6):1750–73. doi: 10.1016/j.fertnstert.2008.12.058
41. Ata B, Yildiz S, Turkgeldi E, Brocal VP, Dinleyici EC, Moya A, et al. The endobiota study: Comparison of vaginal, cervical and gut microbiota between women with stage 3/4 endometriosis and healthy controls. Sci Rep (2019) 9(1):2204. doi: 10.1038/s41598-019-39700-6
42. Chadchan SB, Cheng M, Parnell LA, Yin Y, Schriefer A, Mysorekar IU, et al. Antibiotic therapy with metronidazole reduces endometriosis disease progression in mice: A potential role for gut microbiota. Hum Reprod (2019) 34(6):1106–16. doi: 10.1093/humrep/dez041
43. Hernandes C, Silveira P, Rodrigues Sereia AF, Christoff AP, Mendes H, Valter de Oliveira LF, et al. Microbiome profile of deep endometriosis patients: Comparison of vaginal fluid, endometrium and lesion. Diagnostics (Basel) (2020) 10(3):163. doi: 10.3390/diagnostics10030163
44. Huang L, Liu B, Liu Z, Feng W, Liu M, Wang Y, et al. Gut microbiota exceeds cervical microbiota for early diagnosis of endometriosis. Front Cell Infect Microbiol (2021) 11:788836. doi: 10.3389/fcimb.2021.788836
45. Khan KN, Fujishita A, Kitajima M, Hiraki K, Nakashima M, Masuzaki H. Intra-uterine microbial colonization and occurrence of endometritis in women with endometriosisdagger. Hum Reprod (2014) 29(11):2446–56. doi: 10.1093/humrep/deu222
46. Le N, Cregger M, Fazleabas A, Braundmeier-Fleming A. Effects of endometriosis on immunity and mucosal microbial community dynamics in female olive baboons. Sci Rep (2022) 12(1):1590. doi: 10.1038/s41598-022-05499-y
47. Lee SR, Lee JC, Kim SH, Oh YS, Chae HD, Seo H, et al. Altered composition of microbiota in women with ovarian endometrioma: Microbiome analyses of extracellular vesicles in the peritoneal fluid. Int J Mol Sci (2021) 22(9):4608. doi: 10.3390/ijms22094608
48. Svensson A, Brunkwall L, Roth B, Orho-Melander M, Ohlsson B. Associations between endometriosis and gut microbiota. Reprod Sci (2021) 28(8):2367–77. doi: 10.1007/s43032-021-00506-5
49. Wei W, Zhang X, Tang H, Zeng L, Wu R. Microbiota composition and distribution along the female reproductive tract of women with endometriosis. Ann Clin Microbiol Antimicrob (2020) 19(1):15. doi: 10.1186/s12941-020-00356-0
50. Yuan M, Li D, Zhang Z, Sun H, An M, Wang G. Endometriosis induces gut microbiota alterations in mice. Hum Reprod (2018) 33(4):607–16. doi: 10.1093/humrep/dex372
51. Yuan W, Wu Y, Chai X, Wu X. The colonized microbiota composition in the peritoneal fluid in women with endometriosis. Arch Gynecol Obstet (2022) 305(6):1573–80. doi: 10.1007/s00404-021-06338-7
52. Human Microbiome Project C. Structure, function and diversity of the healthy human microbiome. Nature (2012) 486(7402):207–14. doi: 10.1038/nature11234
53. Salliss ME, Farland LV, Mahnert ND, Herbst-Kralovetz MM. The role of gut and genital microbiota and the estrobolome in endometriosis, infertility and chronic pelvic pain. Hum Reprod Update (2021) 28(1):92–131. doi: 10.1093/humupd/dmab035
54. Akiyama K, Nishioka K, Khan KN, Tanaka Y, Mori T, Nakaya T, et al. Molecular detection of microbial colonization in cervical mucus of women with and without endometriosis. Am J Reprod Immunol (2019) 82(2):e13147. doi: 10.1111/aji.13147
55. Chang CY, Chiang AJ, Lai MT, Yan MJ, Tseng CC, Lo LC, et al. A more diverse cervical microbiome associates with better clinical outcomes in patients with endometriosis: A pilot study. Biomedicines (2022) 10(1):174. doi: 10.3390/biomedicines10010174
56. Walther-Antonio MR, Chen J, Multinu F, Hokenstad A, Distad TJ, Cheek EH, et al. Potential contribution of the uterine microbiome in the development of endometrial cancer. Genome Med (2016) 8(1):122. doi: 10.1186/s13073-016-0368-y
