
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 30 January 2023
Sec. Obesity
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1108618
This article is part of the Research Topic Endocrine and Metabolic Consequences of Childhood Obesity, Volume II View all 21 articles
Context: Severe childhood obesity is associated with increased prevalence of cardiometabolic risk factors (CMRFs). Among children with Class 1 obesity, higher BMI may indicate greater cardiometabolic risk. Class 1 obesity reflects a wide spectrum of BMI values. Each 10% increase in BMI above the 95th percentile is equivalent to an average increase of 2.15 kg/m2 and 2.75 kg/m2 in BMI among children and adolescents, respectively. Such increments may be of clinical importance.
Objectives: The study aimed to determine the prevalence and clustering of CMRFs in children and adolescents with BMI 110%-119% of the 95th BMI percentile.
Methods: A cross-sectional analysis of data, from an Israeli health maintenance organization, of children and adolescents (5-17 years) with overweight or Class 1 obesity, and at least one measurement of lipid profile during Jan/2020-May/2021. CMRFs were defined as abnormal lipid profile, elevated alanine aminotransferase, hypertension, and prediabetes or diabetes. Study groups included overweight and Class 1 Obesity-A (BMI < 110%) and Obesity-B (BMI ≥ 110%) of the 95th BMI percentile.
Results: Of 7211 subjects included, 40.2% were overweight, 50.3% obesity-A, and 9.5% obesity-B. Multivariable analyses showed that children and adolescents from the Obesity-B group had increased odds for higher triglycerides, LDL cholesterol, and ALT levels; and lower HDL cholesterol levels, as compared to Obesity-A. The odds of prediabetes (insignificant) tended to be higher in the Obesity-B group, which was associated with increased CMRFs clustering.
Conclusions: Among children and adolescents with Class 1 obesity, BMI ≥ 110% of the 95th percentile was associated with higher prevalence and clustering of CMRFs.
Obesity among children and adolescents has become a major public health challenge in the twenty-first century. Over the past decade, the prevalence of pediatric obesity in the United States increased from 17.7% to 21.5% (1). Pediatric obesity leads to a significant increase in cardiometabolic morbidity, including type 2 diabetes, hypertension, dyslipidemia, fatty liver, and cardiovascular complications (2–4). Screening tests for children with obesity include fasting lipids, glycated hemoglobin (HbA1c), fasting or random plasma glucose, and liver enzymes (5).
The clustering of cardiometabolic risk factors (CMRFs) in early childhood is of particular concern, given that the majority of children remain obese in adulthood (3). Skinner et al., using National Health and Nutrition Examination Survey (NHANES) data, demonstrated that severe obesity in children and adolescents was associated with increased prevalence of CMRFs, particularly among males (6). They also showed that, as expected, subjects with Class 1 obesity, defined as having a BMI ≥95th percentile to <120% of the 95th percentile for age and sex, had a higher risk of having most risk factors as compared to those with overweight (6, 7).
Each 10% increase in BMI above the 95th percentile is equivalent to an average increase of 2.1 kg/m2 and 2.7 kg/m2 in BMI among male children and adolescents, respectively; and of 2.2 kg/m2 and 2.8 kg/m2 among female children and adolescents, respectively (Table S1) (8). Such increments in BMI may be clinically significant. For example, the Princeton Follow-up Study demonstrated that the risk of metabolic syndrome in adulthood increased by 24% for each 10% increase in age-specific BMI in children and youth (9).
The question of whether the increment in BMI above 109% of the 95th percentile translates into a significant difference in the risk of cardiometabolic morbidity within the Class 1 obesity group has not yet been addressed. Therefore, in the present study, we aimed at assessing the prevalence and clustering of CMRFs in Israeli children and adolescents with a BMI of 110%-119% of the 95th BMI percentile, as compared to those with overweight and Class 1obesity.
This non-interventional, cross-sectional analysis of data was conducted using the electronic medical database of Maccabi Healthcare Services (MHS), a large health maintenance organization in Israel serving over two million patients. All data were collected from the MHS automated database during May 2021. Clinical data included (1): demographic and anthropometric information on age, gender, socioeconomic status (SES) of the place of residence, weight, BMI percentile, and blood pressure (2); laboratory results including fasting serum total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, plasma glucose, glycated hemoglobin (HbA1c), and alanine aminotransferase (ALT); and (3) data from the MHS diabetes registry. The retrieved data included the last available value in the database recorded during January 2020 - May 2021, except for blood pressure for which we included the last two measurements when available, and fasting plasma glucose and HbA1c for which we retrieved all the available data.
We obtained approval from the MHS institutional review board and ethics committee to access and analyze data. Individual patient informed consent was not required because of the anonymized nature of patient records.
We performed a cross-sectional analysis of data from children and adolescents 5-17 years of age with overweight or Class 1 obesity, who had at least one measurement of lipid profile during the study period.
Age was expressed in years and rounded to the nearest year (cutoff points are at the midpoint of the child’s year). BMI was classified according to percentiles established by the Centers for Disease Control and Prevention (CDC). These were validated for Israeli children and adolescents, for age (by month) and sex (10). Overweight was defined as a BMI between the 85th and 94th percentile. Obesity-A was defined as a BMI at or greater than the 95th percentile up to 109% of the 95th percentile. A BMI between 110% and 119% of the 95th percentile was defined as Obesity-B. Subjects with a BMI at or greater than 120% of the 95th percentile (or BMI greater than 35 kg/m2) were not included in this study.
