- 1Department of Metabolic and Bariatric Surgery, Clinical Research Institute, The First Affiliated Hospital, Jinan University, Guangzhou, China
- 2Institute of Obesity and Metabolic Disorders, Jinan University, Guangzhou, China
- 3Laboratory of Metabolic and Molecular Medicine, Guangdong-Hong Kong-Macao Joint University, Guangzhou, China
Background: Postoperative nausea and vomiting (PONV) are common after laparoscopic sleeve gastrectomy (LSG), affecting patient satisfaction and postoperative recovery. The purpose of this study was to investigate the incidence and severity of PONV after LSG and the relationship between Helicobacter pylori (HP) and PONV.
Methods: Patients undergoing LSG in our center from June 1, 2018, to May 31, 2022, were divided into HP-positive and HP-negative groups for retrospective analysis. The independent risk factors of PONV were determined by univariate and binary logistic regression analysis using a 1:1 propensity score matching (PSM) method.
Results: A total of 656 patients was enrolled, and 193 pairs of HP-positive and negative groups were matched after PSM. Both groups of patients had similar clinical features and surgical procedures. PONV occurred in 232 patients (60.1%) after LSG, and the incidence of PONV in HP-positive patients was 61.10%. The incidence and severity of PONV were statistically similar in both groups (P=0.815). Multivariate analysis showed that the female sex (OR=1.644, P=0.042), postoperative pain (OR=2.203, P=0.001) and use of postoperative opioid (OR=2.229, P=0.000) were independent risk factors for PONV after LSG, whereas T2DM (OR=0.510, P=0.009) and OSAS (OR=0.545, P=0.008) independently reduced the incidence rate of PONV. There was no difference either in smoking (P=0.255) or alcohol drinking (P=0.801). HP infection did not affect PONV (P=0.678).
Conclusions: The incidence of PONV following LSG was relatively high. Female sex, postoperative pain and use of postoperative opioid predicted a higher incidence of PONV. Patients with T2DM and OSAS were less likely to have PONV. There was no clear association between HP infection and PONV after LSG.
Introduction
Overweight and obesity are defined as an excess of body fat accumulation that threatens health. According to the updated data from the World Health Organization, in 2016, more than 1.9 billion adults were overweight globally. Of these, over 650 million were in obesity (1, 2). According to epidemiological studies, obesity can progressively cause and/or exacerbate a wide spectrum of chronic diseases, which include type 2 diabetes mellitus, chronic kidney disease (3), cardiovascular disease (3, 4), a range of musculoskeletal disorders (5, 6), and even certain types of cancer (7). Bariatric surgery becomes necessary for people with severe obesity who cannot sustain weight loss by non-surgical means (e.g., diet and exercise). Laparoscopic sleeve gastrectomy (LSG) has become the most common bariatric surgery because of its simple operation, fewer complications, and good effect in reducing weight and alleviating obesity metabolism-related complications (8–11). Of note, there are a variety of side effects and post-op risks related to bariatric surgery, including acid reflux, dilation of the esophagus, obstruction of the stomach, weight gain or failure to lose weight, infection, and postoperative nausea and vomiting (PONV) (12).
PONV, defined as nausea, vomiting, or retching occurring within 24 h following anesthesia, is the most common adverse reaction after LSG. Without preventive antiemetic treatment, its incidence can reach 80% (13). PONV will induce postoperative discomforts and cause serious complications, such as water-electrolyte disorder and aspiration pneumonia, resulting in prolonged hospitalization and increased medical expenses (14). Previous studies have identified the factors affecting the incidence of PONV came from three aspects: patient factors (e.g., female sex, anxiety, infection, metabolic disease, and gastrointestinal disease), medication/anesthesia factors (e.g., opioids, volatile agents, and nitrous oxide), and surgery factors (e.g., surgical time, procedure, and technique) (15, 16). In adults, the known risk factors for PONV include female sex, non-smoking status, use of postoperative opioids, younger age, and history of PONV or motion sickness (17). However, for obese patients, several factors may contribute to the high susceptibility to PONV. Because patients who undergo bariatric surgery are usually younger women and non-smokers, with laparoscopic or robotic surgery lasting more than one hour, and receive perioperative opioid analgesia, all these are risk factors for PONV. Besides, impaired splanchnic perfusion during pneumoperitoneum and gastric volume reduction (especially after LSG) may further lead to PONV (18–20).
Studies have shown that Helicobacter pylori (HP) infection is closely related to digestive tract diseases such as peptic ulcer, gastric cancer, gastric lymphoma, and chronic gastritis (21). There are many studies on the mechanism, prevention, and treatment, but few on the relationship between HP infection and gastrointestinal adverse reactions such as PONV. Several researches have shown that there is an association between HP and hyperemesis gravidarum, which indicates that HP can exacerbate nausea and vomiting during pregnancy (22–25). Thus, the aims of this retrospective study were to investigate the incidence and risk factors of PONV after LSG and to explore whether HP infection affects PONV in subjects receiving LSG using a propensity score matching (PSM) analysis.
Methods
Study population
This study was conducted at the Department of Metabolic and Bariatric Surgery in the First Affiliated Hospital of Jinan University. A preliminary assessment determined surgical qualifications by a multidisciplinary team including surgeons, endocrinologists, anesthesiologists, nutritionists, and nurses. This retrospective study included all patients with obesity who underwent LSG at our bariatric surgery center from June 1, 2018, to May 31, 2022. The exclusion criteria were: (1) age less than 18 years, (2) patients did not undergo HP examination before the operation, (3) patients who were transferred to the intensive care unit (ICU) immediately after the operation, (4) the revision surgery (a repeated surgery due to complications or unsatisfactory results after initial bariatric surgery), (5) patients received HP eradication treatment before the operation, (6) patients received antibiotic treatment within four weeks before the operation, (7) nausea or vomiting before anesthesia.
All Bariatric surgeries were performed by the same well-experienced surgical team. The surgical techniques of LSG and postoperative management were introduced previously (26). On the basis of PONV prophylaxis guidelines, we routinely gave palonosetron and dexamethasone at the end of the operation (13, 27). After surgery, we transferred the patients to post-anesthesia care unit (PACU) until complete recovery and monitored vital signs according to standard clinical practice. In the ward, we used a visual analogue scale (VAS) to evaluate nausea and vomiting or pain (least: 0–10: worst). Depending on the severity of PONV, we decided whether to use antiemetics. For the patients with PONV or cases were intolerable, we usually offered rescue antiemetic agent (including: 5 mg tropisetron, 10 mg metoclopramide or 4 mg ondansetron). On the basis of the level of pain, subjects with postoperative pain received analgesic management, such as flurbiprofen 50 mg, parecoxib 40 mg or tramadol 100 mg (26).