57. Heidarpour M, Derakhshan M, Derakhshan-Horeh M, Kheirollahi M, Dashti S. Prevalence of high-risk human papillomavirus infection in women with ovarian endometriosis. J Obstet Gynaecol Res (2017) 43(1):135–9. doi: 10.1111/jog.13188
58. Oppelt P, Renner SP, Strick R, Valletta D, Mehlhorn G, Fasching PA, et al. Correlation of high-risk human papilloma viruses but not of herpes viruses or chlamydia trachomatis with endometriosis lesions. Fertil Steril (2010) 93(6):1778–86. doi: 10.1016/j.fertnstert.2008.12.061
59. Rocha RM, Souza RP, Gimenes F, Consolaro MEL. The high-risk human papillomavirus continuum along the female reproductive tract and its relationship to infertility and endometriosis. Reprod BioMed Online (2019) 38(6):926–37. doi: 10.1016/j.rbmo.2018.11.032
60. Khan KN, Fujishita A, Hiraki K, Kitajima M, Nakashima M, Fushiki S, et al. Bacterial contamination hypothesis: A new concept in endometriosis. Reprod Med Biol (2018) 17(2):125–33. doi: 10.1002/rmb2.12083
61. Lin WC, Chang CY, Hsu YA, Chiang JH, Wan L. Increased risk of endometriosis in patients with lower genital tract infection: A nationwide cohort study. Med (Baltimore) (2016) 95(10):e2773. doi: 10.1097/MD.0000000000002773
62. Tai FW, Chang CY, Chiang JH, Lin WC, Wan L. Association of pelvic inflammatory disease with risk of endometriosis: A nationwide cohort study involving 141,460 individuals. J Clin Med (2018) 7(11):379. doi: 10.3390/jcm7110379
63. Khan KN, Kitajima M, Hiraki K, Yamaguchi N, Katamine S, Matsuyama T, et al. Escherichia coli contamination of menstrual blood and effect of bacterial endotoxin on endometriosis. Fertil Steril (2010) 94(7):2860–3.e1-3. doi: 10.1016/j.fertnstert.2010.04.053
64. Khan KN, Fujishita A, Masumoto H, Muto H, Kitajima M, Masuzaki H, et al. Molecular detection of intrauterine microbial colonization in women with endometriosis. Eur J Obstet Gynecol Reprod Biol (2016) 199:69–75. doi: 10.1016/j.ejogrb.2016.01.040
65. Baker JM, Al-Nakkash L, Herbst-Kralovetz MM. Estrogen-gut microbiome axis: Physiological and clinical implications. Maturitas (2017) 103:45–53. doi: 10.1016/j.maturitas.2017.06.025
66. Huhtinen K, Stahle M, Perheentupa A, Poutanen M. Estrogen biosynthesis and signaling in endometriosis. Mol Cell Endocrinol (2012) 358(2):146–54. doi: 10.1016/j.mce.2011.08.022
67. Kitawaki J, Kado N, Ishihara H, Koshiba H, Kitaoka Y, Honjo H. Endometriosis: the pathophysiology as an estrogen-dependent disease. J Steroid Biochem Mol Biol (2002) 83(1-5):149–55. doi: 10.1016/S0960-0760(02)00260-1
68. Plottel CS, Blaser MJ. Microbiome and malignancy. Cell Host Microbe (2011) 10(4):324–35. doi: 10.1016/j.chom.2011.10.003
69. Kwa M, Plottel CS, Blaser MJ, Adams S. The intestinal microbiome and estrogen receptor-positive female breast cancer. J Natl Cancer Inst (2016) 108(8):djw029. doi: 10.1093/jnci/djw029
70. Bierne H, Hamon M, Cossart P. Epigenetics and bacterial infections. Cold Spring Harb Perspect Med (2012) 2(12):a010272. doi: 10.1101/cshperspect.a010272
71. Jenner RG, Young RA. Insights into host responses against pathogens from transcriptional profiling. Nat Rev Microbiol (2005) 3(4):281–94. doi: 10.1038/nrmicro1126
72. Koninckx PR, Ussia A, Tahlak M, Adamyan L, Wattiez A, Martin DC, et al. Infection as a potential cofactor in the genetic-epigenetic pathophysiology of endometriosis: A systematic review. Facts Views Vis Obgyn (2019) 11(3):209–16.
73. Campos GB, Marques LM, Rezende IS, Barbosa MS, Abrao MS, Timenetsky J. Mycoplasma genitalium can modulate the local immune response in patients with endometriosis. Fertil Steril (2018) 109(3):549–60.e4. doi: 10.1016/j.fertnstert.2017.11.009
74. Serraino D, Piselli P, Scognamiglio P. Viral infections and cancer: epidemiological aspects. J Biol Regul Homeost Agents (2001) 15(3):224–8.
