- 1Department of Cardiology, The Second Hospital of Jilin University, Changchun, Jilin, China
- 2Department of Cardiology and Cardiovascular Research Institute, Chinese People's Liberation Army, General Hospital of Northern Theater Command, Shenyang, Liaoning, China
Background: The role of fibroblast growth factor 21 (FGF21) in predicting the long-term prognosis of patients with cardiovascular disease (CVD) remains unknown.
Methods: A comprehensive search in PubMed, Embase, and the Cochrane Library was performed to identify studies reporting the association between FGF21 and prognosis among patients with CVD. A meta-analysis was performed, with patients stratified by coronary artery disease (CAD) or heart failure (HF). The endpoint of CAD or HF was major adverse cardiovascular events defined by each study and a composite of death or HF readmission, respectively. The I2 method and linear regression test of funnel plot asymmetry were used to test heterogeneity (I2 > 50% indicates substantial heterogeneity) and publication bias (asymmetry P < 0.05, indicating publication bias).
Results: A total of 807 records were retrieved, and nine studies were finally included. Higher FGF21 levels were significantly associated with the risk of major adverse cardiovascular events in patients with CAD (multivariate hazard ratio [HR]: 1.77, 95% confidence interval [CI]: 1.40–2.23, P < 0.05, I2 = 0%, fixed-effect model). Increased FGF21 levels were also associated with the risk of all-cause death among patients with CAD (multivariate HR: 2.67, 95% CI: 1.25–5.72, P < 0.05, I2 = 64%, random-effect model). No association was found between FGF21 and the endpoint among patients with HF (HR: 1.57, 95% CI: 0.99–2.48, P > 0.05, random-effect model), but a large heterogeneity (I2 = 95%) and potential publication bias (Asymmetry P < 0.05) existed in the analysis.
Conclusion: Increased FGF21 levels were independently associated with poor prognosis of CAD, whereas the role of FGF21 in predicting clinical outcomes of HF requires further investigation.
1 Introduction
Fibroblast growth factor 21 (FGF21), belonging to the FGF19 subclass of the FGF family, is a pleiotropic endocrine hormone (1) that acts in an autocrine/paracrine manner in multiple tissues (2, 3). FGF21 is induced in white adipose tissue (WAT) by fasting and refeeding and can stimulate glucose entry and increase lipolysis and mitochondrial oxidative capacity (2). FGF21 is a key regulator of energy homeostasis (4), which initiates fat mobilization and increases insulin sensitivity (5–7). Many studies have demonstrated that FGF21 protects against pancreatic damage and β-cell dysfunction and increases glucose transport via glucose transporter protein 1 (8). A series of studies have shown that FGF21 has beneficial effects on body weight and glucose and lipid metabolism under physiological conditions (9).
Considering the close association between metabolic syndrome and cardiovascular disease (10), an increasing number of studies have investigated the beneficial effects of FGF21 on the cardiovascular system. Bench et al. reported that FGF21 prevents atherosclerosis (11) and protects against cardiac hypertrophy (12). FGF21 protects against atherosclerosis via two independent mechanisms: regulation of adipocyte adiponectin production and suppression of hepatic expression of the transcription factor sterol regulatory element-binding protein-2 (10, 11). Endogenous FGF21 protects against cardiac hypertrophy via the sirtuin 1 (SIRT1)–peroxisome proliferator-activated receptor α (PPAR-α) pathway (13). Although a protective role of FGF21 in cardiac function and metabolism has been found, the link between FGF21 and cardiovascular disease is controversial. A series of clinical trials and meta-analyses reported that elevated serum FGF21 levels were associated with an increased incidence of cardiovascular diseases (CVD) (14) as well as cardiovascular mortality among patients with diabetes (15). Collectively, these results from bench research and clinical trials created a paradox in determining the predictive value of FGF21 in CVD. Moreover, most studies have focused on the association between FGF21 levels and the primary prevention of CVD, whereas clinical evidence evaluating the role of FGF21 in the prognosis of patients with CVD is limited. Therefore, we conducted a meta-analysis to explore the association between FGF21 and long-term prognosis of patients with established CVD to provide new evidence unveiling the prognostic role of FGF21 in CVD.
2 Materials and methods
2.1 Study eligibility and outcomes
The present meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Review and Meta-analysis Protocols (PRISMA) (16). A comprehensive search was conducted in PubMed, Embase, and the Cochrane Library on June 9, 2022 to identify studies in English that reported the association between FGF21 and the clinical outcomes of patients with CVD. Studies meeting the following inclusion criteria were considered eligible: 1) study population comprising patients with established CVD; 2) studies reporting the relationship between FGF21 levels and CVD prognosis; 3) study endpoints with hard cardiovascular outcomes, such as all-cause or cardiac death, myocardial infarction (MI), and readmission for heart failure (HF); and 4) the follow-up period of studies was at least 6 months from discharge. Using these inclusion criteria and the PICOS (patient, intervention, comparison, outcomes, and study type) principle, we designed the following search terms: “cardiometabolic disease,” “cardiovascular disease,” “coronary artery disease,” “heart failure,” “cardiomyopathy,” “fibroblast growth factor 21,” and “FGF21.” We did not retrieve terms regarding study outcome or type to ensure complete and comprehensive search results (refer to the search strategy in PubMed in the Supplemental Materials).
First, the titles and abstracts of the records were reviewed. If relevant, the full texts and references of each record were manually searched and reviewed to evaluate eligibility. No limitations of study type (cohort or case-control study) were included. Conference abstracts were excluded owing to insufficient information. For patients with coronary artery disease (CAD), the primary endpoint was major adverse cardiovascular events (MACE), defined as the composite of ischemic events from each included study, and secondary endpoints included all-cause death and cardiovascular death. For patients with HF, the endpoint was the composite of all-cause death and readmission for HF.
