- 1Department of Ophthalmology, Peking University Third Hospital, Beijing, China
- 2Beijing Key Laboratory of Restoration of Damaged Ocular Nerve, Peking University Third Hospital, Beijing, China
Purpose: To conduct a network meta-analysis (NMA) comparing the efficacy of anti-vascular endothelial growth factor (VEGF) therapy alone versus laser photocoagulation (LP) therapy alone or anti-VEGF therapy combined with LP therapy for diabetic macular edema (DME).
Methods: PubMed, Embase, Web of Science, and Cochrane Central Register of Controlled Trials were systematically searched for studies comparing anti-VEGF therapy alone versus LP therapy alone or anti-VEGF therapy combined with LP therapy for DME. Primary outcomes were mean best-corrected visual acuity (BCVA) and central macular thickness (CMT) change. Relevant data were collected and pooled using NMA.
Results: A total of 13 randomized controlled trials were included in our NMA. Anti-VEGF therapy significantly improved BCVA the most compared to the combined (mean difference [MD] = 1.5; 95% confidence interval [CI]: 0.084, 2.7) and LP (MD = 6.3; 95% CI: 5.1, 7.6) therapies at six months, while there was no difference in reducing CMT at six months between the anti-VEGF and combined therapies (MD = -16; 95% CI: -46, 13). At 12 months, no significant difference was found between the anti-VEGF and combined therapy in terms of BCVA (MD = 0.1; 95% CI: -1.7, 1.5) and CMT (MD = 21; 95% CI: -3.0, 44).
Conclusion: There was no significant difference between the anti-VEGF therapy and combined therapy. For the long-term treatment of patients with DME, combined therapy is recommended.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42022376401.
Introduction
Diabetic macular edema (DME), a manifestation of diabetic retinopathy (DR) that is diagnosed at any stage of the disease, is defined as retinal oedema and/or thickening, involving, or threatening the fovea. Although the management of diabetes mellitus (DM) has advanced tremendously over the last few decades, DME still accounts for a significant cause of vision loss among patients with DM, and if untreated, can result to blindness. DME affects approximately 7% of patients with DM (1) and represents a substantial public health concern worldwide (2, 3). The prevalence of DME is related to the duration of DM and stage of DR (4).
In recent years, with further understanding of the pathophysiological mechanisms of DME, treatment options for DME have shifted gradually. Laser photocoagulation (LP) was the gold standard treatment for DME prior to the availability of anti-vascular endothelial growth factor (VEGF) treatment (5). The mechanisms of LP include increased oxygen tension and phagocytosis of glial cells and retinal pigment epithelial cells, together with decreased production of vasoactive cytokines (mainly VEGF). LP provides vision stabilization in DME, while the efficacy of providing clinical improvement in patients’ vision seems to be limited (5, 6). Currently, anti-VEGF agents are the first-line treatment option for DME. Ranibizumab, aflibercept, bevacizumab, and pegaptanib have shown significant efficacy in visual improvement in patients with DME in phase II/III clinical trials (7–10). However, anti-VEGF agents cannot treat macular hypoxia; thus, their efficacy is transitory. Additionally, the short half-life of anti-VEGF agents, such as ranibizumab and bevacizumab, in the eyes of 2.75 and 9.8 days, respectively, results in a limited duration of action with consequent high rate of recurrence; thus, requiring frequent injections (11, 12) and imposing a large burden on patients with DME. A combination of anti-VEGF and LP may be more effective than either monotherapy and may reduce the frequency of injections. Additionally, the effectiveness of LP may be improved by LP becoming easier because of the reduction in macular edema caused by anti-VEGF injections. Several studies have evaluated LP as an adjunctive treatment for anti-VEGF agents; however, their conclusions are inconsistent (13–18).
Network meta-analysis (NMA) is a novel data synthesis method that combines direct and indirect evidence from randomized controlled trials (RCTs) using statistical techniques to derive estimates of comparative efficacy (19). Therefore, this study compared the efficacy of anti-VEGF therapy alone, LP therapy alone, or anti-VEGF therapy combined with LP therapy in the treatment of patients with DME within an NMA framework, primarily aimed at assessing the mean best-corrected visual acuity (BCVA) and central macular thickness (CMT) changes.
Methods
The NMA was strictly conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement (20) and the Cochrane Handbook guidelines (21).
Search strategy
RCTs evaluating the efficacy of anti-VEGF therapy alone, LP therapy alone, or anti-VEGF therapy combined with LP therapy in the treatment of DME were systematically searched in PubMed, Embase, Web of Science, and the Cochrane Central Register of Controlled Trials from inception to September 11, 2022. The search strategy (Table S1) was conducted corresponding to the following terms: “diabetic macular edema,” “anti,” “vascular endothelial growth factor,” “vegf,” “ranibizumab,” “bevacizumab,” “aflibercept,” “pegaptanib,” “laser,” and “photocogulation,” which were connected by and/or in different combinations. The search was restricted to human studies. No publication date or language limitation was imposed when searching for the RCTs. Additionally, reference lists of relevant articles were manually examined to identify potentially relevant studies.
Inclusion and exclusion criteria
Studies were eligible if they met the following inclusion criteria: (1) RCT; (2) patients/participants with DME; (3) comparison of at least two of the following comparators: anti-VEGF therapy alone, LP therapy alone, anti-VEGF therapy combined with LP therapy; (4) outcome measures, including the mean BCVA and/or CMT change; and (5) follow-up >6 months.
Exclusion criteria were as follows: (1) review articles, case reports, non-RCTs, meta-analyses, and redundant publications; and (2) studies with insufficient data.
Two authors (J C and H W) independently screened the titles and abstracts of the identified articles. All potentially eligible articles were full-text reviewed to evaluate whether they met the inclusion criteria. Any discrepancies were resolved via discussion. Unsettled discrepancies were arbitrated by a senior reviewer, Prof. Qiu.
