
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 16 February 2023
Sec. Molecular and Structural Endocrinology
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1103949
This article is part of the Research Topic Estrogen Receptors in Endocrine Disorders View all 6 articles
Background: To assess the practice patterns of the recurrence score (RS) based on the 21-gene expression assay on adjuvant chemotherapy recommendations and survival outcomes in estrogen receptor-positive (ER+)/HER2- breast cancer (BC) with one to three positive lymph nodes (N1).
Methods: We included patients with T1-2N1M0 and ER+/HER2- BC diagnosed between 2010 and 2015 in the Surveillance, Epidemiology, and End Results Oncotype DX Database. Breast cancer-specific survival (BCSS) and overall survival (OS) were assessed.
Results: We included 35,137 patients in this study. There were 21.2% of patients who had RS testing in 2010, which was significantly increased to 36.8% in 2015 (P < 0.001). Performance of the 21-gene testing was associated with older age, lower tumor grade, T1 stage, lower number of positive lymph nodes, and progesterone receptor-positive disease (all P < 0.05). In those without 21-gene testing, age was the main factor significantly related to the receipt of chemotherapy, whereas RS was the main factor significantly related to chemotherapy receipt in those with 21-gene testing. The probability of chemotherapy receipt in those without 21-gene testing was 64.1% and was decreased to 30.8% in those with 21-gene testing. On multivariate prognostic analysis, the performance of 21-gene testing was associated with better BCSS (P < 0.001) and OS (P < 0.001) compared with those without 21-gene testing. Similar results were found after propensity score matching.
Conclusions: The 21-gene expression assay is frequently and increasingly used for chemotherapy decision-making in ER+/HER2- BC with N1 disease. Performance of the 21-gene testing is associated with improved survival outcomes. Our study supports the routine use of 21-gene testing in the clinical practice of this population.
Breast cancer (BC) is the most common malignancy diagnosed among women, with approximately 2.2 million new cases annually (1). With the improvement of diagnosis and screening technology, there were 64.2% of the patients who had a node-negative (N0) disease at BC diagnosis, but 35.8% of the patients still had a node-positive (N+) disease. Among those with N+ diseases, 84.5%, 10.4%, and 5.1% had N1, N2, and N3 diseases, respectively (2). More than 70% of BC patients are hormone receptor (HR)-positive (2). For HR-positive early BC with metastasis (N1) of one to three lymph nodes (LNs), endocrine therapy after surgery is the standard treatment strategy, but the role of chemotherapy in this population remains controversial (3, 4). However, approximately 15% of patients with N+ and HR+/HER2- BC receiving endocrine therapy develop tumor recurrence within 5 years of initiating treatment, indicating a requirement for developing novel treatment strategies in this patient subset (5). With increasing knowledge regarding the molecular heterogeneity of BC, the recurrence score (RS) based on the 21-gene expression assay has revolutionized the chemotherapy decision-making in N0 and estrogen receptor-positive (ER+) BC patients (6). The value of RS in N0 and ER+/HER2- early-stage BC has been validated for the first time in a landmark study, indicating that those with RS ≥31 would benefit from additional adjuvant chemotherapy but those with RS <18 would not (7). However, the RS testing in the N+ BC patients has been limited but is steadily increasing, particularly following the recent findings from the data of RxPONDER (8).
For patients with N1 BC, whether treatment decisions can be made according to the results of the 21-gene RS, the current treatment guidelines from the American Society of Clinical Oncology (ASCO) and the National Comprehensive Cancer Network (NCCN) have contradictory opinions (4, 9). However, several previous studies have found that the 21-gene RS could also predict the survival of N1 patients and have an impact on treatment decisions (8, 10–13). In Canada, the 21-gene RS has been publicly funded for patients with N0 and ER+/HER2- BC for several years, whereas the coverage of RS testing for N+ patients has been very limited (14). Given emerging data on the role of RS and the recent discrepant treatment in these patients, this study aimed to clarify the practice patterns and the potential benefit of 21-gene testing in BC patients with ER+/HER2− and one to three LNs.
We identified patients who were diagnosed with BC from the Surveillance, Epidemiology, and End Results (SEER) Oncotype DX Database (2004–2016) with or without SES/Rurality (15), which includes 21-gene RS data for invasive BC patients diagnosed from 2004 to 2015. Patients included in this database had follow-up through the end of 2016. We limited this analysis to pathologic stage T1-2N1M0 and ER+/HER2- BC with the availability of RS diagnosed between 2010 and 2015 because the data regarding HER2 status were only included from the year 2010. We excluded those with missing surgery and radiotherapy information and those with missing tumor grade, progesterone receptor (PR) status, and insurance status. This study did not require institutional review board approval because the data in the SEER program were deidentified.
The following variables were included in the analysis: age, race, tumor grade, histology, tumor (T) stage, PR status, number of positive LNs, surgical procedure, and the receipt of radiotherapy, chemotherapy, and RS testing. Patients who underwent 21-gene testing were further stratified into low (RS <18), intermediate (RS 18–30), and high (RS >30) groups (6). We used the above pre-TAILORx RS categories because the patients included in this study were treated before the publication of the TAILORx study (16). The primary outcome measures were breast cancer-specific survival (BCSS) and overall survival (OS).
Patients’ baseline characteristics were compared according to the receipt of the 21-gene testing using chi-square tests. Multiple logistic regression models were used to determine the predictive factors related to the use of RS testing and chemotherapy. A multicollinearity test was used to assess the data collected. The Kaplan–Meier method was used to assess differences in BCSS and OS and compared by the log-rank test. Multivariable Cox proportional hazards models were performed to determine independent prognostic factors impacting BCSS and OS. A 1:1 propensity score matching (PSM) was used to balance the potential confounders. Statistical analyses were conducted using the SPSS version 25.0. A P value less than 0.05 was considered statistically significant.
We included 35,137 patients in this study (Figure 1 and Table 1). Of these patients, 69.6% (n = 24,466) were non-Hispanic white, 87.5% (n = 30,733) were invasive ductal carcinoma, and 89.5% (n = 31458) were PR positive. There were 23,222 (66.1%), 8,234 (23.4%), and 3,681 (10.5%) patients who had one-, two-, and three-LN metastases, respectively. Regarding treatment, 50.5% (n = 17,762), 55.7% (n = 19,572), and 54.4% (n = 19,118) patients received mastectomy, radiotherapy, and chemotherapy, respectively.
There were 29.2% (n = 10,247) of patients who had RS testing. Of these patients, the median RS was 15 (range, 0–67). In those with RS testing, there were 6,265 (61.1%), 3,405 (33.2%), and 577 (5.6%) patients who had low, intermediate, and high RSs, respectively. The proportion of RS testing over time is listed in Figure 2. There were 21.2% of patients who had RS testing in 2010, which was significantly increased to 36.8% in 2015 (P < 0.001). Moreover, patients aged 50–64 years; are non-Hispanic white; had a lower tumor grade, T1 stage, one positive LN, PR positive; receiving BCS (P < 0.001); and receiving RT were more likely to have RS testing (all P < 0.001) (Table 1). Moreover, patients receiving RS testing were less likely to receive chemotherapy compared with those that did not receive RS testing (30.8% vs. 64.1%, P < 0.001).