Data were obtained on the socioeconomic status (SES) of the place of residence based on the Israeli Central Bureau of Statistics scoring system: low (score 1-4 out of 10), medium (score 5-8 out of 10), and high (score 9-10 out of 10).
Of 26783 children and adolescents (14744 females and 12039 males) aged 5-17 years with documented BMI ≥85th percentile between January 2020 through May 2021, 7211 subjects had a BMI<120% of the 95th percentile and available lipid profile data and thus were included in the study (Figure S1).
We aimed at evaluating children or adolescents for potential comorbidities associated with overweight and obesity. Table 1 summarizes characteristics of the sample age groups and the definition of comorbidities and abnormal values for cardiometabolic variables.
Abnormal lipid profile values were defined using standard cutoff values for levels of total cholesterol (≥200 mg/dL), LDL cholesterol (≥130 mg/dL), HDL cholesterol (<35 mg/dL), and triglycerides (≥150 mg/dL). Serum ALT concentrations above the 95th percentile (>25 U/L for boys and >22U/L for girls) were regarded as abnormal, as proposed by Schwimmer JB et al. (11). In our sample, 6869 children and adolescents had at least one documented ALT value.
Blood pressure was recorded as the mean value of up to two measurements or as a single measurement (3810 of the children and adolescents had two measurements, 1189 had only one measurement, and 2212 had no measurement). For children aged 5–12 years, we used standardized blood pressure tables in which abnormal values were determined according to age and gender and adjusted to the 50th percentile of height; abnormal values were defined as any value at or above the 95th percentile in Table S2. For children aged ≥13 years, we used cutoffs of ≥130 mm Hg for systolic blood pressure and ≥80 mm Hg for diastolic blood pressure (12).
We defined individuals as having prediabetes if they fulfilled the following criteria: at least two separate fasting plasma glucose values between 100 mg/dL and 125mg/dL, or a single measurement of HbA1c between 5.7% and 6.4%; and the person was not included in the MHS diabetes registry. We used data from MHS automated patients’ registry to diagnose diabetes (13). This registry is based on a validated algorithm that collects data from electronic medical records, laboratory results (HbA1c and fasting plasma glucose), dispensed medications (oral glucose-lowering agents or insulin), and clinical diagnoses (more details are available in the Supplementary file: Methods).
