- 1Center for Reproductive Medicine, Department of Obstetrics and Gynecology, Peking University Third Hospital, Beijing, China
- 2National Clinical Research Center for Obstetrics and Gynecology (Peking University Third Hospital), Beijing, China
- 3Key Laboratory of Assisted Reproduction (Peking University), Ministry of Education, Beijing, China
- 4Beijing Key Laboratory of Reproductive Endocrinology and Assisted Reproductive Technology, Peking University Third Hospital, Beijing, China
This prospective cohort study aimed to determine the impact of thyroid autoimmunity and total 25-hydroxyvitamin D concentration on early pregnancy outcomes in women undergoing in vitro fertilization/intracytoplasmic sperm injection who had intact thyroid function. The study included 1,297 women who underwent in vitro fertilization/intracytoplasmic sperm injection cycles, although only 588 patients received fresh embryo transfer. The study endpoints were clinical pregnancy, ongoing pregnancy, ectopic pregnancy, and early miscarriage rates. Our study found that the total 25-hydroxyvitamin D serum concentrations (P<0.001) and anti-Mullerian hormone levels (P=0.019) were lower among patients in the TAI group (n=518) than among those in the non-TAI group (n=779). Additionally, the study population in each group was divided into three subgroups according to the total vitamin D status based on clinical practice guidelines (deficient, <20 ng/mL; insufficient, 21–29 ng/mL; and sufficient, ≥30 ng/mL), TAI group: sufficient, n=144; insufficient, n=187; and deficient, n=187; non-TAI group: sufficient, n=329; insufficient, n=318, and deficient, n=133. In the TAI group, the number of good-quality embryos decreased in patients with vitamin D deficiency (P=0.007). Logistic regression analysis indicated that aging prevented women from achieving clinical (P=0.024) and ongoing pregnancy (P=0.026). The current findings suggest that patients with TAI had reduced serum vitamin D concentration. Furthermore, in the TAI group, the number of good-quality embryos decreased in patients with vitamin D deficiency. Finally, aging adversely impacted achieving clinical and ongoing pregnancy.
1 Introduction
In vitro fertilization (IVF) treatment of infertile couples has helped birth more than 8 million newborns worldwide. Although stimulation protocols and laboratory techniques have improved, the live birth rate per cycle remains between 19% and 22%. After years of practice, clinicians have achieved high treatment success rates (1).
Almost all aspects of biological activity can be correlated with vitamin D, a prohormone first characterized as a vitamin in the 20th century (2). The active metabolite of vitamin D, 1,25(OH)2 vitamin D, is crucial for regulating calcium and phosphate homeostasis and cell proliferation and differentiation. Moreover, the immune and nervous systems and cardiovascular and neurodegenerative diseases can be influenced in delicate ways by vitamin D. The fact that the vitamin D receptor could be detected in several tissues, including the ovary, endometrium, decidua, and placenta, as well as in fallopian epithelial cells (3), indicates that vitamin D may also be vital for the female reproductive system (4).
Thyroid autoimmunity (TAI) is the leading cause of primary hypothyroidism. When damaged by thyroid autoantibodies, thyroid tissue may ultimately be destroyed and lose the ability to maintain normal function (5). Studies have reported that patients with TAI present with decreased 25-hydroxyvitamin D [25(OH)D] levels (6, 7); after vitamin D supplementation, the titer of thyroid autoantibodies in patients with TAI and poor vitamin status decreased significantly (8). The relationship between TAI and the female reproductive system is also noteworthy. Reportedly, the miscarriage rates were higher in euthyroid women who tested positive for thyroid (thyroid peroxidase and thyroglobulin) antibodies in the first trimester of pregnancy (9). Additionally, the number of oocytes retrieved and birth weight in twin pregnancies decreased in patients with TAI (10). Moreover, TAI tends to be associated with IVF failure (11, 12) and various gynecological issues, including unexplained infertility (12, 13).
This study aimed to provide clear clues about the effects of TAI and vitamin D in patients with infertility undergoing IVF who had normal thyroid function.
2 Materials and methods
2.1 Study design and setting
This was a prospective, single-center cohort study. Infertile women undergoing IVF/intracytoplasmic sperm injection (ICSI) cycles between April 2021 and December 2021 were referred to the Reproductive Center of Peking University Third Hospital for IVF/ICSI treatment. The local ethics committee approved the study protocol (registration no. M2021189).
2.2 Participants
A total of 1,297 female patients who underwent IVF/ICSI cycles were enrolled in this study. The inclusion criteria were as follows: 1) age 20–40 years, 2) fresh embryo transfer (ET) recipients, 3) male or tubal factor infertility, and 4) normal thyroid function (defined as a TSH level within the reference range of 0.50–4.78 mIU/L). Patients with any of the following issues were excluded: 1) reproductive endocrinology disorders leading to infertility, including polycystic ovary syndrome, endometriosis, premature ovarian failure, hyperprolactinemia, and diminished ovarian reserve; 2) previous thyroidectomy; 3) autoimmune disorder complications; 4) cardiopulmonary, liver, or kidney diseases; 5) vitamin D supplementation; 6) IVF failure over three times; 7) pelvic/intrauterine adhesion or untreated hydrosalpinx; 8) recurrent abortion; 9) uterine malformation; or 10) uterine myoma (multiple, submucous, or intramural myoma >4 cm).
All the patients underwent IVF/ICSI cycles. The treatment protocols are described in the Supplementary Materials and Methods.
2.3 Study endpoints
The primary outcome was clinical pregnancy, defined as a gestational sac observed on ultrasonography 4 weeks after ET.
Secondary outcomes were as follows: 1) ongoing pregnancy, 2) ectopic pregnancy, and 3) early pregnancy loss. Ongoing pregnancy was defined as a gestational sac observed on ultrasonography 10 weeks after ET. Ectopic pregnancy was diagnosed using ultrasonography, intraoperatively, or histopathologically. Early pregnancy loss was defined as spontaneous abortion of intrauterine pregnancy within 12 weeks of gestation.