Multicollinearity was checked for all predictors by tolerance analysis. All of the predictors’ tolerance was above the cutoff of 0.10 (ranging between 0.918 and 0.980), suggesting that there was no risk of multicollinearity. The predictive factors associated with 21-gene testing were then identified using binomial logistic regression. The results showed that older age, non-Hispanic white, invasive ductal carcinoma subtype, lower tumor grade, T1 stage, lower number of positive LNs, and PR positive disease were the independent predictive factors associated with the receipt of 21-gene testing (all P < 0.05) (Table 2). Those with three positive LNs had the lowest chance of RS testing, with an odds ratio (OR) of 0.307 compared with those with one positive LN (95% CI 0.277–0.339, P < 0.001). There were 34.8%, 20.6%, and 12.7% of patients with one, two, and three positive LNs who had RS testing, respectively (P < 0.001).
In our study, the proportion of patients who did not undergo 21-gene testing who received chemotherapy was 64.1% and the proportion of patients who received 21-gene testing was 30.8%. Two multivariate logistic regression models were used to determine the predictive factors related to the use of chemotherapy (Table 3). The first model included patients without 21-gene testing, and the results showed that younger age, non-Hispanic white, other histological subtypes, higher tumor grade, T2 stage, a higher number of positive LNs, and PR negative diseases were the independent predictive factors associated with the use of chemotherapy (all P < 0.05). Patients aged <50 years had the highest chance of chemotherapy receipt, with an OR of 11.285 compared with those aged ≥65 years (95% confidence interval [CI] 11.359–12.294, P < 0.001). Patients aged 50–64 years were also more likely to receive chemotherapy compared with those aged ≥65 years (OR 6.038, 95% CI 5.653–6.450, P < 0.001). There were 86.2%, 76.5%, and 35.6% of patients aged <50, 50–64, and ≥65 years receiving chemotherapy, respectively (P < 0.001).
The second model included patients with 21-gene testing; the results showed that younger age, higher tumor grade, T2 stage, a higher number of positive LNs, PR negative, and higher RS were the independent predictive factors associated with the use of chemotherapy (all P < 0.05). Patients with high RS had the highest chance of chemotherapy receipt, with an OR of 13.846 compared with those with low RS (95% CI 10.992–17.441, P < 0.001). Patients with intermediate RS were also more likely to receive chemotherapy compared with those with low RS (OR 3.821, 95% CI 3.454–4.228, P < 0.001). There were 18.0% (n = 1,130), 46.3% (n = 1,576), and 78.5% (n = 453) of patients with low, intermediate, and high RS receiving chemotherapy, respectively (P < 0.001) (Figure 3).
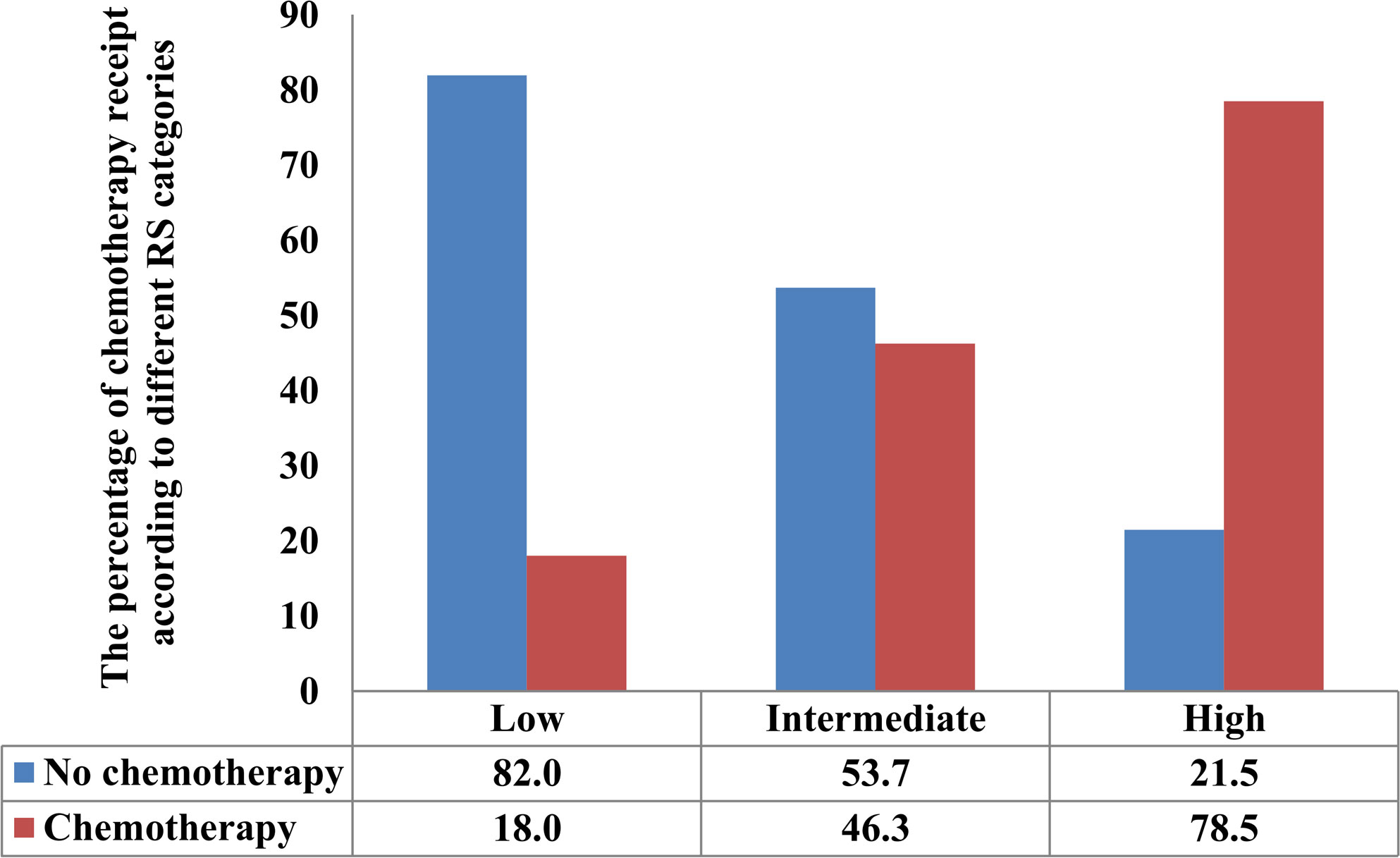
Figure 3 The percentage of receipt of chemotherapy according to different recurrence score categories.
