- Department of Spine Surgery, Beijing Jishuitan Hospital, Beijing, China
Introduction: Intervertebral disc degeneration (IVDD) is an important contributor of low back pain, which represents one of the most disabling symptoms within the adult population. Recently, increasing evidence suggests the potential association between Type 2 diabetes mellitus (T2DM) and IVDD. However, the causal relationship between these two common diseases remains unclear.
Methods: We conducted a two-sample Mendelian randomization (MR) analysis to assess the causal association between T2DM and IVDD. Sensitivity analysis was performed to test for heterogeneity and horizontal pleiotropy. Multivariable MR was also conducted to adjust for the effect of BMI on IVDD.
Results: A total of 128 independent single-nucleotide polymorphisms (SNPs) that were significantly associated with T2DM were selected as instrumental variables in univariable MR analysis. Our results showed that patients with T2DM had a higher risk of developing IVDD (OR, 1.069; 95% CI, 1.026–1.115; p = 0.002). The relationship remained stable in sensitive analysis including multivariable MR, which implicated the direct causal effect of T2DM on IVDD (OR, 1.080; 95% CI, 1.041–1.121; p < 0.001) after adjusting for BMI.
Conclusions: MR analysis indicated a causal effect of T2DM on IVDD, and the effect persisted even when we accounted for the impact of BMI.
Introduction
Intervertebral disc degeneration (IVDD) is currently a common degraded condition in an aging society, referring to an age-dependent, cell-mediated molecular process (1, 2). Degenerated discs are more prone to out-pouching and may press against the nerve roots, which eventually causes low back pain (LBP) or other clinical symptoms. As an increasingly prevalent health problem, IVDD significantly impacts patients’ quality of life and poses a substantial economic burden to countries with rapidly aging populations, such as China (3, 4). To date, in spite of the high prevalence of IVDD, lines of evidence for the risk factors of IVDD have not been fully established yet. Traditionally, IVDD is considered to be a multifactorial disease affected by both genetic and environmental factors including diabetes, obesity, and smoking 5. Recently, increasing evidence has suggested that metabolic disturbances and inflammation might be involved in the development of IVDD, which shifts the focus of research to metabolic risk factors (5).
As the most common metabolic disorder, Type 2 diabetes mellitus (T2DM) threatened aging populations because of its various complications. Apart from being a strong risk factor for cardiovascular diseases and stroke, T2DM may also increase the risk of developing IVDD. To date, the potential relationship between diabetes and IVDD has been recognized in animal and clinical studies. In diabetic models, IVDD-related pathological changes in spine structure such as loss of disc height, decreased vertebral bone mass, and endplate sclerosis were well documented (6–9).However, in contrast to the consistently positive lines of evidence in laboratory studies, clinical studies have produced inconsistent results. Some researchers have inferred that T2DM is a significant risk factor for IVDD using cross-sectional and retrospective studies (10–13). Nevertheless, these cross-sectional or case–control studies failed to examine the independent association between DM and IVDD. It has been challenged that the correlation would disappear when controlling for body mass index (BMI) or other risk factors (14, 15). In fact, these inconsistent results may be due to the limitation of observational studies with susceptibility to bias and an inability to make causal inference.
Recently, Mendelian randomization (MR) studies, which use an epidemiological approach that assesses the causal effect of a risk factor on an outcome, have been increasingly used to overcome the aforementioned limitations and explore causal relationships (16). Since genetic variants are randomly assigned, the confounding factors are minimized by the MR method. Genetic variation significantly associated with exposure can therefore be used as instrumental variables (IVs). There are three assumptions that must be satisfied for instrumental variables: IV1, associated with the exposure; IV2, independent of the outcome given the exposure; and IV3, independent of all confounders known thus far 16. To date, limited evidence for causal factors of IVDD has been reported. In particular, the relationship between diabetes and IVDD has not been fully investigated by MR.
In this regard, we explored the causal effect of T2DM on IVDD using a two-sample MR analysis. Furthermore, as BMI and T2DM are strongly correlated, and because previous observational studies and MR analysis have suggested that causal association may exist between BMI and IVDD (10, 13, 17–19), we therefore conducted a multivariable MR to examine the direct effect of T2DM on IVDD after adjusting for BMI.
Materials and methods
Study design
The study design is shown in Figure 1. We first performed univariable MR to assess the causal relationship between T2DM and IVDD. Then, multivariable MR was conducted to adjust for BMI, which has been suggested to have a causal effect on IVDD (10, 13, 17–19), in order to assess the direct effect of T2DM. We used publicly available GWAS data with the informed consent and ethical approval previously obtained (20–22).