2.4 Laboratory testing
Blood samples for thyroid hormone testing were collected within 6 months before the initiation of controlled ovarian hyperstimulation (COS). The total serum 25(OH)D concentration was measured on the second day of the menstrual cycle. A fully automatic chemiluminescence immunoassay analyzer (ADVIA Centaur XP, Siemens Healthcare Diagnostics) was used to measure the serum free thyroxine (FT4), total thyroxin (TT4), thyroid-stimulating hormone (TSH), thyroid peroxidase antibody (TPOAb), and thyroglobulin antibody (TgAb) levels. Serum vitamin D levels were measured using a fully automatic chemiluminescence immunoassay analyzer (Elecsys Vitamin D total, Roche Diagnostics GmbH, Pleasanton, CA, United States of America). The reference values for TSH and FT4 were 0.55–4.78 µIU/mL and 0.89–1.80 ng/dL, respectively. TPOAb or TGAb levels <60 IU/mL were defined as negative.
2.5 Categorization
After being divided into two groups according to their thyroid antibody positivity, patients in both groups were also divided into three subgroups according to their vitamin D status, defined in the American Endocrine Society’s clinical practice guidelines as follows (14): deficient, <20 ng/mL (50 nmol/L); insufficient, 21–29 ng/mL (52.5–72.5 nmol/L); and sufficient, ≥30 ng/mL (75 nmol/L).
2.6 Statistical analyses
Categorical data are presented as the number of cases (percentage). Continuous data are presented as the mean (standard deviation) for normally distributed data and as the median (interquartile range) for non-normally distributed data. Continuous variables without normal distributions were compared using the Mann–Whitney U test. Student’s t-test/one-way analysis of variance and the chi-square test were used to compare continuous and categorical variables, respectively. A logistic regression model was used to analyze the association between early pregnancy outcomes and relevant factors. Statistical significance was defined as a two-sided P value <0.05. Statistical analyses were performed using SPSS version 24.0.
3 Results
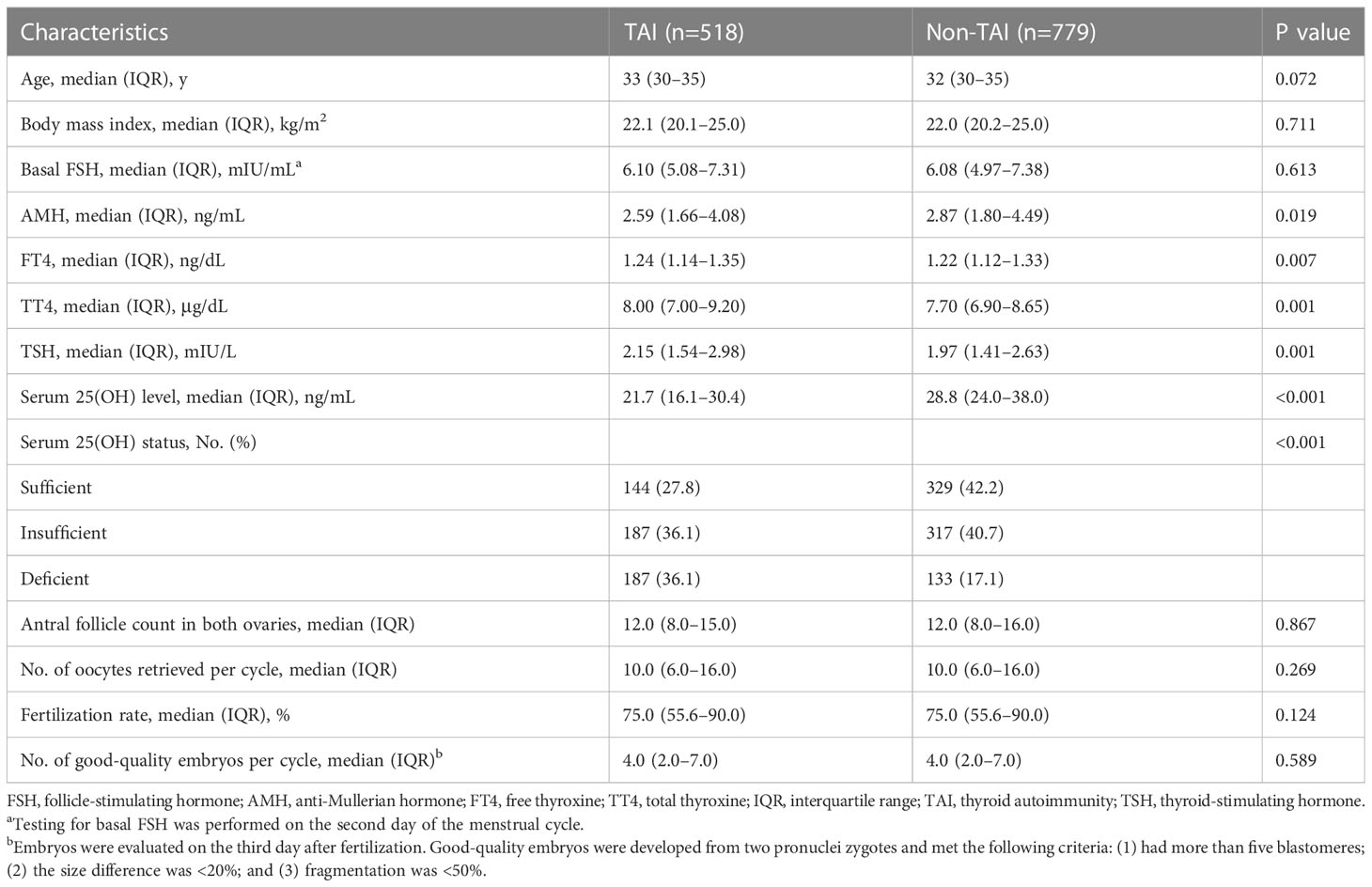
A total of 1,297 participants who met the selection criteria were recruited to initiate the IVF/ICSI cycle. After removing 709 cycles cancelled before ET, 588 patients received fresh ET and continued to be observed for early pregnancy outcomes (clinical pregnancy, ongoing pregnancy, ectopic pregnancy, and early miscarriages). The characteristics of the study population are summarized in Table 1. The total 25(OH)D serum concentration (P<0.001) and anti-Mullerian hormone (AMH) (P=0.019) levels were higher in patients in the non-TAI group than in those in the TAI group. Furthermore, the FT4 (P=0.007), TT4 (P=0.001), and TSH (P=0.001) levels were slightly elevated in the TAI group. Vitamin D status was significantly different between the two groups (P<0.001); vitamin D deficiency was prevalent in 36.1% of the TAI group and 17.1% of the non-TAI group.