Continuous data are presented as mean ± standard deviation (SD) or median (interquartile range). Categorical data are presented as absolute numbers and percentages. Prevalence is reported as a percentage with a 95% confidence interval.
The chi-square test or Fisher’s exact test was used to assess the association between categorical variables. T-test or ANOVA were used to assess the association between continuous variables.
To examine the effect of weight on CMRFs we performed multivariable analyses using generalized linear models (GLMs) with a logarithmic link. We reported the odds ratios (after exponentiation of the coefficients). We also performed logistic regression analyses to evaluate the effect of weight on the clustering of CMRFs. Models were adjusted when appropriate for age and sex. A p-value < 0.05 was considered significant.
All statistical analyses were performed using STATA, version 17 (Stata Corp., Texas, USA).
Among 7211 children and adolescents with a BMI at the 85th percentile or higher, 40.2% were classified as overweight, 50.3% as obesity-A, and 9.5% as obesity-B (Table 2). Figure S2 depicts median BMI values classified by weight categories and age. No significant correlation was found between SES and severity of obesity (Table S3).
Table S4 shows the mean values for each cardiometabolic variable for all subjects. These values increased with the severity of obesity, except for HDL cholesterol.
Table 3 and Figure 1 show the prevalence of CMRFs among children and adolescents, classified by weight category. Except for total cholesterol, diastolic blood pressure, and diabetes, the prevalence of CMRFs (including prediabetes) consistently increased with the severity of obesity in adolescents (Tables 3, S5). This trend was evident albeit to a lesser extent among children for whom only systolic blood pressure and ALT levels differed significantly between weight groups (Tables 3, S6).
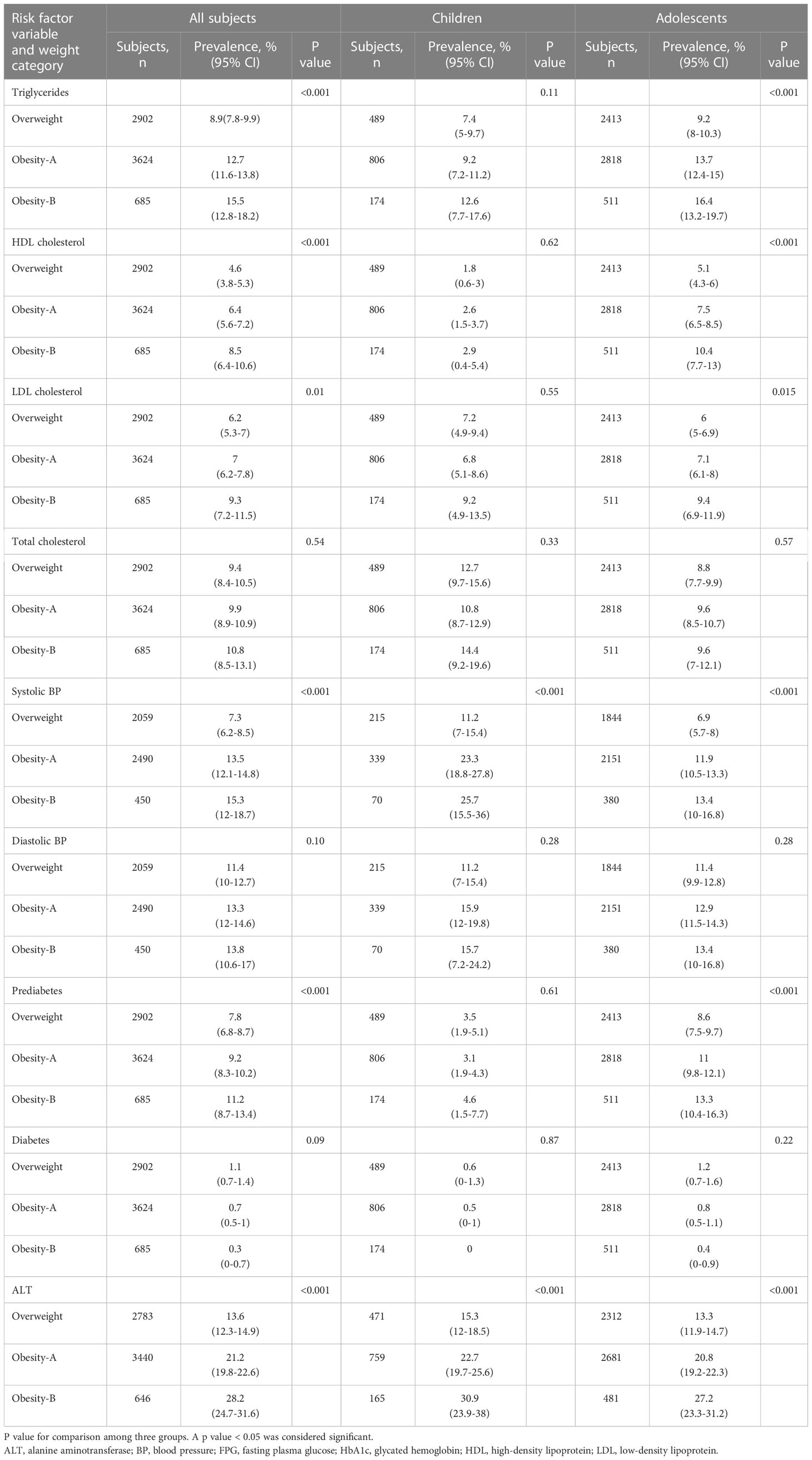
Table 3 Prevalence of comorbidities and abnormal values for cardiometabolic variables by weight among children and adolescents.
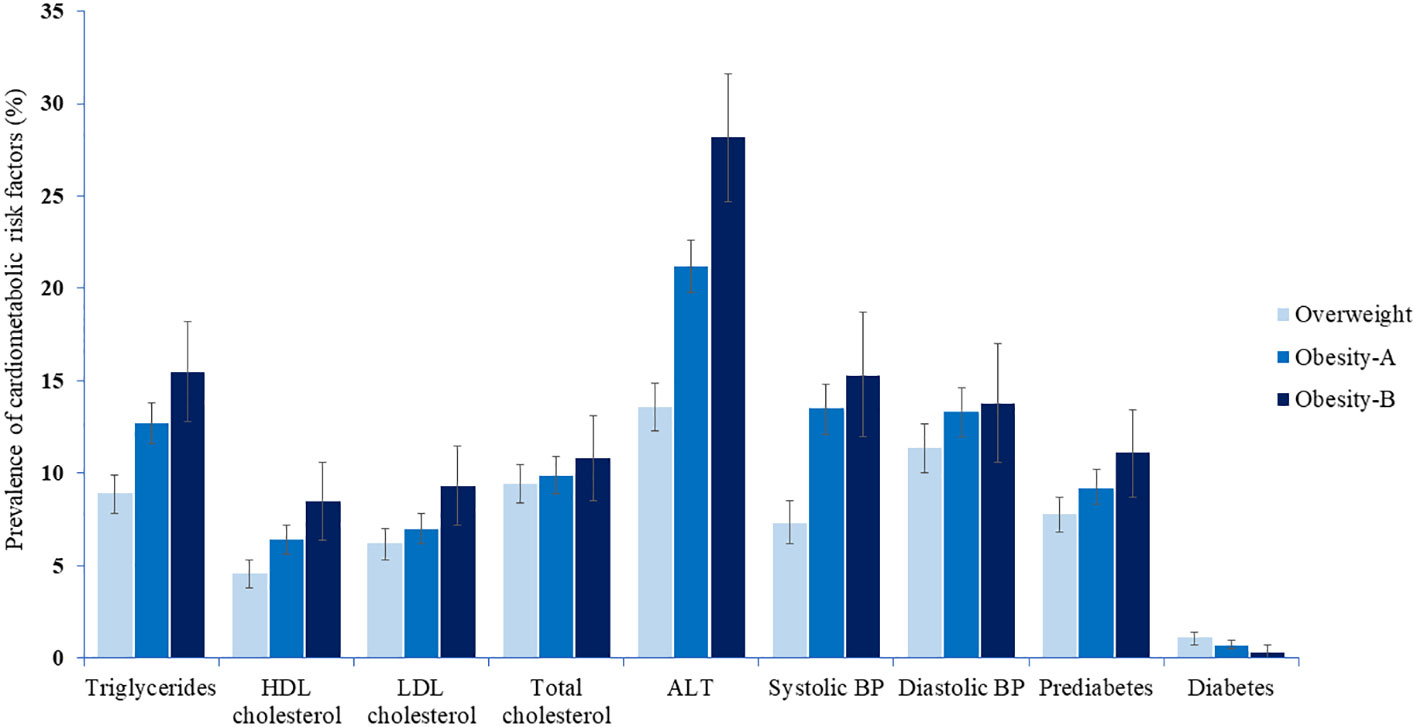
Figure 1 The prevalence of cardiometabolic risk factors among children and adolescents by weight category.
