
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol. , 16 March 2023
Sec. Clinical Diabetes
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1099310
This article is part of the Research Topic Novel Treatments and the Underlying Mechanisms for Diabetic Foot and Related Diseases View all 12 articles
Diabetes has become a global public health problem. Diabetic foot is one of the most severe complications of diabetes, which often places a heavy economic burden on patients and seriously affects their quality of life. The current conventional treatment for the diabetic foot can only relieve the symptoms or delay the progression of the disease but cannot repair damaged blood vessels and nerves. An increasing number of studies have shown that mesenchymal stem cells (MSCs) can promote angiogenesis and re-epithelialization, participate in immune regulation, reduce inflammation, and finally repair diabetic foot ulcer (DFU), rendering it an effective means of treating diabetic foot disease. Currently, stem cells used in the treatment of diabetic foot are divided into two categories: autologous and allogeneic. They are mainly derived from the bone marrow, umbilical cord, adipose tissue, and placenta. MSCs from different sources have similar characteristics and subtle differences. Mastering their features to better select and use MSCs is the premise of improving the therapeutic effect of DFU. This article reviews the types and characteristics of MSCs and their molecular mechanisms and functions in treating DFU to provide innovative ideas for using MSCs to treat diabetic foot and promote wound healing.
Diabetes is a significant global public health problem (1). The number of diabetic patients in 2021 was 536.6 million, and it is expected to increase to approximately 783.2 million people by 2045 (2). With the prolongation and aggravation of the disease, patients with diabetes often present with severe lower extremity vascular disease, leading to DFU. Diabetic foot is one of the most severe complications of diabetes and is the leading cause of surgical non-traumatic amputation (3). Studies have found that approximately 25% of people with diabetes will suffer a DFU in their lifetime, and 30% of people with a diabetic foot will experience disease progression that would eventually leads to amputation (4, 5).
Currently, there is no effective clinical treatment plan for diabetic foot, as conservative medical treatment is only a routine method for diabetic foot treatment. For patients with severe ischemia and unsatisfactory effects of systemic drug treatment, vascular intervention and other operations are necessary to implement blood-flow reconstruction. However, in patients with a diabetic foot, the distal vascular outflow tract is poor, and vascular lesions of the lower extremities are diffuse and multiple. Vascular intervention can only improve stenosis of large vessels to a certain extent, and the improvement effect is limited. Studies have reported that patients with diabetic feet are prone to restenosis after the intervention, the recovery rate of peripheral blood flow is still very low, and the amputation rate is still high (6). Diabetic foot is becoming a worldwide public health problem threatening human health (7, 8). Therefore, a new method to accelerate diabetic wound healing is urgently required.
Previous studies have shown that approximately 50% of diabetic foot cases are caused by neuropathy alone, while peripheral arterial occlusive disease accounts for only 15% of cases. Furthermore, in 35% of cases, diabetic foot is caused by a combination of neuropathy and vascular disease (9, 10). In addition, microvascular diseases, biomechanical abnormalities, joint activity, and infection are increased, and multiple causes can interact (11). As a result, peripheral disease, neuropathy, deformity, previous amputation, and infection are the main factors that lead to DFU development (12).. Currently, conventional treatments—including wound dressing, hyperbaric oxygen therapy (HBOT), negative pressure wound therapy, total contact casting bracing, and wound debridement—can only relieve patients’ symptoms or delay the disease progression. However, they cannot repair damaged blood vessels and nerves. An increasing number of studies have shown that MSCs can promote angiogenesis and re-epithelialization, participate in immune regulation, reduce inflammation, and finally repair DFU, rendering it an effective means of treating diabetic foot disease (13); it is a potential new method for the treatment of the diabetic foot. This article reviews stem cells’ function and molecular mechanisms in treating diabetic foot, to provide innovative ideas for using stem cells to treat diabetic foot and promote wound healing.
Various factors cause the formation of DFU, and the common causes are poor blood sugar control, neuropathy, ischemia, nutritional dysfunction, trauma, and local infection, among others. The advanced glycation end products (AGEs) is a general term for a series of highly active end products formed by non-enzymatic glycosylation (also known as Maillard reaction) between the amino groups of proteins, fatty acids or nucleic acids, and the aldehyde groups of reducing sugars, which is highly associated with the complications of diabetes (14). In diabetic patients, due to metabolic disorders, chronic inflammation and accumulation of AGEs, vascular endothelial injury and hyperplasia, enhanced platelet adhesion, micro-thrombosis, microvascular bleeding, and exudation occur (15). In addition to abnormal glucose metabolism, diabetic patients are often accompanied by abnormal lipid metabolism, which promotes the release of inflammatory mediators, thus inducing the infiltration of macrophages and other immune cells (16). High lipid and sugar promotes the generation of inflammatory mediators, ultimately leading to sustained high inflammation in the body (17). In diabetic patients, the phagocytosis function of white blood cells and related immune cells is down-regulated. The duration of inflammatory factors in diabetic foot ulcer wounds is prolonged to compensate for the decline in white blood cell activity, leading to the downregulated function of fibroblasts and vascular endothelial cells. The formation of granulation tissue is inhibited (18, 19). Under the stimulation of a high glucose environment, the oxidative stress level of the body increases, and a high level of reactive oxygen species (ROS) will lead to the weakened antioxidant effect of the body, and inhibit the release of cytokines and growth factors and the formation of fibroblasts, collagen fibers, and new blood vessels (20, 21). Finally, capillary stenosis or obstruction exacerbates microcirculation disturbance.
Furthermore, metabolic disorders of diabetes lead to degeneration of peripheral nerve axons and nerve membrane cells, motor, sensory, and autonomic nerves dysfunction, resulting in further decline of limb perfusion effect, sensory dysfunction, muscle atrophy, and tendon and ligament sclerosis (22), followed by foot deformities and increased pressure on the forefoot. Metabolic products cannot be excluded, while extremal ischemia and hypoxia, bacterial growth, extremal ulceration, wound healing is challenging, and foot infection can become worsened (23). As blood flow is impaired, it is often difficult for drugs to reach the affected area, and DFU can progress from a simple infection to widespread gangrene (24). The occurrence and development of DFU involve various pathophysiological processes, and these complex processes often transform and superimpose each other, which renders the treatment of DFU a challenge.
Since the occurrence of DFU, people have been looking for the best treatment method. Conventional treatments of DFU mainly include wound debridement, wound dressing, hyperbaric oxygen therapy, negative pressure wound therapy, and off-loading.
Debridement is the most commonly used method, and the widely used types include surgical debridement, enzyme debridement, biological debridement, and ultrasonic debridement (25). The clearance goals include removing deactivated, necrotic, and infected tissue from the ulcer and retaining healthy, blood supply-rich tissue. In addition, debridement promotes healing through the surrounding healthy granulation tissue by eliminating infected tissue, senescent cells, and bacterial biofilms (26). Debridement is the most basic method in the treatment of DFU.
Negative pressure wound therapy involves placing a vacuum device on the ulcer wound after debridement. This vacuum device can collect large amounts of exudate, keep the wound clean and dry, and reduce the frequency of dressing replacement (27). In addition, continuous negative pressure drainage can also provide an irrigation solution to promote wound healing.
Hyperbaric Oxygen Therapy (HBOT) can be divided into two methods: local delivery of oxygen to ulcers and systemic delivery of oxygen. HBOT can improve local tissue perfusion, stimulate collagen synthesis, growth factor production, and neovascularization (28). In DFU patients, local oxygenation of ulcers is impaired. HBOT can also inhibit anaerobic bacteria and reduce the use of antibiotics (29, 30). However, the therapeutic value of HBOT obtained through clinical studies remains controversial. Some studies have suggested that HBOT could improve short-term but not long-term ulcer healing efficacy of DFU and could not reduce the amputation rate of DUF (31, 32).
The primary function of wound dressing is to provide a protective barrier for DFU. Meanwhile, some new bandages can inhibit bacteria and promote the speed of blood vessel and tissue regeneration (33). Hydrogels and alginate are currently used for medical dressings, and silver ions and other nanoparticles can significantly improve the therapeutic effect (34–36). For example, Tsang et al. reported that dressing containing nanocrystalline silver and manuka honey could effectively play an antibacterial role in treating DFU and inhibit the generation of drug-resistant bacteria (37). Wound dressing for various sources is constantly being improved and developed.
Shear stress and vertical pressure on the plantar as the ground surface are adverse factors for DFU healing (38). Therefore, the principle of offloading is to reduce pressure on the plantar and forefoot of the DFU (39). The several ways to relieve foot load include orthopedic walking aids and modified shoes used in DFU treatment (40). Compared with the modified shoes, the total contact casting bracing can reduce the load on the sole, mechanically help to reduce and redistribute the pressure of the DFU, and contribute to the repair of ulcers, and is considered an important means for the treatment of DFU (41, 42). However, the production of total contact casting bracing requires personalization for different patients.
Other considerations, such as glycemic control, vascular assessment, use of sensitive antibiotics, and psychotherapy in patients with DFU, have been fully considered in previous research (43, 44). In addition, amputation may be a life-saving option if the patient’s condition becomes too severe to salvage a limb (45, 46). Although there are many therapeutic methods, treating DFU is still one of the thorny problems in the complications of diabetes.
MSCs are a type of pluripotent stem cells that were first discovered by FriedenStein et al. (47, 48). The term “mesenchymal” refers to the embryonic origin of cells. “Mesenchymal stem cells” were initially named fibroblast colony-forming units or bone marrow stromal cells, and can differentiate into various mesodermal tissues (49). The mesoderm is one of the three main layers formed early in embryonic development. It produces various connective tissues, such as muscle, bone, cartilage, and fat, and cells forming blood vessels, blood cells, and the urogenital system (50). In addition, it has been found that MSCs can be used as ectoderm and endoderm-derived cells, such as liver and nerve cells (51). The differentiation potential of MSCs may depend on the source of stem cells, amplification conditions, and the culture microenvironment. The differentiation process can be induced by specific hormones, growth factors, or specific differentiation agents (52). A complex interaction of genetic and epigenetic factors also controls the differentiation process. Genetic factors include the expression of particular transcription factors and signaling molecules, while epigenetic factors include histone modification, DNA methylation, and altered expression of non-coding RNA (53).
The main feature of stem cells is their diverse origin and potential for self-renewal and multi-differentiation. Moreover, MSCs promote tissue repair by releasing growth factors and cytokines, which help recruit other cells to the damaged site (54). These growth factors and cytokines also promote the formation of new blood vessels necessary for tissue repair. MSCs can also regulate immune system activity, reduce inflammation, and suppress immune responses (55), rendering stem cell therapy a new option for repairing and regenerating tissues. This property renders them promising candidates for cellular therapies for a variety of diseases, such as autoimmune diseases and graft-versus-host diseases.
Numerous studies have found that stem cell transplantation can improve various diseases, such as diabetic retinopathy and keratopathy (56, 57), congenital cataracts (58), ocular surface burns (59, 60), severe skin burns (61, 62), myocardial infarction (63, 64), Parkinson’s disease (65, 66), Huntington’s disease (67, 68), and DFU (48, 69). In addition, MSCs can promote wound healing (70, 71) and serve as a cell source for many tissue engineering applications, including bone regeneration (72, 73), cartilage regeneration (74, 75), neurogenesis (76, 77), myocardial regeneration (78, 79), inflammatory bowel disease (80) and DFU (81, 82).
MSCs are easy to obtain and they belong to a class of immunodeficient cells. In general, allogeneic gene transplantation does not cause immune rejection. Previous studies have shown that most stem cells express low levels of human leukocyte antigen (HLA) class I. They do not express or lower express HLA class II, nor do they express co-stimulator factor (CD40, CD80, and CD86) and surface markers of hematopoietic cells (CD34, CD45, CD79, and CD14) (83–85). This property enables stem cells to be immune-privileged without causing immunological conflict between host and transplanted cells (86). The presence of HLA class I is important because low levels of HLA class I can protect cells from natural killer (NK) cell-mediated cytotoxicity (87). It has been reported that MSCs express HLA class II after being exposed to the pro-inflammatory microenvironment of damaged tissues (86). MSCs have been reported to be highly immunogenic after transplantation into the host (88). More than 90% of undifferentiated MSCS express HLA class II when exposed to IFN-γ (89). In addition, Agudo et al. reported that Hair follicle stem cells downregulate major histocompatibility complex (MHC) class I in the static state to avoid immune surveillance (90). Changes in the immunogenicity of MSCs may depend on many factors, including cell state and microenvironment. Therefore, more studies on the details related to the immunogenicity of MSCs are needed to help improve the efficiency of MSCs transplantation.
Compared with mononuclear cells and endothelial progenitor cells mainly derived from autologous cells, they are suitable for a wide range of clinical applications and the promotion of later stem cell products. MSCs express a series of cell surface immune markers, based on which the International Society for Cellular Therapy (ISCT) formulated a set of identification criteria for MSCs in 2006 (1): plasticity and adherence (2); expression of CD73, CD90, and CD105, and no expression of CD14, CD34, CD45, CD11b, CD79α, CD19, and HLA-DR; (3) capability to differentiate into chondrocytes, osteoblasts, and adipocytes (91). The ISCT guidelines aim to standardize mesenchymal stem cell research and promote collaboration among investigators. Generally, MSCs from different tissue sources can express the typical immunophenotypes of MSCs, but there are slight differences in the expression of the remaining immunophenotypes. It is possible that this standard will be revised in the future as research progresses and new knowledge becomes available.
There are many sources of MSCs. Current research shows that stem cells can be extracted from different tissues. There are more studies on bone marrow MSCs (BM-MSCs), human numerical core MSCs (hUC-MSCs), adipose tissue-derived MSCs (ADSCs), urine-derived stem cells (USCs), and placenta-derived MSCs (PD-MSCs).
BM-MSCs are a group of heterogeneous cells composed of pluripotent adult stem cells with the potential ability for multi-differentiation, including chondrocytic, adipocytic, or osteocytic lineages (92). It represents ~ 0.001–0.01% of bone marrow mononuclear cells (BMMNCs) and expresses CD73, CD90, and CD105 but does not express CD14, CD45, CD34, or CD11b, CD79α, CD19, or HLA-DR surface molecules (93). Due to its low abundance, extensive in vitro culture and amplification are required to obtain sufficient quantities for research or clinical use (94). The acquisition process of BM-MSCs is often invasive and costly. In addition, the cell quality of BM-MSCs decreased significantly with the increase in donor age.
Human umbilical cord MSCs (hUC-MSCs) were separated from Wharton’s Jelly, a colloidal tissue surrounding the umbilical cord blood canal (95). It is usually discarded during childbirth; thus, the collection is non-invasive and poses few ethical problems (96). It has the characteristics of a short doubling time (97), long survival time (98), and strong anti-inflammatory ability (99), and long-term in vitro culture has little influence on its phenotype and genetic stability (100). Compared with BM-MSCs, hUC-MSCs have a higher proliferative ability and lower expression of HLA-ABC and HLA-DR (101).
Adipose tissue-derived MSCs (ADSCs) are rich in tissue sources. It can be obtained by minimally invasive surgery from subcutaneous white adipose tissue separated from the abdomen, thighs, or buttocks/buttocks of animals or humans (102). The isolation of ADSCs is simple, with high yield (~ 100 mL can be collected from 1000 mL adipose tissue) (103). It can differentiate in multiple lineages, including chondrogenesis, osteogenesis, cardiomyocyte, adipogenesis, neurogenic, and hepatic differentiation (104, 105). ADSCs often express CD34 in low-passage cultures, but this decreases with continuous cell passage (106, 107). Unlike BM-MSCs, ASCs do not express the sialoglycoprotein podocalyxin (PODXL) or the adhesion marker CD106 (108, 109).
Tissue sources of placenta-derived MSCs (PD-MSCs) include amniotic fluid, amniotic membrane, chorionic plate, chorionic villi, decidua basalis, complete placenta, and complete placenta (110). Stem cell-like cells in the placenta have higher differentiation potential and self-renewal ability than other tissue-derived MSCs (111). In addition, it has shown low immune properties in vitro and in vivo studies (112). PD-MSCs have also been shown to enhance the differentiation of monocytes from inflammatory M1 macrophages to M2-like macrophages (113), suggesting that PD-MSCs have the potential to improve inflammatory diseases. However, MSCS isolated from different parts of the placenta have different subtle properties. For example, the placental tissue comprises two separate individual tissues (the maternal placental tissues and the fetal). MSCs derived from fetal placental tissues have significantly stronger proliferative capacity than those derived from maternal placental tissues (114). To understand their different characteristics for better use in future research, more research data are needed to clarify the accuracy of their data further.