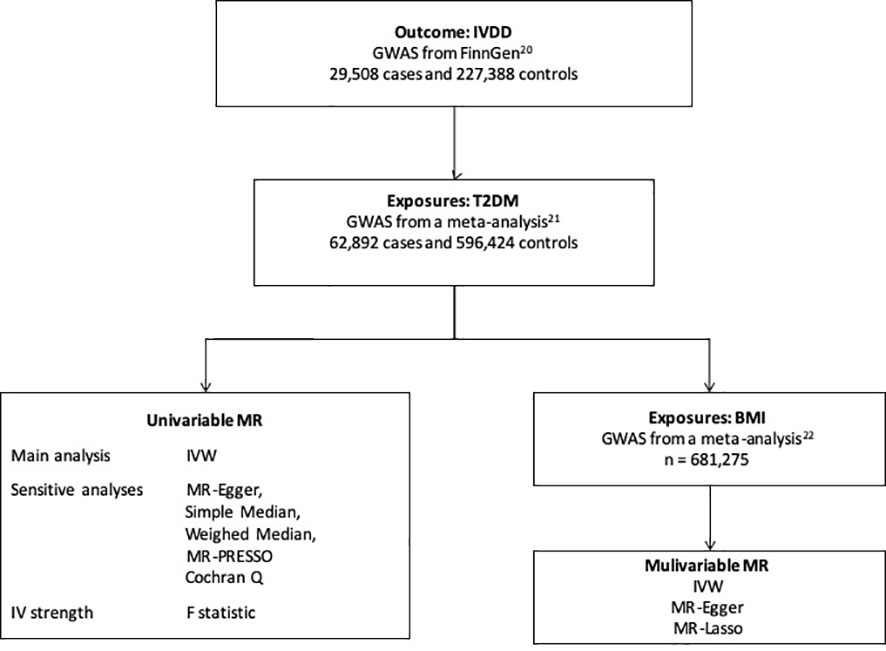
Figure 1 The study design. IVDD, intervertebral disc degeneration; T2DM, Type 2 diabetes mellitus; GWAS, genome-wide association study; MR, Mendelian randomization; BMI, body mass index; IVW, inverse-variance weighted.
GWAS data source
Summary GWAS data for IVDD were available from the FinnGen consortium, including 29,508 cases and 227,388 controls (20). IVDD was diagnosed according to ICD-10 M51, ICD-9 722, and ICD-8 275. Other detailed information of the outcome is presented in Supplementary Table 1.
The GWAS data of T2DM were from a meta-analysis with ~16 million genetic variants in 62,892 T2DM cases and 596,424 controls of European ancestry (21). Analysis was adjusted for age, sex, and the first 20 PCs. Genetic instruments for BMI were identified using results from the largest available meta-analysis of GWAS in 681,275 individuals of European ancestry (22).
Instrumental variable selection
For univariable MR analysis, we first identified independent (linkage disequilibrium, LD clumping r2 threshold = 0.001 and window size = 1,000 kb), genome-wide single-nucleotide polymorphisms (SNPs) significantly associated with T2DM (p < 5 × 10−8). For the multivariable MR analysis, we pooled all genome-wide significant SNPs that were significantly associated with T2DM or BMI and then clumped these SNPs with respect to the lowest p-value corresponding to any of the two using a 1,000-kb window and pairwise LD r2 < 0.001. We calculated the proportion of phenotypic variance explained by instrumental variable SNPs of T2DM and computed the F statistic to verify whether they were strong instruments (23).
MR analysis
We used the inverse-variance weighted (IVW) method as the primary MR approach (16). MR-Egger, weighted median, simple median tests, and MR-PRESSO were further conducted to control horizontal pleiotropy (16). We also used the Cochran Q statistic and MR-Egger (intercept) to test for the heterogeneity and pleiotropy (16).
Next, as BMI and T2DM are strongly correlated and the causal association may exist between BMI and IVDD in previous studies (10, 13, 17–19), we conducted multivariable MR adjusting for BMI to show the casual effect of T2DM on IVDD. The methods we used to conduct multivariable MR included IVW, MR-Egger, and MR-Lasso (24). Moreover, Cochran Q statistic and MR-Egger (intercept) were also conducted for the heterogeneity and pleiotropy of multivariable MR analysis. All statistical analyses were conducted using the “Two Sample MR” (version 0.5.6) and “Mendelian Randomization” (version 0.5.1) in the statistical program R (version 4.1.1). p < 0.05 was considered as statistically significant.