Table 4 shows a direct comparison of CMRFs between weight groups of children and adolescents in multivariable generalized linear models that adjusted for age and sex. Children and adolescents in the obesity-B group had greater odds for higher triglycerides, LDL cholesterol, and ALT and lower HDL cholesterol levels, as compared to the obesity-A group. These differences were significant in females except for the difference in ALT levels, the only observation that was significant in males. The odds of prediabetes tended to be higher in the obesity-B group but did not reach significance. Overweight children and adolescents had lower odds for most of the CMRFs.
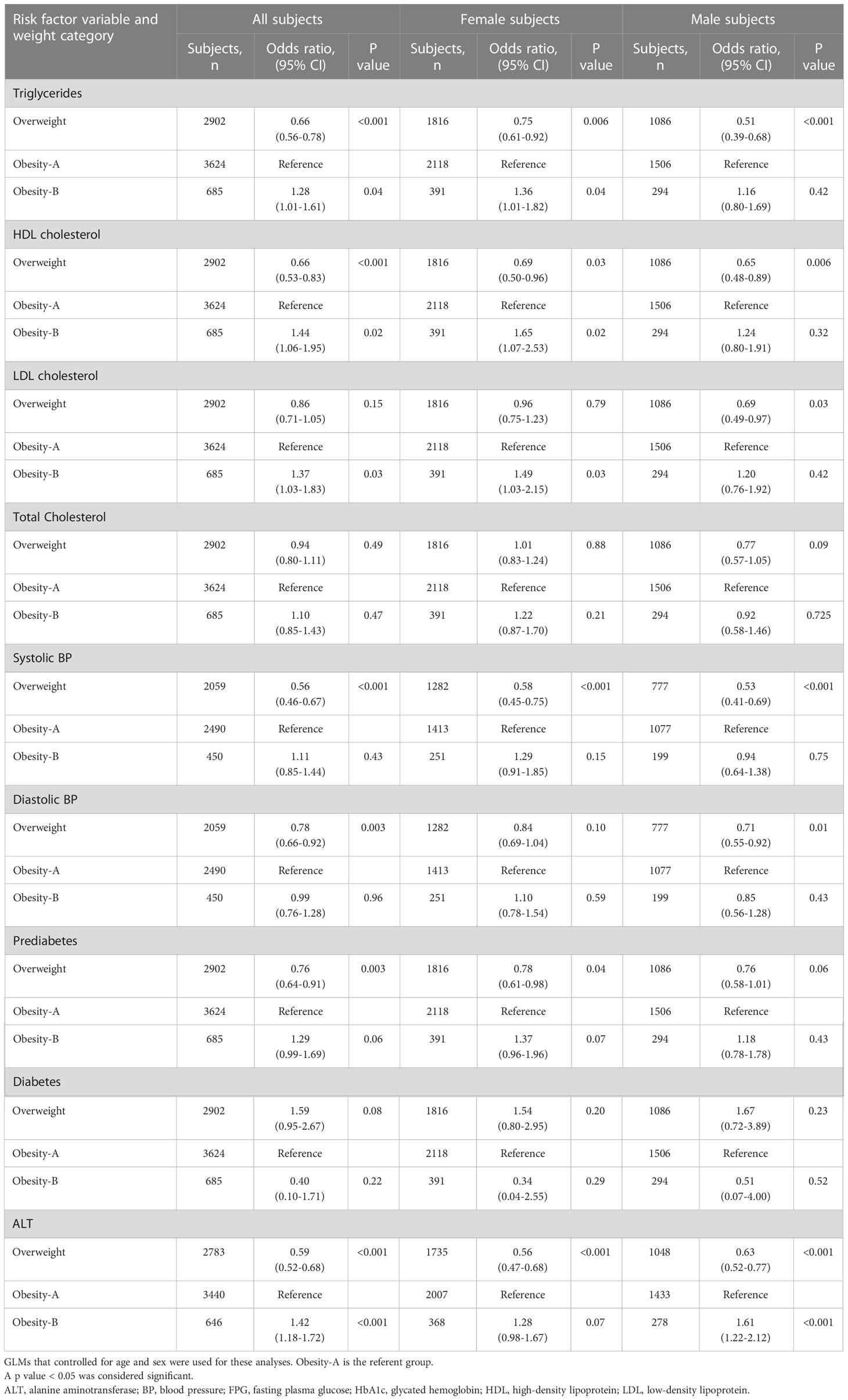
Table 4 Odds ratios for cardiometabolic risk factors among children and adolescents by sex and weight.
The number of CMRFs increased with the severity of obesity in children and adolescents (Table S7; Figure 2). The prevalence of clustering of ≥2 and ≥3 CMRFs were 12.1% and 6.3%, respectively; and 19.8% and 6.7% among children and adolescents from the obesity-B group, respectively (Figure 2). Table 5 shows the adjusted odds ratios for the number of CMRFs across weight groups of children and adolescents. Compared with the obesity-A group, subjects from the obesity-B group were at 52% and 76% higher risk of clustering of ≥ 2 and ≥3 CMRFs, respectively (Table 5).

Figure 2 Distribution of cardiometabolic risk factors among children (A) and adolescents (B) by weight category. Cardiometabolic risk factors include hyperglycemia (prediabetes or diabetes), high triglycerides, LDL cholesterol, ALT, and systolic or diastolic BP, or low HDL cholesterol. The number of cardiometabolic risk factors increased with the severity of obesity in children (p<0.001) and adolescents (p<0.001). P value for difference between groups was determined by chi square test.