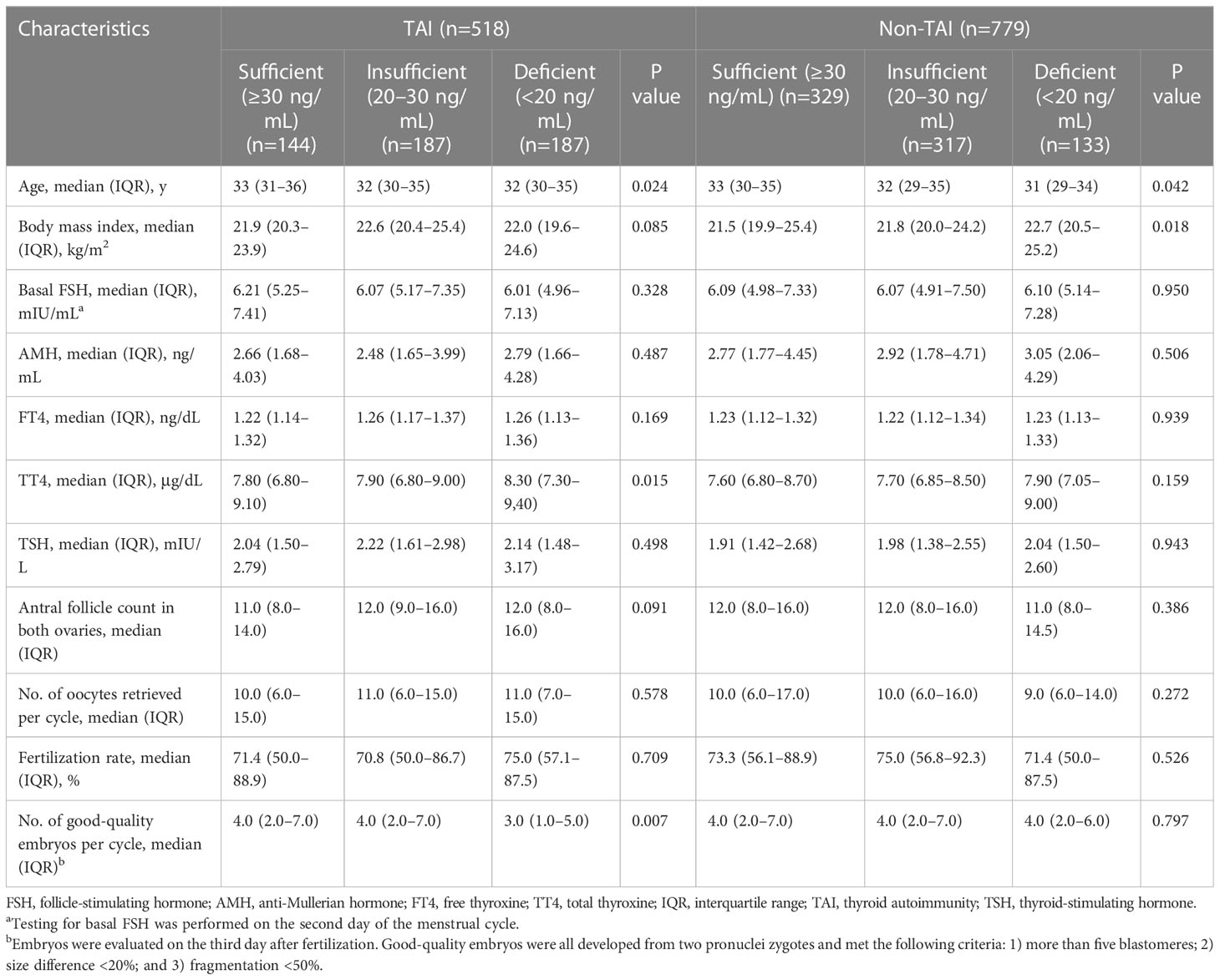
Women in each group were divided into three subgroups based on their 25(OH)D status. The analyses of baseline parameters according to 25(OH)D status are presented in Table 2. All participants with better vitamin D status tended to be older (TAI, P=0.024; non-TAI, 0.042) and had lower TT4 levels (P<0.001). In the TAI group, the TT4 level (P=0.015) and number of good-quality embryos (P=0.007) remained high among patients with better vitamin D status. In the non-TAI group, body mass index (BMI) was inversely correlated with vitamin D status (P=0.018).
Furthermore, the chi-square test showed that patients in the TAI group presented with increased early miscarriage rate (P=0.034). When participants in each group were divided into three subgroups according to their vitamin D status, no significant differences were observed when comparing all the TAI and non-TAI study primary and secondary outcomes (Table 3).