Results
Genetic instruments
We finally identified 128 independent SNPs that are significantly related to T2DM as instrumental variables (Supplementary Table 2). The phenotypic variances they accounted for was 13.9%, calculated by R2. The F statistics of each SNP was greater than 10 (Supplementary Table 2). These findings suggested that there is no potential weak instrument bias, satisfying hypothesis IV1.
Causal relationship between IVDD and T2DM
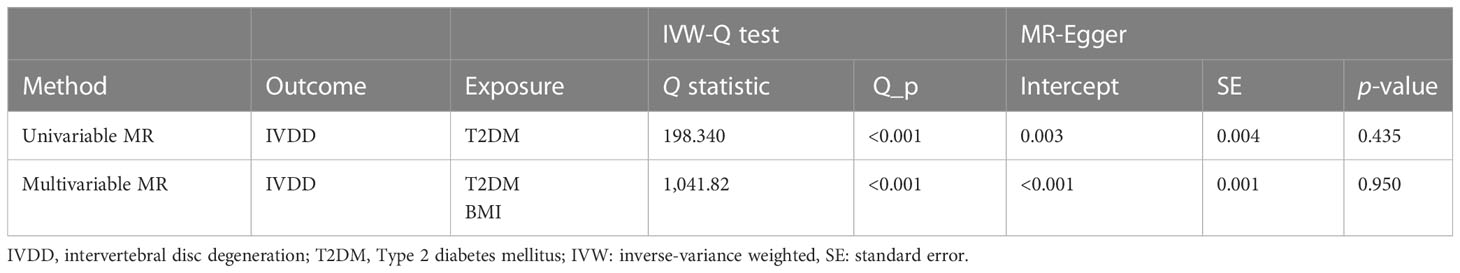
Cochran Q test showed that there was instrumental heterogeneity (p < 0.05) (Table 1). Therefore, we employed the random-effect IVW method. The result showed that patients with T2DM have a 6.9% higher risk of IVDD than those without T2DM (OR, 1.069; 95% CI, 1.026–1.115; p = 0.002) (Figure 2).
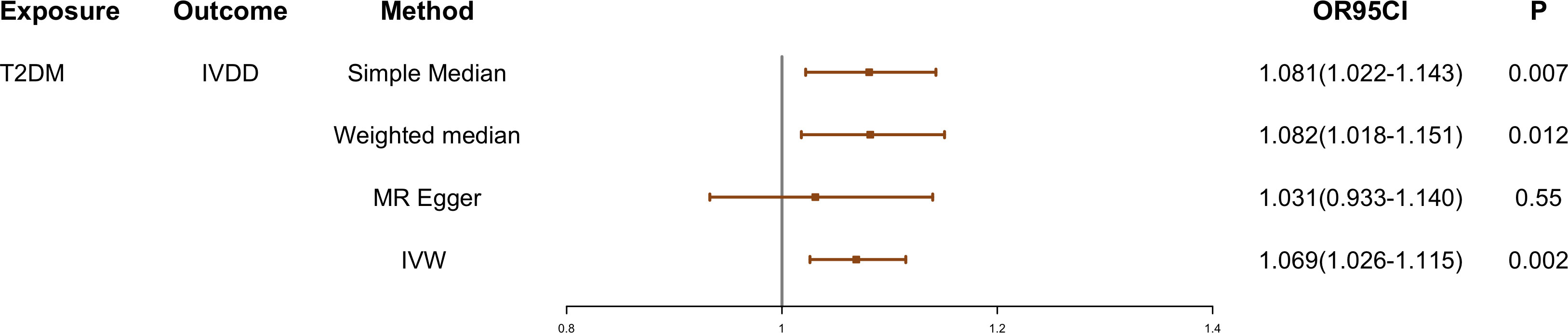
Figure 2 The causal effect of T2DM on IVDD. IVDD, intervertebral disc degeneration; T2DM, Type 2 diabetes mellitus; IVW, inverse-variance weighted. OR, odds ratio; CI, confidence interval.
Sensitivity analysis
The effect values obtained from simple median (OR, 1.081; 95% CI, 1.022–1.143; p = 0.007), weighted median (OR, 1.082; 95% CI, 1.018–1.151; p = 0.012), and MR-Egger (OR, 1.031; 95% CI, 0.933–1.140; p = 0.550) methods were consistent with the IVW estimate (Figures 2 and 3). There was also no significant differences between MR-Egger intercept and 0 (Table 1), which suggested no interference of horizontal pleiotropy in our study.