2.2 Data extraction and quality evaluation
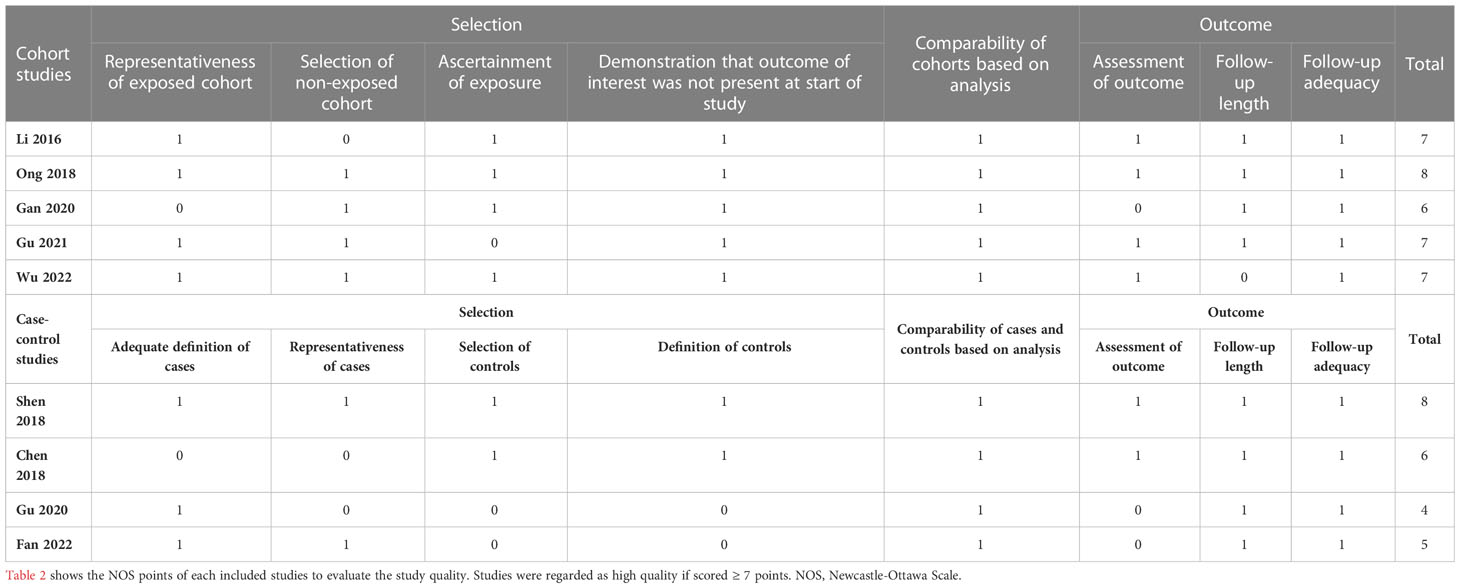
The following items were extracted from each eligible study: first author, study type, year of publication, patient diagnosis and characteristics, sample size, cutoff value of FGF21 levels, endpoints, follow-up duration, and effect size (event and total numbers, univariate or multivariate hazard ratio [HR]). The authors of the included studies were contacted if key data were unavailable. Observational studies stratified by cohort and case-control studies were evaluated using two versions of the modified Newcastle–Ottawa Scale (NOS) (17, 18). Studies were regarded as high-, medium-, or low-quality if the NOS score was ≥ 7, 5–6, or ≤ 4 points, respectively. All processes of study selection, data extraction, and quality evaluation were performed by two independent reviewers (B. Yan and S. Ma), and discrepancies were finally judged by a third reviewer (C. Yan).
2.3 Statistical analyses
The event and total numbers were first calculated as unadjusted risk ratios (RR). Pooled RRs or HRs and 95% confidence intervals (CIs) were synthesized to estimate the impact of FGF21 levels on the observed endpoints using a fixed- or random-effect model if significant heterogeneity existed. Heterogeneity was determined using the Q statistic and I2 method and considered significant for P < 0.10 for Q statistic or I2 > 50%. Publication bias was detected using funnel plots and a linear regression test for funnel plot asymmetry. Subgroup analysis for the primary endpoint of CAD was conducted among MI sub-populations, and sensitivity analyses were stratified by the effect size (RR derived from event and total numbers or HR) or by omitting each study. A two-sided P < 0.05 was considered statistically significant, except for the heterogeneity test (P < 0.10). All analyses were performed using RStudio (Version 1.2.1335) meta-packages.
3 Results
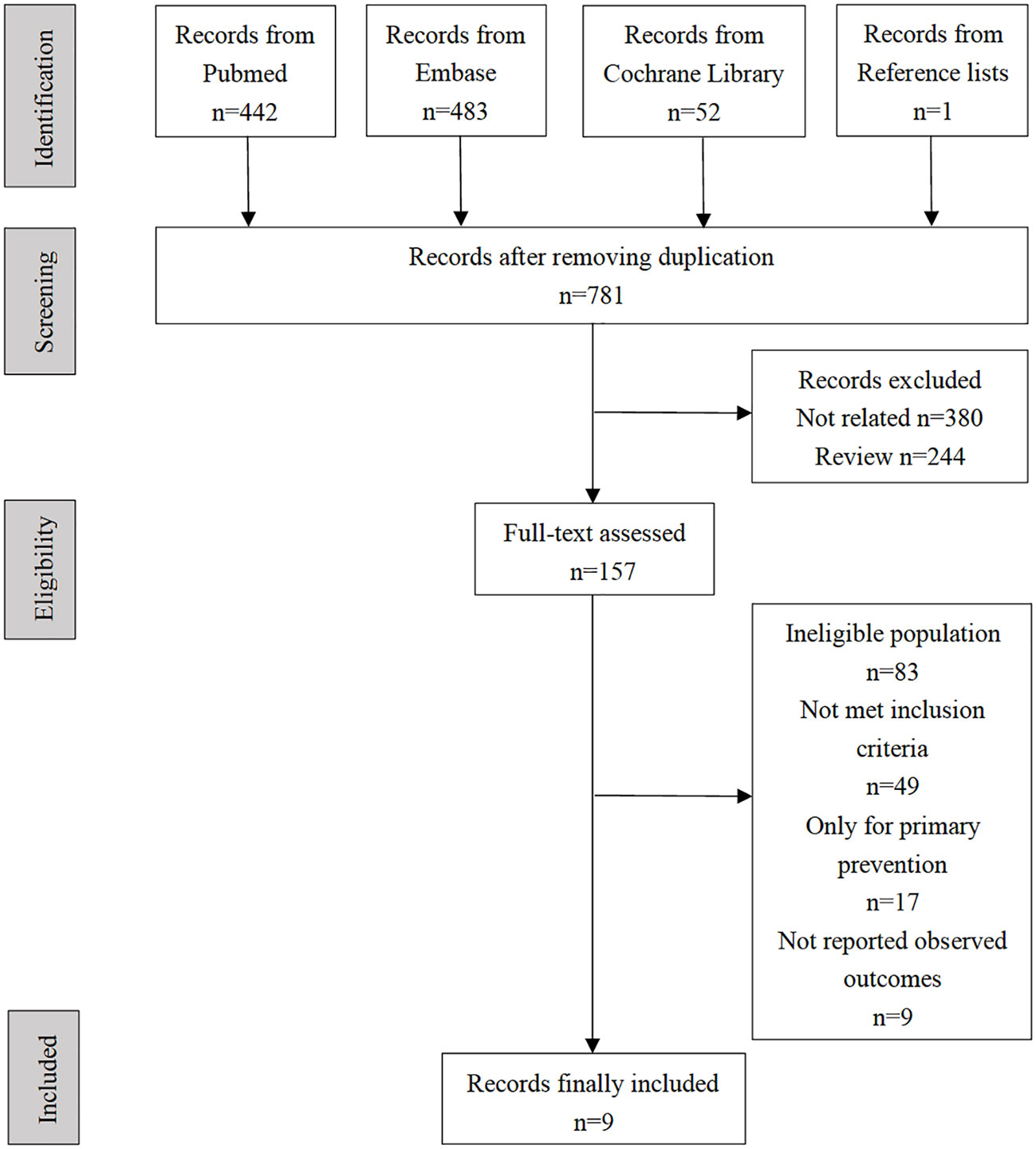
A total of 807 records were identified through a comprehensive retrieval. After removing 27 duplicate studies, titles and abstracts of 781 records were screened. A total of 157 records remained for full-text review, and one record was identified from the reference lists. Finally, nine observational studies that fully met the pre-specified reporting clinical outcomes were included in this meta-analysis (see selection flow diagram in Figure 1). During the full-test review, we found that a series of studies reported both FGF21 and cardiovascular prognosis but were finally excluded from our meta-analysis because they were not in accordance with at least one of the inclusion criteria. Representative excluded studies and their respective reasons for exclusion are presented in Supplemental Materials, Table S1.