The median follow-up of this study was 32 months (range, 0–71 months). A total of 1,938 deaths occurred, including 811 patients who died from BC. Overall, the survival outcomes were excellent in the entire cohort. The 3-year BCSS and OS were 95.0% and 97.8%, respectively. The multivariate prognostic analysis showed that patients who received 21-gene testing had better BCSS (hazard ratio [HR] 0.506, 95% CI 0.406–0.632, P < 0.001) and OS (HR 0.602, 95% CI 0.457–0.793, P < 0.001) than those without 21-gene testing (Tables 4, 5). The 3-year BCSS was 99.2% and 97.4% in those with and without 21-gene testing, respectively (P < 0.001) (Figure 4A). The 3-year OS was 97.3% and 93.6% in those with and without 21-gene testing, respectively (P < 0.001) (Figure 5A).
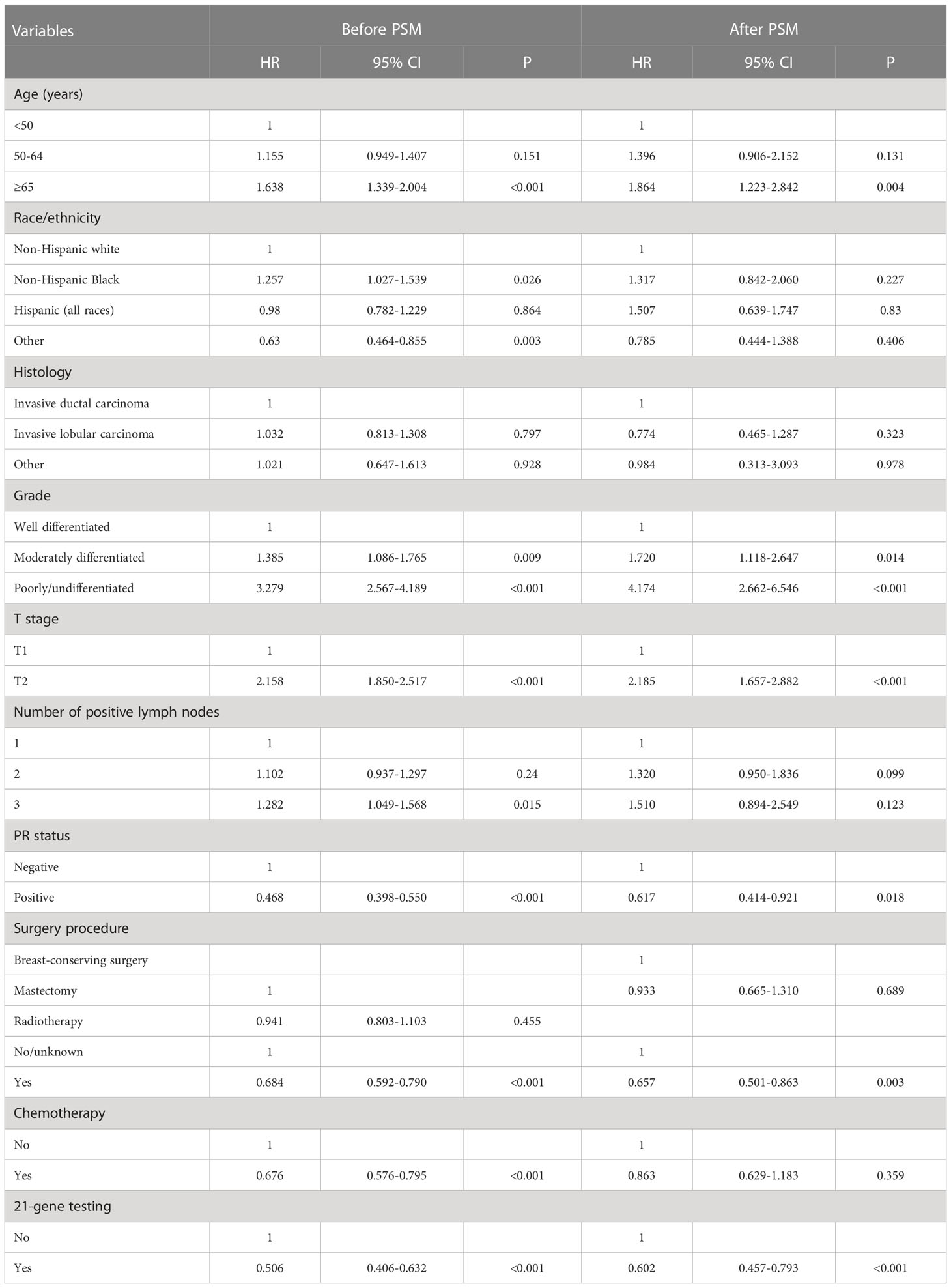
Table 4 Multivariate prognostic analysis for breast cancer-specific survival before and after propensity score matching.
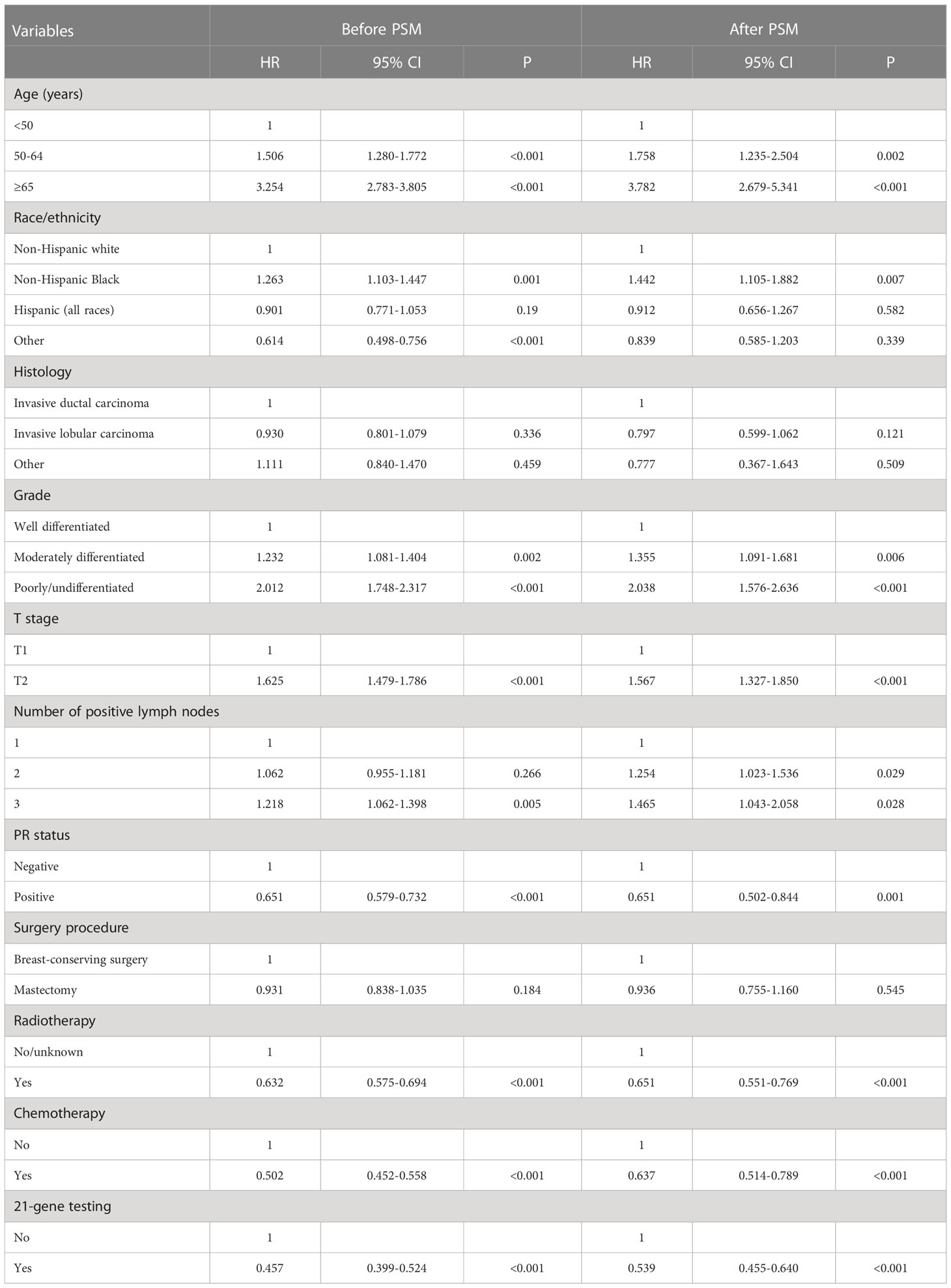
Table 5 Multivariate prognostic analysis for overall survival before and after propensity score matching.