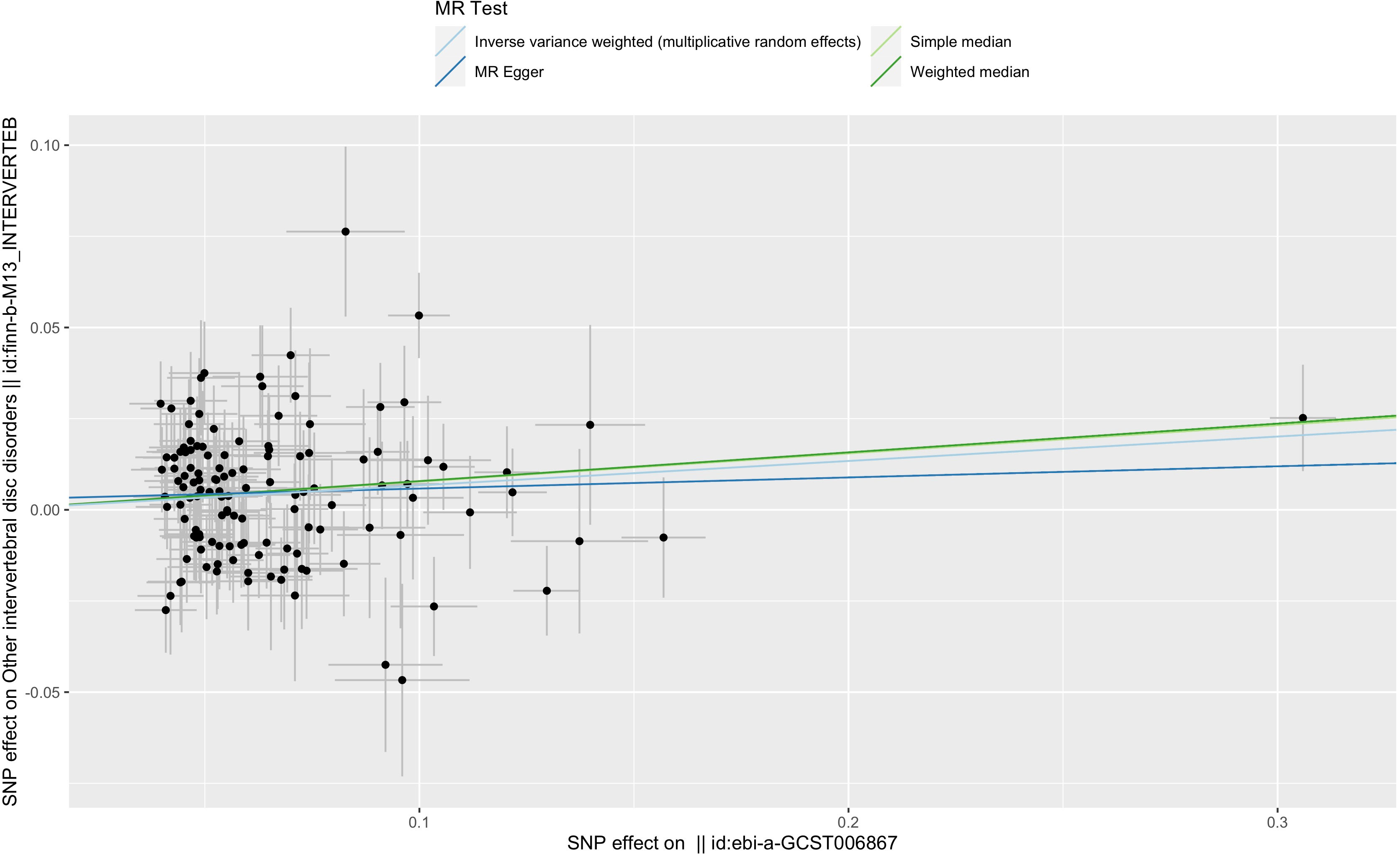
Figure 3 Scatter plot of the relationship between T2DM and IVDD using inverse-variance weighted, simple median, MR-Egger, and weighted median. SNP, single-nucleotide polymorphism; ebi-a-GCST006867, the GWAS ID of BMI; finn-b-M13-INTERVERTEB, the GWAS ID of IVDD.
Furthermore, using MR-PRESSO, three outliers with horizontal pleiotropy were found. After removing these abnormal SNPs, we obtained corrected effect estimate showing similar results (OR, 1.059; 95% CI, 1.017–1.102; p = 0.006). The leave-one-out plot also showed that removing any of the SNPs did not change the results significantly, suggesting the reliability of the results (Supplementary Figure 1).