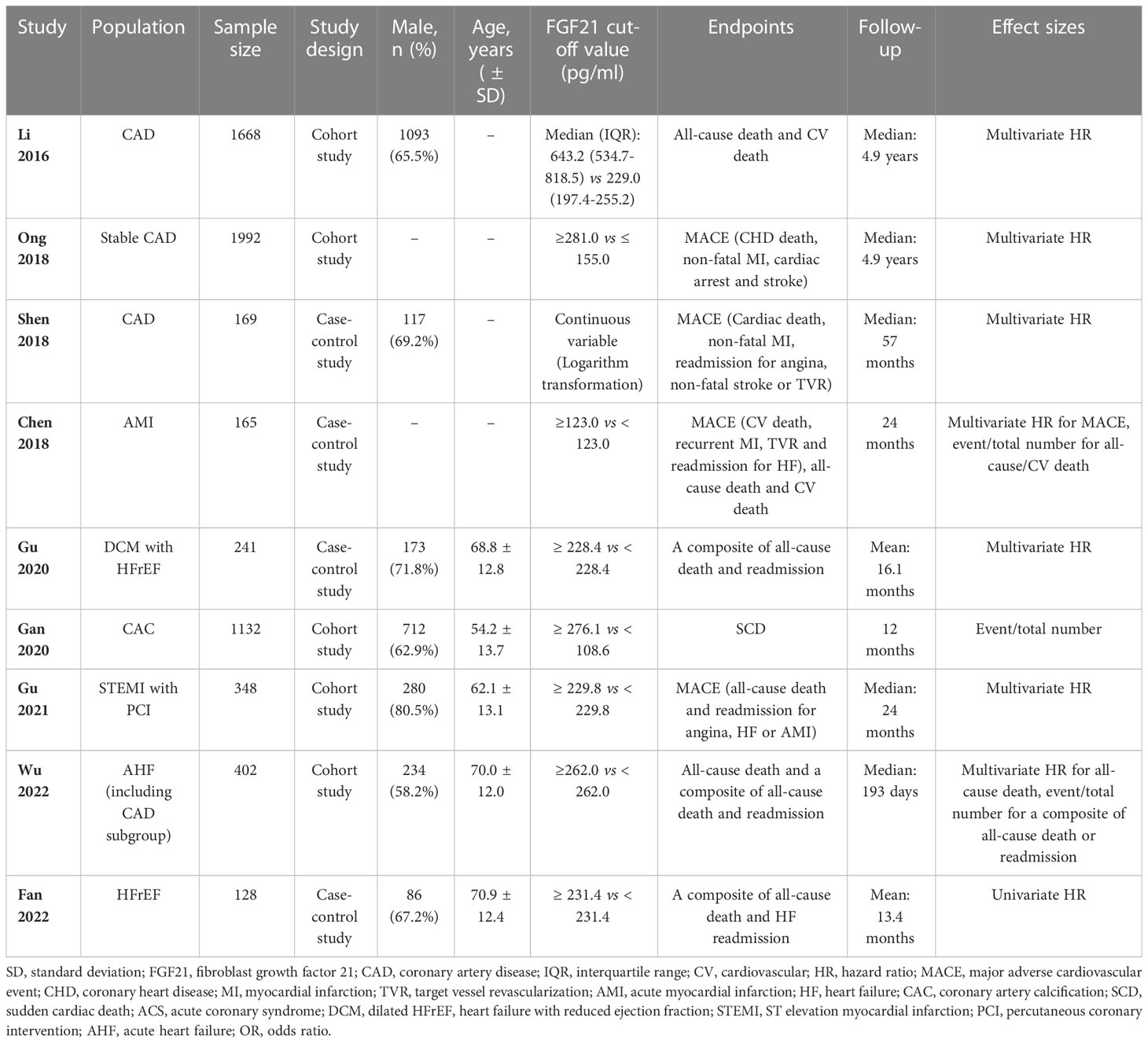
The nine included studies contained five cohort (19–23) and four case-control studies (24–27). Seven studies reported the effect sizes of patients with CAD (19–25), and three studies specifically focused on patients with HF (23, 26, 27). A total of 2674 patients from four studies were included in the analysis of the primary endpoint MACE for CAD (20, 22, 24, 25). A total of 771 patients with HF from three studies were included in the analysis of a composite endpoint of death or readmission for HF (23, 26, 27). Details of the studies included in this meta-analysis are presented in Table 1. All included studies were considered medium or high quality with scores ≥ 5 points in the NOS, except for one case-control study (26) that scored 4 points owing to the potential bias of population selection (Table 2).