Since (1) we had informed all participants receiving LSG that the clinical data which were acquired during the perioperative period may be retrospectively analyzed and published; And (2) in our study, all data were collected as a regular part of surgical care, and none were designed to collect data specifically for the research, so there was no need for written informed consent. This study protocol was approved by the Ethical Committee of the First Affiliated Hospital of Jinan University (no. KY-2021-070).
Anesthesia protocol
All procedures were finished under general anesthesia following a standardized clinical routine. Routine monitoring of electrocardiogram, blood pressure, and pulse oximetry were carried out. General anesthesia was induced with propofol, remifentanil, and rocuronium, and the dosage of drugs depended on the body weight of the patient. The maintenance of anesthesia was implemented by the use of remifentanil and propofol, oxygen, and air (26). In accordance with the PONV prevention guidelines, we routinely provided dexamethasone and palonosetron at the end of surgery (13, 27).
Study outcomes
Nausea is defined as an unpleasant feeling associated with the urge to vomit. Vomiting is defined as successful or unsuccessful (retching) excretion of gastric contents (28). The risk factors and predictors for postoperative nausea and vomiting are generally considered to be almost identical (29). Consequently, nausea or vomiting is not considered as a separate outcome in our research (30). We focused our study on 6 h and 24 h after surgery.
In this study, the primary endpoint was the overall incidence of PONV within 24 h after surgery, with secondary outcomes being the severity of PONV, the type and use of rescue antiemetics, and the time for the first rescue antiemetic and analgesics. Based on the total VAS scores at 6 h and 24 h after operation and the use of rescue antiemetics, two groups were divided (PONV: total VAS score greater than 2 or use of rescue antiemetics; No PONV: total VAS score less than or equal to 2 and no use of rescue antiemetics). Depending on the total postoperative pain VAS (P-VAS) scores at 6 h and 24 h after surgery and the application of rescue analgesics, the definition of postoperative pain was the sum of P-VAS, which was higher than 2 points or applying rescue analgesics. At the same time, for further study, we respectively divided the PONV group and the pain group into three groups: mild (3-6 scores), moderate (7-12 scores) and severe (13-20 scores) (26).
Data collection
A professional researcher reviewed patients’ electronic medical records and extracted the following data which contained demographic data and perioperative factors. The demographic variables included age, BMI, obesity-related comorbidity [type 2 diabetes mellitus (T2DM), hyperlipidemia (HLP), hypertension], and smoking status. Operational details were collected, mainly including duration of surgery, the use of prophylactic antiemetics and anesthesia methods. We used the C13 breath test to detect HP infection.
In our department, the same team performed one standardized questionnaire to all patients. By this way, we acquired the information including PONV score, pain level, alcohol consumption, and smoking status. PONV severity was assessed using the total VAS scores at 6h and 24h after the operation. A higher score indicated more severe nausea and vomiting (31). Pain status was scored with a VAS at 6h and 24h post-operation (32). The alcohol consumption level was quantified before operation using the Alcohol Use Disorders Identification Test (AUDIT) recommended by the World Health Organization. The AUDIT score could be classified into four risk levels: 0 point as a non-drinker; 1-7 points as low risk, 8-15 points as a moderate risk; 16-19 points as high risk; 20 and above as alcohol dependence (33). Smoking status was expressed by the Brinkman index (BI), which is the number of years of smoking multiplied by the number of cigarettes smoked per day. BI results could be divided into four sequential groups: non-smokers as 0; mild smokers as 1-200; moderate smokers as 200-400; and heavy smokers as > 400 (34).