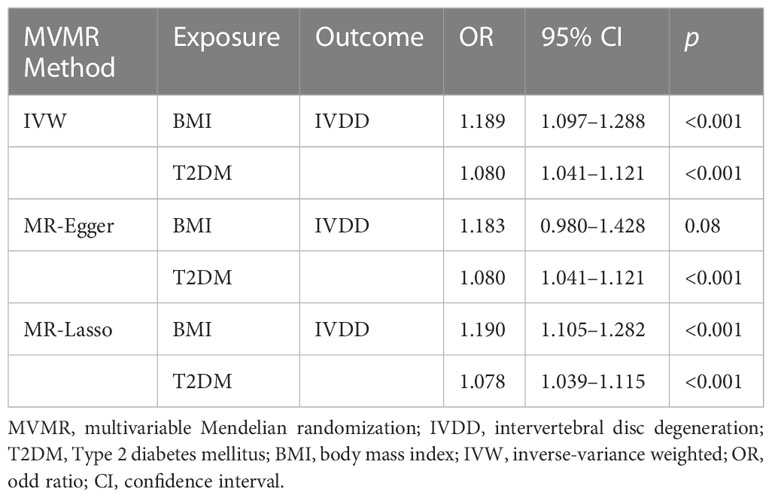
The causal association between BMI and IVDD was suggested in previous studies. Therefore, we conducted a multivariable MR analysis including both BMI and T2DM as exposures to explore the direct effect of T2DM on IVDD. There were 829 independent SNPs selected as instrumental variables for T2DM and BMI (Supplementary Table 3). Although the relationship between BMI and IVDD was confirmed (OR, 1.189; 95% CI, 1.091–1.288; p < 0.001), T2DM still showed a direct effect on IVDD (OR, 1.080; 95% CI, 1.041–1.121; p<0.001) conditioned on BMI (Figure 4) (Table 2). Moreover, multivariable MR-Egger suggested that there was no horizontal pleiotropy in MR analysis (Intercept p > 0.05). Moreover, although the Cochran Q test suggested that there may be heterogeneity (p < 0.01), the result of the MR-Egger test was the same as that of IVW (OR, 1.080; 95% CI, 1.041–1.121; p < 0.001) (Table 2). The result of the MR-Lasso test also remained stable after removing heterogeneous SNPs (OR, 1.078; 95% CI, 1.039–1.115; p < 0.001) (Table 2). Taken together, our results are proven to be reliable.
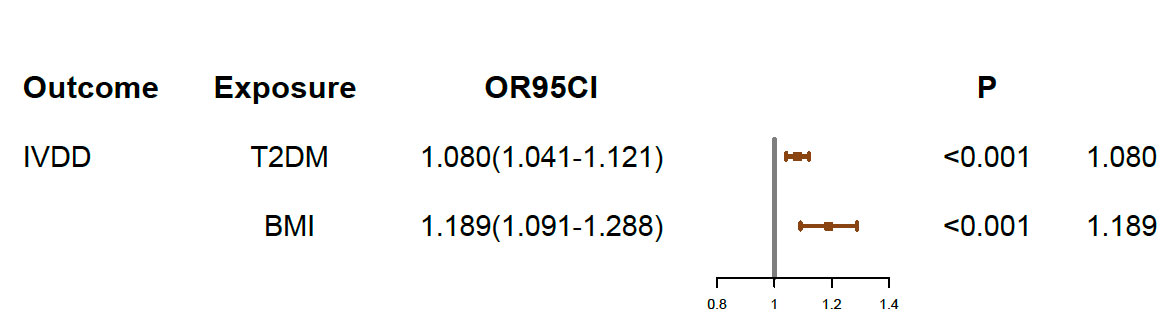
Figure 4 Multivariable MR results. IVDD, intervertebral disc degeneration; T2DM, Type 2 diabetes mellitus; OR, odds ratio; CI, confidence interval.
Discussion
To date, as the role of metabolic characteristics was evident in the development of IVDD, the influence of diabetes on IVDD has aroused widespread attention. However, strong clinical evidence for the direct relationship between diabetes and IVDD remains insufficient. In this study, we demonstrated that T2DM was an important risk factor causally associated with IVDD by using MR analysis. Furthermore, the multivariable MR suggested that the causal association between T2DM and IVDD was independent of BMI.
Our study was in line with five recent studies, which implicated the potential association between diabetes and IVDD. In 2016, Agius et al. first conducted a cross-sectional study on 100 patients with diabetes, investigating the changes in intervertebral disc of patients with T2DM. They found that diabetes might be a risk factor for IVDD since it is associated with significantly lower height of lumbar discs (11). Furthermore, a retrospective single-center study in Chinese patients with diabetes suggested that longer duration and poorly controlled T2DM were risk factors for lumbar disc degeneration. In addition, long-standing diabetes may be a predictor for severe IVDD (p < 0.05) (12).