Zhang et al., in 2008, first identified a urine stem cell population and found that it could expand over ten generations in vitro (115). This stem cell population was named urine-derived stem cells (USCs). USCs are easier to obtain than MSCs. They can be extracted directly from excreted urine and are non-invasive, painless, and low-cost (116). It has the same characteristics as those of USCs isolated from the upper urinary tract. It was found that USCs showed normal karyotypes regardless of passage (117, 118). USCs can differentiate into bone, cartilage, and adipose lineages, as well as urothelial cells, smooth muscle cells, endothelial cells, kidney cells, and podocytes, showing the potential for multidirectional differentiation (119–122). USCs expressed several MSCs markers, including CD44, CD73, and vimentin (123), and also expressed adhesion markers such as CD29 and CD166, but not CD31 (124, 125). It was reported that no teratoma was formed when USCs were injected into immunodeficient mice, showing an absence of the tumorigenic phenotype (126).
Gingival mesenchymal stem cells (GMSCs) can be obtained from periodontal tissue, gingival ligaments, and dental pulp. Similar to MSCs from other sources, GMSCs have MSCs-related cell surfaces markers such as CD73, CD90, CD105, and stromal cell antigen 1 (STRO-1) (127). In addition, studies have shown that GMSCs not only have the potential to differentiate into three lines of mesoderm (adipocytes, osteocytes, and chondrocytes) but can also transdifferentiate into ectoderm and endoderm cell lineages, such as keratinocytes, endothelial cells, and nerve cells (128, 129). In addition, GMSCs also have an anti-inflammatory function and immunomodulatory ability (130, 131), and can promote the differentiation of macrophages (132). Furthermore, GMSCS are homogenous, rapidly proliferating, and not tumorigenic, and have stable morphological and functional characteristics under higher passage (130).
Recently, scientists isolated mixed cell populations with mesenchymal and epithelial features from normal human labial minor salivary glands (133). Subsequently, it was confirmed that human labial gland-derived MSCs (LGMSCs) existed in the lamina propria of the oral mucosa (134). Wang et al. successfully isolated MSCs from adult female salivary gland cysts, identifying their characteristic MSCs expression markers, including CD29, CD44, CD73, CD90, and CD105, using flow cytometry. However, the CD34, CD45, CD106, CD117, and the salivary gland epithelium markers (CD49f) were also negative (135). LGMSCs have the potential for osteogenic and lipogenic differentiation, and their ability to differentiate into salivary gland epithelioid‐like cells is stronger than that of other MSCs. However, its adipogenic differentiation ability is lower than that of ADSCs (136, 137). In addition, LGMSCs have the characteristics of a shallow glandular location, are easy to obtain, expand in vitro, and regulate immune function (138–140).
In addition, MSCs derived from tissues such as the pancreas and the liver are being explored, which will provide options for multi-source pathways of MSCs in the future. It should be noted that MSCs from type 1 diabetes mellitus (T1DM) donors are similar in phenotype and function to healthy donors. They can maintain normal immunomodulatory or secretory functions (141). However, MSCs from type 2 diabetes mellitus (T2DM) donors often show increased apoptosis and senescence, as well as decreased angiogenesis potential (142).
According to the source of MSCs, those used for treating DFU can be divided into autologous and allogeneic MSCs. Due to the different biological characteristics of MSCs from different tissue sources, their therapeutic mechanisms, adapted diseases, preferred lesions, and effects are also different. Furthermore, the methods used to culture MSCs in different laboratories (including enzyme digestion or tissue-advanced methods) are also different (143). Therefore, the quality and degree of cell expansion are different, and the study results may differ. Consequently, it is necessary to establish a quality control system for MSCs to ensure the stability and effectiveness of MSCs.
MSCs are mainly used for the treatment of diabetic foot by local delivery and systemic delivery. Local delivery is divided into topical application, topical injection, scaffold, and gel, systemic delivery is divided into intravenous and arterial administration (13). Previous research has shown that BM-MSCs are most effective by intramuscular injection (144), and the best effect of PD-MSCs was obtained by intraperitoneal injection (145).
Yan et al. found that local injection and intravenous infusion of stem cells were used to treat T2DM rat ulcer models, and both administration methods significantly accelerated wound healing. Moreover, systemic administration also had the potential to ameliorate hyperglycemia (146). However, it has been proposed that MSCs be delivered through the whole body, and most of the cells remain in the lungs, with only a small percentage of the cells moving to the ulcer site (147). In addition, intradermal injection of MSCs into the edge of the ulcer significantly improved the wound healing process. However, local injection of MSCs has the disadvantages of poor cell localization, difficult control of cell density and spacing, and impaired cell vitality due to the influence of local wounds (148, 149).
Furthermore, when MSCs are injected locally into the lesion using a syringe, irreversible damage can be caused to the cell membrane, resulting in decreased cell viability (150). For DFU patients with microvascular complications or arterial occlusion, arterial administration often fails to transport MSCs well to the ulcer site, thus affecting the therapeutic effect. When MSCs are administered to the muscle near the lesion site, the muscle tissue can provide oxygen and nutrients to the injected cells, which contributes to the survival of MSCs and improves their function (148). However, the characteristics of MSCs mean their external preparations are difficult. Therefore, it has been proposed to use scaffolds loaded with MSCs as the primary cell carriers to deliver MSCs, to provide a favorable microenvironment for cell attachment, proliferation, differentiation, and guiding host cell migration, to achieve better healing effects (2). Assi et al. found that compared with the control group with an ordinary injection of MSCs, Rolled collagen scaffolds containing MSCs showed better healing ability and increased vascular endothelial growth factor (VEGF) expression and capillary density in the local ulcers; they found increased numbers of fibroblasts, macrophages, and smooth muscle cells (151).
Assis et al. reported an approach to induce angiogenesis using vascular-inducing devices (VIDs) composed of MSCs derived from healthy donors and decellularized lung-derived micro-fragments. These VIDs express and transcribe the entire library of angiogenic factors in a controlled release manner, induce proliferation of fibroblasts and endothelial cells, and induce local vascular network formation within a week after implantation of non-obese diabetic/severe combined immunodeficiency mice (152). They then transplanted the acellular micro-fragment from the bone marrow of an elderly diabetic patient suffering from lower extremity arterial disease and DFU. They found that the MSCs expressed and secreted angiogenic factors similar to those extracted from healthy individuals (153). This provides a good idea for researching and developing stem cells and scaffolds.
A large number of studies have been devoted to developing excipients that can provide support for MSCs, such as 3D printed collagen, chitosan, polyurethane scaffolds, and cell gels (13, 154, 155), to improve the effective maintenance time for topical application preparations of MSCs (Figure 1). In the actual treatment process, we can choose the most appropriate drug administration route by personalized treatment according to the actual condition of patients and the allocation of medical resources.
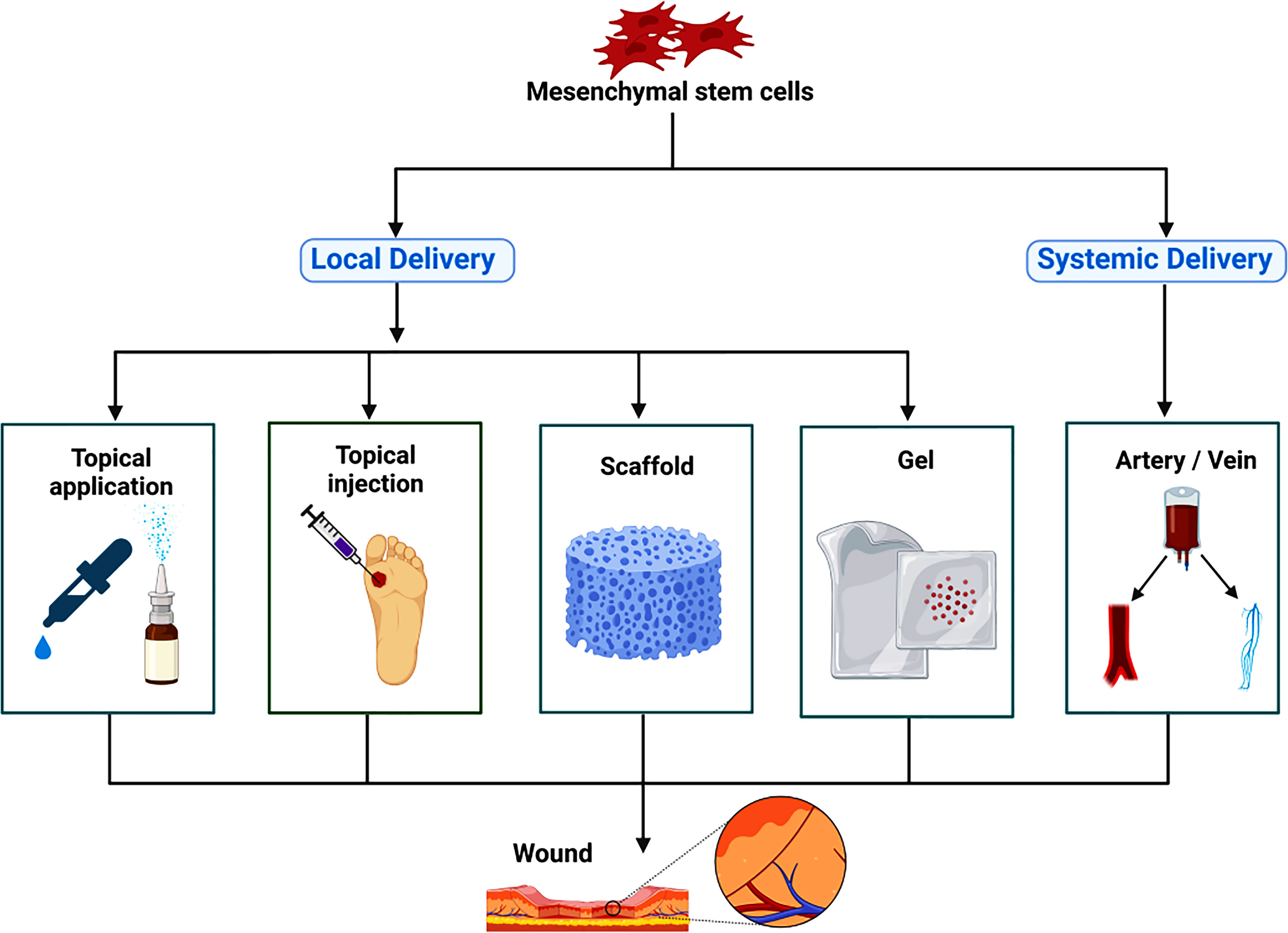
Figure 1 The route of administration for mesenchymal stem cells therapy. Mesenchymal stem cells are mainly used for the treatment of diabetic foot by local delivery and systemic delivery. Local delivery is divided into topical application, topical injection, scaffold, gel and so on; systemic delivery is divided into intravenous administration and arterial administration.
Cell proliferation, differentiation, and migration are crucial for the physiological processes of DFU wound repair and growth. Wounds result from living tissue damage, and coordinating wound repair is initiated immediately upon damage to the tissue surface. During repair, growth factors and cytokines stimulate signal regulation and coordinate intercellular and intracellular signaling to promote cell proliferation, differentiation, migration, and protein synthesis. Recent studies have shown that various growth factors and molecular mechanisms play a vital role in the occurrence and development of DFU (156, 157).
One of the essential reasons for diabetic foot secondary to diabetes is the damage and lesions of blood vessels. The formation and regeneration of new blood vessels in the DFU area provide nutrients for the growth of granulation tissue. Therefore, it is especially important for shrinking ulcers and promoting repair. Studies have shown that MSCs can secrete a variety of cytokines, including VEGF, basic fibroblast growth factor, stromal cell-derived factor-1 (SDF-1), keratinocyte growth factor 2, insulin-like growth factor 1, placental growth factor, and epidermal growth factor (EGF). These factors can promote angiogenesis, enhance microhemodynamics, and promote wound healing (158, 159). Among a series of factors regulating angiogenesis and repair, VEGF is the most potent (160).
Shen et al. showed that BM-MSCs could accelerate wound healing in the feet of diabetic mice by improving the activation of vascular endothelial cells and inducing angiogenesis by the paracrine VEGF and other vasoactive factors (161). After transplanting BM-MSCs into diabetic rat foot wounds, Wan et al. found that the expression of VEGF in wound tissue and angiogenesis was increased, which positively affected wound healing in diabetic rats (144). Furthermore, Badillo et al. showed that Mouse liver-derived MSCs increase local growth factor secretion, such as EGF, VEGF, and SDF-1, thus promoting neovascularization, enhancing wound cell recruitment, and improving wound contraction (162). Moreover, BM-MSCs can significantly promote the secretion of key growth factors, such as EGF and VEGF, for repairing and regenerating damaged tissues. They can increase collagen (types I–V) to promote wound healing in diabetic rats (163). Furthermore, Diao et al. demonstrated that in addition to directly promoting angiogenesis, VEGF can activate transcription factors to regulate endothelial progenitor cells (EPCs), recruit EPCs to the bone marrow, and inhibit the apoptosis of EPCs from promoting wound healing (164). These studies suggest that MSCs may directly or indirectly promote angiogenesis at the injury site via paracrine growth factors, improve blood flow, and promote the healing of diabetic foot wounds.
In vitro studies have shown that MSCs can differentiate into epidermal cells and function as epidermal cells through different induction methods (165, 166). Kato et al. treated the foot wounds of diabetic rats and control rats with BM-MSCs. They found that the reduced phosphorylated focal adhesion kinase levels were restored when human keratinocytes were cultured in a BM-MSCs-conditioned medium containing high glucose. In addition, the levels of matrix metalloproteinase-2, EGF, and insulin-like growth factor 1 were increased, suggesting that BM-MSCs could promote wound healing in diabetic foot model rats by improving keratinocyte function (167). Additionally, BM-MSCs-treated wounds promote the proliferation of keratinocytes and endothelial cells and promote the migration of macrophages, keratinocytes, and endothelial cells into the wounds of model mice, thereby promoting wound healing (168). Wu et al. used genetically diabetic db/db mice to conduct research and found that VEGF, Angiopoietin-1, and keratinocyte-specific protein keratin were higher in wounds treated with BMSCs. Furthermore, Bmscs significantly promoted the growth of keratinocytes at the wound site, stimulated the formation of new blood vessels, promoted epithelial regeneration at wound sites, and accelerated wound healing (169).
Furthermore, hUC-MSCs can specifically localize to the target ulcer tissue in a rat model of diabetic foot ulcer, promote the secretion of cytokeratin 19, stimulate the formation of keratinocytes and extracellular matrix, and promote epithelial regeneration in ulcerated tissues (170). Although numerous studies have confirmed that MSCs can differentiate into keratinocytes and endothelial cells, their engraftment effects remain controversial. It has been suggested that, under special circumstances, MSCs differentiate into keratinocytes but do not have the full set of expression markers that keratinocytes have (171). For example, Schneider et al. reported that BM-MSCs were cultured in air-exposed on dermal equivalents consisting of collagen types I and III with dermal fibroblasts; they found that MSCs possessed obvious vitality and three-dimensional epidermis-like growth patterns and possessed markers of early and mature epithelial cells without expression of E-cadherin or pan-cytokeratin (172).
Thus, an appropriate culture environment should be selected to cultivate BMCs to improve the success rate of differentiation. It should be noted that the current in vivo studies on MSCs observed by DFU models mainly focus on animal models, and the data volume of human models is still small.
Recent studies have shown that various molecular mechanisms, including cell signaling pathways, play important roles in the pathophysiology and healing processes of diabetic foot (173–175). A protein-serine-threonine kinase (AKT) is a serine/threonine kinase that is an important signaling center for various cellular functions. PI13-dependent AKT activation further affects MSC survival, proliferation, migration, and angiogenesis; this pathway plays a core regulatory role (175). The Notch signaling pathway is a short-range communication sensor that regulates stem cell niche maintenance, such as cell differentiation, cell proliferation, and cell death during the development and renewal of adult tissues (176).
Hou et al. found that the conditioned medium of BM-MSCs accelerated the migration and proliferation of human umbilical vein endothelial cells. These processes were closely related to the AKT signaling pathway and independent of the extracellular signal-regulated kinases (ERK) signaling pathway (177). Jun et al. demonstrated that amniotic fluid-derived MSCs (AF-MSCs) promoted wound closure by increasing angiogenic factors while increasing epidermal cell regeneration, and it accelerated the proliferation and migration of dermal fibroblasts and accelerated wound healing through the transforming growth factor-beta (TGF-β)/SMAD2 and PI3K/AKT signaling pathways under hypoxic conditions (178). Liu et al. reported that SDF-1 and chemokine receptor four play important roles in regulating BM-MSCs to promote DFU healing (179). Interestingly, combined treatment with PRP and rat ADSCs promotes angiogenesis, triggers epidermal stem cell proliferation and recruitment by modulating the Notch pathway, and significantly accelerates the healing of experimentally induced diabetic wounds in rats (180). These phenomena suggest that the Notch signaling pathway may be a new potential therapeutic target for diabetic wounds (181, 182).
In addition to their ability to differentiate into different cell types, MSCs also play a regulatory role in inflammatory and immune responses. Many studies have shown that after cell or tissue injury, MSCs can be activated by inflammatory cytokines and control the process of tissue regeneration by releasing a series of factors that may promote the differentiation and proliferation of progenitor cells while participating in immune regulation and inhibiting inflammatory responses (67, 183, 184). (Figure 2).