Data extraction and quality assessment
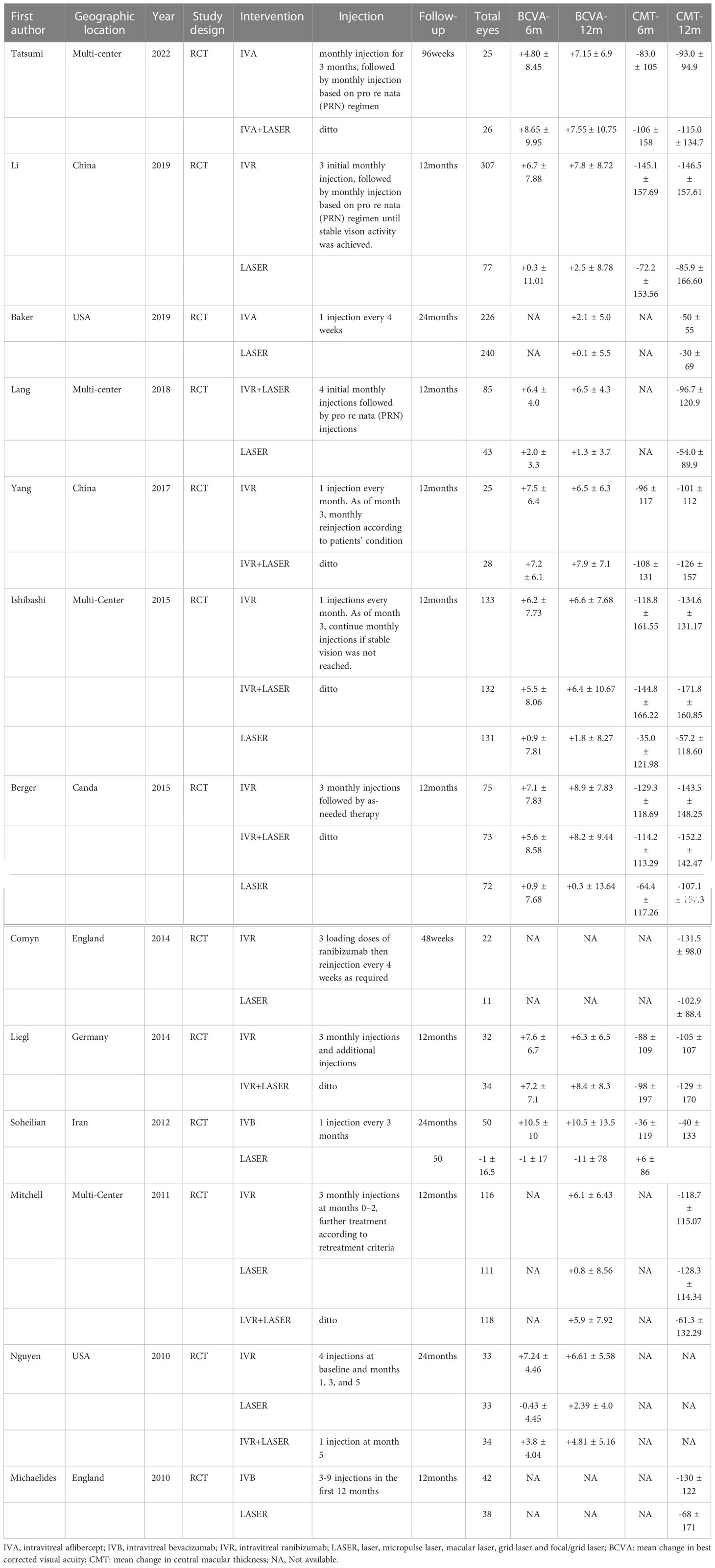
Two authors (J C and H W) independently extracted data from all the included studies. The extracted data included the first author, publication year, geographic location, study design, interventions (including specific injection plan), follow-up time, and total number of eyes of different interventions, together with the details of outcomes, which included the mean BCVA and CMT change from baseline to 6 and 12 months. If any essential information was required for eligibility assessment or data extraction, the corresponding authors of the included studies were contacted. Logarithm of the minimal angle of resolution (logMAR) was converted into the ETDRS letter form when extracting BCVA data. The Cochrane collaboration tool was used to assess risk of bias (21).
Statistical analysis
The NMA was performed within a Bayesian framework to synthesize the mean BCVA and CMT changes from baseline to 6 and 12 months across the RCTs. We used R software (version 4.2.1) with gemtc and rjag packages to create forest plots. Statistical heterogeneity was evaluated using the I2 statistic: <25%, no heterogeneity; 25–50%, low heterogeneity; 50–75%, moderate heterogeneity; and >75%, high heterogeneity (22). The node-splitting method was used to assess the inconsistency between direct and indirect comparisons in NMA (23). Significant heterogeneity was at p < 0.05. Efficacy of the interventions was evaluated using mean difference (MD) with 95% confidence interval (CI). Additionally, we conducted a ranking analysis based on simulations and calculated the rank’s possibility of establishing a hierarchy of different interventions. We also assessed the potential publication bias by creating the funnel plot and conducting the Egger’s test in the traditional meta-analysis.
