- 1Institute of Medical Sciences, Tzu Chi University, Hualien, Taiwan
- 2Tzu Chi University Research Center for Big Data Teaching, Research and Statistic Consultation, Hualien, Taiwan
- 3Department of Ophthalmology, Buddhist Tzu Chi General Hospital, Hualien, Taiwan
- 4Department of Ophthalmology and Visual Science, Tzu Chi University, Hualien, Taiwan
Background: Early Identifying and characterizing patients with diabetic macular edema (DME) is essential for individualized treatment and outcome optimization. This study aimed to timely investigate optical coherence tomography (OCT) biomarkers of DME refractory to intravitreal anti-vascular endothelial growth factor (VEGF) therapy.
Methods: We retrospective reviewed 72 eyes from 44 treatment-naïve patients who were treated with intravitreal anti-VEGF for DME. OCT scans prior to anti-VEGF were evaluated for serous retinal detachment (SRD), size of outer nuclear layer cystoid changes, diffuse retinal thickening, integrity of the inner segment-outer segment (IS-OS) junction, quantity and location of hyperreflective foci, vitreomacular interface abnormalities, and epiretinal membrane (ERM). The Baseline best-corrected visual acuity (BCVA) and central macular thickness was recorded at baseline and 4 months after treatment with anti-VEGF. The main outcome measure was the correlation between spectral-domain OCT measurements and BCVA response at baseline and after anti-VEGF treatment (mean change from baseline; ≥ 10 Early Treatment Diabetic Retinopathy Study letters in BCVA).
Results: Partially continuous IS-OS layers (partially vs. completely continuous: β, -0.138; Wald chi-square, 16.392; P<0.001) was predictor of better response to anti-VEGF treatment. In contrast, ERM (present vs. absent ERM: β, 0.215; Wald chi-square, 5.921; P=0.015) and vitreomacular traction (vitreomacular traction vs. posterior vitreous detachment: β=0.259; Wald chi-square=5.938; P=0.015) were the predictors of poor response. The improvement of BCVA trended toward the OCT predictive value of central macular thickness reduction; however, this was not significant.
Conclusion: Partially continuous IS-OS layers is predictive of better response to anti-VEGF therapy in DME. Meanwhile, ERM is a significant predictor of poor response.
Introduction
Vision loss associated with diabetic retinopathy (DR) is most commonly caused by diabetic macular edema (DME) (1). The Diabetes Control and Complications Trial (DCCT) reported that 27% of patients with type 1 diabetes developed macular edema within 9 years of diabetes onset (2). Other studies indicate that in type 2 diabetes patients, the prevalence increases from 3% within 5 years of diagnosis to 28% after 20 years (3). Although several treatment options are available, no consensus on DME treatment based on patient status has been achieved.
Vascular endothelial growth factor (VEGF) is an important mediator of abnormal vascular permeability in eyes with DME (4). Anti-VEGF injections are generally proposed as first-line therapy for center-involved DME and are effective in improving visual acuity (VA), with 10%–40% of patients achieving significant improvement in VA after 1 year of treatment (5, 6). However, a considerable proportion have unsatisfactory response to anti-VEGF agents; 40% of eyes with DME do not or have suboptimal response to anti-VEGF treatment (7, 8). Nonetheless, there is little information to date about the prognostic factors of poor responders.
Optical coherence tomography (OCT) images are readily available to physicians and provide detailed information. Structural changes presumably reflect part of the complex pathophysiologic processes occurring in DME. Furthermore, anatomical measures on spectral-domain (SD) OCT can predict treatment success or failure of various therapies (9). Distinct structural changes identifiable on SD-OCT could reflect part of the intraocular pathophysiologic process change after anti-VEGF treatments and help predict the treatment response.
Among patients with DME refractory to anti-VEGF therapy after a loading dose of three consecutive monthly injections, those who were switched to other treatment modalities (e.g., corticosteroids) had better visual and anatomical outcomes at 12 months than did those who continued with anti-VEGF therapy (10). Post hoc analysis from the DRCR.net Protocol I study also indicates that early central macular thickness (CMT) response to anti-VEGF is a significant prognostic indicator of medium to long-term anatomical outcomes in DME (11). Accordingly, the early identification of patients who would not benefit from first-line treatment with anti-VEGF therapy is critical. Real-world studies have become increasingly important in providing evidence of treatment effectiveness in clinical practice. They can therefore provide information on the long-term safety, particularly of rare events, and efficacy of drugs in large heterogeneous populations, as well as information on utilization patterns and health and economic outcomes (12). We aimed to investigate whether the characteristics identified on SD-OCT could be predictive markers of treatment response after three monthly anti-VEGF therapies in DME patients.
Research design and methods
Study design and setting
This retrospective study was approved by the Institutional Review Board of the Research Ethics Committee of Hualien Tzu-Chi Hospital and Buddhist Tzu-Chi Medical Foundation (IRB110-188-B) and was conducted in accordance with the guidelines of the Declaration of Helsinki. Data were obtained from Hualien Tzu Chi Hospital Medical Center. Data of patients with DME treated with intravitreal anti-VEGF between April 1, 2013 and April 1, 2021 were reviewed. Written informed consent was obtained from all patients.
Study participants
The inclusion criteria were as follows (1): age≥ 20 years; (2) type 1 or 2 diabetes mellitus; (3) treatment-naïve DME causing visual loss macular edema defined clinically and as retinal thickness of >300 μm in the central subfield and intraretinal or subretinal fluid seen on SD-OCT; and (4) treatment with anti-VEGF agents. The exclusion criteria were (1) another concomitant ocular disease that causes macular edema (i.e., neovascular age-related macular degeneration or choroidal neovascularization due to other reasons, retinal vein occlusion, uveitis, and recent intraocular surgery possibly causing postsurgical macular edema or influence drug absorption, such as cataract surgery or vitrectomy); (2) previous treatment with intraocular corticosteroids or pan-retinal photocoagulation within 6 months before treatment with anti-VEGF agents. For patients who received bilateral treatment, both the eyes were included. Refractory DME was defined as a reduction of less than 10% in retinal thickness on SD-OCT measured 1 month after at least three monthly anti-VEGF injections. Data on demographic data, age, sex, and type of retinopathy (non-proliferative or proliferative) were collected from patient charts.