Statistical analysis
To help overcome the selection bias from the confounding variables, we performed a PSM analysis in each group. The propensity score was calculated by logistic regression analysis. We applied the nearest-neighbor method to match the patients in a 1:1 ratio. As a result, A patient in the HP-positive group was matched with one patient in the HP-negative group. The caliper size was set 0.02 and bad matches were excluded from analysis.
Continuous variables of normal distribution were presented as means ± standard deviations (SD) and were analyzed using an independent t-test. Variables with a skewed distribution were presented as median (interquartile range) and were compared using the Mann-Whitney U-test. Categorical data were presented as percentages and compared using the χ2 and Wilcoxon test. The risk factors of PONV post LSG were firstly analyzed by a univariate analysis. After screening the variables, the likelihood ratio stepwise forward method included the significantly related variables in the binary logistic regression analysis. The analysis indexes included the odds ratio (OR), 95% confidence interval (95% CI), and significance test results (P value).
All data were analyzed using SPSS 26.0 software (SPSS Inc., Chicago, IL). All P values were two-sided, and P < 0.05 was considered statistically significant.
Results
Patient characteristics
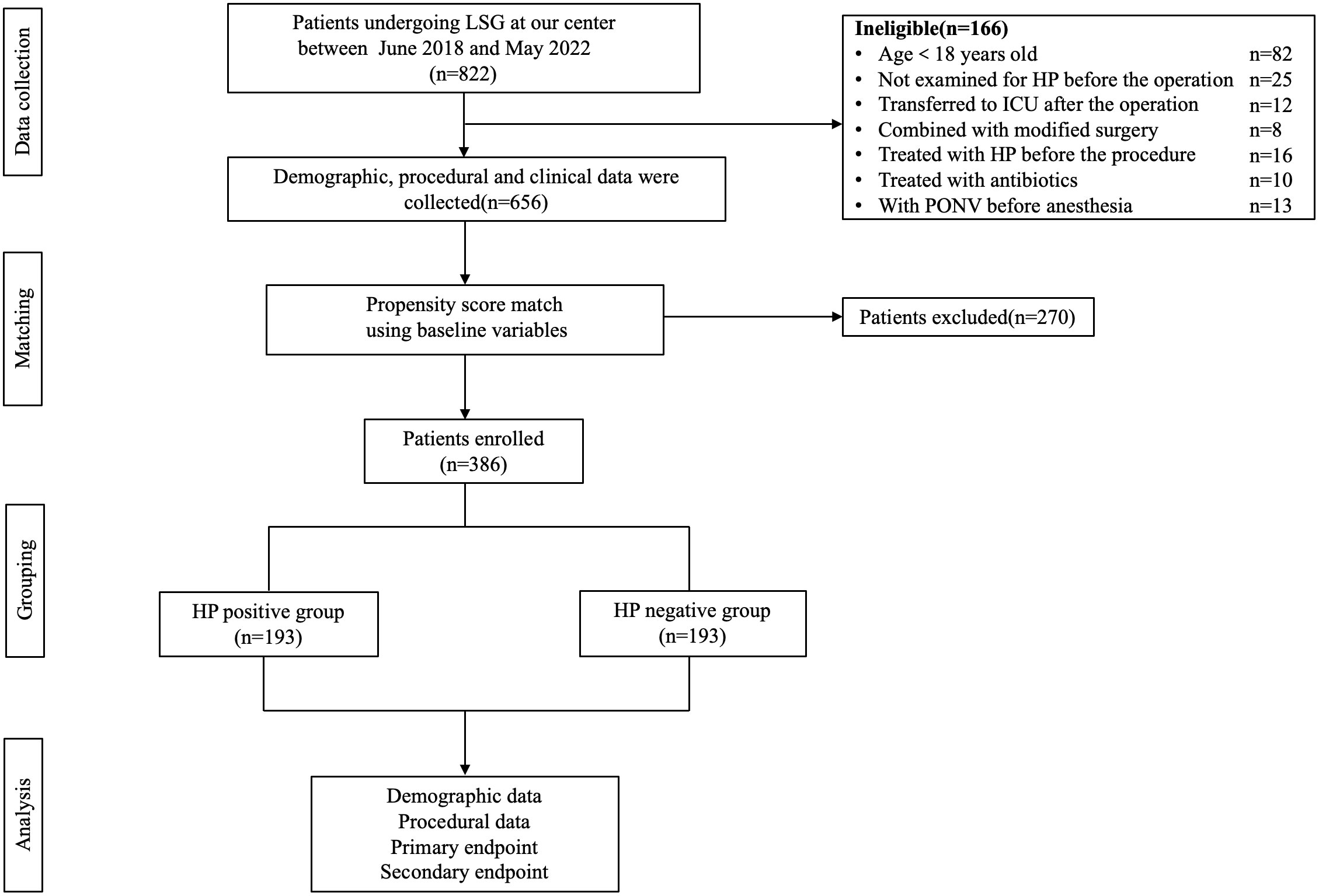
The study reviewed 822 patients (205 males and 617 females) who underwent LSG surgery in our hospital between June 1, 2018, and May 31, 2022. In those patients, 82 were younger than 18 years old, 25 were not examined for HP before the operation, 12 cases were transferred to ICU after the operation, 8 patients received revision surgery, 16 cases were treated for HP before the procedure, 10 cases were treated with antibiotics within four weeks before the operation, and 13 cases had nausea and vomiting before anesthesia. Finally, 656 patients were eligible to enter the study prior to the PSM, and we had 193 matched patients over 1:1 PSM, effectively balancing the preoperative confounding factors of the two groups. The research flow chart was shown in Figure 1. Demographic data and perioperative factors of all patients before and after PSM were shown in Table 1.
Occurrence and severity of PONV in HP-positive group and HP-negative group
Before PSM, there were 390 patients of PONV in 656 patients undergoing LSG, and the infection rate was 59.45%. There was no significant difference in the incidence of PONV between HP-positive and HP-negative patients (P=0.641) (Table 2).
Comparison of covariates before and after PSM in groups
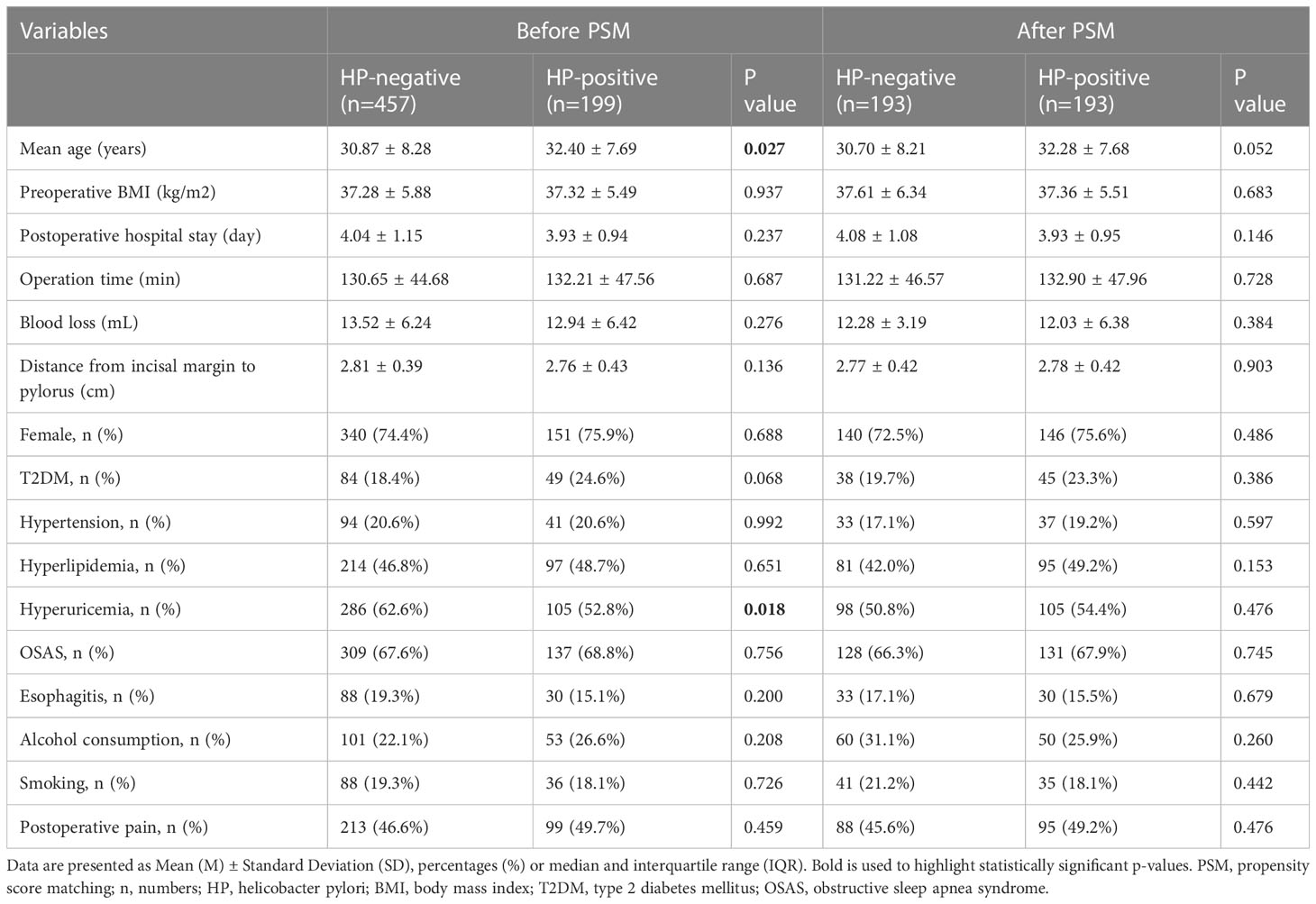
Before PSM, there were 199 cases in the HP-positive group and 457 cases in the HP-negative group, respectively. There were significant differences between the two groups in terms of age (P=0.027) and hyperuricemia (P=0.018); After PSM, the infection of HP was taken as the dependent variable, and the above covariates were taken as the independent variables. After 1:1 matching of the data between the two groups, there were 193 cases in each of the two groups. The distribution of the above covariates between the groups reached equilibrium (all P > 0.05) (Table 1).