Result
Study characteristics
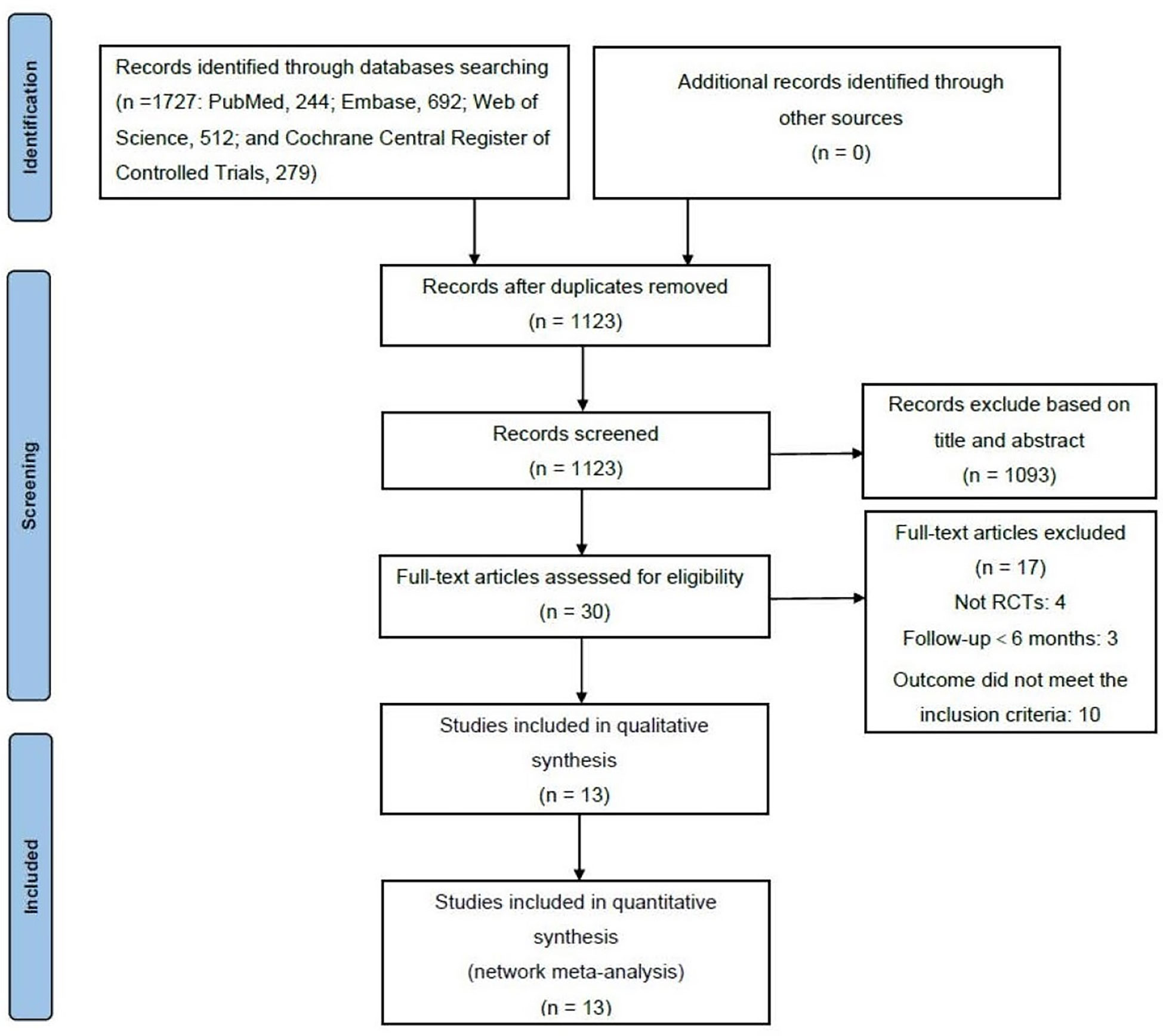
A total of 1,727 articles (PubMed, 244; Embase, 692; Web of Science, 512; and Cochrane Central Register of Controlled Trials, 279) were retrieved from the electronic databases in the primary search, among which 604 articles were removed for duplicates. After screening the titles and abstracts, 1,093 articles were removed. Thirty full-text articles were reviewed to determine whether they met the inclusion criteria. Eventually, 13 articles were included in the NMA (Figure 1) (14, 18, 24–34). Characteristics of all the included RCTs are summarized in Table 1. All the RCTs compared two or more interventions and included a total of 2,432 eyes. The mean BCVA and CMT changes from baseline to 6 and 12 months were recorded for the NMA.
Risk of bias assessment
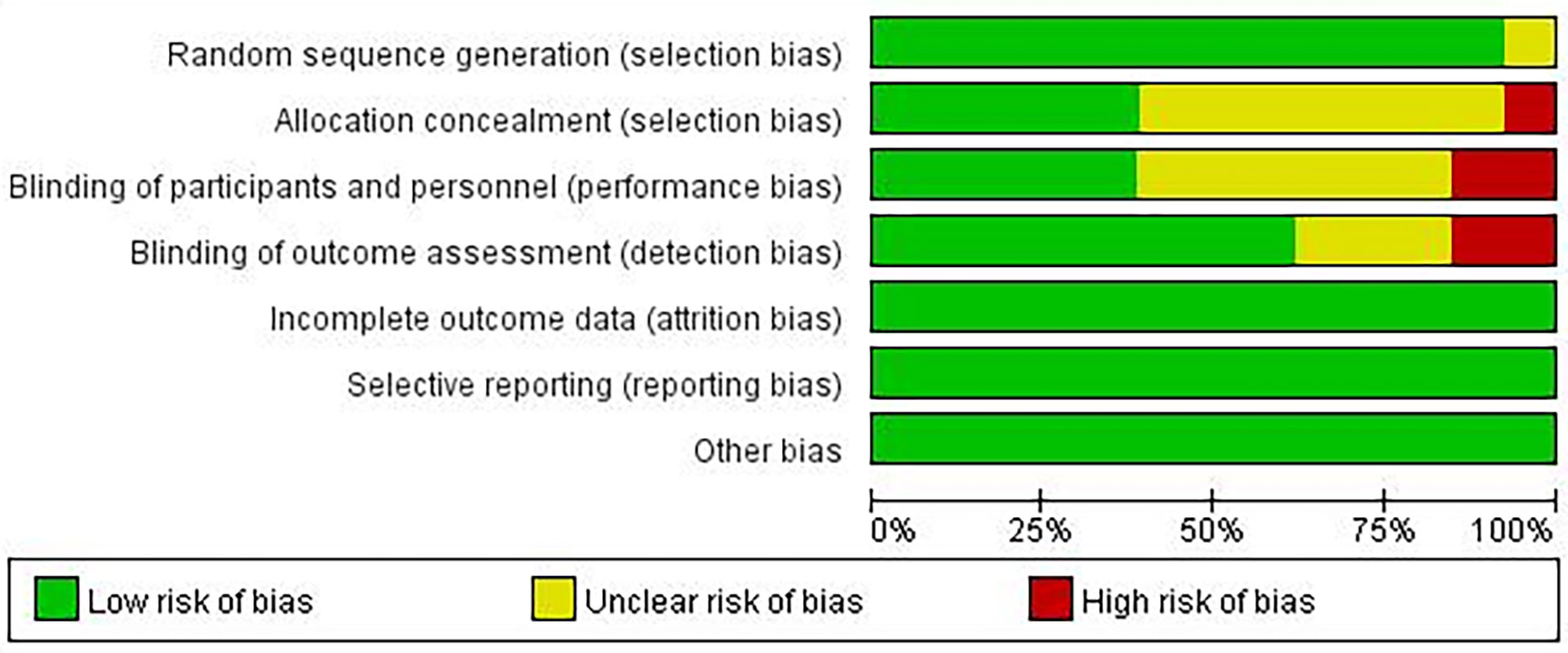
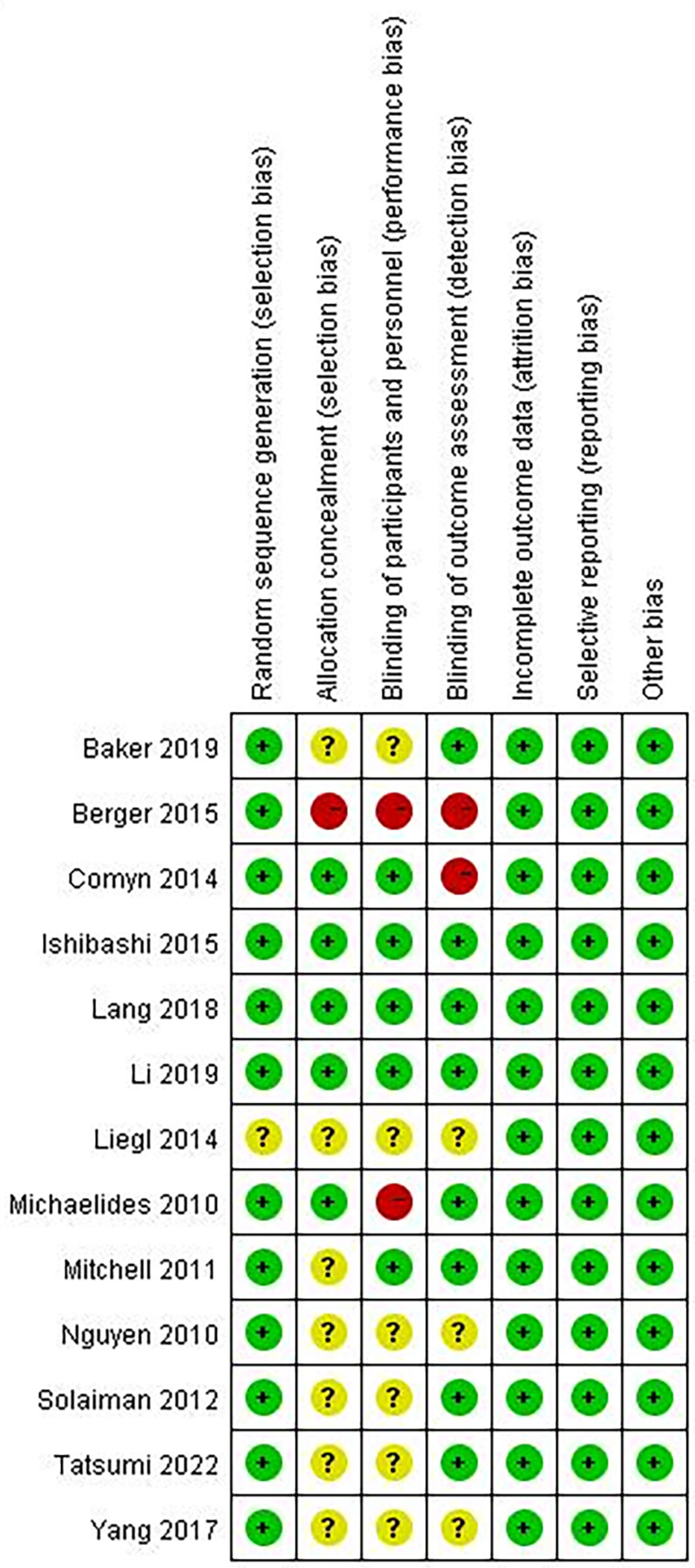
A summary of the risk of bias assessment for the included studies is shown in Figures 2, 3. One of the studies did not mention the method of generating the random allocation sequence, seven did not mention allocation concealment, six did not mention blinding of participants and personnel, and three did not mention blinding of outcome assessment; therefore, the risk of bias assessment was considered unclear. Additionally, one study had a high risk of bias in the random allocation sequence, one featured a high risk of blinding of participants and personnel, and three featured a high risk of blinding outcome assessment. Overall, quality of the included studies was considered high, although the risk of bias in several studies was high or unclear under some conditions.
Network meta-analysis
Mean BCVA change