Optical coherence tomography analysis
Qualitative and quantitative evaluations of SD-OCT images encompassing the fovea were performed at baseline and 4 months after treatment to assess the presence of several morphologic features, including (1) SRD (height at the fovea was measured); (2) cystoid changes in the outer nuclear layer (ONL) and maximal cyst size (small <100μm, large 100-200μm, giant >200μm); (3) continuity of the inner segment-outer segment (IS-OS) layer (completely continuous, partly disrupted, completely disrupted); (4) presence of hyperreflective foci (HRF), as well as quantity (few, 2–10; many >11) and location (between the internal limiting membrane and the inner nuclear layer; between the outer plexiform layer and external limiting membrane; in all retinal layers); (5) status of the vitreomacular interface (detached, vitreomacular adhesion [VMA], vitreomacular traction[VMT]); (6) presence of an epiretinal membrane (ERM); (7) CMT; and (8) presence of diffuse retinal thickening (DRT), as well as the width (≦1, 1–3, 3–6 mm). OCT scans were obtained using SD-OCT (Heidelberg Spectralis, Heidelberg, Germany). The listed features were evaluated using a horizontal b-scan encompassing the fovea. The OCT images were evaluated by two experienced retina specialists (MS He and YC Chang) blinded to the outcome. CMT was recorded at baseline and at 1, 2, 3, and 4 months.
Statistical analysis
Continuous variables and categorical variables were expressed as the mean with standard deviation and as the frequency with proportion, respectively. Both eyes of the patients were included in the analysis. Considering the correlation between eyes, the generalized estimate equation (GEE) was employed for assessing the baseline predictors for the continuous outcome of central macular thickness reduction and central macular thickness reduction <10%. All statistical analyses were performed using SPSS for Windows (version 21.0; IBM, Armonk, NY, USA). All p values were two-sided, and p<0.05 was considered statistically significant.
Results
Study participants
A total of 72 eyes from 44 patients were included in the analysis. The demographic patient characteristics are shown in Table 1. All eyes with DME had no history of anti-VEGF treatment and were treated with three consecutive monthly intravitreal injections of anti-VEGF. Three main types of anti-VEGF drugs were used in our cohort, the most common of which was ranibizumab (n=60 eyes, 83.3%), followed by aflibercept (n=10, 13.9%) and bevacizumab (n=2, 2.8%). A total of 24 eyes (33.3%) had proliferative diabetic retinopathy (PDR), 43 eyes (59.7%) had severe non-proliferative diabetic retinopathy (NPDR), and 5 eyes (6.9%) had moderate NPDR.