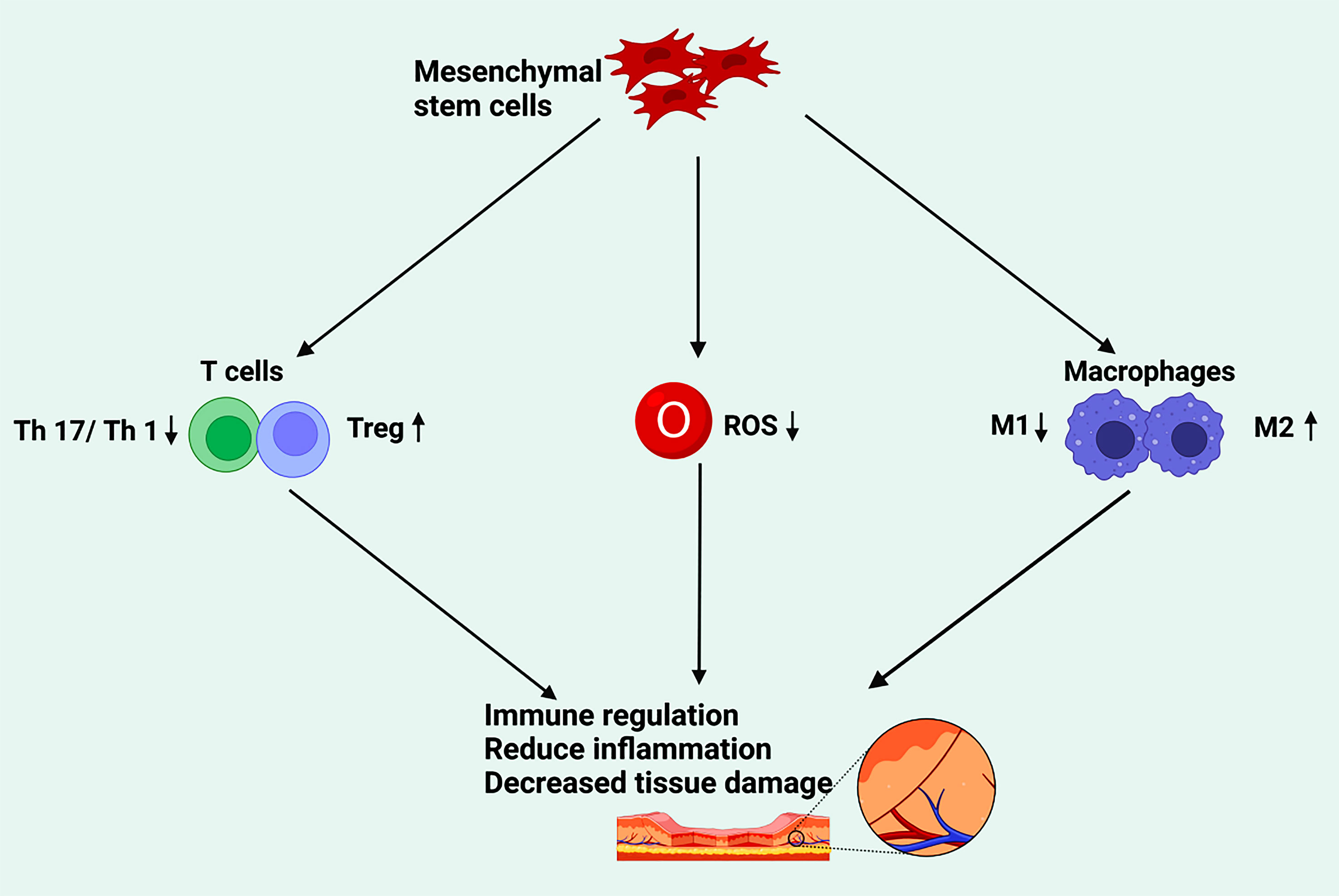
Figure 2 The effect of mesenchymal stem cells on tissue damage through immune regulation. Mesenchymal stem cells participate in immune regulation by inhibiting T17 and T1 cells, promoting Treg cells, downregulating ROS, and accelerating the polarization of M2, so as to reduce inflammation and repair the damage of diabetic foot. (Created in BioRender.com).
T helper cells 17 (Th17) and T helper cells 1 (Th1) can mediate inflammation (185). CD4+ cells, namely regulatory T cells (Treg), are a subset of specialized immunosuppressive T cells that can specifically express CD25 and CTLA-4 on the cell surface and the transcription factor FoxP3 in the nucleus, which can maintain homeostasis and immune self-tolerance (186) (187).. Li et al. confirmed that 15 patients with diabetic foot disease received hUC-MSC transplantation under insulin treatment, after which blood glucose levels and insulin doses were decreased in all 15 patients. Four weeks after transplantation, CD4+CD25 (hi) FoxP3+Treg/Th17 and CD4+CD25 (hi) FoxP3+Treg/Th1 cell ratios increased significantly (p <0.01), while Th17/Th1 cell ratios remained unchanged and VEGF serum levels peaked (188).
ROS are oxygen-free radicals (189, 190). Low ROS levels are beneficial for maintaining cell proliferation, differentiation, and survival, while high ROS levels stimulate immune responses and cause oxidative damage, leading to cell damage and dysfunction (191). When tissues are damaged, phagocytes in the body phagocytose bacteria, apoptotic inflammatory cells, or cell debris to kill pathogens. However, after phagocytosis, long-lived neutrophils generate substantial ROS, causing a respiratory burst that causes tissue damage.
Some studies have suggested that antioxidant activity of MSCs may occur through cell contact or paracrine reduction of lipid peroxidation and protein oxidation (192, 193). MSCs reduce inflammation and oxidative stress in several diseases. These effects include reducing the expression of ROS-producing enzymes myeloperoxidase, inducible nitric oxide synthase, and nitrogen oxides and reducing inflammatory cytokines IL-1β, IL-4, IL-6, IL-9, tumor necrosis factor-alpha (TNF-α), and IFN-γ (194, 195). MSCs can also directly reduce ROS and myeloperoxidase in stimulated monocytes and macrophages, thereby inhibiting their pro-inflammatory phenotypes (196, 197). By enhancing the secretion and expression of stanniocalcin (STC)-1, MSCs significantly inhibited the production of mitochondrial ROS in macrophages, and inhibited nucleotide binding oligomeric domain (NOD)-like receptor pyrin domain containing 3 (NLRP3) inflammasome, Caspase-1 activation, IL-1β production, TNF-α and IL-6 transcription (197). Transplantation of PD-MSCs has also been shown to promote diabetic wound healing by reducing TNF-α, IL-6, and IL-1 pro-inflammatory cytokines and inhibiting NF-κB signal transduction (198). Li et al. showed that mesenchymal stem cell-conditioned medium could reduce the overproduction of ROS in high glucose and/or lipopolysaccharide induced keratinocytes, and reversed the downregulation of mitogen-activated protein kinase (MEK)1/2 and ERK 1/2 phosphorylation induced by high glucose and/or lipopolysaccharide, improving keratinocyte proliferation and migration in diabetes-like microenvironments (199). Raffaghello et al. found that BM-MSCs could prevent excessive or inappropriate oxidative metabolism, activate neutrophils, and inhibit their apoptosis, thereby reducing ROS production without affecting the phagocytic ability of neutrophils (200). Exosomes secreted by human ADSCs can alleviate DFU progression by preventing the senescence of EPCs and inhibiting the expression of ROS and inflammatory cytokines (201).
MSCs play an immunomodulatory role by inhibiting ROS production and enhancing mitochondrial function in macrophages and neutrophils. Therefore, in the future, the role of MSCs in anti-ROS and immune regulation in diseases should be considered to help optimize the therapeutic effect of DFU.
M1 macrophages have traditionally been associated with proinflammatory events. M1 macrophages are defined as macrophages that produce proinflammatory cytokines, which mediate resistance to pathogens, and exhibit powerful bactericidal properties, but also cause tissue destruction and inhibit angiogenesis (202, 203). The M1 macrophages are characterized by an enhanced ability to secrete cytokines such as IL-1β, TNF, IL-12, ROS, and IL-18 (204). On the contrary, M2 macrophages are thought to have anti-inflammatory and pro-regenerative effects. The molecules expressed by M2 macrophages include IL-10, Arginase1 (Arg1), resistin-like-α (also called Fizz1), Mrc1 (also called CD206), and chitinase 3-like 3 (also called Ym1) (205). These molecules may be involved in tissue remodeling, parasitic infections, immunomodulatory functions of tumors, and promote angiogenesis (206). They represent the two ends of the macrophage activation spectrum and can transform into each other in specific microenvironments.
In the first stage of ulcer healing, pro-inflammatory M1 macrophages infiltrate the ulcer to remove bacteria, dead cells, and foreign bodies from the ulcer (207). When tissue begins to repair an acute wound, the M1 macrophage population changes to an M2 phenotype, resulting in anti-inflammatory and regenerative effects (208). In chronic wounds, if proinflammatory macrophages persist with the M1 phenotype, the transformation to the M2 anti-inflammatory phenotype is impeded, which leads to impaired tissue repair (209, 210). A persistent high glucose environment in vivo stimulates macrophages to secrete pro-inflammatory cytokines, such as TNF-α, IL-1β, IL-6 and ROS, leading to a vicious cycle of persistent M1 macrophage phenotypes and a persistently higher state of inflammation in DFU (211). Therefore, it can be inferred that M2 macrophages can promote the healing of DFU. Thus, transforming M1 macrophages into adequate M2 macrophages in the wound-healing process of DFU may be an effective therapeutic idea.
Dayan et al. proposed that co-culture of human BM-MSCs and hUC-MSCs with macrophages reduced the overall macrophage/monocyte levels, including decreased pro-inflammatory M1 macrophages. In contrast, the level of alternately activated anti-inflammatory M2 macrophages was significantly increased (212). In addition, human GMSCs can induce M2 polarization of macrophages to play an immunomodulatory role, thereby enhancing wound repair (213). Yu et al. found that rat ADSCs reduce the number of M1 macrophages and increase the number of CD163 (+) M2 macrophages, delaying the progression of diabetes and its complications (214). Chen et al. used 3D nanofiber scaffolds loaded with mouse BM-MSCs to act on the wounds of diabetic mice. The ratio of alternately activated M2/classically activated M1 macrophages was significantly increased, promoting wound healing in diabetic mice (215). PGE2 secreted by hUC-MSCs rescues endothelial cell dysfunction and improves the local microenvironment of vascular endothelial cells IL-10 and VEGF. It improves angiogenesis to promote wound healing by regulating M1-to-M2 macrophage polarization in diabetic wounds (216).
These reports on the promotion of the polarization of M1 macrophages into M2 macrophages and promotion of the healing of DFU have brought good news to patients; however, they need to be further studied.
With the rapid development of “cell-free therapy,” MSC-derived small extracellular vesicles (EVs) have become a research hotspot for treating various diseases. Exosomes are the smallest extracellular vesicles in the range of 30–150 nm in diameter, with a bilayer structure and disc-like morphology. They mediate signal transduction between adjacent cells, distant cells, and organs by delivering noncoding RNAs, proteins, and DNA (217). Chen et al. found that TNF-α, interleukin 6 (IL-6), and vascular cell adhesion molecule 1 (VCAM-1) induced heterogeneous secretion of exosomes from MSCs. Furthermore, they defined a novel pro-angiogenic miRNA by RNA sequencing, miRNA-21-5p, a novel mechanism and novel biomarker by which exosomes can promote angiogenesis and ischemia tissue repair in DFU (218). In contrast, Chen et al. found that TNF-α and IL-6 down-regulated angiogenic-related miRNA in MSCs-exo, suggesting that the angiogenesis potential of MSCs-exo decreased after TNF-α and IL6 stimulation (219). Moreover, MSCs-EVs can upregulate the expression of the VEGF gene through miRNA-210-3p and activate key pro-angiogenic proteins, such as ERK and AKT, to improve microcirculation and promote angiogenesis (220). In addition, BM-MSCs downregulate the target genes TRAF6 and IRAK1 through exosomal miR-146a, reducing the expression of NF-κB, IL-6, and MIP-2, thereby inhibiting the inflammatory response and promoting the repair of diabetic wounds (221). Finally, Yu et al. demonstrated that BM-MSCs-derived exosomes enhanced the biological function of endothelial cells through the exosomal miRNA-221-3p-mediated AKT/eNOS pathway, thereby promoting the repair of diabetic wounds (222). This suggests that MSCs-EVs can promote angiogenesis and wound healing in treating DFU, but inflammatory factors may inhibit the potential of MSCs-EVs to promote angiogenesis.
In conclusion, MSCs can accelerate the repair of diabetic foot wounds by synergistic effects, such as immunomodulation, upregulation of anti-inflammatory factors, or downregulation of pro-inflammatory factors to reduce the inflammatory response, increase blood supply to ulcers, promote granulation tissue formation, stimulate epidermal regeneration (223), and finally increase the limb salvage rate in diabetic foot patients (224, 225) (Figure 3, Table 1). However, in the context of hyperglycemia and chronic inflammation in DFU patients, AGEs lead to a decline in the survival rate of MSCs and seriously reduce the repair efficiency of MSCs. In addition, inflammatory factors may inhibit the ability of MSCs to promote vascular regeneration and repair. Therefore, good blood glucose control and inflammation control must be considered in treating DUF by MSCs (148, 219, 227). Although the current study has achieved relatively positive clinical results, optimal efficacy still needs to be explored.

Figure 3 The therapeutic mechanism of mesenchymal stem cells (MSCs) for diabetic foot. MSCs are mainly derived from bone marrow, umbilical cord, adipose tissue, placenta, and other parts. During the treatment of diabetic foot, mesenchymal stem cells repair diabetic foot mainly through the proliferation of fibroblasts, keratinocytes and endothelial cells, as well as angiogenesis and polarization of macrophage M2. In this process, paracrine growth factors, related signaling pathways and microRNAs are involved. (Created in BioRender.com).
With the continuous in-depth research and elucidation of the mechanism of action, exploring MSCs or other cell derivatives with a more clear mechanism of action for DFU treatment has become a current research hotspot. The use of these derivatives to treat DFU shows efficacy and characteristics similar to those of MSCs. MSCs or cell derivatives reported in related studies include exosomes, exosome gels, conditioned medium, growth factors, platelet lysates, and platelet-rich plasma (PRP). Among these derivatives, research on exosomes is currently hot. Li et al. suggested that AD-MSCs exosomes could significantly improve inflammation in rat DFU wounds and reduce the expression of oxidative stress-related proteins in the wound while promoting tissue regeneration, the proliferation of EPCs, angiogenesis, and growth factor expression (201).
Yang et al. proposed that the efficient delivery and enhanced exosome capacity of hUC-MSCs-derived exosomes in a Pluronic F-127 hydrogel could accelerate diabetic wound healing. Therefore, MSCs-derived exosome therapy may be a new treatment for chronic wound skin regeneration (226). Dash et al. found that autologous implantation of BM-MSCs in patients with diabetic foot accelerated the lower extremity wound healing process and significantly improved clinical symptoms (228). Furthermore, some research has found that injection of an MSC-conditioned medium promotes wound closure in diabetic mice (156, 169). Growth factors promote wound healing in patients with DFU. Transgenic Lactobacillus merceris secretes platelet-derived growth factor-BB, a dimeric peptide that binds to platelet-derived growth factor receptors and stimulates cell proliferation and survival. This could be a cost-effective method for patients and be used in regenerative medicine strategies to promote tissue repair (229). The combination of MSCs and PRP has been found to enhance wound healing (230). A human clinical study reported that PRP was significantly better than topical antiseptic dressings in cleaning diabetic ulcers and found healing rates of up to 86%, which significantly improved the 68% healing rate of antimicrobial ointment dressings (231). PRP contains growth factors that promote cell proliferation and matrix synthesis and could be considered a candidate treatment for the nonhealing of DFU (232). In conclusion, MSC-related derivatives are a promising new method for treating DFU; however, their exact efficacy remains to be confirmed by further studies.
As mentioned above, conventional treatment of DFU has been mentioned. However, conventional treatment is not always effective. For example, patients with DFU have lower limb artery lesions involving the lower leg arteries and may face amputation if serious vascular diseases occur in the affected limb. MSCs promote tissue repair and regeneration by increasing extracellular matrix, repairing cell activity, promoting angiogenesis at ulcer sites, secreting growth factors, and forming new keratinocytes (233, 234). To date, MSCs have become a hot spot for DFU, and more clinical research has been widely carried out.
Transplanting autologous stem cells into DFU enhances ulcer healing and reduces amputation rates. They include BM-BMSCs, peripheral blood mononuclear cells (PBMNCs), BMMNCs, ADSCs, and an adipose tissue-derived stromal vascular fraction (SVF).