Comparison of occurrence and severity of PONV
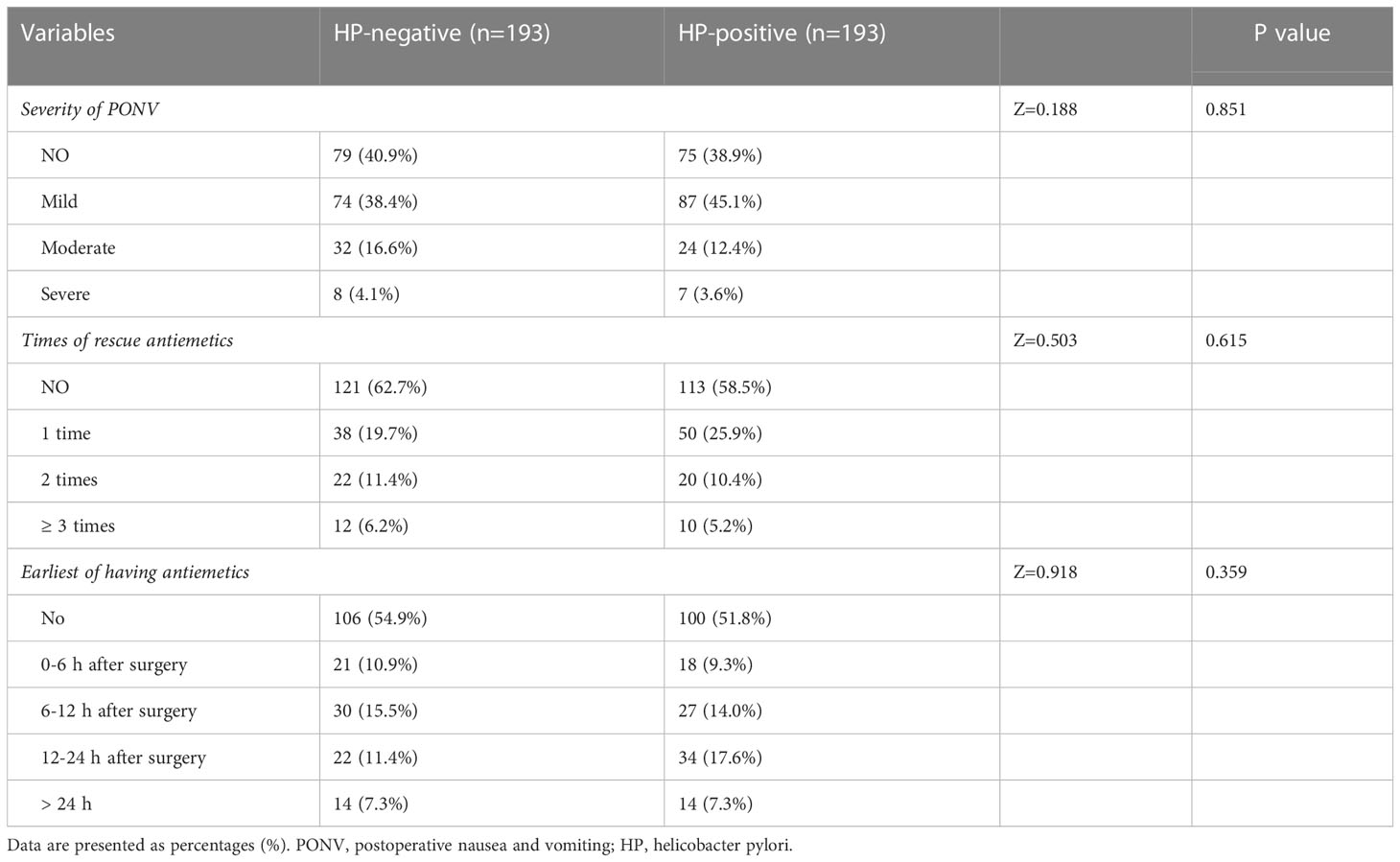
Among the 193 patients in the HP-negative and HP-positive groups, 114 (59.1%) and 118 (61.1%) developed PONV within 24 h after the operation. Most PONV cases were mild. The incidence, severity (P=0.851), frequency of rescue antiemetics (P=0.615), and the earliest antiemetics use (P=0.359) in the two groups were not statistically significant (Table 3).
Univariate analysis
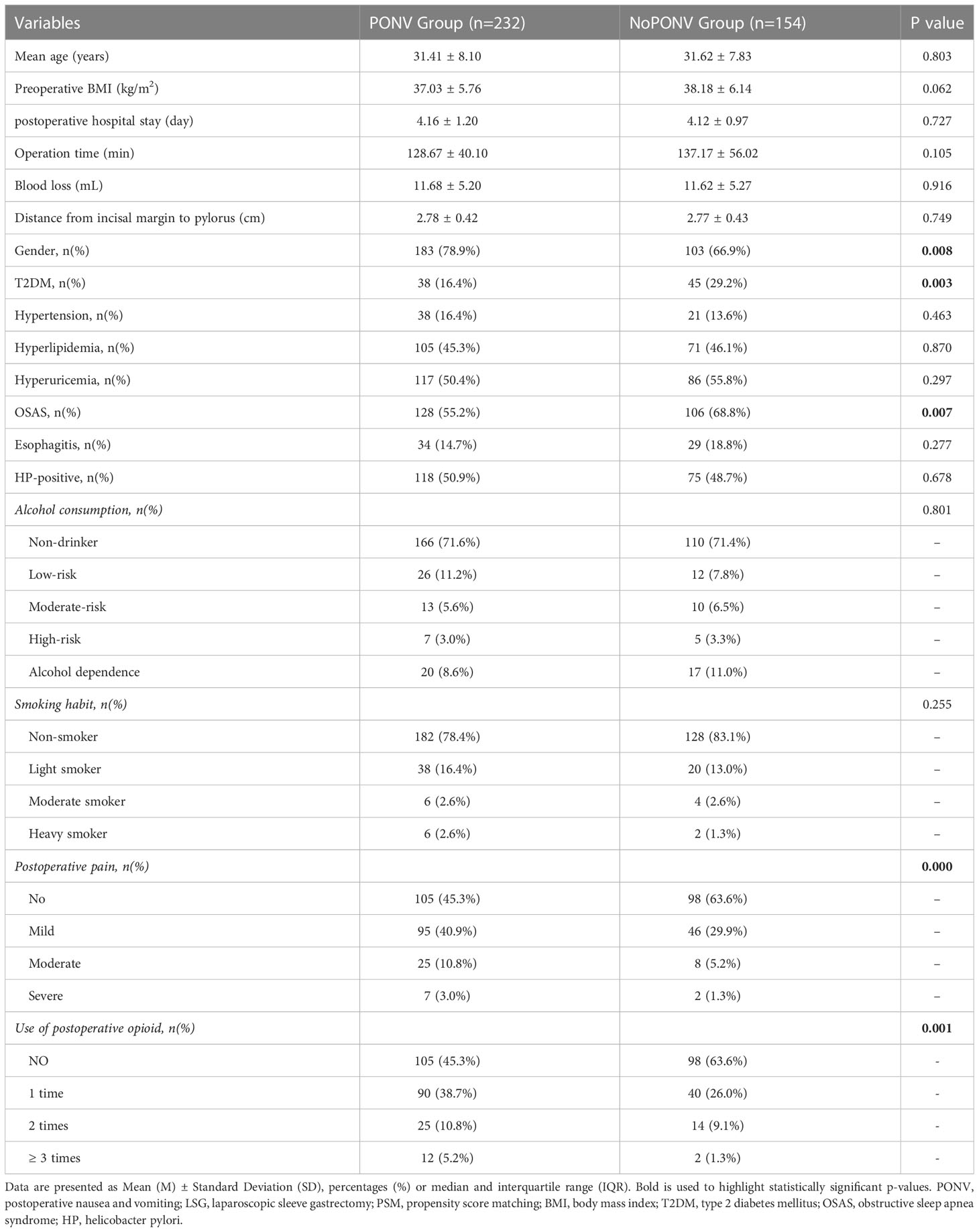
After PSM, 386 patients were finally included, including 100 males and 286 females. A total of 232 occurred PONV, with an incidence rate of 60.1%. According to PONV occurrence, those patients were divided into the PONV group and the no PONV group. The univariate analysis showed that females had a significantly higher risk of PONV than males after LSG (P=0.008). The incidence rate of PONV in patients with diabetes (P=0.003) and OSAS was lower than in those who had not those complications (P=0.007). The incidence of PONV was significantly higher in patients with postoperative pain (P=0.000) and use of postoperative opioid (P=0.001) than in patients without pain (Table 4).