3.1 Association between FGF21 and MACE in CAD
As mentioned previously, four studies including 2674 patients explored the association between FGF21 levels and long-term MACE among patients with CAD (20, 22, 24, 25). All four studies performed Cox regression analysis and reported multivariate HR as the effect size, and the median follow-up length was at least 24 months (Table 1). After effect size synthesis, higher FGF21 levels were independently and significantly associated with the long-term risk of MACE among patients with CAD (multivariate HR: 1.77, 95% CI: 1.40–2.23, P < 0.05, I2 = 0%, fixed-effect model; Figure 2A). For the subgroup analysis of patients with MI, two studies were included in the analysis (22, 25), and the results showed that higher FGF21 levels were also independently associated with an increased risk of MACE in patients with MI (multivariate HR: 1.82, 95% CI: 1.22–2.71, P < 0.05, I2 = 0%, fixed-effect model; Figure 2B). To test the stability of the results, sensitivity analysis was performed by omitting each study from the main analysis. The results demonstrated that higher FGF21 levels were consistently and significantly associated with the risk of MACE, irrespective of the removal of any single study (P < 0.05, Supplemental Materials, Figure S1). Funnel plots and asymmetry tests of the two analyses showed that no publication bias existed (Supplemental Materials, Figures S2A, B).
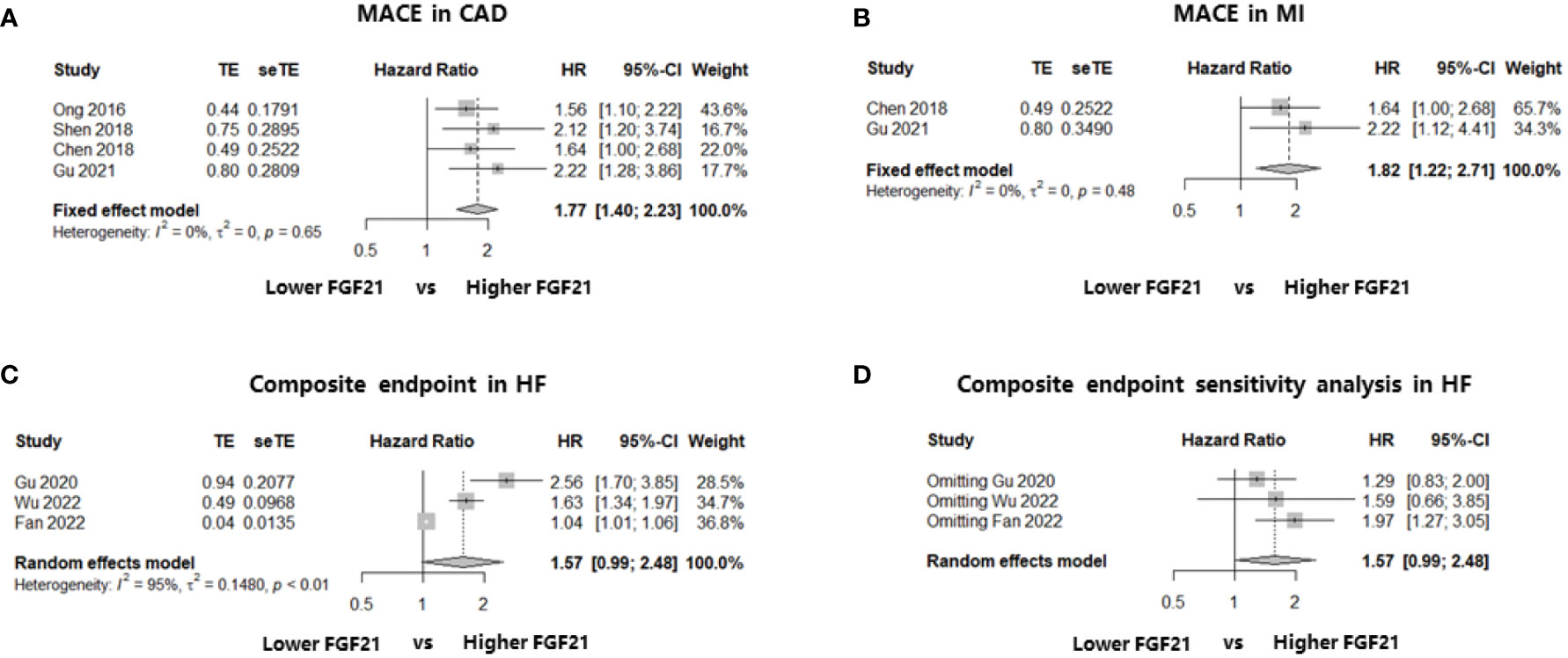
Figure 2 Forest plots of the association of FGF21 with endpoints in patients with CAD or HF. Figure 2 shows the synthesized effect sizes of FGF21 on predicting endpoints among either CAD or HF patients. The endpoint for CAD and HF was MACE and a composite of all-cause death or HF readmission, respectively. (A) FGF21 and MACE in CAD; (B) FGF21 and MACE in MI; (C) FGF21 and a composite of all-cause death or HF readmission in HF; (D) sensitivity analysis of endpoint in HF. FGF21, fibroblast growth factor 21; CAD, coronary artery disease; HF, heart failure; MACE, major adverse cardiovascular event; MI, myocardial infarction.
3.2 Association between FGF21 and death in CAD
Three studies including 2235 patients with CAD identified the relationship between FGF21 levels and all-cause death: two cohort studies reporting multivariate HR (19, 23) and one case-control study reporting the event and total numbers (25). We first calculated the RR in this case-control study and then synthesized the RR using multivariate HRs. The results showed that higher FGF21 levels were not associated with the risk of all-cause death among patients with CAD (HR: 1.86, 95% CI: 0.89–3.87, P > 0.05, I2 = 90%, random-effect model; Figure 3A). However, there was significant heterogeneity (I2 = 90%), which may have been because of the mixture of HRs and RR. In addition, funnel plots and asymmetry tests revealed that publication bias may exist (asymmetry P = 0.02; Supplemental Materials, Figure S3A). Therefore, we performed a sensitivity analysis by excluding the case-control study without multivariate HR (25), and an independent and significant association between higher FGF21 levels and the risk of all-cause death was found in patients with CAD (HR: 2.67, 95% CI: 1.25–5.72, P < 0.05, I2 = 64%, random-effect model; Figure 3B).