Considering that the above samples were sill not sufficient enough, larger populations are required for adequate power. Hence, Jakoi et al. performed a cross-sectional study using a large insurance industry database in USA and discovered that IVDD is correlated with diabetes (10). Similarly, a case–control study that enrolled 160,911 patients with IVDD and 315,225 controls in a group of military members also suggested that diabetes was a risk factor for developing IVDD (13). However, these two large studies still have some limitations such as coding bias. Additionally, since the use of cross-sectional study design cannot confirm the causal relationship, Teraguchi et al. conducted the Wakayama Spine Study in a longitudinal population-based cohort, demonstrating that diabetes was a significant contributor to IVDD in the upper lumbar spine (OR, 6.83; 95% CI, 1.07-133.7) (25). However, these results should also be interpreted with caution, as the sample size of patients with diabetes was small. Therefore, large-scale studies and highly persuasive lines of evidence are needed to further validate the causal relationship between diabetes and IVDD.
With respect to the underlying mechanism, crucial aspects of the linked pathogenesis of IVDD in T2DM are identified using animal models. In general, IVDD consists of three main components: the inner nucleus pulposus (NP), the outer annulus fibrosus (AF), and the cartilaginous endplates (CEPs), which anchor the disc to the adjacent vertebrae (26). In T2DM, irreversible formation and accumulation of advanced glycation end products (AGEs) due to hyperglycemia may result in pathophysiological changes in CEPs and contribute to undermining the nutrient supply, cell viability, matrix homeostasis, and biomechanical properties of the intervertebral disc, leading to structural weakening and, ultimately, IVDD. Interestingly, preclinical evidence from a study of a rat model suggests that T2DM compromises IVDD composition, ECM homeostasis, and biomechanical behavior changes, rather than obesity (6). In summary, diabetic models indicated that hyperglycemia could exert a direct effect on IVDD by multiple diabetic-related pathways (1, 5, 27, 28).
As mentioned above, observational studies could not provide insight into the causal relationship between diabetes and IVDD, even based on a larger sample scale. Furthermore, unmeasured confounding variables, reverse causality, and survival bias may fail to give strong evidence on the relationship of interest. Therefore, we conducted the first MR study of T2DM and IVDD to address this uncertainty. MR analysis used genetic variants as instrumental variables for causal inferences about the effect of modifiable exposures on health- and disease-related outcomes in the presence of unobserved confounding variables (29). In consequence, differences in the outcome can be credited to the difference in the risk factor if the genetic variants are not related to confounders (30).
It should be noted that increasing evidence suggested that higher BMI, especially being overweight or obese, is associated with the risk of IVDD (10, 13, 17–19). Moreover, as higher BMI is interrelated with T2DM (31), the confounding effect of BMI should be paid attention to when discussing the relationship between T2DM and IVDD. As a result, we included both T2DM and BMI in our multivariable MR analysis to explore whether the effect of T2DM on IVDD is independent of BMI. In our study, although the causal association between BMI and IVDD was observed, T2DM was still associated with a higher risk of IVDD after adjusting for BMI in IVW analysis. Hence, we suggested that the causal effect of T2DM on IVDD persisted even when the impact of BMI was accounted for. In addition, the effect of BMI should also be considered when discussing other risk factors for IVDD.
The strengths of the study are as follows: First, a causal association was demonstrated using two large GWAS summary datasets for the first time, which is important for the prevention of IVDD and future clinical research. Second, we used multiple methods to test and account for heterogeneity and horizontal pleiotropy, in order to ensure the reliability of the results. Last, we used multivariable MR to examine the direct effect of T2DM on IVDD adjusting for BMI. However, some limitations should be noted: The GWAS data we used were from the European descent population, and the result cannot be generalized to other populations.
In summary, this is the first MR study to explore the causal effect of T2DM on IVDD, and the effect persisted even when we accounted for the impact of BMI. Moreover, further research is warranted to understand the biological mechanism of this causal effect.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
PJ and WT conceptualized and designed the study. PJ, YX, BX, YW, KY, and JZ performed data analysis. PJ wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Beijing Hospitals Authority Innovation Studio of Young Staff Funding Support, code: 202110.