75. Butel JS. Viral carcinogenesis: revelation of molecular mechanisms and etiology of human disease. Carcinogenesis (2000) 21(3):405–26. doi: 10.1093/carcin/21.3.405
76. Rapp F. Current knowledge of mechanisms of viral carcinogenesis. Crit Rev Toxicol (1984) 13(2):197–204. doi: 10.3109/10408448409034082
77. Hsiao KY, Wu MH, Tsai SJ. Epigenetic regulation of the pathological process in endometriosis. Reprod Med Biol (2017) 16(4):314–9. doi: 10.1002/rmb2.12047
78. Lagana AS, Garzon S, Gotte M, Vigano P, Franchi M, Ghezzi F, et al. The pathogenesis of endometriosis: Molecular and cell biology insights. Int J Mol Sci (2019) 20(22):5615. doi: 10.3390/ijms20225615
79. Perrotta AR, Borrelli GM, Martins CO, Kallas EG, Sanabani SS, Griffith LG, et al. The vaginal microbiome as a tool to predict rASRM stage of disease in endometriosis: A pilot study. Reprod Sci (2020) 27(4):1064–73. doi: 10.1007/s43032-019-00113-5
80. Revised American fertility society classification of endometriosis: 1985. Fertil Steril (1985) 43(3):351–2. doi: 10.1016/s0015-0282(16)48430-x
81. Gholiof M, Adamson-De Luca E, Wessels JM. The female reproductive tract microbiotas, inflammation, and gynecological conditions. Front Reprod Health (2022) 4:963752. doi: 10.3389/frph.2022.963752
82. Itoh H, Sashihara T, Hosono A, Kaminogawa S, Uchida M. Lactobacillus gasseri OLL2809 inhibits development of ectopic endometrial cell in peritoneal cavity via activation of NK cells in a murine endometriosis model. Cytotechnology (2011) 63(2):205–10. doi: 10.1007/s10616-011-9343-z
83. Uchida M, Kobayashi O. Effects of lactobacillus gasseri OLL2809 on the induced endometriosis in rats. Biosci Biotechnol Biochem (2013) 77(9):1879–81. doi: 10.1271/bbb.130319
84. Simopoulos AP. Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr (2002) 21(6):495–505. doi: 10.1080/07315724.2002.10719248
85. Tortosa-Caparros E, Navas-Carrillo D, Marin F, Orenes-Pinero E. Anti-inflammatory effects of omega 3 and omega 6 polyunsaturated fatty acids in cardiovascular disease and metabolic syndrome. Crit Rev Food Sci Nutr (2017) 57(16):3421–9. doi: 10.1080/10408398.2015.1126549
86. Hopeman MM, Riley JK, Frolova AI, Jiang H, Jungheim ES. Serum polyunsaturated fatty acids and endometriosis. Reprod Sci (2015) 22(9):1083–7. doi: 10.1177/1933719114565030
87. Missmer SA, Chavarro JE, Malspeis S, Bertone-Johnson ER, Hornstein MD, Spiegelman D, et al. A prospective study of dietary fat consumption and endometriosis risk. Hum Reprod (2010) 25(6):1528–35. doi: 10.1093/humrep/deq044
88. Attaman JA, Stanic AK, Kim M, Lynch MP, Rueda BR, Styer AK. The anti-inflammatory impact of omega-3 polyunsaturated fatty acids during the establishment of endometriosis-like lesions. Am J Reprod Immunol (2014) 72(4):392–402. doi: 10.1111/aji.12276
89. Riesenfeld CS, Schloss PD, Handelsman J. Metagenomics: Genomic analysis of microbial communities. Annu Rev Genet (2004) 38:525–52. doi: 10.1146/annurev.genet.38.072902.091216
90. Agarwal SK, Chapron C, Giudice LC, Laufer MR, Leyland N, Missmer SA, et al. Clinical diagnosis of endometriosis: A call to action. Am J Obstet Gynecol (2019) 220(4):354.e1–e12. doi: 10.1016/j.ajog.2018.12.039
91. Ghai V, Jan H, Shakir F, Haines P, Kent A. Diagnostic delay for superficial and deep endometriosis in the united kingdom. J Obstet Gynaecol (2020) 40(1):83–9. doi: 10.1080/01443615.2019.1603217
92. Kiesel L, Sourouni M. Diagnosis of endometriosis in the 21st century. Climacteric (2019) 22(3):296–302. doi: 10.1080/13697137.2019.1578743
93. Leonardi M, Uzuner C, Mestdagh W, Lu C, Guerriero S, Zajicek M, et al. Diagnostic accuracy of transvaginal ultrasound for detection of endometriosis using international deep endometriosis analysis (IDEA) approach: Prospective international pilot study. Ultrasound Obstet Gynecol (2022) 60(3):404–13. doi: 10.1002/uog.24936
94. Chowdary P, Stone K, Ma T, Readman E, McIlwaine K, Druitt M, et al. Multicentre retrospective study to assess diagnostic accuracy of ultrasound for superficial endometriosis-are we any closer? Aust N Z J Obstet Gynaecol (2019) 59(2):279–84. doi: 10.1111/ajo.12911
Keywords: endometriosis, microbiome, microbiota, dysbiosis, inflammation, immune response
Citation: Uzuner C, Mak J, El-Assaad F and Condous G (2023) The bidirectional relationship between endometriosis and microbiome. Front. Endocrinol. 14:1110824. doi: 10.3389/fendo.2023.1110824
Received: 29 November 2022; Accepted: 21 February 2023;
Published: 07 March 2023.
Edited by:
Mainak Dutta, Birla Institute of Technology and Science, United Arab EmiratesReviewed by:
Julio Cesar Rosa-e-Silva, University of São Paulo, BrazilXiaona Lin, Sir Run Run Shaw Hospital, China
Copyright © 2023 Uzuner, Mak, El-Assaad and Condous. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cansu Uzuner, cansu.uzuner@gmail.com
 Cansu Uzuner
Cansu Uzuner