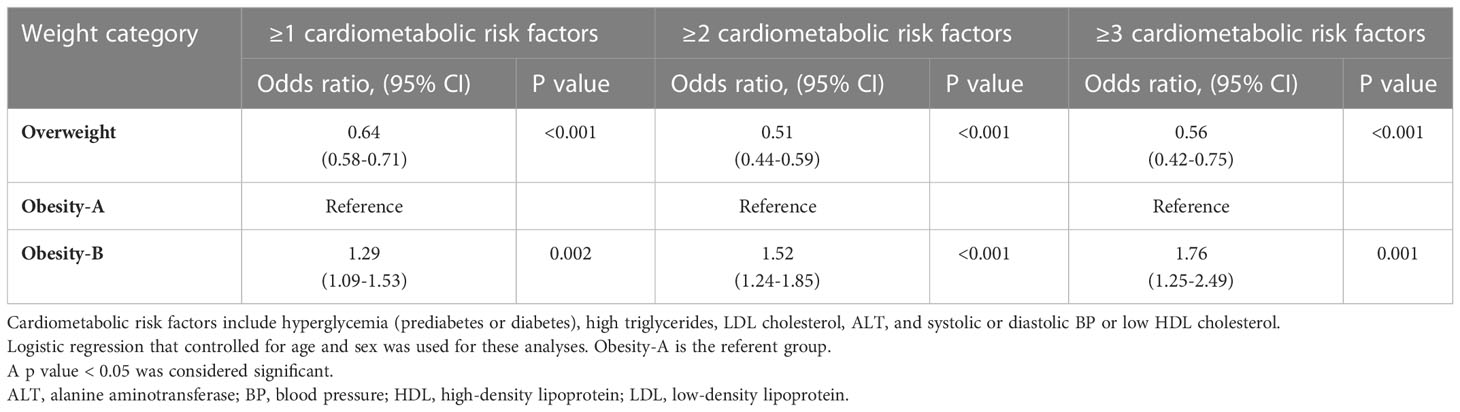
Table 5 Odds ratios of having ≥1, ≥2, and ≥3 (vs zero) cardiometabolic risk factors* by weight category in children and adolescents.
The present study shows a higher prevalence of most of the cardiovascular risk factors in children and adolescents with a BMI between 110%-119% of the 95th BMI percentile (obesity-B group) as compared to individuals with lower degree of obesity. This ten percent increment in BMI within the Class 1 obesity group translates into significantly higher odds for clustering of CMRFs.
Severe obesity in childhood is associated with significant metabolic and cardiovascular morbidity (14). In our study, we aimed at assessing children and adolescents with lower degrees of obesity. Class 1 obesity reflects a wide spectrum of BMI values. Each 10% increase in BMI above the 95th percentile is equivalent to an average increase of 2.15 kg/m2 and 2.75 kg/m2 in BMI among children and adolescents, respectively (Table S1) (8). Such increments may be of high clinical importance. For example, the Princeton Follow-up Study demonstrated that the risk of metabolic syndrome in adulthood increased by 24% for each 10% increase in age-specific BMI in childhood (9). In young adults, each 1-kg/m2 increase in BMI was associated with a 6% higher risk of developing type 2 diabetes before the age of 45 years (15). In a cohort of Danish and Finnish subjects, each z-score increase in BMI at 7 years of age (equivalent to a 1.5 to 2.5 kg/m2 increment) was associated with a 5%-10% greater risk of coronary heart disease in adulthood (16). Moreover, the risk of mortality increases significantly throughout the overweight and obesity range. In adults younger than 50 years of age, every five units higher BMI above 25 kg/m2 was associated with an approximate 52% higher risk of premature death (17). These findings emphasize the importance of losing relative weight at a young age.
We subclassified Class 1 obesity into two groups to “zoom in” and better understand the cardiometabolic morbidity associated with lower degrees of obesity in childhood.
In our study, the prevalence of most CMRFs increased with the severity of obesity, except for diastolic blood pressure, diabetes, and total cholesterol (Table 3).
Our results are in line with Sorof et al. that showed a progressive increase in systolic blood pressure with each increase in BMI percentile, whereas diastolic blood pressure showed no association (18). In our study, the prevalence of prediabetes was higher in the obesity groups as expected. However, the prevalence of diabetes was low with no difference seen between the groups. These registry-derived diagnoses include patients with all forms of diabetes, including type 1 diabetes, which might explain the higher (though insignificant) trend of having diabetes in the overweight group (Table 4). However, Twig et al. showed that the cumulative incidence of early-onset type 2 diabetes was more than 2-fold higher among adolescents with severe obesity than among those with Class 1 obesity (4). This relationship should be regarded as a continuum in which each increment above the 95th BMI increases the risk of prediabetes and the transition toward type 2 diabetes.
Obesity is associated with elevated ALT levels. We defined abnormal serum ALT concentrations as >25 U/L for boys and >22U/L for girls (11). This is because liver biopsy specimens from children with normal or mildly elevated ALT (≥26 to 50 U/L for boys and ≥23 to 44 U/L for girls) were histologically abnormal, including advanced fibrosis (19). In our study, an increment in BMI above 109% of the 95th percentile more than doubled the risk for abnormal ALT values in children and adolescents as compared to those with overweight (Table S5, S6). Despite being nonspecific when used alone, the increased plasma concentrations of ALT with higher severity of obesity might reflect the presence of nonalcoholic fatty liver disease (NAFLD) in a substantial proportion of these children.