Table 1 Descriptive statistics: demographic data and optical coherence tomography baseline measures.
Anatomic baseline characteristics
The baseline OCT characteristics are shown in Table 1. With respect to the DME morphology, DRT was the most common presentation (n=59 eyes, 81.9%), followed by cystoid macular edema (CME) (n=33 eyes, 45.8%) and SRD (n=16 eyes, 22.2%). Furthermore, eyes with DME were more commonly to present with complete continuous IS-OS continuity (52.8%), HRF (86.1%), and VMA (88.9%) in the baseline.
Optical coherence tomography predictors for treatment response
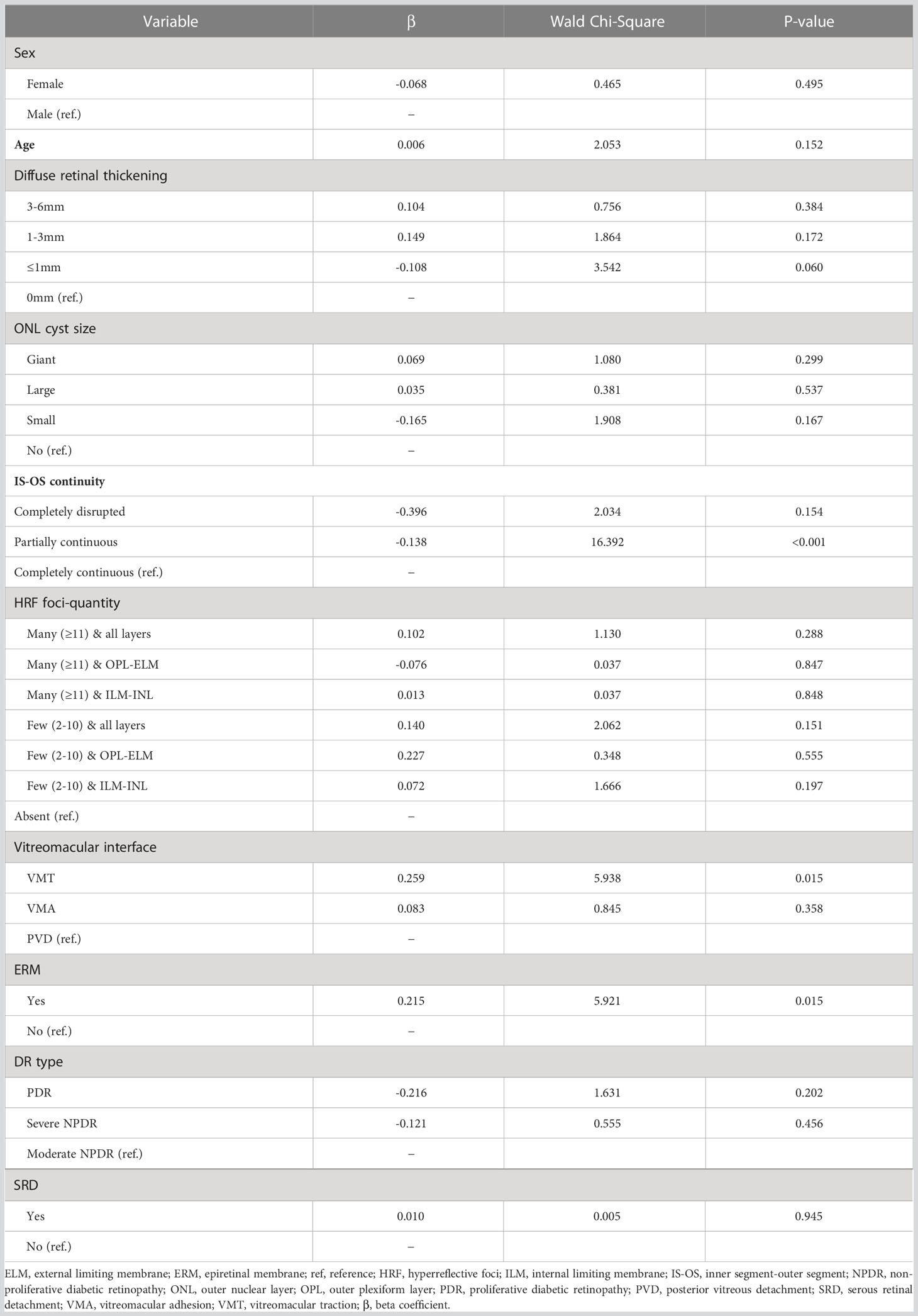
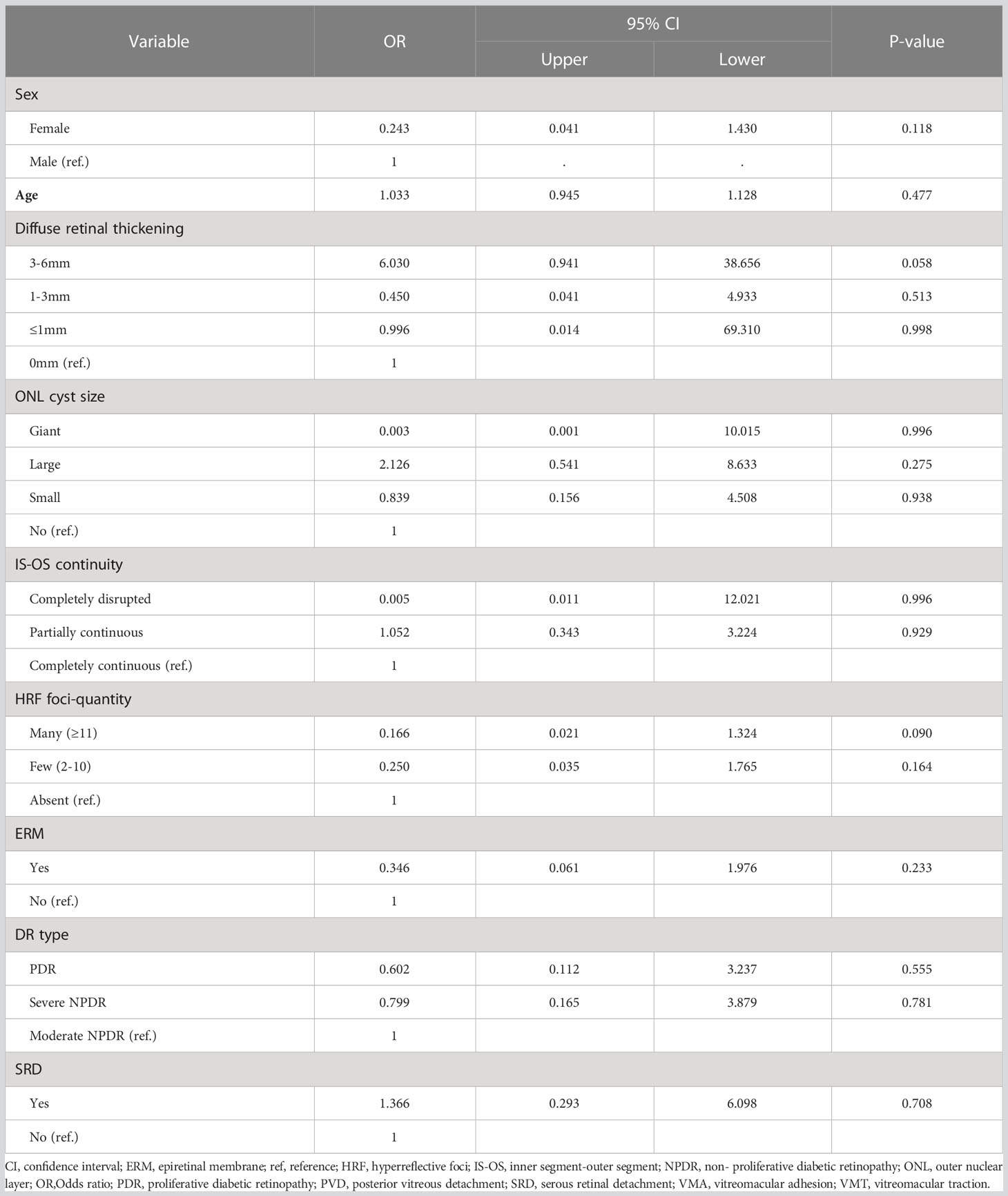
Eyes with partially continuous IS-OS layers had a better treatment response after 4 months (partially vs. completely continuous: β=-0.138; Wald chi-square=16.392; P<0.001). Baseline VMT was a predictor of poor functional treatment response after 4 months (VMT vs. posterior vitreous detachment: β=0.259; Wald chi-square=5.938; P=0.015). Moreover, eyes with ERM at baseline were more likely to have poor response at 4 months (present vs. absent ERM: β=0.215; Wald chi-square=5.921; P=0.015). Figure 1 shows the OCT biomarkers that were predictive of treatment response after 4 months. The predictive values of all OCT measures examined are shown in Table 2. Baseline predictors of mean CMT reduction are shown in Figure 2. Furthermore, the odds of gaining BCVA ≥10 letters at 4 months trended toward the OCT predictive value of CMT reduction; however, this was not significant (Table 3). All OCT biomarkers that were predictive of good BCVA response are shown in Figure 3.

Figure 1 OCT measures. (A), Grading of outer nuclear layer (ONL) cysts: Cystoid diabetic macular edema (DME) with a giant ONL cyst (★). (B), Serous retinal detachment (SRD) with diffuse retinal thickening (DRT, width of 3–6mm) showing retinal elevation between the sensory retina and the retinal pigment epithelium (dashed arrow); the height of SRD is measured. Grading of hyperreflective foci (HRF): A high number of HRF (≥11) are distributed in all layers (located between the ILM and INL [arrowhead] and between OPL and ELM [arrow]). (C, D), Grading of DRT: (C), DME associated with focal DRT (width ≦1 mm, between arrows). (D), DME related to localized DRT (width within 1–3 mm, between arrows). (E, F), Grading of the inner segment-outer segment (IS-OS) integrity. (E), Partially disrupted continuity of the IS-OS layer (between arrows). (F), Complete discontinuity of the IS-OS layer (between arrows). (G), DME associated with epiretinal membrane (arrow). (H), DME associated with vitreomacular traction (arrow). ELM, external limiting membrane; ILM, internal limiting membrane; INL, inner nuclear layer; OPL, outer plexiform layer.