Nine RCTs were included to conduct a NMA for mean BCVA change at six months and 11 RCTs for 12 months. A network of eligible comparisons for the mean BCVA change from baseline to 6 and 12 months is shown in Figure S1. Results of the mean BCVA change at six months from baseline suggested that the anti-VEGF group yielded a better vision improvement compared to the combined (MD = 1.5; 95% CI: 0.084, 2.7) and LP (MD = 6.3; 95% CI: 5.1, 7.6) therapies (Figure 4A). Likewise, the combined therapy yielded better vision improvement compared to the LP therapy (MD = 4.8; 95% CI: 3.7, 6.3). The results of ranking based on simulations suggested that anti-VEGF therapy (97.715%) was the best, followed by combined (2.285%) and LP (0.000%) therapies (Figure S7). However, at 12 months, there was no significant difference between the anti-VEGF and combined therapies (MD = 0.1; 95% CI: -1.7, 1.5). The anti-VEGF (MD = 4.7; 95% CI: 3.3, 6.5) and combined (MD = 4.8; 95% CI: 3.3, 6.7) therapies were significantly superior to the LP therapy (Figure 4B). Ranking based on simulations suggested that combined therapy (56.215%) was the best, followed by anti-VEGF (43.785%) and LP (0.000%) therapies (Figure S8). All the comparisons showed no significant heterogeneity (p>0.05). However, the I2 statistic showed high heterogeneity when comparing the mean BCVA change at 12 months between the anti-VEGF and LP therapies (Figures S3, 4). Funnel plots on the mean BCVA changes at 6 and 12 months were presented in Figures S11, S12. Visual inspection showed no significant asymmetry in plots, while Egger’s tests also suggested that no potential threat of publication bias on the mean BCVA changes at 6 months (p=0.935) and 12 months (p=0.532).