There were differences observed between children and adolescents in terms of their lipid and glucose profiles, with children showing nonsignificant differences across the weight groups (Table 3). This suggests that the duration of obesity may play a stronger role than BMI alone.
There were important differences between male and female subjects in our study. It should be noted that girls constitute 60% of the study sample, although the prevalence of obesity in the general population is greater among boys (20, 21). Given the cross-sectional nature of the study and the eligibility for inclusion based on available data, this gender disparity may have occurred by chance. The present study demonstrates clearly that an increment in BMI above 109% of the 95th percentile is clinically significant, particularly among females. This increment increases the odds of higher triglycerides and LDL cholesterol and lower HDL cholesterol levels as compared to in females with lower degree of obesity (Table 4). This markedly unfavorable lipid profile relative to weight gain in girls has been reported in a previous study which demonstrated slightly stronger indirect effects of weight gain, through childhood adiposity, in girls as compared with boys (22). There was a nonsignificant trend towards increased odds for prediabetes and higher ALT levels in females with a BMI above 109% of the 95th percentile as compared to those with lower degree of obesity. In males, an increment above 109% of the 95th BMI percentile was associated with increased odds for higher ALT levels. This result is in line with previous studies showing that prevalence was generally higher in boys as compared with girls and increased incrementally with greater BMI (23, 24).
Our findings differ from those in other reports. Some studies showed minimal differences between boys and girls and another study (in which 16.7% of the sample had severe obesity) showed a higher prevalence of risk factors in males (6, 25–27).
The clustering of CMRFs in childhood is of high concern, taking into account the fact that around 80% of them remain obese in adulthood (3). Indeed, this observation justifies the evaluation of cardiometabolic variables at a young age, especially among those who are overweight or obese.
Several definitions of metabolic syndrome in children and adolescents have been proposed (28–33). These definitions are diverse and lack uniformity, and there is no clear consensus on which to use. The American Academy of Pediatrics recommends shifting the focus to the concept of clustering of CMRFs rather than defining metabolic syndrome in children and adolescents (34).
In the Bogalusa Heart Study, the clustering of CMRFs was associated with increased severity of asymptomatic coronary atherosclerotic lesions in young people (35). Since the clustering of risk factors is present in childhood and continues into young adulthood, the presence of multiple risk factors such as hyperglycemia, hypertension, and abnormal lipid profile may indicate a faster progression of atherosclerosis in young people.
Using conservative thresholds for prediabetes, dyslipidemia, and hypertension, a large multi-ancestral cohort demonstrated a direct correlation between the severity of obesity and prevalence of CMRFs, whereby each half-unit increase in the BMI z score increased the risk of having cardiovascular risk factors’ clustering by 55% (29). Skinner et al. showed that values for some, but not all, CMRFs were higher with increased severity of obesity in children and adolescents, and demonstrated that greater severity of obesity is associated with a higher risk of lower HDL-cholesterol levels, elevated plasma triglycerides, and high systolic and diastolic blood pressure (6).
In our study, subjects with BMI between 110%-120% of the 95th BMI percentile had a higher prevalence of CMRFs clustering (17.8% and 6.6% for clustering of ≥2 and ≥3 risk factors, respectively) (Table S7). They exhibited a substantially higher risk for CMRFs clustering as compared to those with lower degree of obesity (Table 5). This highlights the need for further stratification of weight groups at a young age to better reflect the risk for metabolic and cardiovascular morbidity.
Previous research has shown that a lower SES during childhood is linked to a higher risk of developing metabolic syndrome in adulthood, even when controlling for other childhood risk factors (36). However, our study did not find a significant association between SES and the level of obesity, thus it cannot be considered a confounding factor.
There are several limitations to our study that should be considered. First, this is a cross-sectional study, which cannot prove the causality between obesity and CMRFs. In addition, we cannot exclude any secondary causes of obesity, such as genetic or hormonal factors. Second, we lacked data on lifestyle, physical activity, and other indicators of obesity such as waist circumference. The latter may be more sensitive than BMI in the context of this study and better define cardiovascular risk in the long term. Third, our sample might differ from the general population in terms of gender representation, as obesity is more common in boys (20). A considerable difference was observed in the number of individuals with obesity-A and obesity-B (50.3% compared to 9.5%). It should be noted that our sample was limited to individuals with available lipid profile data. This limitation, combined with the observational nature of the study, raises the possibility that the representation of obesity-B in our sample may not accurately reflect its prevalence in the general population. Finally, the sample size was relatively small in weight subgroups of children younger than 12 years old, which resulted in some estimates with wide confidence intervals, deeming them insignificant. Thus, some of these estimates should be interpreted with caution.
Our study has certain strengths. The study was based on a relatively large sample of children and adolescents. This permitted the evaluation of a wide variety of CMRFs and the exploration of their clustering among weight subgroups. In conclusion, among children and adolescents with Class 1 obesity, a BMI ≥ 110% of the 95th percentile was associated with a higher prevalence of and greater clustering of CMRFs. The consideration of this group (obesity-B group) in the standard obesity classification may assist in the identification of children and adolescents who could be at a greater risk for abnormal lipid profile, hyperglycemia, and abnormal ALT levels.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Maccabi Healthcare Services institutional review board and ethics committee. Approval number 0183-20-MHS. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
AN and RS were responsible for the design of the study and data acquisition. AN and RS contributed to the analysis and the interpretation of data, drafted the manuscript and revised it critically for important intellectual content. NS and EF contributed to the analysis and the interpretation of data and revised the manuscript critically for important intellectual content. All authors contributed to the article and approved the submitted version.