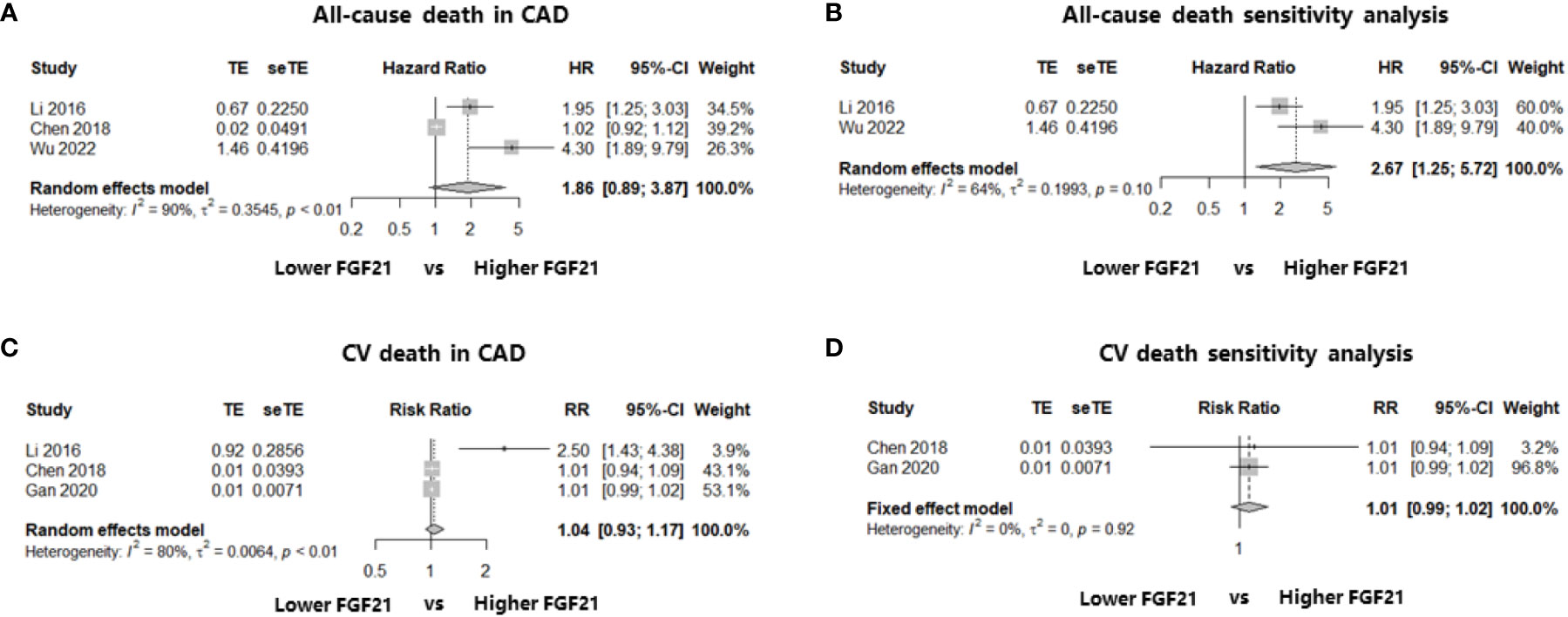
Figure 3 Forest plots of the association of FGF21 with all-cause death or CV death in patients with CAD. Figure 3 shows the synthesized effect sizes of FGF21 on predicting all-cause death or CV death among CAD patients. (A) FGF21 and all-cause death in CAD; (B) sensitivity analysis of all-cause death in CAD; (C) FGF21 and CV death in CAD; (D) sensitivity analysis of CV death in CAD. FGF21, fibroblast growth factor 21; CV death, cardiovascular death.
In terms of FGF21 and CV death, three studies that enrolled patients with CAD were included in the analysis. Two of the three studies reported the event and total numbers (21, 25), and only one cohort study reported the multivariate HR (19). No association was found between FGF21 levels and the risk of CV death among patients with CAD after the effect size synthesis with substantial heterogeneity (RR: 1.04, 95% CI: 0.93–1.17, P > 0.05, I2 = 80%, random-effect model; Figure 3C). To eliminate the heterogeneity from the mixture of RR and HRs, a sensitivity analysis including the two studies (21, 25) reporting event and total numbers was performed, which also found no significant association between FGF21 and the rate of CV death among patients with CAD (RR: 1.01, 95% CI: 0.99–1.02, P > 0.05, I2 = 0%, fixed-effect model; Figure 3D). Funnel plots and asymmetry tests found no potential publication bias in the two meta-analyses of CV death (Supplemental Materials, Figures S3C, D).
3.3 Association between FGF21 and prognosis of HF
Three studies recruiting 771 patients with HF reported the composite endpoint of death or readmission for HF and were included in the meta-analysis (23, 26, 27). The effect sizes of these three studies were event and total numbers (23), univariate HR (27), and multivariate HR (26). The results showed that there was no association between higher FGF21 levels and a composite of death or HF readmission among patients with HF, although there was a statistically significant trend (HR: 1.57, 95% CI: 0.99–2.48, P > 0.05, I2 = 95%, random-effect model; Figure 2C). We also found a large heterogeneity (I2 = 95%) and potential publication bias in this effect size synthesis (asymmetry P < 0.05, funnel plots in Supplemental Materials, Figure S2C). A sensitivity analysis, omitting each study, did not significantly change the negative findings (Figure 2D).
4 Discussion
In this meta-analysis, we explored the association between FGF21 levels and long-term clinical outcomes in patients with CVD stratified by CAD and HF. The results show that higher FGF21 levels were independently associated with the incidence of MACE among patients with CAD. Although the main analysis found no association between FGF21 levels and the rate of all-cause death in CAD, sub-analysis including high-quality studies reporting multivariate HRs showed a significant association between higher FGF21 levels and the risk of all-cause death. In patients with HF, FGF21 was not associated with the rate of a composite of all-cause death or HF readmission, although this outcome should be considered with caution due to the substantial study heterogeneity and variability of effect sizes, including RR, univariate, and multivariate HR. To our knowledge, this is the first meta-analysis to evaluate the association between FGF21 and prognosis of patients with CVD.