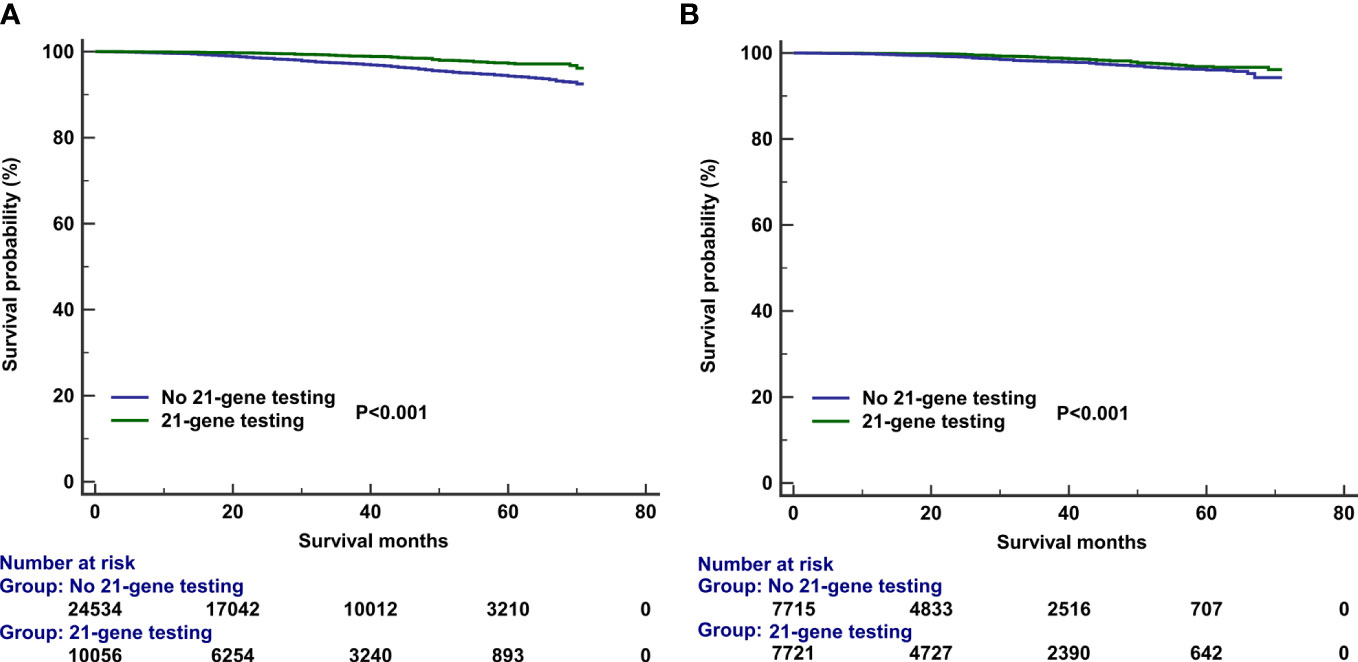
Figure 4 Kaplan–Meier curves of association of 21-gene testing with breast cancer-specific survival before (A) and after (B) propensity score matching.
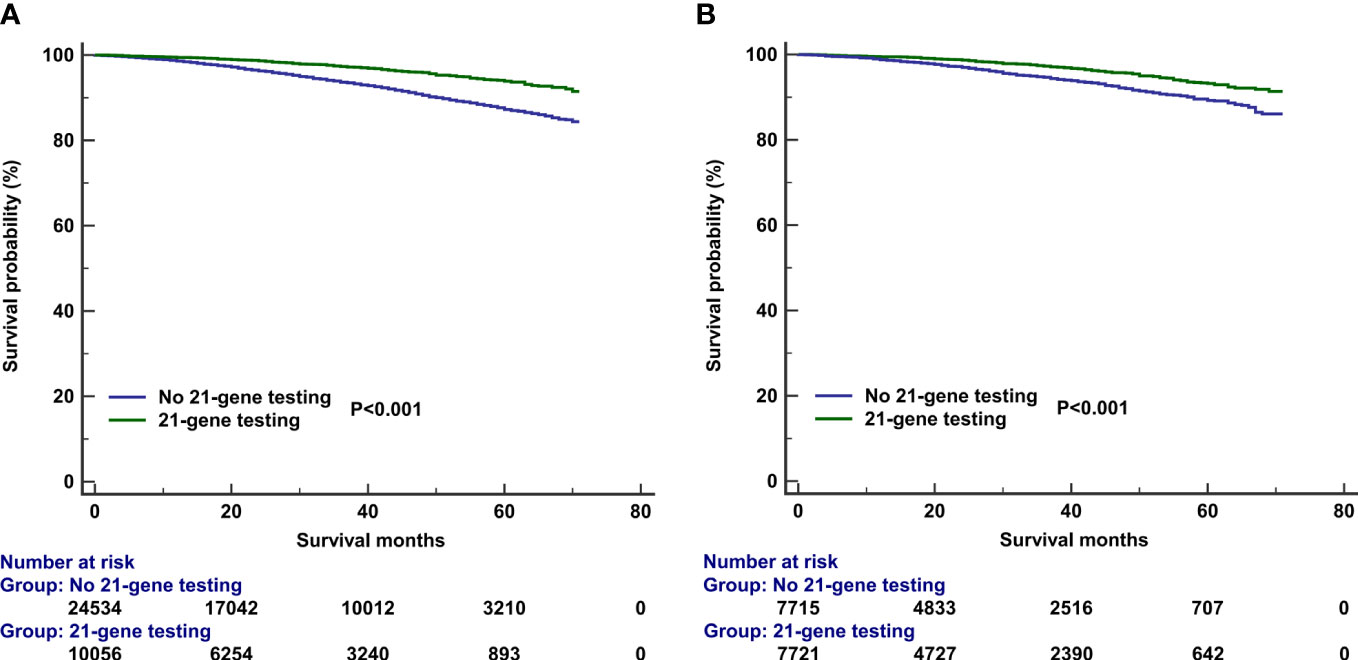
Figure 5 Kaplan–Meier curves of association of 21-gene testing with overall survival before (A) and after (B) propensity score matching.