Yuyama et al. reported autologous BMMNCs transplantation for angiogenesis in patients with limb ischemia (235). A significant proportion of DFU patients suffer from vascular disease. Claeys et al. proposed that percutaneous partial pressure of oxygen could be used as a predictive parameter of DFU associated with vascular disease (236). Kirana et al. included 22 patients with DFU treated with autologous BMMNCs, which resulted in improved wound healing and transcutaneous pressure of oximetry in the affected limb (237). Xu et al. used recombinant human granulocyte colony-stimulating factor (G-CSF) 5–10 µg/kg/day for the proliferation of BM-MSCs in DFU patients for 4–5 consecutive days to promote their release into peripheral blood and then took peripheral blood MSCs and injected them around or at the bottom of ulcers. After 4 weeks of follow-up, the ulcers gradually healed. Digital subtraction angiography (DSA) detection revealed that abundant collateral circulation was established around the lesions of the DFU (238). Huang et al. also used G-CSF to mobilize PBMNCs in treating patients with DFU accompanied by critical limb ischemia (CLI) and achieved significant clinical effect (239). This provides a reference for the diversity of treatment modalities. G-CSF is a growth factor that stimulates bone marrow and mobilizes EPCs, thereby increasing their numbers to cure DFU (240). Lu et al. conducted clinical trials, in which patients with type 2 diabetic feet were given BM-MSCs, BMMNCs, or normal saline (NS). The results illustrated that in promoting the healing of patients with DFU, the BM-MSCs treatment group could be more effective than the BMMNCs treatment group. They also found that the BMMSCs of diabetic patients secret more VEGF, FGF-2, and angiopoietin-1 than BMMNCS under normoxic and hypoxic conditions. Therefore, they believed that BMMSCS is better than BMMNCs in the local vascular generation (241). Procházka et al. performed clinical trials, dividing 96 CLI and DFU patients into two groups. The first group of patients received local treatment of autologous BM-MSCs, while the second group of patients received the standard treatment of medical care. The results suggested that BM-MSCs local treatment can save 79% of the limbs of CLI and DFU patients. Among the 21% of amputation, lymphocytes and platelet reduction may be potentially pathogenic. The primary amputation rate of the control group is 44%. Experiments confirmed that BM-MSCs can greatly improve the prognosis of DFU and reduce the amputation rate. This study found that the low platelet count and the low VEGF level are related to poor healing in bone marrow concentrate. Low platelet and CD34+ cell concentrations were present in most unhealed patients, but moderate platelet and CD34+ cell concentrations were present in most healed patients rather than either of the two extremes. However, for patients with low platelet counts, if they are accompanied by VEGF with high local concentration, the wound healing was satisfactory. Most amputations that are still not saved after using autologous BM-MSCS for treatment are secondary infections. These treatments are proposed to emphasize the importance of debridement and anti-infection (242). Scatena et al. treated 38 patients with DFU and no-option critical limb ischemia (NO-CLI) with intramuscular and perifocal injections of PBMNCs. Patients treated with PBMNCs had a significantly lower rate of amputation than those (38 patients) treated with standard care under the International Working Group on the Diabetic Foot (IWGDF) guidelines (243), and 86.6% of patients in the PBMNCs group recovered during the 2-year follow-up, compared with only one patient in the control group. The results showed that PBMNCs significantly reduced the amputation rate of DFU with NO-CLI (244). In a recent meta-analysis of autologous MSCs in treating DFU, it was also reported that BMMNCs were more effective in healing foot ulcers in DFU than repeated percutaneous transluminal angioplasty (245, 246).
Adipose tissue-derived SVF is a heterogeneous cell fraction. They include mesenchymal progenitor/stem cells, T cells, pericytes, endothelial cells, and macrophages (247). It has also been specifically used to treat DFU. Han et al. were the first to use uncultured processed lipoaspirate cell autografts to treat diabetic ulcers to stimulate the diabetic fibroblasts of activity and obtained a 100% cure rate of DFU (248). Carstens et al. used adipose-derived SVF in treating 10 patients with non-reconstructive peripheral vascular disease in DFU and achieved good results. They also followed the patients for 6 years and found five patients still showed a consistent clinical benefit (249, 250). Subsequent studies have shown that adipose tissue-derived SVF can induce neovascularization in ischemic conditions in treating chronic DFU, increased transcutaneous partial oxygen pressure, and cutaneous microvascular blood flow (251, 252).
Among autologous MSCs, BM-MSCs and PBMNCs are the most commonly used cell types in DFU studies. Mobilized PBMNCs are preferred over BM-MSCs because of the ease of collection and the avoidance of pain and anesthesia associated with bone marrow biopsy. The ease of execution and good clinical efficacy of adipose-derived SVF brings a new choice for treating DFU. However, further studies are needed to explore the exact use of adipose-derived SVF.
Allogeneic stem cells are isolated from an individual of the same species rather than from the recipient, including pluripotent mesenchymal stromal cells from allogeneic sources such as the placenta, umbilical cord, amniotic membrane (148).
Qin et al. included a group of Fontaine II-V DFU patients (28 patients, 34 limbs) with varying degrees of lower extremity arterial disease treated with intravascular infusion and peri-ulcerative injection of the hUC-MSCs after angioplasty. After 3 months of follow-up, the results showed increased neovascularization at the ulcer, ulcer healing, skin temperature, transcutaneous oxygen tension, the ankle-brachial pressure index, and claudication distance were improved noticeably (253). Their study suggests that hUC-MSCs transplantation after angioplasty is a potentially safe and effective clinical treatment for severe DFU. Moon et al. conducted clinical trials, incorporated 59 patients with diabetic foot ulcers, and randomly distributed them to the hydrogel-based allogeneic ADSCs sheets group (n = 30) or the control group of polyurethane film treatment (n = 29). They observed the closure of wounds in the treatment group and control group at weeks 8 and 12, and the median time for the treatment group and the control group was 28.5 days and 63.0 days, respectively. In the 2-year follow-up study, two subjects had a recurrence 6 months after the stem cell therapy ulcer trial, which was different from the site at the beginning of the previous trial. The recurrence was at the toe tip and the plantar foot, susceptible to stress. Later recovery through therapeutic intervention (254). Therefore, in general, they achieved satisfactory results. In addition, there were no serious adverse events related to the treatment of hydrogel-based allogeneic ASC sheets. Therefore, it is proved that hydrogel-based allogeneic ADSCs sheets may be effective and safe for treating DFU (254). Rodríguez et al. launched clinical trials, allowing 28 patients with DFU patients to accept allogeneic BM-MSCs derivatives (n = 12), BM-MSCs (n = 6), or conventional treatment (PolyMem® dress, Ferris, Fort Worth, TX, USA) (n = 10). They conducted a macro assessment of the wound healing process until the ulcers were closed entirely. As a result, no adverse events were reported. Compared with patients receiving conventional treatment, the wound closure rate of DFU patients treated with allogeneic BM-MSCs derivatives or allogeneic BM-MSCs was higher (255). Uzun et al. reported a study that divided 20 patients with DFU accompanied by chronic ulcers into two groups. Patients in the standard group (10 cases) received standard treatment with sterilization, debridement, and dressing coverage. In the study group (10 cases), in addition to routine disinfection and debridement, allogeneic ADSCs were injected into the dermo-epidermal junction and the entire wound surface using intralesional. The results showed that nine patients in the study group had wound healing, while eight patients in the control group had wound healing. The wound healing time of the study group was 31.0 ± 10.7 days, and the wound healing time of the control group was 54.8 ± 15.0 days. In the end, one patient in the study group and two in the control group had their limbs amputated. Allogenic ADSCs were safe for local injection of DFU ulcers with no significant adverse events (256). Their study showed that allogenic ADSCs have a positive therapeutic effect on chronic ulcers of DFU and are superior to standard conventional therapy.
Through the above studies, we can conclude that MSCS and their derivatives and stents delivering MSCs have achieved optimistic clinical effects in the treatment of DFU. However, the role of post-healing patient care, footwear selection, and health education should be considered in preventing the recurrence of ulcers. Furthermore, most of the above clinical trials have a limitation: the sample volume was relatively small. Therefore, multi-center random clinical trial research is recommended to expand the sample volume effectively to obtain more accurate evidence (Table 2).
Following previous studies in humans and animals, MSCs have achieved encouraging efficacy in treating DFU (241, 254, 257). However, with an increase in research, from the results of recent clinical studies, common side effects of MSCs in DFU are diarrhea, fever, increased serum creatinine level, urticaria, nausea, and vomiting (258, 259). After the passage of stem cells for many times in vitro, the multidirectional differentiation potential and paracrine ability may also be reduced, leading to the decline of clinical effect (234). Embryonic stem cells have strong proliferative ability and low differentiation maturity. The introduction of these cells may cause immune rejection and stimulate tumor formation. Therefore, embryonic stem cells should be avoided from DFU treatment as much as possible (260–262). In addition, it has been reported that increasing the number of stem cells applied locally to improve repair efficiency may also increase tumorigenicity (263). Although there may be some side effects of stem cell therapy for diabetic foot, overall, in animal experiments and human studies, BM-MSCs transplantation has achieved positive results in DFU treatment. MSCs transplantation may be a new method that can be used to treat diabetic foot, but the precise utilization of stem cells to control the local microenvironment of DFU to maximize the healing effect is still unknown.
These stem cells, which have clear research results, have a largely positive effect on the treatment of DFU while also having the advantage of being used in combination with other treatments to better exert their effects in the treatment of refractory DFU (234, 264). Although stem cells derived from synovium, urine, amniotic fluid, liver, lung, and gingiva have only been reported in sporadic experiments in the treatment of diabetic foot, these stem cells may still be a potential choice for the treatment of diabetic foot in the future. Researchers can explore the characteristics of MSCs derived from different tissues based on in vivo and in vitro studies. They are expected to clarify their advantages and disadvantages and elucidate the full impact of their therapeutic effects in future studies.
As the first MSCs to be studied for the treatment of DFU, BM-MSCs are relatively convenient to isolate and extract and have achieved good therapeutic effects in many clinical practice applications. Their safety has also been affirmed. After repeated studies, BM-MSCs may be the best choice for treating diabetic foot (265). However, MSCs also have certain shortcomings; for example, the differentiation potential and proliferation ability of BM-MSCs decrease with age (266), and the repair ability is negatively correlated with the number of cell passages (267). This requires us to standardize stem cell therapy for the diabetic foot in the future to maximize its advantages and minimize its disadvantages. So far, different clinical studies have been launched to evaluate the safety and efficacy of MSCs on DFU (NCT03370874, NCT04464213, NCT05610865, NCT04104451, ChiCTR2000036933). It is crucial to standardize the therapeutic efficacy of MSCs products before initiating clinical trials, and this need is driving efforts to develop improved in vitro efficacy assays.
Different MSCs can self-renewal and multi-directional differentiation, which brings hope for the treatment of many intractable diseases, such as Parkinson’s disease, myocardial infarction, and bone defects. It is also expected to obtain gratifying clinical effects in basic and clinical application research on treating diabetic foot. Although stem cell therapy’s efficacy and safety in treating diabetic foot have been preliminarily confirmed, further research is needed regarding the treatment mechanisms, efficacy judgments, individual choices of stem cell source, and promotion norms.
In conclusion, DFU treatment with MSCs is a potential, relatively safe, and effective treatment method, among which BM-MSCs may be an ideal choice. However, in the initial treatment plan of the treatment of DFU, it is necessary to select a certain stem cell to specify the specific treatment method according to the characteristics of each stem cell, such as local applications, meridian transmission, local injection, and intravenous application. This is a key step in obtaining the ideal effect, which is still a considerable challenge facing researchers.
XY, PL and ZL conceived and drafted the manuscript. XY, PL, ZL and ZZ proofread the manuscript and made revisions. XY, PL and ZZ collected the references. ZZ directed the overall design of the manuscript. All authors read and approved the submitted version. All authors contributed to the article and approved the submitted version.
This research was supported by the National Natural Science Foundation of China (82073539); the Project of Sichuan Provincial Department of Science and Technology (2022JDRC0133); the Foundation of The First Affiliated Hospital of Chengdu Medical College (CYFY-GQ35); the Foundation of Chengdu Medical College (CYZYB21-12); and Special Research Project of Sichuan Medical Association (Hengrui) Scientific Research Fund (2021HR56).