FGF21 is a well-known key endocrine hormone that regulates lipolysis in WAT and increases fatty acid oxidation in the liver (28–30). FGF21 increases insulin-independent glucose uptake, improves glucose tolerance, and reduces serum triglyceride levels (31). It has also been recognized that FGF21 has a direct effect on the heart in an endocrine and autocrine manner, which is mediated by the FGFR and co-receptor β-Klotho (32). FGFR1 and FGFR3 are the main FGF21 receptors in the heart (33, 34). FGF21 binds to FGFR and the co-receptor β-Klotho in cardiomyocytes and activates downstream signaling pathways, including ERK, AMPK, and the SIRT1-PPAR-α pathway (35, 36). Among these receptors, FGF21 exerts cardioprotective effects, mainly through FGFR1 (37). A recent study also showed that sodium/glucose cotransporter-2 inhibitors (SGLT2i) can increase serum FGF21 levels, which is one of the mechanisms underlying the cardioprotective effects of SGLT2i (38). Several preclinical trials have also demonstrated that mimics and long-acting derivatives of FGF21 have beneficial effects on body weight, lipoprotein profiles, and metabolic homeostasis (39, 40). However, previous clinical trials have reported that elevated FGF21 levels are associated with increased cardiovascular risk and mortality (41–45). Obviously, a paradox between basic research and clinical studies exists regarding the definite role of FGF21 and CVD; therefore, further comprehensive studies are needed to resolve this issue.
One of the main findings of this meta-analysis was that high FGF21 levels were independently and significantly associated with an increased long-term risk of MACE in patients with CAD (multivariate HR: 1.77, 95% CI: 1.40–2.23, P < 0.05, I2 = 0%, fixed-effect model). Even when focusing on the MI subgroup, the result was consistent (multivariate HR: 1.82, 95% CI: 1.22–2.71, P < 0.05, I2 = 0%, fixed-effect model). No study heterogeneity or publication bias was found in the statistical analyses, indicating that these results were stable and reliable. In terms of all-cause death and FGF21 among patients with CAD, the meta-analysis did not find a significant association (HR: 1.86, 95% CI: 0.89–3.87, P > 0.05, I2 = 90%, random-effect model). However, high study heterogeneity from a mixture of multivariate HRs and RR may discount credibility. Therefore, a sensitivity analysis including studies reporting multivariate HRs was conducted, and an independent and significant association was found between higher FGF21 levels and the risk of all-cause death in CAD. Similar meta-analyses were also performed to determine the relationship between FGF21 and CV death in patients with CAD, but no significant associations were found, irrespective of the main outcome, including three studies (RR: 1.04, 95% CI: 0.93–1.17, P > 0.05, I2 = 80%, random-effect model) or the sensitivity analysis including two studies reporting RR (RR: 1.01, 95% CI: 0.99–1.02, P > 0.05, I2 = 0%, fixed-effect model). Nevertheless, we should note that the study sample size involved in CV death was small; more importantly, two of the three studies only reported event and total numbers without adjusting for other multiple factors, which is inferior to the multivariate HR for authentically reflecting the effect size. Therefore, elevated FGF21 levels are independently associated with poor long-term prognosis in patients with CAD, although more high-level evidence is warranted.
For patients with HF, we pre-specified a composite of all-cause death and HF readmission as endpoints. After statistical analysis, there was no significant association between FGF21 and the long-term endpoint of patients with HF (HR: 1.57, 95% CI: 0.99–2.48, P > 0.05, I2 = 95%, random-effect model). The sensitivity analysis showed that the result was positive only when a case-control study that reported a univariate HR was excluded. The study heterogeneity was also high owing to the effect size variability reported by the included studies (including RR, univariate HR, and multivariate HR). Hence, although a negative relationship was found between FGF21 and clinical outcomes in patients with HF, this result may be insufficient to determine the definite relationship between FGF21 and the prognosis of patients with HF and needs to be reconfirmed by more clinical trials. Overall, our meta-analysis and previous findings (14, 15, 45) collectively identify that increased FGF21 levels may be an independent predictor of poor prognosis among patients with CVD, rather than a protective factor of the heart, which was found in mechanistic studies. The FGF21 paradox not only exists in primary prevention but also in the long-term prognosis of CVD. This paradox may be because of a compensatory response to metabolic stress in patients with CVD. FGF21 resistance may be another underlying mechanism, according to recent findings reporting that stress conditions can decrease FGF21 co-receptor β-Klotho expression in the heart, impair FGF21 signaling, and weaken the protective effect of FGF21 on cardiomyocytes (46). Both underlying mechanism studies and high-level clinical trials are needed to determine this uncertainty to provide potential drug targets.
The present meta-analysis had several limitations. First, although comprehensive retrieval was performed and nine studies were finally included, the study sample size was also relatively small. Second, the effect sizes reported by the included studies were varied and uneven, including event/total number, odds ratio, and univariate and multivariate HR. This diversity increases the study heterogeneity, which may influence data synthesis. Moreover, as shown in Table 1, the cutoff values of FGF21 used in the included studies varied without a uniform criterion. Therefore, a definite FGF21 cutoff value for predicting cardiovascular risk still needs to be explored. Finally, the study endpoints were not abundant because of limited data obtained from the included studies. These deficiencies require further clinical trials to fill the gap.
This meta-analysis demonstrated that increased FGF21 levels were independently associated with the long-term prognosis of patients with CAD. In patients with HF, no association was found between FGF21 levels and prognosis, and the role of FGF21 in predicting clinical outcomes remains unclear. The FGF21 paradox exists in the long-term prognosis of CVD.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
BY designed this study. SM wrote the main manuscript and prepared figures. CY conducted the manuscript reviewing and editing. YH supervised all these procedures of this study. All authors contributed to the article and approved the submitted version.
Funding
Supported by Liaoning S&T Project in Liaoning Province, China (ID: 2020020292-JH1/103-03).
Acknowledgments
We authors are grateful to all the participants of this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1108234/full#supplementary-material
Abbreviations
FGF21, fibroblast growth factor 21; WAT, white adipose tissue; SIRT1, sirtuin 1; PPAR, peroxisome proliferator-activated receptor; CVD, cardiovascular disease; PRISMA, Preferred Reporting Items for Systematic Review and Meta-analysis Protocols; MI, myocardial infarction; HF, heart failure; PICOS, patient, intervention, comparison, outcome, study type; CAD, coronary artery disease; MACE, major adverse cardiovascular event; CV, cardiovascular; HR, hazard ratio; NOS, Newcastle-Ottawa Scale; RR, risk ratio; CI, confidence interval; FGFR, fibroblast growth factor receptor; ERK, extracellular signal regulated kinase; AMPK, adenosine monophosphate activated protein kinase; SGLT2i, sodium/glucose cotransporter-2 inhibitor.