Multivariate regression analysis
Significant and independent predictors of PONV incidence were determined by a multivariate logistic regression analysis. Variables that were statistically significant in the univariate analysis were included in the multivariate logistic regression analysis model. Results showed that female sex and postoperative pain were important independent predictors of the increase in the incidence rate of PONV. At the same time, type 2 diabetes (T2DM) and OSAS significantly and independently reduced the incidence rate of PONV after adjusting for confounding variables. The OR (95% CIs, P value) of PONV incidence after LSG was 1.644 (1.017-2.655, P = 0.042) in females and 2.203 (1.430-3.396, P = 0.001) in the pain group; The group of use of postoperative opioid was 2.229 (1.446-3.434, P = 0.000); T2DM group was 0.510 (0.306-0.848, P = 0.009) and OSAS group was 0.545 (0.349-0.853, P = 0.008) (Table 5).
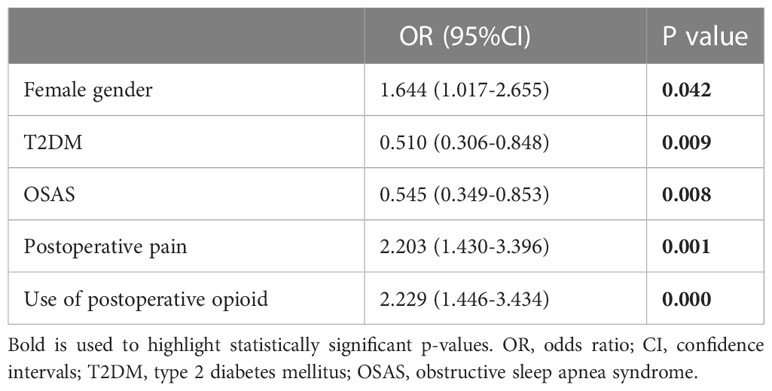
Table 5 Odds ratio (OR) and 95% confidence intervals (CI) of risk factors for PONV after LSG (after PSM).
Discussion
Incidence and severity of PONV
Previous studies have shown that postoperative PONV was the most common adverse effect of weight loss surgery, and its overall incidence exceeded 80% in some types of surgery (35). PONV following LSG was thought to be secondary to the sharp reduction of gastric volume and increased intragastric pressure (36). A retrospective chart review study showed that the incidence of PONV in the LSG group (66.9%) was higher than that in the primary laparoscopic Roux-en-Y gastric bypass group (33.1%) (37). Another study pointed out that 65% of patients experience PONV within the first 24 h following LSG (18). Our study found that the incidence of PONV in Chinese patients at 0-24 h following LSG was 60.1%, lower than the above reported incidences of patients from other countries (38–40). This could be attributed to: in our center, tropisetron hydrochloride (a potent and selective 5-HT3 receptor antagonist) and metoclopramide (a dopamine antagonist) were routinely used during and right after the surgery to prevent PONV (41, 42).
Biological sex and PONV
It was identified that the female sex predicted a higher incidence of PONV following surgery (43–45). Halliday et al. Found that when two or even three preventive drugs were used, the incidence of PONV in female patients was still as high as 78% following weight loss surgery, which was three times than that of male patients during the same period (18). Another retrospective study showed that preventive antiemetic therapy did not have an ideal effect on preventing and treating PONV after weight loss surgery. After drug intervention, the incidence of PONV in female patients was still nearly 1/3 higher than that in male patients (60.4% vs. 42.9%), suggesting that the risk of PONV after bariatric surgery in female patients will not be significantly reduced with the use of preventive drugs (19). The incidence rate of PONV varies with the different phases of the menstrual cycle (19, 46, 47). However, this conclusion was contradicted by a randomized controlled trial study involving more than 5,000 patients in 2007, in which no association between the menstrual cycle stage or menopausal status and the incidence of PONV was identified (48). The molecular mechanism responsible for the correlation between the female sex and the incidence rate of PONV is still largely unknown.
Postoperative pain and PONV
Previous studies had shown that PONV was strongly associated with postoperative pain in LSG (26). Our study also demonstrated that postoperative pain was a risk factor for PONV after LSG. The possible reasons could be (1): in essence, high pain intensity was inclined to increase the risk of PONV, and (2) in our center, opioids, such as tramadol, were preferred for postoperative pain, which may increase the risk of PONV and constituted one of the major risk factors in the scoring system (49, 50). However, further study was warranted to confirm the impact of postoperative pain on PONV after LSG.
OSAS and PONV
A major finding of our research was that patients without OSAS had a higher risk of PONV than patients with OSAS. Obesity is considered the main factor leading to OSAS, of which severity could be measured by the sleep apnea-hypopnea index (AHI). With the increase in BMI, the AHI of both males and females increases, and this trend is tendentious in males (51). Although OSAS did not affect the prognosis of bariatric surgery, it indeed affected the postoperative complication of cardiopulmonary function (52). Continuous positive airway pressure (CPAP) is currently the most effective method for treating moderate to severe OSAS, which improves the respiratory function of patients with morbid obesity and accelerates the reconstruction of preoperative pulmonary function (53). A previous study found that in subjects receiving Roux-en-Y gastric bypass, the no-CPAP group reported a higher incidence of oxygenation disturbance, but a slightly lower incidence, although not statistically significant, of PONV when compared with the CPAP group (54). Thus, a possible reason for the lower incidence of PONV in patients with OSAS in this study is the routine use of CPAP in the perioperative period of LSG. More substantial evidence and molecular pathway for this conclusion warrant further investigations.