Figure 2 Forest plot of baseline predictors of CMT reduction, using generalized estimate equation model. CMT, central macular thickness; ONL, outer nuclear layer; IS-OS, inner segment-outer segment; HRF, hyperreflective foci; OPL, outer plexiform layer; ELM, external limiting membrane; ILM, internal limiting membrane; VMA, vitreomacular adhesion; VMT, vitreomacular traction; PVD, posterior vitreous detachment; ERM, epiretinal membrane; PDR, proliferative diabetic retinopathy; NPDR, non-proliferative diabetic retinopathy; SRD, serous retinal detachment; β, beta coefficient. * Statistically significant at p<0.05.

Figure 3 Forest plot of baseline predictors of a ≥10 letter gain in BCVA, using generalized estimate equation model. BCVA, best-corrected visual acuity; ONL, outer nuclear layer; IS-OS, inner segment-outer segment; HRF, hyperreflective foci; ERM, epiretinal membrane; SRD, serous retinal detachment.
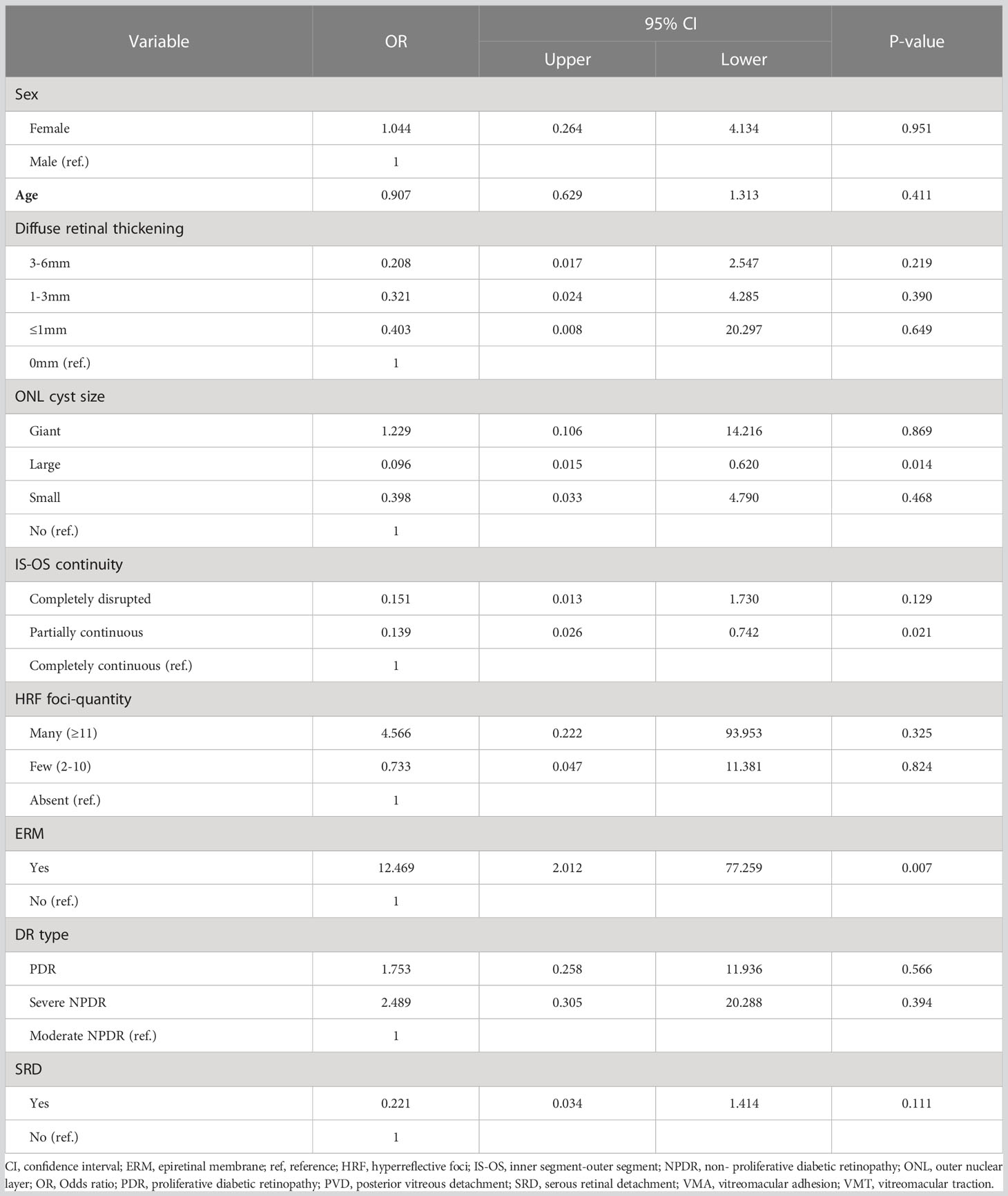
In a subanalysis, eyes with CMT reduction of less than 10% after 4 months were designated to the refractory group (Table 4). The results showed that ERM at baseline predicted highly increased odds of poor response at 4 months (OR, 12.469; 95% CI, 2.012–77.259; P=0.007). In contrast, large ONL cyst sizes at baseline (OR, 0.096; 95% CI, 0.015–0.62; P=0.014) and partially continuous IS-OS layers (OR, 0.139; 95% CI, 0.026–0.742; P=0.021) were less likely to be refractory group after 4 months (Figure 4).

Figure 4 Forest plot of baseline predictors of CMT reduction <10%, using generalized estimate equation model. CMT, central macular thickness; ONL, outer nuclear layer; IS-OS, inner segment-outer segment; HRF, hyperreflective foci; ERM, epiretinal membrane; SRD, serous retinal detachment. *Statistically significant at p<0.05.