References
1. Dolegowska K, Marchelek-Mysliwiec M, Nowosiad-Magda M, Slawinski M, Dolegowska B. FGF19 subfamily members: FGF19 and FGF21. J Physiol Biochem (2019) 75(2):229–40. doi: 10.1007/s13105-019-00675-7
2. Dutchak PA, Katafuchi T, Bookout AL, Choi JH, Yu RT, Mangelsdorf DJ, et al. Fibroblast growth factor-21 regulates PPARγ activity and the antidiabetic actions of thiazolidinediones. Cell (2012) 148(3):556–67. doi: 10.1016/j.cell.2011.11.062
3. Kliewer SA, Mangelsdorf DJ. A dozen years of discovery: Insights into the physiology and pharmacology of FGF21. Cell Metab (2019) 29:246–53. doi: 10.1016/j.cmet.2019.01.004
4. Woo YC, Xu A, Wang Y, Lam KS. Fibroblast growth factor 21 as an emerging metabolic regulator: Clinical perspectives. Clin Endocrinol (Oxf) (2013) 78:489–96. doi: 10.1111/cen.12095
5. Owen BM, Mangelsdorf DJ, Kliewer SA. Tissue-specific actions of the metabolic hormones FGF15/19 and FGF21. Trends Endocrinol Metab (2015) 26:22–9. doi: 10.1016/j.tem.2014.10.002
6. Véniant MM, Hale C, Helmering J, Chen MM, Stanislaus S, Busby J, et al. FGF21 promotes metabolic homeostasis via white adipose and leptin in mice. PloS One (2012) 7(7). doi: 10.1371/journal.pone.0040164
7. Markan KR, Naber MC, Ameka MK, Anderegg MD, Mangelsdorf DJ, Kliewer SA, et al. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes (2014) 63(12):4057–63. doi: 10.2337/db14-0595
8. Liu M, Cao H, Hou Y, Sun G, Li D, Wang W. Liver plays a major role in FGF-21 mediated glucose homeostasis. Cell Physiol Biochem (2018) 45(4):1423–33. doi: 10.1159/000487568
9. Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, Tigno XT, et al. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology (2007) 148(2):774–81. doi: 10.1210/en.2006-1168
10. Baratta F, D’Erasmo L, Bini S, Pastori D, Angelico F, del Ben M, et al. Heterogeneity of non-alcoholic fatty liver disease (NAFLD): Implication for cardiovascular risk stratification. Atherosclerosis (2022) 357:51–9. doi: 10.1016/j.atherosclerosis.2022.08.011
11. Lin Z, Pan X, Wu F, Ye D, Zhang Y, Wang Y, et al. Fibroblast growth factor 21 prevents atherosclerosis by suppression of hepatic sterol regulatory element-binding protein-2 and induction of adiponectin in mice. Circulation (2015) 131(21):1861–71. doi: 10.1161/CIRCULATIONAHA.115.015308
12. Planavila A, Redondo I, Hondares E, Vinciguerra M, Munts C, Iglesias R, et al. Fibroblast growth factor 21 protects against cardiac hypertrophy in mice. Nat Commun (2013) 4(May 2013):2019. doi: 10.1038/ncomms3019
13. Tucker W, Tucker B, Rye KA, Ong KL. Fibroblast growth factor 21 in heart failure. Heart Fail Rev (2022) 28(1):261–272. doi: 10.1007/s10741-022-10268-0
14. Zhang Y, Yan J, Yang N, Qian Z, Nie H, Yang Z, et al. High-level serum fibroblast growth factor 21 concentration is closely associated with an increased risk of cardiovascular diseases: A systematic review and meta-analysis. Front Cardiovasc Med (2021) 8. doi: 10.3389/fcvm.2021.705273
15. Lakhani I, Gong M, Wong WT, Bazoukis G, Lampropoulos K, Wong SH, et al. Fibroblast growth factor 21 in cardio-metabolic disorders: a systematic review and meta-analysis. Metabolism (2018) 83:11–7. doi: 10.1016/j.metabol.2018.01.017
16. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ (2021) 372:n160. doi: 10.1136/bmj.n160
17. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
18. Herzog R, Álvarez-Pasquin MJ, Díaz C, del Barrio JL, Estrada JM, Gil Á. Are healthcare workers intentions to vaccinate related to their knowledge, beliefs and attitudes? a systematic review. BMC Public Health (2013) 13:154. doi: 10.1186/1471-2458-13-154
19. Li Q, Zhang Y, Ding D, Yang Y, Chen Q, Su D, et al. Association between serum fibroblast growth factor 21 and mortality among patients with coronary artery disease. J Clin Endocrinol Metab (2016) 101(12):4886–94. doi: 10.1210/jc.2016-2308
20. Ong KL, Hui N, Januszewski AS, Kaakoush NO, Xu A, Fayyad R, et al. High plasma FGF21 levels predicts major cardiovascular events in patients treated with atorvastatin (from the treating to new targets [TNT] study). Metabolism (2019) 93:93–9. doi: 10.1016/j.metabol.2018.11.006
21. Gan F, Huang J, Dai T, Li M, Liu J. Serum level of fibroblast growth factor 21 predicts long-term prognosis in patients with both diabetes mellitus and coronary artery calcification. Ann Cardiothorac Surg (2020) 9(2):368–74. doi: 10.21037/apm.2020.03.28
22. Gu L, Jiang W, Qian H, Zheng R, Li W. Elevated serum FGF21 predicts the major adverse cardiovascular events in STEMI patients after emergency percutaneous coronary intervention. PeerJ (2021) 9. doi: 110.7717/peerj.12235
23. Wu G, Wu S, Yan J, Gao S, Zhu J, Yue M, et al. Fibroblast growth factor 21 predicts short-term prognosis in patients with acute heart failure: A prospective cohort study. Front Cardiovasc Med (2022) 9:834967. doi: 10.3389/fcvm.2022.834967
24. Shen Y, Zhang X, Xu Y, Xiong Q, Lu Z, Ma X, et al. Serum FGF21 is associated with future cardiovascular events in patients with coronary artery disease. Cardiol (Switzerland) (2018) 139(4):212–8. doi: 10.1159/000486127
25. Chen H, Lu N, Zheng M. Original article a high circulating FGF21 level as a prognostic marker in patients with acute myocardial infarction. Am J Transl Res (2018) 10(9):2958–2966.