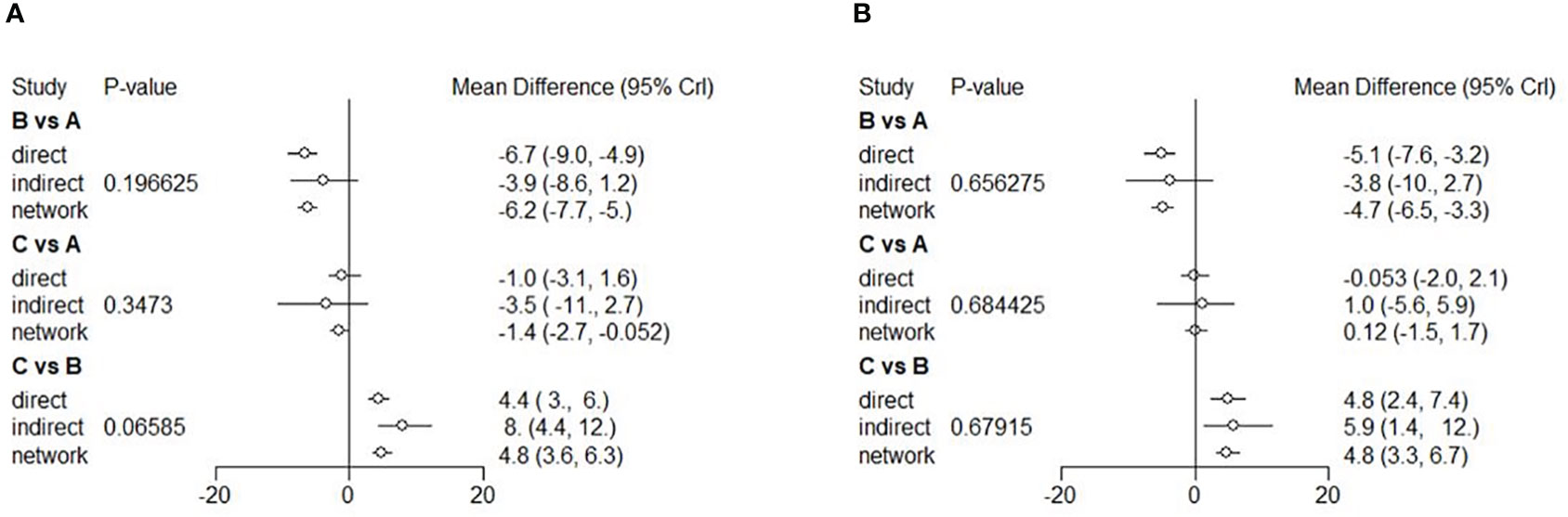
Figure 4 Forest plots of NMA showing mean BCVA change from baseline to 6 (A), and 12 (B) months. Different treatments are indicated with capital letters A, B and C in the forest plots. Treatments are indicated as A [anti-VEGF therapy], B [LP therapy], and C [the combined therapy], respectively.
Mean CMT change
Eight RCTs were included to conduct NMA for the mean BCVA change at six months and 12 RCTs at 12 months. A network of eligible comparisons for the mean CMT change from baseline to 6 and 12 months is shown in Figure S2. The NMA comparing the combined therapy versus anti-VEGF therapy showed no difference in the mean CMT change between the two therapies (MD = -16; 95% CI: -46, -13). Both anti-VEGF (MD = -65; 95% CI: -93, -37) and combined (MD = -81; 95% CI: -0.011, -50) therapies had a better outcome with a significant change in terms of reduced CMT compared to the LP therapy (Figure 5A). Ranking based on simulations suggested that the combined therapy (86.4%) was the best, followed by the anti-VEGF (13.6%) and LP (0.0%) therapies (Figure S9). The NMA of mean CMT change at 12 months showed similar results. There was no difference in the mean CMT change between the anti-VEGF and combined therapies (MD = 21; 95% CI: -3.0, -44). Efficacy of the anti-VEGF (MD = -44; 95% CI: -65, -25) and combined (MD = -65; 95% CI: -90, -41) therapies was better than that of the LP therapy (Figure 5B). Ranking based on simulations suggested that the combined therapy (95.61%) was the best, followed by the anti-VEGF (4.39%) and LP (0.00%) therapies (Figure S10). All the comparisons showed no significant heterogeneity (p>0.05) (Figures S5, 6). Funnel plots on the mean CMT changes at 6 and 12 months were showed in Figures S13, S14. Visual inspection showed little asymmetry in plots, and Egger’s tests suggested the absence of substantial publication bias on the mean CMT changes at 6 months (p=0.739) and 12 months (p=0.680).