The authors would like to thank Mr. Jinhao Zhang for his help with the literature search. Figures were created with BioRender.com.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Liu P, Zhang Z, Li Y. Relevance of the pyroptosis-related inflammasome pathway in the pathogenesis of diabetic kidney disease. Front Immunol (2021) 12:603416. doi: 10.3389/fimmu.2021.603416
2. Du S, Zeugolis DI, O’Brien T. Scaffold-based delivery of mesenchymal stromal cells to diabetic wounds. Stem Cell Res Ther (2022) 13(1):426. doi: 10.1186/s13287-022-03115-4
3. Lim JZ, Ng NS, Thomas C. Prevention and treatment of diabetic foot ulcers. J R Soc Med (2017) 110(3):104–9. doi: 10.1177/0141076816688346
4. Boulton AJ. The diabetic foot: grand overview, epidemiology and pathogenesis. Diabetes Metab Res Rev (2008) 24 Suppl 1:S3–6. doi: 10.1002/dmrr.833
5. Gorden LYT, Ariel YF, Pei H, Meng L, Zhen Yi NG, Graves N, et al. Decision-making for early major amputation in selected diabetic foot ulcer patients with peripheral vascular disease. Health Care Science (2022) 1(2):58–68. doi: 10.1002/hcs2.17
6. Vouillarmet J, Bourron O, Gaudric J, Lermusiaux P, Millon A, Hartemann A. Lower-extremity arterial revascularization: Is there any evidence for diabetic foot ulcer-healing? Diabetes Metab (2016) 42(1):4–15. doi: 10.1016/j.diabet.2015.05.004
7. Colagiuri S, Borch-Johnsen K, Glumer C, Vistisen D. There really is an epidemic of type 2 diabetes. Diabetologia (2005) 48(8):1459–63. doi: 10.1007/s00125-005-1843-y
8. Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet (2005) 366(9498):1719–24. doi: 10.1016/S0140-6736(05)67698-2
9. Monteiro-Soares M, Boyko EJ, Ribeiro J, Ribeiro I, Dinis-Ribeiro M. Risk stratification systems for diabetic foot ulcers: a systematic review. Diabetologia (2011) 54(5):1190–9. doi: 10.1007/s00125-010-2030-3
10. Volmer-Thole M, Lobmann R. Neuropathy and diabetic foot syndrome. Int J Mol Sci (2016) 17(6):917. doi: 10.3390/ijms17060917
11. Sinwar PD. The diabetic foot management - recent advance. Int J Surg (2015) 15:27–30. doi: 10.1016/j.ijsu.2015.01.023
12. Alavi A, Sibbald RG, Mayer D, Goodman L, Botros M, Armstrong DG, et al. Diabetic foot ulcers: Part i. pathophysiology and prevention. J Am Acad Dermatol (2014) 70(1):1 e–18. doi: 10.1016/j.jaad.2013.06.055
13. Lopes L, Setia O, Aurshina A, Liu S, Hu H, Isaji T, et al. Stem cell therapy for diabetic foot ulcers: a review of preclinical and clinical research. Stem Cell Res Ther (2018) 9(1):188. doi: 10.1186/s13287-018-0938-6
14. Sharma C, Kaur A, Thind SS, Singh B, Raina S. Advanced glycation end-products (AGEs): an emerging concern for processed food industries. J Food Sci Technol (2015) 52(12):7561–76. doi: 10.1007/s13197-015-1851-y
15. Barrett EJ, Liu Z, Khamaisi M, King GL, Klein R, Klein BEK, et al. Diabetic microvascular disease: An endocrine society scientific statement. J Clin Endocrinol Metab (2017) 102(12):4343–410. doi: 10.1210/jc.2017-01922
16. Daryabor G, Atashzar MR, Kabelitz D, Meri S, Kalantar K. The effects of type 2 diabetes mellitus on organ metabolism and the immune system. Front Immunol (2020) 11:1582. doi: 10.3389/fimmu.2020.01582
17. Boniakowski AE, Kimball AS, Jacobs BN, Kunkel SL, Gallagher KA. Macrophage-mediated inflammation in normal and diabetic wound healing. J Immunol (2017) 199(1):17–24. doi: 10.4049/jimmunol.1700223
18. Burgess JL, Wyant WA, Abdo Abujamra B, Kirsner RS, Jozic I. Diabetic wound-healing science. Medicina (Kaunas) (2021) 57(10):1072. doi: 10.3390/medicina57101072
19. Boniakowski AM, denDekker AD, Davis FM, Joshi A, Kimball AS, Schaller M, et al. SIRT3 regulates macrophage-mediated inflammation in diabetic wound repair. J Invest Dermatol (2019) 139(12):2528–37 e2. doi: 10.1016/j.jid.2019.05.017
20. Giri B, Dey S, Das T, Sarkar M, Banerjee J, Dash SK. Chronic hyperglycemia mediated physiological alteration and metabolic distortion leads to organ dysfunction, infection, cancer progression and other pathophysiological consequences: An update on glucose toxicity. BioMed Pharmacother (2018) 107:306–28. doi: 10.1016/j.biopha.2018.07.157
21. Chang M, Nguyen TT. Strategy for treatment of infected diabetic foot ulcers. Acc Chem Res (2021) 54(5):1080–93. doi: 10.1021/acs.accounts.0c00864
22. Stino AM, Smith AG. Peripheral neuropathy in prediabetes and the metabolic syndrome. J Diabetes Investig (2017) 8(5):646–55. doi: 10.1111/jdi.12650
23. Baig MS, Banu A, Zehravi M, Rana R, Burle SS, Khan SL, et al. An overview of diabetic foot ulcers and associated problems with special emphasis on treatments with antimicrobials. Life (Basel) (2022) 12(7):1054. doi: 10.3390/life12071054
24. Dayya D, O’Neill OJ, Huedo-Medina TB, Habib N, Moore J, Iyer K. Debridement of diabetic foot ulcers. Adv Wound Care (New Rochelle) (2022) 11(12):666–86. doi: 10.1089/wound.2021.0016
25. Sibbald RG, Elliott JA, Persaud-Jaimangal R, Goodman L, Armstrong DG, Harley C, et al. Wound bed preparation 2021. Adv Skin Wound Care (2021) 34(4):183–95. doi: 10.1097/01.ASW.0000733724.87630.d6
26. Elraiyah T, Domecq JP, Prutsky G, Tsapas A, Nabhan M, Frykberg RG, et al. A systematic review and meta-analysis of debridement methods for chronic diabetic foot ulcers. J Vasc Surg (2016) 63(2 Suppl):37S–45S e1-2. doi: 10.1016/j.jvs.2015.10.002
27. Liu S, He CZ, Cai YT, Xing QP, Guo YZ, Chen ZL, et al. Evaluation of negative-pressure wound therapy for patients with diabetic foot ulcers: systematic review and meta-analysis. Ther Clin Risk Manag (2017) 13:533–44. doi: 10.2147/TCRM.S131193
28. Tejada S, Batle JM, Ferrer MD, Busquets-Cortes C, Monserrat-Mesquida M, Nabavi SM, et al. Therapeutic effects of hyperbaric oxygen in the process of wound healing. Curr Pharm Des (2019) 25(15):1682–93. doi: 10.2174/1381612825666190703162648
29. Lalieu RC, Brouwer RJ, Ubbink DT, Hoencamp R, Bol Raap R, van Hulst RA. Hyperbaric oxygen therapy for nonischemic diabetic ulcers: A systematic review. Wound Repair Regener (2020) 28(2):266–75. doi: 10.1111/wrr.12776
30. Salama SE, Eldeeb AE, Elbarbary AH, Abdelghany SE. Adjuvant hyperbaric oxygen therapy enhances healing of nonischemic diabetic foot ulcers compared with standard wound care alone. Int J Low Extrem Wounds (2019) 18(1):75–80. doi: 10.1177/1534734619829939
31. Kranke P, Bennett MH, Martyn-St James M, Schnabel A, Debus SE, Weibel S. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev (2015) 2015(6):CD004123. doi: 10.1002/14651858.CD004123.pub4
32. Fedorko L, Bowen JM, Jones W, Oreopoulos G, Goeree R, Hopkins RB, et al. Hyperbaric oxygen therapy does not reduce indications for amputation in patients with diabetes with nonhealing ulcers of the lower limb: A prospective, double-blind, randomized controlled clinical trial. Diabetes Care (2016) 39(3):392–9. doi: 10.2337/dc15-2001
33. NA I, Mohd Razip Wee MF, Tabata Y, Bt Hj Idrus R, Nordin A, Fauzi MB. Antibacterial-integrated collagen wound dressing for diabetes-related foot ulcers: An evidence-based review of clinical studies. Polymers (Basel) (2020) 12(9):2168. doi: 10.3390/polym12092168
34. Saco M, Howe N, Nathoo R, Cherpelis B. Comparing the efficacies of alginate, foam, hydrocolloid, hydrofiber, and hydrogel dressings in the management of diabetic foot ulcers and venous leg ulcers: a systematic review and meta-analysis examining how to dress for success. Dermatol Online J (2016) 22(8):13030. doi: 10.5070/D3228032089
35. Wang F, Zhang W, Li H, Chen X, Feng S, Mei Z. How effective are nano-based dressings in diabetic wound healing? a comprehensive review of literature. Int J Nanomed (2022) 17:2097–119. doi: 10.2147/IJN.S361282
36. Tarusha L, Paoletti S, Travan A, Marsich E. Alginate membranes loaded with hyaluronic acid and silver nanoparticles to foster tissue healing and to control bacterial contamination of non-healing wounds. J Mater Sci Mater Med (2018) 29(3):22. doi: 10.1007/s10856-018-6027-7
37. Tsang KK, Kwong EW, To TS, Chung JW, Wong TK. A pilot randomized, controlled study of nanocrystalline silver, manuka honey, and conventional dressing in healing diabetic foot ulcer. Evid Based Complement Alternat Med (2017) 2017:5294890. doi: 10.1155/2017/5294890
38. Jones AD, De Siqueira J, Nixon JE, Siddle HJ, Culmer PR, Russell DA. Plantar shear stress in the diabetic foot: A systematic review and meta-analysis. Diabetes Med (2022) 39(1):e14661. doi: 10.1111/dme.14661
39. Okoli GN, Rabbani R, Lam OLT, Askin N, Horsley T, Bayliss L, et al. Offloading devices for neuropathic foot ulcers in adult persons with type 1 or type 2 diabetes: a rapid review with meta-analysis and trial sequential analysis of randomized controlled trials. BMJ Open Diabetes Res Care (2022) 10(3):e002822. doi: 10.1136/bmjdrc-2022-002822
40. Everett E, Mathioudakis N. Update on management of diabetic foot ulcers. Ann N Y Acad Sci (2018) 1411(1):153–65. doi: 10.1111/nyas.13569
41. Begg L, McLaughlin P, Vicaretti M, Fletcher J, Burns J. Total contact cast wall load in patients with a plantar forefoot ulcer and diabetes. J Foot Ankle Res (2016) 9:2. doi: 10.1186/s13047-015-0119-0
42. Nalisa DL, Moneruzzaman M, Changwe GJ, Mobet Y, Li LP, Ma YJ, et al. Stem cell therapy for diabetic foot ulcers: Theory and practice. J Diabetes Res (2022) 2022:6028743. doi: 10.1155/2022/6028743
43. Bakker K, Apelqvist J, Lipsky BA, Van Netten JJ, International Working Group on the Diabetic F. The 2015 IWGDF guidance documents on prevention and management of foot problems in diabetes: development of an evidence-based global consensus. Diabetes Metab Res Rev (2016) 32 Suppl 1:2–6. doi: 10.1002/dmrr.2694
44. Dogruel H, Aydemir M, Balci MK. Management of diabetic foot ulcers and the challenging points: An endocrine view. World J Diabetes (2022) 13(1):27–36. doi: 10.4239/wjd.v13.i1.27
45. Zhang Z, Liu P, Yang B, Li J, Wang W, Yang H, et al. Necrotizing fasciitis caused by diabetic foot. Int J Infect Dis (2021) 103:3–5. doi: 10.1016/j.ijid.2020.11.132
46. Gorden LYT, Ariel YF, Pei H, Meng L, Yi Zhen NG, Graves N. Decision-making for early major amputation in selected diabetic foot ulcer patients with peripheral vascular disease. Health Care Sci (2022) 1(2):58–68. doi: 10.1002/hcs2.17
47. Gregory CA, Prockop DJ, Spees JL. Non-hematopoietic bone marrow stem cells: molecular control of expansion and differentiation. Exp Cell Res (2005) 306(2):330–5. doi: 10.1016/j.yexcr.2005.03.018
48. Wu Q, Chen B, Liang Z. Mesenchymal stem cells as a prospective therapy for the diabetic foot. Stem Cells Int (2016) 2016:4612167. doi: 10.1155/2016/4612167
50. Ferretti E, Hadjantonakis AK. Mesoderm specification and diversification: from single cells to emergent tissues. Curr Opin Cell Biol (2019) 61:110–6. doi: 10.1016/j.ceb.2019.07.012
51. Li M, Ikehara S. Bone-marrow-derived mesenchymal stem cells for organ repair. Stem Cells Int (2013) 2013:132642. doi: 10.1155/2013/132642
52. Almalki SG, Agrawal DK. Key transcription factors in the differentiation of mesenchymal stem cells. Differentiation (2016) 92(1-2):41–51. doi: 10.1016/j.diff.2016.02.005
53. Yang Y, Liu S, He C, Chen Z, Lyu T, Zeng L, et al. Long non-coding RNA regulation of mesenchymal stem cell homeostasis and differentiation: Advances, challenges, and perspectives. Front Cell Dev Biol (2021) 9:711005. doi: 10.3389/fcell.2021.711005
54. Guillamat-Prats R. The role of MSC in wound healing, scarring and regeneration. Cells (2021) 10(7):1729. doi: 10.3390/cells10071729
55. Ma S, Xie N, Li W, Yuan B, Shi Y, Wang Y. Immunobiology of mesenchymal stem cells. Cell Death Differ (2014) 21(2):216–25. doi: 10.1038/cdd.2013.158
56. Singh MS, Park SS, Albini TA, Canto-Soler MV, Klassen H, MacLaren RE, et al. Retinal stem cell transplantation: Balancing safety and potential. Prog Retin Eye Res (2020) 75:100779. doi: 10.1016/j.preteyeres.2019.100779
57. Le Q, Chauhan T, Yung M, Tseng CH, Deng SX. Outcomes of limbal stem cell transplant: A meta-analysis. JAMA Ophthalmol (2020) 138(6):660–70. doi: 10.1001/jamaophthalmol.2020.1120
58. Shiels A, Hejtmancik JF. Biology of inherited cataracts and opportunities for treatment. Annu Rev Vis Sci (2019) 5:123–49. doi: 10.1146/annurev-vision-091517-034346
59. Basu S, Ali H, Sangwan VS. Clinical outcomes of repeat autologous cultivated limbal epithelial transplantation for ocular surface burns. Am J Ophthalmol (2012) 153(4):643–50. doi: 10.1016/j.ajo.2011.09.016
60. Trounson A, McDonald C. Stem cell therapies in clinical trials: Progress and challenges. Cell Stem Cell (2015) 17(1):11–22. doi: 10.1016/j.stem.2015.06.007
61. Jackson CJ, Tonseth KA, Utheim TP. Cultured epidermal stem cells in regenerative medicine. Stem Cell Res Ther (2017) 8(1):155. doi: 10.1186/s13287-017-0587-1
62. Rodgers K, Jadhav SS. The application of mesenchymal stem cells to treat thermal and radiation burns. Adv Drug Delivery Rev (2018) 123:75–81. doi: 10.1016/j.addr.2017.10.003
63. Miao C, Lei M, Hu W, Han S, Wang Q. A brief review: the therapeutic potential of bone marrow mesenchymal stem cells in myocardial infarction. Stem Cell Res Ther (2017) 8(1):242. doi: 10.1186/s13287-017-0697-9
64. Lee MS, Makkar RR. Stem-cell transplantation in myocardial infarction: a status report. Ann Intern Med (2004) 140(9):729–37. doi: 10.7326/0003-4819-140-9-200405040-00013
65. Parmar M. Towards stem cell based therapies for parkinson’s disease. Development (2018) 145(1):dev156117. doi: 10.1242/dev.156117
66. Andrzejewska A, Dabrowska S, Lukomska B, Janowski M. Mesenchymal stem cells for neurological disorders. Adv Sci (Weinh) (2021) 8(7):2002944. doi: 10.1002/advs.202002944
67. Kerkis I, Haddad MS, Valverde CW, Glosman S. Neural and mesenchymal stem cells in animal models of huntington’s disease: past experiences and future challenges. Stem Cell Res Ther (2015) 6:232. doi: 10.1186/s13287-015-0248-1
68. Tartaglione AM, Popoli P, Calamandrei G. Regenerative medicine in huntington’s disease: Strengths and weaknesses of preclinical studies. Neurosci Biobehav Rev (2017) 77:32–47. doi: 10.1016/j.neubiorev.2017.02.017
69. Berlanga-Acosta JA, Guillen-Nieto GE, Rodriguez-Rodriguez N, Mendoza-Mari Y, Bringas-Vega ML, Berlanga-Saez JO, et al. Cellular senescence as the pathogenic hub of diabetes-related wound chronicity. Front Endocrinol (Lausanne) (2020) 11:573032. doi: 10.3389/fendo.2020.573032
70. Motegi SI, Ishikawa O. Mesenchymal stem cells: The roles and functions in cutaneous wound healing and tumor growth. J Dermatol Sci (2017) 86(2):83–9. doi: 10.1016/j.jdermsci.2016.11.005
71. Mazini L, Rochette L, Admou B, Amal S, Malka G. Hopes and limits of adipose-derived stem cells (ADSCs) and mesenchymal stem cells (MSCs) in wound healing. Int J Mol Sci (2020) 21(4):1306. doi: 10.3390/ijms21041306
72. Lin H, Sohn J, Shen H, Langhans MT, Tuan RS. Bone marrow mesenchymal stem cells: Aging and tissue engineering applications to enhance bone healing. Biomaterials (2019) 203:96–110. doi: 10.1016/j.biomaterials.2018.06.026
73. Arthur A, Gronthos S. Clinical application of bone marrow mesenchymal Stem/Stromal cells to repair skeletal tissue. Int J Mol Sci (2020) 21(24):9759. doi: 10.3390/ijms21249759
74. Le H, Xu W, Zhuang X, Chang F, Wang Y, Ding J. Mesenchymal stem cells for cartilage regeneration. J Tissue Eng (2020) 11:2041731420943839. doi: 10.1177/2041731420943839
75. Iaquinta MR, Lanzillotti C, Mazziotta C, Bononi I, Frontini F, Mazzoni E, et al. The role of microRNAs in the osteogenic and chondrogenic differentiation of mesenchymal stem cells and bone pathologies. Theranostics (2021) 11(13):6573–91. doi: 10.7150/thno.55664
76. Tan YZ, Fei DD, He XN, Dai JM, Xu RC, Xu XY, et al. L-type voltage-gated calcium channels in stem cells and tissue engineering. Cell Prolif (2019) 52(4):e12623. doi: 10.1111/cpr.12623
77. Willerth SM. Neural tissue engineering using embryonic and induced pluripotent stem cells. Stem Cell Res Ther (2011) 2(2):17. doi: 10.1186/scrt58
78. Alonzo M, AnilKumar S, Roman B, Tasnim N, Joddar B. 3D bioprinting of cardiac tissue and cardiac stem cell therapy. Transl Res (2019) 211:64–83. doi: 10.1016/j.trsl.2019.04.004
79. Davidson SM, Padro T, Bollini S, Vilahur G, Duncker DJ, Evans PC, et al. Progress in cardiac research: from rebooting cardiac regeneration to a complete cell atlas of the heart. Cardiovasc Res (2021) 117(10):2161–74. doi: 10.1093/cvr/cvab200
80. Nakamura T, Sato T. Advancing intestinal organoid technology toward regenerative medicine. Cell Mol Gastroenterol Hepatol (2018) 5(1):51–60. doi: 10.1016/j.jcmgh.2017.10.006
81. Sener LT, Albeniz I. Challenge of mesenchymal stem cells against diabetic foot ulcer. Curr Stem Cell Res Ther (2015) 10(6):530–4. doi: 10.2174/1574888x10666150519092931
82. Nolan GS, Smith OJ, Jell G, Mosahebi A. Fat grafting and platelet-rich plasma in wound healing: a review of histology from animal studies. Adipocyte (2021) 10(1):80–90. doi: 10.1080/21623945.2021.1876374
83. Klyushnenkova E, Mosca JD, Zernetkina V, Majumdar MK, Beggs KJ, Simonetti DW, et al. T Cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. J BioMed Sci (2005) 12(1):47–57. doi: 10.1007/s11373-004-8183-7
84. Jacobs SA, Roobrouck VD, Verfaillie CM, Van Gool SW. Immunological characteristics of human mesenchymal stem cells and multipotent adult progenitor cells. Immunol Cell Biol (2013) 91(1):32–9. doi: 10.1038/icb.2012.64
85. Abdal Dayem A, Lee SB, Kim K, Lim KM, Jeon TI, Seok J, et al. Production of mesenchymal stem cells through stem cell reprogramming. Int J Mol Sci (2019) 20(8):1922. doi: 10.3390/ijms20081922
86. Kot M, Baj-Krzyworzeka M, Szatanek R, Musial-Wysocka A, Suda-Szczurek M, Majka M. The importance of HLA assessment in “Off-the-Shelf” allogeneic mesenchymal stem cells based-therapies. Int J Mol Sci (2019) 20(22):5680. doi: 10.3390/ijms20225680
87. Jhunjhunwala S, Hammer C, Delamarre L. Antigen presentation in cancer: insights into tumour immunogenicity and immune evasion. Nat Rev Cancer (2021) 21(5):298–312. doi: 10.1038/s41568-021-00339-z