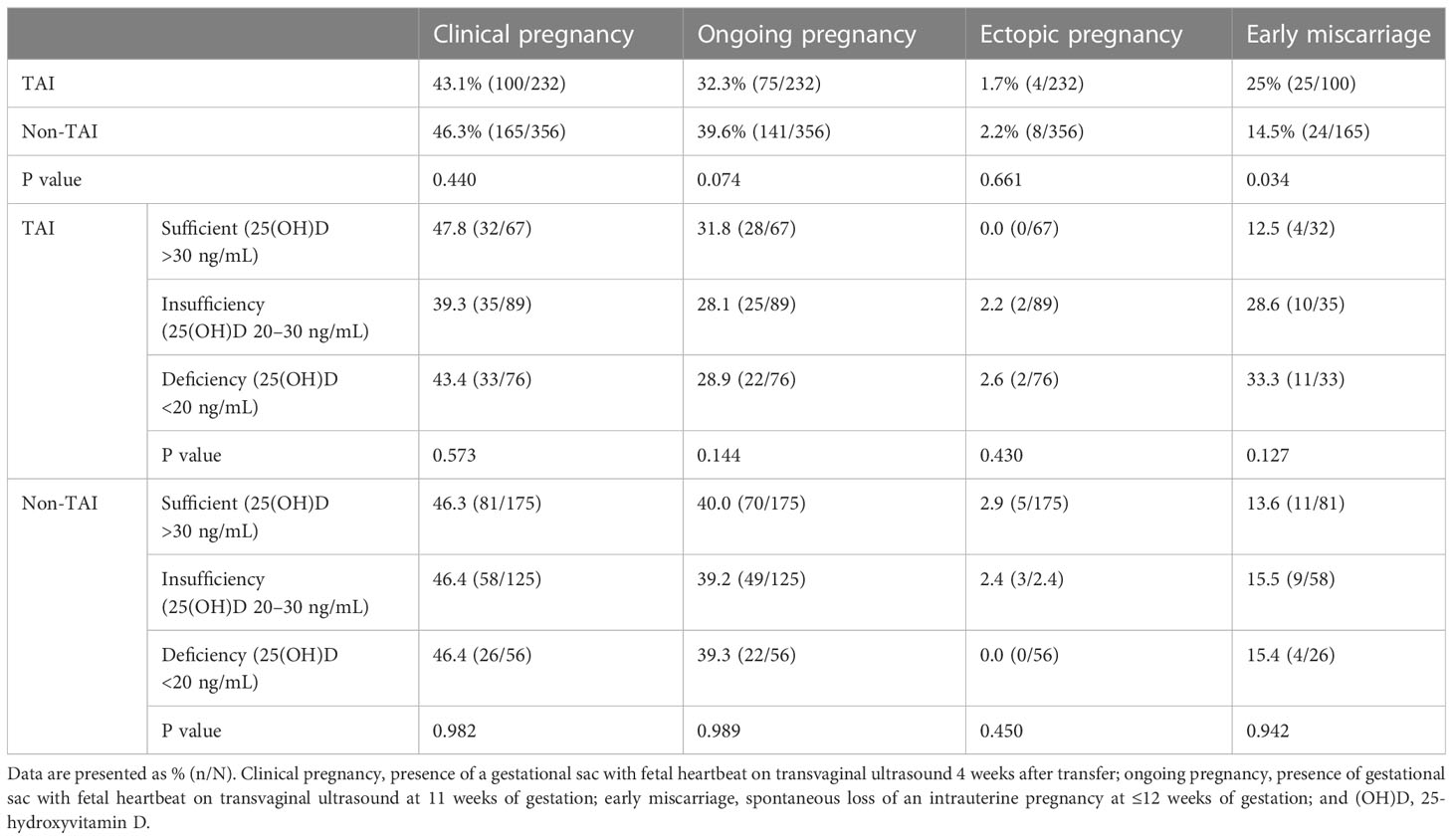
Table 3 Comparison of early pregnancy outcomes according to TAI/non-TAI and total 25(OH)D status in 588 patients who underwent fresh embryo transfer.
Logistic regression analysis for clinical pregnancy, ongoing pregnancy, and early miscarriage considering age, BMI, AMH, serum vitamin D status, infertility type, FT4, TT4, TSH, TAI/non-TAI, protocol of COS, antral follicle count, number of retrieved oocytes and good-quality embryos, and embryo culture duration (day 3 or 5) only indicated that aging prevented women from achieving clinical pregnancy (0.948; 95% confidence interval [CI], 0.905–0.993; P=0.024) and ongoing pregnancy (0.946; 95% CI, 0.901–0.993; P=0.026) (Table 4). TAI, vitamin D status and other relevant factors stated above didn’t influence all early pregnancy outcomes. After considering the interactions between total serum 25(OH)D status and TAI/non-TAI as confounding factors, no significant difference was observed between the interactions and any of the study endpoints (P >0.05) (Table 4).