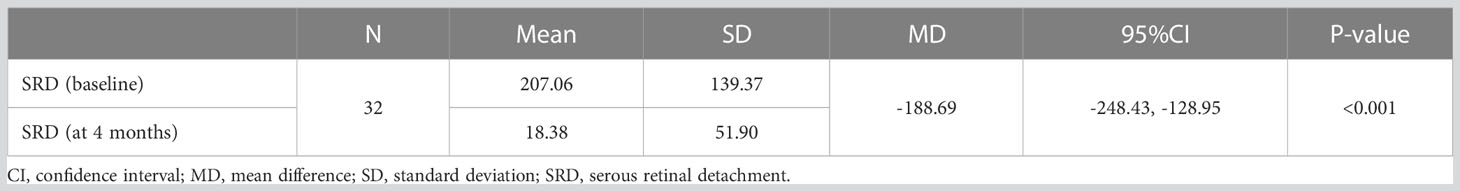
In the univariate analysis, SRD at baseline was significantly associated with treatment response to anti-VEGF agents (MD, -188.69 μm; 95% CI, -128.95 to -248.43; P<0.001) (Table 5). However, in multivariate survival analysis, treatment response to anti-VEGF regimens was not a significant influencing factor in patients with SRD (present vs. absent: β, 0.01; Wald chi-square, 0.005; P=0.945) (Table 2).
Discussion
In this real-world evidence-based study, we identified partially continuous IS-OS layers as biomarkers that predict better response to anti-VEGF therapy in DME. In contrast, ERM is a significant predictor of poor response. DME has a complex pathogenesis, with multiple factors contributing to its pathophysiology, including angiogenic, inflammatory, hypoxic, and hemodynamic processes that lead to the breakdown of the blood-retinal barrier (BRB) and leakage of intraretinal fluid (13). Anti-VEGF injections are generally recommended as the first-line therapy for DME; however, refractory cases are not uncommon. A post-hoc analysis of the DRCR.net Protocol I reported an approximately 40% prevalence of refractory DME after 2 years of monthly intravitreal ranibizumab treatment (7). Combined data from the RIDE/RISE trial found that 23% of eyes receiving intravitreal ranibizumab had persistent macular edema at the end of the study period (14). The real-world prevalence of refractory DME may be higher than estimated in these studies, as rigorous enrolment and follow-up protocols in clinical trials are unlikely to be fully replicated in everyday practice (15). An important issue is the possibility of early identification of patients who would not benefit from first-line anti-VEGF therapy.
VEGF is significantly higher in all types of DME than that in the eyes of non-diabetes patients, indicating that VEGF is equally important for any morphological changes in eyes with DME (16). However, evidence indicates that bioactive factors such as cytokines are also released into the retina. The proposed pathophysiology of each type is quite different; thus, each DME type has its own morphological and topographic characteristics (17). interleukin (IL)-6, a pro-inflammatory cytokine, intraocular levels of IL-6 were significantly higher in eyes with SRD than in eyes with DRT or CME, implying active inflammation. A recent study showed a better response to dexamethasone implants in eyes with SRD (9). The predictive value of SRD at baseline for the treatment response to anti-VEGF agents in DME remains controversial. Although some studies reported a significant improvement in VA in patients with SRD at baseline (18, 19), others found no difference or even worse functional results (20, 21). Univariate analysis to assess whether SRD is responsive to anti-VEGF agents in the current study showed that SRD significantly responded to anti-VEGF agents. However, in the multivariate survival analysis, treatment response to anti-VEGF regimens was not a significant influencing factor in patients with SRD. This can happen when SRD and other covariates are highly correlated. Furthermore, additional variables (ex. ERM) may explain more of the variance in the outcome variable, and thus reduce the impact of the initially significant of SRD.
In DME, the concentration of intraocular VEGF is significantly correlated with IL-6 levels (16). Anti-VEGF therapy reduces intraocular subclinical inflammation, and the aqueous humor concentration of IL-6 is decreased after anti-VEGF treatment (22). This could explain the response to anti-VEGF therapy in eyes with SRD in the current study. Our result was also consistent with that in the study by Sophie et al. (18) who reported that suppression of VEGF effectively eliminated subretinal fluid. Future prospective comparative investigations of the efficacy of anti-VEGF and of dexamethasone implants in eyes with SRD are required to optimize patient management.
In the current study, 45.8% of all patients presented with ONL cysts, and the majority had large ONL cysts (100–200 µm), mainly occurring at a relatively late stage of the disease. We found that large ONL cysts at baseline are less likely to be refractory group after anti-VEGF treatment at 4 months. Previous studies have reported that large ONL cysts negatively affect macular function and are predictive of worse VA outcomes after anti-VEGF therapy (23, 24). Elevated VEGF levels in DR affect the inner BRB, leading to increased vascular permeability, decreased osmotic gradient, extracellular fluid accumulation, and cyst formation (25). Furthermore, liquefaction necrosis of Müller cells and related inflammatory factors result in fluid accumulation in the cystic space (17). However, unlike SRD, IL-6 and IL-8 levels were not significantly increased in eyes with cystic changes (16). This indicated that the eye may not be in an active inflammatory state; rather, it could be a remnant of a previous inflammatory reaction (16). Anti-VEGF agents have been shown to decrease permeability and improve inner BRB by interacting with junctional proteins in the vascular endothelium (23). Our results support this finding and are consistent with a recent report that throughout anti-VEGF treatment, significant regression of ONL cysts accompanied notable improvement of macular function with a substantial decrease in their size (23). Nevertheless, we could not find an association between large ONL cysts and a mean reduction of CMT after anti-VEGF treatment at four months. Furthermore, only 3 eyes presented with giant ONL cysts (>200 µm), and we were unable to find an association between treatment outcomes and anti-VEGF agents in patients with giant ONL cysts.
The pathogenesis of DRT involves the persistent breakdown of the inner BRB and impairment of fluid absorption by Müller cells (17). DRT can be localized or more diffusely encompass the macula. Previous studies have reported that intravitreal anti-VEGF therapy is more effective for the DRT type than for the other types of DME (20, 26). Nevertheless, no study assessed whether the degree of the DRT would interfere with the treatment response or not. To clarify the relationship between DRT and response to anti-VEGF treatment, we first qualitatively evaluated the width of the DRT on a standard horizontal 6-mm B-scan OCT centered through the fovea and further stratified it into three subgroups (≤ 1, 1–3, and 3–6 mm). The results showed that the width of DRT was inversely proportional to the odds of poor response. Specifically, there was a trend indicating that the degree of DRT was proportionally associated with better response, although this was not significant in multivariate analysis.