The authors declare that this study received funding from Novo Nordisk, Israel. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
We would like to thank Mrs. Ateret Malachi for her help in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1108618/full#supplementary-material
1. Hu K, Staiano AE. Trends in obesity prevalence among children and adolescents aged 2 to 19 years in the US from 2011 to 2020. JAMA Pediatr (2022) 176:1037–9. doi: 10.1001/jamapediatrics.2022.2052
2. Weihrauch-Blüher S, Schwarz P, Klusmann JH. Childhood obesity: increased risk for cardiometabolic disease and cancer in adulthood. Metabolism (2019) 92:147–52. doi: 10.1016/j.metabol.2018.12.001
3. Caprio S, Santoro N, Weiss R. Childhood obesity and the associated rise in cardiometabolic complications. Nat Metab (2020) 2:223–32. doi: 10.1038/s42255-020-0183-z
4. Twig G, Zucker I, Afek A, Cukierman-Yaffe T, Bendor CD, Derazne E, et al. Adolescent obesity and early-onset type 2 diabetes. Diabetes Care (2020) 43:1487–95. doi: 10.2337/dc19-1988
5. Styne DM, Arslanian SA, Connor EL, Farooqi IS, Murad MH, Silverstein JH, et al. Pediatric obesity–assessment, treatment, and prevention: An endocrine society clinical practice guideline. J Clin Endocrinol Metab (2017) 102:709–57. doi: 10.1210/jc.2016-2573
6. Skinner AC, Perrin EM, Moss LA, Skelton JA. Cardiometabolic risks and severity of obesity in children and young adults. N Engl J Med (2015) 373:1307–17. doi: 10.1056/NEJMoa1502821
7. Kelly AS, Barlow SE, Rao G, Inge TH, Hayman LL, Steinberger J, et al. Severe obesity in children and adolescents: Identification, associated health risks, and treatment approaches. Circulation (2013) 128:1689–712. doi: 10.1161/CIR.0b013e3182a5cfb3
8. Growth charts - data table of BMI-for-age charts (2001). Available at: https://www.cdc.gov/growthcharts/html_charts/bmiagerev.htm (Accessed 17 October 2022).
9. Morrison JA, Friedman LA, Gray-McGuire C. Metabolic syndrome in childhood predicts adult cardiovascular disease 25 years later: The Princeton lipid research clinics follow-up study. Pediatrics (2007) 120:340–5. doi: 10.1542/peds.2006-1699
10. Goldstein A, Haelyon U, Krolik E, Sack J. Comparison of body weight and height of Israeli schoolchildren with the tanner and centers for disease control and prevention growth charts. Pediatrics (2001) 108:E108. doi: 10.1542/peds.108.6.e108
11. Schwimmer JB, Dunn W, Norman GJ, Pardee PE, Middleton MS, Kerkar N, et al. SAFETY study: alanine aminotransferase cutoff values are set too high for reliable detection of pediatric chronic liver disease. Gastroenterology (2010) 138:1357–64, 1364.e1-2. doi: 10.1053/j.gastro.2009.12.052
12. Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, et al. SUBCOMMITTEE ON SCREENING AND MANAGEMENT OF HIGH BLOOD PRESSURE IN CHILDREN. clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics (2017) 140:e20171904. doi: 10.1542/peds.2017-1904
13. Chodick G, Heymann AD, Shalev V, Kookia E. The epidemiology of diabetes in a large Israeli HMO. Eur J Epidemiol. (2003) 18:1143–6. doi: 10.1023/B:EJEP.0000006635.36802.c8
14. Bendor CD, Bardugo A, Pinhas-Hamiel O, Afek A, Twig G. Cardiovascular morbidity, diabetes and cancer risk among children and adolescents with severe obesity. Cardiovasc Diabetol (2020) 19:1–14. doi: 10.1186/s12933-020-01052-1
15. Hillier TA, Pedula KL. Characteristics of an adult population with newly diagnosed type 2 DiabetesThe relation of obesity and age of onset. Diabetes Care (2001) 24:1522–7. doi: 10.2337/diacare.24.9.1522
16. Andersen LG, Ängquist L, Eriksson JG, Forsen T, Gamborg M, Osmond C, et al. Birth weight, childhood body mass index and risk of coronary heart disease in adults: Combined historical cohort studies. PloS One (2010) 5:e14126. doi: 10.1371/journal.pone.0014126
17. Di Angelantonio E, Bhupathiraju SN, Wormser D, Gao P, Kaptoge S, de Gonzalez AB, et al. Body-mass index and all-cause mortality: Individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet (2016) 388:776–86. doi: 10.1016/S0140-6736(16)30175-1
18. Sorof JM, Lai D, Turner J, Poffenbarger T, Portman RJ. Overweight, ethnicity, and the prevalence of hypertension in school-aged children. Pediatrics (2004) 113:475–82. doi: 10.1542/peds.113.3.475
19. Molleston JP, Schwimmer JB, Yates KP, Murray KF, Cummings OW, Lavine JE, et al. Histological abnormalities in children with nonalcoholic fatty liver disease and normal or mildly elevated alanine aminotransferase levels. J Pediatr (2014) 164:707–713.e3. doi: 10.1016/j.jpeds.2013.10.071
20. Shah B, Tombeau Cost K, Fuller A, Birken CS, Anderson LN. Sex and gender differences in childhood obesity: Contributing to the research agenda. BMJ Nutr Prev Heal (2020) 3:387. doi: 10.1136/bmjnph-2020-000074
21. WHO European regional obesity report 2022 (2022). Copenhagen: WHO Regional Office for Europe; 2022. Licence: CC BY-NC-SA 3.0 IGO.WHO EUROPEAN REGIONAL OBESITY REPORT 2022. Available at: https://apps.who.int/iris/bitstream/handle/10665/353747/9789289057738-eng.pdf (Accessed 8 November 2022).