88. Yang XF, Chen T, Ren LW, Yang L, Qi H, Li FR. Immunogenicity of insulin-producing cells derived from human umbilical cord mesenchymal stem cells. Exp Ther Med (2017) 13(4):1456–64. doi: 10.3892/etm.2017.4096
89. Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringden O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol (2003) 31(10):890–6. doi: 10.1016/s0301-472x(03)00110-3
90. Agudo J, Park ES, Rose SA, Alibo E, Sweeney R, Dhainaut M, et al. Quiescent tissue stem cells evade immune surveillance. Immunity (2018) 48(2):271–85 e5. doi: 10.1016/j.immuni.2018.02.001
91. Galland S, Stamenkovic I. Mesenchymal stromal cells in cancer: a review of their immunomodulatory functions and dual effects on tumor progression. J Pathol (2020) 250(5):555–72. doi: 10.1002/path.5357
92. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science (1999) 284(5411):143–7. doi: 10.1126/science.284.5411.143
93. Gao Q, Wang L, Wang S, Huang B, Jing Y, Su J. Bone marrow mesenchymal stromal cells: Identification, classification, and differentiation. Front Cell Dev Biol (2021) 9:787118. doi: 10.3389/fcell.2021.787118
94. Bhat S, Viswanathan P, Chandanala S, Prasanna SJ, Seetharam RN. Expansion and characterization of bone marrow derived human mesenchymal stromal cells in serum-free conditions. Sci Rep (2021) 11(1):3403. doi: 10.1038/s41598-021-83088-1
95. Kim DW, Staples M, Shinozuka K, Pantcheva P, Kang SD, Borlongan CV. Wharton’s jelly-derived mesenchymal stem cells: phenotypic characterization and optimizing their therapeutic potential for clinical applications. Int J Mol Sci (2013) 14(6):11692–712. doi: 10.3390/ijms140611692
96. Mebarki M, Abadie C, Larghero J, Cras A. Human umbilical cord-derived mesenchymal stem/stromal cells: a promising candidate for the development of advanced therapy medicinal products. Stem Cell Res Ther (2021) 12(1):152. doi: 10.1186/s13287-021-02222-y
97. Bieback K, Kern S, Kluter H, Eichler H. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells (2004) 22(4):625–34. doi: 10.1634/stemcells.22-4-625
98. Jin HJ, Bae YK, Kim M, Kwon SJ, Jeon HB, Choi SJ, et al. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int J Mol Sci (2013) 14(9):17986–8001. doi: 10.3390/ijms140917986
99. Ryu HH, Kang BJ, Park SS, Kim Y, Sung GJ, Woo HM, et al. Comparison of mesenchymal stem cells derived from fat, bone marrow, wharton’s jelly, and umbilical cord blood for treating spinal cord injuries in dogs. J Vet Med Sci (2012) 74(12):1617–30. doi: 10.1292/jvms.12-0065
100. Sabapathy V, Sundaram B V, Mankuzhy P, Kumar S. Human wharton’s jelly mesenchymal stem cells plasticity augments scar-free skin wound healing with hair growth. PloS One (2014) 9(4):e93726. doi: 10.1371/journal.pone.0093726
101. Shang Y, Guan H, Zhou F. Biological characteristics of umbilical cord mesenchymal stem cells and its therapeutic potential for hematological disorders. Front Cell Dev Biol (2021) 9:570179. doi: 10.3389/fcell.2021.570179
102. Mohamed-Ahmed S, Fristad I, Lie SA, Suliman S, Mustafa K, Vindenes H, et al. Adipose-derived and bone marrow mesenchymal stem cells: a donor-matched comparison. Stem Cell Res Ther (2018) 9(1):168. doi: 10.1186/s13287-018-0914-1
103. Gruber HE, Somayaji S, Riley F, Hoelscher GL, Norton HJ, Ingram J, et al. Human adipose-derived mesenchymal stem cells: serial passaging, doubling time and cell senescence. Biotech Histochem (2012) 87(4):303–11. doi: 10.3109/10520295.2011.649785
104. Huang SJ, Fu RH, Shyu WC, Liu SP, Jong GP, Chiu YW, et al. Adipose-derived stem cells: isolation, characterization, and differentiation potential. Cell Transplant (2013) 22(4):701–9. doi: 10.3727/096368912X655127
105. Krawczenko A, Klimczak A. Adipose tissue-derived mesenchymal Stem/Stromal cells and their contribution to angiogenic processes in tissue regeneration. Int J Mol Sci (2022) 23(5):2425. doi: 10.3390/ijms23052425
106. Suga H, Matsumoto D, Eto H, Inoue K, Aoi N, Kato H, et al. Functional implications of CD34 expression in human adipose-derived stem/progenitor cells. Stem Cells Dev (2009) 18(8):1201–10. doi: 10.1089/scd.2009.0003
107. Mazini L, Ezzoubi M, Malka G. Overview of current adipose-derived stem cell (ADSCs) processing involved in therapeutic advancements: flow chart and regulation updates before and after COVID-19. Stem Cell Res Ther (2021) 12(1):1. doi: 10.1186/s13287-020-02006-w
108. Bunnell BA. Adipose tissue-derived mesenchymal stem cells. Cells (2021) 10(12):3433. doi: 10.3390/cells10123433
109. Mohamed-Ahmed S, Yassin MA, Rashad A, Espedal H, Idris SB, Finne-Wistrand A, et al. Comparison of bone regenerative capacity of donor-matched human adipose-derived and bone marrow mesenchymal stem cells. Cell Tissue Res (2021) 383(3):1061–75. doi: 10.1007/s00441-020-03315-5
110. Oliveira MS, Barreto-Filho JB. Placental-derived stem cells: Culture, differentiation and challenges. World J Stem Cells (2015) 7(4):769–75. doi: 10.4252/wjsc.v7.i4.769
111. Macias MI, Grande J, Moreno A, Dominguez I, Bornstein R, Flores AI. Isolation and characterization of true mesenchymal stem cells derived from human term decidua capable of multilineage differentiation into all 3 embryonic layers. Am J Obstet Gynecol (2010) 203(5):495 e9– e23. doi: 10.1016/j.ajog.2010.06.045
112. Siddesh SE, Gowda DM, Jain R, Gulati A, Patil GS, Anudeep TC, et al. Placenta-derived mesenchymal stem cells (P-MSCs) for COVID-19 pneumonia-a regenerative dogma. Stem Cell Investig (2021) 8:3. doi: 10.21037/sci-2020-034
113. Abumaree MH, Al Jumah MA, Kalionis B, Jawdat D, Al Khaldi A, Abomaray FM, et al. Human placental mesenchymal stem cells (pMSCs) play a role as immune suppressive cells by shifting macrophage differentiation from inflammatory M1 to anti-inflammatory M2 macrophages. Stem Cell Rev Rep (2013) 9(5):620–41. doi: 10.1007/s12015-013-9455-2
114. Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood (2001) 98(8):2396–402. doi: 10.1182/blood.v98.8.2396
115. Zhang Y, McNeill E, Tian H, Soker S, Andersson KE, Yoo JJ, et al. Urine derived cells are a potential source for urological tissue reconstruction. J Urol (2008) 180(5):2226–33. doi: 10.1016/j.juro.2008.07.023
116. Zhang W, Hu J, Huang Y, Wu C, Xie H. Urine-derived stem cells: applications in skin, bone and articular cartilage repair. Burns Trauma (2021) 9:tkab039. doi: 10.1093/burnst/tkab039
117. Kang HS, Choi SH, Kim BS, Choi JY, Park GB, Kwon TG, et al. Advanced properties of urine derived stem cells compared to adipose tissue derived stem cells in terms of cell proliferation, immune modulation and multi differentiation. J Korean Med Sci (2015) 30(12):1764–76. doi: 10.3346/jkms.2015.30.12.1764
118. Lang R, Liu G, Shi Y, Bharadwaj S, Leng X, Zhou X, et al. Self-renewal and differentiation capacity of urine-derived stem cells after urine preservation for 24 hours. PloS One (2013) 8(1):e53980. doi: 10.1371/journal.pone.0053980
119. Guan JJ, Niu X, Gong FX, Hu B, Guo SC, Lou YL, et al. Biological characteristics of human-urine-derived stem cells: potential for cell-based therapy in neurology. Tissue Eng Part A (2014) 20(13-14):1794–806. doi: 10.1089/ten.TEA.2013.0584
120. Sun X, Zheng W, Qian C, Wu Q, Hao Y, Lu G. Focal adhesion kinase promotes BMP2-induced osteogenic differentiation of human urinary stem cells via AMPK and wnt signaling pathways. J Cell Physiol (2020) 235(5):4954–64. doi: 10.1002/jcp.29374
121. Liu G, Wu R, Yang B, Deng C, Lu X, Walker SJ, et al. Human urine-derived stem cell differentiation to endothelial cells with barrier function and nitric oxide production. Stem Cells Transl Med (2018) 7(9):686–98. doi: 10.1002/sctm.18-0040
122. Lazzeri E, Ronconi E, Angelotti ML, Peired A, Mazzinghi B, Becherucci F, et al. Human urine-derived renal progenitors for personalized modeling of genetic kidney disorders. J Am Soc Nephrol (2015) 26(8):1961–74. doi: 10.1681/ASN.2014010057
123. Rahman MS, Wruck W, Spitzhorn LS, Nguyen L, Bohndorf M, Martins S, et al. TGFbeta and WNT axis modulate self-renewal of human SIX2(+) urine derived renal progenitor cells. Sci Rep (2020) 10(1):739. doi: 10.1038/s41598-020-57723-2
124. Chen AJ, Pi JK, Hu JG, Huang YZ, Gao HW, Li SF, et al. Identification and characterization of two morphologically distinct stem cell subpopulations from human urine samples. Sci China Life Sci (2020) 63(5):712–23. doi: 10.1007/s11427-018-9543-1
125. He W, Zhu W, Cao Q, Shen Y, Zhou Q, Yu P, et al. Generation of mesenchymal-like stem cells from urine in pediatric patients. Transplant Proc (2016) 48(6):2181–5. doi: 10.1016/j.transproceed.2016.02.078
126. Bharadwaj S, Liu G, Shi Y, Wu R, Yang B, He T, et al. Multipotential differentiation of human urine-derived stem cells: potential for therapeutic applications in urology. Stem Cells (2013) 31(9):1840–56. doi: 10.1002/stem.1424
127. Zhang Q, Shi S, Liu Y, Uyanne J, Shi Y, Shi S, et al. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol (2009) 183(12):7787–98. doi: 10.4049/jimmunol.0902318
128. Li D, Zou XY, El-Ayachi I, Romero LO, Yu Z, Iglesias-Linares A, et al. Human dental pulp stem cells and gingival mesenchymal stem cells display action potential capacity In vitro after neuronogenic differentiation. Stem Cell Rev Rep (2019) 15(1):67–81. doi: 10.1007/s12015-018-9854-5
129. Murugan Girija D, Kalachaveedu M, Ranga Rao S, Subbarayan R. Transdifferentiation of human gingival mesenchymal stem cells into functional keratinocytes by acalypha indica in three-dimensional microenvironment. J Cell Physiol (2018) 233(11):8450–7. doi: 10.1002/jcp.26807
130. Luo Y, Wu W, Gu J, Zhang X, Dang J, Wang J, et al. Human gingival tissue-derived MSC suppress osteoclastogenesis and bone erosion via CD39-adenosine signal pathway in autoimmune arthritis. EBioMedicine (2019) 43:620–31. doi: 10.1016/j.ebiom.2019.04.058
131. De la Rosa-Ruiz MDP, Alvarez-Perez MA, Cortes-Morales VA, Monroy-Garcia A, Mayani H, Fragoso-Gonzalez G, et al. Mesenchymal Stem/Stromal cells derived from dental tissues: A comparative In vitro evaluation of their immunoregulatory properties against T cells. Cells (2019) 8(12):1491. doi: 10.3390/cells8121491
132. Zhang X, Huang F, Li W, Dang JL, Yuan J, Wang J, et al. Human gingiva-derived mesenchymal stem cells modulate Monocytes/Macrophages and alleviate atherosclerosis. Front Immunol (2018) 9:878. doi: 10.3389/fimmu.2018.00878
133. Andreadis D, Bakopoulou A, Leyhausen G, Epivatianos A, Volk J, Markopoulos A, et al. Minor salivary glands of the lips: a novel, easily accessible source of potential stem/progenitor cells. Clin Oral Investig (2014) 18(3):847–56. doi: 10.1007/s00784-013-1056-6
134. Lu L, Li Y, Du MJ, Zhang C, Zhang XY, Tong HZ, et al. Characterization of a self-renewing and multi-potent cell population isolated from human minor salivary glands. Sci Rep (2015) 5:10106. doi: 10.1038/srep10106
135. Wang SQ, Wang YX, Hua H. Characteristics of labial gland mesenchymal stem cells of healthy individuals and patients with sjogren’s syndrome: A preliminary study. Stem Cells Dev (2017) 26(16):1171–85. doi: 10.1089/scd.2017.0045
136. Sato A, Okumura K, Matsumoto S, Hattori K, Hattori S, Shinohara M, et al. Isolation, tissue localization, and cellular characterization of progenitors derived from adult human salivary glands. Cloning Stem Cells (2007) 9(2):191–205. doi: 10.1089/clo.2006.0054
137. Tatsuishi Y, Hirota M, Kishi T, Adachi M, Fukui T, Mitsudo K, et al. Human salivary gland stem/progenitor cells remain dormant even after irradiation. Int J Mol Med (2009) 24(3):361–6. doi: 10.3892/ijmm_00000240
138. Xu J, Su Y, Hu L, Cain A, Gu Y, Liu B, et al. Effect of bone morphogenetic protein 6 on immunomodulatory functions of salivary gland-derived mesenchymal stem cells in sjogren’s syndrome. Stem Cells Dev (2018) 27(22):1540–8. doi: 10.1089/scd.2017.0161
139. Li B, Xing Y, Gan Y, He J, Hua H. Labial gland-derived mesenchymal stem cells and their exosomes ameliorate murine sjogren’s syndrome by modulating the balance of treg and Th17 cells. Stem Cell Res Ther (2021) 12(1):478. doi: 10.1186/s13287-021-02541-0
140. McCoy SS, Giri J, Das R, Paul PK, Pennati A, Parker M, et al. Minor salivary gland mesenchymal stromal cells derived from patients with Sjögren’s syndrome deploy intact immune plasticity. Cytotherapy (2021) 23(4):301–10. doi: 10.1016/j.jcyt.2020.09.008
141. Davies LC, Alm JJ, Heldring N, Moll G, Gavin C, Batsis I, et al. Type 1 diabetes mellitus donor mesenchymal stromal cells exhibit comparable potency to healthy controls. In Vitro. Stem Cells Transl Med (2016) 5(11):1485–95. doi: 10.5966/sctm.2015-0272
142. Savio-Silva C, Beyerstedt S, Soinski-Sousa PE, Casaro EB, Balby-Rocha MTA, Simplicio-Filho A, et al. Mesenchymal stem cell therapy for diabetic kidney disease: A review of the studies using syngeneic, autologous, allogeneic, and xenogeneic cells. Stem Cells Int (2020) 2020:8833725. doi: 10.1155/2020/8833725
143. Guan YT, Xie Y, Li DS, Zhu YY, Zhang XL, Feng YL, et al. Comparison of biological characteristics of mesenchymal stem cells derived from the human umbilical cord and decidua parietalis. Mol Med Rep (2019) 20(1):633–9. doi: 10.3892/mmr.2019.10286
144. Wan J, Xia L, Liang W, Liu Y, Cai Q. Transplantation of bone marrow-derived mesenchymal stem cells promotes delayed wound healing in diabetic rats. J Diabetes Res (2013) 2013:647107. doi: 10.1155/2013/647107
145. Abd-Allah SH, El-Shal AS, Shalaby SM, Abd-Elbary E, Mazen NF, Abdel Kader RR. The role of placenta-derived mesenchymal stem cells in healing of induced full-thickness skin wound in a mouse model. IUBMB Life (2015) 67(9):701–9. doi: 10.1002/iub.1427
146. Yan J, Liang J, Cao Y, El Akkawi MM, Liao X, Chen X, et al. Efficacy of topical and systemic transplantation of mesenchymal stem cells in a rat model of diabetic ischemic wounds. Stem Cell Res Ther (2021) 12(1):220. doi: 10.1186/s13287-021-02288-8
147. Rustad KC, Gurtner GC. Mesenchymal stem cells home to sites of injury and inflammation. Adv Wound Care (New Rochelle) (2012) 1(4):147–52. doi: 10.1089/wound.2011.0314
148. El Hage R, Knippschild U, Arnold T, Hinterseher I. Stem cell-based therapy: A promising treatment for diabetic foot ulcer. Biomedicines (2022) 10(7):1507. doi: 10.3390/biomedicines10071507
149. Marquardt LM, Heilshorn SC. Design of injectable materials to improve stem cell transplantation. Curr Stem Cell Rep (2016) 2(3):207–20. doi: 10.1007/s40778-016-0058-0
150. Wahlberg B, Ghuman H, Liu JR, Modo M. Ex vivo biomechanical characterization of syringe-needle ejections for intracerebral cell delivery. Sci Rep (2018) 8(1):9194. doi: 10.1038/s41598-018-27568-x
151. Assi R, Foster TR, He H, Stamati K, Bai H, Huang Y, et al. Delivery of mesenchymal stem cells in biomimetic engineered scaffolds promotes healing of diabetic ulcers. Regener Med (2016) 11(3):245–60. doi: 10.2217/rme-2015-0045
152. Assis A, Camargo S, Margalit R, Mitrani E. Creation of a vascular inducing device using mesenchymal stem cells to induce angiogenesis. J Biosci Bioeng (2021) 132(4):408–16. doi: 10.1016/j.jbiosc.2021.06.012
153. Assis A, Gellman YN, Cahn A, Haze A, Camargo S, Mitrani E. Angiogenic potential of mesenchymal stem cells derived from patients with diabetes seeded on decellularized micro fragments. J Diabetes Complications (2021) 35(10):108001. doi: 10.1016/j.jdiacomp.2021.108001
154. Ho J, Yue D, Cheema U, Hsia HC, Dardik A. Innovations in stem cell therapy for diabetic wound healing. Adv Wound Care (New Rochelle) (2022). Online ahead of print. doi: 10.1089/wound.2021.0104
155. Chen TY, Wen TK, Dai NT, Hsu SH. Cryogel/hydrogel biomaterials and acupuncture combined to promote diabetic skin wound healing through immunomodulation. Biomaterials (2021) 269:120608. doi: 10.1016/j.biomaterials.2020.120608
156. De Gregorio C, Contador D, Diaz D, Carcamo C, Santapau D, Lobos-Gonzalez L, et al. Human adipose-derived mesenchymal stem cell-conditioned medium ameliorates polyneuropathy and foot ulceration in diabetic BKS db/db mice. Stem Cell Res Ther (2020) 11(1):168. doi: 10.1186/s13287-020-01680-0
157. Shi R, Jin Y, Cao C, Han S, Shao X, Meng L, et al. Localization of human adipose-derived stem cells and their effect in repair of diabetic foot ulcers in rats. Stem Cell Res Ther (2016) 7(1):155. doi: 10.1186/s13287-016-0412-2
158. Schlosser S, Dennler C, Schweizer R, Eberli D, Stein JV, Enzmann V, et al. Paracrine effects of mesenchymal stem cells enhance vascular regeneration in ischemic murine skin. Microvasc Res (2012) 83(3):267–75. doi: 10.1016/j.mvr.2012.02.011
159. Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation (2004) 109(12):1543–9. doi: 10.1161/01.CIR.0000124062.31102.57
160. Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature (2011) 473(7347):298–307. doi: 10.1038/nature10144
161. Shen L, Zeng W, Wu YX, Hou CL, Chen W, Yang MC, et al. Neurotrophin-3 accelerates wound healing in diabetic mice by promoting a paracrine response in mesenchymal stem cells. Cell Transplant (2013) 22(6):1011–21. doi: 10.3727/096368912X657495
162. Badillo AT, Redden RA, Zhang L, Doolin EJ, Liechty KW. Treatment of diabetic wounds with fetal murine mesenchymal stromal cells enhances wound closure. Cell Tissue Res (2007) 329(2):301–11. doi: 10.1007/s00441-007-0417-3
163. Kwon DS, Gao X, Liu YB, Dulchavsky DS, Danyluk AL, Bansal M, et al. Treatment with bone marrow-derived stromal cells accelerates wound healing in diabetic rats. Int Wound J (2008) 5(3):453–63. doi: 10.1111/j.1742-481X.2007.00408.x
164. Diao Y, Lian L, Guo L, Chen H, Chen Y, Song X, et al. A vascular endothelial growth factor activating transcription factor increases the endothelial progenitor cells population and induces therapeutic angiogenesis in a type 1 diabetic mouse with hindlimb ischemia. Chin Med J (Engl) (2014) 127(20):3623–9.