There is a high incidence of vitreomacular interface abnormality (VMIA) among DME patients (27). The current study found a 20.8% incidence of ERM in our cohort. Although DR and its severity are risk factors for developing secondary ERM (27, 28), cases of VMIA are excluded from major clinical trials, even though DME is associated with this condition in 25% of patients (29). Nevertheless, knowledge regarding the effect of VMIA on the response to anti-VEGF treatment in patients with DME has not been thoroughly investigated. Ercalik et al. retrospectively evaluated 56 eyes with or without ERM and found a negative effect of ERM on intravitreal anti-VEGF treatment (30). Wong et al. conducted a prospective study of 104 eyes with DME treated with anti-VEGF and found that ERM was associated with a worsened visual and anatomic response (31). Notably, neither study considered other OCT biomarkers; thus, the findings might not completely represent the true impact of ERM in eyes with DME.
Considering the diversity of OCT morphology in DME, we included various OCT biomarker characteristics and considered ERM as a variable in eyes with refractory DME, despite anti-VEGF therapy. Furthermore, we used a multivariate statistical model to analyze the association between treatment response and each OCT biomarker. Our results showed that ERM significantly increased the odds of poor response. According to previous study on ERM pathology in diffuse DME, multilayered membranes are mainly composed of hyalocytes and myofibroblasts. Hyalocytes were shown to produce VEGF and can transdifferentiate into myofibroblasts, known for their contractive properties (32). Furthermore, contraction of the ERM may cause perifoveal capillary leakage and aggravate macular edema. It has also been demonstrated that glial cells in ERM produce various cytokines and growth factors. VEGF and its receptors, as well as IL-6, are localized to cells in the ERM of patients with DR, thus further increasing inflammation and possibly promoting DME persistence (30, 33). Furthermore, ERM may act as a physical barrier and decrease drug penetration after intravitreal injections of anti-VEGF in DME treatment (34).
The connective tissue growth factor (CTGF) is one of the most potent profibrotic factors. It can stimulate fibroblast proliferation and collagen deposition, resulting in fibrosis (35). Anti-VEGF has been reported to cause hypoxia in vascular endothelial cells and increase CTGF expression, which plays an important role in ERM formation (36). As a result, anti-VEGF therapy may potentially aggravate ERM contractions and interfere with the resolution of macular edema in diabetes. These results may explain the increased likelihood of poor response in this group. Nevertheless, consistent with the guidelines for DME management by retinal specialists, PPV is currently recommended as a therapeutic option in cases of DME associated with VMT (37). In the absence of traction formation, there is no consensus on the role of PPV in the actual treatment of diabetic eyes. Our results call for further comparative studies and treatment modalities other than anti-VEGF in DME patients presenting with ERM-impaired visual and anatomic outcomes.
HRF represents subclinical lipoproteins that extravasate after inner BRB breakdown. It is initially present in the inner retinal layers and subsequently migrates to the outer retinal layers (38). HRF is an important imaging marker for retinal inflammation (39). However, the predictive value of HRF for visual outcomes after anti-VEGF treatment in DME is unclear (9). In our study, we did not find that the presence of HRF was associated with treatment response after anti-VEGF therapy. The integrity of outer retinal layers is a direct indicator of the health of the retinal photoreceptors and retinal pigment epithelium. IS-OS integrities is an important factor for predicting VA after treatment. Subjects with long-standing DME may demonstrate focal or diffuse loss of integrity of the IS-OS junction. Previous studies have reported that IS-OS integrity can be expected to recover after anti-VEGF therapy (17, 23). Our results support this finding and show a better response to anti-VEGF therapy in eyes with partially continuous IS-OS layers.
Our study has some limitations. First, its retrospective and non-randomized design precluded a well-matched control enrollment. Second, the sample size was small, which may have hindered the significance of the results. Third, we prescribed three anti-VEGF agents to treat DME in the real-world clinical practice setting. Although most eyes were treated with ranizucimumab (83.3%), we did not assess each anti-VEGF regimen separately, and thus, the different efficacy between each agent may not have been accounted. Fourth, in our study, despite the odds of gaining BCVA ≥10 letters trended toward the OCT predictive value of CMT reduction, no OCT biomarkers showed significant predictive value for good BCVA response at 4 months. We found that the OCT predictive value of CMT reduction cannot fully translate into the change of VA, which was consistent with the study of a post hoc analysis of the protocol T randomized clinical trial (40). They found changes in CMT appear to account for only a small proportion of the total variation in changes in BCVA, and concluded that changes in CMT cannot support as a surrogate for changes in VA in evaluating anti-VEGF for DME. Despite these limitations, an important strength of our study is that we included various common OCT markers in patients with DME, which yielded ample information and helped us tailor timely and individualized treatment during daily practice.
In conclusion, partial IS-OS continuity is the marker that predicts better response to anti-VEGF treatment in eyes with DME. In contrast, the presence of ERM is a significant predictor of poor response. Our results raise the pertinent issue that DME patients with ERM are significant poor responders to anti-VEGF therapy and may benefit more from other therapeutic approaches.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Review Board of the Research Ethics Committee of Hualien Tzu-Chi Hospital and Buddhist Tzu-Chi Medical Foundation. The patients/participants provided their written informed consent to participate in this study.