Alcohol drinking, smoking, and PONV
A recent study reported decreased risks of PONV in alcoholics than non-drinkers and light-drinkers who underwent abdominal surgery (55). In addition, since chronic alcoholics have higher basal activity of cytochrome P450 2E1 (CYP2E1), which also accelerates the metabolic rate of volatile anesthetics, the main reason of PONV within the first two hours after surgery moderate- or heavy-drinkers (including alcohol dependence patients) may expect a reduced incidence of PONV post-LSG (56, 57). This is not consistent with what we demonstrated here. Previous studies have also built an association between the reduced incidence of PONV and cigarette smoking in Bariatric surgeries (58). However, we did not observe such correlations in our study.
HP and PONV
A previous study has demonstrated no association between HP infection and nausea after general anesthesia (59). Notash et al. Also found no relationship between HP infection and PONV who underwent general and urological surgery (60). The incidence of PONV in our research was similar in HP-positive and negative patients. Although HP may be related to severe pregnancy-related vomiting, it did not exacerbate LSG-related nausea. In bariatric surgery, to our best knowledge, this is the first report showing that HP infection did not affect the prevalence of PONV after LSG. However, since our research is a single center, more extensive cohort studies are needed for the validation of this conclusion.
Strengths and limitations
There are some limitations in this retrospective study: (1) The confirmation of a PONV event was determined by using rescue antiemetics or notating its manifestation in the medical records. This approach raises the possibility that the PONV frequencies were underestimated as some patients might experience untreated PONV; (2) Other potential factors, such as PONV history, migraine, and duration of anesthesia, were not considered, which may bias the results; (3) Since we only observed the PONV incidence within 24 h post-operation, a long-term follow-up study is needed to confirm and expand the conclusions; (4) Mechanistic research is required to investigate the molecular pathways leading to PONV after LSG and other types of bariatric surgery. However, as far as we know, this is the largest reported sample size in the study of PONV in LSG. Those confounding factors could be counterpoised after PSM. The relationship between HP and PONV in populations undergoing LSG was also interpreted. Further basic research is required to investigate the molecular mechanism leading to PONV after LSG and other types of bariatric surgery.
Conclusions
In conclusion, the incidence of PONV after LSG is relatively high. Female sex, postoperative pain and use of postoperative opioid predicted a higher incidence of PONV. Patients with T2DM and OSAS had less likelihood of a related PONV. There was no clear association between HP infection and PONV after LSG.
Data availability statement
The original contributions presented in the study are included in the article/supplementary materials. Further inquiries can be directed to the corresponding authors.
Ethics statement
As all participants receiving LSG were informed that the clinical data which were acquired during the perioperative period may be retrospectively analyzed and published, and all data were collected as a standard part of surgical care, and none were designed to collect data specifically for the research, written informed consent was not required. This study protocol was approved by the Ethical Committee of the First Affiliated Hospital of Jinan University (no. KY-2021-070).
Author contributions
YS, JZ, and WY designed the study. YS collected patients’ data. YS, JZ, and JX performed the analyses and wrote the paper. ZD and CW assisted with the study design and analysis. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lavie CJ, Pandey A, Lau DH, Alpert MA, Sanders P. Obesity and atrial fibrillation prevalence, pathogenesis, and prognosis: Effects of weight loss and exercise. J Am Coll Cardiol (2017) 70(16):2022–35. doi: 10.1016/j.jacc.2017.09.002
2. Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med (2017) 377(1):13–27. doi: 10.1056/NEJMoa1614362
3. Singh GM, Danaei G, Farzadfar F, Stevens GA, Woodward M, Wormser D, et al. The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: A pooled analysis. PloS One (2013) 8(7):e65174. doi: 10.1371/journal.pone.0065174
4. Wormser D, Kaptoge S, Di Angelantonio E, Wood AM, Pennells L, Thompson A, et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: Collaborative analysis of 58 prospective studies. Lancet (2011) 377(9771):1085–95. doi: 10.1016/s0140-6736(11)60105-0
5. Jiang L, Rong J, Wang Y, Hu F, Bao C, Li X, et al. The relationship between body mass index and hip osteoarthritis: A systematic review and meta-analysis. Joint Bone Spine (2011) 78(2):150–5. doi: 10.1016/j.jbspin.2010.04.011
6. Jiang L, Tian W, Wang Y, Rong J, Bao C, Liu Y, et al. Body mass index and susceptibility to knee osteoarthritis: A systematic review and meta-analysis. Joint Bone Spine (2012) 79(3):291–7. doi: 10.1016/j.jbspin.2011.05.015
7. Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body fatness and cancer–viewpoint of the iarc working group. N Engl J Med (2016) 375(8):794–8. doi: 10.1056/NEJMsr1606602
8. Nguyen NT, Varela JE. Bariatric surgery for obesity and metabolic disorders: State of the art. Nat Rev Gastroenterol Hepatol (2017) 14(3):160–9. doi: 10.1038/nrgastro.2016.170
9. Welbourn R, Pournaras DJ, Dixon J, Higa K, Kinsman R, Ottosson J, et al. Bariatric surgery worldwide: Baseline demographic description and one-year outcomes from the second ifso global registry report 2013-2015. Obes Surg (2018) 28(2):313–22. doi: 10.1007/s11695-017-2845-9
10. Khorgami Z, Shoar S, Andalib A, Aminian A, Brethauer SA, Schauer PR. Trends in utilization of bariatric surgery, 2010-2014: Sleeve gastrectomy dominates. Surg Obes Relat Dis (2017) 13(5):774–8. doi: 10.1016/j.soard.2017.01.031
11. Yang W, Zhu S, Cheng Z, Zhang N, Wu L, Chen Y, et al. Laparoscopic roux-En-Y gastric bypass for excess weight and diabetes: A multicenter retrospective cohort study in China. Mini-invasive Surg (2021) 5:11.