165. Li B, Zheng YW, Sano Y, Taniguchi H. Evidence for mesenchymal-epithelial transition associated with mouse hepatic stem cell differentiation. PloS One (2011) 6(2):e17092. doi: 10.1371/journal.pone.0017092
166. Dos Santos JF, Borcari NR, da Silva Araujo M, Nunes VA. Mesenchymal stem cells differentiate into keratinocytes and express epidermal kallikreins: Towards an in vitro model of human epidermis. J Cell Biochem (2019) 120(8):13141–55. doi: 10.1002/jcb.28589
167. Kato J, Kamiya H, Himeno T, Shibata T, Kondo M, Okawa T, et al. Mesenchymal stem cells ameliorate impaired wound healing through enhancing keratinocyte functions in diabetic foot ulcerations on the plantar skin of rats. J Diabetes Complications (2014) 28(5):588–95. doi: 10.1016/j.jdiacomp.2014.05.003
168. Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PloS One (2008) 3(4):e1886. doi: 10.1371/journal.pone.0001886
169. Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells (2007) 25(10):2648–59. doi: 10.1634/stemcells.2007-0226
170. Zhao QS, Xia N, Zhao N, Li M, Bi CL, Zhu Q, et al. Localization of human mesenchymal stem cells from umbilical cord blood and their role in repair of diabetic foot ulcers in rats. Int J Biol Sci (2013) 10(1):80–9. doi: 10.7150/ijbs.7237
171. Afflerbach AK, Kiri MD, Detinis T, Maoz BM. Mesenchymal stem cells as a promising cell source for integration in novel In vitro models. Biomolecules (2020) 10(9):1306. doi: 10.3390/biom10091306
172. Schneider RK, Neuss S, Stainforth R, Laddach N, Bovi M, Knuechel R, et al. Three-dimensional epidermis-like growth of human mesenchymal stem cells on dermal equivalents: contribution to tissue organization by adaptation of myofibroblastic phenotype and function. Differentiation (2008) 76(2):156–67. doi: 10.1111/j.1432-0436.2007.00204.x
173. Guo J, Hu Z, Yan F, Lei S, Li T, Li X, et al. Angelica dahurica promoted angiogenesis and accelerated wound healing in db/db mice via the HIF-1alpha/PDGF-beta signaling pathway. Free Radic Biol Med (2020) 160:447–57. doi: 10.1016/j.freeradbiomed.2020.08.015
174. Zhang E, Gao B, Yang L, Wu X, Wang Z. Notoginsenoside Ft1 promotes fibroblast proliferation via PI3K/Akt/mTOR signaling pathway and benefits wound healing in genetically diabetic mice. J Pharmacol Exp Ther (2016) 356(2):324–32. doi: 10.1124/jpet.115.229369
175. Chen J, Crawford R, Chen C, Xiao Y. The key regulatory roles of the PI3K/Akt signaling pathway in the functionalities of mesenchymal stem cells and applications in tissue regeneration. Tissue Eng Part B Rev (2013) 19(6):516–28. doi: 10.1089/ten.TEB.2012.0672
176. Ilagan MX, Kopan R. SnapShot: notch signaling pathway. Cell (2007) 128(6):1246. doi: 10.1016/j.cell.2007.03.011
177. Hou C, Shen L, Huang Q, Mi J, Wu Y, Yang M, et al. The effect of heme oxygenase-1 complexed with collagen on MSC performance in the treatment of diabetic ischemic ulcer. Biomaterials (2013) 34(1):112–20. doi: 10.1016/j.biomaterials.2012.09.022
178. Jun EK, Zhang Q, Yoon BS, Moon JH, Lee G, Park G, et al. Hypoxic conditioned medium from human amniotic fluid-derived mesenchymal stem cells accelerates skin wound healing through TGF-beta/SMAD2 and PI3K/Akt pathways. Int J Mol Sci (2014) 15(1):605–28. doi: 10.3390/ijms15010605
179. Lau TT, Wang DA. Stromal cell-derived factor-1 (SDF-1): homing factor for engineered regenerative medicine. Expert Opin Biol Ther (2011) 11(2):189–97. doi: 10.1517/14712598.2011.546338
180. Ebrahim N, Dessouky AA, Mostafa O, Hassouna A, Yousef MM, Seleem Y, et al. Adipose mesenchymal stem cells combined with platelet-rich plasma accelerate diabetic wound healing by modulating the notch pathway. Stem Cell Res Ther (2021) 12(1):392. doi: 10.1186/s13287-021-02454-y
181. Huang YW, Zhu QQ, Yang XY, Xu HH, Sun B, Wang XJ, et al. Wound healing can be improved by (-)-epigallocatechin gallate through targeting notch in streptozotocin-induced diabetic mice. FASEB J (2019) 33(1):953–64. doi: 10.1096/fj.201800337R
182. Yang JM, Ryu J, Kim I, Chang H, Kim IK. Dll4 blockade promotes angiogenesis in nonhealing wounds of Sox7-deficient mice. Adv Wound Care (New Rochelle) (2020) 9(11):591–601. doi: 10.1089/wound.2019.1015
183. Yagi H, Soto-Gutierrez A, Parekkadan B, Kitagawa Y, Tompkins RG, Kobayashi N, et al. Mesenchymal stem cells: Mechanisms of immunomodulation and homing. Cell Transplant (2010) 19(6):667–79. doi: 10.3727/096368910X508762
184. Pang QM, Chen SY, Fu SP, Zhou H, Zhang Q, Ao J, et al. Regulatory role of mesenchymal stem cells on secondary inflammation in spinal cord injury. J Inflammation Res (2022) 15:573–93. doi: 10.2147/JIR.S349572
185. Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol (2007) 8(4):345–50. doi: 10.1038/ni0407-345
186. Ohkura N, Sakaguchi S. Transcriptional and epigenetic basis of treg cell development and function: its genetic anomalies or variations in autoimmune diseases. Cell Res (2020) 30(6):465–74. doi: 10.1038/s41422-020-0324-7
187. Luz-Crawford P, Kurte M, Bravo-Alegria J, Contreras R, Nova-Lamperti E, Tejedor G, et al. Mesenchymal stem cells generate a CD4+CD25+Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res Ther (2013) 4(3):65. doi: 10.1186/scrt216
188. Li XY, Zheng ZH, Li XY, Guo J, Zhang Y, Li H, et al. Treatment of foot disease in patients with type 2 diabetes mellitus using human umbilical cord blood mesenchymal stem cells: response and correction of immunological anomalies. Curr Pharm Des (2013) 19(27):4893–9. doi: 10.2174/13816128113199990326
189. Droge W. Free radicals in the physiological control of cell function. Physiol Rev (2002) 82(1):47–95. doi: 10.1152/physrev.00018.2001
190. Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev (2014) 94(3):909–50. doi: 10.1152/physrev.00026.2013
191. Liu P, Wang W, Li Z, Li Y, Yu X, Tu J, et al. Ferroptosis: A new regulatory mechanism in osteoporosis. Oxid Med Cell Longev (2022) 2022:2634431. doi: 10.1155/2022/2634431
192. Liu B, Ding FX, Liu Y, Xiong G, Lin T, He DW, et al. Human umbilical cord-derived mesenchymal stem cells conditioned medium attenuate interstitial fibrosis and stimulate the repair of tubular epithelial cells in an irreversible model of unilateral ureteral obstruction. Nephrol (Carlton) (2018) 23(8):728–36. doi: 10.1111/nep.13099
193. Soria B, Martin-Montalvo A, Aguilera Y, Mellado-Damas N, Lopez-Beas J, Herrera-Herrera I, et al. Human mesenchymal stem cells prevent neurological complications of radiotherapy. Front Cell Neurosci (2019) 13:204. doi: 10.3389/fncel.2019.00204
194. Al-Massri KF, Ahmed LA, El-Abhar HS. Mesenchymal stem cells therapy enhances the efficacy of pregabalin and prevents its motor impairment in paclitaxel-induced neuropathy in rats: Role of Notch1 receptor and JAK/STAT signaling pathway. Behav Brain Res (2019) 360:303–11. doi: 10.1016/j.bbr.2018.12.013
195. Feng J, Lu C, Dai Q, Sheng J, Xu M. SIRT3 facilitates amniotic fluid stem cells to repair diabetic nephropathy through protecting mitochondrial homeostasis by modulation of mitophagy. Cell Physiol Biochem (2018) 46(4):1508–24. doi: 10.1159/000489194
196. Guillen MI, Platas J, Perez Del Caz MD, Mirabet V, Alcaraz MJ. Paracrine anti-inflammatory effects of adipose tissue-derived mesenchymal stem cells in human monocytes. Front Physiol (2018) 9:661. doi: 10.3389/fphys.2018.00661
197. Oh JY, Ko JH, Lee HJ, Yu JM, Choi H, Kim MK, et al. Mesenchymal stem/stromal cells inhibit the NLRP3 inflammasome by decreasing mitochondrial reactive oxygen species. Stem Cells (2014) 32(6):1553–63. doi: 10.1002/stem.1608
198. Wang H, Chen L, Liu Y, Luo B, Xie N, Tan T, et al. Implantation of placenta-derived mesenchymal stem cells accelerates murine dermal wound closure through immunomodulation. Am J Transl Res (2016) 8(11):4912–21.