Acknowledgments
We are grateful to all the studies that have made the public GWAS summary data available, and to all the investigators and participants who contributed to the FinnGen study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1100874/full#supplementary-material
References
1. Alpantaki K, Kampouroglou A, Koutserimpas C, Effraimidis G, Hadjipavlou A. Diabetes mellitus as a risk factor for intervertebral disc degeneration: a critical review. Eur Spine J (2019) 28(9):2129–44. doi: 10.1007/s00586-019-06029-7
2. Fenn J, Olby NJ. Canine spinal cord injury consortium (CANSORT-SCI). classification of intervertebral disc disease. Front Vet Sci (2020) 7:579025. doi: 10.3389/fvets.2020.579025
3. Zhou M, Wang H, Zeng X, Yin P, Zhu J, Chen W, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet (2019) 394(10204):1145–58. doi: 10.1016/S0140-6736(19)30427-1
4. Wu D, Wong P, Guo C, Tam LS, Gu J. Pattern and trend of five major musculoskeletal disorders in China from 1990 to 2017: findings from the global burden of disease study 2017. BMC Med (2021) 19(1):34. doi: 10.1186/s12916-021-01905-w
5. Francisco V, Pino J, González-Gay MÁ, Lago F, Karppinen J, Tervonen O, et al. A new immunometabolic perspective of intervertebral disc degeneration. Nat Rev Rheumatol (2022) 18(1):47–60. doi: 10.1038/s41584-021-00713-z
6. Fields AJ, Berg-Johansen B, Metz LN, Miller S, La B, Liebenberg EC, et al. Alterations in intervertebral disc composition, matrix homeostasis and biomechanical behavior in the UCD-T2DM rat model of type 2 diabetes. J Orthop Res (2015) 33(5):738–46. doi: 10.1002/jor.22807
7. Illien-Junger S, Grosjean F, Laudier DM, Vlassara H, Striker GE, Iatridis JC. Combined anti-inflammatory and anti-AGE drug treatments have a protective effect on intervertebral discs in mice with diabetes. PloS One (2013) 8(5):e64302. doi: 10.1371/journal.pone.0064302
8. Fields AJ, Berg-Johansen B, Metz LN, Miller S, La B, Liebenberg EC, et al. Alterations in intervertebral disc composition, matrix homeostasis and biomechanical behavior in the UCDT2DM rat model of type 2 diabetes. J Orthop Res (2015) 33(5):738–46. doi: 10.1002/jor.22807
9. Illien-Jünger S, Lu Y, Qureshi SA, Hecht AC, Cai W, Vlassara H, et al. Chronic ingestion of advanced glycation end products induces degenerative spinal changes and hypertrophy in aging pre-diabetic mice. PloS One (2015) 10(2):e0116625. doi: 10.1371/journal.pone.0116625
10. Jakoi AM, Pannu G, D'Oro A, Buser Z, Pham MH, Patel NN, et al. The clinical correlations between diabetes, cigarette smoking and obesity on intervertebral degenerative disc disease of the lumbar spine. Asian Spine J (2017) 11(3):337–47. doi: 10.4184/asj.2017.11.3.337
11. Agius R, Galea R, Fava S. Bone mineral density and intervertebral disc height in type 2 diabetes. J Diabetes Complicat (2016) 30(4):644–50. doi: 10.1016/j.jdiacomp.2016.01.021
12. Liu X, Pan F, Ba Z, Wang S, Wu D. The potential effect of type 2 diabetes mellitus on lumbar disc degeneration: a retrospective single-center study. J Orthop Surg Res (2018) 13(1):52. doi: 10.1186/s13018-018-0755-8
13. Steelman T, Lewandowski L, Helgeson M, Wilson K, Olsen C, Gwinn D. Population-based risk factors for the development of degenerative disk disease. Clin Spine Surg (2018) 31(8):E409–12. doi: 10.1097/BSD.0000000000000682
14. Hangai M, Kaneoka K, Kuno S, Hinotsu S, Sakane M, Mamizuka N, et al. Factors associated with lumbar intervertebral disc degeneration in the elderly. Spine J (2008) 8(5):732–40. doi: 10.1016/j.spinee.2007.07.392
15. Videman T, Battié MC, Gibbons LE, Kaprio J, Koskenvuo M, Kannus P, et al. Disc degeneration and bone density in monozygotic twins discordant for insulin- dependent diabetes mellitus. J Orthop Res (2000) 18(5):768–72. doi: 10.1002/jor.1100180514
16. Burgess S, Davey Smith G, Davies NM, Dudbridge F, Gill D, Glymour MM, et al. Guidelines for performing mendelian randomization investigations. Wellcome Open Res (2020) 4:186. doi: 10.12688/wellcomeopenres.15555.2