26. Gu L, Jiang W, Zheng R, Yao Y, Ma G. Fibroblast growth factor 21 correlates with the prognosis of dilated cardiomyopathy. Cardiol (Switzerland) (2021) 146(1):27–33. doi: 10.1159/000509239
27. Fan L, Gu L, Yao Y, Ma G. Elevated serum fibroblast growth factor 21 is relevant to heart failure patients with reduced ejection fraction. Comput Math Methods Med (2022) 2022:7138776. doi: 10.1155/2022/7138776
28. Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab (2007) 5(6):415–25. doi: 10.1016/j.cmet.2007.05.003
29. Li X, Ge H, Weiszmann J, Hecht R, Li Ys, Véniant MM, et al. Inhibition of lipolysis may contribute to the acute regulation of plasma FFA and glucose by FGF21 in ob/ob mice. FEBS Lett (2009) 583(19):3230–4. doi: 10.1016/j.febslet.2009.09.012
30. Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab (2007) 5(6):426–37. doi: 10.1016/j.cmet.2007.05.002
31. Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, et al. FGF-21 as a novel metabolic regulator. J Clin Invest (2005) 115(6):1627–35. doi: 10.1172/JCI23606
32. Tanajak P, Chattipakorn SC, Chattipakorn N. Effects of fibroblast growth factor 21 on the heart. J Endocrinol (2015) 227(2):R13–30. doi: 10.1530/JOE-15-0289
33. Hughes SE. Differential expression of the fibroblast growth factor receptor (FGFR) multigene family in normal human adult tissues. J Histochem Cytochem (1997) 45(7):1005–19. doi: 10.1177/002215549704500710
34. Ahmad I, Iwata T, Leung HY. Mechanisms of FGFR-mediated carcinogenesis. Biochim Biophys Acta (BBA) - Mol Cell Res (2012) 1823(4):850–60. doi: 10.1016/j.bbamcr.2012.01.004
35. Zhang C, Huang Z, Gu J, Yan X, Lu X, Zhou S, et al. Fibroblast growth factor 21 protects the heart from apoptosis in a diabetic mouse model via extracellular signal-regulated kinase 1/2-dependent signalling pathway. Diabetologia (2015) 58(8):1937–48. doi: 10.1007/s00125-015-3630-8
36. Planavila A, Redondo-Angulo I, Ribas F, Garrabou G, Casademont J, Giralt M, et al. Fibroblast growth factor 21 protects the heart from oxidative stress. Cardiovasc Res (2015) 106(1):19–31. doi: 10.1093/cvr/cvu263
37. Furukawa N, Koitabashi N, Matsui H, Sunaga H, Umbarawan Y, Syamsunarno MRAA, et al. DPP-4 inhibitor induces FGF21 expression via sirtuin 1 signaling and improves myocardial energy metabolism. Heart Vessels (2021) 36(1):136–46. doi: 10.1007/s00380-020-01711-z
38. Osataphan S, Macchi C, Singhal G, Chimene-Weiss J, Sales V, Kozuka C, et al. SGLT2 inhibition reprograms systemic metabolism via FGF21-dependent and -independent mechanisms. JCI Insight (2019) 4(5):e123130. doi: 10.1172/jci.insight.123130
39. Talukdar S, Zhou Y, Li D, Rossulek M, Dong J, Somayaji V, et al. A long-acting FGF21 molecule, PF-05231023, decreases body weight and improves lipid profile in non-human primates and type 2 diabetic subjects. Cell Metab (2016) 23(3):427–40. doi: 10.1016/j.cmet.2016.02.001
40. Gaich G, Chien JY, Fu H, Glass LC, Deeg MA, Holland WL, et al. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab (2013) 18(3):333–40. doi: 10.1016/j.cmet.2013.08.005
41. Shen Y, Zhang X, Pan X, Xu Y, Xiong Q, Lu Z, et al. Contribution of serum FGF21 level to the identification of left ventricular systolic dysfunction and cardiac death. Cardiovasc Diabetol (2017) 16(1):106. doi: 10.1186/s12933-017-0588-5
42. Wang JS, Sheu WHH, Lee WJ, Lee I, Lin SY, Lee WL, et al. Associations of fibroblast growth factor 21 with cardiovascular risk and β-cell function in patients who had no history of diabetes. Clinica Chimica Acta (2017) 472:80–5. doi: 10.1016/j.cca.2017.07.017
43. Lenart-Lipińska M, Matyjaszek-Matuszek B, Gernand W, Nowakowski A, Solski J. Serum fibroblast growth factor 21 is predictive of combined cardiovascular morbidity and mortality in patients with type 2 diabetes at a relatively short-term follow-up. Diabetes Res Clin Pract (2013) 101(2):194–200. doi: 10.1016/j.diabres.2013.04.010
44. Lee CH, Woo YC, Chow WS, Yan Cheung CY, Yi Fong CH, Ann Yuen MM, et al. Role of circulating fibroblast growth factor 21 measurement in primary prevention of coronary heart disease among chinese patients with type 2 diabetes mellitus. J Am Heart Assoc (2017) 6(6):e005344. doi: 10.1161/JAHA.116.005344
45. Shen Y, Ma X, Zhou J, Pan X, Hao Y, Zhou M, et al. Additive relationship between serum fibroblast growth factor 21 level and coronary artery disease (2013). Available at: http://www.cardiab.com/content/12/1/124.
Keywords: death, prognosis, coronary artery disease, fibroblast growth factor (FGF 21), heart failure, major adverse cardiac event (MACE)
Citation: Yan B, Ma S, Yan C and Han Y (2023) Fibroblast growth factor 21 and prognosis of patients with cardiovascular disease: A meta-analysis. Front. Endocrinol. 14:1108234. doi: 10.3389/fendo.2023.1108234
Received: 25 November 2022; Accepted: 14 February 2023;
Published: 28 February 2023.
Edited by:
Paola Di Pietro, University of Salerno, ItalyReviewed by:
Rutao Wang, Xijing Hospital, ChinaJun Fang, Fujian Medical University Union Hospital, China
Zhehao Piao, Wenzhou Medical University, China
Copyright © 2023 Yan, Ma, Yan and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chenghui Yan, eWFuY2gxMDI5QDE2My5jb20=; Yaling Han, aGFueWFsaW5nQDE2My5uZXQ=
 Bing Yan
Bing Yan Sicong Ma
Sicong Ma Chenghui Yan2*
Chenghui Yan2* Yaling Han
Yaling Han