199. Li M, Zhao Y, Hao H, Dai H, Han Q, Tong C, et al. Mesenchymal stem cell-conditioned medium improves the proliferation and migration of keratinocytes in a diabetes-like microenvironment. Int J Low Extrem Wounds (2015) 14(1):73–86. doi: 10.1177/1534734615569053
200. Raffaghello L, Bianchi G, Bertolotto M, Montecucco F, Busca A, Dallegri F, et al. Human mesenchymal stem cells inhibit neutrophil apoptosis: a model for neutrophil preservation in the bone marrow niche. Stem Cells (2008) 26(1):151–62. doi: 10.1634/stemcells.2007-0416
201. Li X, Xie X, Lian W, Shi R, Han S, Zhang H, et al. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp Mol Med (2018) 50(4):1–14. doi: 10.1038/s12276-018-0058-5
202. Fleetwood AJ, Lawrence T, Hamilton JA, Cook AD. Granulocyte-macrophage colony-stimulating factor (CSF) and macrophage CSF-dependent macrophage phenotypes display differences in cytokine profiles and transcription factor activities: implications for CSF blockade in inflammation. J Immunol (2007) 178(8):5245–52. doi: 10.4049/jimmunol.178.8.5245
203. Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol (2013) 229(2):176–85. doi: 10.1002/path.4133
204. Arnold CE, Whyte CS, Gordon P, Barker RN, Rees AJ, Wilson HM. A critical role for suppressor of cytokine signalling 3 in promoting M1 macrophage activation and function. Vitro vivo. Immunol (2014) 141(1):96–110. doi: 10.1111/imm.12173
205. Abdelaziz MH, Abdelwahab SF, Wan J, Cai W, Huixuan W, Jianjun C, et al. Alternatively activated macrophages; a double-edged sword in allergic asthma. J Transl Med (2020) 18(1):58. doi: 10.1186/s12967-020-02251-w
206. Al Sadoun H. Macrophage phenotypes in normal and diabetic wound healing and therapeutic interventions. Cells (2022) 11(15):2430. doi: 10.3390/cells11152430
207. Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature (2013) 496(7446):445–55. doi: 10.1038/nature12034
208. Ogle ME, Segar CE, Sridhar S, Botchwey EA. Monocytes and macrophages in tissue repair: Implications for immunoregenerative biomaterial design. Exp Biol Med (Maywood) (2016) 241(10):1084–97. doi: 10.1177/1535370216650293
209. Kimball A, Schaller M, Joshi A, Davis FM, denDekker A, Boniakowski A, et al. Ly6C(Hi) blood Monocyte/Macrophage drive chronic inflammation and impair wound healing in diabetes mellitus. Arterioscler Thromb Vasc Biol (2018) 38(5):1102–14. doi: 10.1161/ATVBAHA.118.310703
210. Pang J, Maienschein-Cline M, Koh TJ. Enhanced proliferation of Ly6C(+) Monocytes/Macrophages contributes to chronic inflammation in skin wounds of diabetic mice. J Immunol (2021) 206(3):621–30. doi: 10.4049/jimmunol.2000935
211. Huang SM, Wu CS, Chiu MH, Wu CH, Chang YT, Chen GS, et al. High glucose environment induces M1 macrophage polarization that impairs keratinocyte migration via TNF-alpha: An important mechanism to delay the diabetic wound healing. J Dermatol Sci (2019) 96(3):159–67. doi: 10.1016/j.jdermsci.2019.11.004
212. Dayan V, Yannarelli G, Billia F, Filomeno P, Wang XH, Davies JE, et al. Mesenchymal stromal cells mediate a switch to alternatively activated monocytes/macrophages after acute myocardial infarction. Basic Res Cardiol (2011) 106(6):1299–310. doi: 10.1007/s00395-011-0221-9
213. Zhang QZ, Su WR, Shi SH, Wilder-Smith P, Xiang AP, Wong A, et al. Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells (2010) 28(10):1856–68. doi: 10.1002/stem.503
214. Yu S, Cheng Y, Zhang L, Yin Y, Xue J, Li B, et al. Treatment with adipose tissue-derived mesenchymal stem cells exerts anti-diabetic effects, improves long-term complications, and attenuates inflammation in type 2 diabetic rats. Stem Cell Res Ther (2019) 10(1):333. doi: 10.1186/s13287-019-1474-8
215. Chen S, Wang H, Su Y, John JV, McCarthy A, Wong SL, et al. Mesenchymal stem cell-laden, personalized 3D scaffolds with controlled structure and fiber alignment promote diabetic wound healing. Acta Biomater (2020) 108:153–67. doi: 10.1016/j.actbio.2020.03.035
216. Zhang S, Chen L, Zhang G, Zhang B. Umbilical cord-matrix stem cells induce the functional restoration of vascular endothelial cells and enhance skin wound healing in diabetic mice via the polarized macrophages. Stem Cell Res Ther (2020) 11(1):39. doi: 10.1186/s13287-020-1561-x
217. Li FX, Lin X, Xu F, Shan SK, Guo B, Lei LM, et al. The role of mesenchymal stromal cells-derived small extracellular vesicles in diabetes and its chronic complications. Front Endocrinol (Lausanne) (2021) 12:780974. doi: 10.3389/fendo.2021.780974
218. Huang C, Luo W, Wang Q, Ye Y, Fan J, Lin L, et al. Human mesenchymal stem cells promote ischemic repairment and angiogenesis of diabetic foot through exosome miRNA-21-5p. Stem Cell Res (2021) 52:102235. doi: 10.1016/j.scr.2021.102235
219. Huang C, Luo WF, Ye YF, Lin L, Wang Z, Luo MH, et al. Characterization of inflammatory factor-induced changes in mesenchymal stem cell exosomes and sequencing analysis of exosomal microRNAs. World J Stem Cells (2019) 11(10):859–90. doi: 10.4252/wjsc.v11.i10.859
220. Gangadaran P, Rajendran RL, Lee HW, Kalimuthu S, Hong CM, Jeong SY, et al. Extracellular vesicles from mesenchymal stem cells activates VEGF receptors and accelerates recovery of hindlimb ischemia. J Control Release (2017) 264:112–26. doi: 10.1016/j.jconrel.2017.08.022
221. Xu J, Wu W, Zhang L, Dorset-Martin W, Morris MW, Mitchell ME, et al. The role of microRNA-146a in the pathogenesis of the diabetic wound-healing impairment: correction with mesenchymal stem cell treatment. Diabetes (2012) 61(11):2906–12. doi: 10.2337/db12-0145
222. Yu M, Liu W, Li J, Lu J, Lu H, Jia W, et al. Exosomes derived from atorvastatin-pretreated MSC accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway. Stem Cell Res Ther (2020) 11(1):350. doi: 10.1186/s13287-020-01824-2
223. Marrotte EJ, Chen DD, Hakim JS, Chen AF. Manganese superoxide dismutase expression in endothelial progenitor cells accelerates wound healing in diabetic mice. J Clin Invest (2010) 120(12):4207–19. doi: 10.1172/JCI36858
224. Chen S, Shi J, Zhang M, Chen Y, Wang X, Zhang L, et al. Mesenchymal stem cell-laden anti-inflammatory hydrogel enhances diabetic wound healing. Sci Rep (2015) 5:18104. doi: 10.1038/srep18104
225. Schiavetta A, Maione C, Botti C, Marino G, Lillo S, Garrone A, et al. A phase II trial of autologous transplantation of bone marrow stem cells for critical limb ischemia: results of the Naples and pietra ligure evaluation of stem cells study. Stem Cells Transl Med (2012) 1(7):572–8. doi: 10.5966/sctm.2012-0021
226. Yang J, Chen Z, Pan D, Li H, Shen J. Umbilical cord-derived mesenchymal stem cell-derived exosomes combined pluronic F127 hydrogel promote chronic diabetic wound healing and complete skin regeneration. Int J Nanomed (2020) 15:5911–26. doi: 10.2147/IJN.S249129
227. Wang Z, Li H, Zhang D, Liu X, Zhao F, Pang X, et al. Effect of advanced glycosylation end products on apoptosis in human adipose tissue-derived stem cells in vitro. Cell Biosci (2015) 5:3. doi: 10.1186/2045-3701-5-3
228. Dash NR, Dash SN, Routray P, Mohapatra S, Mohapatra PC. Targeting nonhealing ulcers of lower extremity in human through autologous bone marrow-derived mesenchymal stem cells. Rejuvenation Res (2009) 12(5):359–66. doi: 10.1089/rej.2009.0872
229. Linger RJ, Belikoff EJ, Yan Y, Li F, Wantuch HA, Fitzsimons HL, et al. Towards next generation maggot debridement therapy: transgenic lucilia sericata larvae that produce and secrete a human growth factor. BMC Biotechnol (2016) 16:30. doi: 10.1186/s12896-016-0263-z
230. Mahmoudian-Sani MR, Rafeei F, Amini R, Saidijam M. The effect of mesenchymal stem cells combined with platelet-rich plasma on skin wound healing. J Cosmet Dermatol (2018) 17(5):650–9. doi: 10.1111/jocd.12512
231. Ahmed M, Reffat SA, Hassan A, Eskander F. Platelet-rich plasma for the treatment of clean diabetic foot ulcers. Ann Vasc Surg (2017) 38:206–11. doi: 10.1016/j.avsg.2016.04.023
232. Del Pino-Sedeno T, Trujillo-Martin MM, Andia I, Aragon-Sanchez J, Herrera-Ramos E, Iruzubieta Barragan FJ, et al. Platelet-rich plasma for the treatment of diabetic foot ulcers: A meta-analysis. Wound Repair Regener (2019) 27(2):170–82. doi: 10.1111/wrr.12690
233. Chiang KJ, Chiu LC, Kang YN, Chen C. Autologous stem cell therapy for chronic lower extremity wounds: A meta-analysis of randomized controlled trials. Cells (2021) 10(12):3307. doi: 10.3390/cells10123307
234. Yu Q, Qiao GH, Wang M, Yu L, Sun Y, Shi H, et al. Stem cell-based therapy for diabetic foot ulcers. Front Cell Dev Biol (2022) 10:812262. doi: 10.3389/fcell.2022.812262
235. Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet (2002) 360(9331):427–35. doi: 10.1016/S0140-6736(02)09670-8
236. Claeys LG, Horsch S. Transcutaneous oxygen pressure as predictive parameter for ulcer healing in endstage vascular patients treated with spinal cord stimulation. Int Angiol (1996) 15(4):344–9.
237. Kirana S, Stratmann B, Prante C, Prohaska W, Koerperich H, Lammers D, et al. Autologous stem cell therapy in the treatment of limb ischaemia induced chronic tissue ulcers of diabetic foot patients. Int J Clin Pract (2012) 66(4):384–93. doi: 10.1111/j.1742-1241.2011.02886.x
238. Xu SM, Liang T. Clinical observation of the application of autologous peripheral blood stem cell transplantation for the treatment of diabetic foot gangrene. Exp Ther Med (2016) 11(1):283–8. doi: 10.3892/etm.2015.2888
239. Huang P, Li S, Han M, Xiao Z, Yang R, Han ZC. Autologous transplantation of granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cells improves critical limb ischemia in diabetes. Diabetes Care (2005) 28(9):2155–60. doi: 10.2337/diacare.28.9.2155
240. Cruciani M, Lipsky BA, Mengoli C, de Lalla F. Granulocyte-colony stimulating factors as adjunctive therapy for diabetic foot infections. Cochrane Database Syst Rev (2013) 8):CD006810. doi: 10.1002/14651858.CD006810.pub3
241. Lu D, Chen B, Liang Z, Deng W, Jiang Y, Li S, et al. Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: a double-blind, randomized, controlled trial. Diabetes Res Clin Pract (2011) 92(1):26–36. doi: 10.1016/j.diabres.2010.12.010
242. Prochazka V, Gumulec J, Jaluvka F, Salounova D, Jonszta T, Czerny D, et al. Cell therapy, a new standard in management of chronic critical limb ischemia and foot ulcer. Cell Transplant (2010) 19(11):1413–24. doi: 10.3727/096368910X514170
243. Lipsky BA, Aragon-Sanchez J, Diggle M, Embil J, Kono S, Lavery L, et al. IWGDF guidance on the diagnosis and management of foot infections in persons with diabetes. Diabetes Metab Res Rev (2016) 32 Suppl 1:45–74. doi: 10.1002/dmrr.2699
244. Scatena A, Petruzzi P, Maioli F, Lucaroni F, Ambrosone C, Ventoruzzo G, et al. Autologous peripheral blood mononuclear cells for limb salvage in diabetic foot patients with no-option critical limb ischemia. J Clin Med (2021) 10(10):2213. doi: 10.3390/jcm10102213
245. Dai J, Jiang C, Chen H, Chai Y. Treatment of diabetic foot with autologous stem cells: A meta-analysis of randomized studies. Stem Cells Int (2020) 2020:6748530. doi: 10.1155/2020/6748530
246. Dubsky M, Jirkovska A, Bem R, Fejfarova V, Pagacova L, Nemcova A, et al. Comparison of the effect of stem cell therapy and percutaneous transluminal angioplasty on diabetic foot disease in patients with critical limb ischemia. Cytotherapy (2014) 16(12):1733–8. doi: 10.1016/j.jcyt.2014.08.010
247. Han S, Sun HM, Hwang KC, Kim SW. Adipose-derived stromal vascular fraction cells: Update on clinical utility and efficacy. Crit Rev Eukaryot Gene Expr (2015) 25(2):145–52. doi: 10.1615/critreveukaryotgeneexpr.2015013057
248. Han SK, Kim HR, Kim WK. The treatment of diabetic foot ulcers with uncultured, processed lipoaspirate cells: a pilot study. Wound Repair Regener (2010) 18(4):342–8. doi: 10.1111/j.1524-475X.2010.00593.x
249. Carstens MH, Gomez A, Cortes R, Turner E, Perez C, Ocon M, et al. Non-reconstructable peripheral vascular disease of the lower extremity in ten patients treated with adipose-derived stromal vascular fraction cells. Stem Cell Res (2017) 18:14–21. doi: 10.1016/j.scr.2016.12.001
250. Carstens MH, Zelaya M, Calero D, Rivera C, Correa D. Adipose-derived stromal vascular fraction (SVF) cells for the treatment of non-reconstructable peripheral vascular disease in patients with critical limb ischemia: A 6-year follow-up showing durable effects. Stem Cell Res (2020) 49:102071. doi: 10.1016/j.scr.2020.102071
251. Moon KC, Chung HY, Han SK, Jeong SH, Dhong ES. Possibility of injecting adipose-derived stromal vascular fraction cells to accelerate microcirculation in ischemic diabetic feet: A pilot study. Int J Stem Cells (2019) 12(1):107–13. doi: 10.15283/ijsc18101
252. Carstens MH, Quintana FJ, Calderwood ST, Sevilla JP, Rios AB, Rivera CM, et al. Treatment of chronic diabetic foot ulcers with adipose-derived stromal vascular fraction cell injections: Safety and evidence of efficacy at 1 year. Stem Cells Transl Med (2021) 10(8):1138–47. doi: 10.1002/sctm.20-0497
253. Qin HL, Zhu XH, Zhang B, Zhou L, Wang WY. Clinical evaluation of human umbilical cord mesenchymal stem cell transplantation after angioplasty for diabetic foot. Exp Clin Endocrinol Diabetes (2016) 124(8):497–503. doi: 10.1055/s-0042-103684
254. Moon KC, Suh HS, Kim KB, Han SK, Young KW, Lee JW, et al. Potential of allogeneic adipose-derived stem cell-hydrogel complex for treating diabetic foot ulcers. Diabetes (2019) 68(4):837–46. doi: 10.2337/db18-0699
255. Arango-Rodriguez ML, Solarte-David VA, Becerra-Bayona SM, Callegari E, Paez MD, Sossa CL, et al. Role of mesenchymal stromal cells derivatives in diabetic foot ulcers: a controlled randomized phase 1/2 clinical trial. Cytotherapy (2022) 24(10):1035–48. doi: 10.1016/j.jcyt.2022.04.002
256. Uzun E, Guney A, Gonen ZB, Ozkul Y, Kafadar IH, Gunay M, et al. Intralesional allogeneic adipose-derived stem cells application in chronic diabetic foot ulcer: Phase I/2 safety study. Foot Ankle Surg (2021) 27(6):636–42. doi: 10.1016/j.fas.2020.08.002
257. Ouyang L, Qiu D, Fu X, Wu A, Yang P, Yang Z, et al. Overexpressing HPGDS in adipose-derived mesenchymal stem cells reduces inflammatory state and improves wound healing in type 2 diabetic mice. Stem Cell Res Ther (2022) 13(1):395. doi: 10.1186/s13287-022-03082-w
258. Rai V, Moellmer R, Agrawal DK. Stem cells and angiogenesis: Implications and limitations in enhancing chronic diabetic foot ulcer healing. Cells (2022) 11(15):2287. doi: 10.3390/cells11152287
259. Wang Y, Yi H, Song Y. The safety of MSC therapy over the past 15 years: a meta-analysis. Stem Cell Res Ther (2021) 12(1):545. doi: 10.1186/s13287-021-02609-x
260. Nishikawa G, Kawada K, Nakagawa J, Toda K, Ogawa R, Inamoto S, et al. Bone marrow-derived mesenchymal stem cells promote colorectal cancer progression via CCR5. Cell Death Dis (2019) 10(4):264. doi: 10.1038/s41419-019-1508-2
261. Han L, He H, Yang Y, Meng Q, Ye F, Chen G, et al. Distinctive clinical and pathologic features of immature teratomas arising from induced pluripotent stem cell-derived beta cell injection in a diabetes patient. Stem Cells Dev (2022) 31(5-6):97–101. doi: 10.1089/scd.2021.0255
262. Jiang XY, Lu DB, Chen B. Progress in stem cell therapy for the diabetic foot. Diabetes Res Clin Pract (2012) 97(1):43–50. doi: 10.1016/j.diabres.2011.12.011
263. Mathew E, Brannon AL, Del Vecchio A, Garcia PE, Penny MK, Kane KT, et al. Mesenchymal stem cells promote pancreatic tumor growth by inducing alternative polarization of macrophages. Neoplasia (2016) 18(3):142–51. doi: 10.1016/j.neo.2016.01.005
264. Riedl J, Popp C, Eide C, Ebens C, Tolar J. Mesenchymal stromal cells in wound healing applications: role of the secretome, targeted delivery and impact on recessive dystrophic epidermolysis bullosa treatment. Cytotherapy (2021) 23(11):961–73. doi: 10.1016/j.jcyt.2021.06.004
265. Cao Y, Gang X, Sun C, Wang G. Mesenchymal stem cells improve healing of diabetic foot ulcer. J Diabetes Res (2017) 2017:9328347. doi: 10.1155/2017/9328347
266. Hua J, Gong J, Meng H, Xu B, Yao L, Qian M, et al. Comparison of different methods for the isolation of mesenchymal stem cells from umbilical cord matrix: proliferation and multilineage differentiation as compared to mesenchymal stem cells from umbilical cord blood and bone marrow. Cell Biol Int (2013). published online ahead of print. doi: 10.1002/cbin.10188
267. Hoang DH, Nguyen TD, Nguyen HP, Nguyen XH, Do PTX, Dang VD, et al. Differential wound healing capacity of mesenchymal stem cell-derived exosomes originated from bone marrow, adipose tissue and umbilical cord under serum- and xeno-free condition. Front Mol Biosci (2020) 7:119. doi: 10.3389/fmolb.2020.00119
Keywords: Diabetic foot, Wound healing, Mesenchymal stem cells, Angiogenesis, mechanism
Citation: Yu X, Liu P, Li Z and Zhang Z (2023) Function and mechanism of mesenchymal stem cells in the healing of diabetic foot wounds. Front. Endocrinol. 14:1099310. doi: 10.3389/fendo.2023.1099310
Received: 15 November 2022; Accepted: 06 March 2023;
Published: 16 March 2023.
Edited by:
Fang Liu, Shanghai General Hospital, ChinaReviewed by:
Yu Kuan Tang, Guangzhou Panyu Central Hospital, ChinaCopyright © 2023 Yu, Liu, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhengdong Zhang, ZG9jdG9yenpkQHZpcC5xcS5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.