To reduce potential selection bias, a PSM was conducted to balance the patients’ clinicopathological and therapeutic characteristics including the following variables: age, race, tumor grade, histology, T stage, number of positive LNs, PR status, surgical procedure, radiotherapy, and chemotherapy. A total of 7,878 pairs of patients were completely matched. Patients who received 21-gene testing also had better BCSS (HR 0.602, 95% CI 0.457–0.793, P < 0.001) and OS (HR 0.539, 95% CI 0.455–0.640, P < 0.001) compared with those without 21-gene testing (Tables 4, 5). Survival curves are listed in Figures 4B, 5B.
In those with 21-gene testing (n = 10,247), the multivariate prognostic analysis indicated that the RS was the independent prognostic factor associated with survival outcomes. Those with high RS had significantly lower BCSS (HR 4.158, 95% CI 2.147–8.082, P < 0.001) and OS (HR 2.079, 95% CI 1.353–3.195, P = 0.001) compared with those with low RS. In addition, patients with intermediate RS also had significantly lower BCSS (HR 2.586, 95% CI 1.604–4.169, P < 0.001) and OS (HR 1.411, 95% CI 1.085–1.836, P = 0.010) compared with those with low RS (Table 6). The survival curves according to the RS cohorts are listed in Figure 6.
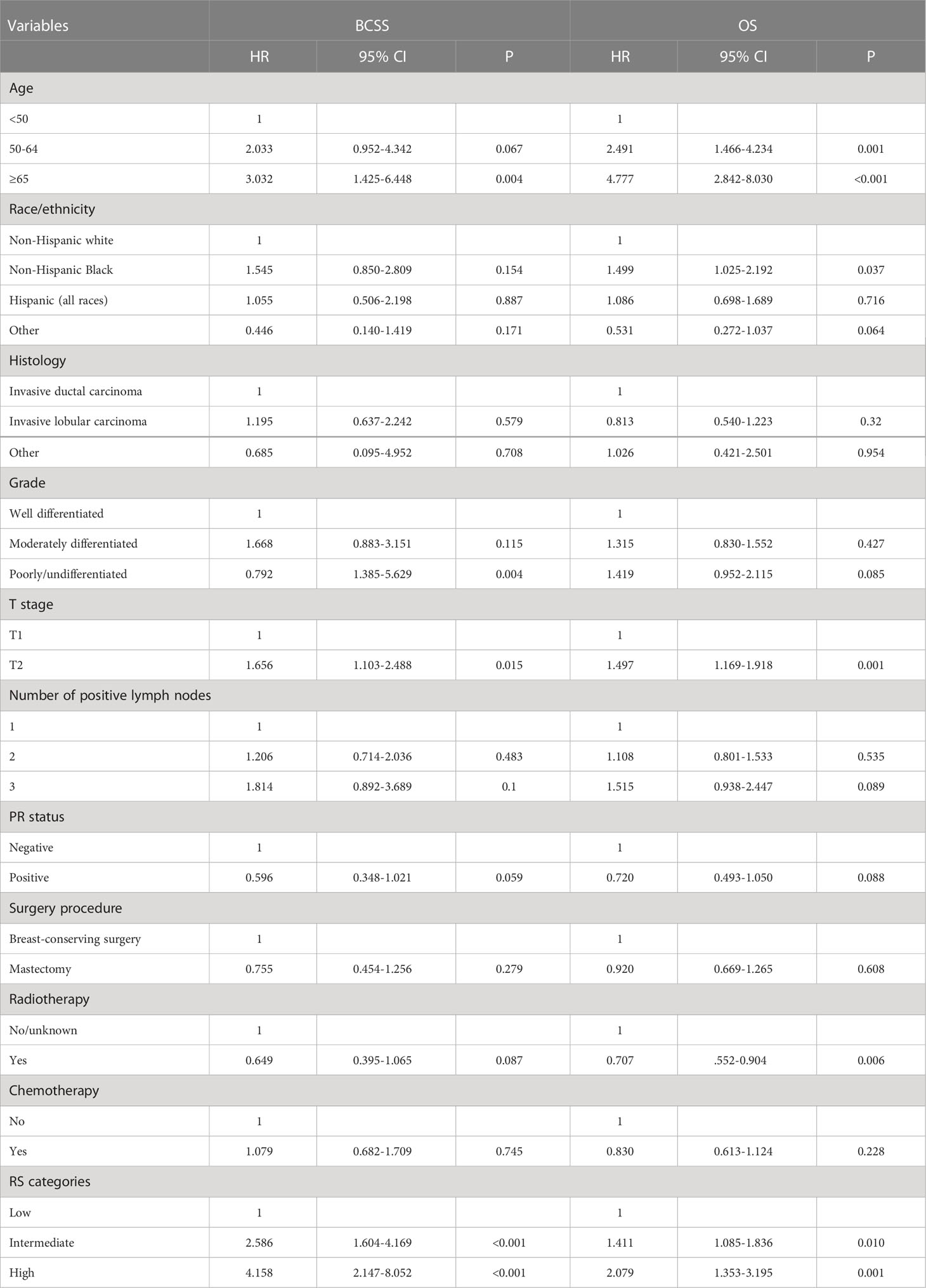
Table 6 Multivariate prognostic analysis for breast cancer-specific survival and overall survival in those with 21-gene testing.