Author contributions
T-CH and M-SH had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: M-SH. Acquisition, analysis, or interpretation of data: T-CH, G-HD, Y-CC, and M-SH. Drafting of the manuscript: T-CH, G-HD, and M-SH. Critical revision of the manuscript for important intellectual content: T-CH, F-LC, and M-SH. Statistical analysis: T-CH and G-HD. Administrative, technical, or material support: G-HD, M-SH, and Y-CC. Supervision: T-CH, M-SH. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors thank the Tzu Chi University (TCU) Research Center for Big Data Teaching, Research and Statistic Consultation for providing statistic consultation assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kempen JH, O’Colmain BJ, Leske MC, Haffner SM, Klein R, Moss SE, et al. The prevalence of diabetic retinopathy among adults in the united states. Arch Ophthalmol (Chicago Ill: 1960). (2004) 122(4):552–63. doi: 10.1001/archopht.122.4.552
2. White NH, Sun W, Cleary PA, Tamborlane WV, Danis RP, Hainsworth DP, et al. Effect of prior intensive therapy in type 1 diabetes on 10-year progression of retinopathy in the DCCT/EDIC: comparison of adults and adolescents. Diabetes (2010) 59(5):1244–53. doi: 10.2337/db09-1216
3. Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin epidemiologic study of diabetic retinopathy. XV. the long-term incidence of macular edema. Ophthalmology (1995) 102(1):7–16. doi: 10.1016/s0161-6420(95)31052-4
4. Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. New Engl J Med (1994) 331(22):1480–7. doi: 10.1056/NEJM199412013312203
5. Massin P, Bandello F, Garweg JG, Hansen LL, Harding SP, Larsen M, et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care (2010) 33(11):2399–405. doi: 10.2337/dc10-0493
6. Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P, Schlingemann RO, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology (2011) 118(4):615–25. doi: 10.1016/j.ophtha.2011.01.031
7. Bressler SB, Ayala AR, Bressler NM, Melia M, Qin H, Ferris FL 3rd, et al. Persistent macular thickening after ranibizumab treatment for diabetic macular edema with vision impairment. JAMA ophthalmol (2016) 134(3):278–85. doi: 10.1001/jamaophthalmol.2015.5346
8. Gonzalez VH, Campbell J, Holekamp NM, Kiss S, Loewenstein A, Augustin AJ, et al. Early and long-term responses to anti-vascular endothelial growth factor therapy in diabetic macular edema: analysis of protocol I data. Am J ophthalmol (2016) 172:72–9. doi: 10.1016/j.ajo.2016.09.012
9. Zur D, Iglicki M, Busch C, Invernizzi A, Mariussi M, Loewenstein A. OCT biomarkers as functional outcome predictors in diabetic macular edema treated with dexamethasone implant. Ophthalmology (2018) 125(2):267–75. doi: 10.1016/j.ophtha.2017.08.031
10. Busch C, Zur D, Fraser-Bell S, Laíns I, Santos AR, Lupidi M, et al. Shall we stay, or shall we switch? continued anti-VEGF therapy versus early switch to dexamethasone implant in refractory diabetic macular edema. Acta Diabetol (2018) 55(8):789–96. doi: 10.1007/s00592-018-1151-x
11. Dugel PU, Campbell JH, Kiss S, Loewenstein A, Shih V, Xu X, et al. ASSOCIATION BETWEEN EARLY ANATOMIC RESPONSE TO ANTI-VASCULAR ENDOTHELIAL GROWTH FACTOR THERAPY AND LONG-TERM OUTCOME IN DIABETIC MACULAR EDEMA: an independent analysis of protocol i study data. Retina (Philadelphia Pa) (2019) 39(1):88–97. doi: 10.1097/IAE.0000000000002110
12. Blonde L, Khunti K, Harris SB, Meizinger C, Skolnik NS. Interpretation and impact of real-world clinical data for the practicing clinician. Adv Ther (2018) 35(11):1763–74. doi: 10.1007/s12325-018-0805-y
13. Das A, McGuire PG, Rangasamy S. Diabetic macular edema: pathophysiology and novel therapeutic targets. Ophthalmology (2015) 122(7):1375–94. doi: 10.1016/j.ophtha.2015.03.024
14. Brown DM, Nguyen QD, Marcus DM, Boyer DS, Patel S, Feiner L, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology (2013) 120(10):2013–22. doi: 10.1016/j.ophtha.2013.02.034
15. Egan C, Zhu H, Lee A, Sim D, Mitry D, Bailey C, et al. The united kingdom diabetic retinopathy electronic medical record users group, report 1: baseline characteristics and visual acuity outcomes in eyes treated with intravitreal injections of ranibizumab for diabetic macular oedema. Br J ophthalmol (2017) 101(1):75–80. doi: 10.1136/bjophthalmol-2016-309313
16. Sonoda S, Sakamoto T, Yamashita T, Shirasawa M, Otsuka H, Sonoda Y. Retinal morphologic changes and concentrations of cytokines in eyes with diabetic macular edema. Retina (Philadelphia Pa) (2014) 34(4):741–8. doi: 10.1097/IAE.0b013e3182a48917
17. Seo KH, Yu SY, Kim M, Kwak HW. Visual and morphologic outcomes of intravitreal ranibizumab for diabetic macular edema based on optical coherence tomography patterns. Retina (Philadelphia Pa). (2016) 36(3):588–95. doi: 10.1097/IAE.0000000000000770
18. Sophie R, Lu N, Campochiaro PA. Predictors of functional and anatomic outcomes in patients with diabetic macular edema treated with ranibizumab. Ophthalmology (2015) 122(7):1395–401. doi: 10.1016/j.ophtha.2015.02.036
19. Fickweiler W, Schauwvlieghe AME, Schlingemann RO, Maria Hooymans JM, Los LI, Verbraak FD. Predictive value of optical coherence tomographic features in the bevacizumab and ranibizumab in patients with diabetic macular edema (brdme) study. Retina (Philadelphia Pa). (2018) 38(4):812–9. doi: 10.1097/IAE.0000000000001626
20. Shimura M, Yasuda K, Yasuda M, Nakazawa T. Visual outcome after intravitreal bevacizumab depends on the optical coherence tomographic patterns of patients with diffuse diabetic macular edema. Retina (Philadelphia Pa) (2013) 33(4):740–7. doi: 10.1097/IAE.0b013e31826b6763
21. Giocanti-Aurégan A, Hrarat L, Qu LM, Sarda V, Boubaya M, Levy V, et al. Functional and anatomical outcomes in patients with serous retinal detachment in diabetic macular edema treated with ranibizumab. Invest Ophthalmol Visual sci (2017) 58(2):797–800. doi: 10.1167/iovs.16-20855