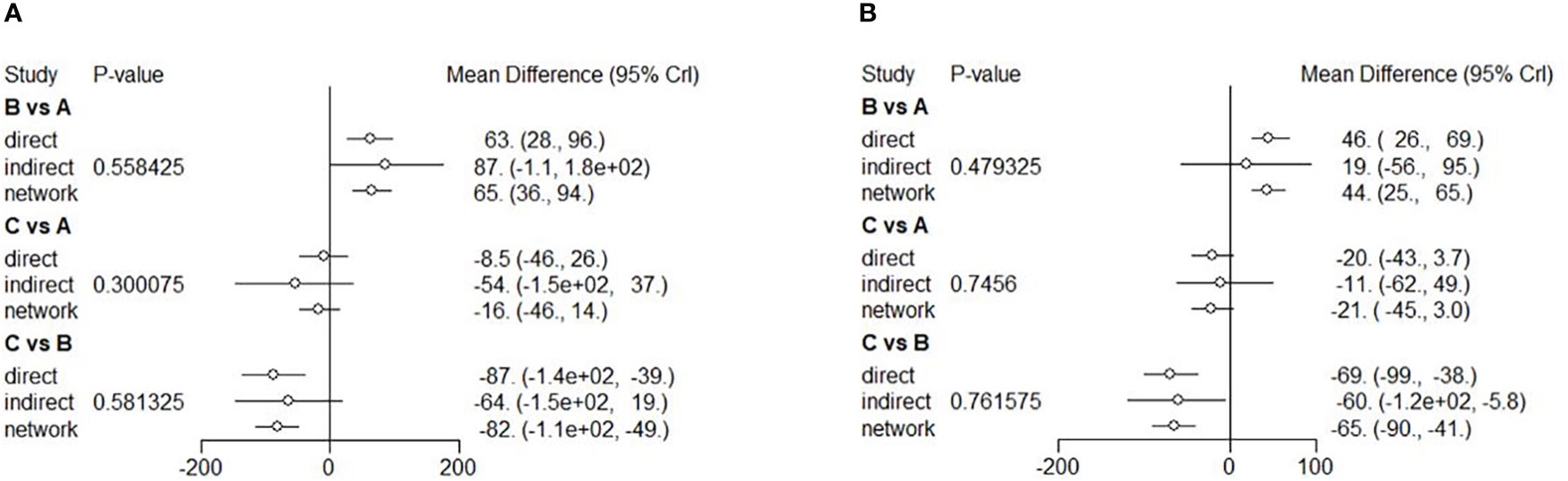
Figure 5 Forest plots of NMA showing mean CMT change from baseline to 6 (A), and 12 (B) months. Different treatments are indicated with capital letters A, B and C in the forest plots. Treatments are indicated as A [anti-VEGF therapy], B [LP therapy], and C [the combined therapy], respectively.
Discussion
In this NMA, which included 13 studies and a total of 2,422 eyes, we systematically reviewed the published literature and compared the efficacy of three different interventions in patients with DME. It was indicated that compared with the LP therapy, both the anti-VEGF therapy alone and combined anti-VEGF therapy with LP therapy were the most efficacious treatments, with no statistical significance based on the mean CMT change at six and 12 months, as well as the mean BCVA change at 12 months. We found that anti-VEGF therapy alone was better than the combined and LP therapies based on the mean BCVA change at six months. One possible reason is that compared with anti-VEGF therapy alone, the combined therapy may have a stronger anti-angiogenic and anti-inflammatory effect in the early stage after injection, which only affects the decrease in CMT, but has no significant improvement in BCVA (35). Additionally, the adverse effects of LP therapy may provide an explanation for the result that the anti-VEGF therapy alone was better than the combined therapy based on the mean BCVA change at six months. Regarding heterogeneity, we suspect that the high heterogeneity of the mean BCVA change at 12 months was mainly due to the large sample size, but limited therapeutic efficacy of the study by Backer et al. (25).
Although intravitreal injection of anti-VEGF agents has been the standard therapy for DME, LP treatment is still often used (2). LP therapy is associated with severe vision loss (36). With the development of novel LP technologies, these adverse effects have reduced (37). This study involved conventional LP (such as grid LP) and novel LP (such as subthreshold LP) therapies. The NMA was based on the assumption that all LP therapies were same and clinicians should pay attention. However, it is also worth mentioning that conventional LP therapy was reported at least as effective as subthreshold LP therapy in the treatment of DME in the previous meta-analysis (38, 39). Moreover, LP therapy has a significant advantage as a long-lasting treatment compared with anti-VEGF therapy, the latter of which is a short-term treatment (40). Patients need to be followed-up for a long time to monitor therapeutic efficacy, and more long-term outcomes are needed to perform analysis and comparison. Owing to repeated injections, anti-VEGF therapy has complications, including intraocular pressure spikes (41) and endophthalmitis (42), to which attention should be paid during treatment. Therefore, anti-VEGF therapy may not be a good treatment option for all patients. A combination of anti-VEGF and LP can reduce the frequency of injections and thus, may solve this problem.
Previous studies have shown that the combined therapy is more effective (39). However, a recent study indicated that anti-VEGF therapy was the most efficacious based on the mean BCVA and CMT changes at 12 months, while anti-VEGF and combined therapies had no significant difference in the decrease of CMT at six months (43). Further studies are required to provide more evidence. According to the present study, anti-VEGF therapy alone and combined therapy are both worth considering. The choice of treatments should consider the patient’s tolerance, adherence, economic situation, and so on.
These therapies in our network meta-analysis are commonly used for the treatment of patients with DME; therefore, the results of our study will be instructive for clinical treatment. However, this study has several limitations. First, the number of included studies was relatively small, although they were generally high-quality studies. Second, the baseline characteristics of the patients in different studies were not balanced, but they were not included in the NMA models. The intervals between anti-VEGF and LP therapies and the types of LP therapy were also inconsistent. This might have potentially influenced the validity of the results of the mean BCVA and CMT changes. Finally, we did not compare the effects of the different anti-VEGF agents. To evaluate the efficacy of these therapies more accurately, more high-quality RCTs are necessary.