17. Zhou J, Mi J, Peng Y, Han H, Liu Z. Causal associations of obesity with the intervertebral degeneration, low back pain, and sciatica: A two-sample mendelian randomization study. Front Endocrinol (Lausanne) (2021) 12:740200. doi: 10.3389/fendo.2021.740200
18. Fabiane SM, Ward KJ, Iatridis JC, Williams FM. Does type 2 diabetes mellitus promote intervertebral disc degeneration? Eur Spine J (2016) 25(9):2716–20. doi: 10.1007/s00586-016-4612-3
19. Samartzis D, Karppinen J, Chan D, Luk KD, Cheung KM. The association of lumbar intervertebral disc degeneration on magnetic resonance imaging with body mass index in overweight and obese adults: a population-based study. Arthritis Rheumatol (2012) 64(5):1488–96. doi: 10.1002/art.33462
20. Kurki MI, Karjalainen J, Palta P, Sipilä TP, Kristiansson K, Donner K, et al. FinnGen: Unique genetic insights from combining isolated population and national health register data. medRxiv (2022). doi: 10.1101/2022.03.03.22271360
21. Xue A, Wu Y, Zhu Z, Zhang F, Kemper KE, Zheng Z, et al. Genome-wide association analyses identify 143 risk variants and putative regulatory mechanisms for type 2 diabetes. Nat Commun (2018) 9(1):2941. doi: 10.1038/s41467-018-04951-w
22. Yengo L, Sidorenko J, Kemper KE, Zheng Z, Wood AR, Weedon MN, et al. Meta-analysis of genome-wide association studies for height and body mass index in ∼700000 individuals of European ancestry. Hum Mol Genet (2018) 27(20):3641–9. doi: 10.1093/hmg/ddy271
23. Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan NA, Thompson JR. Assessing the suitability of summary data for two-sample mendelian randomization analyses using MR-egger regression: the role of the I2 statistic. Int J Epidemiol (2016) 45(6):1961–74. doi: 10.1093/ije/dyw220
24. Burgess S, Thompson SG. Multivariable mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am J Epidemiol (2015) 181(4):251–60. doi: 10.1093/aje/kwu283
25. Teraguchi M, Yoshimura N, Hashizume H, Yamada H, Oka H, Minamide A, et al. Progression, incidence, and risk factors for intervertebral disc degeneration in a longitudinal population-based cohort: the Wakayama spine study. Osteoarthritis Cartilage (2017) 25(7):1122–31. doi: 10.1016/j.joca.2017.01.001
26. Brinjikji W, Luetmer PH, Comstock B, Bresnahan BW, Chen LE, Deyo RA, et al. Systematic literature review of imaging features of spinal degeneration in asymptomatic populations. Am J Neuroradiol (2015) 36(4):811–6:A4173. doi: 10.3174/ajnr
27. Cannata F, Vadalà G, Ambrosio L, Fallucca S, Napoli N, Papalia R, et al. Intervertebral disc degeneration: A focus on obesity and type 2 diabetes. Diabetes Metab Res Rev (2020) 36(1):e3224. doi: 10.1002/dmrr.3224
28. Mahmoud M, Kokozidou M, Auffarth A, Schulze-Tanzil G. The relationship between diabetes mellitus type II and intervertebral disc degeneration in diabetic rodent models: A systematic and comprehensive review. Cells (2020) 9(10):2208. doi: 10.3390/cells9102208
29. Palmer TM, Lawlor DA, Harbord RM, Sheehan NA, Tobias JH, Timpson NJ, et al. Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat Methods Med Res (2012) 21(3):223–42. doi: 10.1177/0962280210394459
30. Sanderson E. Multivariable mendelian randomization and mediation. Cold Spring Harb Perspect Med (2021) 11(2):a038984. doi: 10.1101/cshperspect.a038984
Keywords: type 2 diabetes mellitus, intervertebral disc degeneration, Mendelian randomization (MR) analysis, GWAS data, body mass index
Citation: Jin P, Xing Y, Xiao B, Wei Y, Yan K, Zhao J and Tian W (2023) Diabetes and intervertebral disc degeneration: A Mendelian randomization study. Front. Endocrinol. 14:1100874. doi: 10.3389/fendo.2023.1100874
Received: 17 November 2022; Accepted: 14 February 2023;
Published: 28 February 2023.
Edited by:
Ashim Gupta, Future Biologics, United StatesReviewed by:
Li-Bo Jiang, Fudan University, ChinaFeifei Pu, Huazhong University of Science and Technology, China
Copyright © 2023 Jin, Xing, Xiao, Wei, Yan, Zhao and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Tian, d2VpdGlhbl9zcGluZUAxMjYuY29t
 Peihao Jin
Peihao Jin Yonggang Xing
Yonggang Xing