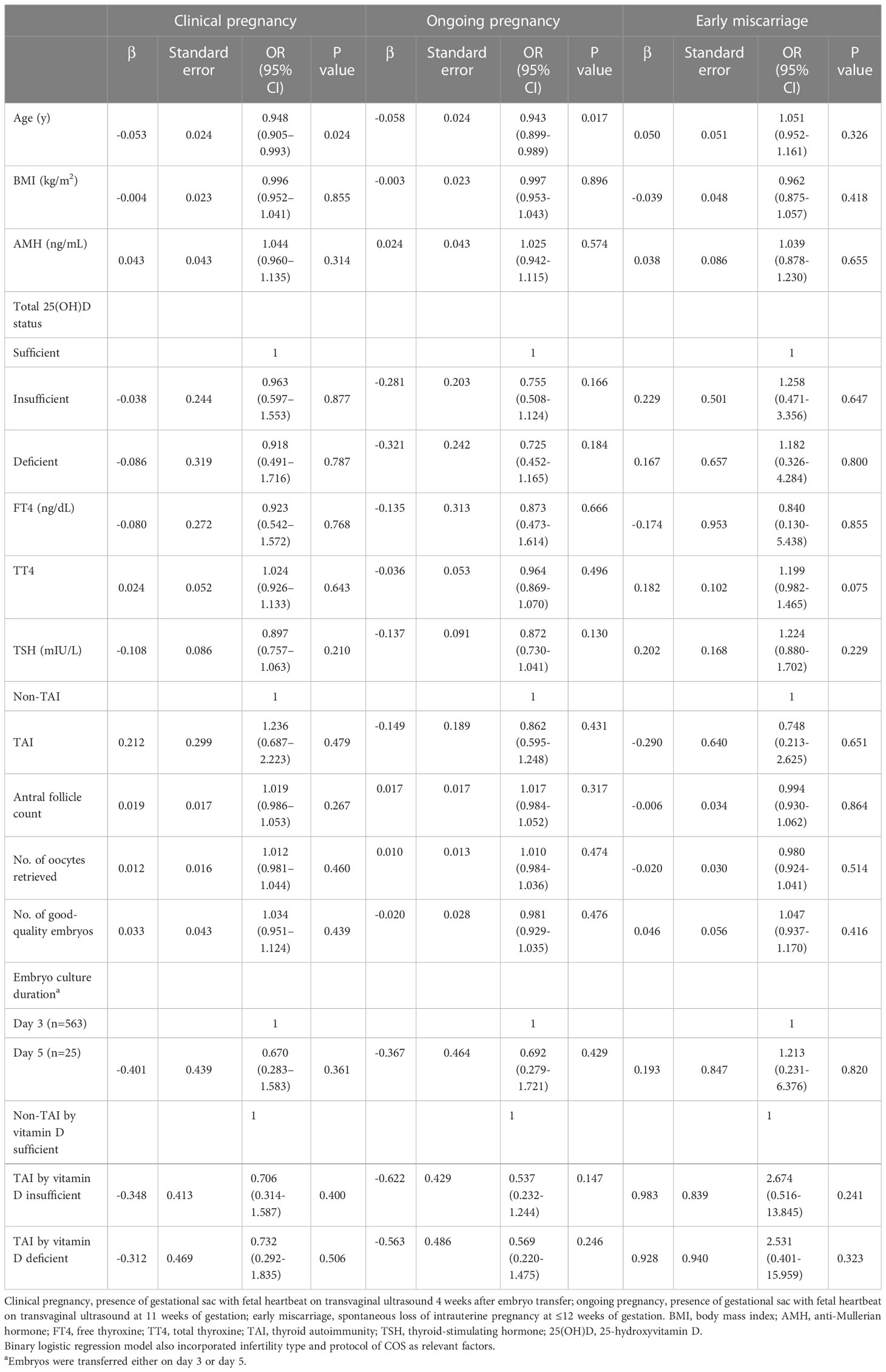
Table 4 Logistic regression analysis of clinical pregnancy, ongoing pregnancy, and early miscarriage according to demographic and metabolic factors.
4 Discussion
Our study’s strengths include being the first prospective cohort study to explore the association among TAI, serum vitamin D level, and early pregnancy outcomes in patients undergoing IVF treatment. Additionally, the sample size was relatively large leading to better-quality results. We found that TAI patients tended to have reduced serum 25(OH)D concentrations. In the TAI group, the number of good-quality embryos decreased in patients with vitamin D deficiency. Furthermore, aging adversely impacted achieving clinical and ongoing pregnancy. Considering the prevalence and potential fertility-damaging impact of TAI, testing for thyroid function and thyroid antibody is recommended for infertile patients in China. A study conducted by Chao et al. (6), involving 5,230 Chinese participants, showed that vitamin D deficiency is prevalent in over 70% in both the Hashimoto’s thyroiditis (HT) and non-HT groups within the Chinese population. These data combined with the findings of our study indicate that treatment with vitamin D may help to improve IVF/ICSI outcomes; large, prospective, randomized controlled studies are warranted to confirm these findings.
Furthermore, there is ambiguous predictability between vitamin D and assisted reproductive technology (ART) outcomes. Pregnancy loss is linked to vitamin D deficiency in women undergoing ART (15). According to a recent Cochrane analysis (16) and another review (17), vitamin D supplementation in pregnant women may reduce the risk of preeclampsia, low birth weight, preterm birth, and wheezing in offspring at 3 years of age. An association between vitamin D deficiency and BMI has also been reported (18). This meta-analysis showed that each 10% increase in BMI leads to a 4% decrease in the 25(OH)D concentration; among obese populations receiving ART, vitamin D deficiency could contribute to a higher miscarriage rate. Similarly, our study showed that the vitamin D concentration was inversely correlated with BMI in the non-TAI group. Several studies investigating the relationship between female vitamin D status and IVF outcomes concluded that higher vitamin D levels lead to a higher clinical pregnancy rate (CPR) (19–22). However, negative results in several studies suggest no link between vitamin D levels and IVF outcomes (23–25). Anifandis et al. (26) found that higher values of vitamin D were associated with a lower possibility of achieving clinical pregnancy. Recent research (27) has shown that women with a sufficient level of vitamin D had higher ongoing pregnancy rate than those with a deficiency or insufficient level of vitamin D. Our study only found that the number of good-quality embryos decreased in patients with vitamin D deficiency in the TAI group, following the negative findings considering early pregnancy outcomes presented above in both TAI and non-TAI groups with different serum vitamin D status. However, we have ruled out potentially vitamin D-relevant diseases leading to infertility, such as polycystic ovary syndrome, endometriosis, premature ovarian failure, and diminished ovarian reserve. The difference in the study participants may have accounted for the discrepancy in the results, noting that participants with other causes of infertility need to be further studied and explained.