22. Sakamoto S, Takahashi H, Tan X, Inoue Y, Nomura Y, Arai Y, et al. Changes in multiple cytokine concentrations in the aqueous humour of neovascular age-related macular degeneration after 2 months of ranibizumab therapy. Br J Ophthalmol (2018) 102(4):448–54. doi: 10.1136/bjophthalmol-2017-310284
23. Reznicek L, Cserhati S, Seidensticker F, Liegl R, Kampik A, Ulbig M, et al. Functional and morphological changes in diabetic macular edema over the course of anti-vascular endothelial growth factor treatment. Acta ophthalmol (2013) 91(7):e529–36. doi: 10.1111/aos.12153
24. Deák GG, Bolz M, Ritter M, Prager S, Benesch T, Schmidt-Erfurth U. A systematic correlation between morphology and functional alterations in diabetic macular edema. Invest Ophthalmol Visual sci (2010) 51(12):6710–4. doi: 10.1167/iovs.09-5064
25. Fine BS, Brucker AJ. Macular edema and cystoid macular edema. Am J Ophthalmol (1981) 92(4):466–81. doi: 10.1016/0002-9394(81)90638-3
26. Usui-Ouchi A, Tamaki A, Sakanishi Y, Tamaki K, Mashimo K, Sakuma T, et al. Factors affecting a short-term response to anti-VEGF therapy in diabetic macular edema. Life (Basel Switzerland) (2021) 11(2). doi: 10.3390/life11020083
27. Ghazi NG, Ciralsky JB, Shah SM, Campochiaro PA, Haller JA. Optical coherence tomography findings in persistent diabetic macular edema: the vitreomacular interface. Am J Ophthalmol (2007) 144(5):747–54. doi: 10.1016/j.ajo.2007.07.012
28. Cheung N, Tan SP, Lee SY, Cheung GCM, Tan G, Kumar N, et al. Prevalence and risk factors for epiretinal membrane: the Singapore epidemiology of eye disease study. Br J Ophthalmol (2017) 101(3):371–6. doi: 10.1136/bjophthalmol-2016-308563
29. Akbar Khan I, Mohamed MD, Mann SS, Hysi PG, Laidlaw DA. Prevalence of vitreomacular interface abnormalities on spectral domain optical coherence tomography of patients undergoing macular photocoagulation for centre involving diabetic macular oedema. Br J Ophthalmol (2015) 99(8):1078–81. doi: 10.1136/bjophthalmol-2014-305966
30. Ercalik NY, Imamoglu S, Kumral ET, Yenerel NM, Bardak H, Bardak Y. Influence of the epiretinal membrane on ranibizumab therapy outcomes in patients with diabetic macular edema. Arquivos brasileiros oftalmol (2016) 79(6):373–5. doi: 10.5935/0004-2749.20160106
31. Wong Y, Steel DHW, Habib MS, Stubbing-Moore A, Bajwa D, Avery PJ, et al. Vitreoretinal interface abnormalities in patients treatedwith ranibizumab for diabetic macular oedema. Graefe’s Arch Clin Exp Ophthalmol (2017) 255(4):733–42. doi: 10.1007/s00417-016-3562-0
32. Hagenau F, Vogt D, Ziada J, Guenther SR, Haritoglou C, Wolf A, et al. Vitrectomy for diabetic macular edema: optical coherence tomography criteria and pathology of the vitreomacular interface. Am J Ophthalmol (2019) 200:34–46. doi: 10.1016/j.ajo.2018.12.004
33. Harada C, Mitamura Y, Harada T. The role of cytokines and trophic factors in epiretinal membranes: involvement of signal transduction in glial cells. Prog retinal eye Res (2006) 25(2):149–64. doi: 10.1016/j.preteyeres.2005.09.001
34. Namba R, Kaneko H, Suzumura A, Shimizu H, Kataoka K, Takayama K, et al. In vitro epiretinal membrane model and antibody permeability: relationship with anti-VEGF resistance in diabetic macular edema. Invest Ophthalmol Visual sci (2019) 60(8):2942–9. doi: 10.1167/iovs.19-26788
35. Kita T, Hata Y, Kano K, Miura M, Nakao S, Noda Y, et al. Transforming growth factor-β2 and connective tissue growth factor in proliferative vitreoretinal diseases: possible involvement of hyalocytes and therapeutic potential of rho kinase inhibitor. Diabetes (2007) 56(1):231–8. doi: 10.2337/db06-0581
36. Marticorena J, Romano MR, Heimann H, Stappler T, Gibran K, Groenewald C, et al. Intravitreal bevacizumab for retinal vein occlusion and early growth of epiretinal membrane: a possible secondary effect? Br J Ophthalmol (2011) 95(3):391. doi: 10.1136/bjo.2009.177287
37. Schmidt-Erfurth U, Garcia-Arumi J, Bandello F, Berg K, Chakravarthy U, Gerendas BS, et al. Guidelines for the management of diabetic macular edema by the European society of retina specialists (EURETINA). Ophthalmol J Int d’ophtalmol Int J Ophthalmol Z fur Augenheilkunde (2017) 237(4):185–222. doi: 10.1159/000458539
38. Markan A, Agarwal A, Arora A, Bazgain K, Rana V, Gupta V. Novel imaging biomarkers in diabetic retinopathy and diabetic macular edema. Ther Adv ophthalmol (2020) 12:2515841420950513. doi: 10.1177/2515841420950513
39. Midena E, Pilotto E, Bini S. Hyperreflective intraretinal foci as an OCT biomarker of retinal inflammation in diabetic macular edema. Invest Ophthalmol Visual sci (2018) 59(13):5366. doi: 10.1167/iovs.18-25611
40. Bressler NM, Odia I, Maguire M, Glassman AR, Jampol LM, MacCumber MW, et al. Association between change in visual acuity and change in central subfield thickness during treatment of diabetic macular edema in participants randomized to aflibercept, bevacizumab, or ranibizumab: a Post hoc analysis of the protocol T randomized clinical trial. JAMA Ophthalmol (2019) 137(9):977–85. doi: 10.1001/jamaophthalmol.2019.1963
Keywords: diabetic macular edema, anti-vegf, optical coherence tomography, epiretinal membrane, diabetic retinopathy
Citation: Hsieh T-C, Deng G-H, Chang Y-C, Chang F-L and He M-S (2023) A real-world study for timely assessing the diabetic macular edema refractory to intravitreal anti-VEGF treatment. Front. Endocrinol. 14:1108097. doi: 10.3389/fendo.2023.1108097
Received: 25 November 2022; Accepted: 08 May 2023;
Published: 17 May 2023.
Edited by:
Rajashekhar Gangaraju, University of Tennessee Health Science Center (UTHSC), United StatesReviewed by:
Guoqiang Zeng, Shenzhen University General Hospital, ChinaCarmen Clapp, National Autonomous University of Mexico, Mexico
Copyright © 2023 Hsieh, Deng, Chang, Chang and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming-Shan He, bWluZ3NoYW5oZXJAZ21haWwuY29t
 Tsung-Cheng Hsieh1
Tsung-Cheng Hsieh1 Ming-Shan He
Ming-Shan He