In conclusion, this NMA showed evidence of comparable efficacy in terms of BCVA and CMT between anti-VEGF therapy alone and anti-VEGF combined with LP therapy, with no overall significant difference. Considering the results of the forest plots and ranking based on simulations of treatments and need for long-term treatment, combined therapy is recommended for the treatment of patients with DME.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
WQ designed the study and revised the manuscript. JC and HW collected and analyzed the data. JC and HW drafted the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The publication of this study was supported by China International Medical Foundation (CIMF), grant Z-2018-40.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1096105/full#supplementary-material
References
1. Neubauer AS, Ulbig MW. Laser treatment in diabetic retinopathy. Ophthalmologica (2007) 221(2):95–102. doi: 10.1159/000098254
2. Kim EJ, Lin WV, Rodriguez SM, Chen A, Loya A, Weng CY. Treatment of diabetic macular edema. Curr Diabetes Rep (2019) 19(9):68. doi: 10.1007/s11892-019-1188-4
3. Browning DJ, Stewart MW, Lee C. Diabetic macular edema: Evidence-based management. Indian J Ophthalmol (2018) 66(12):1736–50. doi: 10.4103/ijo.IJO_1240_18
4. Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. IV. diabetic macular edema. Ophthalmology (1984) 91(12):1464–74. doi: 10.1016/s0161-6420(84)34102-1
5. Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. early treatment diabetic retinopathy study report number 1. early treatment diabetic retinopathy study research group. Arch Ophthalmol (1985) 103(12):1796–806. doi: 10.1001/archopht.1986.01050200021013
6. Beck RW, Edwards AR, Aiello LP, Bressler NM, Ferris F, Glassman AR, et al. Three-year follow-up of a randomized trial comparing focal/grid photocoagulation and intravitreal triamcinolone for diabetic macular edema. Arch Ophthalmol (2009) 127(3):245–51. doi: 10.1001/archophthalmol.2008.610
7. Brown DM, Nguyen QD, Marcus DM, Boyer DS, Patel S, Feiner L, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology (2013) 120(10):2013–22. doi: 10.1016/j.ophtha.2013.02.034
8. Korobelnik JF, Do DV, Schmidt-Erfurth U, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology (2014) 121(11):2247–54. doi: 10.1016/j.ophtha.2014.05.006
9. Scott IU, Edwards AR, Beck RW, Bressler NM, Chan CK, Elman MJ, et al. A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology (2007) 114(10):1860–7. doi: 10.1016/j.ophtha.2007.05.062
10. Sultan MB, Zhou D, Loftus J, Dombi T, Ice KS. A phase 2/3, multicenter, randomized, double-masked, 2-year trial of pegaptanib sodium for the treatment of diabetic macular edema. Ophthalmology (2011) 118(6):1107–18. doi: 10.1016/j.ophtha.2011.02.045
11. Ahn SJ, Ahn J, Park S, Kim H, Hwang DJ, Park JH, et al. Intraocular pharmacokinetics of ranibizumab in vitrectomized versus nonvitrectomized eyes. Invest Ophthalmol Vis Sci (2014) 55(1):567–73. doi: 10.1167/iovs.13-13054
12. Krohne TU, Eter N, Holz FG, Meyer CH. Intraocular pharmacokinetics of bevacizumab after a single intravitreal injection in humans. Am J Ophthalmol (2008) 146(4):508–12. doi: 10.1016/j.ajo.2008.05.036
13. Yan P, Qian C, Wang W, Dong Y, Wan G, Chen Y. Clinical effects and safety of treating diabetic macular edema with intravitreal injection of ranibizumab combined with retinal photocoagulation. Ther Clin Risk Manag (2016) 12:527–33. doi: 10.2147/TCRM.S99224
14. Ishibashi T, Li X, Koh A, Lai TY, Lee FL, Lee WK, et al. The REVEAL study: Ranibizumab monotherapy or combined with laser versus laser monotherapy in Asian patients with diabetic macular edema. Ophthalmology (2015) 122(7):1402–15. doi: 10.1016/j.ophtha.2015.02.006s
15. Lee SJ, Kim ET, Moon YS. Intravitreal bevacizumab alone versus combined with macular photocoagulation in diabetic macular edema. Korean J Ophthalmol (2011) 25(5):299–304. doi: 10.3341/kjo.2011.25.5.299
16. Solaiman KA, Diab MM, Abo-Elenin M. Intravitreal bevacizumab and/or macular photocoagulation as a primary treatment for diffuse diabetic macular edema. Retina (2010) 30(10):1638–45. doi: 10.1097/IAE.0b013e3181e1ed07
17. Huang JD, Song ZY. Clinical study of grid pattern laser photocoagulation with ranibizumab for diabetic macular edema. Int Eye Sci (2016) 16(3):493–5. doi: 10.3980/j.issn.1672-5123.2016.3.23
18. Tatsumi T, Takatsuna Y, Oshitari T, Kaiho T, Kawasaki Y, Shiko Y, et al. Randomized clinical trial comparing intravitreal aflibercept combined with subthreshold laser to intravitreal aflibercept monotherapy for diabetic macular edema. Sci Rep (2022) 12(1). doi: 10.1038/s41598-022-14444-y
19. Bhatnagar N, Lakshmi PV, Jeyashree K. Multiple treatment and indirect treatment comparisons: An overview of network meta-analysis. Perspect Clin Res (2014) 5(4):154–8. doi: 10.1038/s41598-022-14444-y
20. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PloS Med (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097
21. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
22. Higgins JPT, Green S eds. Cochrane handbook for systematic reviews of interventions version 5.1.0. England: The Cochrane Collaboration (2011). Available at: https://www.handbook.cochrane.org.
23. Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med (2010) 29(7-8):932–44. doi: 10.1002/sim.3767
24. Li X, Dai H, Li X, Han M, Li J, Suhner A, et al. Efficacy and safety of ranibizumab 0.5 mg in Chinese patients with visual impairment due to diabetic macular edema: results from the 12-month REFINE study. Graefes Arch Clin Exp Ophthalmol (2019) 257(3):529–41. doi: 10.1007/s00417-018-04213-x
25. Baker CW, Glassman AR, Beaulieu WT, Antoszyk AN, Browning DJ, Chalam KV, et al. Effect of initial management with aflibercept vs laser photocoagulation vs observation on vision loss among patients with diabetic macular edema involving the center of the macula and good visual acuity a randomized clinical trial. Jama-Journal Am Med Assoc (2019) 321(19):1880–94. doi: 10.1001/jama.2019.5790
26. Lang GE, Liakopoulos S, Vögeler J, Weiß C, Spital G, Gamulescu MA, et al. The RELATION study: efficacy and safety of ranibizumab combined with laser photocoagulation treatment versus laser monotherapy in NPDR and PDR patients with diabetic macular oedema. Acta ophthalmolog (2018) 96(3):e377–85. doi: 10.1111/aos.13574
27. Yang M, Li YL, Jiang XG, Meng L, Han XD. Effect of ranibizumab injections combining with 577nm laser macular grid photocoagulation for treatment of severe diabetic macular edema. Int Eye Sci (2017) 17(4):694–7. doi: 10.3980/j.issn.1672-5123.2017.4.26
28. Berger A, Sheidow T, Cruess AF, Arbour JD, Courseau AS, de Takacsy F, et al. Efficacy/safety of ranibizumab monotherapy or with laser versus laser monotherapy in DME. Can J Ophthalmol (2015) 50(3):209–16. doi: 10.1016/j.jcjo.2014.12.014
29. Liegl R, Langer J, Seidensticker F, Reznicek L, Haritoglou C, Ulbig MW, et al. Comparative evaluation of combined navigated laser photocoagulation and intravitreal ranibizumab in the treatment of diabetic macular edema. PloS One (2014) 9(12):e113981. doi: 10.1371/journal.pone.0113981
30. Comyn O, Sivaprasad S, Peto T, Neveu MM, Holder GE, Xing W, et al. A randomized trial to assess functional and structural effects of ranibizumab versus laser in diabetic macular edema (the LUCIDATE study). Am J Ophthalmol (2014) 157(5):960–70. doi: 10.1016/j.ajo.2014.02.019
31. Soheilian M, Garfami KH, Ramezani A, Yaseri M, Peyman GA. Two-year results of a randomized trial of intravitreal bevacizumab alone or combined with triamcinolone versus laser in diabetic macular edema. Retina (2012) 32(2):314–21. doi: 10.1097/IAE.0b013e31822f55de
32. Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P, Schlingemann RO, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology (2011) 118(4):615–25. doi: 10.1016/j.ophtha.2011.01.031
33. Nguyen QD, Shah SM, Khwaja AA, Channa R, Hatef E, Do DV, et al. Two-year outcomes of the ranibizumab for edema of the mAcula in diabetes (READ-2) study. Ophthalmology (2010) 117(11):2146–51. doi: 10.1016/j.ophtha.2010.08.016
34. Michaelides M, Kaines A, Hamilton RD, Fraser-Bell S, Rajendram R, Quhill F, et al. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12-month data: Report 2. Ophthalmology (2010) 117(6):1078–U76. doi: 10.1016/j.ophtha.2010.03.045
35. Zhang XL, Chen J, Zhang RJ, Wang WJ, Zhou Q, Qin XY, et al. Intravitreal triamcinolone versus intravitreal bevacizumab for diabetic macular edema: a meta-analysis. Int J Ophthalmol (2013) 6(4):546–52. doi: 10.3980/j.issn.2222-3959.2013.04.26
36. Gabrielle PH, Massin P, Kodjikian L, Erginay A, Pallot C, Jonval L, et al. Central retinal thickness following panretinal photocoagulation using a multispot semi-automated pattern-scanning laser to treat ischaemic diabetic retinopathy: Treatment in one session compared with four monthly sessions. Acta Ophthalmol (2019) 97(5):e680–7. doi: 10.1097/IAE.0b013e3181e1ed07
37. Reddy SV, Husain D. Panretinal photocoagulation: A review of complications. Semin Ophthalmol (2018) 33(1):83–8. doi: 10.1080/08820538.2017.1353820
38. Chen G, Tzekov R, Li W, Jiang F, Mao S, Tong Y, et al. Subthreshold micropulse diode laser versus conventional laser photocoagulation for diabetic macular edema: A meta-analysis of randomized controlled trials. Retina (2016) 36(11):2059–65. doi: 10.1097/IAE.0000000000001053
39. Wu Y, Ai P, Ai Z, Xu G. Subthreshold diode micropulse laser versus conventional laser photocoagulation monotherapy or combined with anti-VEGF therapy for diabetic macular edema: A Bayesian network meta-analysis[J]. BioMed Pharmacother (2018) 97:293–9. doi: 10.1016/j.biopha.2017.10.078
40. Niwa Y, Kakinoki M, Sawada T, Wang X, Ohji M. Ranibizumab and aflibercept: Intraocular pharmacokinetics and their effects on aqueous VEGF level in vitrectomized and nonvitrectomized macaque eyes. Invest Ophthalmol Vis Sci (2015) 56(11):6501–5. doi: 10.1167/iovs.15-17279
41. Furino C, Grassi MO, Bini V, Nacucchi A, Boscia F, Reibaldi M, et al. Intravitreal injections in arc sterile setting: Safety profile after more than 10,000 treatments. J Ophthalmol (2020) 2020:3680406. doi: 10.1155/2020/3680406
42. Reibaldi M, Avitabile T, Bandello F, Longo A, Bonfiglio V, Russo A, et al. The effectiveness of 0.6% povidone iodine eye drops in reducing the conjunctival bacterial load and needle contamination in patients undergoing anti-VEGF intravitreal injection: A prospective, randomized study. J Clin Med (2019) 8(7):1031. doi: 10.3390/jcm8071031
Keywords: diabetic macular edema, anti-vascular endothelial growth factor, laser photocoagulation, network meta-analysis, combined therapy
Citation: Chen J, Wang H and Qiu W (2023) Intravitreal anti-vascular endothelial growth factor, laser photocoagulation, or combined therapy for diabetic macular edema: A systematic review and network meta-analysis. Front. Endocrinol. 14:1096105. doi: 10.3389/fendo.2023.1096105
Received: 11 November 2022; Accepted: 24 January 2023;
Published: 02 February 2023.
Edited by:
Sobha Sivaprasad, NHS Foundation Trust, United KingdomReviewed by:
Yong Tang, Tianjin Eye Hospital, ChinaHailing Chen, Beijing Jishuitan Hospital, China
Xiaobin Xie, China Academy of Traditional Chinese Medicine Hospital of Ophthalmology, China
Payal Shah, Sankara Eye Hospital, India
Copyright © 2023 Chen, Wang and Qiu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiqiang Qiu, cXdxX2J5c3lAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Jiasheng Chen
Jiasheng Chen Haowei Wang
Haowei Wang Weiqiang Qiu
Weiqiang Qiu