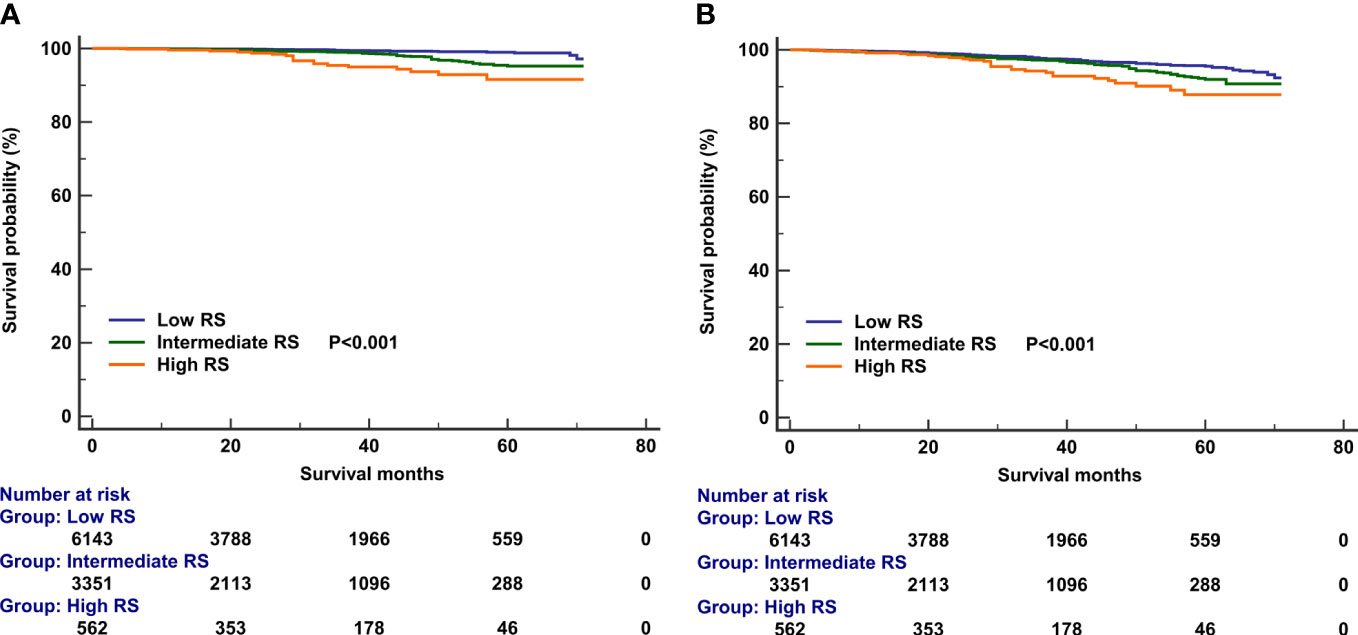
Figure 6 Kaplan–Meier curves of association of recurrence score categories with breast cancer-specific survival (A) and overall survival (B).
In the current study, a large cohort from the SEER Oncotype DX Database was used to investigate the practice patterns and the potential benefit of 21-gene testing in BC patients with ER+/HER2− and one to three positive LNs. Our results showed that adjuvant chemotherapy recommendations for this population mainly relied on genomic profiling assays in the current era. Moreover, the performance of 21-gene testing was associated with improved survival outcomes. Our findings support the routine use of RS testing in this population.
With the rapid progress in the understanding of BC biology, the current treatment of BC is mainly based on the results of genomic profiling assays. As the most widely used genomic profiling assay, 21-gene RS is the most important factor influencing the treatment strategy of N0 early-stage BC. The NCCN guidelines recommend the use of the 21-gene expression assay in the decision-making of both N0 and N+ (one to three LNs) ER+ BC (4). In contrast, the ASCO guidelines state that the clinician should not use the 21-gene expression assay to guide adjuvant chemotherapy decisions in N+ and ER+/HER2− BC patients (9). In a National Cancer Database study including 72,897 patients with N+ BC diagnosed between 2010 and 2013, the receipt of 21-gene testing was 15% in 2013 and 24% in 2013 (17). In our study, there were 21.2% of patients who had 21-gene testing in 2010, which was significantly increased to 36.8% in 2015 (P < 0.001). However, the recommendation of 21-gene testing was also lower than those with N0 disease, with 21.8% having 21-gene testing in the years 2004–2011, and nearly half of patients had 21-gene testing in 2013–2015 in real-world studies (18, 19). This may reflect the practice pattern of most clinicians prescribing chemotherapy to most patients with one to three LNs even with estrogen sensitivity.
The distribution of RS in N0 BC was 48.8%–53.7%, 38.9%–40.7%, and 7.4%–10.4% in patients who had low, intermediate, and high RS, respectively (20, 21). In our study, there were 61.1%, 33.2%, and 5.6% of patients who had low, intermediate, and high RS, respectively. Similar findings were observed in the other studies that included patients from Japan (22), Canada (23), and Israel (24), which suggested that the RS also had a similar distribution in most populations. However, a study from China showed that there were 21.4%, 53.1%, and 25.5% of patients who had low, intermediate, and high RS in those with N1 disease, respectively (11). Only 98 patients who had N1 diseases limited the study to the general population. In our previous study, we found similar distribution, chemotherapy use, and prognostic prediction of the 21-gene RS between Chinese and white American BC patients (25). For Chinese female patients, approximately one-quarter suffered from N1 disease at the time of BC diagnosis (26). Therefore, 21-gene testing could also play an important role in treatment decision-making for the Chinese population with a rapidly growing incidence rate of BC (27).
A review including a series of the study showed that the use of 21-gene testing had an 18%–69% reduction in chemotherapy recommendation in patients with N+ BC (28). In a study by Stemmer et al., they found that 21-gene testing was significantly associated with lower odds of receiving chemotherapy (OR 0.16, P < 0.001) (24). They also found that in those with 21-gene testing, 7.1%, 37.0%, and 100% of patients with low, intermediate, and high RS received chemotherapy, respectively. In our study, the proportion of chemotherapy use in patients without 21-gene testing was 64.1% and 30.8% in those receiving 21-gene testing. In our study, 18.0%, 46.3%, and 78.5% of low-, intermediate-, and high-RS patients received chemotherapy. Our findings demonstrated that the recommended rate for adjuvant chemotherapy was significantly lower in patients undergoing 21-gene testing. For those with N0 disease, the rates of chemotherapy use were 1.4%, 23.7%, and 87.2% in low-, intermediate-, and high-RS patients, respectively (20). There were large differences in the probability of each subgroup receiving chemotherapy after 21-gene testing for patients with N1 and N0 stages, especially for patients with low and intermediate RSs. The above results suggest that although genetic testing technology can better predict the value of being chemotherapy-free in N0 patients, it is still doubtful whether N1 patients can safely avoid chemotherapy.
In our study, there were 29.2% of patients who had 21-gene testing and patients with favorable prognostic factors were more likely to receive 21-gene testing. Moreover, those with three positive LNs had the lowest chance of 21-gene testing. There were 34.8%, 20.6%, and 12.7% of patients with one-, two-, and three-LN metastases who had 21-gene testing, respectively. Similar results were found from the study by Roberts et al., which included 30,410 patients with N+ BC, and the receipt of 21-gene testing was low in patients with high-risk factors (29). Our findings, along with the above study, showed that clinicians may choose not to perform 21-gene testing in more advanced patients with historical indications for chemotherapy.
In the current NCCN guideline, the recommendation of treatment for one to three positive LNs with ER+/HER+- is based on the results of 21-gene testing. In those without 21-gene testing, chemotherapy and endocrine therapy or endocrine therapy alone are recommended, whereas endocrine therapy is recommended in those with RS <26 and chemotherapy and endocrine therapy is recommended for those with RS ≥26 (5). Specifically, age was used as a main predictive factor for chemotherapy recommendation in our study in patients without 21-gene testing; there were 86.2%, 76.5%, and 35.6% of patients aged <50, 50–64, and ≥65 years receiving chemotherapy, respectively (P < 0.001). In those with 21-gene testing, RS was the main predictive factor for chemotherapy in patients; there were 8.0%, 46.3%, and 78.5% of patients with low, intermediate, and high RS receiving chemotherapy, respectively. Several studies showed that in those with one to three positive LNs, the overall change rate of treatment was 49%–55.1%, 27%–59.3%, and 0%–18% in those with low, intermediate, and high RSs, respectively (22, 30). Although the overall change rate of treatment was unavailable in the retrospective analysis, our results showed that there were 61.4% of patients who received chemotherapy in the no 21-gene testing cohort, which was significantly higher than in the patients with 21-gene testing (30.8%) (P < 0.001). This is remarkable in that the decision to omit chemotherapy in those with one to three positive LNs according to the 21-gene testing is similar to those with N0 disease. Therefore, tumor biology with genomic assays has been widely used in treatment-decision making of BC, which has beyond the traditional clinical and pathological characteristics in the selection of adjuvant treatment.