22. Van Hulst A, Barnett TA, Paradis G, Roy-Gagnon MH, Gomez-Lopez L, Henderson M. Birth weight, postnatal weight gain, and childhood adiposity in relation to lipid profile and blood pressure during early adolescence. J Am Heart Assoc (2017) 6:e006302. doi: 10.1161/JAHA.117.006302
23. Villanueva-Ortega E, Garcés-Hernández MJ, Herrera-Rosas A, López-Alvarenga JC, Laresgoiti-Servitje E, Escobedo G, et al. Gender-specific differences in clinical and metabolic variables associated with NAFLD in a Mexican pediatric population. Ann Hepatol (2019) 18:693–700. doi: 10.1016/j.aohep.2019.04.012
24. Anderson EL, Howe LD, Jones HE, Higgins JPT, Lawlor DA, Fraser A. The prevalence of non-alcoholic fatty liver disease in children and adolescents: A systematic review and meta-analysis. PloS One (2015) 10:e0140908. doi: 10.1371/journal.pone.0140908
25. Freedman DS, Mei Z, Srinivasan SR, Berenson GS, Dietz WH. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: The bogalusa heart study. J Pediatr (2007) 150:12–17.e2. doi: 10.1016/j.jpeds.2006.08.042
26. Freedman DS, Dietz WH, Srinivasan SR, Berenson GS. The relation of overweight to cardiovascular risk factors among children and adolescents: The bogalusa heart study. Pediatrics (1999) 103:1175–82. doi: 10.1542/peds.103.6.1175
27. Lambert M, Delvin EE, Levy E, O'Loughlin J, Paradis G, Barnett T, et al. Prevalence of cardiometabolic risk factors by weight status in a population-based sample of Quebec children and adolescents. Can J Cardiol (2008) 24:575–83. doi: 10.1016/S0828-282X(08)70639-1
28. Noubiap JJ, Nansseu JR, Lontchi-Yimagou E, Nkeck JR, Nyaga UF, Ngouo AT, et al. Global, regional, and country estimates of metabolic syndrome burden in children and adolescents in 2020: A systematic review and modelling analysis. Lancet Child Adolesc Heal (2022) 6:158–70. doi: 10.1016/S2352-4642(21)00374-6
29. Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med (2004) 350:2362–74. doi: 10.1056/NEJMoa031049
30. Zimmet P, Alberti KG, Kaufman F, Tajima N, Silink M, Arslanian S, et al. The metabolic syndrome in children and adolescents - an IDF consensus report. Pediatr Diabetes. (2007) 8:299–306. doi: 10.1111/j.1399-5448.2007.00271.x
31. De Ferranti SD, Gauvreau K, Ludwig DS, Neufeld EJ, Newburger JW, Rifai N. Prevalence of the metabolic syndrome in American adolescents: Findings from the third national health and nutrition examination survey. Circulation (2004) 110:2494–7. doi: 10.1161/01.CIR.0000145117.40114.C7
32. Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: Findings from the third national health and nutrition examination survey, 1988-1994. Arch Pediatr Adolesc Med (2003) 157:821–7. doi: 10.1001/archpedi.157.8.821
33. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: An American heart Association/National heart, lung, and blood institute scientific statement. Circulation (2005) 112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404
34. Magge SN, Goodman E, Armstrong SC. COMMITTEE ON NUTRITION; SECTION ON ENDOCRINOLOGY; SECTION ON OBESITY. the metabolic syndrome in children and adolescents: Shifting the focus to cardiometabolic risk factor clustering. Pediatrics (2017) 140:e20171603. doi: 10.1542/peds.2017-1603
35. Berenson GS, Srinivasan SR, Bao W, Newman WP, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. the bogalusa heart study. N Engl J Med (1998) 338:1650–6. doi: 10.1056/NEJM199806043382302
36. Puolakka E, Pahkala K, Laitinen TT, Magnussen CG, Hutri-Kähönen N, Tossavainen P, et al. Childhood socioeconomic status in predicting metabolic syndrome and glucose abnormalities in adulthood: the cardiovascular risk in young finns study. Diabetes Care (2016) 39(12):2311–7. doi: 10.2337/dc16-1565
Keywords: cardiometabolic risk factors, children, adolescents, overweight, Class 1 obesity
Citation: Nakhleh A, Sakhnini R, Furman E and Shehadeh N (2023) Cardiometabolic risk factors among children and adolescents with overweight and Class 1 obesity: A cross-sectional study. Insights from stratification of Class 1 obesity. Front. Endocrinol. 14:1108618. doi: 10.3389/fendo.2023.1108618
Received: 26 November 2022; Accepted: 16 January 2023;
Published: 30 January 2023.
Edited by:
Dénes Molnár, University of Pécs, HungaryReviewed by:
Daniel Weghuber, Paracelsus Medical University, AustriaCopyright © 2023 Nakhleh, Sakhnini, Furman and Shehadeh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Afif Nakhleh, YW5ha2hsZWhAZ21haWwuY29t
†These authors have contributed equally to this work
‡ORCID: Afif Nakhleh, orcid.org/0000-0003-1206-1516
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.