12. Arterburn DE, Telem DA, Kushner RF, Courcoulas AP. Benefits and risks of bariatric surgery in adults: A review. Jama (2020) 324(9):879–87. doi: 10.1001/jama.2020.12567
13. Gan TJ, Belani KG, Bergese S, Chung F, Diemunsch P, Habib AS, et al. Fourth consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg (2020) 131(2):411–48. doi: 10.1213/ane.0000000000004833
14. Shaikh SI, Nagarekha D, Hegade G, Marutheesh M. Postoperative nausea and vomiting: A simple yet complex problem. Anesth Essays Res (2016) 10(3):388–96. doi: 10.4103/0259-1162.179310
15. Ghosh S, Rai KK, Shivakumar HR, Upasi AP, Naik VG, Bharat A. Incidence and risk factors for postoperative nausea and vomiting in orthognathic surgery: A 10-year retrospective study. J Korean Assoc Oral Maxillofac Surg (2020) 46(2):116–24. doi: 10.5125/jkaoms.2020.46.2.116
16. Stoops S, Kovac A. New insights into the pathophysiology and risk factors for ponv. Best Pract Res Clin Anaesthesiol (2020) 34(4):667–79. doi: 10.1016/j.bpa.2020.06.001
17. Tateosian VS, Champagne K, Gan TJ. What is new in the battle against postoperative nausea and vomiting? Best Pract Res Clin Anaesthesiol (2018) 32(2):137–48. doi: 10.1016/j.bpa.2018.06.005
18. Halliday TA, Sundqvist J, Hultin M, Walldén J. Post-operative nausea and vomiting in bariatric surgery patients: An observational study. Acta Anaesthesiol Scand (2017) 61(5):471–9. doi: 10.1111/aas.12884
19. Groene P, Eisenlohr J, Zeuzem C, Dudok S, Karcz K, Hofmann-Kiefer K. Postoperative nausea and vomiting in bariatric surgery in comparison to non-bariatric gastric surgery. Wideochir Inne Tech Maloinwazyjne (2019) 14(1):90–5. doi: 10.5114/wiitm.2018.77629
20. Kushner BS, Freeman D, Sparkman J, Salles A, Eagon JC, Eckhouse SR. Assessment of postoperative nausea and vomiting after bariatric surgery using a validated questionnaire. Surg Obes Relat Dis (2020) 16(10):1505–13. doi: 10.1016/j.soard.2020.05.017
21. Fischbach W, Malfertheiner P. Helicobacter pylori infection. Dtsch Arztebl Int (2018) 115(25):429–36. doi: 10.3238/arztebl.2018.0429
22. Wu CY, Tseng JJ, Chou MM, Lin SK, Poon SK, Chen GH. Correlation between helicobacter pylori infection and gastrointestinal symptoms in pregnancy. Adv Ther (2000) 17(3):152–8. doi: 10.1007/bf02853157
23. Grooten IJ, Den Hollander WJ, Roseboom TJ, Kuipers EJ, Jaddoe VW, Gaillard R, et al. Helicobacter pylori infection: A predictor of vomiting severity in pregnancy and adverse birth outcome. Am J Obstet Gynecol (2017) 216(5):512.e1-.e9. doi: 10.1016/j.ajog.2017.01.042
24. Ng QX, Venkatanarayanan N, De Deyn M, Ho CYX, Mo Y, Yeo WS. A meta-analysis of the association between helicobacter pylori (H. pylori) infection and hyperemesis gravidarum. Helicobacter (2018) 23(1). doi: 10.1111/hel.12455
25. Hussein KS. Hyperemesis gravidarum in first-trimester pregnant Saudi women: Is helicobacter pylori a risk factor? Front Physiol (2020) 11:575. doi: 10.3389/fphys.2020.00575
26. Zhu J, Wu L, Chen G, Zhao X, Chen W, Dong Z, et al. Preoperative reflux or regurgitation symptoms are independent predictors of postoperative nausea and vomiting (Ponv) in patients undergoing bariatric surgery: A propensity score matching analysis. Obes Surg (2022) 32(3):819–28. doi: 10.1007/s11695-021-05859-z
27. Gan TJ, Diemunsch P, Habib AS, Kovac A, Kranke P, Meyer TA, et al. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg (2014) 118(1):85–113. doi: 10.1213/ane.0000000000000002
28. Balaban CD, Yates BJ. What is nausea? a historical analysis of changing views. Auton Neurosci (2017) 202:5–17. doi: 10.1016/j.autneu.2016.07.003
29. Cohen MM, Duncan PG, DeBoer DP, Tweed WA. The postoperative interview: Assessing risk factors for nausea and vomiting. Anesth Analg (1994) 78(1):7–16. doi: 10.1213/00000539-199401000-00004
30. Horn CC, Wallisch WJ, Homanics GE, Williams JP. Pathophysiological and neurochemical mechanisms of postoperative nausea and vomiting. Eur J Pharmacol (2014) 722:55–66. doi: 10.1016/j.ejphar.2013.10.037
31. Myles PS, Wengritzky R. Simplified postoperative nausea and vomiting impact scale for audit and post-discharge review. Br J Anaesth (2012) 108(3):423–9. doi: 10.1093/bja/aer505
32. Chiarotto A, Maxwell LJ, Ostelo RW, Boers M, Tugwell P, Terwee CB. Measurement properties of visual analogue scale, numeric rating scale, and pain severity subscale of the brief pain inventory in patients with low back pain: A systematic review. J Pain (2019) 20(3):245–63. doi: 10.1016/j.jpain.2018.07.009
33. Higgins-Biddle JC, Babor TF. A review of the alcohol use disorders identification test (Audit), audit-c, and usaudit for screening in the united states: Past issues and future directions. Am J Drug Alcohol Abuse (2018) 44(6):578–86. doi: 10.1080/00952990.2018.1456545
34. Hamabe A, Uto H, Imamura Y, Kusano K, Mawatari S, Kumagai K, et al. Impact of cigarette smoking on onset of nonalcoholic fatty liver disease over a 10-year period. J Gastroenterol (2011) 46(6):769–78. doi: 10.1007/s00535-011-0376-z
35. Parisi A, Desiderio J, Cirocchi R, Trastulli S. Enhanced recovery after surgery (Eras): A systematic review of randomised controlled trials (Rcts) in bariatric surgery. Obes Surg (2020) 30(12):5071–85. doi: 10.1007/s11695-020-05000-6
36. Yehoshua RT, Eidelman LA, Stein M, Fichman S, Mazor A, Chen J, et al. Laparoscopic sleeve gastrectomy–volume and pressure assessment. Obes Surg (2008) 18(9):1083–8. doi: 10.1007/s11695-008-9576-x
37. Suh S, Helm M, Kindel TL, Goldblatt MI, Gould JC, Higgins RM. The impact of nausea on post-operative outcomes in bariatric surgery patients. Surg Endosc (2020) 34(7):3085–91. doi: 10.1007/s00464-019-07058-5
38. Fathy M, Abdel-Razik MA, Elshobaky A, Emile SH, El-Rahmawy G, Farid A, et al. Impact of pyloric injection of magnesium sulfate-lidocaine mixture on postoperative nausea and vomiting after laparoscopic sleeve gastrectomy: A randomized-controlled trial. Obes Surg (2019) 29(5):1614–23. doi: 10.1007/s11695-019-03762-2
39. Atif QAA, Al Obaid O, Malik AM. Effect of intravenous scopolamine before stapling, on postoperative nausea and vomiting in sleeve gastrectomy patients: A randomized controlled trial. Surg Endosc (2022) 36(10):7717–21. doi: 10.1007/s00464-022-09075-3
40. Varner KL, March AL. Prevention of nausea and vomiting after laparoscopic sleeve gastrectomy: Are we doing enough? Aana J (2020) 88(2):142–7.