TAI is a chronic autoimmune phenomenon. In COS and early pregnancy, the thyroid gland may fail to respond to aggregated demand after experiencing a subtle deficiency caused by thyroid autoantibodies that may compromise the establishment of immune tolerance during pregnancy (9). However, the association between TAI and IVF outcomes remains controversial. A recent study that aimed to further clarify the association between vitamin D deficiency and HT featuring TAI recruited 5,230 healthy subjects who underwent health examinations. The mean age was 48.95 ± 9.06 years, and 60.1% were male individuals. The researchers discovered that HT patients present with reduced 25(OH)D levels (6), which is consistent with our findings that the total 25(OH)D serum concentration was lower in patients in the TAI group than in those in the TAI group (21.7 [16.1–30.4] ng/mL and 28.8 [24.0–38.0] ng/mL in the TAI and non-TAI groups, respectively), with vitamin D deficiency prevalent in 36.1% of the TAI group and 17.1% of the non-TAI group. Several studies have found that women undergoing TAI may have adverse outcomes. After enrolling 1,556 infertile patients who received their first IVF/ICSI treatment and achieved fresh ET at the Reproductive Center of Peking University Third Hospital, a large-scale retrospective cohort study revealed that the presence of TAI could decrease the number of oocytes retrieved (10). After enrolling 122 patients aged 20–40 years who received IVF/ICSI treatment, a cross-sectional study focusing on the follicular microenvironment of patients with TAI showed that levels of three chemokines (CXCL9/10/11), one cytokine (IFN-γ), and the percentage of CXCR3+ T lymphocyte were elevated, suggesting an occurrence of immunological imbalance (28). It was also reported that TAI is linked to reduced CPR (29), decreased birth or delivery rate (10, 29, 30), and increased miscarriage rate (30, 31); however, evidence from other studies does not support the results mentioned above (32, 33). We found that patients in TAI group presented with increased early miscarriage rate using the chi-square test, but the Logistic regression analysis didn’t prove that TAI contributed to early miscarriage. Besides, the large-scale retrospective cohort study with 1,556 participants at the Reproductive Center of Peking University Third Hospital demonstrated that the early miscarriage rate among patients with TAI wasn’t increased (10), and the sample size of patients with early miscarriage in this study was bigger than ours (100 vs. 49), requiring further prospective study with larger sample size to confirm the impact of TAI on IVF/ICSI outcomes and provide scientific basis for clinical practice. Our study also demonstrated that patients with TAI with vitamin D deficiency tended to have a lower number of good-quality embryos. In this case, vitamin D and TAI likely served as interactive factors that may have influenced the results. This concern is based on the fact that no significant difference between the number of good-quality embryos was found among patients after being divided into two groups according to TAI positivity or into three groups according to vitamin D status. In addition, the lack of studies investigating the interaction between TAI and vitamin D on IVF outcomes has made this a poorly worded question. However, our study found that AMH levels were lower; FT4, TT4, and TSH levels were elevated; and TT4 levels were inversely correlated with the 25(OH)D concentration in the TAI group, suggesting that vitamin D and the thyroid play a possible role in affecting follicle development in TAI patients. A previous study reported that serum TSH concentrations were negatively correlated with IVF oocyte maturation and fertilization rate (34).
Our study also revealed that patients in the TAI group had lower AMH levels. After being secreted by granulosa cells (GCs), AMH regulates the development of early follicular and thus serves as a marker of the ovarian reserve (35). However, studies on AMH levels mostly reported the correlation between vitamin D and AMH levels. Wojtusik et al. (36) found that vitamin D treatment decreased AMH expression in GCs in small follicles, and larger follicles expressed higher level of vitamin D receptor than small follicles. Bednarska-Czerwinska et al. (37) reported that AMH levels were negatively correlated with follicular fluid (FF) vitamin D levels. Similarly, Merhi and colleagues (38) described AMH and AMH receptor (AMHR) gene expression in GCs as being negatively correlated with FF vitamin D among reproductive-aged females. On the other hand, after recruiting 388 premenopausal women in their cross-sectional study, Merhi and colleagues described AMH level as being positively correlated with blood vitamin D levels in women over the age of 40 years (39). However, there are few studies focusing on the correlation between TAI and AMH level and the effect of TAI on AMH expression in GCs.
After being divided into three subgroups, older patients in both the TAI and non-TAI groups presented with sufficient vitamin D levels. One possible explanation is that delayed child wishes often meet with age-related fertility loss, which starts at around 25–30 years of age (40). This delayed child wish pushed women to the reproductive center for fertility assessment, thus introducing them to more opportunities to be tested if they are TAI positive or vitamin D sufficient. Another likely factor is the lack of sun exposure. Sunlight exposure remains a major source of vitamin D (41). Protection from the sun through clothing and glass absorbing all of the ultraviolet B radiation prevents vitamin D production during sun exposure, which could be a matter of concern in China.
This study has some limitations. First, it was a single-center study. However, the quality control checks of the IVF-ET process and laboratory measurements were performed better in a single center than in multiple centers. In addition, our center performs >18,000 cycles of IVF-ET per year; the fact that all patients were scattered across the country makes the study’s population geographically representative. Second, lack of data concerning daylight hours, seasonal factors, outdoor activity conditions, and iodine nutritional status. Third, Han Chinese women only with male or tubal infertility made up the main study population, asking the evaluation of other races or populations with other causes of infertility to ensure the applicability of our findings.
5 Conclusion
This study revealed that patients with TAI tend to have reduced serum 25(OH)D concentration. Additionally, in the TAI group, the number of good-quality embryos decreased in patients with poor vitamin D status. Lastly, among women who underwent fresh ET, aging adversely impacted achieving clinical and ongoing pregnancy.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Reproductive Center of Peking University Third Hospital for IVF/ICSI treatment. The local ethics committee approved the study protocol (registration no. M2021189). The patients/participants provided their written informed consent to participate in this study.
Author contributions
YL, ZH, NH, and HC conceptualized the study, and all the authors contributed to the research discussion. YL, ZH, YW, LZ, and RL took part in patient follow-up and performed data analysis. YL wrote the initial draft of the paper, and all authors contributed to manuscript revision. All authors contributed to the article and approved the submitted version.