In patients with N0 disease, numerous studies have confirmed that RS has an important correlation with patient survival (31, 32). For patients with one to three positive LNs, studies have shown that RS can also affect various survival endpoints, including locoregional control, distant metastasis, and OS (10–13). In our study, we also found that patients with higher RS had worse survival outcomes. Our finding suggests that the RS provides additional prognostic assessment information of tumor biology beyond traditional clinicopathological factors for patients with one to three positive LNs. Given the results of our study and the previous findings, the 21-gene expression assay should be part of routine tests for BC patients with ER+/HER2− and one to three positive LNs.
For clinically high-risk patients, performing 21-gene testing can help clinicians identify subgroups of patients with favorable prognoses who can be safely spared from adjuvant chemotherapy.
To our knowledge, no studies have focused on the effect of 21-gene testing on the survival of T1-2N1 patients. In a previous study by Pomponio et al., they included patients with N0 and tumor size ≤1 cm and found that patients receiving 21-gene testing had better survival compared with those without 21-gene testing (33). Overall, the survival outcomes in our cohort were excellent regardless of the 21-gene testing. The 3-year BC related-death rates were 0.8% and 2.6% in those with and without 21-gene testing, respectively (P < 0.001). Our multivariate analyses showed that the use of RS testing was associated with better survival outcomes after adjustment of known prognostic variables. Similar results were found using PSM. A previous study included patients with N0 disease, which found that 21-gene testing is cost-effective for patients with intermediate- and high-RS cohorts, but not for the low-RS cohort (34). In patients with one to three positive LNs, the use of the 21-gene expression assay could be also a cost-effective strategy in this population (23).
Several limitations should be acknowledged in our study. First, the data of our study were from a retrospective database, which was inherently biased. Second, we were unable to observe clinician influence on patient treatment decisions, only practice patterns. Third, since outcome data regarding locoregional recurrence and distant metastasis are not available in the SEER database, and the median follow-up was only 32 months, the impact of adjuvant chemotherapy on survival outcomes according to different RS categories was not assessed. However, the primary strength of this study was that we used a population-based cohort to investigate the role of 21-gene RS testing in patients with one to three positive LNs. Our study could contribute to the understanding of the role of 21-gene RS testing in chemotherapy decision-making and prognostic prediction in this population.
In conclusion, our study suggests that the 21-gene expression assay is frequently and increasingly used for treatment decision-making in ER+/HER2- BC patients with one to three positive LNs and its use is related to lower rates of adjuvant chemotherapy. Performance of 21-gene testing in BC with one to three positive LNs is associated with better survival outcomes. Our study supports the routine use of 21-gene testing in the clinical practice of this population.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
G-QL and S-JX drafted the manuscript. S-GW acquired the datasets. S-GW and Z-YH conceived the study. S-GW conducted the statistical analyses. G-QL, S-JX, and Z-YH participated in the study design. All authors read and approved the final manuscript.
This work was partly supported by the Commission Young and Middle-aged Talents Training Project of the Fujian Health Commission (No. 2019-ZQNB-25) and the Natural Science Foundation of Fujian Province (No. 2020J011240).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Johansson ALV, Trewin CB, Fredriksson I, Reinertsen KV, Russnes H, Ursin G. In modern times, how important are breast cancer stage, grade and receptor subtype for survival: a population-based cohort study. Breast Cancer Res (2021) 23:17. doi: 10.1186/s13058-021-01393-z
3. Li Y, Ma L. Efficacy of chemotherapy for lymph node-positive luminal a subtype breast cancer patients: An updated meta-analysis. World J Surg Oncol (2020) 18:316. doi: 10.1186/s12957-020-02089-y
4. NCCN. NCCN clinical Practice guidelines in oncology V.4.2022. Breast Cancer Available online at: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf [Access Jul 4, 2022].
5. Salvo EM, Ramirez AO, Cueto J, Law EH, Situ A, Cameron C, et al. Risk of recurrence among patients with HR-positive, HER2-negative, early breast cancer receiving adjuvant endocrine therapy: A systematic review and meta-analysis. Breast (2021) 57:5–17. doi: 10.1016/j.breast.2021.02.009
6. Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med (2004) 351:2817–26. doi: 10.1056/NEJMoa041588
7. Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol (2006) 24:3726–34. doi: 10.1200/JCO.2005.04.7985
8. Kalinsky K, Barlow WE, Gralow JR, Meric-Bernstam F, Albain KS, Hayes DF, et al. 21-gene assay to inform chemotherapy benefit in node-positive breast cancer. N Engl J Med (2021) 385:2336–47. doi: 10.1056/NEJMoa2108873
9. Andre F, Ismaila N, Henry NL, Somerfield MR, Bast RC, Barlow W, et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: ASCO clinical practice guideline update-integration of results from TAILORx. J Clin Oncol (2019) 37:1956–64. doi: 10.1200/JCO.19.00945
10. Penault-Llorca F, Filleron T, Asselain B, Baehner FL, Fumoleau P, Lacroix-Triki M, et al. The 21-gene recurrence score® assay predicts distant recurrence in lymph node-positive, hormone receptor-positive, breast cancer patients treated with adjuvant sequential epirubicin- and docetaxel-based or epirubicin-based chemotherapy (PACS-01 trial). BMC Cancer (2018) 18:526. doi: 10.1186/s12885-018-4331-8
11. Wu J, Gao W, Chen X, Fei C, Lin L, Chen W, et al. Prognostic value of the 21-gene recurrence score in ER-positive, HER2-negative, node-positive breast cancer was similar in node-negative diseases: A single-center study of 800 patients. Front Med (2021) 15:621–8. doi: 10.1007/s11684-020-0738-0
12. Mamounas EP, Liu Q, Paik S, Baehner FL, Tang G, Jeong JH, et al. 21-gene recurrence score and locoregional recurrence in node-Positive/ER-Positive breast cancer treated with chemo-endocrine therapy. J Natl Cancer Inst (2017) 109:djw259. doi: 10.1093/jnci/djw259
13. Woodward WA, Barlow WE, Jagsi R, Buchholz TA, Shak S, Baehner F, et al. Association between 21-gene assay recurrence score and locoregional recurrence rates in patients with node-positive breast cancer. JAMA Oncol (2020) 6:505–11. doi: 10.1001/jamaoncol.2019.5559
14. Zhu X, Dent S, Paquet L, Zhang T, Tesolin D, Graham N, et al. How Canadian oncologists use oncotype DX for treatment of breast cancer patients. Curr Oncol (2021) 28:800–12. doi: 10.3390/curroncol28010077
15. Surveillance, Epidemiology, and End Results (SEER) Program. (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 18 Regs (Excl AK) Custom Data Malignant Breast (with Oncotype DX and Additional Treatment Fields), Nov 2017 Sub (2004-2015) - Linked To County Attributes - Total U.S., 1969-2016 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2018, based on the November 2017 submission.
16. Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med (2018) 379:111–21. doi: 10.1056/NEJMoa1804710
17. Peethambaram PP, Hoskin TL, Day CN, Goetz MP, Habermann EB, Boughey JC. Use of 21-gene recurrence score assay to individualize adjuvant chemotherapy recommendations in ER+/HER2- node positive breast cancer-a national cancer database study. NPJ Breast Cancer (2017) 3:41. doi: 10.1038/s41523-017-0044-4
18. Petkov VI, Miller DP, Howlader N, Gliner N, Howe W, Schussler N, et al. Breast-cancer-specific mortality in patients treated based on the 21-gene assay: A SEER population-based study. NPJ Breast Cancer (2016) 2:16017. doi: 10.1038/npjbcancer.2016.17
19. Kurian AW, Bondarenko I, Jagsi R, Friese CR, McLeod MC, Hawley ST, et al. Recent trends in chemotherapy use and oncologists' treatment recommendations for early-stage breast cancer. J Natl Cancer Inst (2018) 110:493–500. doi: 10.1093/jnci/djx239
20. Stemmer SM, Steiner M, Rizel S, Soussan-Gutman L, Ben-Baruch N, Bareket-Samish A, et al. Clinical outcomes in patients with node-negative breast cancer treated based on the recurrence score results: Evidence from a large prospectively designed registry. NPJ Breast Cancer (2017) 3:33. doi: 10.1038/s41523-017-0034-6
21. Albanell J, Svedman C, Gligorov J, Holt SD, Bertelli G, Blohmer JU, et al. Pooled analysis of prospective European studies assessing the impact of using the 21-gene recurrence score assay on clinical decision making in women with oestrogen receptor-positive, human epidermal growth factor receptor 2-negative early-stage breast cancer. Eur J Cancer (2016) 66:104–13. doi: 10.1016/j.ejca.2016.06.027
22. LeVasseur N, Sun J, Fenton D, Baxter S, Chan A, Roberts S, et al. Impact of the 21-gene recurrence score assay on the treatment of estrogen receptor-positive, HER2-negative, breast cancer patients with 1-3 positive nodes: A prospective clinical utility study. Clin Breast Cancer (2022) 22:e74–9. doi: 10.1016/j.clbc.2021.09.004
23. Masucci L, Torres S, Eisen A, Trudeau M, Tyono I, Saunders H, et al. Cost-utility analysis of 21-gene assay for node-positive early breast cancer. Curr Oncol (2019) 26:307–18. doi: 10.3747/co.26.4769
24. Stemmer SM, Klang SH, Ben-Baruch N, Geffen DB, Steiner M, Soussan-Gutman L, et al. The impact of the 21-gene recurrence score assay on clinical decision-making in node-positive (up to 3 positive nodes) estrogen receptor-positive breast cancer patients. Breast Cancer Res Treat (2013) 140:83–92. doi: 10.1007/s10549-013-2603-1
25. Li GQ, Yao J, Zhou P, Chen DX, Lian CL, Yang SP, et al. Distribution, chemotherapy use, and outcome of the 21-gene recurrence score between Chinese and white breast cancer in the united states. Clin Breast Cancer (2022) 22:279–87. doi: 10.1016/j.clbc.2021.11.003
26. Yu Y, Tan Y, Xie C, Hu Q, Ouyang J, Chen Y, et al. Development and validation of a preoperative magnetic resonance imaging radiomics-based signature to predict axillary lymph node metastasis and disease-free survival in patients with early-stage breast cancer. JAMA Netw Open (2020) 3:e2028086. doi: 10.1001/jamanetworkopen.2020.28086
27. Li T, Mello-Thoms C, Brennan PC. Descriptive epidemiology of breast cancer in China: incidence, mortality, survival and prevalence. Breast Cancer Res Treat (2016) 159:395–406. doi: 10.1007/s10549-016-3947-0
28. Yordanova M, Hassan S. The role of the 21-gene recurrence score® assay in hormone receptor-positive, node-positive breast cancer: The Canadian experience. Curr Oncol (2022) 29:2008–20. doi: 10.3390/curroncol29030163
29. Roberts MC, Kurian AW, Petkov VI. Uptake of the 21-gene assay among women with node-positive, hormone receptor-positive breast cancer. J Natl Compr Canc Netw (2019) 17:662–8. doi: 10.6004/jnccn.2018.7266
30. Eiermann W, Rezai M, Kümmel S, Kühn T, Warm M, Friedrichs K, et al. The 21-gene recurrence score assay impacts adjuvant therapy recommendations for ER-positive, node-negative and node-positive early breast cancer Resulting in a risk-adapted change in chemotherapy use. Ann Oncol (2013) 24:618–24. doi: 10.1093/annonc/mds512
31. Stemmer SM, Steiner M, Rizel S, Ben-Baruch N, Uziely B, Jakubowski DM, et al. Ten-year clinical outcomes in N0 ER+ breast cancer patients with recurrence score-guided therapy. NPJ Breast Cancer (2019) 5:41. doi: 10.1038/s41523-019-0137-3
32. Mamounas EP, Tang G, Fisher B, Paik S, Shak S, Costantino JP, et al. Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: results from NSABP b-14 and NSABP b-20. J Clin Oncol (2010) 28:1677–83. doi: 10.1200/JCO.2009.23.7610
33. Pomponio M, Keele L, Hilt E, Burkbauer L, Goldbach M, Nazarian S, et al. Impact of 21-gene expression assay on clinical outcomes in node-Negative ≤ T1b breast cancer. Ann Surg Oncol (2020) 27:1671–8. doi: 10.1245/s10434-019-08028-w
Keywords: breast cancer, lymph nodes, oncotype, survival, chemotherapy
Citation: Li G-Q, Xie S-J, Wu S-G and He Z-Y (2023) Impact of the 21-gene expression assay on treatment decisions and clinical outcomes in breast cancer with one to three positive lymph nodes. Front. Endocrinol. 14:1103949. doi: 10.3389/fendo.2023.1103949
Received: 21 November 2022; Accepted: 01 February 2023;
Published: 16 February 2023.
Edited by:
Rosamaria Lappano, University of Calabria, ItalyReviewed by:
Can Zhou, The First Affiliated Hospital of Xi’an Jiaotong University, ChinaCopyright © 2023 Li, Xie, Wu and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: San-Gang Wu, dW5vd3UxMjM0NUBob3RtYWlsLmNvbQ==; Zhen-Yu He, aGV6aHlAc3lzdWNjLm9yZy5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.