41. Bataille A, Letourneulx JF, Charmeau A, Lemedioni P, Léger P, Chazot T, et al. Impact of a prophylactic combination of dexamethasone-ondansetron on postoperative nausea and vomiting in obese adult patients undergoing laparoscopic sleeve gastrectomy during closed-loop propofol-remifentanil anaesthesia: A randomised double-blind placebo-controlled study. Eur J Anaesthesiol (2016) 33(12):898–905. doi: 10.1097/eja.0000000000000427
42. Weibel S, Rücker G, Eberhart LH, Pace NL, Hartl HM, Jordan OL, et al. Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: A network meta-analysis. Cochrane Database Syst Rev (2020) 10(10):Cd012859. doi: 10.1002/14651858.CD012859.pub2
43. Apfel CC, Heidrich FM, Jukar-Rao S, Jalota L, Hornuss C, Whelan RP, et al. Evidence-based analysis of risk factors for postoperative nausea and vomiting. Br J Anaesth (2012) 109(5):742–53. doi: 10.1093/bja/aes276
44. Wu YH, Sun HS, Wang ST, Tseng CC. Applicability of risk scores for postoperative nausea and vomiting in a Taiwanese population undergoing general anaesthesia. Anaesth Intensive Care (2015) 43(4):473–8. doi: 10.1177/0310057x1504300409
45. Yi MS, Kang H, Kim MK, Choi GJ, Park YH, Baek CW, et al. Relationship between the incidence and risk factors of postoperative nausea and vomiting in patients with intravenous patient-controlled analgesia. Asian J Surg (2018) 41(4):301–6. doi: 10.1016/j.asjsur.2017.01.005
46. Šimurina T, Mraovic B, Skitarelić N, Andabaka T, Sonicki Z. Influence of the menstrual cycle on the incidence of nausea and vomiting after laparoscopic gynecological surgery: A pilot study. J Clin Anesth (2012) 24(3):185–92. doi: 10.1016/j.jclinane.2011.07.011
47. Harmon D, O'Connor P, Gleasa O, Gardiner J. Menstrual cycle irregularity and the incidence of nausea and vomiting after laparoscopy. Anaesthesia (2000) 55(12):1164–7. doi: 10.1046/j.1365-2044.2000.01719.x
48. Apfel CC, Korttila K, Abdalla M, Kerger H, Turan A, Vedder I, et al. A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. N Engl J Med (2004) 350(24):2441–51. doi: 10.1056/NEJMoa032196
49. Geralemou S, Gan TJ. Assessing the value of risk indices of postoperative nausea and vomiting in ambulatory surgical patients. Curr Opin Anaesthesiol (2016) 29(6):668–73. doi: 10.1097/aco.0000000000000400
50. El Batawi HY. Effect of intraoperative analgesia on children's pain perception during recovery after painful dental procedures performed under general anaesthesia. Eur Arch Paediatr Dent (2015) 16(1):35–41. doi: 10.1007/s40368-014-0143-y
51. Huang KT, Chin CH, Tseng CC, Chang HC, Chen YC, Wang CC, et al. The influence of obesity on different genders in patients with obstructive sleep apnea. ScientificWorldJournal (2014) 2014:487215. doi: 10.1155/2014/487215
52. de Raaff CA, Coblijn UK, de Vries N, Heymans MW, van den Berg BT, van Tets WF, et al. Predictive factors for insufficient weight loss after bariatric surgery: Does obstructive sleep apnea influence weight loss? Obes Surg (2016) 26(5):1048–56. doi: 10.1007/s11695-015-1830-4
53. Drager LF, Togeiro SM, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: A cardiometabolic risk in obesity and the metabolic syndrome. J Am Coll Cardiol (2013) 62(7):569–76. doi: 10.1016/j.jacc.2013.05.045
54. Meng L. Postoperative nausea and vomiting with application of postoperative continuous positive airway pressure after laparoscopic gastric bypass. Obes Surg (2010) 20(7):876–80. doi: 10.1007/s11695-008-9741-2
55. Kao SC, Tsai HI, Cheng CW, Lin TW, Chen CC, Lin CS. The association between frequent alcohol drinking and opioid consumption after abdominal surgery: A retrospective analysis. PloS One (2017) 12(3):e0171275. doi: 10.1371/journal.pone.0171275
56. Cederbaum AI. Alcohol metabolism. Clin Liver Dis (2012) 16(4):667–85. doi: 10.1016/j.cld.2012.08.002
57. Apfel CC, Kranke P, Katz MH, Goepfert C, Papenfuss T, Rauch S, et al. Volatile anaesthetics may be the main cause of early but not delayed postoperative vomiting: A randomized controlled trial of factorial design. Br J Anaesth (2002) 88(5):659–68. doi: 10.1093/bja/88.5.659
58. Brattwall M, Warrén Stomberg M, Rawal N, Segerdahl M, Houltz E, Jakobsson J. Postoperative impact of regular tobacco use, smoking or snuffing, a prospective multi-center study. Acta Anaesthesiol Scand (2010) 54(3):321–7. doi: 10.1111/j.1399-6576.2009.02140.x
59. Woods SD, Chee JB, Sinclair CF, Tremayne AB, Clooney JN. Helicobacter status does not relate to postanesthetic nausea. Helicobacter (2005) 10(5):443–4. doi: 10.1111/j.1523-5378.2005.00352.x
Keywords: nausea, vomiting, sleeve gastrectomy, bariatric surgery, pain, Helicobacter pylori
Citation: Song Y, Zhu J, Dong Z, Wang C, Xiao J and Yang W (2023) Incidence and risk factors of postoperative nausea and vomiting following laparoscopic sleeve gastrectomy and its relationship with Helicobacter pylori: A propensity score matching analysis. Front. Endocrinol. 14:1102017. doi: 10.3389/fendo.2023.1102017
Received: 18 November 2022; Accepted: 10 February 2023;
Published: 22 February 2023.
Edited by:
Mikiko Watanabe, Sapienza University of Rome, ItalyReviewed by:
Yan Li, Southern University of Science and Technology, ChinaHalit Eren Taskin, Istanbul University Cerrahpasa, Türkiye
Copyright © 2023 Song, Zhu, Dong, Wang, Xiao and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jia Xiao, ZWR3aW5zaXVAY29ubmVjdC5oa3UuaGs=; Wah Yang, eWFuZ3dhaEBxcS5jb20=
†These authors share first authorship
 Yali Song
Yali Song Jie Zhu
Jie Zhu Zhiyong Dong1,2,3
Zhiyong Dong1,2,3 Jia Xiao
Jia Xiao Wah Yang
Wah Yang