Funding
The National Natural Science Foundation of China (Grant No. 82171626) and Start-up Fund for Excellent Returnee of Peking University Third Hospital (BYSYLXHG2019003) covered the expense of data collection. The funders had no role in the study design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1098975/full#supplementary-material
References
1. Kamath MS, Mascarenhas M, Franik S, Liu E, Sunkara SK. Clinical adjuncts in in vitro fertilization: a growing list. Fertil Steril (2019) 112:978–86. doi: 10.1016/j.fertnstert.2019.09.019
2. Gil Á, Plaza-Diaz J, Mesa MD. Vitamin d: classic and novel actions. Ann Nutr Metab (2018) 72:87–95. doi: 10.1159/000486536
3. Chen Y, Zhi X. Roles of vitamin d in reproductive systems and assisted reproductive technology. Endocrinology (2020) 161(4):1–12. doi: 10.1210/endocr/bqaa023
4. Nandi A, Sinha N, Ong E, Sonmez H, Poretsky L. Is there a role for vitamin d in human reproduction? Horm Mol Biol Clin Investig (2016) 25:15–28. doi: 10.1515/hmbci-2015-0051
5. Lorini R, Gastaldi R, Traggiai C, Perucchin PP. Hashimoto’s thyroiditis. Pediatr Endocrinol Rev (2003) 1(Suppl 2):205–11.
6. Chao G, Zhu Y, Fang L. Correlation between hashimoto’s thyroiditis-related thyroid hormone levels and 25-hydroxyvitamin d. Front Endocrinol (Lausanne) (2020) 11:4. doi: 10.3389/fendo.2020.00004
7. Štefanić M, Tokić S. Serum 25-hydoxyvitamin d concentrations in relation to hashimoto’s thyroiditis: a systematic review, meta-analysis and meta-regression of observational studies. Eur J Nutr (2020) 59:859–72. doi: 10.1007/s00394-019-01991-w
8. Simsek Y, Cakır I, Yetmis M, Dizdar OS, Baspinar O, Gokay F. Effects of vitamin d treatment on thyroid autoimmunity. J Res Med Sci (2016) 21:85. doi: 10.4103/1735-1995.192501
9. Sinclair D. Clinical and laboratory aspects of thyroid autoantibodies. Ann Clin Biochem (2006) 43:173–83. doi: 10.1258/000456306776865043
10. Huang N, Chen LX, Lian Y, Wang HN, Li R, Qiao J, et al. Impact of thyroid autoimmunity on in vitro fertilization/intracytoplasmic sperm injection outcomes and fetal weight. Front Endocrinol (2021) 12:698579. doi: 10.3389/fendo.2021.698579
11. Geva E, Vardinon N, Lessing JB, Lerner-Geva L, Azem F, Yovel I, et al. Organ-specific autoantibodies are possible markers for reproductive failure: a prospective study in an in-vitro fertilization-embryo transfer programme. Hum Reprod (1996) 11:1627–631. doi: 10.1093/oxfordjournals.humrep.a019458
12. Simopoulou M, Sfakianoudis K, Maziotis E, Grigoriadis S, Giannelou P, Rapani A, et al. The impact of autoantibodies on IVF treatment and outcome: a systematic review. Int J Mol Sci (2019) 20(4):892. doi: 10.3390/ijms20040892
13. van den Boogaard E, Vissenberg R, Land JA, van Wely M, van der Post JAM, Goddijn M, et al. Significance of (sub) clinical thyroid dysfunction and thyroid autoimmunity before conception and in early pregnancy: a systematic review. Hum Reprod Update (2011) 17:605–19. doi: 10.1093/humupd/dmr024
14. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin d deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab (2011) 96:1911–30. doi: 10.1210/jc.2011-0385
15. Pacis MM, Fortin CN, Zarek SM, Mumford SL, Segars JH. Vitamin d and assisted reproduction: should vitamin d be routinely screened and repleted prior to ART? a systematic review. J Assist Reprod Genet (2015) 32:323–35. doi: 10.1007/s10815-014-0407-9
16. De-Regil LM, Palacios C, Lombardo LK, Peña-Rosas JP. Vitamin d supplementation for women during pregnancy. Sao Paulo Med J (2016) 134:274–5. doi: 10.1590/1516-3180.20161343T2
17. Roth DE, Leung M, Mesfin E, Qamar H, Watterworth J, Papp E. Vitamin d supplementation during pregnancy: state of the evidence from a systematic review of randomised trials. BMJ (2017) 359:j5237. doi: 10.1136/bmj.j5237
18. Vimaleswaran KS, Berry DJ, Lu C, Tikkanen E, Pilz S, Hiraki LT, et al. Causal relationship between obesity and vitamin d status: bi-directional mendelian randomization analysis of multiple cohorts. PloS Med (2013) 10:e1001383. doi: 10.1371/journal.pmed.1001383
19. Ozkan S, Jindal S, Greenseid K, Shu J, Zeitlian G, Hickmon C, et al. Replete vitamin d stores predict reproductive success following in vitro fertilization. Fertil Steril (2010) 94:1314–9. doi: 10.1016/j.fertnstert.2009.05.019
20. Rudick B, Ingles S, Chung K, Stanczyk F, Paulson R, Bendikson K. Characterizing the influence of vitamin d levels on IVF outcomes. Hum Reprod (2012) 27:3321–7. doi: 10.1093/humrep/des280
21. Paffoni A, Ferrari S, Viganò P, Pagliardini L, Papaleo E, Candiani M, et al. Vitamin d deficiency and infertility: insights from in vitro fertilization cycles. J Clin Endocrinol Metab (2014) 99:E2372–6. doi: 10.1210/jc.2014-1802
22. Polyzos NP, Anckaert E, Guzman L, Schiettecatte J, Van Landuyt L, Camus M, et al. Vitamin d deficiency and pregnancy rates in women undergoing single embryo, blastocyst stage, transfer (SET) for IVF/ICSI. Hum Reprod (2014) 29:2032–40. doi: 10.1093/humrep/deu156
23. Aflatoonian A, Arabjahvani F, Eftekhar M, Sayadi M. Effect of vitamin d insufficiency treatment on fertility outcomes in frozen-thawed embryo transfer cycles: a randomized clinical trial. Iran J Reprod Med (2014) 12:595–600.
24. van de Vijver A, Drakopoulos P, Van Landuyt L, Vaiarelli A, Blockeel C, Santos-Ribeiro S, et al. Vitamin d deficiency and pregnancy rates following frozen-thawed embryo transfer: a prospective cohort study. Hum Reprod (2016) 31:1749–54. doi: 10.1093/humrep/dew107
25. Firouzabadi RD, Rahmani E, Rahsepar M, Firouzabadi MM. Value of follicular fluid vitamin d in predicting the pregnancy rate in an IVF program. Arch Gynecol Obstet (2014) 289:201–6. doi: 10.1007/s00404-013-2959-9
26. Anifandis GM, Dafopoulos K, Messini CI, Chalvatzas N, Liakos N, Pournaras S, et al. Prognostic value of follicular fluid 25-OH vitamin d and glucose levels in the IVF outcome. Reprod Biol Endocrinol (2010) 8:91. doi: 10.1186/1477-7827-8-91
27. Cozzolino M, Busnelli A, Pellegrini L, Riviello E, Vitagliano A. How vitamin d level influences in vitro fertilization outcomes: results of a systematic review and meta-analysis. Fertil Steril (2020) 114:1014–25. doi: 10.1016/j.fertnstert.2020.05.040
28. Huang N, Liu D, Lian Y, Chi H, Qiao J. Immunological microenvironment alterations in follicles of patients with autoimmune thyroiditis. Front Immunol (2021) 12:770852. doi: 10.3389/fimmu.2021.770852
29. Kilic S, Tasdemir N, Yilmaz N, Yuksel B, Gul A, Batioglu S. The effect of anti-thyroid antibodies on endometrial volume, embryo grade and IVF outcome. Gynecol Endocrinol (2008) 24:649–55. doi: 10.1080/09513590802531112
30. Poppe K, Glinoer D, Tournaye H, Devroey P, van Steirteghem A, Kaufman L, et al. Assisted reproduction and thyroid autoimmunity: an unfortunate combination? J Clin Endocrinol Metab (2003) 88:4149–52. doi: 10.1210/jc.2003-030268
31. Negro R, Mangieri T, Coppola L, Presicce G, Casavola EC, Gismondi R, et al. Levothyroxine treatment in thyroid peroxidase antibody-positive women undergoing assisted reproduction technologies: a prospective study. Hum Reprod (2005) 20:1529–33. doi: 10.1093/humrep/deh843
32. Chai J, Yeung W-YT, Lee C-YV, Li H-WR, Ho P-C, Ng H-YE. Live birth rates following in vitro fertilization in women with thyroid autoimmunity and/or subclinical hypothyroidism. Clin Endocrinol (2014) 80:122–7. doi: 10.1111/cen.12220
33. Mintziori G, Goulis DG, Gialamas E, Dosopoulos K, Zouzoulas D, Gitas G, et al. Association of TSH concentrations and thyroid autoimmunity with IVF outcome in women with TSH concentrations within normal adult range. Gynecol Obstet Investig (2014) 77:84–8. doi: 10.1159/000357193
34. Gao H, Lu X, Huang H, Ji H, Zhang L, Su Z. Thyroid-stimulating hormone level is negatively associated with fertilization rate in patients with polycystic ovary syndrome undergoing in vitro fertilization. Int J Gynaecol Obstet (2021) 155:138–45. doi: 10.1002/ijgo.13581
35. di Clemente N, Racine C, Pierre A, Taieb J. Anti-müllerian hormone in female reproduction. Endocr Rev (2021) 42:753–82. doi: 10.1210/endrev/bnab012
36. Wojtusik J, Johnson PA. Vitamin d regulates anti-mullerian hormone expression in granulosa cells of the hen. Biol Reprod (2012) 86:91. doi: 10.1095/biolreprod.111.094110
37. Bednarska-Czerwińska A, Olszak-Wąsik K, Olejek A, Czerwiński M, Tukiendorf AA. Vitamin d and anti-mullerian hormone levels in infertility treatment: the change-point problem. Nutrients (2019) 11:1053. doi: 10.3390/nu11051053
38. Merhi Z, Doswell A, Krebs K, Cipolla M. Vitamin d alters genes involved in follicular development and steroidogenesis in human cumulus granulosa cells. J Clin Endocrinol Metab (2014) 99:E1137–45. doi: 10.1210/jc.2013-4161
39. Merhi ZO, Seifer DB, Weedon J, Adeyemi O, Holman S, Anastos K, et al. Circulating vitamin d correlates with serum antiMüllerian hormone levels in late-reproductive-aged women: women’s interagency HIV study. Fertil Steril (2012) 98:228–34. doi: 10.1016/j.fertnstert.2012.03.029
40. Vander Borght M, Wyns C. Fertility and infertility: definition and epidemiology. Clin Biochem (2018) 62:2–10. doi: 10.1016/j.clinbiochem.2018.03.012
Keywords: in vitro fertilization, intracytoplasmic sperm injection, thyroid autoimmunity, vitamin D, early pregnancy outcomes
Citation: Liu Y, He Z, Huang N, Zeng L, Wang Y, Li R and Chi H (2023) Impact of thyroid autoimmunity and vitamin D on in vitro fertilization/intracytoplasmic sperm injection outcomes among women with normal thyroid function. Front. Endocrinol. 14:1098975. doi: 10.3389/fendo.2023.1098975
Received: 15 November 2022; Accepted: 12 April 2023;
Published: 08 May 2023.
Edited by:
Faiza Alam, University of Brunei Darussalam, BruneiReviewed by:
Maria Laura Tanda, University of Insubria, ItalyRehana Rehman, Aga Khan University, Pakistan
Copyright © 2023 Liu, He, Huang, Zeng, Wang, Li and Chi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongbin Chi, Q2hpaGJAMTYzLmNvbQ==
 Yalong Liu1,2,3,4
Yalong Liu1,2,3,4 Ning Huang
Ning Huang Lin Zeng
Lin Zeng Hongbin Chi
Hongbin Chi